Abstract
Background: Few low-incidence countries are on track to achieve the ambitious target of reaching TB pre-elimination by 2035. Australia is a high-income country with a low burden of TB, which is particularly concentrated in migrant populations. As part of Australia's migration program, permanent, provisional and humanitarian visa applicants are screened for TB, along with some applicants for temporary visas.
Methods: We calculated the prevalence of all forms of active TB and bacteriologically-confirmed TB among onshore and offshore applicants for visas to Australia from July 2014 to June 2017, and investigated associated risk factors using logistic regression.
Findings: Visa applicants were predominantly young adults from various Asian countries. Among 2,381,217 applicants, 1263 cases of active TB were diagnosed, including 852 cases of bacteriologically-confirmed TB. Overall TB prevalence was 53.0 per 100,000, corresponding to one TB diagnosis for every 1887 applicants screened. TB rates increased with age and were higher among humanitarian applicants and those previously treated for TB, although most cases occurred in applicants without these risk factors. TB prevalence by country of origin was similar to WHO estimates for some countries, but considerably lower for others. For several highly represented countries of origin, rates appear to have fallen relative to earlier comparable studies.
Interpretation: Prevalence of TB among visa applicants to Australia and the consequent risk to the Australian community appear to be declining and remain low. In this context, support for TB control programs overseas and preventive interventions are likely to have the greatest impact on domestic TB burden.
Funding: No specific funding was received for this study. JMT is a recipient of an Early Career Fellowship from the Australian National Health and Medical Research Council (APP1142638).
Research in context
Evidence before this study
The prevalence of TB in migrants to low-burden settings has been infrequently quantified using comprehensive programmatic data sets. A 2009 article describing screening for TB in migrants to the United States described the outcomes of radiological screening and provided less information on the prevalence of TB disease, which requires a holistic clinical assessment for diagnosis. A smaller analysis of offshore assessments for TB in migrants to the United Kingdom found a rate of active TB of 64 cases per 100,000 applicants. Reviews of such studies have found inconsistent effects of estimated TB burden in country of origin on the TB prevalence in migrants.
Added value of this study
We quantify the prevalence of TB in migrants to Australia, using a large and comprehensive data set, spanning both offshore and onshore applications. The number of migrants needed to screen to diagnose a case of TB was 1887 overall, but was higher in some sub-groups with specific risk factors.
Implications of all the available evidence
The rate of TB in migrants appears to be declining over time, consistent with the decreasing estimated global TB incidence. The value of screening for active TB in migrants is falling and programmatic interventions to treat and prevent TB in countries of origin is likely to be a more effective strategy for addressing TB burden in high-income countries.
Introduction
Tuberculosis (TB) has become established as the world's leading killer due to a single infectious pathogen, although rates of disease vary dramatically between countries, with migration a key driver of disease in low-burden settings [1]. The World Health Organization's (WHO) End TB Strategy targets a reduction in TB incidence to below ten cases per 100,000 population per year by 2035, [2] which will necessitate bold programmatic action primarily in high-burden countries. In low-burden countries that have already reached this threshold, an action framework has also been proposed to progress rapidly towards pre-elimination (one case per 100,000 population per year) and elimination (one case per million population per year), [3] with the WHO calling for elimination in low-burden countries to be achieved by 2050 [4]. Addressing special needs of migrants is a priority action area for low-burden countries and incorporates systematic screening for active TB in most such countries [5], [6], [7]. Where screening is undertaken as part of a country's migration program, evaluation of the yield and impact of the screening program is essential. Such activities can both support local capacity building and provide insights into TB epidemiology in high-burden source countries [3,5,8].
As is typical of many high-income countries, [9] Australia has low rates of TB disease and achieved marked reductions in burden during the twentieth century. However since the 1980s, TB notification rates have plateaued at around five to seven cases per 100,000 population per year [10]. Even by comparison to most other low-burden countries, migrants now constitute a particularly high and increasing proportion of Australian TB notifications; [3] now reaching 89% of all cases in 2018 [10]. Over 60% of the global burden of TB occurs in the Asia-Pacific, [13] and this region is highly represented in Australian TB notifications. Hence Australia's TB epidemiology reflects current and historical patterns of immigration and TB burden in migrants’ countries of origin [11,12].
In 2014, the Australian Department of Immigration and Border Protection (now the Department of Home Affairs) replaced its electronic data management system, enabling comprehensive collation of medical data on all new applicants undertaking medical examination as part of their Australian visa application. We analysed these data with the aim to understand characteristics of and associations with all forms of TB among migrants to Australia.
Methods
Australian pre-migration health screening and study population
All applicants for permanent, provisional and humanitarian visas to Australia, as well as some applicants for temporary visas, are required to undertake a health evaluation, termed an Immigration Medical Examination (IME) (Supplementary Material, Full Methods). Onshore (within Australia) and offshore (outside of Australia) applications follow analogous procedures, with applicants residing offshore at the time of application typically undertaking their IME prior to departure. The requirement for an IME for temporary migrants is determined by factors that include intended length of stay, TB prevalence in country of citizenship, TB prevalence in country or countries of residence and other special significance criteria [14]. Physical examination and chest X-ray are the most prominent components of the assessment for active TB. The testing matrix that determines whether an IME is required and the tests required within it are dependent on algorithms that changed from November 20, 2015. Details of IME requirements, including the changes made from November 2015 are presented in Supplementary Figures S1, S2 and S3.
All IMEs are performed by “panel physicians”, whose role is to act as an independent examiner who provides the Department with an objective assessment of their findings. Panel physicians must advise the applicant of any abnormal findings and may also provide referrals as appropriate. In the case of TB only, panel physicians may also provide treatment, which must be completed before the visa is granted.
Under Australia's Migration Act 1958, active TB is the only specifically mentioned condition that would preclude an applicant from being granted a visa. Approved physicians in source countries are directed to consider clinical, radiological and microbiological features in assessing for active TB, along with results of testing for latent tuberculosis infection (LTBI) where available [14]. For applicants with abnormal findings, an Australian Medical Officer of the Commonwealth will assess the information submitted to determine whether the applicant meets Australia's immigration health requirements and record specific diagnoses identified at the IME. Applicants diagnosed with TB must complete appropriate treatment and be “free from active TB” before the application can proceed to further consideration of a visa grant, typically within the same application episode.
Data available for this analysis comprised non-identifiable records for all permanent and humanitarian visa applicants and temporary visa applicants with an intended duration of stay in Australia of at least six months, who completed an IME from July 1, 2014 to June 30, 2017, provided they met the health requirement or were granted a health waiver.[15] This dataset includes nearly all IMEs undertaken by applicants to Australia during the study period, as described in the Supplementary Material.
Data management
Data were extracted from the Department's database by Department staff and provided to the Burnet Institute as de-identified line-listed data by application episode. Data were reviewed internally by the Department's Secrecy and Disclosure and Privacy Departments and Deputy Chief Statistician. From separate datasets pertaining to specific aspects of the pre-migration health screening process, we developed a master database by linking each dataset through a unique identification code. Data were stored on servers of the Burnet Institute and data management complied with relevant legislation and privacy principles.
Occasional inconsistencies in the data provided were revised in consultation with Departmental staff as described in Supplementary Table S2. From four partially complete fields relating to the applicant's country of birth, travel document and residence (Table S1), a single field was derived. Country of birth was used where available; otherwise, country of travel document was used if available and not Australia; otherwise, country of residence was used if available; otherwise, the variable was considered missing (and the record was excluded from relevant analyses, including regression analyses). This composite variable is intended to reflect the country in which each applicant would have spent the longest period of time to reflect the region of potential exposure to and infection with M. tuberculosis.
Measures
Available data fields included visa application characteristics, applicant characteristics, investigation results and medical diagnoses. Variables of relevance to the assessment and management of TB included: results of chest X-ray (CXR) screening, including degree of suspicion of TB; comorbidities such as diabetes, drug addiction and HIV; body mass index; intention to work in health care or childcare; onshore follow-up requirements; results of sputum smear microscopy, mycobacterial culture and drug susceptibility testing; site of TB; past treatment for TB; past exposure to TB; and results of testing for LTBI.
For the purposes of this analysis, active TB was defined by the recorded diagnosis of the assessing physician based on the available IME findings. Bacteriologically-confirmed TB was defined by the recording of any positive sputum smear microscopy or culture result.
Statistical analysis
Prevalence values and associated confidence intervals were calculated as proportions. For comparison of TB prevalence in our study population to WHO-estimated prevalence in country of origin in 2014, [16] only offshore applicants were analysed and rates were age-standardised to the domestic population distribution of each country by 5-year age brackets using data from the United Nations Demographic Statistics Database (Supplementary Materials) [17].
For our main analyses, we performed univariate and multivariate logistic regression to identify correlates of active TB and bacteriologically-confirmed TB. From the large number of explanatory variables available, variables that had previously been found to be associated with TB in migrant populations were selected for analysis. The “past treatment for TB” and “past contact with TB” variables were each not captured in 393,560 records. For regression, we assumed missing results implied absence of past treatment or contact, because these applicants had similar rates of TB as for those with negative entries and panel physician instructions only stipulate that these findings should be noted if positive [14]. These two variables were collinear, such that we present results including TB treatment history only below, and with both variables and an interaction term in the Supplementary Material. Country of origin was categorised into bands according to WHO-estimated prevalence in 2014, as this explained the greatest amount of variation in the response variable and was superior to categorising according to major source countries or region, or treating WHO-estimated prevalence as a continuous variable. Exploratory stepwise regression approaches did not identify further explanatory variables that markedly increased the amount of variation explained by the model.
To ensure consistency and comparability with the past literature, a second regression analysis was undertaken with methods and variables as equivalent as possible to those presented for a study of migrants to the United Kingdom (UK) from 2005 to 2013 [18].
Ethics approval
Ethical approval was provided by the Alfred Hospital Ethics Committee, project number 320/17.
Role of the Funding Source
No direct financial support was received for this project. JMT is a recipient of an Early Career Fellowship from the Australian National Health and Medical Research Council (APP1142638).
Results
Applicant characteristics
During the study period, 2,381,217 applicants successfully completed an IME and met all inclusion criteria (Table 1). Of these applicants, 51.2% were females, most were young to middle-aged adults (74.1% aged 15 to 44) and the rate of applications was approximately constant over the study period (Table 1). Around two-thirds (66.2%) of applications were undertaken offshore, with most applications being for temporary visas (69.0%). Most applicants were from countries with a substantial burden of TB, with around half of applicants originating from a country with a 2014 TB prevalence of 150 cases per 100,000 or higher. The six commonest countries of origin were all located in Asia.
Table 1.
Prevalence of active TB and bacteriologically-confirmed active TB in applicants for migration to Australia, 2014–2017.
| Variable | All applicants | Applicants with active TB |
Applicants with bacteriologically-confirmed active TB |
||
|---|---|---|---|---|---|
| no. (%) | no. (%) | no./100,000 persons (95% CI) | no. | no./100,000 persons (95% CI) | |
| Total | 2,381,217 (100.0) | 1263 (100.0) | 53.0 (50.2–56.1) | 852 (100.0) | 35.8 (33.5–38.3) |
| Sex* | |||||
| Female | 1,218,624 (51.2) | 657 (52.0) | 53.9 (50.0–58.2) | 429 (50.4) | 35.2 (32.0–38.7) |
| Male | 1,162,418 (48.8) | 606 (48.0) | 52.1 (48.1–56.5) | 423 (49.7) | 36.4 (33.1–40.0) |
| Age† | |||||
| 0–4 | 110,375 (4.6) | 8 (0.6) | 7.2 (3.6–14.5) | 0 | 0 |
| 5–14 | 123,673 (5.2) | 21 (1.7) | 17.0 (11.1–26.0) | <5 | <5 |
| 15–24 | 689,328 (29.0) | 303 (24.0) | 44.0 (39.3–49.2) | 211 (24.8) | 30.6 (26.8–35.0) |
| 25–44 | 1,073,981 (45.1) | 596 (47.2) | 55.5 (51.2–60.1) | 397 (46.6) | 37.0 (33.5–40.8) |
| 45–64 | 280,670 (11.8) | 208 (16.5) | 74.1 (64.7–84.9) | 153 (18.0) | 54.5 (46.5–63.9) |
| ≥65 | 103,169 (4.3) | 127 (10.1) | 123.1 (103.5–146.5) | 89 (10.5) | 86.3 (70.1–106.2) |
| Year | |||||
| 2014 | 374,771 (15.7) | 189 (15.0) | 50.4 (43.7–58.2) | 129 (15.1) | 34.4 (29.0–40.9) |
| 2015 | 763,196 (32.1) | 411 (32.5) | 53.9 (48.9–59.3) | 279 (32.8) | 36.6 (32.5–41.1) |
| 2016 | 828,771 (34.8) | 385 (30.5) | 46.5 (42.0–51.3) | 258 (30.3) | 31.1 (27.6–35.2) |
| 2017 | 414,479 (17.4) | 278 (20.0) | 67.1 (59.6–75.4) | 186 (21.8) | 44.9 (38.9–51.8) |
| Visa class | |||||
| Permanent | 668,117 (28.1) | 371 (29.4) | 55.5 (50.2–61.5) | 242 (28.4) | 36.2 (31.9–41.1) |
| Temporary | 1,642,516 (69.0) | 763 (60.4) | 46.5 (43.3–49.9) | 520 (61.0) | 31.7 (29.1–34.5) |
| Humanitarian | 70,584 (3.0) | 129 (10.2) | 182.8 (153.8–217.1) | 90 (10.6) | 127.5 (103.7–156.7) |
| Application location‡ | |||||
| Onshore | 803,071 (33.7) | 258 (20.4) | 32.1 (28.4–36.3) | 184 (21.6) | 22.9 (19.8–26.5) |
| Offshore | 1,577,053 (66.2) | 1002 (79.3) | 63.5 (59.7–67.6) | 667 (78.3) | 42.3 (39.2–45.6) |
| Past treatment for TB | |||||
| Yes | 9622 (0.4) | 206 (16.3) | 2140.9 (1870.0–2450.1) | 131 (15.4) | 1361.5 (1148.3–1613.5) |
| No | 1,978,035 (83.1) | 823 (65.2) | 41.6 (38.9–44.6) | 557 (65.4) | 28.2 (25.9–30.6) |
| Not recorded | 393,560 (16.5) | 234 (18.5) | 59.5 (52.3–67.6) | 164 (19.3) | 41.7 (35.8–48.6) |
| Reported past contact with TB | |||||
| Yes | 7451 (0.3) | 42 (3.3) | 563.7 (416.8–761.9) | 27 (3.2) | 362.4 (248.6–527.9) |
| No | 1,980,206 (83.2) | 987 (78.2) | 49.8 (46.8–53.1) | 661 (77.6) | 33.4 (30.9–36.0) |
| Not recorded | 393,560 (16.5) | 234 (18.5) | 59.5 (52.3–67.7) | 164 (19.3) | 41.7 (35.8–48.6) |
| WHO-estimated TB prevalence in country of origin in 2014 (per 100,000)§ | |||||
| 0–39 | 337,973 (14.2) | 23 (1.8) | 6.8 (4.5–10.2) | 13 (1.6) | 3.9 (2.2–6.6) |
| 40–149 | 846,260 (35.5) | 212 (16.8) | 25.1 (21.9–28.7) | 135 (16.4) | 16.0 (13.5–18.9) |
| 150–349 | 818,555 (34.4) | 552 (43.7) | 67.4 (62.0–73.3) | 437 (53.1) | 53.4 (48.6–58.6) |
| ≥350 | 222,804 (9.4) | 442 (35.0) | 198.8 (180.7–217.7) | 238 (28.9) | 106.8 (94.1–121.3) |
| Country of origin¶ | |||||
| India | 475,792 (20.0) | 174 (13.8) | 36.6 (31.5–42.4) | 116 (13.6) | 24.4 (20.3–29.3) |
| China | 466,401 (19.6) | 123 (9.7) | 26.4 (22.1–31.5) | 77 (9.0) | 16.5 (13.2–20.6) |
| South Korea | 127,775 (5.4) | 26 (2.1) | 20.4 (13.9–29.9) | 19 (2.2) | 14.9 (9.5–23.3) |
| The Philippines | 99,036 (4.1) | 300 (23.8) | 302.9 (270.6–339.2) | 170 (20.0) | 171.7 (147.7–199.5) |
| Taiwan | 93,217 (3.9) | <5 | <5 | <5 | <5 |
| Nepal | 85,700 (3.6) | 84 (6.7) | 98.0 (79.2–121.4) | 74 (8.7) | 86.4 (68.8–108.4) |
| United Kingdom | 75,724 (3.2) | 6 | 7.9 (3.6-17.6) | <5 | <5 |
| Vietnam | 72,001 (3.0) | 137 (10.9) | 190.3 (161.0–224.9) | 128 (15.0) | 177.8 (150.0–211.4) |
| Other | 885,571 (37.2) | 409 (32.4) | 46.2 (41.9–50.9) | 263 (37.2) | 29.7 (26.3–33.5) |
171 unknown, 4 indeterminate.
21 implausible values.
1093 unknown, including three cases of active TB.
32,397 country of origin could not be derived and a further 123,228 could not be mapped to a country from Global TB Report.
Top eight countries by number of applicants. Cells with fewer than five records are presented as “<5″ for privacy reasons.
Screening
Of all applicants, 2,103,259 (91.7%) received a CXR as part of their assessment, including 99.6% of all adults aged 15 and over, reflecting high levels of compliance with screening requirements (Online Data Supplement, Full Methods). Of permanent and humanitarian applicants aged 15 to 69 who met the criteria to complete a CXR throughout the study period, 99.8% of applicants completed this examination, with the lowest completion rates observed in European countries with a low TB burden from which small numbers of applications were received.
Prevalence of active TB
In total, 1263 cases of active TB were diagnosed, for a rate of 53.0 cases per 100,000 applicants (95% CI 50.2–56.1, Table 1), or 1887 applicants needed to be screened per TB case diagnosed. The greatest number of active TB diagnoses was seen in the 25 to 44 year age group, which constituted nearly half of all diagnoses, but prevalence rates increased steadily with increasing age. Although only 16.3% of all cases of active TB occurred in applicants who reported past treatment for TB, the prevalence of TB was considerably higher in this group (2140.9 per 100,000). The proportion of active TB cases with bacteriological confirmation in this group (63.6%) was similar to those without a past history of treatment (67.7%). Similarly, although humanitarian entrants constituted only 10.2% of all diagnosed TB, prevalence of TB was also considerably higher in this population (182.8 per 100,000).
Of the 1263 TB cases, 1190 (94.2%) had pulmonary involvement. Evidence of TB was noted on 39,113 CXRs, of which 1040 applicants were ultimately diagnosed with TB (2.7%), while 2985 CXRs were assessed as strongly suspicious for TB, of which 502 were diagnosed with TB (16.8%).
Bacteriological confirmation was achieved in 852 cases (67.5%), giving a rate of 35.8 (95% CI 33.5–38.3) cases per 100,000 for bacteriologically-confirmed TB. Of these, 215 cases (25.2%) were smear-positive and culture-positive, while 585 (68.7%) were smear-negative but culture-positive. The remaining 52 (6.1%) were smear-positive but culture-negative, although it was not possible to determine from the data available whether this reflected inability to access culture-based diagnostics, commencement of anti-tuberculous treatment prior to specimen collection, laboratory error, or other factors. Of the 800 applicants with positive culture results, 736 (92.0%) had information recorded on the drug susceptibility profile of the Mycobacterium tuberculosis isolate, with 77 (10.5%) recording resistance to at least one anti-tuberculous agent. Of these resistant isolates, 10 were resistant to both rifampicin and isoniazid (multidrug-resistant TB, MDR-TB), and 20, 53, 8, 14 and 10 were resistant to rifampicin, isoniazid, ethambutol, pyrazinamide and any second-line agent respectively (including 10 that were resistant to rifampicin but not isoniazid, and 43 that were resistant to isoniazid but not rifampicin). The greatest number of isoniazid-monoresistant isolates were from applicants from Vietnam (14), the Philippines (9), and China (8), while the Philippines and China had three cases of MDR-TB each.
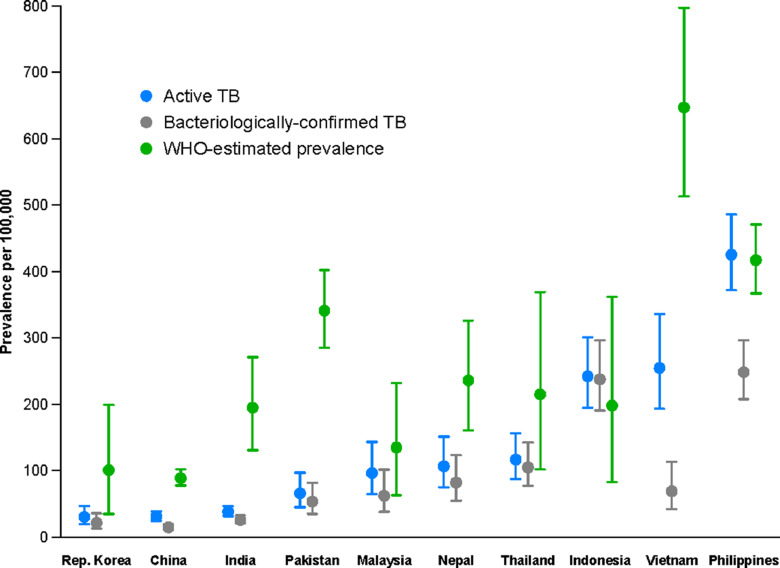
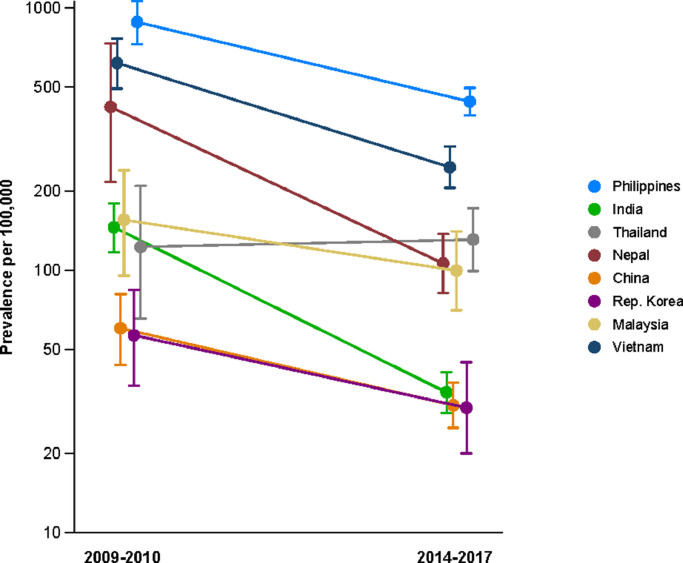
Partly reflecting application caseload (Table 1), the greatest absolute numbers of TB cases were observed in applicants from the Philippines (300), India (174), Vietnam (137), China (123), Nepal (84), and Indonesia (81). There appeared to be a steadily increasing gradient in TB prevalence with WHO-estimated prevalence of TB in 2014 by country of origin (Table 1). The age-standardised prevalence of active TB in offshore applicants closely matched the WHO-estimated prevalence in 2014 for several countries from which a significant number of TB cases were diagnosed, but was considerably lower in several others (China, India, Pakistan, Nepal and Vietnam, Fig. 1). Compared with an analysis of offshore applicants in 2009–2010 by manual review of records, rates of disease in offshore applicants from most countries of origin were lower (Fig. 2).
Fig. 1.
Comparison of the age-standardised prevalence of active TB and bacteriologically-confirmed TB in offshore applicants of the study cohort to the WHO-estimated prevalence of active TB in 2014, for the ten countries for which the greatest total number of cases of active TB were identified.
Fig. 2.
Comparison of prevalence of active TB from previous and current analyses in offshore applicants only, [20] logarithmic y-axis scale. Arbitrary horizontal jitter added to visually separate closely located points.
Regression analyses
The analysis of associations with active TB showed a similar profile to the analysis for bacteriologically-confirmed TB. Independent associations were observed for: increasing estimated TB prevalence in country of origin in 2014, increasing age, humanitarian visa application status, offshore application location and past treatment for TB (Table 2). We found a significant interaction between past treatment for and past contact with active TB, such that past contact conferred a particularly increased risk for those individuals who had not been previously treated.
Table 2.
Logistic regression analyses for outcomes of all forms of active TB and bacteriologically-confirmed TB.
| Active TB |
Bacteriologically-confirmed TB |
|||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Sex | ||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 0.97 (0.87–1.08) | 1.03 (0.92–1.16) | 1.03 (0.90–1.18) | 1.11 (0.96–1.26) |
| Age group | ||||
| 0–4 yr | 0.13 (0.06–0.26) | 0.10 (0.05–0.21) | N/A* | N/A* |
| 5–14 yr | 0.31 (0.20–0.47) | 0.17 (0.11–0.27) | 0.04 (0.01–0.18) | 0.01 (0.00–0.09) |
| 15–24 yr | 0.79 (0.69–0.91) | 0.93 (0.80–1.08) | 0.83 (0.70–0.98) | 0.97 (0.81–1.16) |
| 25–44 yr | 1.00 | 1.00 | 1.00 | 1.00 |
| 45–64 yr | 1.34 (1.14–1.56) | 1.08 (0.92–1.28) | 1.47 (1.22–1.78) | 1.21 (0.99–1.47) |
| ≥65 yr | 2.22 (1.83–2.69) | 1.66 (1.36–2.04) | 2.33 (1.86–2.94) | 1.80 (1.41–2.29) |
| Visa class | ||||
| Temporary | 1.00 | 1.00 | 1.00 | 1.00 |
| Permanent | 1.20 (1.06–1.35) | 1.44 (1.26–1.65) | 1.14 (0.98–1.33) | 1.47 (1.25–1.74) |
| Humanitarian | 3.94 (3.27–4.75) | 8.03 (6.52–9.90) | 4.03 (3.22–5.04) | 8.33 (6.49–10.7) |
| Location | ||||
| Offshore | 1.00 | 1.00 | 1.00 | 1.00 |
| Onshore | 0.51 (0.44–0.58) | 0.52 (0.45–0.60) | 0.54 (0.46–0.64) | 0.57 (0.48–0.68) |
| Past treatment for TB | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 49.1 (42.2–57.0) | 25.7 (21.9–30.2) | 45.4 (37.6–57.4) | 24.4 (20.0–29.9) |
| WHO-reported TB prevalence in country of origin in 2014 (per 100,000) | ||||
| 0–39 | 1.00 | 1.00 | 1.00 | 1.00 |
| 40–149 | 3.68 (2.39–5.66) | 5.23 (3.37–8.12) | 4.15 (2.35–7.33) | 5.83 (3.27–10.4) |
| 150–349 | 9.92 (6.53–15.0) | 12.7 (8.34–19.5) | 13.9 (8.00–24.1) | 17.8 (10.2–31.1) |
| ≥350 | 29.2 (19.2–44.4) | 36.2 (23.6–55.6) | 27.8 (15.9–48.6) | 35.0 (19.8–61.8) |
No cases of bacteriologically-confirmed TB were diagnosed in this age group.
We observed increased risk among applicants from high-incidence countries, contrasting with the opposite finding from the earlier study of UK migrants (Table 3).
Table 3.
Poisson regression analysis for bacteriologically-confirmed TB, with comparison to previous study of migrants to the UK 2005 to 2013.
| Current study | Previous study of UK migrants [17] | |
|---|---|---|
| Multivariate IRR | Multivariate odds ratio | |
| Age | ||
| 0–14/15 yr* | 0.00 (0.00–0.00) | 0.3 (0.2–0.7) |
| 15/16–44 yr† | 1.00 | 1.00 |
| 45–64 yr | 1.44 (1.12–1.84) | 1.2 (0.7–2.0) |
| ≥65 yr | 2.29 (1.70–3.09) | 3.2 (1.6–6.3) |
| Gender | ||
| Female | 1.00 | 1.00 |
| Male | 1.25 (1.07–1.47) | 1.0 (0.8–1.3) |
| Close contact with TB | ||
| No | 1.00 | 1.00 |
| Yes | 5.01 (3.12–8.06) | 11.6 (7.0–19.3) |
| Visa category | ||
| Student | 1.00 | 1.00 |
| Family | 3.18 (2.49–4.05) | |
| Permanent subclass | N/A | |
| Humanitarian | 11.3 (8.54–15.0) | |
| Skilled | 0.87 (0.67–1.13) | |
| Visitor | 0.94 (0.71–1.26) | |
| Settlement and dependant | 1.3 (1.0–1.6) | |
| Work | 0.9 (0.5–1.6) | |
| Working holiday maker | 1.2 (0.5–2.8) | |
| Family reunion | 0.4 (0.1–1.7) | |
| Other | 0.9 (0.4–2.1) | |
| WHO-reported TB prevalence in country of origin (per 100,000)‡ | ||
| 40–149 | 0.35 (0.28–0.43) | 0.1 (0.1–0.3) |
| 150–349 | 1.00 | 1.00 |
| 350+ | 2.40 (2.01–2.88) | 0.3 (0.3–0.4) |
Age bracket is 0 to 14 in current study, but 0 to 15 in UK migrants. †Age bracket is 15 to 44 in current study, but 16 to 44 to UK migrants. ‡2014 prevalence estimates used for our analysis, 2010 prevalence estimates used in the UK analysis.
Discussion
We found a prevalence of active TB of 53 cases per 100,000 in a large population of applicants for visas to Australia. Rates increased steadily with age and were higher in offshore and humanitarian applicants, and those reporting past treatment for active TB. Prevalence of TB also increased with increasing disease burden in country of origin, closely matching official estimates in some countries but not others.
An earlier study of recipients of visas to the United States diagnosed around 1000 cases of “smear-negative TB” per 100,000 on the basis of a chest X-ray suggestive of active TB and three negative sputum smears [19]. By comparison, for every 100,000 applicants in our study population, 2013 applicants with negative sputum smears were reported to have evidence of TB and 150 were strongly suspicious for TB based on radiology alone. Our findings are likely to have greater relevance to migration programs seeking to identify symptomatic and infectious forms of TB, which is most accurately diagnosed based on a combination of clinical, radiological and microbiological findings. For comparison to earlier studies of TB prevalence in offshore applicants to Australia, rates for several countries of origin appear to have fallen considerably over recent years [20], [21], [22]. We found somewhat lower rates of active TB in offshore applicants (64 per 100,000) compared to those reported in a recent study of offshore applicants for long-term visas to the UK, in which 92 cases per 100,000 applicants diagnosed with active TB[18]. Comparison of our regression analysis to that from this previous study suggests that the strong effect of contact with active TB previously observed may be partly attributable to confounding by prior treatment for active TB. The differences in the effect of WHO-estimated TB prevalence by country of origin may be attributable to the marked differences in the distribution of migrants according to this variable between these two studies. In a recent study of pre-entry screening of refugees to the UK, prevalence of TB was 92 per 100,000 (95%CI 48–177), [23] which is considerably lower than our finding of 183 per 100,000 (95%CI 154–217).
A systematic review of pre-entry screening of migrants to low-incidence countries suggested an increase in the yield of screening for culture-positive TB with increasing TB prevalence in the country of origin [24]. However, when this review was updated to incorporate results from the British study introduced above, this effect was no longer observed [18]. Our results are more consistent with the original finding of TB burden in country of origin being an important factor. The markedly lower prevalence of disease in applicants from China, India, Pakistan, Nepal and Vietnam by comparison to the reported population-wide prevalence in these countries could reflect sampling bias, such that applicants for visas to Australia from these countries may be less representative of the wider community with regards TB exposure. Other possible explanations include inaccurate official estimates or issues with the screening process.
Although we describe an analysis of comprehensive, population-level data, the following minor considerations could introduce some bias. A small proportion of applications was unavailable for analysis because they did not meet health requirements, with the commonest conditions listed as associated being chronic, incurable conditions, not including TB. This consideration is only relevant with regards to generalisability to the entire application cohort and not to the cohort of migrants arriving to Australia. Whether applicants subsequently migrated is unknown and a very small proportion of records would pertain to non-migrating family members required to undertake an IME as part of their family member's application. Further, data were provided at the level of application rather than applicant and repeated applications from the same individual during the study period could not be identified. However, patients diagnosed with and treated for active TB would typically complete treatment and continue their application during a single application episode, such that our approach is very similar to past studies that included repeat screening episodes that occurred after more than 12 months [18]. As described in the Supplementary Material, data were revised for consistency in a very small number of cases, and it was necessary to derive a country of origin field from the fields available.
Although practices differ, Australia is among the majority of high-income countries that screen for TB in immigrants [7]. Screening of migrants to Australia explicitly focuses on protecting the existing resident population and health system of the recipient country from health threats [14]. Although such programs have some impact, [6] evidence from Australia and elsewhere indicates that onshore transmission to domestic populations is infrequent [25,26]. In this context, migrant screening should ideally be seen as an opportunity to assess and enhance each migrant's health for their own benefit [27,28] and to strengthen health systems in countries of origin [8,29]. Current Australian migration health policy for LTBI screening focuses on children aged two to ten years, with screening of older migrants only recommended for close contacts of TB patients. The importance of reactivation of overseas-acquired LTBI and the high quality of data available on those at risk of TB raises the prospect of scaling up post-migration interventions to reduce TB burden in Australia. Such interventions could include awareness raising, expanded access to preventive treatments and mitigation of the socio-economic disadvantages that are likely to contribute to TB reactivation risk in such populations [3,[30], [31], [32].
Our findings are consistent with low and declining rates of active TB in applicants for visas to Australia from a mix of predominantly Asian countries. By comparison to previous work, we report findings from a comprehensive assessment to diagnose TB that incorporated clinical, radiological and microbiological findings and are able to compare onshore and offshore applicants in a single dataset. Given the importance of migration to the burden of TB in Australia, these low and falling rates imply that screening for active TB at entry alone is unlikely to achieve elimination targets set for low-burden countries.
Declaration of Competing Interest
Prof Hellard reports grants from Gilead Sciences, Abbvie, and BMS, outside the submitted work; Ms Soares Caplice is Senior Director of the Immigration Health Policy and Assurance Branch, on behalf of the Health Services Division of the Department of Home Affairs. The remaining authors have nothing to disclose.
Acknowledgments
Acknowledgements
We acknowledge the support of the Australian Government Department of Home Affairs in assisting with data extraction and interpretation.
Author Contributions
BMW, ILL and DH managed and collated data. JMT performed analysis with advice from ESM. SM, SMG and MEH provided advice on interpretation. LVSC advised on departmental policy and practice. JMT drafted the manuscript, which was finalised following input from all authors.
Funding statement
No direct financial support was received for this project. The corresponding author and DH are a recipients of Early Career Fellowships from the Australian National Health and Medical Research Council, APP1142638 and APP1092077 respectively.
Data sharing statement
Due to the sensitivity of this large data set, under the terms of our agreement with the Australian Department of Home Affairs, the individual-level data cannot be publicly released.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100135.
Appendix. Supplementary materials
References
- 1.World Health Organization . World Health Organization; 2019. Global tuberculosis report 2019. [Google Scholar]
- 2.Uplekar M., Weil D., Lonnroth K. WHO's new End TB Strategy. Lancet. 2015;385:1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 3.Lönnroth K., Migliori G.B., Abubakar I. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45 doi: 10.1183/09031936.00214014. ERJ-02140-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO targets elimination of TB in over 30 countries. https://www.who.int/mediacentre/news/releases/2014/tb-elimination/en/ (Accessed 2021).
- 5.Kunst H., Burman M., Arnesen T.M. Tuberculosis and latent tuberculous infection screening of migrants in Europe: comparative analysis of policies, surveillance systems and results. Int J Tuberc Lung Dis. 2017;21:840–851. doi: 10.5588/ijtld.17.0036. [DOI] [PubMed] [Google Scholar]
- 6.Zenner D., Hafezi H., Potter J., Capone S., Matteelli A. Effectiveness and cost-effectiveness of screening migrants for active tuberculosis and latent tuberculous infection. Int J Tuberc Lung Dis. 2017;21:965–976. doi: 10.5588/ijtld.16.0935. [DOI] [PubMed] [Google Scholar]
- 7.Pareek M., Baussano I., Abubakar I., Dye C., Lalvani A. Evaluation of immigrant tuberculosis screening in industrialized countries. Emerg Infect Dis. 2012;18:1422–1429. doi: 10.3201/eid1809.120128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas P., Posey D.L., Zenner D., Robson J., Abubakar I., Giovinazzo G. Capacity strengthening through pre-migration tuberculosis screening programmes: IRHWG experiences. Int J Tuberc Lung Dis. 2017;21:737–745. doi: 10.5588/ijtld.17.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lönnroth K., Mor Z., Erkens C. Tuberculosis in migrants in low-incidence countries: epidemiology and intervention entry points. Int J Tuberc Lung Dis. 2017;21:624–636. doi: 10.5588/ijtld.16.0845. [DOI] [PubMed] [Google Scholar]
- 10.Bright A., Denholm J.T., Coulter C., Waring J., Stapledon R. Tuberculosis notifications in Australia, 2015-2018. Commun Dis Intell. 2020;44 doi: 10.33321/cdi.2020.44.88. [DOI] [PubMed] [Google Scholar]
- 11.Lavender C.J., Globan M., Kelly H. Epidemiology and control of tuberculosis in Victoria, a low-burden state in south-eastern Australia, 2005–2010. Int J Tuberc Lung Dis. 2013;17:752–758. doi: 10.5588/ijtld.12.0791. [DOI] [PubMed] [Google Scholar]
- 12.NSW TB Program . Vol. 2019. Surveillance Report; 2017. Communicable Diseases Branch. (Tuberculosis in new south wales). [Google Scholar]
- 13.World Health Organization. World Heal Organ; 2020. Global Tuberculosis Report 2019. 1037//0033-2909.I26.1.78. [Google Scholar]
- 14.Panel Member Instructions. Australian Immigration Medical Instructions. July 2016. Issued by the Department of Immigration and Border Protection Immigration Health Branch, Australia.
- 15.Australian Government Department of Home Affairs Meeting our requirements. Health. 2019 https://immi.homeaffairs.gov.au/help-support/meeting-our-requirements/health accessed March 18, 2019. [Google Scholar]
- 16.World Health Organization. Global Tuberculosis Report 2015. 2015; 2015. http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1.
- 17.United Nations. UNdata, Population by age, sex and urban/rural residence. 2019. http://data.un.org/Data.aspx?d=POP&f=tableCode%3A22 (Accessed 8 March 2019).
- 18.Aldridge R.W., Zenner D., White P.J. Prevalence of and risk factors for active tuberculosis in migrants screened before entry to the UK: a population-based cross-sectional study. Lancet Infect Dis. 2016;16:962–970. doi: 10.1016/S1473-3099(16)00072-4. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Weinberg M.S., Ortega L.S., Painter J.A., Maloney S.A. Overseas screening for tuberculosis in U.S.-bound immigrants and refugees. N Engl J Med. 2009;360:2406–2415. doi: 10.1056/NEJMoa0809497. [DOI] [PubMed] [Google Scholar]
- 20.King K., Douglas P.J., Beath K. Is premigration health screening for tuberculosis worthwhile? Med J Aust. 2011;195:534–537. doi: 10.5694/mja11.11395. [DOI] [PubMed] [Google Scholar]
- 21.Plant A.J., Watkins R.E., Motus N. Results of tuberculosis screening in applicants for migration in Vietnam and Cambodia. Int J Tuberc Lung Dis. 2005;9:157–163. [PubMed] [Google Scholar]
- 22.Watkins R., Plant A., Sang D., Eltom M.A., Streeton M.J., Gushulak F.B. The association between subjective and clinical indicators of health in prospective Vietnamese migrants. Asia Pac J Public Heal. 2005;17:46–50. doi: 10.1177/101053950501700111. [DOI] [PubMed] [Google Scholar]
- 23.Crawshaw A.F., Pareek M., Were J. Infectious disease testing of UK-bound refugees: a population-based, cross-sectional study. BMC Med. 2018;16:143. doi: 10.1186/s12916-018-1125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldridge R.W., Yates T.A., Zenner D., White P.J., Abubakar I., Hayward A.C. Pre-entry screening programmes for tuberculosis in migrants to low-incidence countries: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:1240–1249. doi: 10.1016/S1473-3099(14)70966-1. [DOI] [PubMed] [Google Scholar]
- 25.Dahle U.R., Eldholm V., Winje B.A., Mannsåker T., Heldal E. Impact of Immigration on the Molecular Epidemiology of Mycobacterium tuberculosis in a Low-Incidence Country. Am J Respir Crit Care Med. 2007;176:930–935. doi: 10.1164/rccm.200702-187OC. [DOI] [PubMed] [Google Scholar]
- 26.Globan M., Lavender C., Leslie D. Molecular epidemiology of tuberculosis in Victoria, Australia, reveals low level of transmission. Int J Tuberc Lung Dis. 2016;20:652–658. doi: 10.5588/ijtld.15.0437. [DOI] [PubMed] [Google Scholar]
- 27.Abubakar I., Aldridge R.W., Devakumar D. The UCL–Lancet Commission on Migration and Health: the health of a world on the move. Lancet. 2018;392:2606–2654. doi: 10.1016/S0140-6736(18)32114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhavan P., Dias H.M., Creswell J., Weil D. An overview of tuberculosis and migration. Int J Tuberc Lung Dis. 2017;21:610–623. doi: 10.5588/ijtld.16.0917. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzman K., Oxlade O., Barr R.G. Domestic returns from investment in the control of tuberculosis in other countries. N Engl J Med. 2005;353:1008–1020. doi: 10.1056/NEJMsa043194. [DOI] [PubMed] [Google Scholar]
- 30.Shete P.B., Boccia D., Dhavan P. Defining a migrant-inclusive tuberculosis research agenda to end TB. Int J Tuberc Lung Dis. 2018;22:835–843. doi: 10.5588/ijtld.17.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seedat F., Hargreaves S., Nellums L.B., Ouyang J., Brown M., Friedland J.S. How effective are approaches to migrant screening for infectious diseases in Europe? A systematic review. Lancet Infect Dis. 2018;18:E259–E271. doi: 10.1016/S1473-3099(18)30117-8. [DOI] [PubMed] [Google Scholar]
- 32.McBryde E.S., Denholm J.T. Risk of active tuberculosis in immigrants: effects of age, region of origin and time since arrival in a low-exposure setting. Med J Aust. 2012;197:458–461. doi: 10.5694/mja12.10035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.