Abstract
The foundation of preventive cardiology begins with knowing the patient’s baseline cardiovascular disease (CVD) risk from which the patient-clinician risk discussion informs on the best ways to lower risk through lifestyle management, as well as a decision about the initiation and intensity of pharmacologic therapy. Global CVD risk assessment involves estimation of cardiovascular risk using a basic panel of risk factors. The Framingham Heart Study championed the first such risk scores, followed by others around the world. Most recently, the Pooled Cohort Equations (PCE) have been recommended in the United States as a starting point in CVD risk assessment. Persons at low (<5%) 10-year risk are generally recommended for lifestyle management only and those at highest (>20%) 10-year risk are recommended for both lifestyle and pharmacologic therapy to reduce risk. Assessing the presence of one or more “risk enhancing” factors is intended to inform the treatment decision in those at borderline (5-<7.5%) or intermediate (7.5–20%) risk, with the use of coronary calcium scores to further refine the treatment decision. Moreover, not all those with ASCVD are treated equal, and recent guidelines provide criteria for identifying those at very high risk. While current techniques best predict long-term risk of CVD events, biomarkers strategies are being developed to predict near-term events, and other imaging techniques such as coronary CT angiography and vascular MRI hold promise to identify vulnerable plaque. Validation and incorporating into clinical practice such state of the art techniques will be vital to moving CVD risk assessment to the next level.
Keywords: Global risk assessment, Cardiovascular disease, Risk factors, Biomarkers, Coronary calcium, CT angiography
The Framingham Heart Study which began 1948 is the longest running study of cardiovascular disease (CVD) in the world, and the one that has taught us the most about the etiology of CVD. In 1961, with just two words “risk factors” the field of preventive cardiology was born when former Framingham Heart Study director Dr. William B. Kannel published the article “Factors of Risk in the Development of Coronary Heart Disease: Six Year Follow-up Experience: The Framingham Study” [1]. This key study described how elevated cholesterol, elevated blood pressure, and left ventricular hypertrophy predicted the subsequent development of coronary heart disease (CHD) events. Perhaps just as important, the study also showed how the number of risk factors was directly related to the risk of development of CHD. These data, nearly 60 years ago, probably was the first demonstration of the concept we now know as “global risk”. The need and rationale for assessing cardiovascular risk was stated as early as 1976 by Dr. Kannel who indicated that risk functions provide an “economic and efficient method of identifying persons at high cardiovascular risk who need preventive treatment …” [2]. The American College of Cardiology Bethesda Conference two decades later noted the intensity of treatment should match a person’s risk [3]. Studies show a physician’s estimate is only accurate 24% of the time [4] and routine use of global risk scores leads to greater use of guideline-based therapy and modest improvements in intermediate outcomes with no harm identified [5].
This review covers the use and role of global risk estimation, followed by the role of biomarkers and other risk enhancing factors in CVD risk assessment, as well as the evidence and role of established and newer methods of subclinical atherosclerosis evaluation in the determination of CVD risk.
1. Original cardiovascular risk scores and incorporation into cardiovascular prevention guidelines
The Framingham Heart Study championed the development of the first risk scores, initially for the prediction of CHD events in 10 years [6] and involved a simple addition of points in separate scales for men and women corresponding to different levels of age, total and HDL-cholesterol, blood pressure, smoking and diabetes status from which the summed number of points corresponded to a 10-year risk estimation. This was important since the Third Adult Treatment Panel of the National Cholesterol Education Program in 2001 [7] was the first guideline to recommend use of these risk equations for stratification of persons into low (<10%), intermediate (10–20%), or high (>20% or with known CHD or other CHD risk equivalents) 10-year risk of CHD for the purposes of identifying treatment initiation and target levels of LDL-cholesterol. Other Framingham Heart Study scores, including those for individual cardiovascular events such as stroke or heart failure, as well as for total CVD, reflecting the myriad of both fatal and non-fatal cardiovascular sequelae, have also been published, including those with and without the use of laboratory measures [8]. It is crucial for the user to understand risk scores can differ by endpoint predicted – e.g., hard versus all CHD or CVD mortality, 10-year vs. 30-year or lifetime prediction and whether they are designed for prediction of primary (most are) or secondary events. Moreover, certain algorithms utilize equations incorporating the actual measured value of one or more risk factors as compared to assigning points based on what category of each factor the patient fits into.
With the realization that Framingham risk scores were developed based on a primary Caucasian middle-aged cohort in a small town outside of Boston, Massachusetts and may not be generalizable to more diverse patient populations, and in particular to other parts of the world, other risk scores have been developed globally over the past few decades. Most notably, the European Risk Scores are created for both high and low risk countries in Europe, and in fact have also been recently calibrated for use in most individual countries in Europe [9]. This risk score has served as the foundation for risk estimation in the European Society of Cardiology Cardiovascular Disease Prevention guidelines [10], providing cutpoints for defining low to very high risk categories for which depending on levels of blood pressure or LDL-C, certain treatment approaches and/or targets are recommended. The European SCORE differs from Framingham and other US-based scores in that it focuses on the prediction of CVD mortality only, thus does not incorporate estimation of non-fatal events. Moreover, it treats all those with diabetes as a high-risk equivalent and thus diabetes is not one of the factors in the algorithm. More recently, in an effort to unify a single scoring system that can be used globally, the Globorisk group [11] has developed a large set of country specific risk scores using a very limited set of risk factors.
2. Current recommended US global risk scoring
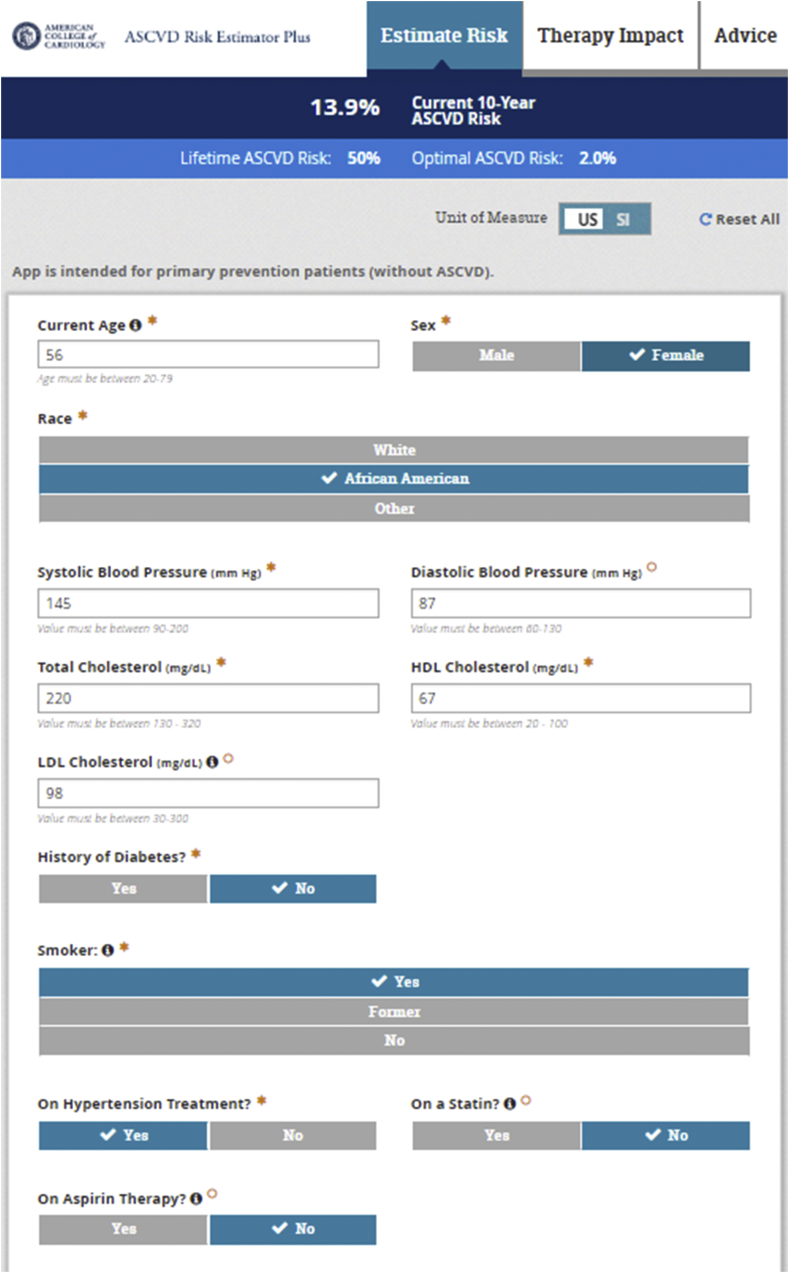
The most recent ACC/AHA Primary Prevention Guidelines [12] as well as Multisociety Cholesterol Management Guidelines [13] recommend the Pooled Cohort Risk Estimator Plus (tools.acc.org/ascvd-risk-estimator-plus) (Fig. 1) for initial CVD risk assessment in persons without known CVD or familial hypercholesterolemia (e.g., those with LDL-C ≥190 mg/dl who are suspected to have familial hypercholesterolemia with a lifetime burden of elevated LDL-C; these persons are assumed to be at high or very high risk due to their lifetime of significantly elevated LDL-C). The Pooled Cohort Risk Estimator Plus was developed from four major US cohorts consisting of more than 30,000 individuals with at least 10-years of follow-up for CVD events; it specifically predicts 10-year (for those aged 40–79 years) and lifetime (for those 20–59 years of age) risk of atherosclerotic cardiovascular disease (ASCVD) consisting of fatal and nonfatal coronary heart disease and stroke only. Like Framingham, it relies on a limited set of risk factors, namely, age, sex, systolic blood pressure, antihypertensive treatment, total and HDL-cholesterol, diabetes, and cigarette smoking; however, also has an input for ethnicity and because of the large number of African-Americans in the combined derivation cohort, providing reasonable estimates for this ethnic group. 10-year risk of future ASCVD is calculated and categorized into those at low (<5%), borderline (5-<7.5%), intermediate (7.5–20%), and high (>20%) risk. Importantly, the Risk Estimator Plus also provides inputs to examine the effect on risk of setting a different blood pressure or cholesterol or other risk factor level, so can be an invaluable tool in teaching patients what the impact of a certain change in a given risk factors might be on their risk. The clinician-patient discussion to utilize the tool to discuss the best ways to lower CVD risk is emphasized in the guidelines.
Fig. 1.
US pooled cohort risk estimator plus.
3. Identification and use of risk enhancing factors
From the above calculation of 10-year ASCVD risk, the latest US guidelines [12,13] recommend the use of lifestyle management alone for those at low <5% risk and a combination of both lifestyle and concurrent pharmacotherapy (e.g., statin medication) for those at high >20% risk (Fig. 2). For those at borderline (5-<7.5%) or intermediate (7.5–20%) 10-year risk, it is recommended to consider the presence of one or more risk enhancing factors (Table 1) for informing the treatment decision. All these factors (e.g., presence of metabolic syndrome, chronic kidney disease, premature family history, other lipids including triglycerides) should be known and available at the time of risk assessment. While there is no specific guidance given to the number or severity of such factors that should result in a definite treatment recommendation (e.g., initiation or intensification of statin therapy), this is left up to clinical judgement and must importantly be part of the clinician-patient risk discussion. For example, a premature family history encompassing several first degree relatives with an early CVD event would weigh much more heavily than a single relative with such a history (this is also a reminder to do a complete family history of all first degree relatives and not to only define premature family history as a simple yes or no). Besides primary hypercholesterolemia, for the first time those with chronic kidney disease, chronic inflammatory disorders including HIV, female specific factors such as pre-eclampsia or premature menopause, as well as high risk ethnicities such as South Asians are pointed out as risk enhancing factors to guide the treatment decision. Other factors, if measured, can also inform the treatment decision – this includes an elevated hs-CRP (>2 mg/L) as well as low ankle brachial index (<0.9 indicative of peripheral artery disease) as been noted in prior guidelines, as well as elevated lipoprotein(a) (50 mg/dL or higher) and apolipoprotein B (≥130 mg/dL).
Fig. 2.
Risk Stratification Algorithm from the ACC/AHA Primary Prevention Guidelines (adapted from Arnett et al. [12]). ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium.
Table 1.
Risk enhancing factors for the clinician-patient discussion.
| Adapted from Arnett et a., 2019 [12]. |
|---|
|
4. Rationale and criteria for measuring subclinical atherosclerosis
Despite the value and continued role for global risk scoring as the first step in CVD risk assessment, it is well-established that many persons experiencing CVD events often have one or fewer traditional risk factors [14] and hence the accuracy of global risk assessment has been called into question, as many persons experiencing CVD events are actually at low or moderate calculated risk of such an event. While the consideration of most of the aforementioned risk enhancing factors is an improvement, many experts believe directly measuring atherosclerosis in its subclinical phase before the occurrence of a clinical event is the best way to improve the prediction of future CVD events. Important criteria for a new screening test for subclinical atherosclerosis includes 1) the test must detect the disease of interest with adequate sensitivity and specificity, 2) must be sufficiently reproducible, 3) detect persons where early intervention can be beneficial, and 4) must provide predictive value over office-based risk assessment [15].
5. Coronary calcium in risk assessment
Twenty-five years ago, we were involved with some of the early investigations examining the prognostic significance of coronary artery calcium (CAC) [16,17], including the Multiethnic Study of Atherosclerosis (MESA) which was the first to document the significance of CAC as a predictor of CHD in four major US ethnic groups [18]. In a more recent report, a CAC score of >100 was identified as being associated with a >7.5% 10-year risk in these same ethnic groups, indicating such levels of CAC to be “statin eligible” regardless of ethnicity [19]. CAC was also shown in MESA to improve risk prediction over standard risk factors and a number of other screening tests, including carotid ultrasound and brachial artery reactivity testing [20]. The ACC/AHA Cardiovascular Risk Assessment guideline in 2013 noted CAC to be the most promising of the subclinical disease modalities to improve CVD risk assessment [21].
The work of Nasir and colleagues [22] in 2015 laid the groundwork for how CAC testing was to be incorporated later into the cholesterol guidelines. They showed from examining CAC scores in those who were eligible for statin treatment in the MESA study that as long as the 10-year ASCVD risk based on the pooled cohort risk calculator was <20%, that a CAC score of 0 was associated with a projected 10-year risk that was actually below the 7.5% "statin eligibility" threshold documented in the 2013 cholesterol guidelines to be the cutpoint for which the net clinical benefit for considering statin therapy was positive [23]. Conversely, if the CAC score was ≥100, this was identified with a projected risk that was near or above the 7.5% threshold, even if the calculated ASCVD risk was 5-<7.5%. These data in part led the 2018 guidelines committee to consider as appropriate the “de-risking” of individuals in the 5–20% risk group range who had a 0 calcium score, withholding statin therapy (as long as a premature family history, diabetes, or cigarette smoking were not present), and those with CAC scores of 100 or higher as a definite indication for statin therapy. For those who were in the range of 1–100, however, within the 5–20% risk groups, it was noted that statin therapy may be considered. Ultimately, the clinician must consider not only the CAC score but the patient’s baseline risk and presence of other risk enhancing factors, and most importantly discussion with the patient as to the appropriateness of starting or intensifying therapy in any case. Further, there are no guidelines for repeating CAC screening for the purposes of monitoring the effects of any therapy. While the progression of CAC does predict future CHD event risk [24], the baseline CAC score is far more important in CVD risk assessment, thus its role in the guidelines as a risk stratifier.
Perhaps one of the greatest attributes of CAC screening is its ability to motivate improved lifestyle behaviors and use of preventive therapies. Nearly 25 years ago, we showed persons who received CAC scans and the greater the degree of CAC identified, the greater the initiation of cholesterol-lowering medication, healthy diet, smoking cessation, and even seeing the physician [25]. The EISNER study [26] subsequently documented those who were scanned vs. not scanned to have a halting in their progression of estimated 10-year CVD risk, as well as reduced healthcare costs.
6. Other imaging methods and risk assessment
While CAC screening receives the most attention in the US as the most valued tool for assessing CVD risk, other modalities can and have been shown to be useful. Investigations going back over 30 years have shown the value of carotid intimal medial thickness (CIMT) in assessing future cardiovascular risk [27,28]; however, a meta-analysis in 2012 showed its clinical utility to be limited due to a negligible improvement in the net reclassification index (NRI) on top of standard risk factors [29]. Thus, CIMT screening alone was not recommended in the 2013 nor in the most recent US cardiovascular risk assessment guidelines. However, the identification of plaque, and more recently the ability to distinguish and even quantitate different types of plaque (e.g., fatty, fibrotic) have led to the continued recommendation for CIMT screening along with plaque assessment at least in the European guidelines [10]. Its use specifically as a risk stratifier for consideration of treatment, however, was not addressed in the most recent US guidelines, although it would seem reasonable that the presence of increased CIMT accompanied by carotid plaque, in particular, should be considered a risk enhancer. We have shown in US adults a substantial age-related increase in the prevalence of mixed or soft plaque with over 60% of older men and women having such features [30].
Efforts involving the use of carotid and aortic magnetic resonance imaging [31,32], as well as CT angiography [33], and most recently the use of 3D vascular ultrasound [34] are also being investigated not only to assess vulnerable plaque risk, but also the effects of different medical therapies on affecting the progression of plaque. It will be of importance to know whether quantification of vulnerable plaque components beyond that of CAC further improves risk assessment. The role of CT angiography for assessing CVD event risk beyond that of CAC in asymptomatic persons has also been evaluated, although with mixed results [35].
7. CVD risk assessment in diabetes
While persons with diabetes were designed as CHD risk equivalents in past guidelines [7], a meta-analyses of prospective studies [36], as well as data on global risk assessment [37] and CVD event risk stratified by coronary calcium levels [38] show this not to be the case. While there is no US-based pooled cohort diabetes risk score currently available, the latest guidelines recommend the use of the PCE which utilizes diabetes as binary factor and does not include diabetes-specific factors such as duration of diabetes or glycated hemoglobin. The PCE is recommended to stratify ASCVD risk in diabetes, where a high intensity statin is recommended for those with multiple risk factors or >20% 10-year risk, with ezetimibe considered in the latter case to ensure a 50% reduction in LDL-C [13]. For persons aged 20–39 years of age with diabetes, since the PCE cannot be used in such individuals, certain risk enhancing factors such as a long duration of diabetes (10 years with Type 2 diabetes or 20 years with Type 1 diabetes), albuminuria, or microalbuminuria are used to inform the treatment decision.
8. Considerations for risk assessment in persons with established ASCVD
While prior guidelines have lumped all those with ASCVD into one group often with a single LDL-C goal or treatment strategy (e.g., high intensity statin), this has undergoing significantly revision recently as we understand not all persons with ASCVD are equal. The recent 2018 Cholesterol Management Guideline identifies as “very high risk” those who have two or more major ASCVD event or one major event and two or more high risk conditions (Table 2) [13]. Those with ASCVD who otherwise do not fit one of these criteria are deemed to be “not at very high risk”. Recently published data provide a rationale for this distinction where those defined to be at very high risk have a 3-fold or greater risk of subsequent events as compared those not at very high risk [39]. Those defined to be at very high risk also have been shown to be among those who benefitted more (greater absolute risk reduction) from PCSK9 therapy [40]. While the recent 2019 European Society of Cardiology Dyslipidemia Guidelines [10] take a different approach, noting all those with ASCVD to be at very high risk, they do distinguish those who have had a recurrent ASCVD event within the past two years as being a more extreme risk (although not specifically designating as such) where an even lower LDL-C target of <40 mg/dL (compared to <55 mg/dL for all others with ASCVD) is specified.
Table 2.
Criteria for Very High Risk Status (Adapted from Grundy et al., 2019) [13].
| Major ASCVD Events |
|---|
| - Recent ACS (within the past 12 mo) |
| - History of MI (other than recent ACS event listed above) |
| - History of ischemic stroke |
| - Symptomatic peripheral arterial disease |
|
High-Risk Conditions |
| - Age ≥65 y |
| - Heterozygous familial hypercholesterolemia |
| - History of prior coronary artery bypass surgery or percutaneous coronary intervention outside of the major ASCVD event(s) |
| - Diabetes mellitus |
| - Hypertension |
| - CKD (eGFR 15–59 mL/min/1.73 m2) |
| - Current smoking |
| - Persistently elevated LDL-C (LDL-C ≥100 mg/dL [≥2.6 mmol/L]) despite maximally tolerated statin therapy and ezetimibe |
| - History of congestive HF |
Very high-risk status is defined as two or more major ASCVD events or one major ASCVD event and multiple high risk conditions.
9. Multiple biomarker approaches to CVD risk assessment
Since the advent of high sensitivity C-reactive protein (hs-CRP) was identified to be a stronger risk factor than most traditional risk factors and even the total cholesterol/HDL-C ratio nearly twenty years ago [41], there has been great interest in not only identifying single biomarkers, but biomarkers of multiple mechanisms to improve CVD risk assessment [42,43]. A panel of biomarkers, namely, BNP, hs-CRP, urine/albumin-creatinine, homocysteine, and renin, while shown in Framingham to independently predict future CVD events, only modestly improved the c-statistic from 0.80 to 0.82 [42]. Efforts by other investigators also identified sets of biomarkers, namely BNP, troponin, and hs-CRP, which also only modestly improved the c-statistic [43]. More recent efforts, such as the develop of a multiple biomarker protein unstable lesion signature (PULS) test identifying factors related to acute coronary syndrome, clinical NRI was 40% in intermediate risk MESA subjects, suggesting it to be a promising test for identification of near-term acute coronary syndrome [44]. While no current guidelines recommend measuring a specific set of biomarkers, and with some being proprietary and not readily available, future investigation is needed to better assess their role in CVD risk assessment and in what specific populations of patients.
10. Use of current risk stratification in treatment guidelines
The concept of intensity of treatment matching level of risk as proposed nearly 25 years ago [3] remains relevant to current treatment guidelines. First, the 2017 ACC/AHA blood pressure guideline [45] now recommends use of the ASCVD risk estimator, where for those with Stage 1 hypertension (systolic blood pressure of 130–139 mmHg or diastolic blood pressure of 80–89 mmHg, while lifestyle management is recommended for all, if the 10-year ASCVD risk is ≥ 10%, concurrent pharmacologic treatment is recommended. Also, in the case of antiplatelet therapy, recent primary prevention guidelines [12] have given a modest recommendation for the use of low dose aspirin in those who are at higher risk (e.g., multiple risk factors or ≥10% 10-year ASCVD risk), while the net clinical benefit considering the risk of bleeding still remains a priority. Moreover, the 2018 Multisociety Cholesterol Management guideline [13] recommends lifestyle management only if the 10-year ASCVD risk is <5% and concurrent pharmacologic treatment (e.g., statin to reduce LDL-C by at least 50%) if the ASCVD risk is >20%, with the consideration of risk enhancing factors (and coronary calcium scores if the decision is still uncertain) for those with a calculated risk of 5–20%. For secondary prevention, if despite maximally tolerated statin therapy, the patient is still above a “threshold” LDL-C level of 70 mg/dL or higher, ezetimibe should be added, and if the patient is at very high risk (as previously defined), a PCSK9 inhibitor may also be added. The term threshold is used here to refer to a point where if one is at or above it, additional therapy would be warranted. This can be distinguished from a goal which is a broader objective, such as to lower LDL-C, or a target which is more specific and usually has a numerical value, such as LDL-C<70 mg/dL.
11. Conclusions
An understanding of the patient’s risk for CVD is the foundation of preventive cardiology from which the intensity of treatment is based on the identified risk. Global risk scoring is the starting point in CVD risk assessment, resulting in the calculation of 10-year risk from a set of standard office-based risk factors from which a clinician-patient risk discussion is used to discuss the best ways to reduce CVD risk. The presence of one or more risk enhancing factors further informs the treatment decision. The presence and extent of subclinical atherosclerosis from CAC in particular, as well as evidence from other imaging modalities, can be used to further decide on the therapeutic approach to take. The identification of atherosclerosis from CAC, in particular can also be a potent motivator for improved lifestyle and/or adherence to preventive therapies. Finally, the identification of newer imaging modalities and biomarker approaches for identifying the vulnerable plaque and near-term CVD event risk is an active area of investigation, but needs further validation before they can be considered in guidelines.
Author disclosures
Dr. Nathan Wong notes research support form Amgen, Amarin, Novartis, Boehringer Ingelheim, and Novo Nordisk, as well as speaker bureau/advisory board participation from Amarin, Esperion, and Sanofi, all not related to the topic of this manuscript. No funding was received for the preparation of this article.
References
- 1.Kannel W.B., Dawber T.R., Kagan A., Revotskie N., Stokes J., 3rd Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 2.Kannel W.B., McGee D., Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38(1):46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 3.Califf R.M., Armstrong P.W., Carver J.R., D’Agostino R.B., Strauss W.E. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 5. Stratification of patients into high, medium and low risk subgroups for purposes of risk factor management. J Am Coll Cardiol. 1996;27(5):1007–1019. doi: 10.1016/0735-1097(96)87733-3. [DOI] [PubMed] [Google Scholar]
- 4.Pignone M., Phillips C.J., Elasy T.A., Fernandez A. Physicians’ ability to predict the risk of coronary heart disease. BMC Health Serv Res. 2003;3(1):13. doi: 10.1186/1472-6963-3-13. Published 2003 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheridan S.L., Crespo E. Does the routine use of global coronary heart disease risk scores translate into clinical benefits or harms? A systematic review of the literature. BMC Health Serv Res. 2008;8:60. doi: 10.1186/1472-6963-8-60. Published 2008 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson K.M., Wilson P.W., Odell P.M., Kannel W.B. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83(1):356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 7.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the Third report of the national cholesterol education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) J Am Med Assoc. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.D’Agostino RB Sr, Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 9.Conroy R.M., Pyörälä K., Fitzgerald A.P. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 10.Mach F., Baigent C., Catapano A.L. ESC scientific document group, 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and European atherosclerosis society (EAS) Eur Heart J. 1 January 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 11.Mayor S. New scoring system predicts cardiovascular disease risk worldwide. BMJ. 2015;350 doi: 10.1136/bmj.h1670. h1670. Published 2015 Mar 26. [DOI] [PubMed] [Google Scholar]
- 12.Arnett D.K., Blumenthal R.S., Albert M.A. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. 2019 Sep 10;140(11):e649-e650] [published correction appears in Circulation. 2020 Jan 28;141(4):e60] Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of cardiology/American heart association task force on clinical practice guidelines [published correction appears in J Am coll cardiol. 2019 jun 25;73(24):3237-3241] J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Khot U.N., Khot M.B., Bajzer C.T. Prevalence of conventional risk factors in patients with coronary heart disease. J Am Med Assoc. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 15.Redberg R.F., Vogel R.A., Criqui M.H., Herrington D.M., Lima J.A., Roman M.J. 34th Bethesda Conference: task force #3--What is the spectrum of current and emerging techniques for the noninvasive measurement of atherosclerosis? J Am Coll Cardiol. 2003;41(11):1886–1898. doi: 10.1016/s0735-1097(03)00360-7. [DOI] [PubMed] [Google Scholar]
- 16.Detrano R.C., Wong N.D., Tang W. Prognostic significance of cardiac cinefluoroscopy for coronary calcific deposits in asymptomatic high risk subjects. J Am Coll Cardiol. 1994;24(2):354–358. doi: 10.1016/0735-1097(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 17.Wong N.D., Hsu J.C., Detrano R.C., Diamond G., Eisenberg H., Gardin J.M. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86(5):495–498. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 18.Detrano R., Guerci A.D., Carr J.J. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 19.Budoff M.J., Young R., Burke G. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA) Eur Heart J. 2018;39(25):2401–2408. doi: 10.1093/eurheartj/ehy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeboah J., McClelland R.L., Polonsky T.S. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. J Am Med Assoc. 2012;308(8):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of cardiology/American heart association task force on practice guidelines [published correction appears in circulation. 2014 jun 24;129(25 suppl 2):S74-5] Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 22.Nasir K., Bittencourt M.S., Blaha M.J. Implications of coronary artery calcium testing among statin candidates according to American College of cardiology/American heart association cholesterol management guidelines: MESA (Multi-Ethnic study of atherosclerosis) [published correction appears in J Am coll cardiol. Dec 15;66(23):2686. Miemdema, michael D [corrected to miedema, michael D]] J Am Coll Cardiol. 2015;66(15):1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 23.Stone N.J., Robinson J.G., Lichtenstein A.H. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):3024-3025] [published correction appears in J Am Coll Cardiol. 2015 Dec 22;66(24):2812] J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Budoff M.J., Young R., Lopez V.A. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61(12):1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong N.D., Detrano R.C., Diamond G. Does coronary artery screening by electron beam computed tomography motivate potentially beneficial lifestyle behaviors? Am J Cardiol. 1996;78(11):1220–1223. doi: 10.1016/s0002-9149(96)00599-1. [DOI] [PubMed] [Google Scholar]
- 26.Rozanski A., Gransar H., Shaw L.J. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57(15):1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambless L.E., Heiss G., Folsom A.R. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997 Sep 15;146(6):483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary D.H., Polak J.F., Kronmal R.A., Manolio T.A., Burke G.L., Wolfson S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999 Jan 7;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 29.Den Ruijter H.M., Peters S.A., Anderson T.J. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis [published correction appears in JAMA. 2013 Oct 23;310(16):1739] J Am Med Assoc. 2012;308(8):796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 30.Boulos N.M., Gardin J.M., Malik S., Postley J., Wong N.D. Carotid plaque characterization, stenosis, and intima-media thickness according to age and gender in a large registry cohort. Am J Cardiol. 2016 Apr 1;117(7):1185–1191. doi: 10.1016/j.amjcard.2015.12.062. Epub 2016 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J., Zhao X.Q., Balu N. Carotid magnetic resonance imaging for monitoring atherosclerotic plaque progression: a multicenter reproducibility study. Int J Cardiovasc Imag. 2015;31(1):95–103. doi: 10.1007/s10554-014-0532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J., Zhao X.Q., Balu N. Carotid plaque lipid content and fibrous cap status predict systemic CV outcomes: the MRI substudy in AIM-HIGH. JACC Cardiovasc Imaging. 2017;10(3):241–249. doi: 10.1016/j.jcmg.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahal S., Budoff M.J. Implications of serial coronary computed tomography angiography in the evaluation of coronary plaque progression. Curr Opin Lipidol. 2019;30(6):446–451. doi: 10.1097/MOL.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Melgar B., Fernandez-Friera L., Oliva B. Short-term progression of multiterritorial subclinical atherosclerosis: the PESA Study. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.02.026. (in press) [DOI] [PubMed] [Google Scholar]
- 35.Cho I., Chang H.J., Ó Hartaigh B. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the COronary CT Angiography EvaluatioN for Clinical Outcomes InteRnational Multicenter (CONFIRM) study [published correction appears in Eur Heart J. 2015 Dec 7;36(46):3287] Eur Heart J. 2015;36(8):501–508. doi: 10.1093/eurheartj/ehu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulugahapitiya U., Siyambalapitiya S., Sithole J., Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26(2):142–148. doi: 10.1111/j.1464-5491.2008.02640. [DOI] [PubMed] [Google Scholar]
- 37.Wong N.D., Glovaci D., Wong K. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res. 2012;9(2):146–152. doi: 10.1177/1479164112436403. [DOI] [PubMed] [Google Scholar]
- 38.Malik S., Budoff M.J., Katz R. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the multi-ethnic study of atherosclerosis. Diabetes Care. 2011;34(10):2285–2290. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colantonio L.D., Shannon E.D., Orroth K.K. Ischemic event rates in very-high-risk adults. J Am Coll Cardiol. 2019;74(20):2496–2507. doi: 10.1016/j.jacc.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 40.Vallejo-Vaz A.J., Ray K.K., Ginsberg H.N. Associations between lower levels of low-density lipoprotein cholesterol and cardiovascular events in very high-risk patients: pooled analysis of nine ODYSSEY trials of alirocumab versus control. Atherosclerosis. 2019;288:85–93. doi: 10.1016/j.atherosclerosis.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Ridker P.M., Hennekens C.H., Buring J.E., Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 42.Wang T.J., Gona P., Larson M.G. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 43.Blankenberg S., Zeller T., Saarela O. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121(22):2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 44.Cross D.S., McCarty C.A., Hytopoulos E. Coronary risk assessment among intermediate risk patients using a clinical and biomarker based algorithm developed and validated in two population cohorts. Curr Med Res Opin. 2012;28(11):1819–1830. doi: 10.1185/03007995.2012.742878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018 Jun;71(6):e140-e144] Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]