Abstract
Background
Nontuberculous mycobacteria (NTM) are opportunistically pathogenic bacteria that are found abundantly in the soil and water. Susceptible individuals exposed to NTM-containing aerosols from environmental sources may develop NTM pulmonary disease (NTM-PD). Reported survival after NTM-PD diagnosis varies widely among existing studies. Prior work has suggested that mortality among persons with NTM-PD is primarily driven by comorbidities rather than NTM-PD.
Methods
We retrospectively identified a cohort of patients in the Duke University Health System who were diagnosed with NTM-PD between 1996 and 2015. Hospitalizations and survival were compared among patients with NTM-PD with and without other comorbidities. Additionally, survival among patients with NTM-PD was compared with standardized mortality data for a similar cohort of the general population.
Results
Patients with NTM-PD without other comorbidities had 0.65 hospitalizations/1000 patient-days compared with 1.37 hospitalizations/1000 patient-days for patients with other comorbidities. Compared with a cohort of the general population, expected survival decreased by approximately 4 years for a diagnosis of NTM-PD without comorbidities and 8.6 years for a diagnosis of NTM-PD with comorbidities. Mortality 5 years after diagnosis was 25.0% and 44.9% among NTM patients without and with comorbidities, respectively, compared with 5.7% in the general-population cohort.
Conclusions
NTM-PD was associated with significant morbidity that was worse in patients with comorbidities. Patients with NTM-PD, even without comorbidities, had worse survival than expected.
Keywords: nontuberculous mycobacterium, nontuberculous mycobacterium pulmonary disease, survival
Nontuberculous mycobacterial pulmonary disease (NTM-PD) is a prevalent problem with a varying spectrum of illness. Reduction in expected survival associated with NTM-PD is still unclear. Our retrospective study has shown that survival can be reduced by up to 8.6 years.
Nontuberculous mycobacteria (NTM) are opportunistically pathogenic bacteria that are found abundantly in the soil and water [1]. Susceptible individuals exposed to NTM-containing aerosols from these environmental sources may develop NTM pulmonary disease (NTM-PD) [1, 2]. NTM-PD is an emerging problem worldwide, with rising prevalence and associated healthcare costs [3, 4].
NTM-PD has a broad spectrum of severity: some patients spontaneously clear infections with no therapeutic intervention [5] and other patients experience substantial morbidity and mortality [6]. Treatment often requires long courses of antimicrobials and is frequently complicated by adverse effects, resulting in early cessation/incomplete treatment [7]. Furthermore, even after long treatment courses, as many as 75% of patients experience recurrence of disease [8, 9]. While a recent review reported an average 5-year mortality after NTM-PD diagnosis of 27%, there was high variability (10–48%) among studies [10]. Prior work has suggested that mortality among persons with NTM-PD is primarily driven by comorbidities rather than NTM-PD itself, although a recent analysis suggested that deaths attributed to NTM in the United States are increasing over time [11–14].
At present, the prognosis of NTM-PD is not well understood, and a frequent question among patients with NTM-PD is, “How long will I live with this infection?” Such patients are frequently counseled that they will “die with it [NTM-PD], not of it.” Assessing the truth of this statement is challenging because ascertaining an exact cause of death and the contribution of NTM-PD to premature mortality is inexact at best. We sought to improve understanding of the morbidity and mortality of NTM-PD by examining comorbidity-stratified NTM-PD patient hospitalization and survival.
METHODS
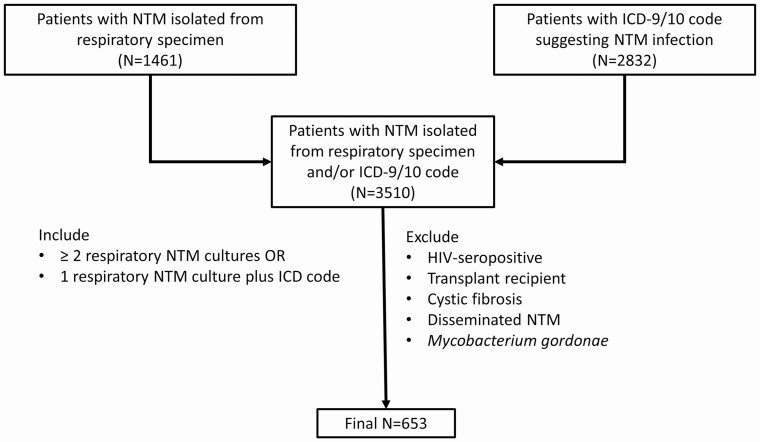
We retrospectively identified a cohort of patients with NTM-PD who were seen in the Duke University Health System (DUHS) from 1 January 1996 through 30 June 2015. Duke University Health System includes 3 hospitals in central North Carolina: Duke University Hospital (a 957-bed tertiary care hospital) and 2 community hospitals, Duke Regional Hospital (369 beds), and Duke Raleigh Hospital (186 beds). Patients were identified by querying the DEDUCE (Duke Enterprise Data Unified Content Explorer) system, which is a self-service interface to clinical data for all DUHS patients [15]. Patients 18 years and older who, during the study period, had at least 1 culture of a respiratory (eg, sputum, bronchoscopy, lung tissue) specimen that grew an NTM other than Mycobacterium gordonae or had an International Classification of Diseases (ICD), version 9 or 10, diagnosis code for nontuberculous mycobacterial infection (031.0, 031.2, 031.8, 031.9, A31.0, A31.2, A31.8, or A31.9) associated with an encounter during the study period were considered for inclusion in the study. Patients with an ICD code indicating prior infection with the human immunodeficiency virus, solid-organ transplantation, hematopoietic stem cell transplantation, or cystic fibrosis were excluded. Patients were also excluded from the cohort if a positive culture from a site suggesting disseminated NTM infection (eg, blood, musculoskeletal sites, cerebrospinal fluid, mediastinum, pericardium, peritoneal fluid, sternum, or bone marrow) was obtained. Patients were included in the final cohort if they had either (1) 1 positive culture for an NTM from a respiratory specimen plus an ICD code indicating NTM infection or (2) 2 or more positive respiratory cultures for the same NTM (Figure 1) [16]. The use of these inclusion criteria was validated by performing chart review on a 10% simple random sample of patients included in the final cohort (N = 65). Of patients included in the random sample, 55 met American Thoracic Society (ATS)/European Respiratory Society (ERS)/European Society of Clinical Microbiology and Infectious Diseases (ESCMID)/Infectious Diseases Society of America (IDSA) criteria for NTM-PD, 1 met the radiographic and microbiologic criteria but was asymptomatic, and another had no documentation of symptoms but met radiographic and microbiologic criteria; the other 8 patients did not meet either radiographic or microbiologic criteria [17]. Extrapolating to the entire cohort, an estimated 84.6% (95% confidence interval [CI], 73.9–91.4%) of patients would have met ATS/ERS/ESCMID/IDSA NTM-PD criteria.
Figure 1.
Selection of patients with NTM pulmonary disease. Abbreviations: HIV, human immunodeficiency virus; ICD, International Classification of Diseases; NTM, nontuberculous mycobacterial/mycobacteria.
The date of NTM diagnosis was designated as the earlier of the date of collection of the first positive respiratory culture or the first date on which an ICD code indicating a diagnosis of NTM disease was applied. A set of specific comorbidities included in the Charlson comorbidity index and known to be associated with reduced survival (diabetes mellitus, cancer, chronic obstructive pulmonary disease, chronic renal failure, prior cerebrovascular accident, prior myocardial infarction, peptic ulcer disease, autoimmune disease, cirrhosis) as well as 2 additional comorbidities (idiopathic pulmonary fibrosis, sarcoidosis) were assessed using ICD codes [18]. Bronchiectasis was not considered a separate comorbidity for purposes of this study because it frequently accompanies NTM-PD and it is challenging to determine whether it is a risk factor for NTM-PD, a consequence of NTM-PD, or both. A comorbidity was determined to be pre-existing if the first date of the ICD code indicating this comorbidity predated the ICD diagnosis of NTM-PD. In an attempt to capture the additional health burden of NTM-PD, DUHS hospitalizations during the study period were classified as “possibly NTM-related” if the primary or secondary admission diagnoses (per ICD codes) were diseases of the respiratory tract (eg, bronchiectasis, pneumonia, NTM pulmonary disease) and as “probably not NTM-related” if neither the primary nor secondary admission diagnoses were diseases of the respiratory tract. Survival was measured starting at the date of NTM diagnosis and was censored at the time of death, the time of the last known visit to DUHS, or 30 June 2015, whichever came first.
Statistical analysis was performed using R version 3.5.3 (R Core Team, Vienna, Austria) via the RStudio version 1.1.463 interface. Continuous variables were summarized using means and interquartile ranges (IQRs), and categorical variables were summarized with frequency counts. Expected population survival was based on life tables for the US population for 2015 [19]. Expected survival for a cohort of similar demographic composition to our cohort was obtained by using the annual probabilities of dying for the age, race/ethnicity (stratified into black, white, Hispanic, and other), and gender-stratified survival estimates from US life tables, with patients in the “other” category assigned the expected survival based on their age and gender only. Concretely, a simulated cohort of size of 10 000 was created to have the exact age, gender, and race/ethnicity distribution of our cohort at the time of NTM-PD diagnosis. Survival of cohort members was assigned based on the US population life tables over a 20-year time horizon. Survival among groups was compared using the log-rank test, with a P value of less than .05 deemed statistically significant.
RESULTS
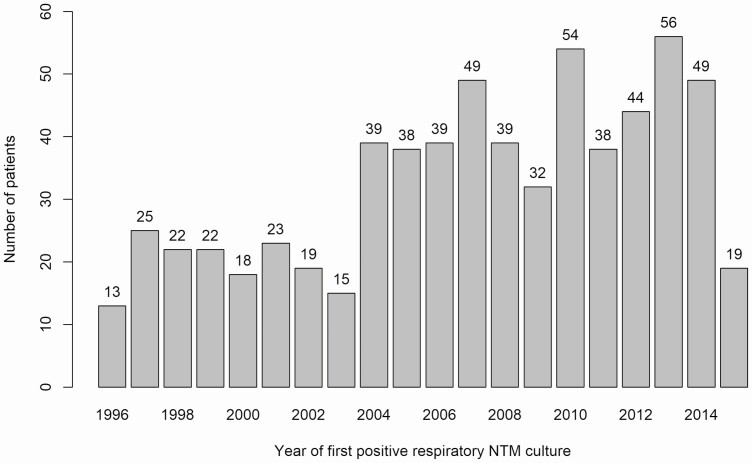
We identified a total of 3510 patients with NTM isolated from a respiratory specimen (n = 1461) and/or with an ICD-9/10 code suggesting NTM-PD (n = 2832). Of those patients, 653 patients met the study inclusion criteria (Figure 1). Of the 653 patients comprising the final study cohort, 451 (69%) were female, 548 (84%) were Caucasian, and the median age was 69 years (IQR, 59–76 years) (Table 1). A total of 351 (53.8%) had an ICD diagnosis of bronchiectasis; 326 patients (49.9%) had none of the listed comorbidities prior to NTM diagnosis; 544 (83.3%) had only Mycobacterium avium complex (MAC) isolates in cultures; 39 (6.0%) had only Mycobacterium abscessus; 33 (5.1%) had both MAC and M. abscessus; and 37 (5.7%) had other NTM isolates. The number of patients with a first positive respiratory NTM culture increased during the latter decade of the study period (Figure 2), in part attributable to an outbreak of M. abscessus associated with a new hospital building [20]. Median follow-up time was 1252 days (IQR, 449–2688 days).
Table 1.
Characteristics of 653 Patients With Nontuberculous Mycobacterial Pulmonary Disease
| Values | |
|---|---|
| Age at diagnosis (median, IQR), years | 69 (20–96) |
| Female sex, n (%) | 451 (69) |
| Race/ethnicity, n (%) | |
| Asian, non-Hispanic | 5 (0.8) |
| Black, non-Hispanic | 65 (10.0) |
| Hispanic (any race) | 5 (0.8) |
| Multiple, non-Hispanic | 5 (0.8) |
| Native American, non-Hispanic | 5 (0.8) |
| White, non-Hispanic | 548 (83.9) |
| Unknown | 20 (3.1) |
| Diagnostic criteria, n (%) | |
| At least 2 positive respiratory NTM cultures | 270 (41.3) |
| One positive respiratory NTM culture + ICD code | 84 (12.9) |
| At least 2 positive respiratory NTM cultures + ICD code | 299 (45.8) |
| Comorbidities, n (%) | |
| Malignancy | 141 (21.6) |
| Chronic obstructive pulmonary disease | 117 (18.0) |
| Diabetes mellitus | 50 (7.7) |
| Prior stroke | 49 (7.5) |
| Prior myocardial infarction | 23 (3.5) |
| Dementia | 10 (1.5) |
| Peptic ulcer disease | 14 (2.1) |
| Autoimmune disease | 46 (7.0) |
| Liver disease | 84 (12.9) |
| Pulmonary fibrosis | 81 (12.4) |
| Sarcoidosis | 9 (1.4) |
| No comorbidities | 326 (49.9) |
| NTM species group, n (%) | |
| Mycobacterium avium complex only | 544 (83.3) |
| Mycobacterium abscessus only | 39 (6.0) |
| M. avium complex and M. abscessus | 33 (5.1) |
| Other | 37 (5.7) |
Abbreviations: ICD, International Classification of Diseases; IQR, interquartile range; NTM, nontuberculous mycobacteria.
Figure 2.
Number of patients per year with first positive respiratory culture for NTM. Abbreviation: NTM, nontuberculous mycobacteria.
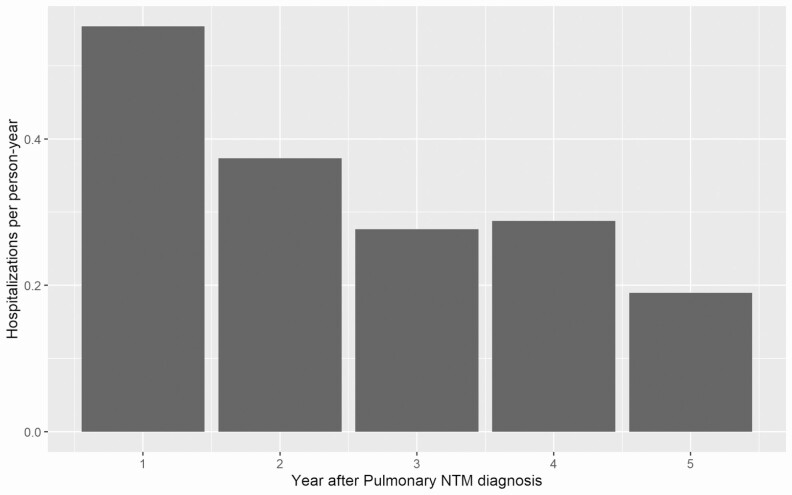
The 653 patients required a total of 1030 hospitalizations with a median length of stay of 4.0 days (IQR, 2.5–6.9 days), for an overall hospitalization rate of 0.92 per 1000 patient-days. The 326 patients with no prior comorbidities accounted for 447 hospitalizations, with a hospitalization rate of 0.65 per 1000 patient-days and a median of 0 hospitalizations per patient (IQR, 0–2), while the 327 patients with prior comorbidities were hospitalized 583 times, with a hospitalization rate of 1.37 per 1000 patient-days and a median of 1 hospitalization per patient (IQR, 0–2). The hospitalization rate tended to decline over time after NTM diagnosis (Figure 3). Among patients without comorbidities, 375 of 447 (85%) admissions were possibly NTM related, while among patients with comorbidities, 491 of 583 (84%) admissions were possibly NTM related (P = .92).
Figure 3.
Incidence of all-cause hospitalization during the first 5 years after diagnosis of NTM pulmonary disease. Abbreviation: NTM, nontuberculous mycobacteria/mycobacterial.
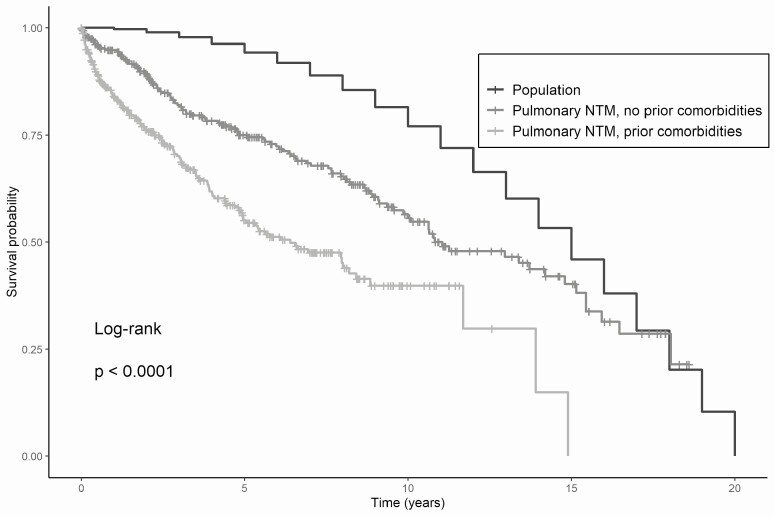
The median expected population survival of a general-population cohort matched by age and gender was 5479 days, while median survival in our cohort was 4038 days (95% CI, 3602–5533 days) and 2337 days (95% CI, 1805–3233 days) among persons without and with comorbidities, respectively (P < .0001) (Figure 4). Therefore, expected survival decreased by 1441 days (4.0 years) for a diagnosis of NTM-PD without comorbidities and by 3142 days (8.6 years) for a diagnosis of NTM-PD with comorbidities. Mortality 5 years after diagnosis was 25.0% (95% CI, 19.5–30.1%) and 44.9% (95% CI, 38.0–51.1%) among patients with NTM without and with comorbidities, respectively, versus an expected 5.7% in a demographically matched general-population cohort.
Figure 4.
Survival of patients with NTM pulmonary disease, with and without selected prior comorbidities, compared with expected survival for a demographically matched general population cohort. Abbreviation: NTM, nontuberculous mycobacteria/mycobacterial.
Discussion
Our data suggest that there is a clinically meaningful reduction in survival associated with NTM-PD. While some of this reduction may be driven by other medical conditions, comorbidities did not explain all of the reduction in expected survival among patients with NTM-PD. A diagnosis of NTM-PD reduced expected survival of patients by 4.0 and 8.6 years for patients with a diagnosis of NTM-PD without and with comorbidities, respectively. Furthermore, compared with an expected population survival of a demographically similar cohort, 5-year mortality was 19.3% higher among patients with NTM-PD with no comorbidities and 39.2% higher among patients with NTM-PD with comorbidities. Hospitalization among patients with NTM-PD was frequently associated with a respiratory diagnosis, providing indirect evidence that a significant proportion of the morbidity associated with NTM-PD was attributable to the NTM-PD as opposed to other illnesses. Patients with NTM-PD required frequent hospitalizations, often for respiratory illness, with 85% of hospitalizations attributable to NTM-PD, and patients with comorbidities required more than twice the hospitalizations (hospitalization rate of 1.37/1000 patient-days) than patients without comorbidities (hospitalization rate of 0.65/1000 patient-days).
Our findings are consistent with an earlier study that assessed a US cohort of patients from 2007 to 2016. This cohort comprised patients covered by commercial insurance (including patients enrolled in Medicare Advantage plans) and showed that patients with NTM-PD had more associated comorbidities and had approximately double the all-cause mortality rate, at 20.7 per 1000 person-years versus 5.6 per 1000 person-years in patients with NTM-PD versus controls, respectively, with a statistically significant hazard ratio of 2.06. Although the population was geographically diverse, this cohort excluded patients with no insurance coverage or those receiving only government plans, and there was concern for underreporting of mortality [21]. In addition, a nationwide population study from Korea evaluated approximately 46 000 patients with NTM and showed that the 5-year mortality of patients with NTM was 17.8%, with a standardized mortality ratio of 2.16 compared with the general population; however, approximately 60% of those patients had a Charlson comorbidity index of 3 or higher, and mortality was not stratified by Charlson index. The cause of death was primarily respiratory, with a total of 38.5% of deaths attributed to tuberculosis, pneumonia, chronic lower respiratory disease, or lung cancer [22].
Teasing out the relative contribution of NTM-PD to morbidity and mortality has posed a significant challenge. For example, a retrospective cohort of patients with NTM isolated from respiratory specimens in Oregon found that 5-year mortality was high (35.1%) among all patients with NTM, and that meeting the ATS/IDSA criteria for NTM-PD was only marginally associated with increased mortality once age and comorbidities were taken into account [23]. Similarly, another retrospective cohort of Finnish patients found that mortality among patients with NTM isolated from respiratory specimens was driven by underlying comorbidities rather than whether the patient met criteria for NTM-PD [11]. A US-based nationwide survey of mortality attributed to NTM-PD (based on ICD codes) reported comorbid chronic obstructive pulmonary disease among 24% of persons whose death was attributed to NTM-PD, but other comorbidities were relatively uncommon [24]. A Japanese cohort study, which reported a relatively low 10% 10-year mortality among 309 patients with NTM-PD, attributed the mortality to the NTM-PD in fewer than half (13/31, 42%) of cases [25]. In addition to the uncertainties around directly attributing mortality to NTM-PD, bronchiectasis itself has been associated with increased mortality [26]. The incompletely understood causality chain between bronchiectasis and NTM-PD makes attributing deaths to NTM infection even more challenging. However, at least 1 carefully controlled, large-cohort study has demonstrated that NTM-PD was associated with reduced survival compared with respiratory NTM isolation without disease, which, in turn, was associated with reduced survival compared with an age- and sex-matched population cohort [27].
Our data must clearly be interpreted in the context of their limitations. This was a retrospective cohort study, with potential for incomplete ascertainment of patients with NTM-PD, referral bias, and incomplete ascertainment of outcomes. In addition, given the retrospective nature of the study and availability of radiographic and symptom data in the data extract used, ascertaining NTM-PD using standard ATS/ERS/ESCMID/IDSA criteria was challenging. However, the random sample of patients who underwent manual chart review suggested that concordance between our case definition and standard criteria was high. Distinguishing between incident and prevalent cases of NTM-PD was not possible in our cohort; cases newly incident to our health system may well have been diagnosed elsewhere and referred. Fortunately, the comparison with expected population mortality addresses the clinically relevant question of survival after initial diagnosis in our health system, so did not depend on an accurate date of original NTM-PD diagnosis. Furthermore, our classification of NTM-related DUHS hospitalizations used a broad definition in an attempt to capture the upper bound of morbidity associated with NTM-PD, which may have resulted in an overestimation of NTM-related hospitalization. We did not attempt to assess the effect of NTM treatment on survival given the significant (and probably analytically insurmountable) biases surrounding treatment decisions and tolerability.
In conclusion, there is a significant health burden associated with NTM-PD, which may suggest that some patients with NTM-PD are likely dying “of” their NTM infection and not just “with” it. Further studies on predictors of mortality among patients with NTM-PD who do not have significant comorbidities, and on the impact of NTM-PD treatment on mortality, are needed to inform patients and clinicians.
Notes
Financial support. This work was supported by a resident research grant from the Department of Medicine, Duke University Medical Center.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Faria S, Joao I, Jordao L. General overview on nontuberculous mycobacteria, biofilms, and human infection. J Pathog 2015; 2015:809014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ; ATS Mycobacterial Diseases Subcommittee, American Thoracic Society ; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 3. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diel R, Jacob J, Lampenius N, et al. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J 2017; 49. [DOI] [PubMed] [Google Scholar]
- 5. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 2017; 49. [DOI] [PubMed] [Google Scholar]
- 6. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in non-tuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis 2015; 34:1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balavoine C, Blanc F-X, Lanotte P, Meurice JC, Andrejak C, Marchand-Adam S. Adverse events during treatment of nontuberculous mycobacterial lung disease: do they really matter? Eur Respir J 2018; 52:PA2664. [Google Scholar]
- 8. Philley JV, DeGroote MA, Honda JR, et al. Treatment of non-tuberculous mycobacterial lung disease. Curr Treat Options Infect Dis 2016; 8:275–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuuchi K, Morimoto K, Kurashima A, et al. Treatment duration and disease recurrence following the successful treatment of patients with mycobacterium avium complex lung disease. Chest 2020; 157:1442–5. [DOI] [PubMed] [Google Scholar]
- 10. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis 2018; 18:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Prognostic value of American Thoracic Society criteria for non-tuberculous mycobacterial disease: a retrospective analysis of 120 cases with four years of follow-up. Scand J Infect Dis 2013; 45:194–202. [DOI] [PubMed] [Google Scholar]
- 12. Hayashi M, Takayanagi N, Kanauchi T, Miyahara Y, Yanagisawa T, Sugita Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012; 185:575–83. [DOI] [PubMed] [Google Scholar]
- 13. Gochi M, Takayanagi N, Kanauchi T, Ishiguro T, Yanagisawa T, Sugita Y. Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ Open 2015; 5:e008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinnard C, Longworth S, Mezochow A, Patrawalla A, Kreiswirth BN, Hamilton K. Deaths related to nontuberculous mycobacterial infections in the United States, 1999–2014. Ann Am Thorac Soc 2016; 13:1951–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horvath MM, Rusincovitch SA, Brinson S, Shang HC, Evans S, Ferranti JM. Modular design, application architecture, and usage of a self-service model for enterprise data delivery: the Duke Enterprise Data Unified Content Explorer (DEDUCE). J Biomed Inform 2014; 52:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winthrop KL, Baxter R, Liu L, et al. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf 2011; 20:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 2020; 71:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 19. Arias E. United States life tables, 2011. Natl Vital Stat Rep 2015; 64:1–63. [PubMed] [Google Scholar]
- 20. Baker AW, Lewis SS, Alexander BD, et al. Two-phase hospital-associated outbreak of mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 2017; 64:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marras TK, Vinnard C, Zhang Q, et al. Relative risk of all-cause mortality in patients with nontuberculous mycobacterial lung disease in a US managed care population. Respir Med 2018; 145:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park SC, Kang MJ, Han CH, et al. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: a nationwide population-based study. BMC Pulm Med 2019; 19:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novosad SA, Henkle E, Schafer S, et al. Mortality after respiratory isolation of nontuberculous mycobacteria. a comparison of patients who did and did not meet disease criteria. Ann Am Thorac Soc 2017; 14:1112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999–2010: a population-based comparative study. PLoS One 2014; 9:e91879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc 2014; 11:1–8. [DOI] [PubMed] [Google Scholar]
- 26. Sin S, Yun SY, Kim JM, et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir Res 2019; 20:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marras TK, Campitelli MA, Lu H, et al. Pulmonary nontuberculous mycobacteria-associated deaths, Ontario, Canada, 2001–2013. Emerg Infect Dis 2017; 23:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]