Abstract
Background
Completion of tuberculosis (TB) preventive treatment is important to optimize efficacy; treatment-related adverse events (AEs) sometimes result in discontinuation. This study describes the occurrence of AEs and their risk factors during a 6-month, 2-drug, fluoroquinolone-based preventive treatment for household contacts of patients with drug-resistant TB in Karachi, Pakistan.
Methods
The primary outcome was development of any clinical AE during preventive treatment. Adverse events were categorized using the AE grading tables of the National Institutes of Health. Time-to-event analysis with Kaplan-Meier curves and Cox proportional hazards models accounting for recurrence were used to analyze associated risk factors.
Results
Of the 172 household contacts on preventive treatment, 36 (21%) developed 64 AEs during 813 months of treatment. The incidence of AEs over 6 months of treatment was 7.9 per 100 person-months; 16 per 100 person-months with a fluoroquinolone and ethionamide, and 4.4 per 100 person-months with a fluoroquinolone and ethambutol. There were 53 (83%) grade 1 and 11 grade 2 AEs, with no grade 3 or 4 AEs. In multivariable analysis, the risk of AEs was higher in contacts prescribed ethionamide as compared to ethambutol adjusting for age, sex, and body mass index (adjusted hazard ratio, 2.1 [95% confidence interval {CI}, 1.2–3.6]). Overall, there was no notable difference in treatment completion among the contacts who experienced an AE and those who did not (crude odds ratio, 1.1 [95% CI, .52–2.5]).
Conclusions
A fluoroquinolone-based preventive treatment regimen for drug-resistant TB exposure is well tolerated. Regimens with ethionamide are more likely to result in AEs.
Keywords: drug-resistant tuberculosis infection, TB, preventive therapy, fluoroquinolone, adverse events
This study assessed the adverse events (AEs) and associated risk factors of preventive treatment for drug-resistant tuberculosis exposure. We found preventive treatment to be safe, with no serious AEs and no association between AEs and treatment discontinuation.
(See the Editorial Commentary by Reuter and Furin on pages 1716–8.)
In 2017, 10.4 million people developed tuberculosis (TB) disease, of whom 1.3 million died; almost 558 000 cases of TB globally were due to drug-resistant TB (DR-TB) [1]. Among household contacts of patients with DR-TB, almost half are infected [2].
Approximately 10%–20% of infected individuals progress to active TB disease from reactivation of latent TB over their lifetime [3, 4]. As such, household contact tracing and preventive treatment for exposed contacts have long been key elements of TB control [2, 5]. Preventive treatment for TB can reduce incidence of TB among contacts by 60%–90% [6], but currently available preventive treatment would not be effective in contacts exposed to DR-TB as the organism is resistant to these drugs.
Observational studies have suggested that fluoroquinolone-based preventive treatment may be effective in preventing DR-TB; the World Health Organization recommends treating DR-TB infection in high-risk contacts although the quality of available evidence is considered quite low, because observational studies are given a lower quality score than experimental studies [7–21]. There is concern about development of adverse events (AEs) because some preventive treatment regimens for DR-TB make use of second-line TB drugs that have been shown to be toxic in patients with DR-TB [1]. However, AEs have not been systematically documented across studies of preventive treatment. Bamrah et al showed that 53% of the contacts in the Federated States of Micronesia experienced some adverse effects, but only 4% stopped preventive treatment [8]. A systematic review and meta-analysis found that a mean of 19% of TB contacts started on preventive treatment eventually discontinued treatment because of AEs [22].
Completion of TB preventive treatment is important to optimize efficacy, with data showing that those who do not complete treatment are at 5 times the risk for developing TB disease compared to people who complete preventive treatment (hazard ratio [HR], 5.4 [95% confidence interval {CI}, 2.1–14]) [23]. However, it can be challenging to persuade healthy people to take preventive treatment when it may result in an AE [22].
Studies providing preventive treatment to persons exposed to DR-TB have suggested that regimens combining a fluoroquinolone with either ethambutol or ethionamide may be effective in preventing active TB disease [22]. The rate of AEs using a fluoroquinolone with ethambutol is lower (16%) as compared to a regimen of fluoroquinolone and ethionamide (58%) [22]. However, the AE profiles and individual-level factors associated with them have not been well-described or compared. Improved identification of individuals at risk of developing AEs during preventive treatment could allow for public health programs to prevent poor outcomes and plan for resources accordingly.
Here we compare the AE profiles between 2 preventive treatment regimens: (1) fluoroquinolone with ethambutol and (2) fluoroquinolone with ethionamide. These regimens were offered to eligible household contacts of DR-TB patients in Karachi, Pakistan. We also examine the association between development of AEs and treatment discontinuation.
METHODS
Setting and Study Population
This study utilized data collected from a cohort established in Karachi, Pakistan, to evaluate the feasibility, safety, and effectiveness of TB preventive therapy for individuals exposed to DR-TB (defined as resistance to either isoniazid and/or rifampicin). Details of the cohort are reported elsewhere [24]. In brief, 800 household contacts (defined as those sleeping under the same roof at time of treatment initiation) of 100 consecutive (index) patients initiating treatment for culture-confirmed DR-TB disease at the Indus Hospital were evaluated. Contacts were eligible for the study if the index patient (1) did not have extensively drug-resistant TB, defined as resistant to isoniazid, rifampin, any fluoroquinolone, and at least 1 of 3 injectable second-line drugs; (2) lived in Karachi, Pakistan; and (3) consented to participate in the study. If the index TB patient’s isolate was resistant to a fluoroquinolone on drug susceptibility testing but not resistant to any of the second-line injectables, the household contacts were eligible for the study. TB disease–free contacts (1) younger than 5 years; (2) between 5 and 17 years of age with a positive tuberculin skin test, diabetes, human immunodeficiency virus, or malnutrition (weight for age less than third percentile); or (3) 18 years and older with diabetes, HIV, or malnutrition (body mass index [BMI] < 18.5 kg/m2) were eligible to start a 6-month fluoroquinolone-based preventive treatment regimen based on expert consensus [12]. Levofloxacin was the fluoroquinolone of choice unless the index patient’s TB strain was resistant to it on culture. In such cases, moxifloxacin was prescribed. Ethambutol was the companion drug of choice unless it was not available in the correct dosing form due to drug supply chain disruption, in which case it was replaced by ethionamide. Contacts started on any given regimen were continued on that regimen throughout the course of treatment. Clinicians managed AEs by providing symptomatic treatment including giving ethionamide in 2 divided doses per day.
Overall, 215 household contacts were eligible to receive a fluoroquinolone-based TB preventive treatment of which 172 were started on 1 of the following 6-month, 2-drug combinations:
Levofloxacin (15–20 mg/kg for children 5 years and younger; 7.5–10 mg/kg for individuals older than 5 years; maximum dose: 1000 mg/day) and ethambutol (15–25 mg/kg; maximum dose: 2000 mg/day)
Levofloxacin (15–20 mg/kg for children 5 years and younger; 7.5–10 mg/kg for individuals older than 5 years; maximum dose: 1000 mg/day) and ethionamide (15–20 mg/kg; maximum dose: 750 mg/day)
Moxifloxacin (7.5–10 mg/kg; maximum dose: 400 mg/day) and ethambutol (15–25 mg/kg; maximum dose: 2000 mg/day)
Moxifloxacin (7.5–10 mg/kg; maximum dose: 400 mg/day) and ethionamide (15–20 mg/kg; maximum dose: 750 mg/day)
A clinical psychologist called these contacts 15 days after treatment initiation to monitor for treatment adherence and AEs. Contacts were then followed up in clinic every 2 months for the duration of treatment. In between clinic visits, a study health worker visited the household to monitor treatment adherence and AEs monthly. All contacts completing at least 5 months of treatment were considered to have completed treatment.
Adverse Events
A structured questionnaire to assess AEs was administered to patients or parents/caregivers at follow-up visits either at clinic or home. The questionnaire was adapted from “How to Care for People Exposed to Drug-Resistant Tuberculosis: a Practical Guide” [25]. These AEs were classified retrospectively by the study team using the AEs grading tables of the National Institute of Allergy and Infectious Diseases’ Division of AIDS. As cough, increased frequency of urination, and disturbed menstruation are not categorized in this classification, we used the National Institutes of Health’s Common Terminology Criteria for Adverse Events to classify these 3 types of events.
Analysis
The primary outcome of interest was the development of any clinical AE during preventive treatment at any follow-up visit. Descriptive analysis was performed with frequency counts reported for each AE. For each contact on treatment, the total number of AEs over the 6-month course of preventive treatment was calculated. The incidence of AEs was calculated by dividing the sum of all AEs in all contacts on treatment by the total person-months of follow-up measured and expressed as events per 100 person-months of follow-up. We also calculated the rate of AEs in each of the 6 months of treatment.
Different covariates were examined between those who developed AEs compared with those who did not develop AEs using χ 2 or Fisher exact test for dichotomous, and t test or nonparametric Wilcoxon tests for continuous variables.
Time-to-event analysis with Kaplan-Meier curves and Cox proportional hazards modeling was used to analyze associated risk factors for AEs. Participants were censored at time of treatment completion or discontinuation of treatment. As AEs are a recurrent outcome, we used the Prentice, Williams, and Peterson with total time to event (PWP-TT) extension to the Cox proportional hazards model for analysis to account for all AEs. This model analyzes ordered multiple events by stratification with only those persons with an event in the previous stratum at risk. All participants are at risk of an event in the first stratum [26].
We repeated the multivariable analysis using just gastrointestinal, respiratory, and dermatological AEs as a sensitivity analysis as these are more objectively reported. We completed this sensitivity analysis to address the concern that very young children may not always be able to express some of the subjective symptoms recorded as AEs.
We constructed a matched analysis with matching with respect to analysis time, age category, and sex to analyze the effect of AEs on treatment discontinuation. Each contact discontinuing treatment was matched with 3 contacts who completed treatment for this analysis.
Data were analyzed using Stata version 15 software (StataCorp, College Station, Texas).
Ethical Approval
The parent study was approved by the institutional review boards (IRBs) of Interactive Research and Development and Harvard Medical School. The AE analysis presented in this manuscript was also approved by the IRB of Emory University.
RESULTS
Of the 215 household contacts who were eligible to start preventive treatment, 172 (80%) started preventive treatment; the median age was 7 years (interquartile range [IQR], 3–15 years), and 53% were male (Table 1). Contacts who started preventive treatment were younger compared to those who did not start preventive treatment (median age, 16 years [IQR, 3–22 years]). Otherwise these contacts did not differ from each other with respect to demographics and clinical features.
Table 1.
Demographics and Clinical Characteristics of Drug-Resistant Tuberculosis Contacts Enrolled on Preventive Treatment at the Indus Hospital
| Characteristic | On Treatment | No AE | With AE |
|---|---|---|---|
| (n = 172) | (n = 136) | (n = 36) | |
| Age, y, median (IQR) | 7 (3–15) | 5.5 (2.5–12) | 11.5 (6–17.5) |
| Age categories | |||
| < 5 y | 61 (35) | 55 (40) | 6 (17) |
| 5–9 y | 44 (26) | 35 (26) | 9 (25) |
| 10–19 y | 48 (28) | 34 (25) | 14 (39) |
| > 19 y | 19 (11) | 12 (9) | 7 (19) |
| Sex, male | 91 (53) | 72 (53) | 19 (53) |
| (n = 171) | (n = 135) | (n = 36) | |
| BMI, kg/m2, median (IQR) | 14.8 (13.4–16.9) | 14.7 (13.4–16.9) | 15.2 (13.3–16.7) |
| Presence of symptoms | |||
| Cough | 3 (2) | 3 (2) | 0 (0) |
| Fever | 1 (1) | 1 (1) | 0 (0) |
| Weight loss | 1 (1) | 0 (0) | 1 (3) |
| Additional TB risk factors | |||
| History of TB | 0 (0.0) | 0 (0) | 0 (0) |
| TST ≥ 5 mm | 6/64 (9) | 5/50 (10) | 1/14 (7) |
| Index case resistant to FQ | 16 (9) | 12 (9) | 4 (11) |
| Regimen given | |||
| Levofloxacin/ethambutol | 102 (59) | 90 (66) | 12 (33) |
| Levofloxacin/ethionamide | 54 (31) | 34 (25) | 20 (56) |
| Moxifloxacin/ethambutol | 11 (6) | 7 (5) | 4 (11) |
| Moxifloxacin/ethionamide | 5 (3) | 5 (4) | 0 (0) |
Data are presented as no. (column %) unless otherwise indicated.
Abbreviations: AE, adverse events; BMI, body mass index; FQ, fluoroquinolone; IQR, interquartile range; TB, tuberculosis; TST, tuberculin skin test.
Of the 172 household contacts started on preventive treatment, 113 (65%) received ethambutol as a companion drug, and the rest received ethionamide (Table 1). Thirty-six of the 172 (21%) contacts developed 64 AEs during 813 months of treatment with a median of 2 AEs per contact experiencing an AE. The incidence of AEs over 6 months of treatment was 7.9 events per 100 person-months.
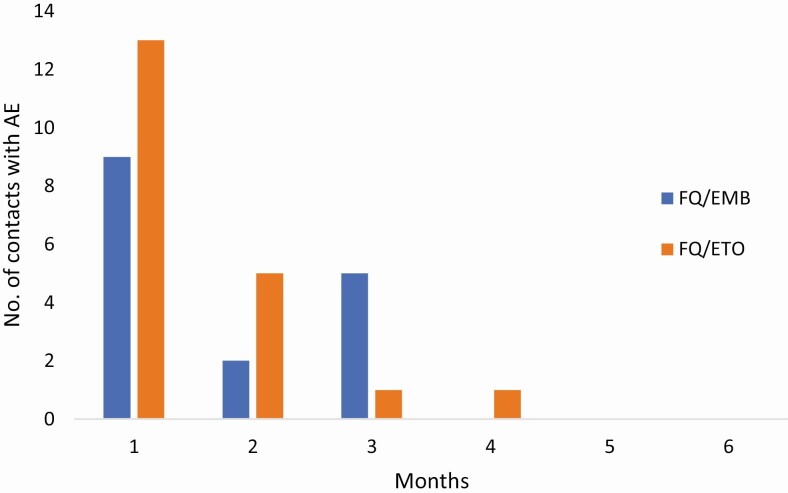
Of the 36 contacts experiencing an AE, 22 (61%) experienced the first AE within the first month of treatment. Almost all contacts had experienced their first AE by 3 months of treatment (Figure 1).
Figure 1.
Time to first AE in months, by regimen. Abbreviations: AE, adverse event; EMB, ethambutol; ETO, ethionamide; FQ, fluoroquinolone.
The most common AE was vertigo/dizziness, with 19 (11%) of the contacts experiencing it (Table 2), whereas the most common system involved was gastrointestinal, with 21 (12%) contacts experiencing at least 1 gastrointestinal AE (eg, vomiting, nausea). Of the 64 clinical AEs recorded, 53 (83%) were grade 1 while 11 were grade 2. There was no grade 3 or 4 AE observed during treatment (Table 2).
Table 2.
Severity of Reported Adverse Eventsa
| Ethambutol (n = 28) | Ethionamide (n = 36) | ||||
|---|---|---|---|---|---|
| Adverse Event | Grade 1 (n = 24) | Grade 2 (n = 4) | Grade 1 (n = 29) | Grade 2 (n = 7) | Total Adverse Events (n = 64) |
| Vertigo/dizziness | 8 (42) | 0 (0) | 11 (58) | 0 (0) | 19 (30) |
| Vomiting | 4 (36) | 0 (0) | 6 (55) | 1 (9) | 11 (17) |
| Anxiety | 0 (0) | 2 (20) | 2 (20) | 6 (60) | 10 (16) |
| Nausea | 4 (50) | 0 (0) | 4 (50) | 0 (0) | 8 (13) |
| Arthralgia | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 3 (4) |
| Pain | 2 (67) | 0 (0) | 1 (33) | 0 (0) | 3 (4) |
| Abdominal distention | 1 (100) | 0 (0) | 0 (0) | 0 | 1 (2) |
| Bloating | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (2) |
| Cough | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Disturbed menstruation | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (2) |
| Fatigue | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Headache | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (2) |
| Insomnia | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (2) |
| Myalgia | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (2) |
| Polyuria | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (2) |
| Yellow discoloration of skin | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (2) |
Data are presented as no. (row %).
aThere were no grade 3 or 4 adverse events.
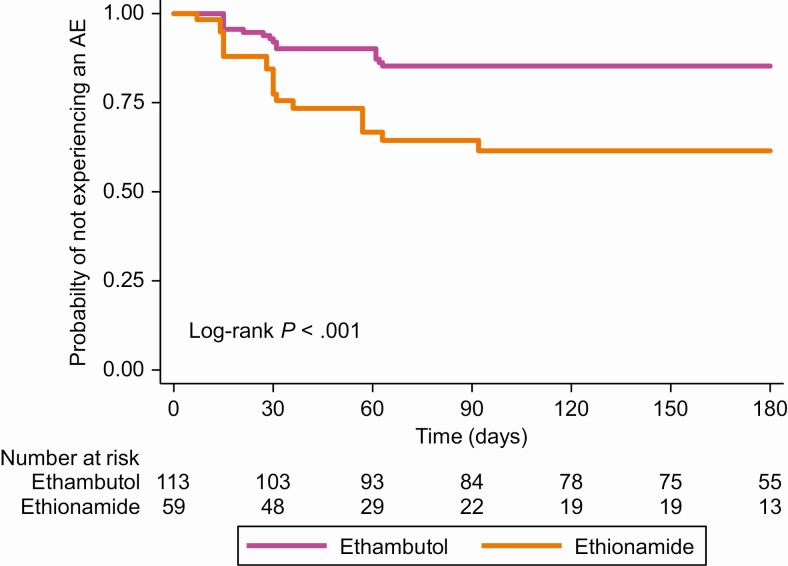
Household contacts who received ethionamide with a fluoroquinolone had an incidence rate of 16 AEs per 100 person-months while those who received ethambutol with a fluoroquinolone had an incidence rate of 4.4 AEs per 100 person-months (incidence rate ratio, 3.7 [95% CI, 2.2–6.3]). Figure 2 shows the probability of an AE by companion drug during treatment.
Figure 2.
Time to first AE, by companion drug. Abbreviation: AE, adverse event.
In bivariate Cox proportional hazards analysis accounting for recurrence (PWP-TT model), risk of AE was 2-fold higher among contacts prescribed ethionamide as compared to ethambutol as the companion drug (HR, 2.2 [95% CI, 1.2–3.8]) (Table 3). Similarly, older children and adults were at a higher risk for AEs as compared to children < 5 years of age (5–9 years: HR, 2.7 [95% CI, 1.1–6.5]; 10–19 years: HR, 3.9 [95% CI, 1.8–8.6]; > 19 years: HR, 4.1 [95% CI, 1.7–9.7]) (Table 3). In multivariable analysis, the risk remained higher for the group exposed to ethionamide as compared to ethambutol after adjusting for age, sex, and BMI (adjusted HR, 2.1 [95% CI, 1.1–3.9]) (Table 3).
Table 3.
Risk of Recurrent Adverse Events
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Characteristic | HR | (95% CI) | HR | (95% CI) |
| Ethionamide | 2.2 | (1.2–3.8) | 2.1 | (1.1–3.9) |
| Age < 5 y (Ref) | … | … | … | … |
| Age 5–9 y | 2.7 | (1.1–6.5) | 2.9 | (1.2–7.1) |
| Age 10–19 y | 3.9 | (1.8–8.6) | 3.2 | (1.5–6.9) |
| Age > 19 y | 4.1 | (1.7–9.7) | 4.3 | (1.7–11) |
| Sex (male) | 0.91 | (.56–1.5) | 0.72 | (.44–1.2) |
| BMI | 1.0 | (.95–1.0 ) | 0.99 | (.92–1.1) |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; Ref, reference value.
To assess interaction between age and companion drug, we performed an age-stratified analysis. The HRs for AEs for ethionamide as compared to ethambutol were as follows: < 5 years: 2.4 (95% CI, .54–11); 5–9 years: 5.2 (95% CI, 2.1–13); 10–19 years: 4.5 (95% CI, 1.1–18); > 19 years: 0.16 (95% CI, .03–.80). We were unable to account for this interaction in our multivariable analysis because of collinearity.
We repeated the multivariable analysis using just gastrointestinal, respiratory, and dermatological AEs as a sensitivity analysis. Of the 64 total AEs, only 23 were included in this model. In this analysis, the HR for AEs in contacts prescribed ethionamide was 1.7 (95% CI, .62–4.6). Age-stratified HRs for ethionamide as compared to ethambutol were as follows: < 5 years: 3.1 (95% CI, .26–37); 5–9 years: 4.7 (95% CI, 1.2–19); 10–19 years: 3.3 (95% CI, .28–36). There were no observable AEs with ethionamide in the age group > 19 years.
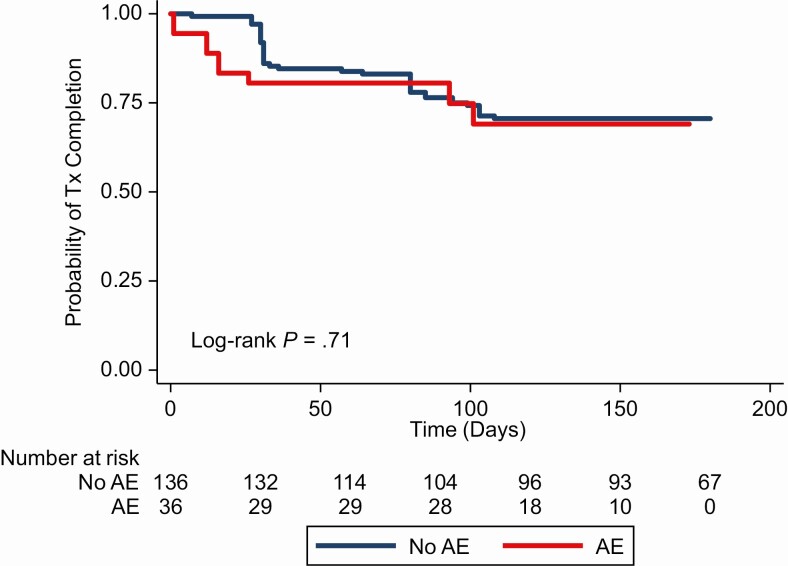
There were 11 (31%) contacts experiencing AEs and 40 (29%) contacts not experiencing an AE who did not complete treatment. Overall, there was a near null difference in treatment completion among the contacts who experienced an AE and those who did not (crude odds ratio, 1.1 [95% CI, .52–2.5]) (Figure 3).
Figure 3.
Probability of treatment completion, by adverse event (AE) status. Time scale for the graph is as follows: start of treatment for no AE group; time of AE occurrence for AE group. Abbreviations: AE: adverse event; Tx, treatment.
DISCUSSION
We examined the incidence of AEs and their risk factors among participants in a prospective study treating household contacts of DR-TB index cases with a fluoroquinolone-based, 2-drug preventive therapy regimen. Overall, the incidence of AEs was low, with 7.9 instances occurring in 100 person-months of follow-up; there was no grade 3 or 4 event or any AE requiring hospitalization during 6 months of preventive treatment. The rate of AEs and proportion of participants who experienced them was lower than found in previous studies [8, 11, 22]. In contacts who experienced any AEs, the first instance of an AE occurred within the first month of treatment 61% of the time, and almost all AEs occurred within 3 months of treatment initiation, suggesting that these events occur early during the treatment. Finally, AEs were less common among children, who are the risk group that is most likely to benefit from preventive treatment, lending strength to the recommendation of using a fluoroquinolone-based regimen for pediatric contacts of DR-TB index cases.
Subjects in our study received either ethambutol or ethionamide as the companion drug along with a fluoroquinolone for treatment. Contacts who received ethionamide had an almost 2-fold increase in risk of an AE as compared to those who received ethambutol. This risk decreased slightly after adjusting for age, sex, and BMI. Ethionamide is generally not well tolerated, and our results are consistent with the reports of AEs observed with ethionamide use for DR-TB treatment showing gastrointestinal AEs in 50% of the patients and neuropsychotoxic effects in 25%–30% of the patients [27]. A meta-analysis on preventive treatment of DR-TB suggested that fluoroquinolone with ethionamide might be the most effective regimen for preventing active TB disease development, while the combination of a fluoroquinolone and ethambutol may be the most cost-effective treatment option after consideration of both AEs and treatment discontinuation [22].
On age-stratified analysis, adults (> 19 years of age) were more likely to have an AE when prescribed ethambutol in contrast to children, suggesting an interaction between age and companion drug. We were unable to account for this interaction in our multivariable analysis because of collinearity. Because of the limited availability of ethambutol in the right dosage form, most adults were prescribed ethionamide in our study, which may have led to age and drug variables being collinear. Further research is needed to understand this association further.
Our results show that younger children tolerate preventive treatment better than older children and adults, with 10% of children < 5 years of age experiencing an AE and 37% of contacts > 19 years of age experiencing an AE. The literature suggests that children generally tolerate DR-TB treatment better than adults, and our results are consistent with this observation [28, 29]. This is an important finding as children aged < 5 years are at an increased risk of developing active TB disease after exposure and should receive preventive treatment [21, 30]. There is a possibility of ascertainment bias, with younger children not being able to communicate more subjective AEs like dizziness. On sensitivity analysis with just gastrointestinal, respiratory, and dermatological AEs, the directionality and magnitude of the effect was maintained, although precision was necessarily diminished by restricting to half the total events.
We observed no association between treatment discontinuation and AEs. Contacts who experienced any AE were as likely to complete treatment as those who did not experience an AE. Reports from other studies have shown that 1%–4% of the contacts of a fluoroquinolone-based treatment (excluding regimens with pyrazinamide) discontinue treatment because of AEs, and our results follow similarly [22]. This may be because there were no serious AEs in our study, and also the treatment program had a strong counseling component in which contacts and their parents/guardians were counseled before starting treatment and during each follow-up visit along with phone counseling by a certified psychologist. This counseling may have helped contacts experiencing an AE to continue treatment.
Our study had several limitations. Although contacts were systematically followed up clinically, there was no systematic laboratory testing. Hence, we are only able to report clinical AEs. We did not test for ocular toxicity in our study, an AE observed with ethambutol; therefore, there may be underreporting of AEs of ethambutol. Clinical AEs relied on self-reporting with possible variations in cultural attributes among families and ascertainment in younger children. For an assessment of adherence to treatment, we relied on self-reports by participants or their family members, which could have resulted in overreporting. However, adherence was cross-checked on home visits through pill counts by the healthcare workers.
Strengths of our study include a prospective design and excellent follow-up, with > 70% of the contacts on treatment completing the full course of treatment.
Overall, in this cohort of contacts of patients with DR-TB from Pakistan, we found that using a fluoroquinolone-based, 2-drug regimen for TB preventive treatment was well tolerated, without any serious AEs (grade 3 or 4). Adverse events were even less common in younger children, who are a high-risk group for TB disease following exposure and should be prioritized for preventive therapy. Our results can guide programs looking to implement preventive treatment for DR-TB exposure.
Notes
Author contributions. A. A. M., M. C. B., H. H., and N. R. G. conceptualized the study and wrote the protocol; A. A. M., J. F., S. S., and M. J. collected data under supervision from H. H., N. S., F. A., and S. K.; A. A. M., M. C. B., T. L. L., L. M. C., S. B. O., and N. R. G. performed and reviewed the analysis; A. A. M., M. C. B., and N. R. G. wrote the initial draft of the manuscript. All authors helped interpret the findings and read and approved the final version of the manuscript.
Disclaimer. The funder of this study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. The authors had full access to the data and made the decision to publish the manuscript.
Financial support. This work was supported by a Dubai Harvard Foundation for Medical Research grant to the Harvard Medical School Center for Global Health Delivery–Dubai. It was also supported in part by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number K24AI114444 to N. R. G.); the Emory University Center for AIDS Research (grant number P30AI050409); and the TB Research Unit ASTRa (grant number U19AI111211).
Potential conflicts of interest. F. A. is the Chair of the World Health Organization’s Child and Adolescent Tuberculosis Working Group. F. A. and M. C. B. are part of the writing group that wrote the 2015 consensus recommendations for the use of presumed drug-resistant tuberculosis infection treatment in household contacts. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis control report 2018. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 2. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2014; 58:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vynnycky E, Fine PE. Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol 2000; 152:247–63. [DOI] [PubMed] [Google Scholar]
- 4. Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet 2003; 362:887–99. [DOI] [PubMed] [Google Scholar]
- 5. Vella V, Racalbuto V, Guerra R, et al. Household contact investigation of multidrug-resistant and extensively drug-resistant tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis 2011; 15:1170–5, i. [DOI] [PubMed] [Google Scholar]
- 6. Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection—the promise and the challenges. Int J Infect Dis 2017; 56:68–76. [DOI] [PubMed] [Google Scholar]
- 7. Adler-Shohet FC, Low J, Carson M, Girma H, Singh J. Management of latent tuberculosis infection in child contacts of multidrug-resistant tuberculosis. Pediatr Infect Dis J 2014; 33:664–6. [DOI] [PubMed] [Google Scholar]
- 8. Bamrah S, Brostrom R, Dorina F, et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis 2014; 18:912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feja K, McNelley E, Tran CS, Burzynski J, Saiman L. Management of pediatric multidrug-resistant tuberculosis and latent tuberculosis infections in New York City from 1995 to 2003. Pediatr Infect Dis J 2008; 27:907–12. [DOI] [PubMed] [Google Scholar]
- 10. Schaaf HS, Gie RP, Kennedy M, Beyers N, Hesseling PB, Donald PR. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics 2002; 109:765–71. [DOI] [PubMed] [Google Scholar]
- 11. Seddon JA, Hesseling AC, Finlayson H, et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis 2013; 57:1676–84. [DOI] [PubMed] [Google Scholar]
- 12. Seddon JA, Amanullah F, Schaaf HS, et al. Post-exposure management of multidrug-resistant tuberculosis contacts: evidence-based recommendations. Policy Brief No. 1. Dubai, UAE: Harvard Medical School Center for Global Health Delivery–Dubai, 2015. [Google Scholar]
- 13. Trieu L, Proops DC, Ahuja SD. Moxifloxacin prophylaxis against MDR TB, New York, New York, USA. Emerg Infect Dis 2015; 21:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Attamna A, Chemtob D, Attamna S, et al. Risk of tuberculosis in close contacts of patients with multidrug resistant tuberculosis: a nationwide cohort. Thorax 2009; 64:271. [DOI] [PubMed] [Google Scholar]
- 15. Ridzon R, Meador J, Maxwell R, Higgins K, Weismuller P, Onorato IM. Asymptomatic hepatitis in persons who received alternative preventive therapy with pyrazinamide and ofloxacin. Clin Infect Dis 1997; 24:1264–5. [DOI] [PubMed] [Google Scholar]
- 16. Williams B, Ramroop S, Shah P, et al. Management of pediatric contacts of multidrug resistant tuberculosis in the United Kingdom. Pediatr Infect Dis J 2013; 32:926–7. [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Prats AJ, Zimri K, Mramba Z, Schaaf HS, Hesseling AC. Children exposed to multidrug-resistant tuberculosis at a home-based day care centre: a contact investigation. Int J Tuberc Lung Dis 2014; 18:1292–8. [DOI] [PubMed] [Google Scholar]
- 18. Papastavros T, Dolovich LR, Holbrook A, Whitehead L, Loeb M. Adverse events associated with pyrazinamide and levofloxacin in the treatment of latent multidrug-resistant tuberculosis. CMAJ 2002; 167:131–6. [PMC free article] [PubMed] [Google Scholar]
- 19. Lou HX, Shullo MA, McKaveney TP. Limited tolerability of levofloxacin and pyrazinamide for multidrug-resistant tuberculosis prophylaxis in a solid organ transplant population. Pharmacotherapy 2002; 22:701–4. [DOI] [PubMed] [Google Scholar]
- 20. Denholm J, Leslie D, Jenkin G, et al. Long-term follow-up of contacts exposed to multidrug-resistant tuberculosis in Victoria, Australia, 1995–2010. Int J Tuberc Lung Dis 2012; 16:1320–5. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, Switzerland: WHO, 2018. [PubMed] [Google Scholar]
- 22. Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis 2017; 64:1670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morán-Mendoza O, Marion SA, Elwood K, Patrick D, FitzGerald JM. Risk factors for developing tuberculosis: a 12-year follow-up of contacts of tuberculosis cases. Int J Tuberc Lung Dis 2010; 14:1112–9. [PubMed] [Google Scholar]
- 24. Malik AA, Fuad J, Siddiqui S, et al. TB preventive therapy for individuals exposed to drug-resistant tuberculosis: feasibility and safety of a community-based delivery of fluoroquinolone-containing preventive regimen [manuscript published online ahead of print 12 June 2019]. Clin Infect Dis 2019. doi:10.1093/cid/ciz502. [DOI] [PubMed] [Google Scholar]
- 25. et al. How to care for people exposed to drug-resistant tuberculosis: a practical guide. 1st ed. Boston, MA: Sentinel Project on Pediatric Drug-Resistant Tuberculosis, 2018. [Google Scholar]
- 26. Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika 1981; 68:373–9. [Google Scholar]
- 27. Thee S, Garcia-Prats AJ, Donald PR, Hesseling AC, Schaaf HS. A review of the use of ethionamide and prothionamide in childhood tuberculosis. Tuberculosis (Edinb) 2016; 97:126–36. [DOI] [PubMed] [Google Scholar]
- 28. Schaaf HS, Marais BJ. Management of multidrug-resistant tuberculosis in children: a survival guide for paediatricians. Paediatr Respir Rev 2011; 12:31–8. [DOI] [PubMed] [Google Scholar]
- 29. Swanson DS, Starke JR. Drug-resistant tuberculosis in pediatrics. Pediatr Clin North Am 1995; 42:553–81. [DOI] [PubMed] [Google Scholar]
- 30. Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:278–85. [PubMed] [Google Scholar]