Abstract
Background
Early-life risk factors, including maternal hyperglycaemia and birthweight, are thought to contribute to the high burden of cardiometabolic disease experienced by Indigenous populations. We examined rates of pre-existing diabetes in pregnancy, gestational diabetes mellitus (GDM) and extremes of birthweight over three decades in the Northern Territory (NT) of Australia.
Methods
We performed a retrospective cohort analysis of the NT Perinatal Data Collection from 1987 to 2016, including all births >20 weeks gestation, stratified by maternal Aboriginal identification. Key outcomes were annual rates of pre-existing diabetes, GDM, small-for-gestational-age, large-for-gestational-age, low birthweight (<2500 g), and high birthweight (>4000 g). Logistic regression was used to assess trends and interactions.
Findings
109 349 babies were born to 64 877 mothers, 36% of whom identified as Aboriginal ethnicity. Among Aboriginal women, rates of GDM and pre-existing diabetes, respectively, were 3 · 4% and 0 · 6% in 1987 and rose to 13% and 5 · 7% in 2016 (both trends p<0 · 001). Among non-Aboriginal women, rates of GDM increased from 1 · 9% in 1987 to 11% in 2016 (p<0 · 001), while pre-existing diabetes was uncommon (≤0 · 7% throughout). Rates of small-for-gestational-age decreased, while rates of large-for-gestational-age and high birthweight increased in both groups (all trends p<0 · 001). Multivariable modelling suggests that hyperglycaemia was largely responsible for the growing rate of large-for-gestational-age births among Aboriginal women.
Interpretation
The burden of hyperglycaemia in pregnancy has grown substantially in the NT over three decades and is impacting birthweight trends. The prevalence of pre-gestational diabetes in Aboriginal women is among the highest in the world.
Funding
Diabetes Australia Research Program.
Research in context.
Evidence before this study
Indigenous populations are disproportionately burdened by cardiometabolic conditions and there is growing interest in the role of intergenerational and early-life risk factors for adult disease. Pre-existing diabetes and gestational diabetes (GDM) are associated with adverse pregnancy and neonatal outcomes as well as long-term risk of cardiometabolic disease in both the mother and offspring. Low birthweight and high birthweight are also associated with future risk of diabetes and cardiovascular disease. A recent meta-analysis confirmed that Indigenous women from populations with similar histories of colonisation are at increased risk of pre-existing and gestational diabetes. However, existing evidence regarding contemporary trends in rates of diabetes during pregnancy and adverse birthweight in Indigenous populations is limited.
We searched PubMed with no language restrictions for human studies published prior to 29th February 2020 relating to Indigenous populations and either birthweight or hyperglycaemia in pregnancy. Search terms for Indigenous populations included “Indigenous Peoples”[MeSH], “Oceanic Ancestry Group”[MeSH], “Indians, North American”[MeSH], “Aboriginal”[Text], “Indigenous”[Text] or “First Nations”[Text]. Diabetes search terms included “Diabetes, Gestational”[MeSH], “Pregnancy in Diabetics”[MeSH], “Diabetes in pregnancy”[Text], “Pre-existing diabetes”[Text], “Pre-gestational diabetes”[Text], “Hyperglycemia in pregnancy”[Text] or “Hyperglycaemia in pregnancy”[Text]. Birthweight search terms included “Birth Weight”[MeSH], “birth weight”[Text] or “birthweight”[Text]. The search identified 721 publications, of which 55 reported relevant data regarding hyperglycaemia in pregnancy, high birthweight, or large-for-gestational-age. Of those, 13 reported on trends over time, including nine focusing on diabetes, three on macrosomia and one on indications for induction of labour. Only one of these included diabetes data from within the last decade. While studies confirmed that maternal hyperglycaemia was associated with higher birthweights, none investigated associations between the population-level trends in diabetes and large-for-gestational-age.
Added value of this study
In this population-based study of 109 349 births (36% to Aboriginal mothers) in the Northern Territory (NT) of Australia, we report increased rates of GDM, high birthweight and large-for-gestational-age between 1987 and 2016. Rates of pre-existing diabetes among pregnant Aboriginal women increased substantially. We believe Aboriginal women in the Central Australia region of the NT now have the highest ever reported prevalence of pre-existing diabetes in pregnancy (8 · 4% in 2016). The trends in large-for-gestational-age among births to Aboriginal mothers were strongly associated with the growing burden of GDM and pre-existing diabetes. We also observed favourable trends among pregnancies to Aboriginal women in relation to maternal age, stillbirth, preeclampsia, engagement with antenatal care and small-for-gestational-age.
Implications of all the available evidence
While recent decades have seen improvements in some perinatal outcomes among Indigenous populations, the growing burden of metabolic disease during pregnancy is concerning. High rates of hyperglycaemia in pregnancy has the potential to drive an escalating cycle of intergenerational cardiometabolic risk. Effective public health strategies, developed in partnership with Indigenous communities, are required to address this challenge.
Alt-text: Unlabelled box
Introduction
The global epidemic of diabetes over recent decades has disproportionately affected Indigenous peoples, who have some of the highest prevalence rates of diabetes and diabetes-related complications [1]. In Australia, Aboriginal and Torres Strait Islander people experience high rates of morbidity and premature mortality due to chronic disease and there is growing interest in the role of intergenerational and early-life cardiometabolic risk factors [2].
In multiple different populations, in-utero exposure to maternal hyperglycaemia has been associated with increased risk of diabetes later in childhood [2,3]. Similarly, both low and high birthweight are associated with future risk of diabetes and cardiovascular disease in adult life [2,4]. While birthweight is not causative in these associations, it is a useful marker of fetal wellbeing and growth, affected by numerous factors, many relating to maternal health and health behaviours. Investigating population trends in birthweight and hyperglycaemia in pregnancy improves understanding of recent epidemiological transitions and current burden of disease. It may also have implications for the health of future generations. Examining these trends together is of additional interest because maternal hyperglycaemia contributes to excess fetal growth [5]. Hyperglycaemia is also associated with numerous other pregnancy complications, including risk of preterm delivery and, thus, risk of low birthweight [5].
Hyperglycaemia in pregnancy can be due to pre-existing diabetes, including type 1 (T1D) and type 2 diabetes (T2D), or gestational diabetes mellitus (GDM). Each has significant implications for the short and long-term health of the mother and the baby [2,5,6]. Furthermore, the burden of hyperglycaemia in pregnancy on healthcare systems is growing due to the increasing prevalence of overweight and obesity, later age at childbearing and changes to screening practices and diagnostic criteria [7].
Aboriginal and Torres Strait Islander peoples have lived in Australia for over 60 000 years and now constitute 3% of the national population. Since colonisation, Aboriginal people have experienced intergenerational trauma, systematised discrimination, socioeconomic disadvantage and rapid loss of traditional lifestyles [8]. The Northern Territory (NT) of Australia spans an area of 1.35 million km2 and has a population of 246 000 people [9]. A large proportion of the population live in remote or rural areas and 30% identify as Aboriginal people [9]. Given its remoteness, some areas of the NT were not substantially affected by European colonisation until the mid-20th Century and there remains great strength of culture, diversity of language and connection to country. Studies of long-term health trends among Indigenous populations globally are often limited by historical deficiencies in data recording and changing propensity to identify as Indigenous. The NT is unique in that reliable health data for Aboriginal people have been recorded for many years and individuals’ propensity to identify as Aboriginal has been relatively stable [10].
In this context, we examined rates of pre-existing diabetes, GDM, large-for-gestational-age, high birthweight, small-for-gestational-age and low birthweight over three decades among all births to Aboriginal and non-Aboriginal women in the NT.
Methods
Data source
We retrospectively analysed data from the NT Perinatal Data Collection between 1st January 1987 and 31st December 2016. The NT Perinatal Data Collection is a population-based census of all births in the NT, including hospital and non-hospital births. The dataset includes extensive information relating to demographics, antenatal care, pregnancy, labour and childbirth, perinatal outcomes, and maternal health, including diabetes status. The data are collected and entered by midwives shortly following a delivery. The dataset is maintained by the NT Department of Health. Hyperglycaemia in pregnancy status has been cross-referenced against hospital admission records since 2008. Since 2014, additional data from the NT Diabetes in Pregnancy Clinical Register have also been incorporated [11].
Study population
Data from births of at least 20 weeks gestation were included. For mothers who had more than one pregnancy during the study period, each pregnancy was included. In the event of a multiple pregnancy (i.e. twins and triplets), the pregnancy was only counted once for the hyperglycaemia in pregnancy trend analyses. Analyses of birthweight were conducted among singleton livebirths to maintain consistency with birthweight centile reference data. Records missing a maternal identification number (n = 32), baby's date of birth (n = 8) or ethnicity (n = 30) were excluded.
Outcome definitions
Hyperglycaemia in pregnancy was recorded as pre-existing diabetes or GDM. Diagnostic criteria for GDM and T2D have changed over time. In 1991, the Australasian Diabetes in Pregnancy Society (ADIPS) developed guidelines for universal GDM screening [12]. Prior to that, diverse approaches were used across Australia [13]. In 2013, ADIPS adopted the International Association for Diabetes in Pregnancy Study Groups (IADPSG) recommendations to implement a one-step screening process with revised diagnostic criteria [14]. Details of the different diagnostic thresholds used over time for GDM and T2D are summarised in appendix Tables 1 and 2. Information on type of pre-existing diabetes was only available from 2014. Small-for-gestational-age was defined as <10th percentile and large-for-gestational-age as >90th percentile, according to national birthweight percentiles by sex and gestational age [15]. Low birthweight was defined as <2500 g [16]. High birthweight was defined as >4000 g given the lower birthweights of Aboriginal babies on average, as per previous NT Government reports [16].
Table 1.
Demographic factors, maternal clinical history and pregnancy outcomes stratified by decade among Aboriginal and non-Aboriginal mothers in the Northern Territory 1987–2016.
| Pregnancies of Aboriginal mothers(n = 39 327) |
Pregnancies of non-Aboriginal mothers(n = 68 753) |
|||||
|---|---|---|---|---|---|---|
| 1987–1996 | 1997–2006 | 2007–2016 | 1987–1996 | 1997–2006 | 2007–2016 | |
| Denominator – Pregnancies | n = 11 936 | n = 13 532 | n = 13 859 | n = 21 311 | n = 22 353 | n = 25 089 |
| Rural/remote location | 8836 (74) | 9779 (72) | 9532 (69)* | 7645 (36) | 3856 (17) | 3138 (13)* |
| Top end region | 7693 (64) | 9038 (67) | 9248 (67)* | 16 822 (79) | 18 636 (83) | 21 500 (86)* |
| Maternal age | 23 · 5 ± 5 · 7 | 24 · 1 ± 5 · 9 | 25 · 4 ± 6 · 1* | 28 · 2 ± 5 · 3 | 29 · 4 ± 5 · 7 | 30 · 3 ± 5 · 5* |
| Smoked during pregnancy | NA | 6174 (48) | 6888 (51)* | NA | 4740 (22) | 2841 (12)* |
| Nulliparous | 3774 (32) | 4373 (32) | 4427 (32) | 9222 (43) | 9709 (43) | 11 269 (45)* |
| Pre-existing renal disease | NA | 291 (2 · 2) | 345 (2 · 5) | NA | 119 (0 · 5) | 68 (0 · 3)* |
| Pre-existing cardiac disease | NA | 649 (4 · 8) | 763 (5 · 5)† | NA | 185 (0 · 8) | 137 (0 · 6)* |
| Twin pregnancy | 94 (0 · 8) | 110 (0 · 8) | 161 (1 · 2) * | 260 (1 · 2) | 315 (1 · 4) | 311 (1 · 2) |
| Pre-term delivery | 1641 (14) | 2005 (15) | 2137 (15)* | 1378 (6 · 5) | 1706 (7 · 6) | 1672 (6 · 7) |
| Preeclampsia | 915 (7 · 7) | 784 (5 · 8) | 662 (4 · 8)* | 1191 (5 · 6) | 958 (4 · 3) | 794 (3 · 2)* |
| Gestation at 1st antenatal review | 20 (14–26) | 18 (10–24) | 13 (8–20)* | 13 (9–19) | 12 (8–17) | 10 (7–12)* |
| Caesarean delivery | 2412 (20) | 3210 (24) | 3945 (29)* | 3598 (17) | 5736 (26) | 7984 (32)* |
| Induction of labour | 1426 (12) | 2263 (17) | 3242 (23)* | 4413 (21) | 5229 (23) | 6511 (26)* |
| Denominator – Babies | n = 12 036 | n = 13 644 | n = 14 020 | n = 21 577 | n = 22 668 | n = 25 404 |
| Stillbirth | 244 (2 · 0) | 187 (1 · 4) | 191 (1 · 4)* | 177 (0 · 8) | 156 (0 · 7) | 153 (0 · 6)† |
| Denominator – Singleton livebirths | n = 11 607 | n = 13 238 | n = 13 517 | n = 20 888 | n = 21 894 | n = 24 640 |
| Gestation at delivery | 40 (38–40) | 39 (38–40) | 39 (38–40)* | 40 (39–40) | 40 (38–40) | 39 (38–40)* |
| Male offspring | 6006 (52) | 6826 (52) | 6995 (52) | 10 742 (51) | 11 228 (51) | 12 665 (51) |
| Birthweight, grams | 3084 ± 614 | 3124 ± 650 | 3136 ± 670* | 3337 ± 541 | 3379 ± 559 | 3403 ± 539* |
Data presented as n (%), mean ± SD, median (IQR). *P ≤ 0 · 001, †P<0 · 05 for trend over decades. NA = not available.
Table 2.
Odds ratios for large-for-gestational-age over time (per 10 years*) among singleton livebirths with and without adjustment for hyperglycaemia in pregnancy between 1987 and 2016.
| OR (95% CI, p-value) for large-for-gestational-age over time* |
||||
|---|---|---|---|---|
| Model 1† | Model 1 + GDM | Model 1 + pre-existing diabetes | Model 1 + GDM + pre-existing diabetes | |
| Aboriginal mothers (n = 33 714) | 1 · 12 (1 · 06–1 · 19, p<0 · 001) | 1 · 08 (1 · 02–1 · 14, p = 0 · 010) | 1 · 07 (1 · 01–1 · 14, p = 0 · 016) | 1 · 02 (0 · 96–1 · 08, p = 0 · 510) |
| Non-Aboriginal mothers (n = 61 479) | 1 · 12 (1 · 08–1 · 16, p<0 · 001) | 1 · 11 (1 · 07–1 · 15, p<0 · 001) | 1 · 12 (1 · 08–1 · 16, p<0 · 001) | 1 · 11 (1 · 07–1 · 15, p<0 · 001) |
ORs calculated using multivariable logistic regression. *Year of birth as a continuous variable divided by 10. †Adjusted for parity, gestation at first antenatal review, remote or rural location, and maternal age. OR = odds ratio, GDM = gestational diabetes mellitus.
Covariate definitions
Aboriginal ethnicity is self-identified. The authors recognise the importance of using acceptable terminology to identify the diverse cultures and peoples who make up Australia's First Peoples. The term “Aboriginal” people is generally preferred in the NT context, whereas “Aboriginal and Torres Strait Islander” people is the preferred collective term nationally [17]. Maternal age is the mother's age in years on the day she gave birth. Pre-term delivery indicates birth prior to 37 weeks gestation. Urban and rural/remote location is based on the usual residence of the mother, rather than the location of the birth. Urban locations included the Darwin and Alice Springs urban districts, with other districts considered rural/remote. NT region was based primarily on the hospital of birth or, for non-hospital births, on the health district of the mother's usual residential location [16]. Smoking status incorporates current smoking noted at any timepoint in pregnancy. Pre-eclampsia, maternal history of renal disease and maternal history of cardiac disease are as recorded in the dataset by midwives, without specific definitions. These are likely based on diagnoses listed in the hospital clinical record. Smoking status and history of renal or cardiac disease have only been recorded since 1996.
Statistical methods
All analyses were stratified by maternal Aboriginal identification. Descriptive analyses of the cohort were further stratified by decade. Descriptive data are presented as number (%), mean ± standard deviation or median (interquartile range). Trends in descriptive variables over decades were assessed using linear regression for normally distributed continuous variables, logistic regression for binary variables and the Cuzick non-parametric test for trend for non-normally distributed continuous variables. Hyperglycaemia and birthweight trends data are presented as annual rates (%) with 95% confidence intervals (CI). Statistical significance of trends over time within each ethnic group were assessed using logistic regression. Differences in the trends between the ethnic groups were assessed using ethnicity-year interaction terms in logistic regression models. Given known demographic, historical and epidemiological differences, additional subgroup analyses examined findings for Aboriginal women separately for the two broad NT regions (Top End or Central Australia). The Aboriginal population in the NT incorporates numerous distinct people groups, with over 100 languages spoken [18]. The Top End and Central Australia are the northern and southern regions, respectively, of the NT and are serviced by two separate publicly-funded health services. Previous research has demonstrated differences in social factors, environment, and health outcomes between these regions [19,20]. Hyperglycaemia in pregnancy rates were also assessed separately for urban or remote/rural-dwelling mothers. The contribution of hyperglycaemia in pregnancy to the association between large-for-gestational-age and time (in decades) was assessed using multivariable logistic regression with and without hyperglycaemia status in the model. Other variables were selected for inclusion in these multivariable models if an association with birthweight was plausible, a significant association was seen on univariable analysis and the rate or distribution of the variable changed over time. To account for mothers who had more than one pregnancy during the study period being included in these models on multiple occasions, cluster-robust variance estimates were used.
Data were non-missing in >99% of records for all variables aside from smoking status and gestation at first antenatal review. Smoking status was missing (since 1996) for 3573 (7 · 5%) of non-Aboriginal mothers and 4106 (15%) of Aboriginal mothers. If a mother with missing smoking data for a pregnancy had more than one pregnancy during the study period, her smoking status was assumed to be the same as in her most recent pregnancy in which it was recorded. Following this adjustment, 897 (3 · 3%) pregnancies to Aboriginal mothers and 1749 (3 · 7%) pregnancies to non-Aboriginal mothers were missing smoking data and were excluded from the descriptive analyses. The 3223 (8 · 2%) pregnancies to Aboriginal mothers and 5426 (7 · 9%) pregnancies to non-Aboriginal mothers missing gestation at first antenatal review were excluded from the descriptive analyses and multivariable logistic regression models.
Analyses were performed in Stata v.15 · 1 (StataCorp, Texas).
Ethics
The study was approved by the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research, including the Aboriginal Ethics Sub-Committee, and the Central Australian Human Research Ethics Committee.
Role of the funding source
The study was supported by a competitive grant from the Diabetes Australia Research Program. The funding body had no other input into the study. The corresponding author had full access to all data and final responsibility for the decision to submit for publication.
Results
Cohort description
From 1987 to 2016, 109 349 babies were born to 64 877 mothers (Fig. 1). Of these, 39 700 (36%) were born to Aboriginal mothers, 83 998 (77%) were born in the Top End region and 43 199 (40%) were born to mothers from rural or remote areas. Mean maternal age and birthweight increased over time among pregnancies of both Aboriginal and non-Aboriginal mothers, while rates of preeclampsia and stillbirth decreased (Table 1). Rates of smoking during pregnancy increased among Aboriginal mothers and decreased among non-Aboriginal mothers. Over the three decades, women completed their first antenatal review at earlier gestations and induction of labour and Caesarean delivery became more common.
Fig. 1.
Flow chart showing cohort numbers of babies, mothers, pregnancies and singleton livebirths included in the study.
Descriptions of the cohort stratified by region (Top End vs. Central Australia) are presented in Appendix Tables 3 and 4. Notable differences include lower rates of smoking and higher rates of induction of labour in Central Australia for Aboriginal women. Among non-Aboriginal women, Caesarean delivery and induction of labour were more frequent in the Top End.
Hyperglycaemia in pregnancy trends
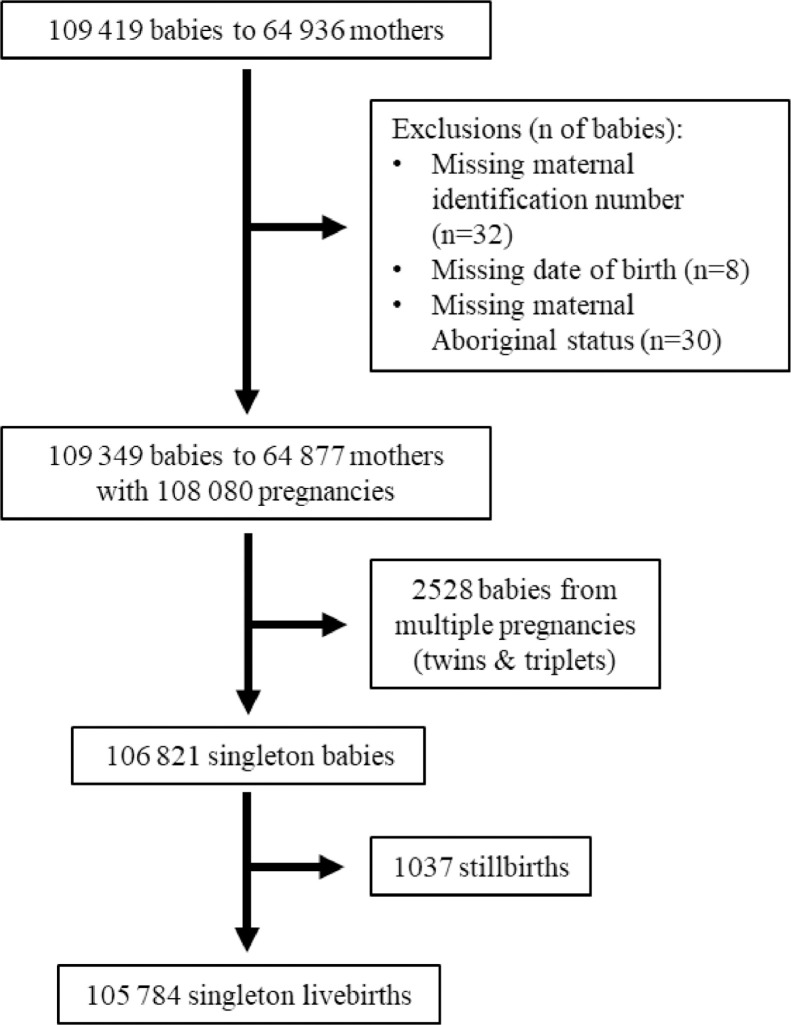
Rates of hyperglycaemia in pregnancy increased over time. The rate of change varied between the ethnic groups as evidenced by ethnicity-year interactions for both GDM and pre-existing diabetes (both p<0 · 001). Among Aboriginal women, the rate of GDM rose from 3 · 4% in 1987 to 13% in 2016 and the rate of pre-existing diabetes during pregnancy rose from 0 · 6% in 1987 to 5 · 7% in 2016 (Fig. 2A). GDM complicated 1 · 9% of pregnancies to non-Aboriginal women in 1987 compared to 11% in 2016, while pre-existing diabetes was infrequent (≤0 · 7% throughout, Fig. 2B). In the three most recent years of the study, T2D accounted for 99% and 55% of pre-existing diabetes among Aboriginal women and non-Aboriginal women, respectively (Appendix Table 5).
Fig. 2.
Annual rates of gestational diabetes (GDM) and pre-existing diabetes among pregnancies to (A) Aboriginal and (B) non-Aboriginal women between 1987 and 2016. Shaded areas are 95% confidence intervals. All trends p<0 · 001. Point estimates for trends, as odds ratios per 10 years (95% confidence interval), were: 1 · 66 (1 · 58–1 · 74) for GDM and 2 · 54 (2 · 31–2 · 81) for pre-existing diabetes among Aboriginal women; and 1 · 88 (1 · 80–1 · 96) for GDM and 1 · 36 (1 · 17–1 · 57) for pre-existing diabetes among non-Aboriginal women. Mean total number of pregnancies per annum for Aboriginal and non-Aboriginal mothers, respectively, were 1194 and 2131 for the first decade, 1353 and 2235 for the second decade, and 1386 and 2509 in the third decade.
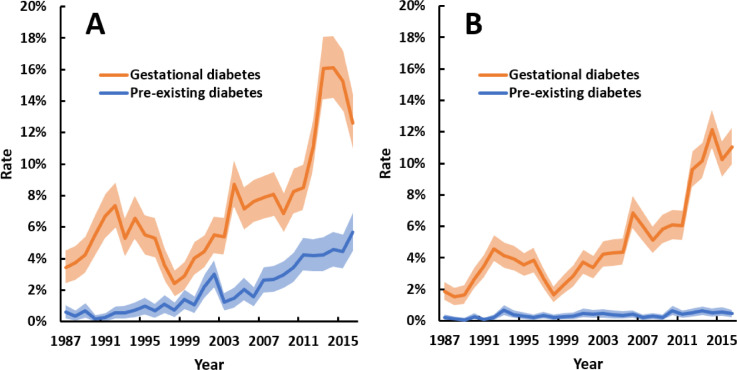
There were differences in GDM trends over time among Aboriginal women between regions (p<0 · 001 for interaction). GDM was initially more frequently reported in Central Australia, but since 2010 has been more common in the Top End (Fig. 3). Nevertheless, contemporary overall rates of hyperglycaemia in pregnancy are higher in Central Australia due to an increase in pre-existing diabetes from 0 · 9% in 1987 to 8 · 4% in 2016 (all of which were T2D). There were more diagnoses of GDM and pre-existing diabetes in urban-dwelling Aboriginal women in the early years of the study, but for the last decade both conditions were more commonly reported among women from rural or remote areas (Appendix Fig. 1).
Fig. 3.
Annual rates of gestational diabetes and pre-existing diabetes among pregnancies to Aboriginal women in (A) Top End and (B) Central Australia regions of the NT between 1987 and 2016. Shaded areas are 95% confidence intervals. All trends p<0 · 001. Point estimates for trends, as odds ratios per 10 years (95% confidence interval), were: 1 · 97 (1 · 85–2 · 10) for GDM and 2 · 40 (2 · 09–2 · 75) for pre-existing diabetes in the Top End; and 1 · 29 (1 · 20–1 · 39) for GDM and 2 · 74 (2 · 37–3 · 16) for pre-existing diabetes in Central Australia. Mean total number of pregnancies per annum for Aboriginal women in the Top End and Central Australia regions, respectively, were 769 and 422 for the first decade, 904 and 449 for the second decade, and 925 and 461 in the third decade.
Birthweight trends
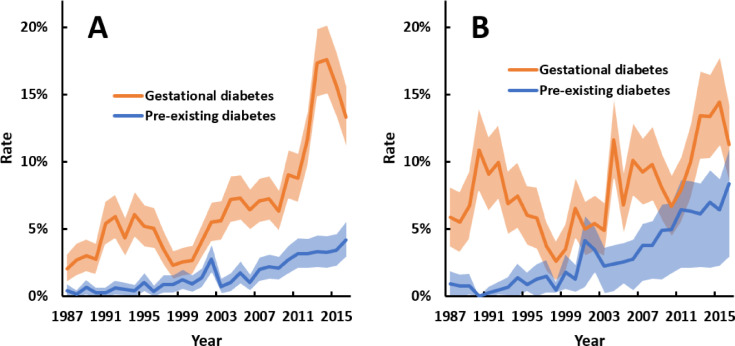
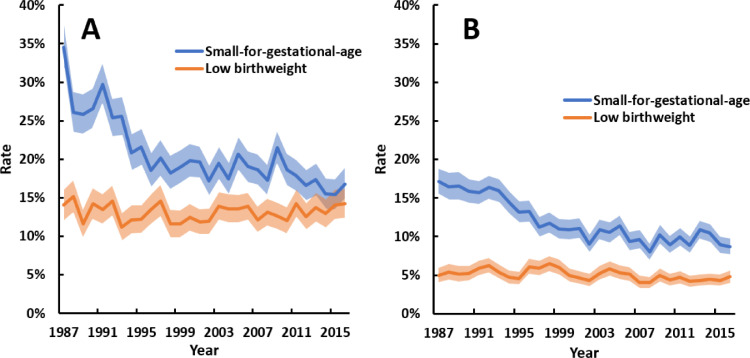
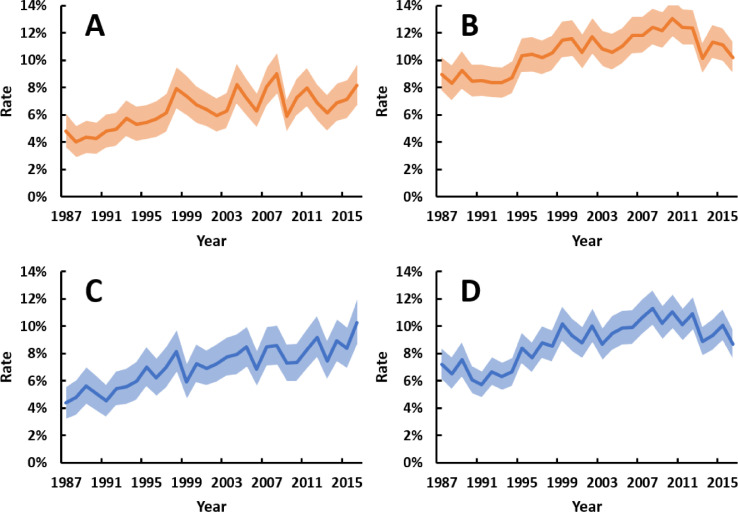
Trends in small-for-gestational-age were similar among births to Aboriginal and non-Aboriginal mothers (p = 0 · 297 for ethnicity-year interaction), with substantial reductions over time (Fig. 4). The rate of low birthweight did not change among births to Aboriginal mothers but decreased slightly among births to non-Aboriginal mothers. High birthweight and large-for-gestational age became more common over time in both ethnic groups (Fig. 5). The average increase in high birthweight and large-for-gestational-age rates over time was greater among births to Aboriginal mothers (ethnicity-year interactions: p = 0 · 041 for high birthweight, p = 0 · 072 for large-for-gestational-age). In 1987, 4 · 4% of births to Aboriginal mothers were large-for-gestational-age, compared to 10% in 2016.
Fig. 4.
Annual rates of small-for-gestational-age (SGA) and low birthweight (<2500 g) among singleton livebirths with (A) Aboriginal and (B) non-Aboriginal mothers between 1987 and 2016. Shaded areas are 95% confidence intervals. All trends p<0 · 001, except low birthweight in panel A (p = 0 · 793). Point estimates for trends, as odds ratios per 10 years (95% confidence interval), were: 0 · 78 (0 · 76–0 · 80) for SGA and 1 · 00 (0 · 97–1 · 04) for low birthweight among Aboriginal births; and 0 · 76 (0 · 74–0 · 78) for SGA and 0 · 91 (0 · 87–0 · 95) for low birthweight among non-Aboriginal births. Mean total number of singleton livebirths per annum for Aboriginal and non-Aboriginal mothers, respectively, were 1161 and 2089 for the first decade, 1324 and 2189 for the second decade, and 1352 and 2464 in the third decade.
Fig. 5.
Annual rates of singleton liveborn babies with (A) high birthweight (>4000 g) and Aboriginal mothers, (B) high birthweight (>4000 g) and non-Aboriginal mothers, (C) large-for-gestational-age (LGA) and Aboriginal mothers, and (D) LGA and non-Aboriginal mothers between 1987 and 2016. Shaded areas are 95% confidence intervals. All trends p<0 · 001. Point estimates for trends, as odds ratios per 10 years (95% confidence interval), were: 1 · 20 (1 · 14–1 · 26) and 1 · 13 (1 · 10–1 · 16) for high birthweight among Aboriginal and non-Aboriginal births, respectively; and 1 · 25 (1 · 19–1 · 31) and 1 · 19 (1 · 15–1 · 23) for LGA among Aboriginal and non-Aboriginal births, respectively. Mean total number of singleton livebirths per annum for Aboriginal and non-Aboriginal mothers, respectively, were 1161 and 2089 for the first decade, 1324 and 2189 for the second decade, and 1352 and 2464 in the third decade.
Associations between hyperglycaemia and large-for-gestational-age rates
Multivariable logistic regression models were used to assess whether trends in large-for-gestational-age over time were related to changing rates of hyperglycaemia in pregnancy (Table 2). Among births to Aboriginal mothers, there was a 12% increase per decade in the odds of large-for-gestational-age after adjustment for parity, gestation at first antenatal review, remote or rural location and maternal age (OR 1 · 12, 95% CI: 1 · 06–1 · 19, p<0.001). Accounting for GDM in this model partially attenuated the increase in large-for-gestational-age over time (OR 1 · 08, 95% CI: 1 · 02–1 · 14, p = 0.010). Alternatively, adding pre-existing diabetes to the model also partially attenuated the increase in large-for-gestational-age over time (OR 1 · 07, 95% CI: 1 · 01–1 · 14, p = 0.016). When both forms of hyperglycaemia in pregnancy were incorporated in the model, the relationship between large-for-gestational-age and time was no longer significant (OR 1 · 02, 95% CI: 0 · 96–1 · 08, p = 0 · 510). This was not the case among births to non-Aboriginal mothers. There was a similar 12% increase per decade in the odds of large-for-gestational-age (OR 1 · 12, 95% CI: 1 · 08–1 · 16, p<0.001). However, accounting for hyperglycaemia in pregnancy in the model had minimal effect on the point estimate and the relationship between large-for-gestational-age and time remained significant (OR 1 · 11, 95% CI: 1 · 07–1 · 15, p<0 · 001).
Discussion
Over three decades, the NT has experienced substantial epidemiological transitions in relation to hyperglycaemia in pregnancy and birthweight. Large increases in the rate of GDM were observed among both Aboriginal and non-Aboriginal women. We found that approximately one in five pregnancies to Aboriginal women in recent years has been complicated by hyperglycaemia despite relatively young maternal ages. The epidemic of pre-existing diabetes among pregnant Aboriginal women is particularly concerning, with the rate having increased almost 10-fold. The rate of pre-existing T2D in pregnancy observed among Aboriginal women in Central Australia (8 · 4% in 2016) is, to our knowledge, the highest ever reported in any population-based cohort. This growing burden of hyperglycaemia in pregnancy in the NT is impacting the next generation, accounting for much of the observed rise in large-for-gestational-age observed over the study period. Despite the lower absolute number of women affected, pre-existing diabetes is as strongly associated with the trends in large-for-gestational-age at a population level as GDM.
Small-for-gestational-age affected more than a third of births to Aboriginal women in the late 1980s. The rate has now decreased to under 17%, which still indicates a disproportionate number of babies classified below the national 10th centile of birthweight [15]. Rates of small-for-gestational-age also approximately halved among non-Aboriginal births, with the 2016 rate of 8 · 7% being low compared to the national reference population. At the other end of the spectrum, rates of high birthweight and large-for-gestational-age increased over the 30 years. The rate of large-for-gestational-age among births to Aboriginal mothers more than doubled. Our multivariable regression analyses suggest that this trend is primarily driven by increasing rates of hyperglycaemia in pregnancy in this population.
The observed trends are in keeping with the growing prevalence of diabetes globally and the high prevalence of T2D across the life course among Aboriginal and Torres Strait Islander Australians [21]. A recent systematic review and meta-analysis confirmed that Indigenous populations with a similar history of colonisation have increased risk of both pre-existing diabetes during pregnancy and GDM compared to non-Indigenous people [22]. The highest prevalence of pre-existing diabetes reported in this review was 4 · 0% among Aboriginal women in the NT in 2013, based on the same dataset used for our study, without stratification by region. The next highest prevalence rates were 3 · 9% among two First Nations populations in Quebec and Ontario, Canada. Seminal research among the high-risk Pima Indian population of Arizona in the 1960–70s reported a 6 · 3% prevalence of pre-existing diabetes among pregnant women [23]. The high burden of pre-existing diabetes we have observed among Aboriginal women in Central Australia is consistent with studies among the non-pregnant population of this region, which show a higher burden of cardiometabolic conditions, including diabetes, hypertension, and renal disease [20].
Studies reporting trends in hyperglycaemia in pregnancy among Indigenous populations are limited. Growth in the burden of GDM among Aboriginal and Torres Strait Islander women nationally in Australia was reported from 1990 to 2009 [24]. We now show that these trends have continued in the NT and additionally report on pre-existing diabetes, which has increased to an even greater extent. Within the First Nations population of Saskatchewan, Canada, from 1980 to 2009 the rate of GDM increased from 1 · 0% to 6 · 6% and the rate of pre-existing diabetes increased from 0 · 7% to 2 · 0% [25]. Data from First Nations women in neighbouring Alberta, show similar contemporary rates of GDM and pre-existing diabetes, but rates were stable between 2000 and 2009 [26]. In the same population and time period, the prevalence of high birthweight (>4000 g) decreased, while low birthweight (<2500 g) remained stable [27]. GDM and maternal weight ≥91 kg were risk factors for high birthweight in the Alberta study but contribution to trends over time was not assessed. In Québec, rates of extreme high birthweight (>5000 g) and large-for-gestational-age (>97th percentile) increased between 1981 and 2008 for births to First Nations but not Inuit women [28]. This study had no data on hyperglycaemia in pregnancy. Our study was able to investigate birthweight and hyperglycaemia trends concurrently, showing increases in both high birthweight and large-for-gestational-age over time. Furthermore, we showed that the trends in large-for-gestational-age were strongly associated with the increase in frequency of hyperglycaemia in pregnancy.
Positive changes over this time period, such as improved antenatal care, have likely contributed to the trends in small-for-gestational-age. This is evidenced by the earlier gestation of first antenatal review over time, as well as improving rates of other pregnancy complications including stillbirth and preeclampsia. Fewer births to teenage mothers may also have contributed to reductions in small-for-gestational-age [29]. A previous study showed that babies of teenage Aboriginal mothers in the Northern Territory had lower birthweights than babies of adult mothers, but that this difference was related to factors other than age, such as remoteness, engagement with antenatal care and health behaviours [30].
The lack of change in low birthweight rates among births to Aboriginal women has multiple potential explanations. Low birthweight is highly correlated with pre-term birth, which was essentially stable in frequency over the study period. This may relate to improved neonatal intensive care services in Darwin, meaning that fewer high-risk pregnancies were transferred to specialised centres outside the NT. However, persistent high rates of smoking during pregnancy, food insecurity and other socioeconomic disparities experienced by Aboriginal women are other potential contributing factors to the persistent rates of low birthweight and pre-term birth [5,31]. Hyperglycaemia in pregnancy may be another contributing factor. The prospective PANDORA (Pregnancy And Neonatal Diabetes Outcomes in Remote Australia) study observed pre-term birth rates of 39% among Aboriginal women with T2D compared to 5 · 1% among those with normoglycaemia [5].
Multiple factors are likely to be driving the epidemic of diabetes during pregnancy in the NT population, with social determinants of health and obesity playing considerable roles. Almost half of pregnant women in Australia now have overweight or obesity [32]. To some extent the increase in GDM diagnoses among both Aboriginal and non-Aboriginal women will also relate to improved uptake of screening, the change from two-step screening to a single oral glucose tolerance test, and changes to diagnostic criteria. In the Aboriginal population, the trends we have observed are consistent with other research that has shown substantial epidemiological transitions over recent decades, with reductions in infant and childhood mortality and the emergence of high rates of chronic non-communicable diseases [33]. The growing burden of cardiometabolic disease has followed a relatively rapid transition towards more sedentary lifestyles and access to high caloric Western foods. This emergence of non-communicable diseases has occurred at different times across the NT, usually reflecting relative time of contact with Western lifestyles as a result of colonisation.
The contemporary high rates of pre-existing diabetes in pregnancy among Aboriginal women reflect the growing burden of youth-onset type 2 diabetes in this population [34]. It has been noted that with each generation, the age of onset of type 2 diabetes continues to decrease, with children as young as 5 years now being diagnosed [35,36]. This public health challenge is rooted in the social determinants of health, including poverty, discrimination, and rapid loss of traditional cultures and lifestyles post-colonisation [35]. The high cost of fresh food and food insecurity are significant barriers to healthy diets, particularly in remote communities [31]. We believe that these factors are contributing to an escalating intergenerational cycle of adverse metabolic health. In previous generations, high rates of restricted fetal growth were reported, but now we are observing increasing rates of hyperglycaemia in pregnancy, which are likely contributing to more youth-onset type 2 diabetes in each subsequent generation. This hypothesis aligns with observational evidence from other cohorts showing associations of both low-birth weight and in-utero exposure to maternal hyperglycaemia with future diabetes risk [2–4]. The causes of this intergenerational risk are unproven but may be mediated by changes in gene expression, controlled by epigenetic mechanisms [37].
Strengths of this study include the use of a population-wide dataset which minimises selection bias and improves generalisability. The NT provides a unique opportunity to investigate high risk groups for hyperglycaemia in pregnancy, given the high proportion of Aboriginal women compared to other Australian jurisdictions and the availability of reliable data. The accuracy of Aboriginal identification in NT Department of Health hospital data was estimated at 94% in 1997 and 98% in 2011 [38]. An earlier validation study by the NT Department of Health of Aboriginal identification in death records between 1979 and 1991 showed 97% accuracy [39]. Our study is also strengthened by the inclusion of broad descriptive data as well as concurrent investigation of birthweight and hyperglycaemia trends.
The limitations of our study relate to the retrospective approach, using existing data. First, there were no data on maternal obesity which is likely to be a driver of both hyperglycaemia and birthweight and has increased over time in the general Aboriginal and non-Aboriginal female population [31]. Unmeasured confounding from maternal obesity could explain some of the observed association between hyperglycaemia and large-for-gestational-age trends. Alternatively, hyperglycaemia may be on a causal pathway between maternal obesity and large-for-gestational-age. Nevertheless, the PANDORA study confirmed that maternal hyperglycaemia, independent of obesity, was associated with risk of large-for-gestational-age [5]. Second, capture of known cases of hyperglycaemia in pregnancy in the NT Perinatal Data Collection will have varied over time, likely being more complete in recent years due to increasing validation against other data sources. This could have exaggerated the observed trends showing increasing rates of hyperglycaemia in pregnancy over time. Third, while universal GDM screening has been recommended in Australia since 1991, screening rates may have increased over time [12]. It remains likely that current rates of hyperglycaemia in pregnancy are underestimates due to evidence of incomplete screening uptake. A 2015 audit in the Central Australia region found that 23% of pregnant women had no screening for diabetes during pregnancy [40]. Fourth, the approach to screening for GDM has changed multiple times as noted in the Methods section. Overall, these changes in screening practices are likely to have increased rates of GDM over time as seen in other cohorts [7]. Finally, estimates of gestational age are likely to have some limitations. Presentation for antenatal care occurred later in pregnancy in the early years of the study, meaning both sonographic estimation of gestational age and recollection of the last menstrual period would be less reliable.
In summary, the burden of GDM and pre-existing diabetes in pregnancy has grown substantially in the NT over three decades. Rates of T2D among Aboriginal women are particularly concerning. There have been numerous improvements in antenatal care and perinatal outcomes over the same period. However, there are increasing numbers of large-for-gestational-age babies. Among births to Aboriginal women, this trend is related to hyperglycaemia in pregnancy. This study could not assess the role of maternal overweight and obesity in these associations, which highlights the need for improved capture of maternal weight data in both clinical practice and research databases. The epidemic of hyperglycaemia in pregnancy has both short and long-term implications for the health of mothers, babies and future generations. Further research into these intergenerational effects among Aboriginal people in Australia is warranted to guide public health priorities. There is also an ongoing need for preventative health initiatives with intersectoral collaboration to address and reverse these trends. The importance of working with Aboriginal communities to co-design culturally appropriate strategies focussing on the metabolic health of young people and women, particularly aiming for a healthy weight, before, during and after pregnancy, cannot be underestimated.
Declaration of Competing Interest
MJLH, FB, SG, RFD, ELMB, GS, VW, JES and LJMB report a competitive grant from the Diabetes Australia Research Program for this study. MJLH also reports scholarship support from the National Health and Medical Research Council (NHMRC), Diabetes Australia and the Australian Academy of Science. JAB, JES and LJMB also report competitive fellowships or grants from the NHMRC. JES has received honoraria for lectures and consultancies unrelated to this study from AstraZeneca, Eli Lilly, Mylan, Novo Nordisk, Sanofi, Merck Sharp and Dohme, Abbott and Boehringer Ingelheim.
Acknowledgments
Contributions
MJLH undertook the analyses and wrote the manuscript. LJMB supervised all aspects of the study. FB, LJMB, SG and RFD conceived the project. FB led funding, ethics and data access applications. MJLH and FB designed and interpreted the analyses. JAB, SG, RFD, ELMB, GS, HF, JES and LJMB revised the analysis plan and assisted in interpreting the data. VW coordinated input from the Diabetes across the Lifecourse: Northern Australia Partnership Indigenous Reference Group and critically revised the manuscript from an Aboriginal perspective. All authors critically revised the manuscript for important intellectual content and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge the NT Government Department of Health as the data owner of the NT Perinatal Data Collection, Dr Shu Qin Li and Leanne O'Neil for provision of the data and maintaining the collection, and the many midwives who have entered data over many years. We also acknowledge the Diabetes across the Lifecourse: Northern Australia Partnership Indigenous Reference Group for their advice on this work. This project was supported by a Diabetes Australia Research Program General Grant. MJLH is supported by National Health and Medical Research Council (NHMRC) Postgraduate Scholarship #1169091, the NHMRC/Diabetes Australia Postgraduate Award and the Australian Academy of Science Douglas and Lola Douglas Scholarship in Medical Science. JES is supported by NHMRC Investigator Grant #1173952. JAB is supported by a NHMRC Career Development Fellowship. LMB is supported by NHMRC Practitioner Fellowship #1078477. Funding bodies had no role in the study design, analysis, interpretation, manuscript preparation or decision to submit the manuscript for publication.
Data sharing
The Northern Territory Government Department of Health is the owner of the NT Perinatal Data Collection. Any access to the deidentified study dataset would require relevant approvals from the Department of Health and the support of the study investigators.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2020.100005.
Appendix. Supplementary materials
References
- 1.Naqshbandi M., Harris S.B., Esler J.G., Antwi-Nsiah F. Global complication rates of type 2 diabetes in Indigenous peoples: a comprehensive review. Diabetes Res Clin Pract. 2008;82(1):1–17. doi: 10.1016/j.diabres.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 2.McNamara B.J., Gubhaju L., Chamberlain C., Stanley F., Eades S.J. Early life influences on cardio-metabolic disease risk in aboriginal populations – what is the evidence? A systematic review of longitudinal and case-control studies. Int J Epidemiol. 2012;41(6):1661–1682. doi: 10.1093/ije/dys190. [DOI] [PubMed] [Google Scholar]
- 3.Lowe W.L., Jr., Scholtens D.M., Kuang A. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372–380. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knop M.R., Geng T.T., Gorny A.W. Birth weight and risk of Type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta-analysis of 7 646 267 participants from 135 studies. J Am Heart Assoc. 2018;7(23) doi: 10.1161/JAHA.118.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maple-Brown L., Lee I.L., Longmore D. Pregnancy and neonatal diabetes outcomes in remote Australia: the PANDORA study-an observational birth cohort. Int J Epidemiol. 2019;48(1):307–318. doi: 10.1093/ije/dyy245. [DOI] [PubMed] [Google Scholar]
- 6.Kramer C.K., Campbell S., Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–914. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y., Zhang C. Prevalence of gestational diabetes and risk of progression to Type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United Nations Department of Economic and Social Affairs. State of the World's Indigenous Peoples. ST/ESA/328. New York: United Nations, 2009.
- 9.Northern Territory Government Department of Treasury and Finance. Northern Territory Economy – Population. 2020. https://nteconomy.nt.gov.au/population (accessed 28th January 2020).
- 10.Condon J.R., Zhang X., Dempsey K., Garling L., Guthridge S. Trends in cancer incidence and survival for Indigenous and non-Indigenous people in the Northern Territory. Med J Aust. 2016;205(10):454–458. doi: 10.5694/mja16.00588. [DOI] [PubMed] [Google Scholar]
- 11.Kirkham R., Whitbread C., Connors C. Implementation of a diabetes in pregnancy clinical register in a complex setting: findings from a process evaluation. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0179487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin F.I. The diagnosis of gestational diabetes. Ad Hoc Working Party. Med J Aust. 1991;155(2):112. [PubMed] [Google Scholar]
- 13.Hunter A., Doery J.C., Miranda V. Diagnosis of gestational diabetes in Australia: a national survey of current practice. Med J Aust. 1990;153(5):290–292. doi: 10.5694/j.1326-5377.1990.tb136904.x. [DOI] [PubMed] [Google Scholar]
- 14.Australasian Diabetes in Pregnancy Society. ADIPS Consensus Guidelines for the Testing and Diagnosis of Gestational Diabetes Mellitus in Australia, 2013.
- 15.Dobbins T.A., Sullivan E.A., Roberts C.L., Simpson J.M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust. 2012;197(5):291–294. doi: 10.5694/mja11.11331. [DOI] [PubMed] [Google Scholar]
- 16.Li L., O'Neil L. Department of Health, Northern Territory; Darwin: 2018. Mothers and babies 2015: Northern territory midwives’ collection. [Google Scholar]
- 17.The Lowitja Institute . The Loitja Institute; Carlton: 2017. The Lowitja Institute and The Lowitja Institute aboriginal and Torres strait islander health CRC style guide v14. [Google Scholar]
- 18.Northern Territory Government. Aboriginal languages in NT, 2020. Accessed via:https://nt.gov.au/community/interpreting-and-translating-services/aboriginal-interpreter-service/aboriginal-languages-in-nt; accessed date: 15th June 2020.
- 19.Munoz E., Powers J.R., Nienhuys T.G., Mathews J.D. Social and environmental factors in 10 aboriginal communities in the Northern Territory: relationship to hospital admissions of children. Med J Aust. 1992;156(8):529–533. doi: 10.5694/j.1326-5377.1992.tb121412.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Connors C., Wright J., Guthridge S., Bailie R. Estimating chronic disease prevalence among the remote Aboriginal population of the Northern Territory using multiple data sources. Aust N Z J Public Health. 2008;32(4):307–313. doi: 10.1111/j.1753-6405.2008.00245.x. [DOI] [PubMed] [Google Scholar]
- 21.Australian Institute of Health and Welfare . AIHW; Canberra: 2015. The health and welfare of Australia's aboriginal and Torres Strait islander peoples 2015. Cat. no. IHW 147. [Google Scholar]
- 22.Voaklander B., Rowe S., Sanni O., Campbell S., Eurich D., Ospina M.B. Prevalence of diabetes in pregnancy among Indigenous women in Australia, Canada, New Zealand, and the USA: a systematic review and meta-analysis. Lancet Glob Health. 2020;8(5):PE681–PEE98. doi: 10.1016/S2214-109X(20)30046-2. [DOI] [PubMed] [Google Scholar]
- 23.Pettitt D.J., Knowler W.C., Baird H.R., Bennett P.H. Gestational diabetes: infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care. 1980;3(3):458–464. doi: 10.2337/diacare.3.3.458. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain C., Banks E., Joshy G. Prevalence of gestational diabetes mellitus among Indigenous women and comparison with non-Indigenous Australian women: 1990-2009. Aust N Z J Obstet Gynaecol. 2014;54(5):433–440. doi: 10.1111/ajo.12213. [DOI] [PubMed] [Google Scholar]
- 25.Dyck R.F., Karunanayake C., Pahwa P., Stang M., Osgood N.D. Epidemiology of Diabetes in pregnancy among first nations and non-first nations women in Saskatchewan, 19802013. Part 1: populations, methodology and frequencies (19802009); results from the DIP: ORRIIGENSS project. Can J Diabetes. 2019 doi: 10.1016/j.jcjd.2019.10.005. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 26.Oster R.T., King M., Morrish D.W., Mayan M.J., Toth E.L. Diabetes in pregnancy among First Nations women in Alberta, Canada: a retrospective analysis. BMC Pregnancy Childbirth. 2014;14:136. doi: 10.1186/1471-2393-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oster R.T., Toth E.L. Longitudinal rates and risk factors for adverse birth weight among first nations pregnancies in Alberta. J Obstet Gynaecol Can. 2016;38(1):29–34. doi: 10.1016/j.jogc.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Auger N., Park A.L., Zoungrana H., Fon Sing M., Lo E., Luo Z.C. Widening inequality in extreme macrosomia between Indigenous and non-Indigenous populations of Quebec, Canada. Aust N Z J Public Health. 2013;37(1):58–62. doi: 10.1111/1753-6405.12011. [DOI] [PubMed] [Google Scholar]
- 29.Ganchimeg T., Ota E., Morisaki N. Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG. 2014;121(Suppl1):40–48. doi: 10.1111/1471-0528.12630. [DOI] [PubMed] [Google Scholar]
- 30.Steenkamp M., Boyle J., Kildea S., Moore V., Davies M., Rumbold A. Perinatal outcomes among young indigenous Australian mothers: a cross-sectional study and comparison with adult indigenous mothers. Birth. 2017;44(3):262–271. doi: 10.1111/birt.12283. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson M., Brown C., Georga C., Miles E., Wilson A., Brimblecombe J. Traditional food availability and consumption in remote Aboriginal communities in the Northern Territory, Australia. Aust N Z J Public Health. 2017;41(3):294–298. doi: 10.1111/1753-6405.12664. [DOI] [PubMed] [Google Scholar]
- 32.Australian institute of Health and Welfare . AIHW; Canberra: 2017. A picture of overweight and obesity in Australia. Cat. No: PHE 216. [Google Scholar]
- 33.Hoy W.E., Mott S.A., McLeod B.J. Transformation of mortality in a remote Australian Aboriginal community: a retrospective observational study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titmuss A., Davis E.A., Brown A., Maple-Brown L.J. Emerging diabetes and metabolic conditions among Aboriginal and Torres Strait Islander young people. Med J Aust. 2019;210(3):111–113. doi: 10.5694/mja2.13002. [DOI] [PubMed] [Google Scholar]
- 35.Maple-Brown L.J., Hampton D. Indigenous cultures in countries with similar colonisation histories share the challenge of intergenerational diabetes. Lancet Glob Health. 2019;8(5):E619–E620. doi: 10.1016/S2214-109X(20)30072-3. [DOI] [PubMed] [Google Scholar]
- 36.Kevat D., Wilson D., Sinha A. A 5-year-old girl with type 2 diabetes. Lancet. 2014;383:1268. doi: 10.1016/S0140-6736(14)60487-6. [DOI] [PubMed] [Google Scholar]
- 37.Hjort L., Novakovic B., Grunnet L.G. Diabetes in pregnancy and epigenetic mechanisms – how the first 9 months from conception might affect the child's epigenome and later risk of disease. Lancet Diabetes Endocrinol. 2019;7(10):796–806. doi: 10.1016/S2213-8587(19)30078-6. [DOI] [PubMed] [Google Scholar]
- 38.Foley M., Zhao Y., Condon J. Department of Health; Darwin: 2012. Demographic data quality assessment for northern territory public hospitals 2001. [Google Scholar]
- 39.Plan A., Condon J.R., Durling G. Northern Territory Department of Health and Community Services; Darwin: 1995. Northern territory health outcomes, morbidity and mortality 1979–1991. [Google Scholar]
- 40.Wicks M., Van Dokkum P. Not so sweet: diabetes in pregnancy – the central australian experience. Proceedings of the Australasian diabetes in pregnancy society annual scientific meeting; Canberra,; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.