Abstract
Background
The COVID-19 pandemic challenges neurologists in counselling patients with multiple sclerosis (pwMS) regarding their risk by SARS-CoV-2 and in guiding disease-modifying treatment (DMT).
Objective
To characterize the prevalence and outcome of COVID-19 in pwMS specifically associated with different DMT in a nationwide population-based study.
Methods
We included patients aged ≥18 years with a confirmed diagnosis of MS and a diagnosis of COVID-19 established between January 1, 2020 and December 31, 2020. We classified COVID-19 course as either mild, severe or fatal. Impact of DMT and specifically immunosuppressants (alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab) on COVID-19 outcome was determined by multivariable models, adjusted for a-priori-risk.
Results
Of 126 MS patients with COVID-19 (mean age 43.2 years [SD 13.4], 71% female), 86.5% had a mild course, 9.5% a severe course and 3.2% died from COVID-19. A-priori-risk significantly predicted COVID-19 severity (R2 0.814; p<0.001) and mortality (R2 0.664; p<0.001). Adjusting for this a-priori-risk, neither exposure to any DMT nor exposure to specific immunosuppressive DMT were significantly associated with COVID-19 severity (odds ratio [OR] 1.6; p = 0.667 and OR 1.9; p = 0.426) or mortality (OR 0.5; p = 0.711 and 2.1; 0.233) when compared to no DMT.
Conclusions
In a population-based MS cohort, COVID-19 outcome was not associated with exposure to DMT and immunosuppressive DMT when accounting for other already known risk factors. This provides reassuring evidence that COVID-19 risk can be individually anticipated in MS and–except for a very small proportion of high-risk patients–treatment decisions should be primarily focused on treating MS rather than the pandemic.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused more than 83 million confirmed infections worldwide and approximately 1.8 million have died from the consequences of the virus-associated respiratory disease (CoronaVirus-Disease 2019, COVID-19) as by December 31st, 2020. Mortality and clinical severity of COVID-19 are strongly dependent of age and preexisting comorbidities [1, 2].
Multiple sclerosis (MS) is often considered as a disease affecting young adults, but a substantial number of patients with MS (pwMS) are older than 60 years and might thus be at an increased risk of severe morbidity and mortality from COVID-19 [2–4].
Further, there is particular concern whether immunomodulatory or immunosuppressive disease-modifying treatments (DMT), which are to some extent associated with a greater risk of infection, also increase the risk for COVID-19 severity and mortality [5, 6]. Although initial reports indicated a low proportion of pwMS at high risk of COVID-19 mortality, evidence regarding the effect of DMT on the course of COVID-19 is as scarce as direly needed for guiding pwMS through the pandemic [5, 7, 8].
The objective of this study was to characterize the prevalence, severity and overall mortality of SARS-CoV-2 infections in pwMS specifically associated with different DMT in a nationwide population-based study.
Methods
Patients and data collection
In this nationwide multicenter observational study, we included patients with a confirmed diagnosis of MS aged ≥18 years and with a diagnosis of COVID-19 (defined by either a positive SARS-CoV-2 polymerase chain reaction [PCR] or clinical diagnosis supported by i) a subsequent positive SARS-CoV-2 antibody test or b) a positive SARS-CoV-2 PCR in a close contact person of the patient) established between January 1, 2020 and December 31, 2020 into the Austrian MS-COVID-19 registry (AUT-MuSC) [9].
Patients were recruited through the Austrian MS network, which is a collaboration of MS centers certified by the Austrian Neurological Society adhering to a common and controlled high-quality standard of managing and documenting about 13.500 patients with MS in Austria [10]. The study was designed and conducted in accordance with the Declaration of Helsinki, the General Data Protection Regulation and the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) guidelines and was approved by the ethics committee of the Medical University Vienna (ethical approval number: EK 1338–2020).
When the treating neurologist was informed about a diagnosis of COVID-19 or a confirmed SARS-CoV-2 infection in an MS patient either at an on-site visit or remotely, data were collected retrospectively by fulfilling a pseudonymized case report form from a review of medical records, which was then sent to the coordinating study center at the Department of Neurology, Medical University of Vienna. Patients included were informed about the objective of the study and written informed consent was obtained.
Data collected included demographic data, details of MS course, DMT history, a detailed documentation of prior and current comorbidities and a detailed description of source, course, available laboratory and radiographic diagnostics, treatment and outcome of COVID-19.
Definitions and endpoints
Patients were classified regarding their a-priori risk of COVID-19 severity and mortality according to a recently developed risk score (MS-COV-risk, see Table 1), categorizing MS patients based on age, physical disability (Expanded Disability Status Scale [EDSS] score), smoking status, obesity (body-mass-index ≥30), and presence of cardiovascular disease (coronary heart disease and/or ischemic heart failure and/or cardiac valve disease), chronic pulmonary disease (asthma, obstructive pulmonary disease [COPD] or pulmonary fibrosis), diabetes mellitus, chronic kidney disease and current malignancy as having either low (<1%), mild (<5%), moderate (~15%), high (~30%) or very high risk (~50%) of COVID-19 mortality [7].
Table 1. MS-COV-risk score.
| Factor | Score |
| Diabetes AND age <40 years | 5 |
| Age ≥65 years | 3 |
| Chronic kidney disease | 3 |
| Chronic obstructive pulmonary disease | 1 |
| Cardiovascular disease | 1 |
| Current Malignancy | 1 |
| Obesity (BMI ≥30) | 1 |
| Diabetes | 1 |
| Smoking | 1 |
| Severe physical disability (EDSS >6) | 1 |
| Age <40 | -6 |
| Risk category | Score Interval |
| Low risk | ≤0 |
| Mild risk | 1–3 |
| Moderate risk | 4–7 |
| High risk | 8–11 |
| Very high risk | ≥12 |
BMI: body mass index. EDSS: expanded disability status scale. See reference [7].
Patients were grouped according to their DMT status at the time of SARS-CoV-2 infection as either receiving no DMT (N-DMT); immunomodulating DMT (IM-DMT) comprising dimethyl fumarate, glatiramer acetate, interferon-beta preparations, natalizumab, and teriflunomide; or immunosuppressive DMT (IS-DMT) comprising alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab [8, 11].
The primary endpoint was COVID-19 severity defined as the clinical status at the most severe point of COVID-19 course on a 4-point ordinal scale, where 0 indicates an asymptomatic course; 1 a mild course (no pneumonia or mild pneumonia without hospitalization); 2 a severe course requiring hospitalization and fulfilling at least one of five criteria (breathing rate >30/minute, SpO2 ≤93%, PaO2/FiO2-Ratio <300, Pulmonary infiltrate >50% within 24-48h, requirement of noninvasive ventilation, high-flow oxygen, mechanical ventilation or extracorporeal membrane oxygenation), and 3 death. The secondary endpoint was defined as mortality from COVID-19.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (SPSS Inc, Chicago, IL). Categorical variables were expressed in frequencies and percentages. Continuous variables were tested for normal distribution by Shapiro-Wilk test and expressed as mean and standard deviation (SD) or median and range as appropriate. Univariate group comparisons were conducted by t-test, ANOVA, Mann-Whitney-U test, Kruskal-Wallis test or chi-square test as appropriate.
First, we investigated the strength of association between a-priori risk scoring and COVID-19 outcome (severity and mortality) by performing univariable correlation analyses (Spearman-rho) and multivariable logistic regression models with COVID-19 severity/mortality as the dependent variable and MS-COV-risk score (absolute score and risk categories) as the independent variable adjusting for sex (age is already included in the MS-COV-risk score) and lymphopenia before COVID-19 as a possible risk factor not included in the MS-COV-risk score [8]. Then, we tested the independent impact of overall DMT as well as IM-DMT and IS-DMT on COVID-19 outcome by calculating multivariate logistic regression models with COVID-19 severity/mortality as the dependent variable and DMT categories (reference category: N-DMT) as the independent variable adjusted for a-priori-risk as expressed by MS-COV-risk score adjusting for sex and lymphopenia before COVID-19. Finally, we conducted sensitivity analyses evaluating the robustness of results to the impact of any single DMT substance by stepwise removal from analyses. Robustness of the statistically significant differences to unidentified confounders not accounted for by MS-COV-risk score was quantified with Rosenbaum sensitivity test for Hodges–Lehmann Γ [12]. Missing values were handled by multiple (20 times) imputation using the missing not at random (MNAR) approach with pooling of estimates according to Rubin’s rules [13]. A two-sided p-value <0.05 was considered statistically significant.
Results
We included 126 patients with a mean age of 43.2 years [SD 13.4] and a female predominance of 71%. Overall characteristics of the study cohort are given in Table 2. A detailed description of every patient included is presented in (S1 Table).
Table 2. Characteristics of the AUT-MuSC-19 cohort.
| n = 126 | |
|---|---|
| Femalea | 90 (71.4) |
| Age (years)b,c | 43.2 (13.4; 21–79) |
| BMI c | 24.1 (17.4–41.0) |
| Smokersa | 17 (3.5) |
| Ethnicitya | |
| Caucasiana | 123 (97.6) |
| Othera | 3 (2.4) |
| No of comorbidities associated with increased COVID-19 morbidityc* | 0 (0–5) |
| Disease duration (years) b | 12.0 (9.3) |
| Disease coursea | |
| RRMSa | 98 (77.8) |
| SPMSa | 19 (15.1) |
| PPMSa | 9 (7.1) |
| EDSSc | 2.0 (0–8.5) |
| On DMTa | 90 (71.4) |
| IM-DMT | 48 (38.1) |
| Interferon-betaa | 6 (4.8) |
| Glatiramer acetatea | 11 (8.7) |
| Dimethyl fumaratea | 19 (15.1) |
| Teriflunomidea | 2 (1.6) |
| Natalizumaba | 10 (7.9) |
| IS-DMT | 41 (32.5) |
| Fingolimoda | 16 (12.7) |
| Ocrelizumab/Rituximaba | 12 (9.5) |
| Alemtuzumaba | 2 (1.6) |
| Cladribine | 2 (1.6) |
| Azathioprina | 1 (0.8) |
| Lymphopenia at last lab before SARS-CoV2 infectiona** | 19 (18.4) |
| Grade 3 or lowera | 7 (6.8) |
BMI: body mass index. DMT: disease modifying treatment. EDSS: Expanded Disability Status Scale. IM-DMT: Immunomodulating DMT = dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab, and teriflunomide. IS-DMT: Immunosuppressive DMT = alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab. MS: multiple sclerosis. PPMS: primary progressive MS. RRMS: relapsing-remitting MS. SPMS: secondary progressive MS.
aabsolute number and percentage.
bmean and standard deviation.
cmedian and minimum-maximum range.
*defined as cardiovascular diseases, chronic obstructive pulmonary disease, chronic kidney disease, diabetes and concurrent malignancy.
**available from 103 patients.
The number of comorbidities associated with increased COVID-19 morbidity (cardiovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, diabetes and concurrent malignancy) as well as MS-associated physical disability (EDSS) significantly increased with age (rho = 0.176, p = 0.049 and rho = 0.576, p<0.001, respectively). Frequency of obesity and smoking was not associated with age, but pwMS receiving DMT were significantly younger than those without DMT (mean age 39.0 vs. 53.6; p<0.001). Of the 103 patients with available differential blood count before COVID-19 onset, 19 (18.4%) had lymphopenia, with 7 (6.8%) grade 3 or lower.
Overall, 5 (4.0%) were asymptomatically infected with SARS-CoV-2, 109 (86.5%) had mild COVID-19 and 12 (9.5%) had a severe course, of whom 4 (3.2%) died from COVID-19.
A-priori-risk and COVID-19 outcome
Overall, a-priori risk categories were distributed as follows: 75 (59.5%) low risk, 39 (3.0%) mild risk, 8 (6.3%) moderate risk and 4 (3.2%) high risk of COVID-19 mortality. We therefore condensed patients with moderate and high risk into one group to facilitate further categorical analyses.
MS-COV-risk score was strongly correlated with COVID-19 outcome (rho = -0.426, p<0.001). Among the 103 patients for whom lymphocyte counts were available before COVID-19 onset, we did not find an association between lymphopenia and COVID-19 severity or mortality.
In the multivariable models adjusting for sex and lymphopenia, MS-COV-risk score significantly predicted COVID-19 severity (odds ratio [OR]: 1.4 per 1 point increase; 95% confidence interval [CI]: 1.2–3.7; p = 0.001; R2 0.814; p<0.001) and mortality (OR: 2.4; CI: 1.2–5.4; p = 0.007; R2 0.664; p<0.001).
The impact of DMT on COVID-19 outcome
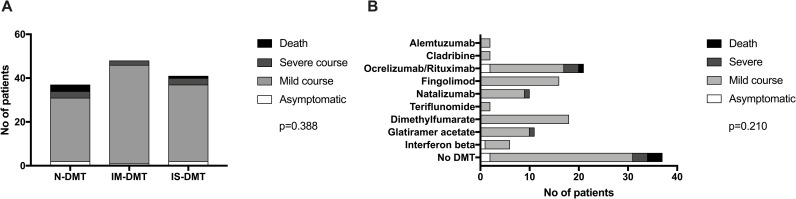
COVID-19 outcome according to DMT categories and single DMT substances is shown in Fig 1. We did not find any significant association between DMT and COVID-19 morbidity, neither overall, nor when stratifying according to a-priori risk categories (Table 3).
Fig 1.
COVID-19 outcome according to DMT classes (A) and single substances (B). DMT: disease modifying treatment. IM-DMT: Immunomodulating DMT = dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab, and teriflunomide. IS-DMT: Immunosuppressive DMT = alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab. p-values calculated by chi-square test. N-DMT: no DMT.
Table 3. COVID-19 severity and mortality according to a-priori risk and DMT class.
| Risk category | At risk | Severe COVID-19 | p-value | Fatal COVID-19 | p-value | |
|---|---|---|---|---|---|---|
| Low risk (Score ≤0) N = 75 | N-DMT | 17 | 0 | 0.4551 | 0 (0) | NA |
| IM-DMT | 31 | 1 | 0 (0) | |||
| IS-DMT | 27 | 2 | 0 (0) | |||
| Mild risk (Score 1–3) N = 39 | N-DMT | 11 | 0 | 0.3151 | 0 (0) | NA |
| IM-DMT | 16 | 0 | 0 (0) | |||
| IS-DMT | 12 | 1 | 0 (0) | |||
| Moderate/high risk (Score ≥4) N = 12 | N-DMT | 9 | 6 | 0.6871 | 3 | 0.6771 |
| IM-DMT | 1 | 1 | 0 | |||
| IS-DMT | 2 | 1 | 1 |
DMT: disease modifying treatment. IM-DMT: Immunomodulating DMT = dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab, and teriflunomide. IS-DMT: Immunosuppressive DMT = alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab.
1calculated by chi-square test. N-DMT: no DMT.
Adjusting for MS-COV-risk score, sex and lymphopenia before SARS-CoV-2 infection, exposure to any DMT was neither associated with COVID-19 severity (OR: 1.6; CI: 0.2–11.9; p = 0.667) nor with mortality (OR: 0.5; CI: 0.2–19.6; p = 0.711, Table 4) when compared to no DMT. Similarly, exposure to IS-DMT was not associated with COVID-19 severity (OR: 1.9; CI: 0.5–9.4; p = 0.426) and mortality (OR 2.1; CI: 0.8–12.3; p = 0.233).
Table 4. COVID-19 severity and mortality depend on a priori risk but not DMT class.
| COVID-19 severity | COVID-19 mortality | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| MS-COV-risk score (per point) | 1.5 | 1.2–3.7 | 0.005 | MS-COV-risk score (per point) | 2.4 | 1.2–7.3 | 0.021 |
| DMT1 | 1.6 | 0.2–11.9 | 0.667 | DMT1 | 0.5 | 0.2–19.6 | 0.711 |
| IM-DMT | 1.1 | 0.2–7.3 | 0.943 | IM-DMT | 0.2 | 0.0–3.4 | 0.122 |
| IS-DMT | 1.9 | 0.5–9.4 | 0.426 | IS-DMT | 2.1 | 0.8–12.3 | 0.233 |
| R square 0.832; p<0.001 | R square 0.683; p<0.001 | ||||||
1reference category: no DMT.
IM-DMT: Immunomodulating DMT = dimethyl fumarate, glatiramer acetate, interferon beta preparations, natalizumab, and teriflunomide. IS-DMT: Immunosuppressive DMT = alemtuzumab, cladribine, fingolimod, ocrelizumab or rituximab. OR: odds ratio. 95% CI: confidence interval. Calculated by binary linear regression models with severe COVID-19 and fatal COVID-19 adjusted for sex and lymphopenia before COVID-19.
Discussion
In this nationwide population-based study of SARS-CoV-2 infections in pwMS, we report several key findings: 1) prevalence, severity and mortality in pwMS in Austria are comparable to the general population, 2) COVID-19 severity and mortality can be predicted by applying a score of known a-priori risk factors and 3) COVID-19 severity and mortality are not associated with exposure to DMT and specifically immunosuppressive DMT when accounting for a-priori risk.
Prevalence
Given a population of approximately 13,500 MS patients in Austria, the 126 pwMS included in this population-based study would translate to a prevalence of 0.93% at the end of 2020.[10] This would seem well below the prevalence of 4.2% of COVID-19 in the general population (360,815 confirmed cases in 8.5 million people) during the same period [14, 15]. However, the prevalence in the general population is based on the number of positive SARS-CoV-2 tests rather than symptomatic COVID-19. Considering that only about 20% of seropositive MS patients appear to have symptomatic COVID-19 and that only 5/126 (3.9%) patients were asymptomatic in our cohort, the true prevalence of SARS-CoV-2 infection in the Austrian MS population can be estimated at 4.1% and, thus, lies well within the range of the general population [16]. This is in line with previous studies applying various definitions of SARS-CoV-2 infection/COVID-19 and pharmacoepidemiological techniques adding to the mounting evidence that pwMS are neither more nor less likely to contract SARS-CoV-2 [17–19]. Thus, it can be inferred that the AUT-MuSC-19 registry is likely to include most of pwMS with a symptomatic SARS-CoV-2 infections in Austria and is likely representative of a central European, primarily Caucasian MS population.
COVID severity and mortality
Evidence regarding the specific morbidity and mortality from COVID-19 in pwMS is still scarce. In our population-based cohort, we found 9.5% with severe COVID-19 course and 3.2% mortality. Encouragingly, this is within the range of mortality reports both in Austria (1.7%; 6,222 of 360,815 confirmed cases) and globally (2.2%) in the general population and in line with previous studies suggesting that MS patients do not have an increased risk of COVID-19 fatality compared with the population at large [8, 14, 20–22].
It is well established that in the general population COVID-19 severity and mortality increases with older age and in the presence of comorbidities, i.e. cardiovascular disease, pulmonary disease, chronic kidney disease, malignancy, obesity and smoking [1, 2, 23, 24]. In MS patients, advanced physical disability represents an additional factor associated with poor COVID-19 outcome [8, 22]. We have recently introduced a score (MS-COV-risk) to cumulatively quantify these a-priori risk factors in order to predict COVID-19 severity and mortality in pwMS [7]. Here, we found that by applying the MS-COV-risk score, 81% of the variation in COVID-19 severity (R2 0.862; p<0.001) and 66% of mortality (R2 0.663; p<0.001) could be predicted. This both validates the predictive ability of the MS-COV-risk score and confirms that COVID morbidity in pwMS is largely determined by factors independent of MS and, thus, can be anticipated.
The role of DMT
One of the most pressing questions in managing pwMS during the pandemic is whether immunomodulatory or immunosuppressive DMT increase the risk for COVID-19 severity and mortality. While MS is generally not associated with increased morbidity from viral pathogens, some DMTs are to some extent associated with a greater risk of infection [21]. Therefore, initial recommendations of various expert committees at the beginning of the pandemic in early 2020 have offered very conservative advice with some even suggesting discontinuation of DMT. As the number of cases grew over the subsequent months, evidence is reassuringly growing that poor outcome in pwMS contracting COVID-19 is primarily dependent on age, obesity, comorbidities and degree of physical disability rather than DMT use in general [5, 8, 22, 25–28]. Our data confirm and extend these findings showing that when accounting for a-priori risk, COVID-19 severity and mortality are independent of both overall exposure to DMT as well immunosuppressive DMT. This has also been recently shown in a large pharmacoepidemiological study [18]. However, there is some concern regarding a possibly increased risk under treatment with anti-CD20 B cell depleting agents as indicated by a recent large observational study [28]. Also, there have been reports of a decreased serologic response in pwMS after SARS-CoV-2 infection [29, 30]. Our study is not sufficiently powered to determine the effect of single DMT substances on COVID outcome. However, sensitivity analyses did not indicate a significant change of results when removing single DMT substances. Similar to other studies, we also did not find an association between lymphopenia and COVID-19 morbidity, neither in univariable analyses nor when accounting for a-priori risk and DMT [8, 26].
Therefore, in pwMS with low to moderate risk of COVID-19 mortality, the benefit-risk ratio is clearly in favor of both continuing and initiating DMT when indicated by the MS course in the respective individual patient. The majority of the small group of pwMS displaying a high a-priori risk of having severe COVID-19 does not even receive DMT (in our cohort 0 of 4 patients) and is unlikely to display significant disease activity, hence, the question of stopping or delaying DMT hardly arises [7]. Consequently, most expert committees have now adopted less cautious guidelines emphasizing the paramount need for ensuring optimal treatment of MS individually integrating MS specific parameters as well as comorbidities, social circumstances, personal risk perception etc., especially during the pandemic [31, 32]. In this light, reports of significant drops in patient rate and changed/deferred DMT regimens are particularly concerning as quality of life of pwMS greatly depends on prompt access to a broad range of health and care service [33, 34]. It is essential for MS caregivers to uphold the standard of care for pwMS, including continuation of safety monitoring procedures as far as possible depending on local circumstances [21, 32].
Strengths and limitations
The main strengths of this study are its population-based approach and the detailed characterization of the study cohort provided by the high-quality data from certified specialized MS centers. We have to acknowledge some potential limitations inherent to the study design.
MS patients with asymptomatic SARS-CoV-2 infection may have been systematically missed due to the study design. As already noted earlier, our study is not sufficiently powered to determine the effect of single DMT substances on COVID outcome. Thus, we had to use a categorization in immunomodulating and immunosuppressive DMTs in the primary analyses, resulting in an estimated power of 73% for detecting an increased risk of COVID severity by an odds ratio of 2. However, we conducted sensitivity analyses evaluating the robustness of results to the impact of any single DMT substance by stepwise removal, which did not indicate a significant change of results. In addition, there may be a referral bias as severe COVID-19 courses may be more likely to be reported. On the other hand, patients with advanced and progressive MS or patients not receiving DMT are less frequently seeing a neurologist regularly and, thus, this cohort might be underrepresented in this study. There may also be confounders influencing COVID severity/mortality in pwMS unaccounted for by MS-COV-risk score and DMT. However, Rosenbaum bounds did indicate only a small potential impact of hidden bias not accounted for in the multivariable models [12].
Conclusion
We showed in a population-based MS cohort that COVID-19 severity and mortality are not associated with exposure to DMT and immunosuppressive DMT when accounting for unmodifiable risk factors. This provides reassuring evidence that the COVID-19 risk can be anticipated in MS and–except for a very small proportion of high-risk patients–treatment decisions should be primarily focused on treating MS rather than the pandemic.
Supporting information
ATZ: alemtuzumab, CHD: coronary heart disease., CKD: Chronic kidney disease, CLA: cladribine, COPD: chronic obstructive pulmonary disease, DMF: dimethyl fumarate, DMT: disease modifying treatment, EDSS: Expanded disability status scale, FTY: fingolimod, F: female, GLA: glatiramer acetate, IFN: interferon beta, M: male, NTZ: natalizumab, OCR: ocrelizumab, PCR: SARS-CoV-2-polymerase-chain-reaction, RTX: rituximab, TERI: teriflunomide.
(DOCX)
Acknowledgments
We thank all the AUT-MuSC-19 investigators, clinical research staff, and especially the patients for helping to collect these data. The named individuals were not compensated for their help. Lead AUT-MuSC investigator: Gabriel Bsteh, MD, PhD; Email: gabriel.bsteh@meduniwien.ac.at.
AUT-MuSC investigators in alphabetical order: Assar, Hamid (Kepler University Hospital, Linz, Austria); Berger, Thomas (Medical University of Vienna, Vienna, Austria); Böck, Klaus (Kepler University Hospital, Linz, Austria); Bsteh, Christian (Private practice neurologist, Salzburg, Austria); Bsteh, Gabriel (Medical University of Vienna, Vienna, Austria); Di Pauli, Franziska (Medical University of Innsbruck, Innsbruck, Austria); Enzinger, Christian (Medical University of Graz, Graz, Austria); Gradl, Christiane (Medical University of St. Pölten, St. Pölten, Austria); Guger, Michael (Med Campus III, Kepler University Hospital GmbH, Linz, Austria); Hegen, Harald (Medical University of Innsbruck, Innsbruck, Austria); Heschl, Bettina (Medical University of Graz, Graz, Austria); Hiller, Marie-Sophie (Barmherzige Brüder Hospital, Eisenstadt, Austria); Kornek, Barbara (Medical University of Vienna, Vienna, Austria); Leutmezer, Fritz (Medical University of Vienna, Vienna, Austria); Mayr, Markus (District Hospital Kufstein, Kufstein, Austria); Morgenstern, Gabriele (Private practice neurologist, Lienz, Austria); Rommer, Paulus (Medical University of Vienna, Vienna, Austria); Schnabl, Peter (Private practice neurologist, Velden, Austria); Schneider-Koch, Gabriela (Ottakring Hospital, Vienna, Austria); Schrotter, Gabriele (LKH West Graz, Graz, Austria); Traxler, Gerhard (Clinic for Neurology 2, Med Campus III, Kepler University Hospital GmbH, Linz, Austria); Wipfler, Peter (Paracelsus Medical University of Salzburg, Salzburg, Austria); Zulehner, Gudrun (Medical University of Vienna, Vienna, Austria); Zrzavy, Tobias (Medical University of Vienna, Vienna, Austria).
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and can be accessed from and upon approval by the ethics committee of the Medical University Vienna (contact via letter to Borschkegasse 9, 1090 Vienna, Austria) since data contain potentially sensitive information.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. Published online 2020. doi: 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minden SL, Frankel D, Hadden LS, Srinath KP, Perloff JN. Disability in elderly people with multiple sclerosis: An analysis of baseline data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. Neurorehab. 2004;19:55–67. doi: 10.3233/nre-2004-19107 [DOI] [PubMed] [Google Scholar]
- 5.Möhn N, Konen FF, Pul R, et al. Experience in Multiple Sclerosis Patients with COVID-19 and Disease-Modifying Therapies: A Review of 873 Published Cases. J Clin Med. 2020;9(12):4067. doi: 10.3390/jcm9124067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grebenciucova E, Pruitt A. Infections in Patients Receiving Multiple Sclerosis Disease-Modifying Therapies. Curr Neurol Neurosci. 2017;17(11):88. doi: 10.1007/s11910-017-0800-8 [DOI] [PubMed] [Google Scholar]
- 7.Bsteh G, Bitschnau C, Hegen H, et al. Multiple sclerosis and COVID-19: how many are at risk? Eur J Neurol. Published online 2020. doi: 10.1111/ene.14555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louapre C, Collongues N, Stankoff B, et al. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020;77(9). doi: 10.1001/jamaneurol.2020.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 10.Salhofer-Polanyi S, Cetin H, Leutmezer F, et al. Epidemiology of Multiple Sclerosis in Austria. Neuroepidemiol. 2017;49(1–2):40–44. doi: 10.1159/000479696 [DOI] [PubMed] [Google Scholar]
- 11.Brownlee W, Bourdette D, Broadley S, Killestein J, Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94(22):10.1212/WNL.0000000000009507. doi: 10.1212/WNL.0000000000009507 [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. J Am Stat Assoc. 1984;79(387):516–524. doi: 10.2307/2288398 [DOI] [Google Scholar]
- 13.Council National Research (CNR). The Prevention and Treatment of Missing Data in Clinical Trials. National Academies Press (US); 2010. doi: 10.17226/12955 [DOI] [PubMed]
- 14.Agentur für Gesundheit und Ernährungssicherheit (AGES). AGES Dashboard COVID19. Published December 31, 2020. Accessed December 31, 2020. https://covid19-dashboard.ages.at
- 15.Asamer E-M, Astleithner F, Cetkovic P, et al. Quality assessment for register-based statistics—Results for the Austrian census 2011. Blauensteiner S, ed. Austrian J Stat. 2014;45(2):3–14. doi: 10.17713/ajs.v45i2.97 [DOI] [Google Scholar]
- 16.Capasso N, Palladino R, Montella E, et al. Prevalence of SARS-CoV-2 Antibodies in Multiple Sclerosis: The Hidden Part of the Iceberg. J Clin Med. 2020;9(12):4066. doi: 10.3390/jcm9124066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evangelou N, Garjani A, dasNair R, et al. Self-diagnosed COVID-19 in people with multiple sclerosis: a community-based cohort of the UK MS Register. J Neurology Neurosurg Psych. 2021;92(1):107–109. doi: 10.1136/jnnp-2020-324449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovvuru S, Nalleballe K, Onteddu SR, et al. Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic. J Neurol Sci. Published online 2020:117230. doi: 10.1016/j.jns.2020.117230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talaat F, Ramadan I, Aly S, Hamdy E. Are multiple sclerosis patients and their caregivers more anxious and more committed to following the basic preventive measures during the COVID-19 pandemic? Mult Scler Relat Dis. 2020;46:102580. doi: 10.1016/j.msard.2020.102580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Published December 31, 2020. Accessed December 31, 2020. https://covid19.who.int
- 21.Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e761. doi: 10.1212/NXI.0000000000000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sormani MP, sclerosis ISG on C-19 infection in multiple. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481–482. doi: 10.1016/S1474-4422(20)30147-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. Published online 2020. doi: 10.1016/S1470-2045(20)30309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle Risk Factors, Inflammatory Mechanisms, and COVID-19 Hospitalization: A Community-Based Cohort Study of 387,109 Adults in UK. Brain Behav Immun. Published online 2020. doi: 10.1016/j.bbi.2020.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrotta E, Kister I, Charvet L, et al. COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol- Neuroimmunol Neuroinflamm. 2020;7(5):e835. doi: 10.1212/NXI.0000000000000835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loonstra FC, Hoitsma E, Kempen ZL van, Killestein J, Mostert JP. COVID-19 in multiple sclerosis: The Dutch experience. Mult Scler J. 2020;26(10):1256–1260. doi: 10.1177/1352458520942198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharifian-Dorche M, Sahraian MA, Fadda G, et al. COVID-19 and Disease-Modifying Therapies in Patients with Demyelinating Diseases of the Central Nervous System: A Systematic Review. Mult Scler Relat Dis. 2021;50:102800. doi: 10.1016/j.msard.2021.102800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sormani MP, Rossi ND, Schiavetti I, et al. Disease-Modifying Therapies and Coronavirus Disease 2019. Severity in Multiple Sclerosis. Ann Neurol. Published online 2021. doi: 10.1097/WCO.0000000000000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maillart E, Papeix C, Lubetzki C, Roux T, Pourcher V, Louapre C. Beyond COVID-19: do MS/NMO-SD patients treated with anti-CD20 therapies develop SARS-CoV2 antibodies? Mult Scler Relat Dis. 2020;46:102482. doi: 10.1016/j.msard.2020.102482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker D, Roberts CAK, Pryce G, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149–161. doi: 10.1111/cei.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bsteh G, Monz E, Zamarian L, et al. Combined evaluation of personality, risk and coping in MS patients: A step towards individualized treatment choice–The PeRiCoMS-Study I. J Neurol Sci. Published online March 3, 2017:1–22. doi: 10.1016/j.jns.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 32.Amor S, Baker D, Khoury SJ, Schmierer K, Giovanonni G. SARS-CoV-2 and Multiple Sclerosis: Not All Immune Depleting DMTs are Equal or Bad. Ann Neurol. 2020;87(6):794–797. doi: 10.1002/ana.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manacorda T, Bandiera P, Terzuoli F, et al. Impact of the COVID-19 pandemic on persons with multiple sclerosis: Early findings from a survey on disruptions in care and self-reported outcomes. J Health Serv Res Po. Published online 2020:135581962097506. doi: 10.1177/1355819620975069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mateen FJ, Rezaei S, Alakel N, Gazdag B, Kumar AR, Vogel A. Impact of COVID-19 on U.S. and Canadian neurologists’ therapeutic approach to multiple sclerosis: a survey of knowledge, attitudes, and practices. J Neurol. 2020;267(12):3467–3475. doi: 10.1007/s00415-020-10045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]