Summary
Background
Myasthenia gravis (MG) is the most common primary disorder of neuromuscular transmission, but the incidence of MG in China is unknown. We conducted the first nationwide study to determine the incidence and mortality rates of MG in all age groups at the national level in China.
Methods
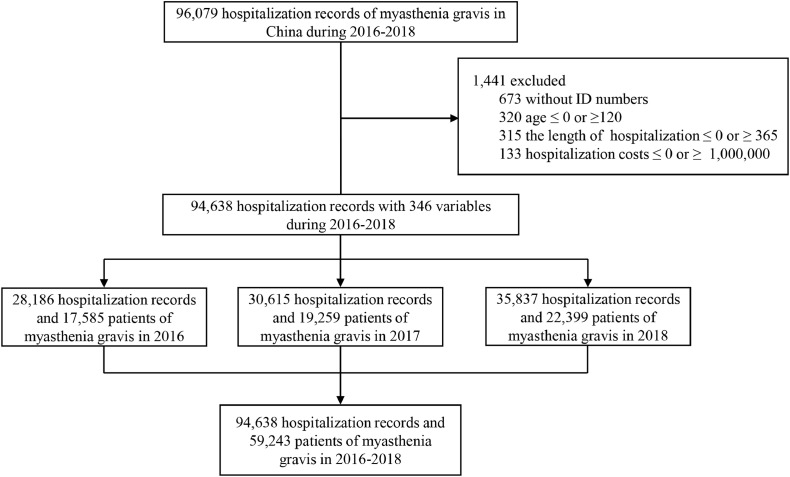
This national population-based registry study is based on the database of the Hospital Quality Monitoring System of National Health Commission, which covers 1665 hospitals providing myasthenia gravis care in 31 provinces and municipalities across China. 94,638 hospital admissions for 59,243 myasthenia gravis patients were identified from January 1st, 2016 to December 31st, 2018. Myasthenia gravis was identified by ICD-10 codes (G70). Incidence of myasthenia gravis was stratified by age, sex, and province.
Findings
Of 59,243 patients, 30,503 individuals with myasthenia gravis were newly diagnosed. Age and sex adjusted incidence of myasthenia gravis was 0.68 per 100,000 person-years, with highest in the age group of 70–74 years. The incidence in females was 0.76 per 100,000 and 0.60 per 100,000 in males. The admission mortality rate was 14.69‰. Respiratory failure was the leading cause of death in patients with myasthenic crisis. There were 14,840 patients with thymomas, encompassing 14,636 (26.5%) adults and 204 (7.1%) juveniles. 9453 (63.7%) patients with thymomas underwent thymomectomy. The median length of hospital stay was 8 days (interquartile range (IQR) 4 to 15 days) with median hospitalization cost $1037 (IQR $493 to $2925). The Basic Medical Insurance was the most common payment method, covering 67.4% of patients.
Interpretation
The age and sex adjusted incidence of MG was 0.68 per 100,000 person-years in China. The admission mortality rate was 14.69‰. Most cases of new onset MG occurred in the seventh decade of life.
Funding
National Science Foundation of China (91642205, and 81830038); Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing
Research in context.
Evidence before this study
We searched PubMed and Google Scholar for studies published before Mar 31, 2020, using the terms “myasthenia gravis”, “China OR Chinese”, and “incidence OR prevalence”. Of the 137 articles identified, there were only three relevant articles on the estimation of myasthenia gravis (MG) incidence in local regions in China. According to their reports, the estimated prevalence of myasthenia gravis was 5.09 in Henan province, 6.47 in Hubei province, and 2.19–11.07 per 100,000 in Guangzhou city. However, these studies were based either on one hospital or limited local healthcare insurance data. Comprehensive epidemiological data on myasthenia gravis is extremely sparse or lacking in China, a country that spans vast regions across Asia and encompasses 19% of the world population. In order to determine the incidence and mortality rates of myasthenia gravis in all age groups at the national level in China, we conducted this national population-based study by using data from the Hospital Quality Monitoring System of National Health Commission, which covers 1665 hospitals providing myasthenia gravis care in 31 provinces and municipalities across China.
Added value of this study
This study provides the first estimates of age and sex adjusted incidence rate and admission mortality rate of myasthenia gravis in mainland China. We also compile a map of the incidence rates of 31 provinces and municipalities in mainland China. The hospitalization burden and comorbidities of myasthenia gravis were also evaluated.
Implication of all the available evidence
The age and sex adjusted incidence of myasthenia gravis was 0.68 per 100,000 person-years in China, and the peak age of onset was in the seventh decade. The admission mortality rate was 14.69‰. The Basic Medical Insurance covered 67.4% of patients. This study urges efforts to improve the accessibility and utilization rate of health services and reduce the out-of-pocket spending. Our study fills the gaps in epidemiologic data in China and enriches the global map of myasthenia gravis incidence.
Alt-text: Unlabelled box
1. Introduction
Myasthenia gravis (MG) is an organ-specific autoimmune disorder of the neuromuscular junction, characterized by specific autoantibodies against skeletal muscular antigens in the postsynaptic membrane [1,2]. Muscle weakness is the predominant manifestation of MG, and 85% of all patients experience generalized myasthenia gravis affecting proximal muscles of the extremities and the trunk. Myasthenic crisis occurs due to weakness of respiratory muscles and is a potentially life-threatening complication. Mechanical ventilation is usually required to rescue patients from respiratory failure. It is estimated that myasthenia gravis affects more than 700,000 people worldwide and approximately 36,000 to 60,000 patients in the United States.
The incidence rate of myasthenia gravis varies with age, gender, and ethnic groups [3]. Estimates of incidence range from 0.3 to 2.8 per 100,000 worldwide, and the median global estimated prevalence is 10 per 100,000 [4]. In European countries the annual incidence ranges from 0.4 (Norway) to 2.1 (Italy) per 100,000. The incidence is expected to be 1.9 per 100,000 in Australia [5]. In Asia, the incidence rate of MG in Japan is 0.69–0.87/100,000 [6], similar to that in Korea where the incidence rate of is 0.69 per 100,000 [7]. However, as the world's most populous country, a national survey of MG in China does not exist. Importantly, data on myasthenic crisis and estimates of mortality resulting from crisis are lacking. There is an urgent need for the investigation that can largely promote the policy implementation and insurance support. This study fills the gaps in epidemiologic data in China of an approximately 1.4 billion population and enriches the global map of MG incidence.
2. Methods
2.1. Data sources and collection
This study was conducted based on the Nationwide Mandatory Database of the Hospital Quality Monitoring System (HQMS), which is maintained by the National Health Commission to monitor the quality of medical care in public hospitals [8]. The HQMS was established in 2011 and has been gradually improved with the implementing of medical and health reforms. The system, by the end of 2015, covered all public tertiary hospitals in the country. Each tertiary hospital is required to submit a summary of individual hospitalization information to the HQMS medical records homepage over a unified data transfer protocol (appendix). The HQMS homepage contains 346 variables including demographic characteristics, diagnoses, procedures, complications, and expenses etc.
2.2. Study population
We identified 94,638 hospitalization records from the HQMS database between January 1st, 2016 to December 31st, 2018, based on the diagnosis of myasthenia gravis. According to China Health Statistics Yearbook 2018, there were 2340 tertiary hospitals in China. 1665 hospitals surveyed covered 98.5 percent of tertiary public western medicine hospitals, where MG patients were diagnosed and managed. After excluding 25 hospitals that do not treat neuroimmune diseases, the remaining 228 private hospitals and 422 Chinese medicine hospitals do not accommodate these patients. All hospitals with neurologists treating MG in China were included. Almost all patients with MG in China were referred to tertiary public hospitals. To avoid replicate registration, patients were identified by their unique resident identity numbers in the HQMS. Supplementary materials Section 1 displays the distribution of the hospitals in mainland China.
2.3. Case ascertainment
Myasthenia gravis patients were determined based on the International Classification of Diseases, Tenth Revision (ICD-10) code G70 in principle or other discharge diagnoses. The diagnoses of MG were based on compatible clinical features together with one or more of the following criteria: (a) seropositive assay for autoantibodies directed against the acetylcholine receptor (AChR-ab), muscle-specific kinase (MuSK-ab), or lipoprotein-related protein 4 (LRP4-ab); (b) electrophysiological study findings compatible with a postsynaptic neuromuscular junction disorder (repetitive stimulation, single-fiber electromyography, or both); and (c) a response to cholinesterase inhibitors. In cases of seronegativity, MG diagnosis was confirmed by abnormal findings from neurophysiological studies [2,9,10]. MG-related antibodies (AChR-ab, MuSK-ab, LRP4-ab) were detected in accordance with international standard by third-party testing facilities. Chest CT was routinely administered for diagnosis. Follow up in all 1665 tertiary hospitals in China HQMS required a “Quality Assurance Physician” and coder for each registered record, to review the diagnosis in accordance with ICD-10 code. Patients who were initially discharged with a principle diagnosis of MG were defined as “new-onset cases” in HQMS during 2016 to 2018. New-onset patients in each province or municipality were determined based on a "from province" HQMS parameter. Patients in the HQMS database come from all departments including neurology, emergency, respiratory, pediatrics, and rehabilitation, ensuring maximum coverage for MG patients. Protocol details are provided in the appendix. In alignment with WHO guidelines, this study defines an adult as a person older than 19 years of age, and a juvenile as a person younger than 19 years old.
2.4. Statistical analysis
The annual incidence rates are defined as the fraction of new MG cases, the numerator, over the total national population in 2016, 2017, and 2018. The population according to annual report of the National Bureau of Statistics of Chinaprovided precise information on China's mainland patient population. Incidence rates stratified by age, sex, and provinces and municipalities were calculated per 100,000 person-years at risk. Admission mortality rates were defined as the number of total deaths as numerator, divided by total admissions as denominator. Data were presented as mean or median with interquartile range for the number of inpatient hospitalizations, number of days in the hospital, and cost of each encounter. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
2.5. Patients and public involvement
Due to study design, there was no patient or public involvement in the experimental design. No patients were asked to advise on interpretation or drafting of results. The results of this paper will be shared publicly through the China National Clinical Research Center for Neurological Diseases website. The study was approved by Institutional Review Board of China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University (KY 2020–013–01).
2.6. Role of the funding source
The funding sources of this study did not influence nor participate in study design, data collection, data analysis, data interpretation, or drafting of the report. The corresponding author had full access to all the data in the study as well as final responsibility for the decision to submit for publication.
3. Results
94,638 hospital admissions for 59,243 patients of MG from 2016 to 2018 were identified. Amongst these patients, 30,503 MG patients were newly diagnosed: 10,188 in 2016, 9659 in 2017, and 10,656 in 2018. The overall estimated age and sex adjusted incidence of MG per 100,000 person-years was 0.68: 0.38 in juveniles and 0.74 in adults. Table 1 summarizes MG incidence across age groups. The incidence in females (0.76 per 100,000 person-years,) was higher than that in males (0.60 per 100,000 person-years); the female to male incidence ratio was 1.3. The incidence rate of different age groups in females and males separately is shown in Appendix Fig. 2. The proportion of new male cases increased by year. The incidence peak was observed in the 70–74 years age-group (1.89 per 100,000 person-years) in adults. Correspondingly, the incidence in children reached a nadir in the 10–14 years-old group (0.28 per 100,000 person-years). For groups aged from 1 to 7 decades, the incidence of MG increased steadily with age.
Table 1.
Incidence and admission mortality rates of myasthenia gravis in China.
| Variables | 2016 | 2017 | 2018 | Annual | |||||
|---|---|---|---|---|---|---|---|---|---|
| New diagnosed, n (%) | Death, n (%) | New diagnosed, n (%) | Death, n (%) | New diagnosed, n (%) | Death, n (%) | Incidence rate, per 105 | Admission mortality rate, per 103 | ||
| Age group | |||||||||
| 0–4 | 363 (3.6) | 1 (0.4) | 373 (3.9) | 1 (0.3) | 553 (5.2) | 0 | 0.52 | 1.26 | |
| 5–9 | 219 (2.1) | 1 (0.4) | 217 (2.2) | 1 (0.3) | 309 (2.9) | 0 | 0.32 | 2.06 | |
| 10–14 | 190 (1.9) | 0 | 211 (2.2) | 2 (0.6) | 223 (2.1) | 3 (1.1) | 0.28 | 5.88 | |
| 15–19 | 270 (2.7) | 4 (1.4) | 222 (2.3) | 1 (0.3) | 255 (2.4) | 3 (1.1) | 0.35 | 6.86 | |
| 20–24 | 349 (3.4) | 4 (1.4) | 319 (3.3) | 2 (0.6) | 334 (3.1) | 4 (1.4) | 0.38 | 6.15 | |
| 25–29 | 540 (5.3) | 11 (4.0) | 449 (4.6) | 1 (0.3) | 517 (4.9) | 2 (0.7) | 0.41 | 5.58 | |
| 30–34 | 515 (5.1) | 4 (1.4) | 453 (4.7) | 8 (2.6) | 470 (4.4) | 4 (1.4) | 0.44 | 6.25 | |
| 35–39 | 502 (4.9) | 4 (1.4) | 433 (4.5) | 5 (1.6) | 490 (4.6) | 6 (2.1) | 0.48 | 5.67 | |
| 40–44 | 664 (6.5) | 10 (3.6) | 590 (6.1) | 13 (4.2) | 600 (5.6) | 12 (4.3) | 0.58 | 9.96 | |
| 45–49 | 902 (8.9) | 15 (5.4) | 889 (9.2) | 10 (3.2) | 929 (8.7) | 17 (6.0) | 0.72 | 8.13 | |
| 50–54 | 1,130 (11.1) | 19 (6.9) | 1,095 (11.3) | 25 (8.0) | 1,029 (9.7) | 14 (5.0) | 0.92 | 9.02 | |
| 55–59 | 917 (9.0) | 27 (9.8) | 829 (8.6) | 23 (7.4) | 941 (8.8) | 21 (7.4) | 1.17 | 13.20 | |
| 60–64 | 1,172 (11.5) | 23 (8.3) | 1,092 (11.3) | 38 (12.2) | 1,204 (11.3) | 31 (11.0) | 1.41 | 13.21 | |
| 65–69 | 946 (9.3) | 27 (9.8) | 1,039 (10.8) | 31 (9.9) | 1,128 (10.6) | 30 (10.6) | 1.66 | 13.61 | |
| 70–74 | 741 (7.3) | 38 (13.8) | 693 (7.2) | 40 (12.8) | 825 (7.7) | 30 (10.6) | 1.89 | 22.37 | |
| 75–79 | 470 (4.6) | 34 (12.3) | 465 (4.8) | 39 (12.5) | 509 (4.8) | 38 (13.5) | 1.76 | 30.59 | |
| 80–84 | 229 (2.2) | 35 (12.7) | 225 (2.3) | 31 (9.9) | 251 (2.4) | 37 (13.1) | 1.33 | 48.70 | |
| ≥85 | 69 (0.7) | 19 (6.9) | 65 (0.7) | 41 (13.1) | 89 (0.8) | 30 (10.6) | 0.68 | 107.40 | |
| Female | 5,758 (56.5) | 130 (47.1) | 5,222 (54.1) | 158 (50.6) | 5,696 (53.5) | 134 (47.5) | 0.76 | 16.52 | |
| Male | 4,430 (43.5) | 146 (52.9) | 4,437 (45.9) | 154 (49.4) | 4,960 (46.5) | 148 (52.5) | 0.60 | 12.86 | |
| Total | 10,188 | 276 | 9,659 | 312 | 10,656 | 282 | 0.68 | 14.69 | |
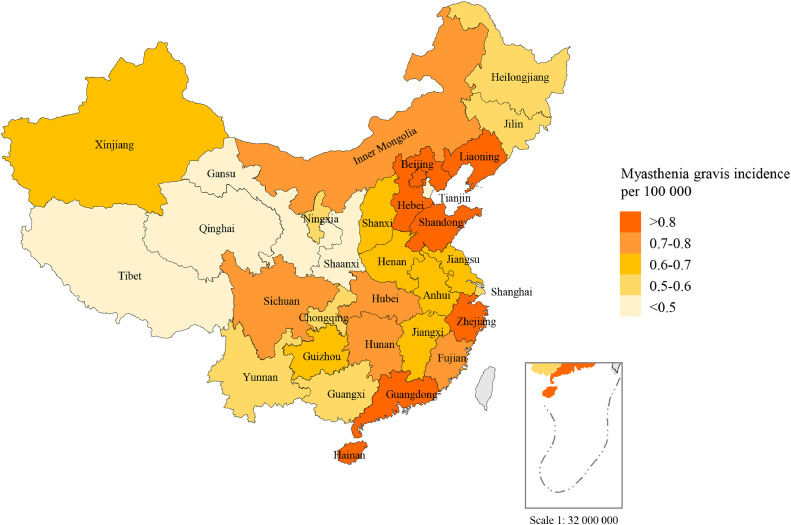
Fig. 2.
Incidence rates of myasthenia gravis for 31 provinces and administrative division in mainland China. The study was conducted in mainland China. Hong Kong and Macao were not included.
Regional differences in incidence were found across China. The estimated incidence of myasthenia gravis per 100,000 persons-years varied from 0.10in Tibet to 1.17 in Beijing. The incidence of MG was higher in coastal areas than that in inland areas, especially in circum-Bohai Sea Zone, including Liaoning, Beijing, Hebei, and Shandong provinces (Fig. 1).
Fig. 1.
Flow chart of participant inclusion in the study.
The characteristics of all the patients with MG are listed in Table 2. 54,670 (92.3%) patients were adult (aged >19 years) and 4573 (7.7%) were children (aged ≤19 years). The mean age of disease diagnosis was 52.2 (19.8) years, with adult 55.9 (15.8) years and children 8.9 (6.1) years. The proportion of female patients reached 54.7%, slightly more than male. 17,512 (29.6%) patients with MG were concomitant with hypertension, followed by diabetes mellitus (13.1%) and hyperlipidemia (12.1%). Remote comorbidities include osteoporosis (3.0%), depression/anxiety (2.1%), and autoimmune diseases (1.5%). In children with MG there were no conspicuous comorbidities, even in those with diabetes, hyperlipidemia, and autoimmune diseases. There were 14,840 patients with thymomas, 14,514 (26.5%) adults and 326 (7.1%) children. 9,453 (63.7%) patients with thymomas underwent thymomectomy.
Table 2.
Characteristics of myasthenia gravis patients admitted to hospital in China (2016–2018).
| Characteristics | Adult (n = 54,670) | Juvenile (n = 4,573) | Total (n = 59,243) |
|---|---|---|---|
| Age, mean (SD), y | 55.9 (15.8) | 8.9 (6.1) | 52.2 (19.8) |
| Sex (female), n (%) | 30,005 (54.9) | 2,425 (53.0) | 32,430 (54.7) |
| Comorbidity, n (%) | |||
| Hypertension | 17,500 (32.0) | 12 (0.3) | 17,512 (29.6) |
| Diabetes | 7,725 (14.1) | 26 (0.6) | 7,751 (13.1) |
| Hyperlipidemia | 7,134 (13.0) | 40 (0.9) | 7,174 (12.1) |
| Osteoporosis | 1,769 (3.2) | 7 (0.2) | 1,776 (3.0) |
| Depression/anxiety | 1,259 (2.3) | 8 (0.2) | 1,267 (2.1) |
| Autoimmune diseases | 878 (1.6) | 23 (0.5) | 901 (1.5) |
| Thymoma | 14,514 (26.5) | 326 (7.1) | 14,840 (25.0) |
| Length of hospital stay, median (IQR), d | 10.0 (6.0 to 16.0) | 6.5 (3.0 to 11.0) | 8.0 (4.0 to 15.0) |
| Hospitalization cost, median (IQR), US$ | 1,433 (785 to 3,382) | 786 (426 to 1,512) | 1,037 (493 to 2,925) |
| Payment methods, n (%) | |||
| UEBMI | 19,756 (36.1) | 295 (6.5) | 20,051 (33.8) |
| URRBMI | 17,955 (32.8) | 1,976 (43.2) | 19,931 (33.6) |
| Commercial Health Insurance | 219 (0.4) | 17 (0.4) | 236 (0.4) |
| Out-of-pocket health expenses | 8,797 (16.1) | 1,809 (39.6) | 10,606 (17.9) |
| Other | 7,943 (14.5) | 476 (10.4) | 8,419 (14.2) |
Abbreviation: IQR, interquartile range; SD, standard deviation; UEBMI, Urban Employee Basic Medical Insurance; URRBMI, Urban Rural Resident Basic Medical Insurance.
The median length of hospital stay was 8 days (interquartile range (IQR) 4 to 15 days) with median hospitalization cost $1037 (IQR $493 to $2,925) among all MG patients. On average, the average length of stay (6.5, IQR 3 to 11 days) was shorter for juveniles than adults and corresponding hospitalization cost ($786, IQR $426 to $1,512) were lower. In regards to payment methods, the Urban Employee Basic Medical Insurance (UEBMI) and the Urban and Rural Resident Basic Medical Insurance (URRBMI) covered about 67.4% patients, and 17.9% patients paid out-of-pocket for treatment and medications. The proportion of out-of-pocket payment accounts for children were relatively higher, at 39.6%. Only 0.4% of MG patients purchased commercial health insurance.
Among the 59,243 patients, the admission mortality was 870 (14.69‰). Male mortality rate (16.52‰) was higher than that of females (12.86‰). From 2016 to 2018, 1468 patients were hospitalized with myasthenic crisis and 95 patients with myasthenic crisis died: 31 in 2016, 31 in 2017, and 33 in 2018. The average age of these patients who died was 61.2 ± 18.5, with no gender difference. Approximately 70% of MG deaths were admitted to the critical care medicine and neurology departments, in which most of deaths were treated. Respiratory failure was the most common causes of death (Table 3).
Table 3.
Characteristics of myasthenic crisis (2016–2018).
| Characteristics | 2016 (n = 442) | 2017 (n = 418) | 2018 (n = 608) |
|---|---|---|---|
| Age, mean (SD), y | 51.9 ± 18.4 | 53.0 ± 17.3 | 50.2 ± 19.6 |
| Sex (female), n (%) | 256 (57.9) | 243 (58.1) | 346 (56.9) |
| Length of hospital stay, median (IQR) | 15.0 (8.0 to 23.0) | 15.0 (8.0 to 25.0) | 14.0 (7.0 to 25.0) |
| Hospitalization cost, median (IQR), US$ | 3,521 (1,075 to 9,311) | 3,473 (1,044 to 9,076) | 3,408 (1,039 to 8,762) |
| Mortality characteristics | n = 31 | n = 31 | n = 33 |
| Age, mean (SD), y | 65.8 ± 13.1 | 61.1 ± 16.8 | 58.9 ± 22.5 |
| Sex (female), n (%) | 15 (48.4) | 13 (41.9) | 17 (51.5) |
| Department, n (%) | |||
| Neurology | 12 (38.7) | 12 (38.7) | 12 (36.4) |
| Critical Care Medicine | 10 (32.3) | 11 (35.5) | 9 (27.3) |
| Respiratory | 2 (6.5) | 1 (3.2) | 1 (3.0) |
| Oncology | 2 (6.5) | 1 (3.2) | 0 |
| Emergency | 0 | 1 (3.2) | 3 (9.1) |
| Thoracic Surgery | 1 (3.2) | 2 (6.5) | 1 (3.0) |
| Other | 4 (12.9) | 3 (9.7) | 7 (21.2) |
| Causes*, n (%) | |||
| Respiratory failure | 17 (54.8) | 24 (77.4) | 19 (61.3) |
| Electrolyte disorders | 19 (61.3) | 13 (41.9) | 24 (77.4) |
| Lung infection | 5 (16.1) | 5 (16.1) | 6 (19.4) |
| Diabetic ketoacidosis | 7 (22.6) | 3 (9.7) | 6 (19.4) |
| Hypoproteinemia | 9 (29.0) | 6 (19.4) | 10 (32.3) |
Top 5 death related diseases.
4. Discussion
Given its complete coverage and emphasis on diagnoses, HQMS serves as a unique and unparalleled resource for determining the incidence of relatively rare diseases such as myasthenia gravis.
4.1. MG incidence in China
This is the first nationwide MG incidence study in mainland China ever conducted. The age and incidence of MG in China is 0.68 per 100,000 person-years, falling into the incidence range between 3.0 and 30.0 per million worldwide [11]. The annual average number of new occurrences over 10,000 from 2016 to 2018 remains the largest reported globally. The incidence of MG among children and adults was like African data, although a smaller sample with only AChR-ab positive cases was investigated [12]. This is interesting as the African sample, unlike European children, also showed a higher incidence. Pediatric MG patients comprised 5.7% of all patients in China with a lower incidence than adults (0.38 vs. 0.74 per 100,000). Childhood patients (0–10 years) seem to have a higher incidence (0.42 per 100,000) than peri-pubertal patients (0.32 per 100,000). However, it is higher than reports from western countries, where the incidence rate was 1.5 per million in the UK [13], 0.3–2.2 per million in a Danish population [14], 0.9–2.0 in Canada [15], and 1.6 per million in Norway [16]. The studies indicate that potential geographical and ethnic differences in risk complicate direct comparisons.
A linear distribution of MG incidence in males is frequently described, increasing with age and peaking between 60 and 80 years. Linear and bimodal patterns have both been reported for female MG. A peak of disease onset occurs at 60 to 80 years; another peak may occur at a range of 20 to 30 years but may also not appear [17,18]. We found that women diagnosed with MG have a higher incidence than men, and both sexes most often occur in the seventh decade. The incidence peaks once in both sexes and differs from bimodal gender distribution.
With the aging of the population, the age of onset of MG increases. It was thought to be more common in women under 40 years, but men and older patients have increased in recent decades [19]. Recent studies found that patients with over 65 years of age has been increasing in MG [20]. Late-onset MG has been associated with the presence of thymoma and more severe forms of the disease, suggesting the possibility of changes in demographic distributions among patients [21,22].
4.2. Economic evaluation
The share of medical expenses paid by all MG patients with and without the scope of basic medical insurance system, which aims to promote public health, plays the indispensable roles in maintaining the health of adult MG patients. However, the higher out-of-pocket health expenses in adolescent patients pose an inevitable expenditure in patients’ families. Raising the proportion of total health expenditure by basic medical insurance may contribute to improving the level of medical security for MG patients. Increasing effort in healthcare outreach have resulted in an increasing level of insurance coverage. Correspondingly, the hospitalization cost was lower than previously reported [23].
4.3. Coexisting disorders
We found that the percentages of patients with hypertension, diabetes mellitus, hyperlipidemia come close to the general population, and is more common in men with late-onset MG. Since corticosteroids act as the first-line agents for induction treatment and also for long-term maintenance, it is clear that higher administered doses and long-term use of corticosteroids increase the risk of hypertension, impaired glucose tolerance, and osteoporosis [24], [25], [26]. Further, treatment with statins may cause exacerbations of MG [27]. In a large population-based study, patients who received corticosteroid doses higher than 7.5 mg/day were 2.5 times more likely than control subjects to experience a cardiovascular event, even after adjustment of known co-variates [28,29]. As these comorbidities are closely related to steroid treatment, it is a challenge how to manage patients with these comorbidities in MG. Complete avoidance of the use of corticosteroids may decrease mortality caused by cardiovascular events and stroke [30]. New clinical trials that focus on effective treatment with reducing doses of corticosteroids are necessary, especially for MG in elderly patients.
10–20% of MG patients have a thymoma which typically occurs after 50 years of age. We found a higher occurrence of thymoma in Chinese adult patients with MG than reported in the literature [31], [32], [33]. One of the explanations may be the loss of data on out-patient patients, as HQMS database only covers in-patient data from registered hospitals. Patients from the out-patient clinic with mild symptoms or follow up office visits were not included into HQMS. Most of these patients did not have thymomas. Due to the advantages of HQMS, the study included MG patients who were prepared to undergo thymus surgery in the thoracic surgery department. Though children are rarely be affected, 6% had thymoma here. There is no major sex bias. 46.3% of adult patients received thymectomy. As thymomectomy was performed when patients’ symptoms are stabilized, many patients underwent thymomectomy after 2018. They were not included in the current survey that only covered the hospitalised patients from 2016 to 2018 from HQMS. In part, this contributed to the relatively low frequency of thymectomy for patients enrolled in this study. Among patients who underwent thymectomy, the proportion of patients who performed thoracotomy or thoracoscopic surgery was similar, and the choice of the two procedures was not tendentious.
4.4. Myasthenic crisis
The incidence of myasthenic crisis was estimated at between 15% to 20% in all patients with MG. The overall mortality rate (1.5%) was similar to previous reports [34,35]. In China, we found a lower incidence of myasthenic crisis (4.81%) and also a lower mortality rate (6.4%) for patients in myasthenic crisis compared to the USA [36]. The respiratory failure that characterizes myasthenic crisis was the most common cause of death. Respiratory tract disease has ever been a major cause of death among MG patients, which was more pronounced in males in Norway [37]. However, with improved treatment of MG and its related complications in the past two decades, the mortality of patients dying of respiratory failure has reduced.
4.5. Limitations
Given its complete coverage and emphasis on diagnoses, HQMS serves as a unique and unparalleled resource for determining the incidence of relatively rare diseases such as myasthenia gravis. For the first time, our study captures the incidence of MG in all age groups in almost all Chinese patients, filling vital gaps in global MG epidemiology. Based on China's 1.4 billion people, HQMS has a full coverage and continuity of diagnostic institutions, positioning a unique advantage for the study of rare diseases. Strengths of our study include the national level population-based study, centralized hospitalization records from administrative hospital quality monitor system, and coverage of all tertiary hospitals and departments. However, our study also had some limitations. First, this study may not include records from all tertiary, secondary hospitals as well as outpatients. Reportedly, about 50% of Chinese MG patients have pure ocular manifestations during their entire lifetime and are more likely to be diagnosed and followed up in out-patient rather than in-patient, as the pure ocular type is usually milder than generalized patients [38]. As a result, the exclusion of these out-patient data may underestimate the incidence of MG. Second, results of MG-related antibodies tests were not collected in this study. While these data are helpful for the description and classification of diseases, it is not an indispensable indicator for determination of the incidence of MG. Third, in this general survey, the severity grade of MG was not recorded because HQMS system did not stratify the severity in raw data. Lastly, incidence rate is generally calculated from a long-term cohort follow-up study, wherein participants are tracked over time and the occurrence of new cases is recorded. However, due to the low incidence rate of MG and the large population in China, it is extremely difficult to conduct a cohort study with a substantial sample size.
5. Contributors
F.-D.S., H.L., and Y.W. conceived and designed this study; F.-D.S., Z.L., D.-C.T., C.Z., J.C., Y.X. and Y.Z. acquired and analyzed the data; and F.-D.S., D.-C.T., C.Z., and J.C. drafted the manuscript and prepared the figures; H.G. was involved in statistics analysis; F.-D.S. obtained funding; F.-D.S. made critical revisions of the manuscript and important intellectual contributions. All authors reviewed the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
We thank colleagues from National Center for Quality Control of Neurological Diseases for technical and logistic support; Samuel X. Shi for English editing; Yingyu Jiang for statistical analysis.
Data sharing statement
The study protocol, statistical analysis plan, and deidentified data that underlie the results of this Article will be available for investigators after approval by the Institutional Review Board of China National Clinical Research Center for Neurological Diseases (Beijing, China). Please email the corresponding author for more information.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2020.100063.
Contributor Information
Hao Li, Email: Li_hao71@aliyun.com.
Yongjun Wang, Email: yongjunwang@ncrcnd.org.cn.
Fu-Dong Shi, Email: fshi@tmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Gilhus N.E. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus N.E., Verschuuren J.J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 3.Meriggioli M.N., Sanders D.B. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deenen J.C., Horlings C.G., Verschuuren J.J., Verbeek A.L., van Engelen B.G. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis. 2015;2(1):73–85. [PubMed] [Google Scholar]
- 5.Gattellari M., Goumas C., Worthington J.M. A national epidemiological study of myasthenia gravis in Australia. Eur J Neurol. 2012;19(11):1413–1420. doi: 10.1111/j.1468-1331.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 6.Matsui N., Nakane S., Nakagawa Y. Increasing incidence of elderly onset patients with myasthenia gravis in a local area of Japan. J Neurol Neurosurg Psychiatry. 2009;80(10):1168–1171. doi: 10.1136/jnnp.2008.152637. [DOI] [PubMed] [Google Scholar]
- 7.Lee H.S., Lee H.S., Shin H.Y., Choi Y.C., Kim S.M. The epidemiology of myasthenia gravis in Korea. Yonsei Med J. 2016;57(2):419–425. doi: 10.3349/ymj.2016.57.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L., Krumholz H.M., Li X., Li J., Hu S. Achieving best outcomes for patients with cardiovascular disease in China by enhancing the quality of medical care and establishing a learning health-care system. Lancet. 2015;386(10002):1493–1505. doi: 10.1016/S0140-6736(15)00343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew W.K., Powell C.A., Sloan S.R. Comparison of plasmapheresis and intravenous immunoglobulin as maintenance therapies for juvenile myasthenia gravis. JAMA Neurol. 2014;71(5):575–580. doi: 10.1001/jamaneurol.2014.17. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C., Bu B., Yang H. Immunotherapy choice and maintenance for generalized myasthenia gravis in China. CNS Neurosci Ther. 2020 doi: 10.1111/cns.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGrogan A., Sneddon S., de Vries C.S. The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology. 2010;34(3):171–183. doi: 10.1159/000279334. [DOI] [PubMed] [Google Scholar]
- 12.Mombaur B., Lesosky M.R., Liebenberg L., Vreede H., Heckmann J.M. Incidence of acetylcholine receptor-antibody-positive myasthenia gravis in South Africa. Muscle Nerve. 2015;51(4):533–537. doi: 10.1002/mus.24348. [DOI] [PubMed] [Google Scholar]
- 13.Parr J.R., Andrew M.J., Finnis M., Beeson D., Vincent A., Jayawant S. How common is childhood myasthenia? The UK incidence and prevalence of autoimmune and congenital myasthenia. Arch Dis Child. 2014;99(6):539–542. doi: 10.1136/archdischild-2013-304788. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen E.G., Hallas J., Hansen K., Jensen P.E., Gaist D. Late-onset myasthenia not on the increase: a nationwide register study in Denmark, 1996–2009. Eur J Neurol. 2013;20(2):309–314. doi: 10.1111/j.1468-1331.2012.03850.x. [DOI] [PubMed] [Google Scholar]
- 15.VanderPluym J., Vajsar J., Jacob F.D., Mah J.K., Grenier D., Kolski H. Clinical characteristics of pediatric myasthenia: a surveillance study. Pediatrics. 2013;132(4):e939–e944. doi: 10.1542/peds.2013-0814. [DOI] [PubMed] [Google Scholar]
- 16.Popperud T.H., Boldingh M.I., Brunborg C. Juvenile myasthenia gravis in Norway: a nationwide epidemiological study. Eur J Paediatr Neurol. 2017;21(2):312–317. doi: 10.1016/j.ejpn.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Andersen J.B., Heldal A.T., Engeland A., Gilhus N.E. Myasthenia gravis epidemiology in a national cohort; combining multiple disease registries. Acta Neurol Scand Suppl. 2014;(198):26–31. doi: 10.1111/ane.12233. [DOI] [PubMed] [Google Scholar]
- 18.Carr A.S., Cardwell C.R., McCarron P.O., McConville J. A systematic review of population based epidemiological studies in myasthenia gravis. BMC Neurol. 2010;10:46. doi: 10.1186/1471-2377-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aarli J.A. Myasthenia gravis in the elderly: is it different. Ann N Y Acad Sci. 2008;1132:238–243. doi: 10.1196/annals.1405.040. [DOI] [PubMed] [Google Scholar]
- 20.Aarli J.A. Late-onset myasthenia gravis: a changing scene. Arch Neurol. 1999;56(1):25–27. doi: 10.1001/archneur.56.1.25. [DOI] [PubMed] [Google Scholar]
- 21.Cortes-Vicente E., Alvarez-Velasco R., Segovia S. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology. 2020;94(11):e1171–e1e80. doi: 10.1212/WNL.0000000000008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casetta I., Groppo E., De Gennaro R. Myasthenia gravis: a changing pattern of incidence. J Neurol. 2010;257(12):2015–2019. doi: 10.1007/s00415-010-5651-z. [DOI] [PubMed] [Google Scholar]
- 23.Omorodion J.O., Pines J.M., Kaminski H.J. Inpatient cost analysis for treatment of myasthenia gravis. Muscle Nerve. 2017;56(6):1114–1118. doi: 10.1002/mus.25624. [DOI] [PubMed] [Google Scholar]
- 24.Gilhus N.E., Nacu A., Andersen J.B., Owe J.F. Myasthenia gravis and risks for comorbidity. Eur J Neurol. 2015;22(1):17–23. doi: 10.1111/ene.12599. [DOI] [PubMed] [Google Scholar]
- 25.Wakata N., Nemoto H., Konno S. Myasthenia gravis and diabetes mellitus: a 35-year retrospective study. Intern Med. 2007;46(9):557–559. doi: 10.2169/internalmedicine.46.6237. [DOI] [PubMed] [Google Scholar]
- 26.Misra U.K., Kalita J., Singh V.K., Kumar S. A study of comorbidities in myasthenia gravis. Acta Neurol Belg. 2020;120(1):59–64. doi: 10.1007/s13760-019-01102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh S.J., Dhall R., Young A., Morgan M.B., Lu L., Claussen G.C. Statins may aggravate myasthenia gravis. Muscle Nerve. 2008;38(3):1101–1107. doi: 10.1002/mus.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tannemaat M.R., Verschuuren J. Emerging therapies for autoimmune myasthenia gravis: towards treatment without corticosteroids. Neuromuscul Disord. 2020;30(2):111–119. doi: 10.1016/j.nmd.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Wei L., MacDonald T.M., Walker B.R. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141(10):764–770. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 30.Roubille C., Richer V., Starnino T. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(3):480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent A., Palace J., Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357(9274):2122–2128. doi: 10.1016/S0140-6736(00)05186-2. [DOI] [PubMed] [Google Scholar]
- 32.Marx A., Pfister F., Schalke B., Saruhan-Direskeneli G., Melms A., Strobel P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev. 2013;12(9):875–884. doi: 10.1016/j.autrev.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Tsinzerling N., Lefvert A.K., Matell G., Pirskanen-Matell R. Myasthenia gravis: a long term follow-up study of Swedish patients with specific reference to thymic histology. J Neurol Neurosurg Psychiatry. 2007;78(10):1109–1112. doi: 10.1136/jnnp.2006.109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann B., Angstwurm K., Mergenthaler P. Myasthenic crisis demanding mechanical ventilation: a multicenter analysis of 250 cases. Neurology. 2020;94(3):e299–e313. doi: 10.1212/WNL.0000000000008688. [DOI] [PubMed] [Google Scholar]
- 35.Thomas C.E., Mayer S.A., Gungor Y. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48(5):1253–1260. doi: 10.1212/wnl.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 36.Alshekhlee A., Miles J.D., Katirji B., Preston D.C., Kaminski H.J. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. 2009;72(18):1548–1554. doi: 10.1212/WNL.0b013e3181a41211. [DOI] [PubMed] [Google Scholar]
- 37.Owe J.F., Daltveit A.K., Gilhus N.E. Causes of death among patients with myasthenia gravis in Norway between 1951 and 2001. J Neurol Neurosurg Psychiatry. 2006;77(2):203–207. doi: 10.1136/jnnp.2005.072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Yang M., Xu J. Clinical and serological study of myasthenia gravis in HuBei Province, China. J Neurol Neurosurg Psychiatry. 2007;78(4):386–390. doi: 10.1136/jnnp.2006.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.