Abstract
Glyco-engineered recombinant antibodies are currently being developed as the next generation therapeutics to treat human diseases, including cancer, autoimmunity and infection. Antibodies lacking core fucosylation show great increase in affinity for FcγRIIIA, leading to an improved receptor-mediated effector function. While afucosyl human IgG1 exhibits 50–100-fold increase in antibody-mediated cellular cytotoxicity (ADCC), a key immune effector mechanism underlying the anti-cancer effect of some approved therapeutic antibodies, it is not clear whether such glyco-engineered antibodies would find similar use for infectious disease. Due to the species difference, human antibodies may have different binding properties towards corresponding IgG receptors from animals used for modeling infection and intoxication. During the course of studying a recombinant human IgG1 in neutralizing diphtheria toxin (DT) in Guinea pigs (Cavia porcellus), we identified a previously uncharacterized Guinea pig protein H0VDZ8 from UNIPROT database that shows high sequence homologies to human FcγRIIIA and mouse FcγRIV. This Fcγ receptor, which we named as gpFcγRIV, also demonstrates functional similarity although not to the same extent as the human and mouse counterparts, in that it binds to afucosyl human and mouse IgG much stronger than to the wild type antibodies. Thus, Guinea pigs can be used to compare the efficacies of wild type vs. afucosyl anti-DT human IgG1 in toxin removal and animal protection. Molecular and functional characterization of human FcγRIIIA and mouse FcγRIV equivalents in other species could expand the list of preclinical animal models for testing afucosyl human antibodies in treating various human diseases.
Keywords: Immunoglobulin G, Fc Receptor, Afucosylation, Guinea Pig
Introduction
Monoclonal antibodies (Mabs) are being developed as therapeutics for treating various human diseases. The vast majority of marketed IgG Mabs are produced in mammalian cells, especially Chinese hamster ovary (CHO) cells. IgG1 antibodies produced from wild type CHO cells have a fucose residue in a 1,6 linkage to the first GlcNAc of the oligosaccharide core (“core fucosylation”) in their bi-antenna glycan attached to asparagine (Asn) residue 297 in the Fc region. It has been well established by many laboratories that the addition of core fucose diminishes the affinity of IgG Fc to the human FcγRIIIA receptor expressed on natural killer cells, macrophages, neutrophils, and other immune cells. As a result, IgG molecules lacking a core fucose residue bind with 50-fold enhanced affinity to FcγRIIIA and exhibit 50–100-fold greater cellular immune functions, for example, antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) [1].
Currently, afucosyl antibodies with enhanced ADCC towards target cells are mainly being pursued in the immuno-oncology area. For example, Mogamulizumab is an afucosyl humanized Mab targeting CC chemokine receptor 4 (CCR4), approved in Japan in 2012 for the treatment of relapsed or refractory adult T-cell leukaemia-lymphoma (ATL) [2]. Obinutuzumab against CD20 is the second afucosyl Mab approved by US FDA in 2013 for treating B cell lymphoma [3]. Besides these two approved, more afucosyl antibodies are in the research pipeline, and the pharmaceutical industry has recognized the advantages of fucose-free therapeutic antibodies [3]. While the benefits of afucosyl Mabs in cancer immunotherapy are obvious, so far there are only two reports in infectious disease area supporting their potential application, where afucosyl antiviral antibodies show enhanced cell-mediated antiviral potency against the HIV and Ebola viruses [4, 5].
The main reason for this deficiency is due to the lack of knowledge on the binding properties of human IgGs with or without fucose towards corresponding antibody receptors from animals used for modeling infection and intoxication. Among the human Fcγ receptors, only FcγRIII is sensitive to IgG defucosylation. In mice, mFcγRIV is regarded as the functional homologue of hFcγRIIIA. Accordingly, only mFcγRIV is sensitive to IgG defucosylation among all the murine Fcγ receptors ([6] and our unpublished observation). Little is known on the counterparts of hFcγRIIIA and mFcγRIV in other species, as well as their binding sensitivity towards afucosyl antibodies.
During the course of studying a human anti-diphtheria toxin IgG1 Mab in Guinea pigs (Cavia porcellus), we first asked: 1) whether hIgG1 can bind to Guinea pig FcγR, and 2) whether there is an equivalent of hFcγRIIIA and mFcγRIV in Guinea pigs that exhibits enhanced binding to afucosyl hIgG1. Our studies show that a previously uncharacterized Guinea pig protein H0VDZ8 from UNIPROT database is a structural and functional homologue to human FcγRIIIA and mouse FcγRIV.
Materials and Methods
Cell line generation:
CHO cell lines expressing the functional hFcγRIIIA, mFcγRIV or gpFcγRIV complexes on the cell surface were generated with the “Toggle-In” CHO system (Antagen Pharmaceuticals, Inc., Boston, MA). The establishment of the anchor “Toggle-In” CHO cell line and the construction of pTOG3 and pTOG4 expression vectors with alternate use of Hygromycin B and Puromycin as selection markers will be published in more detail elsewhere. Briefly, an anchor “Toggle-In” CHO cell line was selected by FACS sorting on d1EGFP expression at a CHO genomic “hot-spot”. Several genes involved in enhancing protein expression, e.g., SRP54, SRP9, SRP14, ERO1-L and FGF9 were sequentially integrated at this “hot-spot” by replacing d1EGFP with Cre-LoxP recombination-mediated cassette exchange (RMCE). A CHO cell line thus generated is named as the master “Toggle-In” line, which has been used for establishing other cell line models. Herein for example, human FcR common γ chain in pTOG4 was first transfected into the master “Toggle-In” line, and selected with Puromycin (10 μg/mL). An FcRγ-positive clone was picked and further transfected with hFcγRIIIA-V158, mFcγRIV or gpFcγRIV, all cloned in pTOG3, respectively, and selected with Hygromycin B (1 mg/mL). Single CHO clones of double transfectants were screened and confirmed by FACS staining with hIgG1 or mIgG2a.
Expression of wild type and afucosyl antibodies:
A ScFv-hIgG1 construct, where its ScFv part utilizes anti-TNFα Humira sequence to fuse with human IgG1 Fc, was cloned into pDirect4.0 expression vector (Antagen), and transfected into either wild type or proprietary Fut8−/− CHO-K1 cells (Antagen). This Fut8−/− CHO cell line was established in-house with TALEN technology. Detailed method and genomic sequences of the two TALEN-targeted Fut8 alleles will be disclosed elsewhere. A mouse hybridoma secreting IgG2a antibody (PK136, ATCC, Manassas, Virginia) against mouse NK1.1 was transfected with two CRISPR constructs targeting the mouse Fut8 gene (Antagen). The Fut8−/− CHO or hybridoma cells were selected by MACS depletion of fucose-positive cells, and confirmed by negative staining with FITC-labeled Lens culinaris agglutinin (LCA, Vector Laboratories, Inc., Burlingame, CA), recognizing the α−1,6 fucosylated tri-mannnosyl core structure on N-glycans on the cell surface.
Comparing wild type and afucosyl antibodies in binding to FcγR:
Both CHO-derived wild type and afucosyl hIgG1, as well as hybridoma-derived wild type and afucosyl mIgG2a, were purified with Protein A column from culture supernatants. Protein concentrations were determined by OD280, and titrated amounts of wild type and afucosyl antibodies were assayed for receptor binding with goat anti-human or mouse secondary antibody in flow cytometry, using CHO cell lines expressing hFcγRIIIA, mFcγRIV or gpFcγRIV.
Results and Discussion
To search for Guinea pig homologue(s) of hFcγRIIIA and mFcγRIV, we blasted UNIPROT database and found a few candidate Guinea pig proteins that show various homologies. One uncharacterized protein H0VDZ8 that has not been assigned a gene name shows the highest homologies: 55.3% identical and 72.5% similar amino acids with hFcγRIIIA; 54.9% identical and 71.4% similar amino acids with mFcγRIV (Figure 1). H0VDZ8 could be the potential Guinea pig equivalent of hFcγRIIIA and mFcγRIV.
Figure 1.
Alignment of Guinea pig protein H0VDZ8 with hFcγRIIIA (P08637, upper) or mFcγRIV (Q8R477, lower).
Based on its amino acid sequence, we ordered full gene synthesis for H0VDZ8 as “gpFcγRIV” with CHO codon optimization (Integrated DNA Technologies, Inc., Coralville, Iowa), and cloned it into the pTOG3 vector (Antagen). As FcR common γ chain co-expression is required for proper protein folding and cell surface display of hFcγRIIIA or mFcγRIV, we took the advantage of our “Toggle-In” system (Antagen), where Cre-LoxP based sequential integration of exogenous genes into the same genomic locus is exploited for isogeneic co-expression of the hFcγRIIIA+hFcRγ, mFcγRIV+hFcRγ or gpFcγRIV+hFcRγ complex (See Methods).
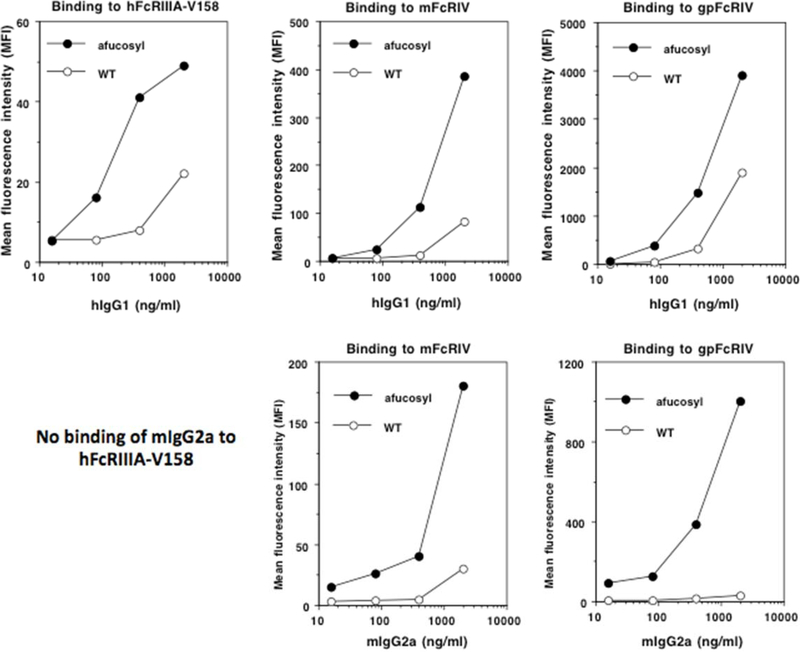
When titrated amounts of wild type and afucosyl hIgG1 were added to hFcγRIIIA-V158 expressing CHO cells, afucosyl hIgG1 demonstrated much better binding than wild type hIgG1. The EC50 of afucosyl hIgG1 binding to hFcγRIIIA-V158 is about 200 ng/mL (1.25 nM), whereas that of the wild type hIgG1 is >2000 ng/mL (12.5 nM) (Figure 2, upper left panel). We estimate that by our method, there is >10–20-fold increase in hIgG1 binding to hFcγRIIIA-V158 after defucosylation. Mouse IgG2a did not show any binding to hFcγRIIIA (Figure 2, lower left panel), emphasizing that species difference will have to be taken into consideration when directly testing in vitro ADCC activities of mouse antibodies using hFcγRIIIA-expressing effector cells.
Figure 2.
Flow cytometry analysis of binding of wild type or afucosyl hIgG1 (upper) and mIgG2a (lower) to hFcγRIIIA, mFcγRIV or gpFcγRIV (H0VDZ8). Data are representative of two similar experiments.
Interestingly, when wild type and afucosyl hIgG1 or mIgG2a antibodies were added to mFcγRIV-expressing CHO cells, very similar binding patterns were obtained (Figure 2, upper and lower middle panel). This suggests that murine immune system could largely recapitulate the benefits of hIgG1 defucosylation. In other words, to demonstrate the enhanced therapeutic values of afucosylated humanized murine antibody (hIgG1 isotype) in mouse models of disease, researchers can directly take the advantage of CRISPR knockout of mouse Fut8 gene in hybridoma, and compare hybridoma-derived wild type and afucosyl parental mouse antibodies if they are of ADCC-enabling IgG2a/2b/2c isotypes. This could be an alternative to cloning the VH VL genes from the hybridoma and expressing the chimeric antibody genes with hIgG1 Fc in wild type and Fut8−/− CHO cells.
However, the binding of hIgG1 to gpFcγRIV does not show much enhancement after defucosylation (Figure 2, upper right panel). We repeated the assay and got very similar results: The EC50 of afucosyl hIgG1 binding to gpFcγRIV is about 289.3 ng/mL (1.81 nM), whereas that of the wild type hIgG1 is 712.1 ng/mL (4.45 nM) (data not shown), a mere 2.5 fold enhancement. For mIgG2a, although it does not bind to hFcγRIIIA-V158, its binding to gpFcγRIV is dramatically enhanced after defucosylation (Figure 2, lower right panel).
Our studies answered the initial questions that hIgG1 can bind to Guinea pig FcγR and H0VDZ8 is a structural and functional homologue to human FcγRIIIA and mouse FcγRIV. In fact, based on the mean fluorescence intensity (MFI) data in flow cytometry, the binding affinity of hIgG1 to this gpFcγRIV is about 100-fold and 10-fold over the binding affinity of hIgG1 to hFcγRIIIA and mFcγRIV, respectively. We believe these are not due to the cell line differences in receptor surface expression because of integration site and copy number variation seen in the conventional transfection method, as our “Toggle-In” system can eliminate such factors by site-specific isogeneic expression of exogenous genes with equal copy number. Perhaps also due to this stronger base line binding of hIgG1 to gpFcγRIV, the difference between the wild type and the afucosyl forms of hIgG1 in binding to gpFcγRIV is not as dramatic. Therefore, the benefit of afucosyl hIgG1 in Guinea pig model could be under-manifested as in humans. From proof-of-concept point of view, it remains to be tested whether murinized human therapeutic IgG with mIgG2a/b/c Fc tail would better exemplify the benefits of afucosyl antibodies in disease models where Guinea pigs are used as host.
Acknowledgement
This study is supported by SBIR contracts from CDC (200-2014-M-59655, 2015-N-17179) and NIH/NIDA (HHSN271201100015C).
Abbreviations:
- CHO
Chinese hamster ovary
- ADCC
Antibody-mediated cellular cytotoxicity
- ADCP
antibody-dependent cellular phagocytosis
- Fut8
Alpha-(1,6)-fucosyltransferase 8
- TALEN
Transcription activator-like effector nuclease
Reference
- 1.Listinsky JJ, Siegal GP, Listinsky CM (2013) Glycoengineering in cancer therapeutics: a review with fucose-depleted trastuzumab as the model. Anticancer Drugs 24: 219–227. http://www.ncbi.nlm.nih.gov/pubmed/23059384 [DOI] [PubMed] [Google Scholar]
- 2.Beck A, Reichert JM (2012) Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs 4: 419–425. http://www.ncbi.nlm.nih.gov/pubmed/22699226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small S (2013) Approval of obinutuzumab as a breakthrough therapy for chronic lymphocytic leukemia. Clin Adv Hematol Oncol 11: 809–810. http://www.ncbi.nlm.nih.gov/pubmed/25016628 [PubMed] [Google Scholar]
- 4.Forthal DN, Gach JS, Landucci G, Jez J, Strasser R, et al. (2010) Fc-glycosylation influences Fcγ receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J Immunol 185: 6876–6882. http://www.ncbi.nlm.nih.gov/pubmed/21041724 [DOI] [PubMed] [Google Scholar]
- 5.Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, et al. (2011) Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA 108: 20690–20694. http://www.ncbi.nlm.nih.gov/pubmed/22143789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimmerjahn F, Ravetch JV (2005) Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310: 1510–1512. http://www.ncbi.nlm.nih.gov/pubmed/16322460 [DOI] [PubMed] [Google Scholar]