Abstract
Objective
Atherosclerotic cardiovascular disease (ASCVD) and chronic obstructive pulmonary disease (COPD) are among the leading causes of morbidity, mortality, and economic burden in the United States (US). While previous reports have shown that an optimal cardiovascular risk factor (CRF) profile is associated with improved outcomes among COPD patients, the impact of ASCVD and CRF on healthcare costs and resource utilization is not well described.
Methods
The Medical Expenditure Panel Survey (MEPS) database was used from 2011 to 2016 to study healthcare expenditure for COPD patients with and without ASCVD and across CRF profiles in a nationally representative population of adults in the United States.
Results
The study population consisted of 14,807 adults with COPD, representing 28 million cases annually. Presence of ASCVD was associated with higher reported expenditure across the spectrum of CRF profiles among those with COPD. On average, after adjusting for confounders, presence of ASCVD represented a mean difference per capita of $5438 (95% CI $4121 - $6754; p < 0.001). Mean per capita expenditures were significantly higher comparing poor vs optimal CRF profiles, with marginal expenditures of $8552 and $6531 among those with and without ASCVD, respectively. When comparing individuals with ASCVD and poor CRF profile versus individuals without ASCVD and optimal CRF profile, those in the latter group used 13% fewer prescription medications and required 24% fewer hospitalizations. Furthermore, an optimal CRF profile was associated with lower odds of most sources of healthcare utilization regardless of ASCVD status.
Conclusion
An absence of ASCVD and a favorable CRF profile was associated with lower healthcare expenditure and resource utilization among patients with COPD. These results provide robust estimates for potential healthcare savings as preemptive strategies continue to become integrated into new healthcare delivery models, for increased awareness and the need for improvement of CRF profiles among high-risk patients.
Keywords: ASCVD, COPD, Healthcare expenditure, Medications
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) and chronic obstructive pulmonary disease (COPD) are among the leading causes of morbidity, mortality, and economic burden in the United States (US) [1]. The prevalence of COPD and concurrent ASCVD is expected to increase over the next decade [[2], [3], [4]] and the combined annual direct and indirect cost of ASCVD and COPD in the US (~$605 billion) is projected to increase 2-fold by 2035 [[5], [6], [7]].
While ASCVD and COPD have been viewed as distinct conditions, there is growing evidence that common modifiable risk factors are associated with an increased risk of both. Therefore, enhanced ASCVD primary prevention may reduce economic burden and morbidity among COPD patients. Although previous reports have shown that an optimal cardiovascular risk factor (CRF) profile is associated with improved outcomes among COPD patients, the impact of ASCVD and CRF on healthcare costs and resource utilization is not well described [[8], [9], [10]]. Accordingly, we studied healthcare expenditure for patients with COPD with and without ASCVD and across CRF profiles in a nationally representative population of adults in the United States.
2. Methods
The Medical Expenditure Panel Survey (MEPS) database was used from 2011 to 2016. The Household Component (HC) of the MEPS collects data regarding utilization of healthcare services along with their frequency and cost, and source of payment [11]. The sampling frame for the MEPS-HC is drawn from respondents to the National Health Interview Survey and the design includes sampling weights, stratification, and clustering. Full-year consolidated data files were used to assess demographics on an individual-level, while medical conditions files include individual diagnostic information. This study was exempt from review by the Houston Methodist institutional review board committee since MEPS data are deidentified and publicly available.
ASCVD, COPD, and CRFs were ascertained using self-reported information from the questionnaires in the HC survey and/or ICD-9-CM or ICD-10 codes, based on MEPS switching from ICD-9-CM to ICD-10 codes in 2016. ASCVD diagnoses included: coronary artery disease (self-reported coronary heart disease, heart attack or angina, and/or ICD-9: 410, 413, 414/ICD-10: I20, I21, I25), stroke (self-reported stroke, and/or ICD-9: 433–437/ICD-10: I63, G45), and peripheral vascular disease (ICD-9: 440, 443/ICD-10: I70, I73, I79). COPD diagnoses included self-reported emphysema or chronic bronchitis, and/or ICD-9: 490–493, 496/ICD-10: J40-J45.
In a similar fashion, CRFs were ascertained using self-reported information and/or ICD-9-CM or ICD-10 codes (when applicable), and included: hypertension (ICD-9: 401/ICD-10: I10), diabetes (ICD-9: 250/ICD-10: E11), dyslipidemia (ICD-9: 272/ICD-10: E78), lack of exercise (defined as not participating in moderate-vigorous physical activity for 30 min at least 5 times per week), smoking, and obesity (body mass index [BMI] ≥30 kg/m2, using self-reported height and weight). Based on the presence of these individual CRFs, survey participants were profiled as having “Poor” (≥4 CRF), “Average” (2–3 CRF), or “Optimal” (0–1 CRF) profiles. Further, a modified Charlson Comorbidity Index (without the cardiovascular components) was used to adjust for comorbid conditions.
Total annual direct medical expenditures and resource utilization per person were calculated based on expenditures from all payer groups and out-of-pocket spending, including information from hospitalizations, prescribed medications, outpatient visits, emergency department (ED) visits and other sources of expenditure (dental visits, vision aid, home healthcare, and other medical supplies).
Due to the right-skewedness of expenditure data, two-part models were utilized: 1) the probability that any given individual had any expenditure, and 2) their mean expenditure. The first part of the model consists of a probabilistic regression model (probit), which estimates the probability of zero versus positive expenditure. Contingent upon having a positive annual healthcare expenditure, a generalized linear model (glm) with gamma distribution and a logarithmic-link function estimates the average expenditure per capita; we determined the distribution of the glm using the modified Park test. For all analyses, a two-sided p < 0.05 was considered statistically significant. Analyses were performed using Stata®, version 16 (StataCorp, LP, College Station, Texas, USA).
3. Results
The study population consisted of 14,807 adults with COPD, representing 28 million cases annually. Among those with COPD, 44.1% of adults were between 40 and 64 years of age, 62.2% were female, and 61.7% had private insurance (Table 1). Moreover, 23% reported having concurrent ASCVD, translating into 9.5 million adults annually. Prevalence of a suboptimal CRF profile was higher among those with ASCVD when compared to those without.
Table 1.
Characteristics of adults with COPD from the Medical Expenditure Panel Survey 2011–16 overall and by ASCVD status.
| Overall | No ASCVD | ASCVD | p-value | |
|---|---|---|---|---|
| Sample (N) | 14,807 | 11,336 | 3471 | |
| Weighted sample | 28,217,994 | 18,763,209 | 9,454,785 | |
| Age Category, n (weighted %) | <0.001 | |||
| 18–39 | 3855 (25.4) | 3695 (31.8) | 160 (3.7) | |
| 40–64 | 6845 (44.1) | 5314 (45.7) | 1531 (38.4) | |
| 65–74 | 2359 (17.1) | 1428 (13.7) | 931 (28.9) | |
| 75 & Above | 1748 (13.4) | 899 (8.8) | 849 (29.0) | |
| Sex, n (weighted %) | <0.001 | |||
| Female | 9586 (62.2) | 7540 (64.0) | 2046 (55.9) | |
| Male | 5221 (37.8) | 3796 (36.0) | 1425 (44.1) | |
| Race/Ethnicity, n (weighted %) | <0.001 | |||
| Non-Hispanic White | 7943 (76.2) | 5995 (75.5) | 1948 (78.4) | |
| Non-Hispanic Black | 3170 (11.3) | 2423 (11.5) | 747 (10.6) | |
| Non-Hispanic Asian | 538 (2.6) | 444 (2.8) | 94 (1.8) | |
| Hispanic | 2653 (10.0) | 2072 (10.2) | 581 (9.1) | |
| Education level, n (weighted %) | <0.001 | |||
| Less than High School | 3084 (14.9) | 977 (21.5) | 2107 (13.0) | |
| High School/GED & Equivalent | 5052 (33.7) | 1285 (39.2) | 3767 (32.1) | |
| Some College or Higher | 6607 (51.4) | 1191 (39.3) | 5416 (54.9) | |
| Family Income, n (weighted %) | <0.001 | |||
| Low Income | 7074 (36.4) | 5012 (33.0) | 2062 (47.9) | |
| Middle Income | 3987 (28.2) | 3168 (28.3) | 819 (27.7) | |
| High Income | 3746 (35.5) | 3156 (38.7) | 590 (24.4) | |
| Health Insurance Type, n (weighted %) | <0.001 | |||
| Private | 7706 (61.7) | 6403 (66.1) | 1303 (46.5) | |
| Public Only | 5809 (31.4) | 3813 (26.1) | 1996 (50.0) | |
| Uninsured | 1292 (6.9) | 1120 (7.8) | 172 (3.9) | |
| Region, n (weighted %) | <0.001 | |||
| Northeast | 2747 (18.9) | 2149 (19.4) | 598 (16.8) | |
| Midwest | 3319 (23.7) | 2531 (23.6) | 788 (24.0) | |
| South | 5505 (37.5) | 4008 (36.3) | 1497 (42.0) | |
| West | 3236 (20.0) | 2648 (20.7) | 588 (17.2) | |
| CRF Profile, n (weighted %) | <0.001 | |||
| Optimal | 4266 (31.5) | 4023 (38.3) | 243 (8.1) | |
| Average | 6598 (44.3) | 5094 (44.0) | 1504 (45.4) | |
| Poor | 3943 (24.2) | 2219 (17.7) | 1724 (46.5) | |
| Modified Charlson Comorbidity Index, n (weighted %) | <0.001 | |||
| 0 | 1386 (10.2) | 1013 (9.5) | 373 (12.7) | |
| 1 | 10,292 (83.4) | 8107 (85.8) | 2185 (75.0) | |
| ≥2 | 757 (6.4) | 432 (4.7) | 325 (12.3) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; GED, general education diploma; CRF, cardiovascular risk factor.
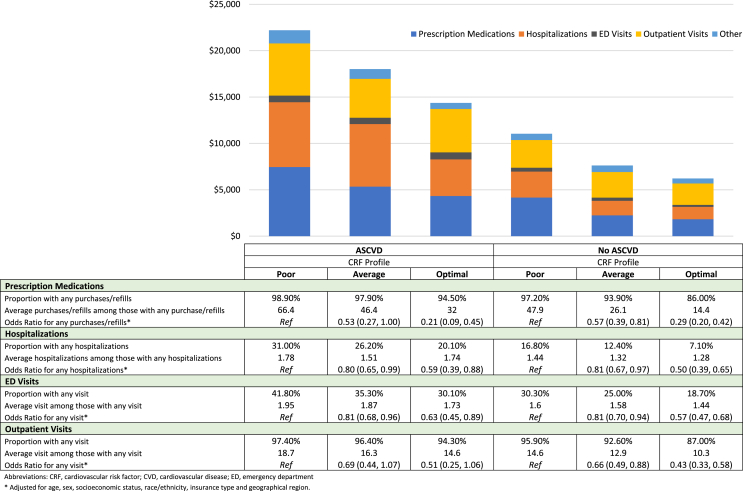
Presence of ASCVD was associated with higher reported expenditure across the spectrum of CRF profiles among those with COPD. On average, after adjusting for confounders, presence of ASCVD represented a mean difference per capita of $5438 (95% CI $4121 - $6754; p < 0.001). Mean per capita expenditures were significantly higher comparing poor vs optimal CRF profiles, with marginal expenditures of $8552 and $6531 among those with and without ASCVD, respectively. Similarly, a more favorable CRF profile was associated with less prescription medications, fewer hospitalizations and ED and outpatient visits (Fig. 1). When comparing individuals with ASCVD and poor CRF profile versus individuals without ASCVD and optimal CRF profile, those in the latter group used 13% fewer prescription medications and required 24% fewer hospitalizations. Furthermore, an optimal CRF profile was associated with lower odds of most sources of healthcare utilization regardless of ASCVD status.
Fig. 1.
Healthcare expenditures and resource utilization, among individuals with chronic obstructive pulmonary disease, with and without atherosclerotic cardiovascular disease, by cardiovascular risk factor profile.
4. Discussion
In our nationally representative sample of adults in the US, patients with COPD and ASCVD with favorable CRF profile had significantly lower overall healthcare expenditures and resource utilization with the average healthcare cost varying significantly among COPD patients. While the lowest annual expenditure was observed among COPD individuals without ASCVD and with optimal CRF profile, the highest annual expenditure was seen for those with both ASCVD and poor CRF profile. These insights strongly support the expansion of screening and prevention counseling for modifiable CRFs to bend the curve of rising healthcare costs as well as to limit ASCVD-related morbidity and mortality among COPD patients.
Several limitations should be noted. First, we could not differentiate cost by type or stage of COPD, or whether active treatment for COPD was being delivered at the time of survey completion. Second, MEPS data only capture a fraction of total national health expenditures for COPD patients. Therefore, the overall expenditure and the absolute differences between groups were most likely underestimated. Third, because COPD, ASCVD, and modifiable CRF were self-reported, there is likely an underrepresentation of the true national prevalence. Lastly, the risk of residual confounding from unmeasured characteristics could not be controlled.
In summary, we found that absence of ASCVD and a favorable CRF profile were associated with significantly lower healthcare expenditure and resource utilization among patients with COPD. These results provide robust estimates for potential healthcare savings as preemptive strategies continue to become integrated into new healthcare delivery models, for increased awareness and prevention of ASCVD, and the continued need for improvement of CRF profiles among high-risk patients such as those presenting with COPD.
Declaration of competing interest
Dr. Nasir is supported by the Katz Academy for Translational Research.
Acknowledgments
Z.J., J.V.E., G.J.S., I.A., T.Y., S.M., R.M., S.U.K., and M.C.A. contributed to the discussion of results, statistical analysis, writing of the manuscript, finalization of the manuscript, and approval of the submitted article. K.N. conceived the research project; contributed in clinical work, integrity, and accuracy of data; and preparing and approval of the submitted article. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajpc.2020.100084.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kochanek K.D., Murphy S.L., Xu J., Arias E. Deaths: final data for 2017. Natl Vital Stat Rep. 2019;68(9):1–76. [PubMed] [Google Scholar]
- 2.Wheaton A., Cunningham T., Ford E., Croft J. Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:289–295. [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan A.D., Zakeri R., Quint J.K. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis. 2018;12 doi: 10.1177/1753465817750524. 1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelstein J., Cha E., Scharf S.M. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis. 2009;4:337–349. doi: 10.2147/copd.s6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020 doi: 10.1161/CIR.0000000000000757. CIR0000000000000757. [DOI] [PubMed] [Google Scholar]
- 6.Guarascio A.J., Ray S.M., Finch C.K., Self T.H. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford E.S., Murphy L.B., Khavjou O., Giles W.H., Holt J.B., Croft J.B. Total and state-specific medical and absenteeism costs of COPD among adults aged >/= 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi: 10.1378/chest.14-0972. [DOI] [PubMed] [Google Scholar]
- 8.Mannino D.M., Thorn D., Swensen A., Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 9.Dalal A.A., Shah M., Lunacsek O., Hanania N.A. Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir Med. 2011;105(10):1516–1522. doi: 10.1016/j.rmed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Mannino D.M., Higuchi K., Yu T.C., Zhou H., Li Y., Tian H., Suh K. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148(1):138–150. doi: 10.1378/chest.14-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz M.J., Powers M.A., Leontos C., Holzmeister L.A., Kulkarni K., Monk A., Wedel N., Gradwell E. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc. 2010;110(12):1852–1889. doi: 10.1016/j.jada.2010.09.014. [DOI] [PubMed] [Google Scholar]