Summary
Background
Understanding patient pathways can help align patient preferences and tuberculosis (TB) related services. We investigated patient pathways, and diagnostic and treatment delays among TB patients in Indonesia, which has one of the highest proportions of non-notified TB cases globally.
Methods
We conducted a study of TB patients recruited from Community Health Centers (CHCs), public and private hospitals, and private practitioners from 2017 to 2019 in Bandung City, regarding general characteristics and symptoms, and health-seeking, diagnostic and treatment pathways.
Findings
We recruited 414 TB patients: 138 (33%) in CHCs, 210 (51%) in hospitals, 66 (20%) in private practitioners. Most patients (74·6%) first sought care at an informal or private provider and experienced a complex pathway visiting both public and private providers to obtain a diagnosis. The median number of health provider visits pre-diagnosis was 6 (IQR 4–8). From start of symptoms, it took a median 30 days (IQR 14–61) to present to a health provider, 62 days (IQR 35–113) to reach a TB diagnosis, and 65 days (IQR 37–119) to start treatment. Patient delay was longer among male, lowly-educated and uninsured individuals. There were longer diagnostic delays among uninsured individuals, those who initially visited private providers, and those with multiple visits prior to diagnosis. Longer treatment delays were found in those with multiple pre-diagnosis visits or diagnosed by private practitioners.
Interpretation
Patient pathways in Indonesia are complex, involving the public and private sector, with multiple visits and long delays, especially to diagnosis. A widely available accurate diagnostic test for TB could have a dramatic effect on reducing delays, onward transmission and mortality.
Funding
This project was funded by the Partnership for Enhanced Engagement in Research (PEER) grant under Prime Agreement Number AID-OAA-A-11–00,012 by National Academy of Sciences (NAS); the United States Agency for International Development (USAID); University of Otago, New Zealand, and the Indonesian Endowment Fund for Education (LPDP).
Keywords: Patient delay, Diagnostic delay, Treatment delay, TB, Private practitioners
Evidence before this study.
Globally almost 3 million TB cases are thought to be under-diagnosed, or detected but not reported. Providing accurate, accessible and timely diagnosis and treatment, and knowing where those cases are, is crucial for TB control. Patient pathway analysis (PPA) is an approach to identify where missing cases occur and possible reasons for delay to diagnosis and treatment. Delay to diagnosis and treatment of TB can contribute to late presentation, more severe disease, ongoing transmission of M. tuberculosis, and increased out-of-pocket costs.
Indonesia has a high prevalence of TB. Previous studies have shown the role of private health care providers in TB patient care seeking patient pathways. However, evidence is lacking to respond to variances in care-seeking patterns and the capacities of public and private providers for TB control.
Added value of this study
From our study which recruited TB patients from different health care settings, we were able to identify key factors related to delay in presentation and diagnosis of TB. In particular the pathways involving informal, and formal private and public health care providers contributed to the complexity and long delays.
Implications of all the available evidence
Greater partnership between, and strengthening of, the public and private sectors for diagnosis and reporting of TB is essential for the control of TB in Indonesia. Patient-centered, easy to access TB diagnosis and treatment centres with all available tools in one place is essential and would have a dramatic effect on TB control in Indonesia.
Alt-text: Unlabelled box
1. Introduction
The large number of undiagnosed or non-notified tuberculosis (TB) cases is a global public health concern. In 2018, an estimated three million TB cases were thought to be under-diagnosed, or detected but not reported, many of whom were in low- and middle-income countries (LMICs) [1]. India, Indonesia, and Nigeria are the top three countries contributing almost 50% of the total undiagnosed or non-notified cases worldwide [1]. Finding these TB patients, and providing accurate and accessible diagnosis and treatment according to international standards in a timely way are crucial for disease control [2].
Patient pathway analysis (PPA) is an approach to identify barriers to all TB patients’ access to diagnostic and treatment services. PPA has proved to be a valuable tool informing stakeholders for allocating TB diagnostic and treatment facilities from a patient centered-care perspective [3]. Previous PPA studies have demonstrated that there are substantial differences between patients’ care-seeking behavior and TB service availability [3]. In a recent study in Taiwan, Ku et.al. used longitudinal routine national health insurance data to generate individual TB patients’ care-seeking pathways and reported considerable heterogeneity and complex pathways leading to TB diagnosis [5].
Delay to diagnosis and treatment of TB can contribute to late presentation, more severe disease, ongoing transmission of M. tuberculosis, and increased out-of-pocket costs [6,7]. Delay can be classified as patient delay or health system delay. A range of factors have been associated with both these delays [8]. In a modeling study, delay before a patient's presentation to a healthcare provider appeared to account for most of the delay in the patient pathway to care [4]. Unemployment, low income, low education level, pulmonary abnormalities, having HIV, and seeking initial care from an informal provider were associated with patient delay across studies and locations [9,10]. Consulting multiple health care providers and seeking initial care from a private provider have been consistently associated with health system delay [11,12]. Other factors that have been found, but less consistently, include patient factors such as gender and residence type, and health system factors such as consultation at a public, rather than a private, hospital [[8], [9], [10],12].
We aimed to describe patient trajectories, measure time from presentation to TB diagnosis and from diagnosis to treatment, and identify factors associated with delays, in an urban setting in Indonesia, which has a complicated public-private health care provider network and one of the highest proportions of undiagnosed or non-notified TB cases globally [1].
2. Methods
2.1. Study design and setting
This cross-sectional study was conducted over a two-year period in Bandung, the capital of West Java province, Indonesia, a city with 2·5 million inhabitants. In 2018, the TB case notification rate in Bandung was 402/100,000/ year and the treatment success rate was 79% [13]. TB diagnosis and treatment are provided according to the National Guidelines for TB Control issued by the Ministry of Health. Diagnosis mostly relies on passive case finding, whereby patients present to a health facility when they are unwell [14]. TB related services in Bandung are provided through a network of 73 Community Health Centers (CHCs), one lung clinic, one lung hospital, 16 secondary level hospitals, four prison clinics, and one tertiary level hospital. Of these services, 88% are publicly funded. In CHCs and hospitals, TB diagnosis is mostly through sputum smear examination. According to the National Tuberculosis Program (NTP) guideline, presumptive TB patients with negative sputum smear result, and suggestive chest x-ray findings of TB are diagnosed as clinical TB [14]. Thirty of the 73 CHCs (41·1%) have sputum smear microscopy facilities. No CHC and only five out of sixteen hospitals used Xpert/MTB-RIF as a molecular test for TB diagnosis within the study period. Culture and drug-sensitivity test (DST) are only performed for rifampicin-resistant TB patients and conducted at the provincial reference laboratory. TB treatment can be obtained in either the public sector (CHCs, public hospitals) or in private hospitals that are parts of Directly Observed Treatment Short-course (DOTS) network. There is also a relatively large number of approximately 2435 private practitioners (PPs) in Bandung, either working in solo practices or in clinics with other PPs. Some of these PPs are engaged in the NTP for referring presumptive TB cases, diagnosing or providing treatment. However, little is known about the exact number of PPs managing TB cases. A national TB inventory study conducted in 2017 showed that approximately 39% of detected TB cases from PPs, private clinics and laboratories were not notified to the NTP [13].
2.2. Study population
Thirty sub-districts in Bandung were randomly selected in proportion to population size. These contained 30 CHCs, seven public hospitals, and 10 private hospitals, from which we randomly selected 10 CHCs and purposively selected two public and three private hospitals according to the number of new TB cases detected, as well as willingness of hospital management to collaborate in the study. Subsequently, we conducted a survey to identify PPs in the CHC areas who had diagnosed and treated TB patients in the preceding six months (n = 282/939). Out of 282 PPs, 145 PPs were willing to participate in this study. Based on the 2015 TB report from the Bandung Municipal Health Office, a total of 2087 positive TB patients were notified by the 73 CHCs (average of 28 patients per CHC per year), and a further 3560 from DOTS linkage hospitals and lung clinics. From these numbers, we estimated that 37% of TB patients were registered in CHCs and 63% in hospitals. As information about the number and proportion of TB patients who visit PPs was limited, we assumed this to be at least 30%. We therefore recruited 30% of patients from CHCs, 40% from hospitals and 30% from PPs. Taking into account a 10% non-response rate, we aimed for a minimum sample size of 400 TB patients comprising 120, 160 (80 public and 80 private), and 120 patients from CHCs, hospitals, and PPs, respectively.
We interviewed adults with newly diagnosed pulmonary TB and receiving treatment for less than six months from each recruitment site. We consecutively enrolled consenting patients from 1 October 2017 to 31 January 2019 until the sample size was met. We excluded patients who had a previous history of TB treatment (retreatment cases), extrapulmonary TB and those who were not living in Bandung. This study was approved by the Health Research Ethics Committee at Faculty of Medicine, Universitas Padjadjaran under number 687/UN6.C.10/PN/2017. Before study enrollment, all patients provided written informed consent.
2.3. Definitions
We adopted the definition of health care providers (HCPs) developed by Lei, et al. [15]. Public HCPs belonged to national, provincial, district or municipal governments which implement the NTP and provide TB related-services (CHCs, lung clinics, lung hospitals, and public hospitals). Private HCPs were not owned by the government and provide TB or general health services for profit, both formal and informal, regulated or not by the government (solo practice, private clinics or hospitals, and informal HCPs such as traditional healers, unauthorised practitioners, and chemists or pharmacists).
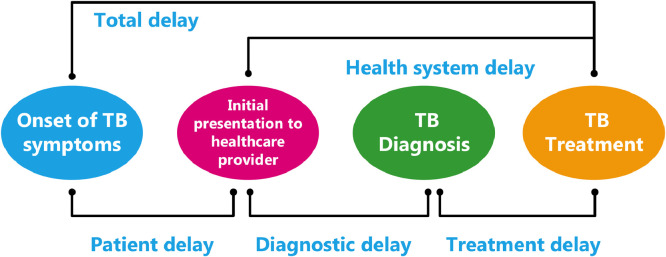
We used the conceptual framework for delays based on Sreeramareddy et al. [12,16] as depicted in Fig. 1. Patient delay was defined as the time from onset of TB symptoms to first visit to any HCP. Diagnostic delay was defined as the duration between first HCP consultation and TB diagnosis. Treatment delay was defined as time between TB diagnosis and commencement of treatment. Health system delay was calculated as time from the first HCP consultation to TB treatment initiation. Total delay was defined as the sum of patient and health system delays.
Fig. 1.
As PPA was used to describe the alignment between patient care seeking and the availability of TB diagnostic and treatment services, we defined the capacity for TB diagnosis and treatment for healthcare facilities in the health system according to Surya et al. [17], as shown in Table 1.
Table 1.
TB diagnosis and treatment capacity in Indonesia according to healthcare setting.
| Level | Availability of TB diagnostic and treatment | Example |
|---|---|---|
| L0 | Non-public services for basic triage, health information, and basic care, but without laboratory testing or TB treatment available. | Pharmacy, drug sellers, drug stores, community-based organizations (with health cadres) |
| L1a | Government-owned facilities that provide primary health care, generally on an outpatient basis. Sputum smear microscopy and TB treatment may be available. | Community Health centre (CHC) |
| L1b | Private facilities that provide primary health care, generally on an outpatient basis. Sputum smear microscopy (and other TB diagnostics) are mostly absent, but TB treatment may be available. | Private practitioners, private clinics |
| L2a | Government-owned secondary level health facilities for outpatient and inpatient care, generally with more diagnostic services (sputum smear microscopy, x-ray, and Xpert MTB/RIF) as well as TB treatment. | Public hospitals |
| L2b | Private facilities that provide secondary level health outpatient and inpatient care, generally with more diagnostic services (sputum smear microscopy, x-ray, and Xpert MTB/RIF) as well as TB treatment. | Private hospitals |
| L3 | Specialized health care facilities with large inpatient capacity, specialized doctors and more sophisticated diagnostic capabilities including sputum culture with drug-susceptibility test, and treatment services for drug-sensitive or drug-resistant TB. | Tertiary care hospitals |
2.4. Procedures
Four trained nurses interviewed patients using a structured questionnaire adapted from the “Tool to estimate patients costs” [18]. The questionnaire collected information on socio-demographic characteristics, TB symptoms, diagnostic pathways from onset of symptoms until TB diagnosis and treatment, type and sequence of providers visited, and associated costs. The questionnaire was pre-tested in a pilot study on 20 TB patients. Dates for the commencement of symptoms and health-seeking events were self-reported. Dates of TB diagnosis and treatment start were obtained from medical records. Knowledge about TB was measured through 15 questions adapted from Naidoo et.al. [19]. A summary score defined “good knowledge” as ≥80% correctly answered questions or “poor” if ˂80%. All results were cross-checked to identify errors, missing data, or inconsistencies.
2.5. Analysis
We performed descriptive analyses to summarize the data on patient trajectories and presented the number and type of visits for presumptive cases prior to a TB diagnosis, stratified by type of recruitment site. Bivariate analysis was carried out to study the association between patients’ characteristics and type of HCP at initial presentation. We defined patient delay as more than 30 days from onset of symptoms to first visit to any HCP [9]; diagnostic delay as more than seven days between first HCP consultation to TB diagnosis [9,12,20]; and treatment delay as more than two days between TB diagnosis and commencing treatment [12] to dichotomize these delays into acceptable or longer delay. For diagnostic delay, a secondary analysis using a cut-off of 30 days was also estimated taking into account time variability on diagnostic procedures taken by the patients [9,12]. We used logistic regression models to examine the association between possible risk factors to each delay type. We excluded distance to the closest CHC as a covariate in our analysis, since the majority of patients (94%) lived within 5 km from a CHC. Variables were considered in the final models if they were associated with the outcome variable (p<0·2). We assessed correlation between covariates, calculating correlation coefficients. Results are presented as adjusted odds ratios (aOR) with 95% confidence intervals (CI) and a p-value <0.05 was considered statistically significant.
2.6. Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Socio-demographic and clinical disease characterisistics
From 591 TB patients assessed, 131 (22·2%) did not meet the eligibility criteria, mostly due to them having extrapulmonary TB or living outside of Bandung city. Thirty (5·1%) refused to participate, and 16 (2·7%) were unable to be interviewed, leaving 414 available for analysis (CHC n = 138; public hospital n = 106; private hospital n = 104; PP n = 66). Of these 414 patients, 57·5% were males, the median age was 35 years, 49·5% were high-school graduates, and 60·9% were employed at the time of interview. Almost all patients lived within 5 km of their closest CHC (93·7%), and 28·5% lacked private or government health insurance (Table 2). Most patients (88·6%) presented with a cough, 51·2% were diagnosed by smear microscopy (no culture or Xpert was done) and the remaining were diagnosed by clinical symptoms and chest X-ray (Table 2). One third (29·2%) had prior contact history with a TB patient, 67·6% took antibiotics before being diagnosed with TB, and 43·5% smoked cigarettes within the past year. Based on the interview [19], half of the patients (51·2%) had good knowledge about TB. We found only weak correlations between any of the variables and type of HCP at initial presentation (Appendix 2).
Table 2.
Patient characteristics that contribute to delays according to the site of recruitment (N = 414).
| Characteristic | Community Health Center n = 138 (33·3%) |
Public hospital n = 106 (25·6%) |
Private hospital n = 104 (25·1%) |
Private Practitioner n = 66 (15·9%) |
p-value |
|---|---|---|---|---|---|
| Age, median (IQR) | 36·2 (27–49) | 31·3 (23–46) | 39·7 (24–52) | 31·8 (24–47) | 0·08 |
| Male | 80 (58·0) | 61 (57·5) | 61 (58·7) | 36 (54·5) | 0·96 |
| Education | |||||
| Primary school or less | 32 (23·2) | 20 (18·9) | 23 (22·1) | 17 (25·8) | 0·27 |
| Secondary school | 27 (19·6) | 12 (11·3) | 22 (21·2) | 9 (13·6) | |
| Senior high school | 66 (47·8) | 61 (57·5) | 50 (48·1) | 28 (42·4) | |
| University or higher education | 13 (9·4) | 13 (12·3) | 9 (8·6) | 12 (18·2) | |
| Employment status | |||||
| Employed | 87 (63·0) | 64 (60·4) | 58 (55·8) | 43 (65·2) | 0·88 |
| Unemployed | 14 (10·1) | 13 (12·3) | 15 (14·4) | 8 (12·1) | |
| Other (student/housewife/retired) | 37 (26·8) | 29 (27·4) | 31 (29·8) | 15 (22·7) | |
| Household income/month, USDa | |||||
| ≤230 | 80 (58·0) | 38 (35·8) | 48 (46·2) | 16 (24·2) | <0·001 |
| >230 | 48 (34·8) | 51 (48·1) | 44 (42·3) | 43 (65·2) | |
| Refused to answer/ don't know | 10 (7·2) | 17 (16·1) | 12 (11·5) | 7 (10·6) | |
| Marital status | |||||
| Married | 87 (63·0) | 57 (53·8) | 66 (63·5) | 35 (53·0) | 0·27 |
| Single/divorced/widowed | 51 (37·0) | 49 (46·2) | 38 (36·5) | 31 (47·0) | |
| Insurance | 74 (53·6) | 84 (79·2) | 94 (90·4) | 44 (66·7) | <0·001 |
| Distance to closest CHC | |||||
| ≤ 5 km | 132 (95·7) | 92 (86·8) | 101 (97·1) | 63 (95·5) | 0·01 |
| > 5 km | 6 (4·3) | 14 (13·2) | 3 (2·9) | 3 (4·5) | |
| Level of TB knowledge | |||||
| Poor | 56 (40·6) | 41 (38·7) | 62 (59·6) | 43 (65·2) | <0·001 |
| Good | 82 (59·4) | 65 (61·3) | 42 (40·4) | 23 (34·8) | |
| History of contact with a TB patient | 56 (40·6) | 29 (27·4) | 20 (19·2) | 16 (13·2) | 0·002 |
| Smoking within the past year | 69 (50·0) | 38 (35·8) | 49 (47·1) | 24 (36·4) | 0·08 |
| Comorbiditiesb | |||||
| HIV | 1 (0·7) | 2 (1·9) | 1 (1·0) | 0 (0·0) | 0·64 |
| Diabetes | 10 (7·2) | 7 (6·6) | 11 (10·6) | 6 (9·1) | 0·71 |
| Cardiovascular disease* | 6 (4·3) | 14 (13·2) | 21 (20·2) | 3 (4·5) | 0·02 |
| TB symptomsc | |||||
| Cough | 131 (94·9) | 97 (91·5) | 80 (76·9) | 59 (89·4) | <0·001 |
| Hemoptysis | 39 (28·3) | 22 (20·8) | 18 (17·3) | 14 (21·2) | 0·21 |
| Fever | 119 (86·2) | 79 (74·5) | 68 (65·4) | 39 (59·1) | <0·001 |
| Weight loss | 118 (85·5) | 83 (78·3) | 61 (58·7) | 46 (69·7) | <0·001 |
| Night sweats | 93 (67·4) | 74 (69·8) | 58 (55·8) | 41 (62·1) | 0·14 |
| No symptoms | 2 (1·4) | 2 (1·9) | 6 (5·8) | 3 (4·5) | 0·20 |
| TB diagnosis | |||||
| Smear positive | 103 (74·6) | 57 (53·8) | 30 (28·8) | 55 (45·5) | <0·001 |
| Smear negative, x-ray positive | 35 (25·4) | 42 (39·6) | 42 (40·4) | 38 (31·4) | |
| X-ray positive, no smear examination | 0 | 5 (4·7) | 29 (27·9) | 27 (22·3) | |
| Other⁎⁎ | 0 | 2 (1·9) | 3 (2·9) | 1 (0·8) | |
| Duration of cough prior to diagnosis, days, median (IQR) | 68 (36–125) | 35 (16–66) | 63 (34–122) | 53 (32–95) | <0·001 |
| Duration of cough prior to presentation to formal HCP, days, median (IQR) | 31 (30–92) | 30 (7–55) | 15 (7–31) | 30 (14–31) | <0·001 |
| Received antibiotics prior to TB diagnosis | 99 (71·7) | 73 (68·9) | 65 (62·5) | 43 (65·2) | 0·71 |
USD=United States dollar (1 USD = 13,755 Indonesian Rupiah).
Comorbidities were self-reported by the patients.
More than one TB symptom may be indicated.
Cardiovacular disease includes hypertension, heart disease, dyslipidemia.
3 (0·7%) patients were diagnosed using PCR-TB (Xpert) and 3 (0·7%) by IGRA test. CHC Community Health Center, HCP=Healthcare provider, IQR=Interquartile range, TB=tuberculosis.
3.2. Patient pathways from seeking care to diagnosis and treatment
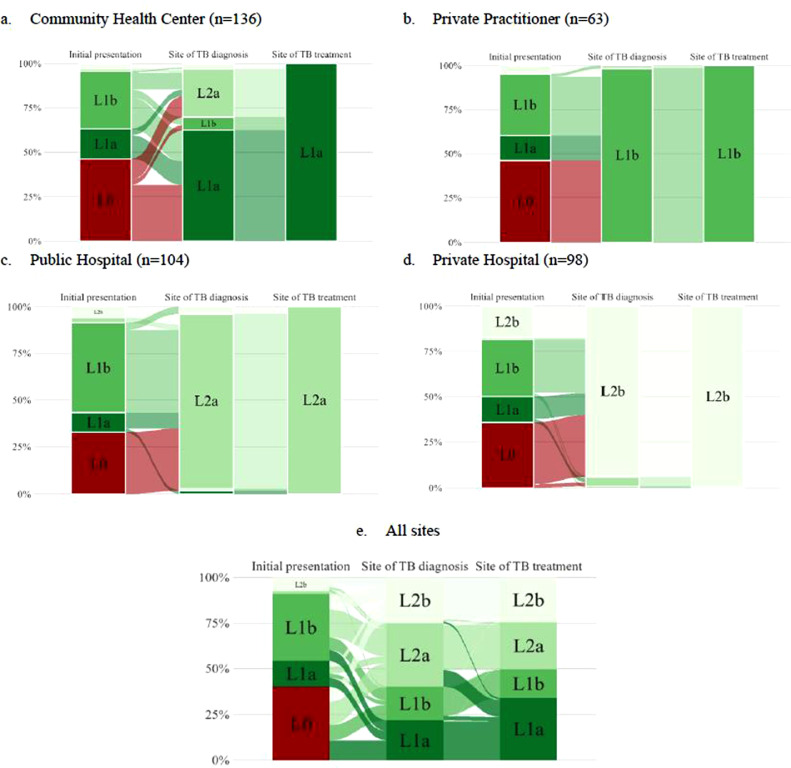
Overall, three quarters (76·8%) of patients initially visited an informal or private provider and a minority first visited a CHC (Fig. 2). For those recruited from CHCs, most had first visited informal (46·3%) or private providers (32·4%), but diagnosis was usually (62·5%) made at CHCs (Fig. 2a). For those recruited from PPs, the site of initial presentation was similar, but almost all (98·4%) were diagnosed by PPs (Fig. 2b). For those recruited at public and private hospitals, initial presentation showed a similar pattern, but almost all diagnoses (93–94%) were made at a hospital (Figs. 2c and 2d). With respect to treatment, those who were treated at public HCPs had been diagnosed at a variety of different types of HCPs, whereas for those who were treated in private care almost all had been diagnosed by private HCPs. Overall, only one in five patients received their TB diagnosis, and less than a quarter started their TB treatment, at healthcare facilities where they first presented (Fig. 2e).
Fig. 2.
Pathways undertaken by tuberculosis patients for diagnosis and treatment according to site of recruitment (N = 401). TB=tuberculosis. 13 people did not present with symptoms and were therefore excluded from this pathway analysis. L0 = informal providers (pharmacy, drug sellers, drug store, etc.); L1a = Community Health Center; L1b = private practitioner or private clinic; L2a = public hospital; L2b = private hospital.
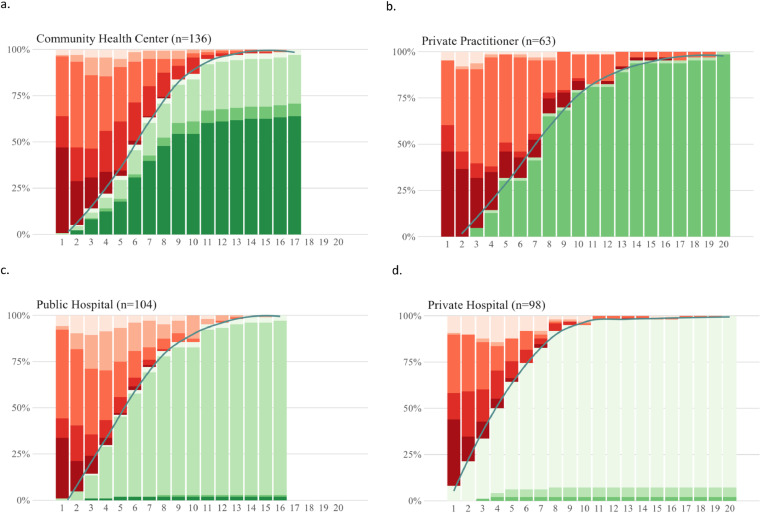
Specifically, with respect to obtaining a diagnosis, patients generally underwent a complex journey visiting both private and public providers. Fig. 3 displays the number and nature of the visits of TB patients before a diagnosis was made, stratified by recruitment site. The median number of visits until a TB diagnosis was made was six (Interquartile Range (IQR) 4–8), and for some patients that it took up to 20 visits before a diagnosis was made. Furthermore, only about 50% of cases were bacteriologically confirmed. A first visit to an informal provider before going to a formal (private or public) HCP was reported by 46·3%, 32·7%, 35·7% and 46·0% of participants recruited from CHC, public or private hospitals, and PPs, respectively. Patients recruited at the primary care level (Figs. 3a and 3b) had slightly more visits compared to those recruited at the hospital level (Figs. 3c and 3d).
Fig. 3.
Proportion of patients diagnosed, or with a missed diagnosis, over sequential visits, stratified by site of recruitment (N = 401). 13 patients were excluded because they didn't have TB symptoms. Horizontal axis indicates sequence of visit and vertical axis shows the proportion of diagnosed or missed opportunity to be diagnosed as TB at that particular visit based on type of HCP. The black line indicates the cumulative proportion of patients diagnosed with TB. HCP = healthcare provider, TB=tuberculosis.
 = visited informal provider (L0),
= visited informal provider (L0),  = missed opportunity to be diagnosed as TB at CHC (L1a),
= missed opportunity to be diagnosed as TB at CHC (L1a),  = missed opportunity to be diagnosed as TB at private practitioner (L1b),
= missed opportunity to be diagnosed as TB at private practitioner (L1b),  = missed opportunity to be diagnosed as TB at public hospital (L2a),
= missed opportunity to be diagnosed as TB at public hospital (L2a),  = missed opportunity to be diagnosed as TB at private hospital (L2b),
= missed opportunity to be diagnosed as TB at private hospital (L2b),  = diagnosed at CHC (L1a),
= diagnosed at CHC (L1a),  = diagnosed at private practitioner (L1b),
= diagnosed at private practitioner (L1b),  = diagnosed at public hospital (L2a),
= diagnosed at public hospital (L2a),  = diagnosed at private hospital (L2b).
= diagnosed at private hospital (L2b).
3.3. Delays to care seeking, diagnosis and initiation of treatment, and associated risk factors
There were substantial patient and diagnostic delays, while time to treatment initiation after diagnosis was generally short. Of patients recruited from CHC, public hospital, private hospital, and PP, 63·2%; 41·3%; 32·7%; and 47·6%, respectively, experienced a delay of more than 30 days before visiting any HCP for their TB symptoms (Table 3). Patient delay was associated with being male (aOR 1·6, 95% CI 1·0–2·7), having a lower level of education (aOR 1·9, 95% CI 1·1–3·1), and not having health insurance (aOR 2·1, 95% CI 1·2–3·5; Table 4). The median time from the first presentation to any HCP to their TB diagnosis was 23 days (IQR 9–52); 75·6% (313/414) experienced delay of more than 7 days. Diagnostic delay was associated with not having insurance (aOR = 1·9, 95%CI 1·0–3·6), multiple visits to HCPs (aOR=12·4, 95%CI 6·3–24·6), and an initial visit to a PP (aOR=2·6, 95%CI 1·3–5·2). When using a 30-day cut-off, being male (aOR 1·7, 95% CI 1·0–2·8), multiple visits to HCPs (aOR 9·4, 95% CI 5·5–16·1) and first visiting a PP (aOR 3·2, 95% CI 1·8–5·6) were factors associated with diagnostic delay. After receiving a TB diagnosis, 278 (69·3%) patients were started on TB treatment within one day, while almost a quarter (21·9%) experienced treatment delay for more than two days. Having multiple visits before TB diagnosis (aOR 2·2, 95% CI 1·2–3·9) and being diagnosed with TB by a PP (aOR 5·7, 95% CI 1·6–21·1) were associated with longer treatment delay. The median total time from first onset of TB symptoms to treatment initiation was 65 days (IQR 37–119).
Table 3b.
Median delay in days to care-seeking, diagnosis and treatment according to first health care provider encountered (N = 414).
| Delay type | Type of HCP first visited prior to presentation at site of recruitment |
|||||
|---|---|---|---|---|---|---|
| Informal providers/ pharmacy/drug store n = 161 | Community Health Center n = 60 | Public hospital n = 9 | Private hospital n = 36 | Private Practitioner n = 148 | p-value | |
| Patient delay (n = 401)* | 31 (15–61) | 31 (14–31) | 47 (30–110) | 30 (7–61) | 30 (14–61) | 0·08 |
| Diagnostic delay | 23 (10–45) | 18 (7–47) | 10 (1–22) | 7 (0–22) | 31 (14–65) | <0·001 |
| Treatment delay | 1 (0–2) | 1 (0–2) | 1 (0–4) | 1 (0–3) | 1 (0–2) | 0·15 |
| Health system delay | 28 (12–47) | 19 (10–53) | 11 (2–27) | 10 (2–29) | 34 (14–68) | <0·001 |
| Total delay (n = 401)* | 63 (41–107) | 57 (34–132) | 72 (46–111) | 37 (18–104) | 71 (37–123) | 0·31 |
13 people did not present with symptoms and were therefore excluded from this delay calculation. Time delay is presented as a median and IQR (interquartile range). HCP=Healthcare provider.
Table 3a.
Median delay in days to care-seeking, diagnosis and treatment according to site of recruitment (N = 414).
| Delay type | Site of recruitment |
|||||
|---|---|---|---|---|---|---|
| Community Health Center n = 138 | Public hospital n = 106 | Private hospital n = 104 | Private Practitioner n = 66 | p-value | ||
| Patient delay (n = 401)* | 31 (30–68) | 30 (10–55) | 15 (7–31) | 30 (14–31) | <0·001 | |
| Diagnostic delay | 28 (13–55) | 30 (12–68) | 19 (5–38) | 15 (3–46) | 0·05 | |
| Treatment delay | 1 (0–3) | 0 (0–1) | 1 (0–3) | 0 (0–2) | 0·001 | |
| Health system delay | 32 (15–60) | 30 (14–68) | 22 (9–39) | 16 (7–46) | 0·02 | |
| Total delay (n = 401)* | 80 (43–137) | 69 (38–127) | 45 (29–76) | 56 (34–102) | 0·001 | |
Table 4.
Factors associated with patient, diagnostic and treatment delay (N = 414).
| Patient delay⁎⁎ (>30 days) |
Diagnostic delay (>7 days) |
Treatment delay (>2 days) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Crude OR (CI) | p value | Adjusted OR* (CI) | p value | Crude OR (CI) | p value | Adjusted OR* (CI) | p value | Crude OR (CI) | p value | Adjusted OR* (CI) | p value |
| Age, median (IQR) | 1·0 (0·9–1·0) | 0·5 | 1·0 (0·9–1·0) | 0·6 | 1·0 (0·9–1·0) | 0·5 | 0·9 (0·9–1·0) | 0·3 | 1·0 (0·9–1·0) | 0·1 | 1·0 (0·9–1·0) | 0·9 |
| Sex | ||||||||||||
| Male | 1·2 (0·8–1·8) | 0·4 | 1·6 (1·0–2·7) | 0·05 | 1·3 (0·8–2·1) | 0·3 | 0·7 (0·5–1·8) | 0·9 | 0·7 (0·5–1·2) | 0·2 | 0·9 (0·5–1·5) | 0·7 |
| Female | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Education | ||||||||||||
| Up to secondary school | 1·8 (1·2–2·6) | 0·01 | 1·9 (1·1–3·1) | 0·02 | 0·8 (0·5–1·3) | 0·4 | 0·6 (0·4–1·1) | 0·1 | 1·6 (1·0–2·6) | 0·04 | 1·6 (0·9–2·9) | 0·1 |
| High school or greater | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Employment | ||||||||||||
| Unemployed | 1·1 (0·8–1·7) | 0·6 | 1·4 (0·8–2·3) | 0·3 | 0·7 (0·5–1·2) | 0·2 | 0·7 (0·4–1·2) | 0·2 | 0·9 (0·6–1·5) | 0·8 | 0·9 (0·5–1·5) | 0·6 |
| Employed | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Average household income/month, USD | ||||||||||||
| 0–230 | 1·3 (0·83–1·90) | 0·3 | 0·9 (0·6–1·4) | 0·6 | 0·9 (0·4–2·1) | 0·9 | 1·2 (0·4–3·1) | 0·8 | 1·4 (0·9–2·3) | 0·2 | 1·2 (0·7–2·0) | 0·6 |
| ≥231 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Insurance | ||||||||||||
| Yes | Reference | Reference | Reference | Reference | ||||||||
| No | 2·4 (1·5–3·7) | <0·01 | 2·1 (1·2–3·5) | 0·01 | 1·2 (0·8–1·9) | 0·4 | 1·9 (1·0–3·6) | 0·04 | 0·9 (0·5–1·5) | 0·6 | 0·8 (0·4–1·4) | 0·4 |
| Comorbidities (HIV/DM/Hypertension) | ||||||||||||
| Yes | 0·9 (0·5–1·5) | 0·6 | 1·5 (0·8–3·2) | 0·3 | 1·3 (0·7–2·4) | 0·5 | 1·4 (0·7–3·0) | 0·4 | 1·3 (0·7–2·5) | 0·4 | 1·0 (0·5–2·3) | 0·9 |
| No | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Level of TB knowledge | ||||||||||||
| Poor | 1·0 (0·7–1·5) | 0·9 | 0·9 (0·5–1·7) | 0·7 | 0·9 (0·6–1·5) | 0·9 | 1·4 (0·8–2·5) | 0·2 | 1·1 (0·7–1·7) | 0·7 | 1·0 (0·6–1·7) | 0·9 |
| Good | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Number of visits to HCP before diagnosis | ||||||||||||
| <6 visits | Reference | Reference | Reference | Reference | ||||||||
| ≥6 visits | 10·9 (6·0–19·8) | <0·001 | 12·4 (6·3–24·6) | <0·001 | 1·9 (1·2–2·9) | 0·01 | 2·2 (1·2–3·9) | 0·01 | ||||
| Type of HCP visited at initial presentation prior to TB diagnosis | ||||||||||||
| Informal provider | Reference | Reference | Reference | Reference | ||||||||
| Private practitioner | 1·2 (0·7–2·1) | 0·6 | 2·6 (1·3–5·2) | 0·01 | 0·9 (0·5–1·5) | 0·7 | 0·7 (0·4–1·4) | 0·3 | ||||
| CHC | 0·6 (0·3–1·2) | 0·2 | 1·2 (0·6–2·7) | 0·6 | 0·9 (0·5–1·9) | 0·9 | 0·6 (0·3–1·4) | 0·2 | ||||
| Public or private hospital | 0·2 (0·1–0·4) | <0·001 | 0·6 (0·3–1·4) | 0·2 | 1·9 (0·9–3·9) | 0·07 | 1·9 (0·8–4·6) | 0·2 | ||||
| Site of TB diagnosis | ||||||||||||
| CHC | Reference | Reference | ||||||||||
| Private practitioner | 1·0 (0·5–2·1) | 0·9 | 5·7 (1·6–21·1) | 0·01 | ||||||||
| Public hospital | 0·7 (0·4–1·4) | 0·3 | 1·3 (0·5–3·2) | 0·6 | ||||||||
| Private hospital | 1·3 (0·7–2·4) | 0·7 | 0·8 (0·2–4·1) | 0·8 | ||||||||
Adjusted for site of recruitment.
13 people did not present with symptoms and were therefore excluded from this delay calculation. CHC Community Health Center, HCP=Healthcare provider, OR=Odds ratio, CI=Confidence interval, TB=tuberculosis, USD=United States dollar.
4. Discussion
In this study, patient pathways to TB care were complex and characterized by multiple visits to HCPs. The majority of TB patients first sought care from informal providers and PPs, who mostly lack TB diagnostic capacity. Only a small proportion of TB patients were diagnosed where they first presented and many encountered significant diagnostic and treatment delays, with an average total delay of two months. Patient delays were longer for those who had no health insurance or a lower education level, and diagnostic and treatment delays were longer for those who first went to PPs. The complexities of patient health seeking and the mix of private and public providers in this setting present signifant challenges for the provision of efficient TB diagnosis and treatment.
In Indonesia, approximately 74% of all HCPs at the primary care level are working outside the public system [13]. Informal HCPs and the private sector are therefore key entry points for patients seeking health care, even though TB diagnosis and treatment services are mainly available (and free-of-charge) in the public health system. Early on, patients may have non-specific symptoms, and prefer to visit informal and private providers [21], often for reasons of convenience [22] Our findings are consistent with previous studies in other high TB-burden settings such as in India [23,24], China [25], Uganda [26], and Tanzania [27], which were also found to have complex pathways generating diagnostic delays.
That few patients were successfully diagnosed at their initial visit, indicates a big gap between patient preference and diagnostic capacity of the HCP where they first present. Less than a quarter of HCPs where presumptive TB cases first present have sputum microscopy available and none use molecular testing or culture. Even for those patients that access a public provider early, repeated visits are common before referral for sputum smear examination occurs. Our analyses show that most patients with typical TB symptoms first visited primary level HCPs, but TB diagnosis was mostly established at secondary level healthcare indicating lack of confidence or familiarity in diagnosing TB among clinicians at the primary level [5]. A systematic review from India [28] showed that only half of HCPs knew that people with unexplained cough should be evaluated for TB. Moreover, prescribing antibiotics before ordering a sputum test is common among PPs and associated with diagnostic delay. Antibiotic use was more common among TB patients who first presented to informal providers and PPs. This practice of using medication as a diagnostic tool is worrisome [29]. Beside low awareness of unexplained cough as a key symptom of pulmonary TB among PPs, insensitive diagnostic tools such as smear microscopy also lead to delayed diagnosis [30].
Almost half of the patients in this study had at least a one-month delay in seeking care for their symptoms and only 50% had good knowledge about TB. Consistent with other studies we also found an association of lower education level with longer delay [10], indicating a need for interventions to increase awareness and knowledge about TB in the general population. Our findings of longer delay among men is supported by some [31,32], but not all studies [11,12]. The association between health insurance ownership and earlier presentation and treatment is consistent with the findings of a recent study in Vietnam; having health insurance influences patients’ behavior towards seeking care from a formal HCP [33]. Patients diagnosed by PPs reported longer delays. In Indonesia, most anti-TB treatment resources are within the public sector, while only 36% of the formal private sector has TB treatment available [22].
Our study is the first of its type in Indonesia, combining patient pathway and time to TB diagnosis and treatment from both the public and private sectors at different levels of healthcare facilities. Prior studies have been conducted only on patients treated under the public system [17,34]. Our study has several limitations. First, we relied on patients’ recall about their pathways. However, patients were asked to relate their experiences to specific events or dates to prompt their recall and where possible dates were checked against available medical records. Second, the recruitment numbers were not in proportion to the actual numbers of patients treated in the different recruitment facilities, although we made some attempt to estimate relative proportions. To take this into account, site of recruitment was included as an adjusted variable in the logistic regression analysis. Third, this study was conducted in Bandung City, an urban setting in West Java, and therefore may not be representative of TB patients throughout Indonesia.
A number of recommendations can be made from our study. First, partnerships between the public and private sectors should be strengthened. Interventions aiming to better engage the private sector with the TB program are worth exploring, including realigning patients’ preferences with allocation of diagnostic resources. Improving TB care and control requires a patient-centered approach and removal of crucial barriers for accessing care [35]. Second, universal health insurance coverage would likely improve timely TB diagnosis and treatment. Health insurance can facilitate more direct patient journeys to a TB diagnosis and improve treatment outcomes. Targeted interventions for social minorities to encourage demand for TB services for example by providing social protection for TB patients could reduce patient delay [4]. Third, patient access to TB diagnostic tools needs to be optimised. For example, increasing provision of Xpert MTB/RIF as a point-of-care test at healthcare facilities, strengthening the referral system for PPs to prioritize presumptive cases, and establishing systems to transport specimens from both the public and private sector to a central laboratory [26], may help streamline pathways to care. So far, roll out of Xpert MTB/RIF in Indonesia has been limited. Chest X-ray might also be mandated as a screening tool to prioritize patients for further microbiological testing [36]. Only about 50% of patients were bacteriologically confirmed, and by all means, a simple and sensitive point-of-care test, preferably using blood or urine rather than sputum, could dramatically transform patient pathways in Indonesia and similar TB-endemic settings.
Financial support
This project was funded by the Partnership for Enhanced Engagement in Research (PEER) grant under Prime Agreement Number AID-OAA-A-11-00012 by National Academy of Sciences (NAS); the United States Agency for International Development (USAID); University of Otago, New Zealand, and the Indonesian Endowment Fund for Education (LPDP). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Authors contribution
BWL, SM, PFH, MM, RvC, PH, BA designed the study. BWL, PFH, NA, and IDJ carried out the data collection. BWL wrote the first draft. BWL and SM analyzed the data. BWL, SM, MM, PH, RvC, and BA critically read and modified the draft. All authors read and approved the final version.
Data sharing statement
After publication, the data will be made available to others upon written requests to the corresponding author. Deidentified participant data will be provided subject to a written proposal with detailed description of study objectives and data analysis plan and a signed data sharing agreement.
Declaration of Competing Interest
No conflicts of interest exist.
Acknowledgment
We would like to thank the staff in the Bandung City Health Office, public and private hospitals, community health centers (Puskesmas), and private practitioners for their support during data collection of this study. The authors also thank the entire staff at INSTEP study for their assistance in conducting this study, and the patients who agreed to participate for the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at 10.1016/j.lanwpc.2020.100059.
Appendix. Supplementary materials
References
- 1.Global Tuberculosis Report 2019. Geneva: world Health Organization; 2019. https://www.who.int/tb/publications/global_report/en/.
- 2.Reid M.J.A., Arinaminpathy N., Bloom A., Bloom B.R., Boehme C., Chaisson R. Building a tuberculosis-free world: the Lancet commission on tuberculosis. Lancet North Am editor. 2019;393(10178):1331–1384. doi: 10.1016/S0140-6736(19)30024-8. [DOI] [PubMed] [Google Scholar]
- 3.Hanson C., Osberg M., Brown J., Durham G., Chin D.P. Finding the missing patients with tuberculosis: lessons learned from patient-pathway analyses in 5 countries. J Infect Dis. 2017;216(suppl_7):S686–SS95. doi: 10.1093/infdis/jix388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vesga J.F., Hallett T.B., Reid M.J.A., Sachdeva K.S., Rao R., Khaparde S. Assessing tuberculosis control priorities in high-burden settings: a modelling approach. Lancet Glob Health. 2019;7(5):e585–ee95. doi: 10.1016/S2214-109X(19)30037-3. [DOI] [PubMed] [Google Scholar]
- 5.Ku C.-.C., Chen C.-.C., Dixon S., Lin H.H., Dodd P.J. Patient pathways of tuberculosis care-seeking and treatment: an individual-level analysis of national health insurance data in Taiwan. BMJ Glob Health. 2020;5(6) doi: 10.1136/bmjgh-2019-002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuady A., Houweling T.A.J., Mansyur M., Burhan E., Richardus J.H. Cost of seeking care for tuberculosis since the implementation of universal health coverage in Indonesia. BMC Health Serv Res. 2020;20(1):502. doi: 10.1186/s12913-020-05350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAllister S.M., Wiem Lestari B., Sullivan T., Fortuna Hadisoemarto P., Afifah N., Arosdiani Apip R. Out-of-pocket costs for patients diagnosed with tuberculosis in different healthcare settings in Bandung, Indonesia. Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.19-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai J., Wang X., Ma A., Wang Q., Han X., Li Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storla D.G., Yimer S., Bjune G.A. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8(1):15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getnet F., Demissie M., Assefa N., Mengistie B., Worku A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: systematic review and meta-analysis. BMC Pulm Med. 2017;17(1):202. doi: 10.1186/s12890-017-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Ehiri J., Tang S., Li D., Bian Y., Lin H. Factors associated with patient, and diagnostic delays in Chinese TB patients: a systematic review and meta-analysis. BMC Med. 2013;11(1):156. doi: 10.1186/1741-7015-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreeramareddy C.T., Qin Z.Z., Satyanarayana S., Subbaraman R., Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis. 2014;18(3):255–266. doi: 10.5588/ijtld.13.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health Indonesia. Tuberculosis Inventory Study in Indonesia 2016-2017. Jakarta.2018. https://www.who.int/tb/advisory_bodies/impact_measurement_taskforce/meetings/tf7_p04_Indonesia_inventory_study_results.pdf?ua=1. Accessed November 12, 2019.
- 14.Ministry of Health Republic of Indonesia. National guideline for TB control. Jakarta,; 2016. http://hukor.kemkes.go.id/uploads/produk_hukum/PMK_No._67_ttg_Penanggulangan_Tuberkolosis_.pdf. Accessed April 26, 2017.
- 15.Lei X., Liu Q., Escobar E., Philogene J., Zhu H., Wang Y. Public–private mix for tuberculosis care and control: a systematic review. Int J Infect Dis. 2015;34:20–32. doi: 10.1016/j.ijid.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 16.WHO; Geneva: 2006. Diagnostic and treatment delay in tuberculosis: an in-depth analysis of the health-seeking behaviour of patients and health system response in seven countries of the Eastern Mediterranean region. [Google Scholar]
- 17.Surya A., Setyaningsih B., Suryani Nasution H., Gita Parwati C., Yuzwar Y.E., Osberg M. Quality tuberculosis care in indonesia: using patient pathway analysis to optimize public-private collaboration. J Infect Dis. 2017;216(suppl_7):S724–SS32. doi: 10.1093/infdis/jix379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The TB coalition for technical assistance. The tool to estimate patients' costs. USAID; 2015. http://www.stoptb.org/wg/dots_expansion/tbandpoverty/assets/documents/Tool%20to%20estimate%20Patients'%20Costs.pdf. Accessed August 15, 2017.
- 19.Naidoo P., Simbayi L., Labadarios D., Ntsepe Y., Bikitsha N., Khan G. Predictors of knowledge about tuberculosis: results from SANHANES I, a national, cross-sectional household survey in South Africa. BMC Public Health. 2016;16(1):276. doi: 10.1186/s12889-016-2951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad R.A., Mahendradhata Y., Cunningham J., Utarini A., de Vlas S.J. How to optimize tuberculosis case finding: explorations for Indonesia with a health system model. BMC Infect Dis. 2009;9(1):87. doi: 10.1186/1471-2334-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X.L., Liang X.Y., Walley J.D., Liu F.Y., Dong B.Q. Analysis of care-seeking pathways of tuberculosis patients in Guangxi, China, with and without decentralised tuberculosis services. Int J Tuberc Lung Dis. 2009;13(4):514–520. [PubMed] [Google Scholar]
- 22.Ministry of Health, Republic of Indonesia . National Institute of Health Research and Development; Jakarta: 2015. Indonesia tuberculosis prevalence survey 2013-2014. [Google Scholar]
- 23.Kapoor S.K., Raman A.V., Sachdeva K.S., Satyanarayana S. How did the TB patients reach DOTS services in Delhi? A study of patient treatment seeking behavior. PLoS ONE. 2012;7(8):e42458. doi: 10.1371/journal.pone.0042458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bronner Murrison L., Ananthakrishnan R., Swaminathan A., Auguesteen S., Krishnan N., Pai M. How do patients access the private sector in Chennai, India? An evaluation of delays in tuberculosis diagnosis. Int J Tuberc Lung Dis. 2016;20(4):544–551. doi: 10.5588/ijtld.15.0423. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Ehiri J., Oren E., Hu D., Luo X., Liu Y. Are we doing enough to stem the tide of acquired MDR-TB in countries with high TB burden? Results of a mixed method study in Chongqing, China. PLOS ONE. 2014;9(2):e88330. doi: 10.1371/journal.pone.0088330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shete P.B., Haguma P., Miller C.R., Ochom E., Ayakaka I., Davis J.L. Pathways and costs of care for patients with tuberculosis symptoms in rural Uganda. Int J Tuberc Lung Dis. 2015;19(8):912–917. doi: 10.5588/ijtld.14.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mhalu G., Hella J., Mhimbira F., Said K., Mosabi T., Mlacha Y.P. Pathways and associated costs of care in patients with confirmed and presumptive tuberculosis in Tanzania: a cross-sectional study. BMJ Open. 2019;9(4) doi: 10.1136/bmjopen-2018-025079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satyanarayana S., Subbaraman R., Shete P., Gore G., Das J., Cattamanchi A. Quality of tuberculosis care in India: a systematic review. Int J Tuberc Lung Dis. 2015;19(7):751–763. doi: 10.5588/ijtld.15.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDowell A., Pai M. Treatment as diagnosis and diagnosis as treatment: empirical management of presumptive tuberculosis in India. Int J Tuberc Lung Dis. 2016;20(4):536–543. doi: 10.5588/ijtld.15.0562. [DOI] [PubMed] [Google Scholar]
- 30.Drain P.K., Bajema K.L., Dowdy D., Dheda K., Naidoo K., Schumacher S.G. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. 2018;31(4):e00021. doi: 10.1128/CMR.00021-18. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad R.A., Richardus J.H., de Vlas S.J. Care-seeking behaviour among individuals with TB symptoms in Jogjakarta Province, Indonesia: a community-based study. Int Health. 2012;5(1):51–57. doi: 10.1093/inthealth/ihs002. [DOI] [PubMed] [Google Scholar]
- 32.Ebrahimi Kalan M., Yekrang Sis H., Kelkar V., Harrison S.H., Goins G.D., Asghari Jafarabadi M. The identification of risk factors associated with patient and healthcare system delays in the treatment of tuberculosis in Tabriz, Iran. BMC Public Health. 2018;18(1):174. doi: 10.1186/s12889-018-5066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giang N.H., Oanh T.T.M., Anh Tuan K., Hong Van P., Jayasuriya R. Is health insurance associated with health service utilization and economic burden of non-communicable diseases on households in Vietnam? Health Systems Reform. 2019:1–15. doi: 10.1080/23288604.2019.1619065. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad R.A., Mahendradhata Y., Utarini A., de Vlas S.J. Diagnostic delay amongst tuberculosis patients in Jogjakarta Province, Indonesia is related to the quality of services in DOTS facilities. Trop Med Int Health. 2011;16(4):412–423. doi: 10.1111/j.1365-3156.2010.02713.x. [DOI] [PubMed] [Google Scholar]
- 35.Odone A., Roberts B., Dara M., van den Boom M., Kluge H., McKee M. People- and patient-centred care for tuberculosis: models of care for tuberculosis. Int J Tuberc Lung Dis. 2018;22(2):133–138. doi: 10.5588/ijtld.17.0608. [DOI] [PubMed] [Google Scholar]
- 36.Furin J., Cox H., Pai M. Tuberculosis. Lancet North Am editor. 2019;393(10181):1642–1656. doi: 10.1016/S0140-6736(19)30308-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.