Abstract
Objective
On-treatment levels of high sensitivity C-reactive protein (hsCRP) in statin-treated patients predict plaque progression and the prospective risk of atherosclerotic cardiovascular events. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors produce additional LDL-C lowering, reduce plaque burden and improve cardiovascular outcomes in statin-treated patients. It is unknown whether residual systemic inflammation attenuates their favorable effects on plaque burden.
Methods
GLAGOV compared the effects of treatment for 78 weeks with evolocumab or placebo on progression of coronary atherosclerosis in statin-treated patients with coronary artery disease.
Clinical demographics, biochemistry and changes in both the burden (percentage atheroma volume (PAV), total atheroma volume (TAV), n = 413) and composition (n = 162) of coronary plaque were evaluated in evolocumab-treated patients according to baseline hsCRP strata (<1, 1–3, >3 mg/L).
Results
The study cohort comprised 413 evolocumab-treated patients (32% low [<1 mg/L], 41% intermediate [1–3 mg/L] and 27% high [>3 mg/L] baseline hsCRP levels). Patients in the highest hsCRP stratum were more likely to be female and had a higher prevalence of diabetes, hypertension, and the metabolic syndrome. LDL-C levels were similar across the groups, however participants with higher hsCRP levels had higher triglyceride and lower HDL-C levels at baseline. At follow-up, the change in PAV from baseline (−0.87% [low] vs. −0.84% [intermediate] vs. −1.22% [high], p = 0.46) and the proportion of patients experiencing any degree of regression (65.9% vs. 63.5% vs. 63.1%, p = 0.88) was similar across hsCRP strata and when evaluated by levels of achieved LDL-C. There were no serial differences in plaque composition by hsCRP strata.
Conclusion
The ability of evolocumab to induce regression in statin-treated patients is not attenuated by the presence of enhanced systemic inflammation. This underscores the potential benefits of intensive lipid lowering, even in the presence of heightened inflammatory states.
Keywords: PCSK9 inhibitor, Atherosclerosis, C-reactive protein, Intravascular imaging, IVUS
Abbreviations
- ACE
Angiotensin converting enzyme
- ASCVD
Atherosclerotic cardiovascular disease
- CABG
Coronary artery bypass grafting
- CeVD
Cerebrovascular disease
- DBP
Diastolic blood pressure
- EEM
External elastic membrane
- HDL-C
High-density lipoprotein cholesterol
- hsCRP
High sensitivity C-reactive protein
- IVUS
Intravascular ultrasound
- LDL-C
Low-density lipoprotein cholesterol
- LSM
Least-squares mean
- MI
Myocardial infarction
- PAD
Peripheral arterial disease
- PAV
Percentage atheroma volume
- PCI
Percutaneous coronary intervention
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- SBP
Systolic blood pressure
- TAV
Total atheroma volume
- VH
Virtual histology
1. Introduction
A large body of translational data has established the causal role of low-density lipoprotein cholesterol (LDL-C) in the development of atherosclerotic cardiovascular disease [1,2]. Statin therapy has been the cornerstone of preventive cardiology through the ability of these drugs to lower LDL-C, favourably effect coronary atheroma burden, and reduce cardiovascular events [3]. More recently, the introduction of proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors has further supported the relationship between LDL-C lowering and reduction in cardiovascular events in statin-treated patients [[4], [5], [6]]. In parallel, multiple lines of evidence implicate inflammation in the genesis, progression and instability of atherosclerotic plaque [7]. Elevated levels of high sensitivity C-reactive protein (hsCRP), a circulating marker of inflammation, have consistently identified patients at increased risk for future cardiovascular events. However it remains of interest whether cholesterol and inflammatory cardiovascular risk are independent or synergistic, and whether they differentially impact response to therapies.
Current understanding from statin trials suggest that the salutary effects of lipid lowering on plaque burden and composition occur independent of baseline inflammatory status [8]. However less is known about these relationships in a contemporary era of treatment. In particular, it remains uncertain if some of the consistency of benefit may be specific to statins, and further, whether the presence of enhanced “inflammatory risk” may be more significant at lower achieved LDL-C. The GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound) trial evaluated the effect of administering evolocumab for 78 weeks on coronary plaque burden in statin-treated patients [9]. In this post hoc analysis of the GLAGOV trial, we sought to determine the effect of baseline inflammatory status on the propensity for plaque regression and changes in plaque composition in statin-treated patients.
2. Methods
2.1. Trial design
GLAGOV was a multicenter, double-blind placebo controlled randomized trial designed by the Cleveland Clinic Coordinating Centre for Clinical Research (C5Research) in collaboration with the sponsor [10]. Local institutional review boards approved the protocol and written informed consent was obtained from each subject. The study design has been previously published [10], as have results from primary [9] and subsequent analyses [11]. In brief, patients aged 18 years or older were eligible for participation following a clinically indicated coronary angiogram. Inclusion required subjects to have at least one epicardial stenosis of 20% or greater in addition to a target vessel suitable for imaging with 50% or less visual stenosis. Subjects had to be on a stable dose of statin (4 weeks) with an LDL-C of ≥80 mg/dL or between 60 and 80 mg/dL with additional risk factors (one major or three minor). Major risk factors included non-coronary atherosclerotic vascular disease, hospitalisation for acute coronary syndrome (myocardial infarction or unstable angina) in the preceding 2 years or type 2 diabetes. Minor risk factors included current cigarette smoking, hypertension, low HDL (male <40 mg/dL, female <50 mg/dL), family history of premature coronary disease (1st degree male <55years or 1st degree female <65 years), hsCRP ≥2 mg/L or increased age (male ≥50 years, female ≥55years). Exclusion criteria included heart failure, renal dysfunction, liver disease or either uncontrolled hypertension or diabetes. Subjects were randomized 1:1 in blocks of 4 to treatment with evolocumab (420 mg) or placebo administered monthly via subcutaneous injection for 76 weeks. Intravascular imaging was undertaken at baseline and at week 78 (i.e. two weeks following the final administration).
2.2. Intravascular ultrasound image acquisition
Following coronary angiography, baseline intravascular ultrasound imaging was performed in a single coronary artery and analyzed as previously described [[12], [13], [14], [15]]. Repeat imaging was performed at 78 weeks in the same coronary artery. Analysis was performed by the core laboratory on digitised images by personnel blinded to treatment status and sequence of imaging studies.
2.3. Plaque burden analysis
In brief, leading edges of the lumen and external elastic membrane (EEM) were manually planimetered on images with 1 mm spacing, each subtending regions of interest. Images were excluded if there was acoustic artefact extending >90° across the outer vessel wall. Reproducibility has previously been reported [16]. Measures of plaque burden were then determined. Percent atheroma volume (PAV) was calculated as follows:
where EEMarea is the cross-sectional area of the external elastic membrane and Lumenarea is the cross-sectional area of the lumen. The change in PAV was calculated as the PAV at 78 weeks minus the PAV at baseline. Normalized total atheroma volume (TAV) was calculated as follows:
where the average plaque area in each image (plaque area divided by number of images in pullback)was multiplied by the median number of images analyzed in the entire cohort to compensate for differences in segment length between patients. Change in normalized TAV was calculated as the TAV at 78 weeks minus TAV at baseline. Regression of PAV or TAV was defined in any reduction in that parameter from baseline.
2.4. Plaque composition analysis
Plaque compositional analysis off the ultrasonic radiofrequency backscatter signal was performed on studies acquired with the 45 MHz rotational catheter (Revolution, Volcano Corporation, California). Methods have been described in detail elsewhere [11,[17], [18], [19]], however in brief, the radiofrequency signal enables reconstruction of a color-coded map distinguishing necrotic core, dense calcium, fibrofatty and fibrotic plaque components. Off-line grayscale and radiofrequency IVUS analysis was performed using echoPlaque 4.0 (Indec Medical Systems, Santa Clara, California). External elastic membrane and lumen borders were generated for each image gated at the time of the peak of the R-wave, and the acoustic shadow due to the catheter was excluded from plaque analysis. Absolute and percentage plaque measures of each VH parameter was performed using the trapezoidal rule [20].
2.5. Statistical analysis
The entire cohort was categorized by strata of baseline hsCRP (low ‘<1 mg/L’, intermediate ‘1–3 mg/L’ and high ‘>3 mg/L’) as per AHA consensus guidelines which represent tertiles of approximate population distribution [21]. Continuous variables are expressed as mean ± SD or median (IQR) where not normally distributed. Categorical parameters are expressed as percentage. Two-sample t tests were used for normally distributed continuous variables, Wilcoxon rank sum tests were used for non-normally distributed continuous variables, and Chi-squared tests were used for categorical variables. Tests of trend were performed in baseline characteristics across hsCRP categories. Baseline and serial changes in the TAV and PAV of evolocumab-treated patients were evaluated by analysis of variance, adjusting for their baseline measurement and geographical region, and were reported as least-squares mean ± standard error. Chi-square test for proportion of individuals demonstrating coronary atheroma regression (shown as percentages) was utilized across hsCRP stratum.
Evolocumab treated patients were then divided by achieved LDL strata (≤20, 20–40, ≥40 mg/dL, which were approximate tertiles). These groups were then dichotomized initially by baseline hsCRP (≤2 or >2 mg/L), and then by achieved hsCRP; this threshold has been used in previous trials to identify a population with inflammatory risk [22]. Comparisons between hsCRP groups (≤2 or >2 mg/L) at each level of achieved LDL were performed with Wilcoxon rank sum test. Baseline and serial change in each of the four normalized VH parameters (dense calcium volume, necrotic core, fibrofatty and fibrous plaque) were evaluated by analysis of covariance, adjusting for baseline volume and geographical region, and reported as least-square means and corresponding 95% confidence intervals. Tests of trend were evaluated at baseline and follow up across hsCRP strata. All p values are 2-sided, and p < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
3. Results
3.1. Baseline characteristics
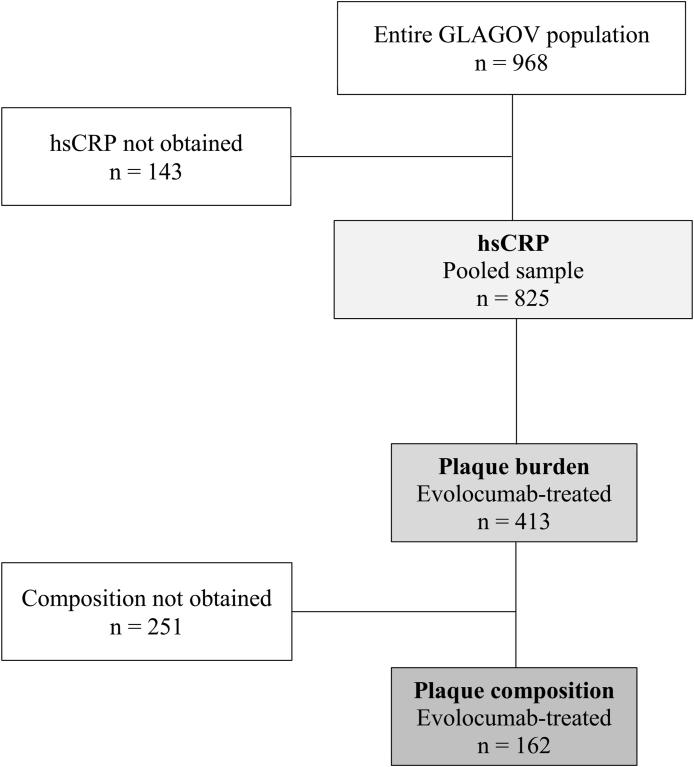
The study cohort with evaluable imaging and baseline hsCRP values comprised 413 evolocumab-treated patients [Fig. 1]; 132 (32%) with low hsCRP (<1 mg/L) levels, 170 (41%) patients with intermediate (1–3 mg/L) levels and 111 (27%) participants with high (>3 mg/L) levels [Table 1]. Patients with higher baseline hsCRP levels were more frequently female, had a higher prevalence of diabetes, hypertension and the metabolic syndrome, and had less commonly undergone prior PCI. Use of lipid lowering therapy and cardiovascular secondary prevention medications was consistent across the groups. While baseline LDL-C was similar, those with higher hsCRP levels had higher triglycerides and lower HDL-C levels. Patients with higher hsCRP levels had higher BMI values and higher HbA1c levels.
Fig. 1.
Flow diagram of cohort build.
Table 1.
Baseline characteristics stratified by baseline hsCRP.
| hsCRP |
P value | |||
|---|---|---|---|---|
| <1 mg/L [n = 132] | 1 – 3 mg/L [n = 170] | >3 mg/L [n = 111] | ||
| Age (years) | 59.7 ± 9.3 | 59.0 ± 9.6 | 60.0 ± 9.4 | 0.62 |
| Female, % (N) | 22.7 (30) | 27.6 (47) | 33.3 (37) | 0.18 |
| Medical history | ||||
| - MI, % (N) | 35.6 (47) | 32.4 (55) | 30.6 (34) | 0.70 |
| - CABG, % (N) | 0.8 [1] | 0.6 [1] | 0.9 [1] | 1.00 |
| - PCI, % (N) | 37.9 (50) | 45.9 (78) | 29.7 (33) | 0.02 |
| - CeVD, % (N) | 3.0 [4] | 2.9 [5] | 2.7 [3] | 1.00 |
| - PAD, % (N) | 2.3 [3] | 1.8 [3] | 3.6 [4] | 0.66 |
| Risk factors | ||||
| - Type II DM, % (N) | 14.4 [19] | 23.5 (40) | 24.3 [27] | 0.09 |
| - Current smoker, % (N) | 25.0 (33) | 28.2 (48) | 23.4 [26] | 0.64 |
| - Hypertension, % (N) | 72.7 (96) | 83.5 (142) | 86.5 (96) | 0.01 |
| - FHx CAD, % (N) | 41.7 (55) | 33.5 (57) | 29.7 (33) | 0.13 |
| - Metabolic Syndrome, % (N) | 28.0 (37) | 35.3 (60) | 41.4 (46) | 0.09 |
| Anthropometric/BP | ||||
| - SBP, mmHg ± SD | 130 ± 15 | 131 ± 14 | 133 ± 15 | 0.32 |
| - DBP mmHg ± SD | 78 ± 9 | 78 ± 9 | 78 ± 10 | 0.90 |
| - BMI, kg/m2 (Q1,Q3) | 26.8 (24.5,29.7) | 28.8 (26.3,32.6) | 30.2 (27.0,34.3) | <0.01 |
| Medications | ||||
| - Statin, % (N) | 99.2 (131) | 98.8 (168) | 98.2 (109) | 0.74 |
| - Ezetimibe, % (N) | 2.3 [3] | 2.9 [5] | 0 (0) | 0.20 |
| - Antiplatelets, % (N) | 93.9 (124) | 95.3 (162) | 90.1 (100) | 0.22 |
| - Beta blockers, % (N) | 68.2 (90) | 78.8 (134) | 75.7 (84) | 0.10 |
| - ACE/ARB, % (N) | 60.1 (80) | 72.9 (124) | 82.9 (92) | 0.08 |
| Metabolic | ||||
| - LDL-C, mg/dL ± SD | 90.1 ± 24.7 | 92.7 ± 28.6 | 92.7 ± 27.9 | 0.65 |
| - HDL-C, mg/dL ± SD | 49.4 ± 13.5 | 47.0 ± 12.7 | 42.3 ± 10.2 | <0.01 |
| - TG, mg/dL (Q1,Q3) | 118.6 (79,137) | 125.0 (93,167) | 128.0 (95,195) | <0.01 |
| - HbA1c, % ± SD | 5.7 ± 0.6 | 5.9 ± 0.8 | 5.9 ± 0.8 | 0.03 |
Characteristics presented as median (Q1,Q3) or mean ± SD.
ACE/ARB – angiotensin converting enzyme inhibitor or angiotensin-II receptor antagonist; BMI – body mass index; CABG – coronary artery bypass surgery; CeVD – cerebrovascular disease; DBP - diastolic blood pressure; FHx CAD – family history of coronary artery disease; HDL-C – high density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; MI – myocardial infarction; PAD – peripheral arterial disease; PCI – percutaneous coronary intervention; SBP – systolic blood pressure; TG - triglycerides.
3.2. Effect of evolocumab on hsCRP and lipids
Baseline and follow-up hsCRP levels were similar in the cohort of evolocumab-treated participants (baseline; 1.6 mg/L [0.8,3.4] vs. follow-up; 1.4 mg/L [0.7,3.0], p = 0.47). With respect to lipids, evolocumab therapy consistently reduced LDL-C by >55% regardless of baseline hsCRP [Table 2].
Table 2.
LDL cholesterol in evolocumab treated patients stratified by baseline hsCRP.
| hsCRP |
P value | |||
|---|---|---|---|---|
| <1 mg/L [n = 132] | 1 – 3 mg/L [n = 170] | >3 mg/L [n = 111] | ||
| Baseline, mg/dL ± SD | 90.1 ± 24.7 | 92.7 ± 28.6 | 92.7 ± 27.9 | 0.65 |
| Weighted average, mg/dL ± SD | 32.1 ± 19.9 | 37.3 ± 25.1 | 35.9 ± 19.7 | 0.12 |
| Absolute Change, % ± SD | −58.0 ± 22.8 | −55.4 ± 30.7 | −56.8 ± 21.3 | 0.69 |
3.3. Effect of evolocumab on plaque burden by hsCRP
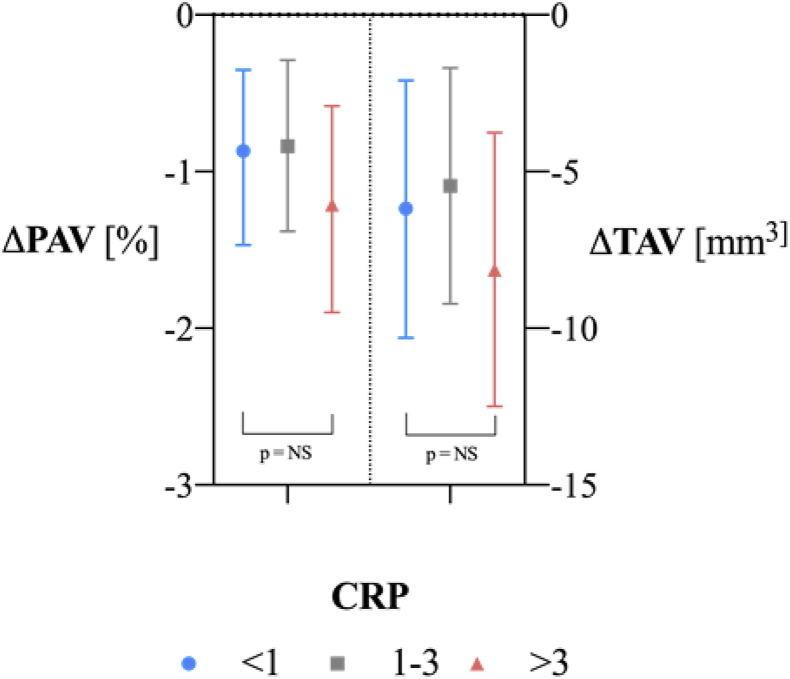
Measures of plaque burden stratified by baseline hsCRP levels are presented in Table 3. Patients with the highest baseline hsCRP in the evolocumab-treated cohort had numerically lower PAV and TAV than patients with lower hsCRP levels, although neither comparison reached statistical significance. Overall, participants across hsCRP strata achieved significant reductions in plaque burden with similar proportions of regressors (defined as ΔPAV<0) observed in each group [Fig. 2]. Adjusted for baseline levels, the magnitude of PAV and TAV reduction with evolocumab was similar regardless of baseline hsCRP.
Table 3.
Effect of evolocumab on markers of atheroma burden stratified by baseline hsCRP.
| hsCRP |
P value | |||
|---|---|---|---|---|
| <1 mg/L [n = 132] | 1–3 mg/L [n = 170] | >3 mg/L [n = 111] | ||
| PAV | ||||
| - baseline, % | 37.2 ± 8.2 | 36.9 ± 9.0 | 34.9 ± 8.8 | 0.10 |
| - LSM, % | −0.87 (−1.46,-0.27) | −0.84 (−1.38,-0.29) | −1.22 (−1.85,-0.58) | 0.46 |
| - p value | 0.005 | 0.003 | <0.001 | |
| TAV | ||||
| - baseline, mm3 | 180.4 (129.2,257.2) | 174.2 (131.1,240.0) | 163.7 (124.5,214.6) | 0.08 |
| - LSM, mm3 | −6.18 (−10.3,-2.1) | −5.46 (−9.22,-1.70) | −8.13 (−12.5,-3.76) | 0.49 |
| - p value | 0.003 | 0.005 | <0.001 | |
| ‘Regressors’ | ||||
| - PAV, % | 65.9 | 63.5 | 63.1 | 0.88 |
| - TAV, % | 62.1 | 59.4 | 63.1 | 0.80 |
LSM – least squared mean (95%CI); PAV – percentage atheroma volume, mm3 ± SD; TAV – total atheroma volume, mm3 (IQR).
Fig. 2.
Change in PAV and TAV in evolocumab treated participants stratified by baseline hsCRP.
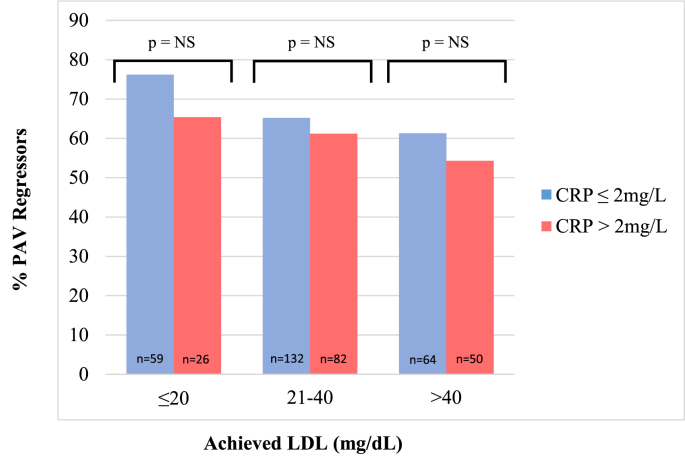
As seen in the main GLAGOV analysis, a stepwise inverse relationship was observed between achieved LDL-C and the proportion of evolocumab-treated patients experiencing PAV regression. When comparing participants by strata of achieved LDL-C, the proportion experiencing regression was statistically no different in those with high (>2 mg/L) or low levels (≤2 mg/L) of inflammation at baseline [Fig. 3], or at follow up (data not shown).
Fig. 3.
Proportion of evolocumab-treated participants experiencing PAV regression (‘Regressors’). Participants are presented in groups of achieved LDL (≤20, 20–40, >40 mg/dL) and then further dichotomized by baseline hsCRP (≤2 = blue, >2 = red). Number of participants in each group depicted numerically at base of column (n). Analysis shows consistent proportion of ‘Regressors’ at each level of achieved LDL-C irrespective of baseline hsCRP.
3.4. Effect of evolocumab on plaque composition by hsCRP
Plaque composition was determined in 331 (34%) patients from GLAGOV, of which 162 were treated with evolocumab [Fig. 1]. Measures of plaque composition, stratified by baseline hsCRP, are presented in Table 4. Compared to those with the lowest hsCRP, patients with the highest hsCRP levels had similar amounts of necrotic core and dense calcium, however there was proportionally less fibrofatty (35.7% (24.3,42.2) vs. 44.5% (33.6,53.4), p = 0.03) and fibrous plaque (38.9% (33.9,47.5) vs. 33.6% (28.0,41.2), p = 0.03). There were no significant differences in plaque composition on serial imaging when evaluated by baseline hsCRP.
Table 4.
Effect of evolocumab on plaque composition stratified by baseline hsCRP. Data presented as median (Q1,Q3) or mean ± SD.
| hsCRP |
P value | |||
|---|---|---|---|---|
| <1 mg/L [n = 46] | 1–3 mg/L [n = 70] | >3 mg/L [n = 46] | ||
| Normalized volume | ||||
| Necrotic core, mm3 | ||||
| - baseline | 8.0 (4.8,14.8) | 12.2 (3.8,19.7) | 8.3 (3.9,13.9) | 0.51 |
| - follow-up | 7.9 (3.3,14.5) | 11.0 (3.5,19.3) | 5.2 (2.3,12.0) | 0.08 |
| - nominal change | −0.43 ± 1.04 | −0.39 ± 0.85 | −2.40 ± 1.05 | 0.12 |
| - p value | 0.68 | 0.65 | 0.02 | |
| Fibrofatty, mm3 | ||||
| - baseline | 23.6 (12.9,44.5) | 20.9 (8.2,49.4) | 15.9 (6.5,28.5) | 0.21 |
| - follow-up | 19.3 (5.8,32.6) | 17.6 (5.6,38.5) | 12.2 (3.7,24.7) | 0.18 |
| - nominal change | −5.41 ± 1.74 | −3.82 ± 1.41 | −5.37 ± 1.76 | 0.71 |
| - p value | <0.01 | <0.01 | <0.01 | |
| Fibrous, mm3 | ||||
| - baseline | 20.5 (10.1,29.9) | 24.5 (10.0,39.8) | 18.9 (8.6,29.5) | 0.45 |
| - follow-up | 14.9 (7.6,25.4) | 20.2 (7.7,35.4) | 14.1 (5.5,27.0) | 0.24 |
| - nominal change | −1.08 ± 1.33 | −2.94 ± 1.09 | −4.90 ± 1.34 | 0.13 |
| - p value | 0.42 | <0.01 | <0.001 | |
| Dense calcium, mm3 | ||||
| - baseline | 1.7 (0.5,6.2) | 2.6 (0.3,6.2) | 2.1 (0.8,5.1) | 0.94 |
| - follow-up | 2.3 (0.6,7.6) | 4.1 (0.5,7.0) | 1.6 (0.5,5.0) | 0.36 |
| - nominal change | 1.04 ± 0.60 | 1.45 ± 0.48 | 0.17 ± 0.60 | 0.26 |
| - p value | 0.08 | <0.01 | 0.78 | |
| Percent plaque | ||||
| Necrotic core, % | ||||
| - baseline | 14.5 (10.5,21.9) | 16.5 (11.5,20.8) | 17.9 (12.6,22.0) | 0.44 |
| - follow-up | 17.5 (10.3,21.8) | 17.8 (11.6,24.4) | 16.5 (8.3,21.8) | 0.38 |
| - nominal change | 0.11 ± 1.06 | 2.19 ± 0.88 | −0.94 ± 1.08 | 0.07 |
| - p value | 0.914 | 0.01 | 0.38 | |
| Fibrofatty, % | ||||
| - baseline | 44.5 (33.6,53.4) | 38.2 (28.1,49.4) | 35.7 (24.3,42.2) | 0.03 |
| - follow-up | 40.3 (23.7,57.5) | 34.9 (25.1,49.0) | 37.2 (25.1,44.1) | 0.52 |
| - nominal change | −0.85 ± 2.25 | −2.52 ± 1.84 | 0.58 ± 2.27 | 0.56 |
| - p value | 0.71 | 0.17 | 0.80 | |
| Fibrous, % | ||||
| - baseline | 33.6 (28.0,41.2) | 39.2 (31.5, 45.9) | 38.9 (33.9,47.5) | 0.03 |
| - follow-up | 34.7 (27.3,42.0) | 34.9 (42.0, 43.5) | 38.3 (29.8, 47.6) | 0.22 |
| - nominal change | −2.13 ± 1.47 | −2.01 ± 1.20 | −0.75 ± 1.47 | 0.76 |
| - p value | 0.15 | 0.10 | 0.61 | |
| Dense calcium, % | ||||
| - baseline | 3.5 (1.2,8.7) | 5.0 (1.4,8.3) | 4.8 (1.8,8.9) | 0.67 |
| - follow-up | 5.6 (1.2,12.3) | 6.7 (2.1,11.8) | 5.5 (2.9,10.5) | 0.77 |
| - nominal change | 2.26 ± 0.74 | 2.62 ± 0.61 | 1.31 ± 0.75 | 0.40 |
| - p value | <0.01 | <0.001 | 0.08 | |
4. Discussion
This post hoc analysis of both the primary GLAGOV trial and the plaque composition sub-study demonstrates that the presence of enhanced systemic inflammation, as evidenced by greater hsCRP levels, is not associated with a diminution of the favorable effects of evolocumab on LDL-mediated plaque regression. In this analysis, both the proportion of patients experiencing atheroma regression and the magnitude of reduction in atheroma burden were consistent, regardless of baseline hsCRP.
Markers of inflammation have consistently and independently identified individuals at higher risk of future adverse atherosclerotic cardiovascular outcomes. Despite achieving guideline-recommended LDL-C treatment targets with the use of statin therapy, elevated hsCRP levels have been shown to retain prognostic capacity for adverse cardiovascular outcomes [23]. Until recently, it was unknown whether the incremental benefits associated with achieving very low LDL-C levels may be attenuated in individuals with higher levels of inflammation. In separate post hoc analyses of both the SPIRE (Studies of PCSK9 Inhibition and the Reduction of Vascular Events) and FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trials of bococizumab and evolocumab respectively, individuals were stratified by baseline hsCRP and their outcomes compared [24,25]. In both of the SPIRE (median hsCRP = 1.9 mg/L) and FOURIER (median hsCRP = 1.8 mg/L) analyses, PCSK9 inhibitor therapy was associated with similar relative risk reduction for the individual and composite cardiovascular outcomes, regardless of hsCRP. Our analysis provides mechanistic support for these clinical observations by demonstrating the same degree of plaque regression at the arterial wall independent of baseline inflammatory status.
Our lack of association between hsCRP and any measure of plaque composition at baseline, or on serial assessment, question links between measures of systemic inflammation and VH-derived measures of plaque composition in the post-statin era. Studies in patients who are statin-naïve or have high LDL-C concentrations (>100 mg/dL) have shown elevated levels of hsCRP to associate with greater proportions of necrotic core and fibrofatty plaque, albeit with slightly different VH imaging techniques (radiofrequency backscatter) [26,27]. Similarly, in patients treated with high-intensity statin therapy, both on-treatment and change in hsCRP have been associated with favorable reductions in plaque volume as well as increases in dense calcium and/or reductions in necrotic core [19]. Whether plaque composition is less modifiable at lower levels of LDL-C (i.e. lower residual ‘cholesterol’ risk), or is predominantly determined by the anti-inflammatory effects of statins in the previously mentioned studies, or the compositional changes are too subtle for this imaging modality, remains uncertain.
There are several implications from this analysis. The presence of elevated inflammatory status does not mitigate the favorable effects of further LDL-C lowering on plaque burden. Furthermore, our findings that uncouple inflammatory status from both atheroma burden and plaque composition support the independence of ‘cholesterol risk’ from ‘inflammatory risk’. Thus, while patients appear to derive similar benefit from ‘cholesterol-risk’ reduction in GLAGOV and FOURIER, their ‘inflammatory risk’ remains unaddressed through an LDL-lowering approach. In this context, patients may derive independent, and presumably additional benefit from selectively reducing inflammation as tested with an IL-1β monoclonal antibody in CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study [28]), however whether these benefits are accrued at lower levels of residual cholesterol risk (LDL-C = 84 mg/dL in CANTOS), and whether they are discernible at the arterial wall, would require 2 × 2 factorial study of these approaches.
Several limitations of this study should be considered. Despite being the largest serial plaque imaging study evaluating the effects of a PCSK9 inhibitor, the invasive, time-consuming and expensive nature of the modality means our sample size is modest compared with the large phase III outcome trials evaluating these agents. The median baseline hsCRP for the GLAGOV cohort was 1.6 mg/L, compared to 4 mg/L in the CANTOS trial of canakinumab. Thus, our findings may not be generalizable to larger numbers of patients or those with on-average, higher hsCRP levels. To be eligible for inclusion in GLAGOV, patients were required to be treated with stable statin therapy; the plaque features studied in this analysis may have behaved differently in the absence of statin therapy.
In summary, evolocumab administration was associated with favorable reductions in atheroma burden regardless of baseline hsCRP levels. Consistent relative reductions in the plaque burden of patients supports the results of the phase 3 outcomes studies of PCSK9 inhibitors where consistent reductions in cardiovascular events were observed in patients with elevated systemic inflammation.
Funding
The GLAGOV trial was sponsored by Amgen.
Acknowledgements
Plaque measurements were made by the members of the Atherosclerosis Imaging Core Laboratory at the Cleveland Clinic.
References
- 1.Cholesterol Treatment Trialists C., Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman M.G., Ference B.A., Im K., Wiviott S.D., Giugliano R.P., Grundy S.M. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. J Am Med Assoc. 2016;316(12):1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 4.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 5.Scherer D.J., Nelson A.J., O’Brien R., Kostner K.M., Hare D.L., Colquhoun D.M. Status of PCSK9 monoclonal antibodies in Australia. Heart Lung Circ. 2019;28(10):1571–1579. doi: 10.1016/j.hlc.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 7.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri R., Nissen S.E., Shao M., Uno K., Kataoka Y., Kapadia S.R. Impact of baseline lipoprotein and C-reactive protein levels on coronary atheroma regression following high-intensity statin therapy. Am J Cardiol. 2014;114(10):1465–1472. doi: 10.1016/j.amjcard.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls S.J., Puri R., Anderson T., Ballantyne C.M., Cho L., Kastelein J.J. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. J Am Med Assoc. 2016;316(22):2373–2384. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 10.Puri R., Nissen S.E., Somaratne R., Cho L., Kastelein J.J., Ballantyne C.M. Impact of PCSK9 inhibition on coronary atheroma progression: rationale and design of global assessment of plaque regression with a PCSK9 antibody as measured by intravascular ultrasound (GLAGOV) Am Heart J. 2016;176:83–92. doi: 10.1016/j.ahj.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls S.J., Puri R., Anderson T., Ballantyne C.M., Cho L., Kastelein J.J.P. Effect of evolocumab on coronary plaque composition. J Am Coll Cardiol. 2018;72(17):2012–2021. doi: 10.1016/j.jacc.2018.06.078. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls S.J., Ballantyne C.M., Barter P.J., Chapman M.J., Erbel R.M., Libby P. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 13.Nicholls S.J., Hsu A., Wolski K., Hu B., Bayturan O., Lavoie A. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010;55(21):2399–2407. doi: 10.1016/j.jacc.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Nissen S.E., Nicholls S.J., Sipahi I., Libby P., Raichlen J.S., Ballantyne C.M. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. J Am Med Assoc. 2006;295(13):1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 15.Nissen S.E., Tuzcu E.M., Schoenhagen P., Brown B.G., Ganz P., Vogel R.A. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. J Am Med Assoc. 2004;291(9):1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 16.Nissen S.E., Tsunoda T., Tuzcu E.M., Schoenhagen P., Cooper C.J., Yasin M. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. J Am Med Assoc. 2003;290(17):2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Garcia H.M., Mintz G.S., Lerman A., Vince D.G., Margolis M.P., van Es G.A. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5(2):177–189. doi: 10.4244/eijv5i2a29. [DOI] [PubMed] [Google Scholar]
- 18.Maehara A., Cristea E., Mintz G.S., Lansky A.J., Dressler O., Biro S. Definitions and methodology for the grayscale and radiofrequency intravascular ultrasound and coronary angiographic analyses. JACC Cardiovasc Imag. 2012;5(3 Suppl):S1–S9. doi: 10.1016/j.jcmg.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Puri R., Libby P., Nissen S.E., Wolski K., Ballantyne C.M., Barter P.J. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imag. 2014;15(4):380–388. doi: 10.1093/ehjci/jet251. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson K.E. second ed. Wiley and Sons; New York: 1989. An introduction to numerical analysis. [Google Scholar]
- 21.Myers G.L., Rifai N., Tracy R.P., Roberts W.L., Alexander R.W., Biasucci L.M. CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: report from the laboratory science discussion group. Circulation. 2004;110(25):e545–e549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 22.Ridker P.M. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37(22):1720–1722. doi: 10.1093/eurheartj/ehw024. [DOI] [PubMed] [Google Scholar]
- 23.Ridker P.M., Cannon C.P., Morrow D., Rifai N., Rose L.M., McCabe C.H. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 24.Bohula E.A., Giugliano R.P., Leiter L.A., Verma S., Park J.G., Sever P.S. Inflammatory and cholesterol risk in the FOURIER trial. Circulation. 2018;138(2):131–140. doi: 10.1161/CIRCULATIONAHA.118.034032. [DOI] [PubMed] [Google Scholar]
- 25.Pradhan A.D., Aday A.W., Rose L.M., Ridker P.M. Residual inflammatory risk on treatment with PCSK9 inhibition and statin therapy. Circulation. 2018;138(2):141–149. doi: 10.1161/CIRCULATIONAHA.118.034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataoka Y., Puri R., Nicholls S.J. Inflammation, plaque progression and vulnerability: evidence from intravascular ultrasound imaging. Cardiovasc Diagn Ther. 2015;5(4):280–289. doi: 10.3978/j.issn.2223-3652.2015.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo T., Matsuo Y., Hayashi Y., Yamano T., Tanimoto T., Ino Y. High-sensitivity C-reactive protein and plaque composition in patients with stable angina pectoris: a virtual histology intravascular ultrasound study. Coron Artery Dis. 2009;20(8):531–535. doi: 10.1097/MCA.0b013e328332a6b0. [DOI] [PubMed] [Google Scholar]
- 28.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]