Abstract
Comprehensive management of coronary artery disease (CAD) includes physical exercise as a part of daily lifestyle therapy. Still CAD patients generally have low physical activity (PA) and high sedentary behaviour (SB). This review summarizes the effect of exercise training and habitual PA and SB on physical fitness and quality of life (QoL) as well as on rehospitalizations and mortality in patients with stable CAD, recent acute coronary syndrome (ACS) or recent revascularization. A literature review of the influence of exercise, and PA and SB profiles in secondary prevention of CAD was performed using PubMed. All articles published between January 2001 and April 2019, meeting the inclusion criteria were considered. A total of 25 cross-sectional or prospective studies or randomized controlled trials (RCT) were included to this review. Exercise training was found to improve maximal oxygen consumption, QoL, and to reduce rehospitalizations and mortality among patients with established CAD. Remote PA interventions have not been as effective as the supervised exercise sessions in reducing the clinical endpoints. High SB, especially when combined to low PA, is associated with poor cardiorespiratory fitness and worse long-term prognosis among patients with ACS. In conclusion, exercise training and high PA are beneficial for patients with stable CAD, recent ACS or recent revascularization. High SB is associated with poor cardiopulmonary fitness and increased mortality in ACS patients. Novel tools using online applications and smart devices are promising means to offer remote guidance for PA among patients unable to participate in regular exercise sessions.
Keywords: Coronary artery bypass grafting, Coronary heart disease, Exercise, Myocardial infarction, Percutaneous coronary intervention, Rehabilitation, Sitting
1. Introduction
Coronary artery disease (CAD) is the most common cardiovascular disease (CVD) with prevalence of over 110 million cases globally [1]. The clinical manifestations of CAD are angina pectoris, acute coronary syndrome (ACS), including myocardial infarction (MI), and sudden cardiac death.
Physical activity (PA), including activities consuming 1.5 or more METs (Metabolic equivalent = 3.5 ml/kg/min of O2 consumption), can be divided into following categories: light PA (1.5–3.0 MET) and moderate-to vigorous PA (MVPA; >3.0 MET) [2]. Insufficient PA, defined as not meeting the PA guidelines, has been identified as an independent risk factor for CAD in large population-based studies [3,4]. It has been predicted that low PA accounts for as much as 6% of the prevalence of CAD globally [3]. In addition, there seems to be no major differences in accumulated PA profiles between patients who have established CAD and those at high risk of CVD [5].
Besides low PA, more recently, high sedentary behaviour (SB, including sitting and lying with energy consumption less than 1.5 METs) has also been recognized as an independent risk factor of cardiovascular diseases [4,6,7].
Medical therapy targeting lipid and blood pressure control as well as antiplatelet and antianginal therapy and, in certain cases, revascularization, are the cornerstones of treating CAD patients’ symptoms and secondary prevention [8,9]. In addition, lifestyle guidance including regular PA (at least 2 h 30 min moderate PA/week), healthy nutrition and smoking cessation are recommended for secondary prevention of CAD [10,11]. For example, two types of interventions have been used to promote PA among CAD patients: 1) Interventions that are based on regular, structured exercise either supervised or non-supervised according to rather strict instructions (exercise intervention), and 2) Interventions in which the participants are given daily PA/exercise goals (e.g. for step count), and the attainment of the goals is monitored (e.g. with device-based approaches) and personalized feedback is provided (PA intervention). The advantage for the latter approach is that all daily activities, such as walking or cycling to workplace or using stairs instead of an elevator, are taken into account.
This review summarizes the knowledge about benefits of increasing PA and reducing SB in patients with stable CAD, patients recovering from recent ACS and those who have undergone a recent coronary revascularization procedure. Furthermore, the different strategies for organizing PA interventions for CAD patients will be discussed.
2. Methods
A systematic review of the scientific literature on the influence of increasing PA and reducing SB in secondary prevention of CAD was conducted using PubMed database. All relevant articles published between January 2001 and April 2019 were considered for this review.
Following Medical Subject Heading (MESH) -terms were used to select the eligible articles:
(“Coronary artery disease” OR “Coronary Heart Disease” OR “Ischemic Heart Disease” OR “Acute Coronary Syndrome” OR “Myocardial Infarction” OR “Percutaneous Coronary Intervention” OR “Percutaneous Coronary Angioplasty” OR “Coronary Artery Bypass Grafting” OR “PCI” OR “PTCA” OR “CABG”) AND (“Physical Activity” OR “PA” OR “Sedentary Behaviour” OR “SB” OR “Sedentary Time” OR “Exercise” OR “Accelerometer” OR “Physical Fitness”) AND (“Cardiac Rehabilitation” OR “Secondary Prevention”).
The selection of articles was performed in two steps. At first, the publication titles obviously not matching the inclusion criteria were excluded. Secondly, the abstracts of the remaining articles were read and the articles were selected according to following inclusion criteria:
-
1.
The study population included patients with established CAD (stable CAD, recent ACS, recent revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)) diagnosed with electrocardiography, exercise test, echocardiography, computed tomography angiography, invasive coronary angiography, or stress perfusion, stress magnetic resonance imaging or stress echocardiography.
-
2.
The study design was cross-sectional, observational or randomized/non-randomized controlled trial (RCT), and published between January 2001 and April 2019 (i.e. during the era of modern medical therapy for CAD).
-
3.
The study evaluated associations between PA and/or SB with relevant outcomes in secondary prevention of CAD; or compared the standard and exercise-based rehabilitation in secondary prevention of CAD.
-
4.
The study employed one or more of the following endpoints: step count, maximal oxygen consumption, PA time, SB time, physical fitness, quality of life (QoL), echocardiography measurements (e.g. left ventricular ejection fraction (LVEF)), incidence of rehospitalizations, incidence of myocardial infarction, incidence of revascularizations and/or mortality.
-
5.
The study was published in English.
3. Results
3.1. Results of the review
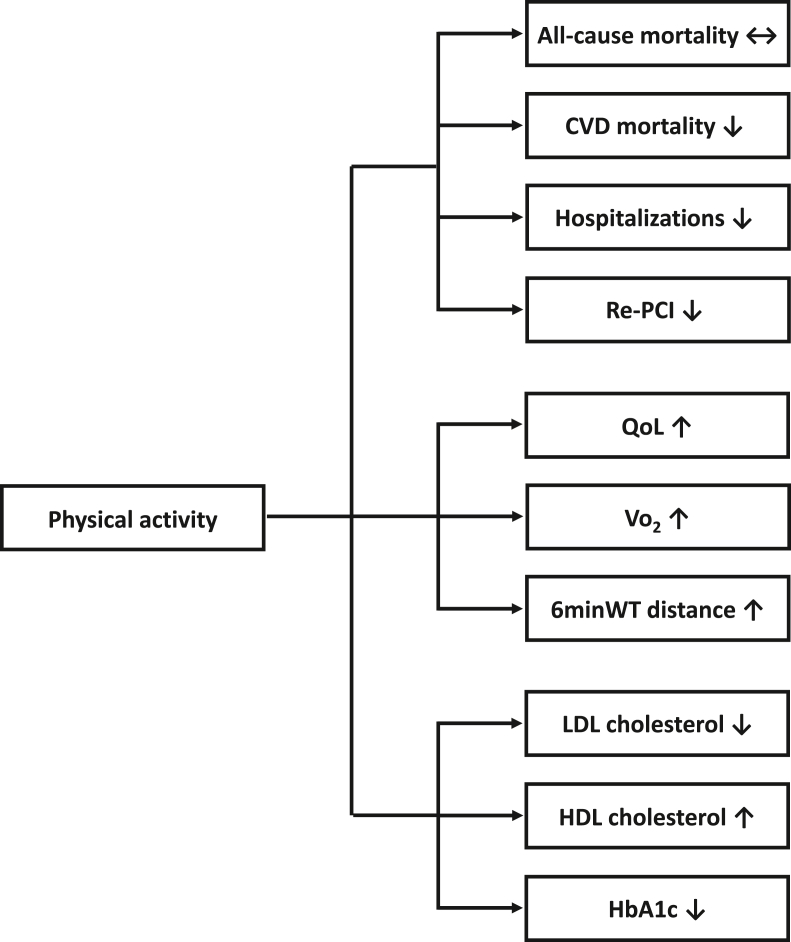
A total of 25 studies, 1 cross-sectional study (n = 263), 3 prospective observational studies (n = 5992) and 21 RCTs (n = 27 324) were included to this review (Table 1, Table 2). The results are presented below according to participants’ CAD status: stable disease, recent ACS or recent revascularization. The results on SB in CAD patients are reported in separately. The main effects of PA on different health outcomes are summarized in Fig. 1.
Table 1.
Studies reporting exercise or physical activity interventions in coronary artery disease patients.
| CAD status | Author; year | Study design | N | Follow-up | Control group | Intervention group | Symptomatic outcomes (e.g. 6MWT, QoL) | LVEF | Prognostic outcomes (e.g. hospitalizations) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Stable CAD | The difference between baseline and follow-up: intervention vs. control group | |||||||||
| or recent ACS/revasc | Anderson et al., 2016 | Meta-analysis | 14 486 | >6mo | Usual care | Exe int. | QoL: Improved |
Hospitalizations: −28% CVD mortality: −26% Total mortality: No difference |
||
| or PCI | Gomes-Neto et al., 2017 | Meta-analysis | 609 | Exe int | HIIT int | VO2 peak: +1.3 ml/kg/min QoL: No difference |
||||
| Hambrecht et al., 2004 | RCT | 101 | 12mo | PCI | Exe int. (no PCI) | VO2peak: +3.6 vs. +0.5 ml/kg/min | Ischemia-free survival: +26% Total mortality: No difference |
|||
| Maddison et al., 2015 | RCT | 171 | 6mo | Usual care | PA int. | Daily walking time: +63 vs. −56 min | ||||
| Oerkild et al., 2012 | RCT | 40 | 12mo | Usual care | Exe int. | 6MWT at 3mo: +12% vs. +3% QoL: No difference |
||||
| or recent ACS/revasc | Rawstorn et al., 2016 | Meta-analysis | 1189 | 3mo | Usual care Exe int |
Telehealth Exe int | PA:Improved VO2 peak: No difference |
|||
| Post-ACS | ||||||||||
| Briffa et al., 2005 | RCT | 113 | 12mo | Usual care | Exe int. | QoL: Improved |
||||
| and PCI or CABG |
Frederix et al., 2015 | RCT | 80 | 18 week | Usual care | PA int. | VO2peak: +4 vs. +1 ml/kg/min | Hospitalizations: -53% | ||
| La Rovere et al., 2002 | RCT | 95 | 10y | Usual care | Exe int. | CVD mortality: 12% vs. 26% |
||||
| Lawler et al., 2011 | RCT | 6111 | 3mo – 5y | Usual care | Exe int. | Mortality: −26% CVD mortality: −40% |
||||
| Marchionni et al., 2003 | RCT | 270 | 14mo | Usual care | Exe int. In 1. Hospital or 2. Home | QoL: Improved in older patients Total work capacity: Improved (both in hospital and home –based) |
||||
| Reid et al., 2012 | RCT | 223 | 12mo | Usual care | PA int. | Daily step count: 7392 vs. 6750 |
||||
| Xu et al., 2016 | RCT | 52 | 4 week | Usual care | Exe int. | LVEF: +4.1% vs. −1.7% | ||||
| or PCI | Yu et al., 2004 | RCT | 269 | 24mo | Usual care | Exe int. | QoL: Improved |
Re-PCI: 13% vs. 26% Total mortality: No difference |
||
| Wang et al., 2012 | RCT | 160 | 6mo | Usual care | Exe int. | QoL: Improved Depression: No difference |
||||
| West et al., 2012 | RCT | 1813 | 24mo | Usual care | Exe int. | No difference in any outcome above. | ||||
| Post-PCI/CABG | ||||||||||
| PCI | Belardinelli et al., 2001 | RCT | 118 | 33mo | Usual care | Exe int. | VO2 peak: +5 vs. −1 ml/kg/min QoL: Improved |
Hospitalizations: −60% Restenosis: No difference |
||
| CABG | Moholdt et al., 2009 | RCT | 59 | 6mo | Exe int. | Aerobic interval int. | VO2peak at 6mo: 18.8% vs. 12.6% |
|||
| PCI | Munk et al., 2009 | RCT | 40 | 6mo | Usual care | Exe int. | VO2peak: 16.8% vs. 7.8% |
Late luminal loss: 0.1 vs. 0.4 mm |
||
| CABG | Mutwalli et al., 2012 | RCT | 49 | 6mo | Usual care | Exe int. | QoL: Improved |
|||
| PCI | Higgins et al., 2001 | RCT | 99 | 12mo | Usual care | Exe int. | Functional capability: Improved Sick leave: Shorter |
|||
Abbreviations: ACS: Acute coronary syndrome; CABG: Coronary artery bypass grafting; CAD: Coronary artery disease; CVD: Cardiovascular disease; Exe int: Interventions based on structured or supervised exercise sessions; HIIT: High-intensity interval training; LVEF: Left ventricular ejection fraction; N: Number of study participants; PA: Physical activity; PA Int: Intervention based on patient’s daily PA goals of which fulfillment is monitored; PCI: Percutaneous coronary intervention; QoL: Quality of life; RCT: Randomized controlled trial; Rehab.: Rehabilitation; Rehosp.: Rehospitalizations; Revasc: Revascularization; Review: Review of RCTs; VO2peak: Maximal oxygen consumption; 6MWT: 6-min walk test; +: Increased/Improved; -: Decreased.
Table 2.
Studies reporting physical activity or sedentary behaviour in coronary artery disease patients.
| CAD status | Author; year | Study design | N | Follow-up | 6MWT | Mortality | |
|---|---|---|---|---|---|---|---|
| PA & CAD | |||||||
| Booth et al., 2014 | Pros. observ. | 4174 | 54 mo | Intensive PA 4 times/week vs. no intensive PA/week after ACS: | −29% | ||
| Gorczyca et al., 2017 | Pros. observ. | 856 | 86 mo | Increased PA (>150 min PA/week) vs. inactive PA (<150 min PA/week) after ACS: |
−46% | ||
| SB & CAD | |||||||
| Prince et al., 2016 | Cross-sectional | 263 | NA | High SB vs low SB: | VO2 peak: Lower |
||
| Wu et al., 2019 | Pros. observ. | 989 | 84 mo | High SB (4–8 h/day) vs. low SB (<4 h/day): | +62% | ||
Abbreviations: ACS: Acute coronary syndrome; CAD: Coronary artery disease; N: Number of study participants; Observ: Observational; PA: Physical activity; Pros: Prospective; SB: Sedentary behaviour; VO2peak: Maximal oxygen consumption; 6MWT: 6-min walk test.
Fig. 1.
The effects of physical activity in coronary artery disease.
3.2. Exercise intervention and physical activity in stable coronary artery disease
Hambrecht et al. compared bicycle exercise training with PCI in 101 male CAD patients with stable one vessel CAD (one native coronary artery with stenosis ≥75%) amenable to PCI [12]. The patients were randomly assigned to either optimal medical therapy plus exercise intervention or optimal medical therapy plus PCI with stenting. In the exercise intervention group, the patients exercised with bicycle ergometer targeting 70% of their maximal heart rate for 20 min per day and participated in one 60-min group training of aerobic exercise per week. After 12 months, the exercise intervention group had significantly lower resting heart rate, better exercise capacity and better maximal oxygen consumption compared to the PCI group. In addition, exercise training reduced the incidence of combined clinical endpoint (cardiac death, stroke, resuscitation from cardiac arrest, CABG, PCI, and worsening angina resulting in hospitalization).
The effect of home-based exercise training was addressed by Oerkild et al. [13]. 40 elderly (>65 years) CAD patients were randomized to either a 3-month exercise training intervention at home including medical assessment and risk factor modification (n = 21) or usual care (n = 19). Exercise (e.g. slow walking) was based on the recommendations advising a 30-min exercise performed 6 days per week and individualized for each patient. Exercise training improved 6-min walk test (walking distance) by 12% compared to baseline, whereas only 3% improvement was reported in the usual care group. However, the positive effect of training was not permanent, but disappeared after the follow-up of 3-month training period. After 12 months, the walking distance was 17% lower compared to baseline in both groups. In addition, exercise training had no effects on QoL.
Anderson et al. performed a meta-analysis of exercise-based cardiac rehabilitation including 63 RCT studies and more than 14 000 CAD patients with stable disease, recent ACS or recent revascularization [14]. The studies published since the 70s were included. Exercise intervention was defined as supervised or unsupervised inpatient, outpatient, community-based, or home-based intervention including some form of exercise training and the follow-up was at least 6 months. Exercise training improved QoL, and most importantly, reduced the incidence of rehospitalizations by 28% and cardiovascular mortality by 26%. However, it had no effect on all-cause mortality, myocardial infarctions or revascularizations.
High-intensity interval training and continuous exercise training were compared in a meta-analysis of 12 studies and 609 patients with stable CAD, recent MI and/or recent PCI [15]. A mean improvement of 1.3 ml/kg/min in maximal oxygen consumption was reported in high-intensity interval training group when compared to continuous exercise whereas no significant difference between the groups were observed in QoL.
Modern technology consisting of exercise guidance with text messages and videos aiming to increase weekly exercise was studied by Maddison et al. in 171 patients with stable CAD [16]. 85 patients were randomized to a 24-week PA intervention and 86 to usual care. Patients in the usual care group were encouraged to be physically active and attend a cardiac club. Compared to baseline, patient’s weekly walking time increased by 63 min in the PA group whereas decreased by 56 min in the usual care group. In addition, PA intervention improved general health score but had no effect on exercise capacity. Influence on clinical events was not reported.
Telehealth exercise-based rehabilitation intervention was compared with center-based exercise rehabilitation intervention and usual care in a meta-analysis of 11 trials and 1189 patients with stable CAD or recent ACS [17]. Telehealth intervention included 2 to 5 tele-guided sessions per week and center-based intervention 2 to 3 supervised exercise-sessions with duration of 0.5–1 h. Telehealth and supervised exercise interventions were effective in improving PA when compared to usual care. However, telehealth intervention was associated with better adherence to the intervention and lower blood pressure and LDL cholesterol than the supervised exercise. Due to the low incidence of clinical events, intervention effect on prognosis was not discussed.
The above cited meta-analysis included not only stable CAD patients, but also post-ACS and post-revascularization patients [14,15,17]. Thus, the results may not apply entirely to patients with stable CAD.
3.3. Exercise intervention and physical activity after acute coronary syndrome
La Rovere et al. randomized 95 post-ACS patients into exercise intervention or usual care [18]. CVD mortality was significantly lower among the intervention patients when compared to control peers (12% vs. 26%, respectively).
In the study by Yu et al.; 269 CAD patients recovering from MI or elective PCI were randomized to 2-year rehabilitation program or usual care [19]. The rehabilitation program consisted of 7 days inpatient ambulating program, followed by an 8-week twice-weekly outpatient education class and exercise training consisting of 2 h aerobic exercise intervention, 6 months home exercise training and maintenance period until the end of 2-year rehabilitation. The usual care patients attended the first 7-day phase only. Intervention resulted in significant improvement in 6/8 dimensions of the 36-Item Short-Form Health Survey (SF-36) representing patient’s QoL. Only 4/8 dimensions were improved among the control peers, and the positive development was observed after longer follow-up when compared to the intervention patients. During a follow-up of 2 years, the health care costs (15 292 vs. 15 707 dollars) and the incidence of subsequent PCI (13% vs. 26%) were lower among the intervention patients. However, no significant effects on mortality (3% vs. 5%) or rehospitalizations (26% vs. 22%) were reported.
The effect of a 5-week, home-based exercise rehabilitation intervention on left ventricular ejection fraction (LVEF) was studied among patients recovering from ST-elevation MI [20]. 52 patients were randomized into early cardiac rehabilitation (n = 26) or usual care (n = 26). Intervention included walking (during inpatient week), and 30-min exercise sessions comprising warm-up, aerobic exercise and cool-down (during 4 outpatient weeks). LVEF improved significantly among the intervention patients when compared to the control peers (mean change +4.1% and −1.7%, respectively).
In the study by Marchionni et al. 270 patients with recent ACS were randomized to hospital- or home-based exercise rehabilitation intervention or usual care [21]. During the follow-up, the patients in both intervention groups improved total work capacity when compared to control peers. Especially, the older (>65 years) participants benefitted from home-based exercise program. In addition, among the older patients, QoL improved only in those participating in the exercise intervention.
Personalized 6-month website-based PA intervention was evaluated by Reid et al. [22]. 223 ACS patients were randomized to PA intervention (n = 115) or usual care (n = 108). The intervention was based on personalized PA plan and website platform for PA planning and monitoring. In addition, as a part of the intervention, the patients were in email contact with an exercise specialist. After the follow-up of 6 months, the intervention patients had almost 1000 steps higher daily step count when compared to the control peers. Furthermore, the emotional and physical dimensions of CAD health-related QoL were reported to be better among the intervention patients.
Frederix et al. also evaluated the personalized PA intervention in 80 ACS patients [23]. The patients were randomized to either personalized PA intervention (n = 40) or usual care (n = 40). PA intervention was based on weekly progressing, personalized daily step count goals monitored with readable tri-axial accelerometers during 18 weeks. After the study period, maximal oxygen consumption in the intervention patients increased on average 4 ml/min/kg when compared to baseline, whereas no significant change was found among the control patients. In addition, the intervention patients had lower incidence of rehospitalizations when compared to the control peers (12.5% vs. 26.5%). The intervention was also found cost-effective: The total costs for an intervention patient were on average 564.40 € lower than for a control patient [24].
Lawler et al. reported a meta-analysis of 64 studies and 6111 post-infarction patients randomized to minimum of 2 weeks exercise-based rehabilitation intervention or usual care [25]. Rehabilitation intervention was associated with 47% reduction of reinfarctions and 26% reduction of all-cause mortality.
Despite the potentially positive effects of exercise, there are also contradictory studies. In a multicenter RAMIT trial of 1813 patients, cardiac rehabilitation, including exercise training as well as education and counseling about CAD and its risk factors during 6–8 weeks, was evaluated [26]. After a 24-month follow-up, rehabilitation was not found to have significant effect on morbidity or mortality.
In prospective observational studies, physically active lifestyle has also been found beneficial among ACS patients [27,28]. In a sample of 856 post-ACS women, Gorczyca et al. found that the patients who were able to increase their PA to the level suggested by PA guidelines (from <150 min/week to at least 150 min moderate PA/week) had on average 46% lower mortality than their peers who remained inactive (<150 min moderate PA/week) [27]. Furthermore, those who remained physically active after ACS (at least 150 min moderate PA/week) had 48% lower mortality than their physically inactive peers. In a sample of 4174 post-ACS patients, Booth et al. observed that physically active patients (intensive PA at least 4 times weekly) had 29% lower mortality than their low PA peers (no intensive PA weekly) after a follow-up of 54 months [28]. Furthermore, PA was found especially beneficial when it was combined to healthy diet and smoking cessation.
3.4. Exercise intervention and physical activity after revascularization
Moholdt et al. compared the effectiveness of continuous exercise training (n = 28) and aerobic interval training (n = 31), performed at 70% and 90% of maximum heart rate, respectively, in CAD patients with recent CABG (4–16 weeks previously) [29]. Training was scheduled 5 times/week during 4 weeks in supervised conditions and at home thereafter for 6 months. After 4-weeks of intervention, maximal oxygen consumption was significantly improved in both groups (Continuous exercise: from 26.2 ml/kg/min to 28.5 ml/kg/min and Interval training: from 27.1 ml/kg/min to 30.4 ml/kg/min). In the interval training group maximal oxygen consumption increased further at 6 months (32.2 ml/kg/min) but no more in the continuous exercise group (29.5 ml/kg/min). Neither continuous training nor aerobic interval training was associated with improvement of cardiac systolic or diastolic function.
The effect of exercise intervention was studied among 118 CAD patients scheduled for elective PCI [30]. Patients undergoing PCI of one or two native coronary arteries were randomized to exercise intervention (n = 59) or usual care (n = 56). The intervention included supervised exercise sessions, e.g. bicycle ergometer training, three times a week in the hospital gym over 6 months. The incidence of CAD events and rehospitalizations was significantly lower among the intervention patients when compared to the control peers during a follow-up of 33 months (11.9% vs. 32.2%, respectively). In addition, exercise intervention was associated with improved functional capacity and QoL, whereas no change was found in the control group.
Munk et al. randomized 40 post-PCI patients to high-intensity interval exercise intervention or usual care [31]. After a 6-month follow-up, the intervention patients had lower late luminal loss of their stented coronary segment than their control peers (0.1 vs. 0.4 mm, respectively). In addition, a greater improvement in maximal oxygen consumption was reported among intervention patients (16.8% vs. 7.8%).
In the study by Higgins et al. post-PCI patients were randomized to rehabilitation intervention at home (n = 50) or usual care (n = 49) [32]. Rehabilitation included individualized exercise, risk factor modification and psychosocial counseling. During a follow-up of 12 months, the patients randomized to intervention had improved functional capacity and shorter sick leaves when compared to their control peers.
Mutwalli et al. compared the effects of 6-month home-based cardiac rehabilitation intervention (n = 28) and usual care (n = 21) among post-CABG patients [33]. When compared to usual care, the intervention significantly improved patients’ risk factor profiles (e.g. fasting glucose, triglycerides, high-density lipoprotein cholesterol) and QoL.
3.5. The association of physical activity with biomarkers and cardiac structure
Bouillon et al. studied the influence of PA on serum low-density lipoprotein (LDL) cholesterol in a long-term prospective cohort study with 4469 civil servants [34]. PA was associated with 0.10 mmol/l decrease in serum LDL cholesterol. As comes to HDL, a moderate, 0.042 mmol/l increase in serum HDL cholesterol was reported for each additional hour of weekly intensive exercise [35]. In the study by Bakrania et al. each 30min/day increase in moderate to vigorous PA (MVPA) was associated with 0.07% decrease in plasma HbA1c among non-diabetic subjects [36].
In a large population-based cohort (n = 125 402) Byambasukh et al. studied the effect of MVPA on blood pressure [37]. They found that the participants in the highest MVPA tertile had −2.90 mmHg lower systolic blood pressure and −1.50 mmHg diastolic blood pressure when compared to their peers with no MVPA after adjustment with other risk factors of hypertension. In addition to blood pressure, light exercise at least once a week reduced the risk for developing LVH by 76% when compared to peers with no regular exercise [38].
3.6. Sedentary behavior in patients with coronary artery disease
In a cross-sectional sample of 263 CAD patients with recent ACS, PCI or CABG, Prince et al. found a significant association between high accumulated SB time and poor postoperative cardiorespiratory fitness when adjusted for age, accumulated moderate-to vigorous PA and drug therapy [39].
In a prospective observational sample of more than 100 000 MI survivors, the patients with high SB (4–8 h/day) had 62% higher mortality when compared to their peers with low SB (<4 h/day) [40]. Furthermore, the post-MI patients with high SB time and inadequate PA had 174% higher mortality compared to their physically active peers with low SB. Supporting these results, Gorczyca et al. found in a prospective observational study of 856 post-ACS women that each additional daily hour of SB was associated with 9% higher mortality [27].
4. Discussion
The positive influence of exercise (e.g. including walking, bicycle ergometer training) is widely recognized as an essential part of the lifestyle therapy of patients with CAD [14,19,29,[41], [42], [43]]. However, some of them, e.g. the meta-analysis by Anderson et al. have included publications from the 70’s or 80’s [14]. Since then the secondary prevention of CAD has enhanced with marked effect on patient’s prognosis. Therefore, in this review article, we included only studies published since 2001, i.e. during the era of modern medical therapy for CAD including the treatments with aspirin, statins, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, ADP-receptor inhibitors and direct oral anticoagulants.
Exercise training has been found to induce positive effects on cardiorespiratory fitness, and QoL among patients with stable CAD as well as in patients with recent ACS or recent revascularization [12,13,19,29,41,42]. Aerobic interval training or high intensity interval training has been considered especially beneficial [15,29]. However, the results indicating positive influence of exercise training are mostly based on rather limited follow-up, such as 12 months or less. In addition, the patients often do not continue training after the exercise intervention period and, as a result, the beneficial effect tends to disappear [13]. Thus, there seems to be a need for training programs that motivate the patients to continue training in long-term.
There is some evidence that exercise intervention, compared to standard care, improves cardiac systolic function in patients recovering from ACS [20]. However, the effects, on e.g. LVEF, have remained only modest but potentially positive effects of exercise training on cardiac systolic function have been reported among patients with heart failure [44]. Contradictory results have also been reported. Moholdt et al. were not able to find exercise-induced improvement in cardiac function in patients recovering from CABG [29]. In addition, spontaneous recovery of LVEF occurs frequently after acute myocardial infarction. A mean improvement of 8% in LVEF was reported among patients recovering from myocardial infarction during the first eight post-MI weeks [45]. Thus, also in this context, longer exercise/PA interventions are needed to reveal the possible effects of exercise on ACS patient’s cardiac systolic, and especially, diastolic function.
There is also some evidence that regular moderate exercise can reduce rehospitalizations among patients with stable CAD, those recovering from recent ACS or recent revascularization [12,14,23,30]. Reduced need for rehospitalizations has further led to lower costs and improved cost-effectiveness of CAD therapy [12,23,24,30]. In addition, somewhat lower health care costs have been reported also in those studies without significant changes in incidence of rehospitalizations or mortality [19].
Most importantly, in a large meta-analysis, exercise training was found to reduce cardiovascular mortality among CAD patients [14]. However, the meta-analysis included studies also from the 70s and 80s. The development of CAD management, e.g. modern medical therapy, has improved since those days. However, the most recent CAD exercise guidelines by European Society of Cardiology (ESC) support the concept of exercise-induced prognosis-enhancing effects on CAD patients [43]. Updated information about exercise training’s effects on CAD patient’s prognosis is still required. Additionally, there are studies that have not found exercise or rehabilitation interventions to influence morbidity or mortality [19,26]. However, some of them are based on relatively short interventions [26]. So far, the prognostic effect of exercise intervention among CAD patients (varying from zero effect to 26% reduction in cardiovascular mortality) has remained only modest when compared to other commonly applied prognostic therapies, such as statins (30% reduction in mortality) or acetylsalicylic acid (35–50% reduction in mortality) [14,46,47]. However, the benefit of exercise training is close to 17% reduction in cardiovascular mortality reported with angiotensin convertase enzyme inhibitors and angiotensin receptor blockers in CAD patients with preserved ejection fraction [48]. More long-term evidence from the era of modern medical therapy for CAD is required to assess the symptomatic and especially prognostic effects of exercise training among patients with CAD and recent ACS or revascularization.
Exercise and PA have been reported to be safe in CAD patients, also among the ones recovering from ACS [[49], [50], [51]]. In a sample of 2351 patients recovering from coronary stenting, submaximal exercise training on the first postoperative day did not increase stent thrombosis or other complications in one-month follow-up compared to controls [49]. In a smaller sample of 47 post-ACS patients, both maximal-intensity and high-intensity aerobic training were not associated with cardiovascular complications (e.g. arrythmias) after adaptation training of two post-ACS weeks [50]. Exercise testing was also found safe when started within 3 days after acute myocardial infarction [51]. In addition, aerobic exercise training started two weeks after cardiac surgery has been reported to be safe [52].
However, it should be noted that myocardial ischemia is most commonly responsible for cardiac events occurring during exercise [43]. Especially, exercise requiring vigorous effort can increase the acute risk of ischemic cardiac events among CAD patients, even ones with stable/chronic disease. In addition, as suggested by recent ESC guidelines, the potential benefits of PA and exercise are far more important than the risk of exercise-induced adverse events among CAD patients. This perspective has been supported by most of the previous studies, reporting favorable outcomes and low incidence of adverse cardiac events among CAD patients randomized to exercise interventions [14,15,17,50,51].
According to the recent ESC guidelines, exercise-related risks should be assessed when designing exercise interventions for CAD patients both before beginning of the exercise but also during the follow-up [43]. This should be done particularly when there are any signs of disease progression. The guidelines also recommend tailored exercise programs for CAD patients with different demographical background and disease severity [43]. Up-to-date technology may provide tools for providing personalized exercise programs based on individual risk analysis of exercise-induced adverse events [23,53].
Patients with recent ACS or stroke are at high risk of being sedentary [40,54]. However, there are still only limited data addressing the association between SB and clinical endpoints, such as rehospitalizations, morbidity and mortality [14,23,27]. In addition, the causative role of SB on clinical outcome has remained elusive.
Most solid evidence about the benefits of exercise intervention is based on supervised or highly structured rehabilitation interventions [12,14,26]. However, the intensity of PA in these rehabilitation programs often do not take into account patient’s habitual activity (e.g. walking or cycling to work, choosing stairs instead of an elevator, etc.) or SB profile. In addition, supervised exercise interventions require remarkable financial resources for organizing training facilities and exercise professionals. More recently, there are reports suggesting that besides structured and supervised exercise interventions, habitual PA is beneficial and associated with lower mortality among CAD patients, especially those with recent ACS [27,28]. Some recent studies have used remote access tools, such as telerehabilitation and Internet applications, to supervise exercise and provide PA guidance for CAD patients [16,22]. Despite their beneficial effect on PA behavior, there is only limited data on the effects of remote PA interventions on patient’s morbidity or mortality. Only recently, PA interventions based on personalized PA goals and objective measurement of their fulfillment have reduced rehospitalizations and improved cost-effectiveness of therapy [23,24]. Novel interventions applying online connections (e.g. Bluetooth-based devices) to provide PA guidance and accelerometer data to monitor attainment of these goals should be devised to facilitate postoperative rehabilitation after revascularization [53]. In addition, online applications should be used to forward data on PA and SB to the health care providers. Objective accelerometer-based techniques are considered as the state-of-the-art technology in modern activity monitoring [2, 55].
5. Conclusions
Regular physical activity has been found beneficial and safe in different CAD groups, such as patients with stable CAD, recent ACS, or recent revascularization. Exercise intervention is an effective tool not only to increase PA, but also to improve cardiorespiratory fitness and QoL, reduce rehospitalizations, and most importantly improve prognosis. However, supervised exercise interventions as a part of large-scale rehabilitation program carry several challenges. They require training facilities, professional exercise specialists and major financial resources. In addition, motivating patients, often elderly, to participate regularly in supervised exercise programs can be difficult. For example, long distance to rehabilitation center can be a major limitation. The development of online applications and smart phones provides novel tools for effective and cost-effective online tutoring of CAD patients at home. Furthermore, novel accelerometer and cloud-based services and applications allow objective monitoring of patient’s habitual activity online, which further enhance the accuracy of activity tracking. In addition, by employing these modern approaches, patient’s habitual activity and SB profile can be monitored over long period of time. Interventions using modern information technologies, such as smartphone and interactive accelerometers that allow 24/7 follow-up and personalized PA and SB goals, may well facilitate exercise therapy programs of CAD patients. Adequately-powered long-term RCTS of CAD patients are needed to demonstrate these anticipated benefits.
Author contributions
Ville Vasankari: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Roles/Writing - original draft; Jari Halonen: Conceptualization, Project administration, Writing - review & editing; Tommi Vasankari: Conceptualization, Project administration, Writing - review & editing. Vesa Anttila: Conceptualization, Project administration, Writing - review & editing. Juhani Airaksinen: Conceptualization, Project administration, Writing - review & editing. Harri Sievänen: Conceptualization, Methodology, Project administration, Writing - review & editing. Juha Hartikainen: Conceptualization, Methodology, Project administration, Writing - review & editing.
Funding
This research did not receive any specific grant from any funding agencies.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None to declare.
Abbreviations
- CABG
Coronary artery bypass grafting
- CVD
Cardiovascular disease
- HbA1c
Glycated haemoglobin
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- PCI
Percutaneous coronary intervention
- QoL
Quality of life
- VO2
Maximal oxygen consumption
- WT
Walk test
References
- 1.Roth G.A., Johnson C., Abajobir A. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017 Jul 4;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strath S.J., Kaminsky L.A., Ainsworth B.E. American heart association physical activity committee of the council on lifestyle and cardiometabolic health and cardiovascular, exercise, cardiac rehabilitation and prevention committee of the council on clinical cardiology, and council. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American heart association. Circulation. 2013 Nov 12;128(20):2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 3.Lee I.M., Shiroma E.J., Lobelo F. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012 Jul 21;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasankari V., Husu P., Vähä-Ypyä H. Association of objectively measured sedentary behaviour and physical activity with cardiovascular disease risk. Eur J Prev Cardiol. 2017 Aug;24(12):1311–1318. doi: 10.1177/2047487317711048. [DOI] [PubMed] [Google Scholar]
- 5.Vasankari V., Husu P., Vähä-Ypyä H. Subjects with cardiovascular disease or high cardiovascular disease risk are more sedentary and less physically active than their healthy peers. BMJ Open Sport Exerc Med. 2018;4 doi: 10.1136/bmjsem-2018-000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey A., Salahuddin U., Garg S. Continuous dose-response association between sedentary time and risk for cardiovascular disease: a meta-analysis. JAMA Cardiol. 2016 Aug 1;1(5):575–583. doi: 10.1001/jamacardio.2016.1567. [DOI] [PubMed] [Google Scholar]
- 7.Qi Q., Strizich G., Merchant G. Objectively measured sedentary time and cardiometabolic biomarkers in US hispanic/latino adults: the hispanic community health study/study of latinos (HCHS/SOL) Circulation. 2015 Oct 20;132(16):1560–1569. doi: 10.1161/CIRCULATIONAHA.115.016938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Task Force Members. Montalescot G., Sechtem U., Achenbach S., ESC Committee for Practice Guidelines. Zamorano J.L., Achenbach S., Baumgartner H., Bax J.J., Bueno H., Dean V. ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. Oct. [DOI] [PubMed] [Google Scholar]
- 9.Fihn S.D., Blankenship J.C., Alexander K.P. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. 2014;130(19):1749–1767. doi: 10.1161/CIR.0000000000000095. 2014 Nov 4. [DOI] [PubMed] [Google Scholar]
- 10.Piercy K.L., Troiano R.P., Ballard R.M. The physical activity guidelines for Americans. J. Am. Med. Assoc. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mok Y., Sang Y., Ballew S.H. American heart association’s life’s simple 7 at middle age and prognosis after myocardial infarction in later life. J Am Heart Assoc. 2018 Feb 17;7(4) doi: 10.1161/JAHA.117.007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hambrecht R., Walther C., Möbius-Winkler S. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004 Mar 23;109(11):1371–1378. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 13.Oerkild B., Frederiksen M., Hansen J.F., Prescott E. Home-based cardiac rehabilitation is an attractive alternative to no cardiac rehabilitation for elderly patients with coronary heart disease: results from a randomised clinical trial. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson L., Oldridge N., Thompson D. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane systematic review and meta-analysis. J. Am. Coll. Cardiol. 2016 Jan 5;67(1):1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Gomes-Neto M., Duraes A., Reis H., Neves V., Martinez B., Carvalho V. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017 Nov;24(16):1696–1707. doi: 10.1177/2047487317728370. [DOI] [PubMed] [Google Scholar]
- 16.Maddison R., Pfaeffli L., Whittaker R. A mobile phone intervention increases physical activity in people with cardiovascular disease: results from the HEART randomized controlled trial. Eur J Prev Cardiol. 2015;22:701–709. doi: 10.1177/2047487314535076. [DOI] [PubMed] [Google Scholar]
- 17.Rawstorn J.C., Gant N., Direito A., Beckmann C., Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183–1192. doi: 10.1136/heartjnl-2015-308966. [DOI] [PubMed] [Google Scholar]
- 18.La Rovere T., Bersano J., Gnemmi J., Specchia J., Schwartz J. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106(8):945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 19.Yu C.M., Lau C.P., Chan J. A short course of cardiac rehabilitation program is highly cost effective in improving long-term quality of life in patients with recent myocardial infarction or percutaneous coronary intervention. Arch. Phys. Med. Rehabil. 2004;85:1915–1922. doi: 10.1016/j.apmr.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Xu L., Cai Z., Xiong M. Efficacy of an early home-based cardiac rehabilitation program for patients after acute myocardial infarction: a three-dimensional speckle tracking echocardiography randomized trial. Medicine (Baltim.) 2016 Dec;95(52) doi: 10.1097/MD.0000000000005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchionni N., Fattiolli F., Fumagalli S. Improved exercise tolerance and quality of life with cardiac rehabilitation of older patients after myocardial infarction. Circulation. 2003 May 6;107:2201–2206. doi: 10.1161/01.CIR.0000066322.21016.4A. [DOI] [PubMed] [Google Scholar]
- 22.Reid R.D., Morrin L.I., Beaton L.J. Randomized trial of an internet-based computer tailored expert system for physical activity in patients with heart disease. Eur J Prev Cardiol. 2012;19:1357–1364. doi: 10.1177/1741826711422988. [DOI] [PubMed] [Google Scholar]
- 23.Frederix I., Van Driessche N., Hansen D. Increasing the medium-term clinical benefits of hospital-based cardiac rehabilitation by physical activity telemonitoring in coronary artery disease patients. Eur J Prev Cardiol. 2015 Feb;22(2):150–158. doi: 10.1177/2047487313514018. [DOI] [PubMed] [Google Scholar]
- 24.Frederix I., Hansen D., Coninx K. Effect of comprehensive cardiac telerehabilitation on one-year cardiovascular rehospitalization rate, medical costs and quality of life: a cost-effectiveness analysis. Eur J Prev Cardiol. 2016 May;23(7):674–682. doi: 10.1177/2047487315602257. [DOI] [PubMed] [Google Scholar]
- 25.Lawler P.R., Filion K.B., Eisenberg M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction A systematic review and meta-analysis of randomized controlled trials. Am. Heart J. 2011 Oct;162(4):71–84. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 26.West R.R., Jones D.A., Henderson A.H. Rehabilitation after myocardial infarction trial (RAMIT): multi-center randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart. 2012;98:637–644. doi: 10.1136/heartjnl-2011-300302. [DOI] [PubMed] [Google Scholar]
- 27.Gorczyca A.M., Eaton C.B., LaMonte M.J. Change in physical activity and sitting time after myocardial infarction and mortality among postmenopausal women in the women’s health initiative-observational study. J Am Heart Assoc. 2017 May 15;6(5) doi: 10.1161/JAHA.116.005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booth J.N., 3rd, Levitan E.B., Brown T.M., Farkouh M.E., Safford M.M., Muntner P. Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the REasons for Geographic and Racial Differences in Stroke Study) Am. J. Cardiol. 2014 Jun 15;113(12):1933–1940. doi: 10.1016/j.amjcard.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moholdt T.T., Amundsen B.H., Rustad L.A. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am. Heart J. 2009 Dec;158(6):1031–1037. doi: 10.1016/j.ahj.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Belardinelli R., Paolini I., Cianci G., Piva R., Georgiou D., Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J. Am. Coll. Cardiol. 2001;37:1891–1900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- 31.Munk P.S., Staal E.M., Butt N., Isaksen K., Larsen A.I. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation: a randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am. Heart J. 2009 Nov;158(5):734–741. doi: 10.1016/j.ahj.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Higgins H.C., Hayes R.L., McKenna K.T. Rehabilitation outcomes following percutaneous coronary intervention. Patient Educ. Counsel. 2001;43:219–230. doi: 10.1016/s0738-3991(00)00164-6. [DOI] [PubMed] [Google Scholar]
- 33.Mutwalli H.A., Fallows S.J., Arnous A.A., Zamzami M.S. Randomized controlled evaluation shows the effectiveness of a home-based cardiac rehabilitation program. Saudi Med. J. 2012;33:152–159. [PubMed] [Google Scholar]
- 34.Bouillon K., Singh-Manoux A., Jokela M. Decline in low-density lipoprotein cholesterol concentration: lipid-lowering drugs, diet, or physical activity? Evidence from the Whitehall II study. Heart. 2011 Jun;97(11):923–930. doi: 10.1136/hrt.2010.216309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raitakari O.T., Taimela S., Porkka K.V., Viikari J.S. Effect of leisure-time physical activity change on high-density lipoprotein cholesterol in adolescents and young adults. Ann. Med. 1996 Jun;28(3):259–263. doi: 10.3109/07853899609033128. [DOI] [PubMed] [Google Scholar]
- 36.Bakrania K., Yates T., Edwardson C.L. Associations of moderate-to-vigorous-intensity physical activity and body mass index with glycated haemoglobin within the general population: a cross-sectional analysis of the 2008 Health Survey for England. BMJ Open. 2017 Apr 3;7(4) doi: 10.1136/bmjopen-2016-014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byambasukh O., Snieder H., Corpeleijn E. Relation between leisure time, commuting, and occupational physical activity with blood pressure in 125 402 adults: the lifelines cohort. J Am Heart Assoc. 2020 Feb 18;9(4) doi: 10.1161/JAHA.119.014313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palatini P., Visentin P., Dorigatti F. Regular physical activity prevents development of left ventricular hypertrophy in hypertension. Eur. Heart J. 2009 Jan;30(2):225–232. doi: 10.1093/eurheartj/ehn533. [DOI] [PubMed] [Google Scholar]
- 39.Prince S.A., Blanchard C.M., Grace S.L., Reid R.D. Objectively-measured sedentary time and its association with markers of cardiometabolic health and fitness among cardiac rehabilitation graduates. Eur J Prev Cardiol. 2016 May;23(8):818–825. doi: 10.1177/2047487315617101. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z., Huang Z., Wu Y. Sedentary time, metabolic abnormalities, and all-cause mortality after myocardial infarction: a mediation analysis. Eur J Prev Cardiol. 2019;26(1):96–104. doi: 10.1177/2047487318804611. [DOI] [PubMed] [Google Scholar]
- 41.Wang W., Chair S.Y., Thompson D.R., Twinn S.F. Effects of home-based rehabilitation on health-related quality of life and psychological status in Chinese patients recovering from acute myocardial infarction. Heart Lung. 2012;41:15–25. doi: 10.1016/j.hrtlng.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Briffa T.G., Eckermann S.D., Griffiths A.D. Cost-effectiveness of rehabilitation after an acute coronary event: a randomized controlled trial. Med. J. Aust. 2005;183:450–455. doi: 10.5694/j.1326-5377.2005.tb07121.x. [DOI] [PubMed] [Google Scholar]
- 43.Pelliccia A., Sharma S., Gati S. ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021 Jan 1;42(1):17–96. doi: 10.1093/eurheartj/ehaa605. 2020 Aug 29: ehaa605. [DOI] [PubMed] [Google Scholar]
- 44.Haykowsky M.J., Liang Y., Pechter D., Jones L.W., McAlister F.A., Clark A.M. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J. Am. Coll. Cardiol. 2007 Jun 19;49(24):2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 45.Exner D.V., Kavanagh K.M., Slawnych M.P. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J. Am. Coll. Cardiol. 2007 Dec 11;50(24):2275–2284. doi: 10.1016/j.jacc.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 46.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994 Nov 19;344(8934):1383–1389. [PubMed] [Google Scholar]
- 47.Gum P.A., Thamilarasan M., Watanabe J., Blackstone E.H., Lauer M.S. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: a propensity analysis. J. Am. Med. Assoc. 2001 Sep 12;286(10):1187–1194. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]
- 48.Hoang V., Alam M., Addison D., Macedo F., Virani S., Birnbaum Y. Efficacy of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers in coronary artery disease without heart failure in the modern statin era: a meta-analysis of randomized-controlled trials. Cardiovasc. Drugs Ther. 2016 Apr;30(2):189–198. doi: 10.1007/s10557-016-6652-7. [DOI] [PubMed] [Google Scholar]
- 49.Soga Y., Yokoi H., Ando K. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur. J. Cardiovasc. Prev. Rehabil. 2010 Apr;17(2):230–234. doi: 10.1097/HJR.0b013e3283359c4e. [DOI] [PubMed] [Google Scholar]
- 50.Kim C., Choi H.E. The effect and safety of aerobic interval training according to exercise intensity in acute coronary syndrome. J Cardiopulm Rehabil Prev. 2020 May;40(3):178–182. doi: 10.1097/HCR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 51.Senaratne M.P., Smith G., Gulamhusein S.S. Feasibility and safety of early exercise testing using the Bruce protocol after acute myocardial infarction. J. Am. Coll. Cardiol. 2000 Apr;35(5):1212–1220. doi: 10.1016/s0735-1097(00)00545-3. [DOI] [PubMed] [Google Scholar]
- 52.Doyle M., Indraratna P., Tardo D., Peeceeyen S., Peoples G. Safety and efficacy of aerobic exercise commenced early after cardiac surgery: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(1):36–45. doi: 10.1177/2047487318798924. [DOI] [PubMed] [Google Scholar]
- 53.Vasankari V., Halonen J., Husu P. Personalised eHealth intervention to increase physical activity and reduce sedentary behaviour in rehabilitation after cardiac operations: study protocol for the PACO randomised controlled trial ( NCT03470246) BMJ Open Sport Exerc Med. 2019;5 doi: 10.1136/bmjsem-2019-000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tieges Z., Mead G., Allerhand M. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch. Phys. Med. Rehabil. 2015 Jan;96(1):15–23. doi: 10.1016/j.apmr.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Sievänen H., Kujala U.M. Accelerometry-Simple, but challenging. Scand. J. Med. Sci. Sports. 2017 Jun;27(6):574–578. doi: 10.1111/sms.12887. [DOI] [PubMed] [Google Scholar]