Abstract
With the global spread of abdominal obesity, cardiovascular disease continues to spread to all countries of the world. Given the large population, the challenges presented by cardiometabolic risk in the Asia Pacific region are considerable. In addition to the clinical consequences of cardiovascular disease, in terms of its morbidity and mortality, the diversity of the Asia Pacific region brings heterogeneity in approaches to prevention, diagnosis and treatment of cardiometabolic risk. In this manuscript, we will review the current state of knowledge of cardiometabolic risk in Asia Pacific and highlight the needs moving forward to tackle this public health challenge.
Keywords: Cardiovascular disease, Risk factors, Hypertension, Diabetes, Dyslipidemia, Asia pacific
1. Introduction
Cardiovascular disease (CVD) continues to be a leading cause of morbidity and mortality worldwide (Fig. 1). On the basis of large epidemiology studies, a number of metabolic risk factors have been identified to associate with CV risk and have been the primary focus of efforts to predict and prevent the risk of clinical complications. The beneficial effects of randomized controlled trials of therapies targeting cardiometabolic risk factors have been increasingly incorporated into clinical guidelines for disease prevention and treatment.
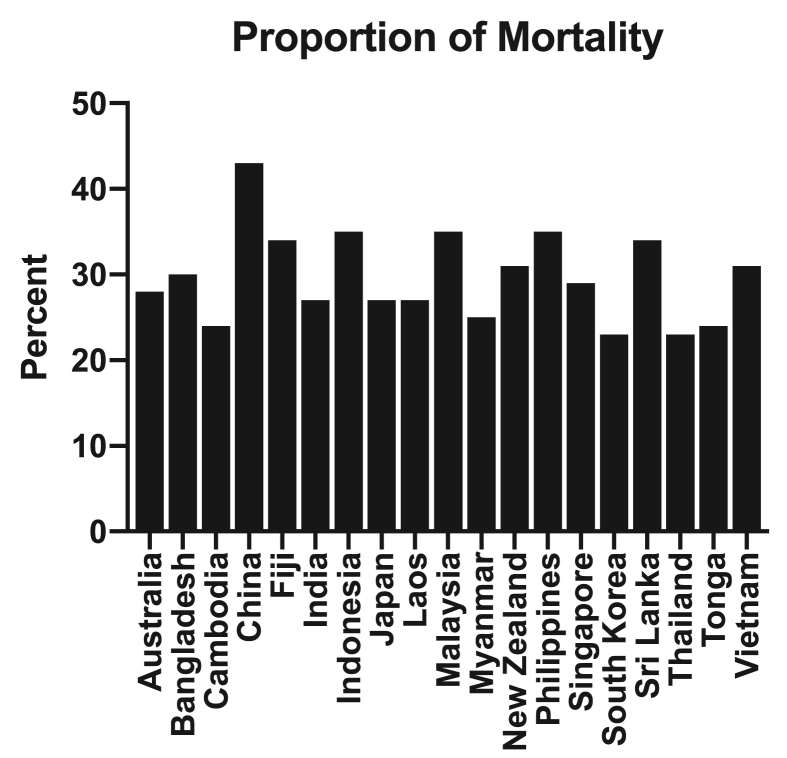
Fig. 1.
Proportion of all deaths attributed to cardiovascular death annually in a range of countries across the Asia Pacific region [96].
In more recent years, increasing concerns have been raised due to the rise in presence of cardiometabolic disease in the Asia Pacific region, accompanying the adoption of western lifestyle habits and the spread of abdominal adiposity (Fig. 2, Fig. 3, Fig. 4). Whether this relates specifically to the diagnosis of the metabolic syndrome, which can vary according to definition, or simply reflects the contribution of the individual risk factors is unknown. This has implications as the metabolic syndrome is an actionable diagnosis in Japan, with initiatives in place to facilitate both its detection and preventing its progression [1]. Given the large population of this region, the potential health, economic and social consequences are substantial. Furthermore, the diversity of the region, in terms of language, economics and access to appropriate preventive and therapeutic strategies, highlights the need to develop more effective and comprehensive approaches to the management of cardiometabolic risk. As a result, we convened a group of academic physicians with a major interest in cardiometabolic risk to discuss strategies to more effectively target cardiometabolic risk in Asia Pacific. In this review, we have attempted to define the scope of the problem, what is known in the region and importantly limitations in our knowledge, in order to identify novel steps to improve disease prevention.
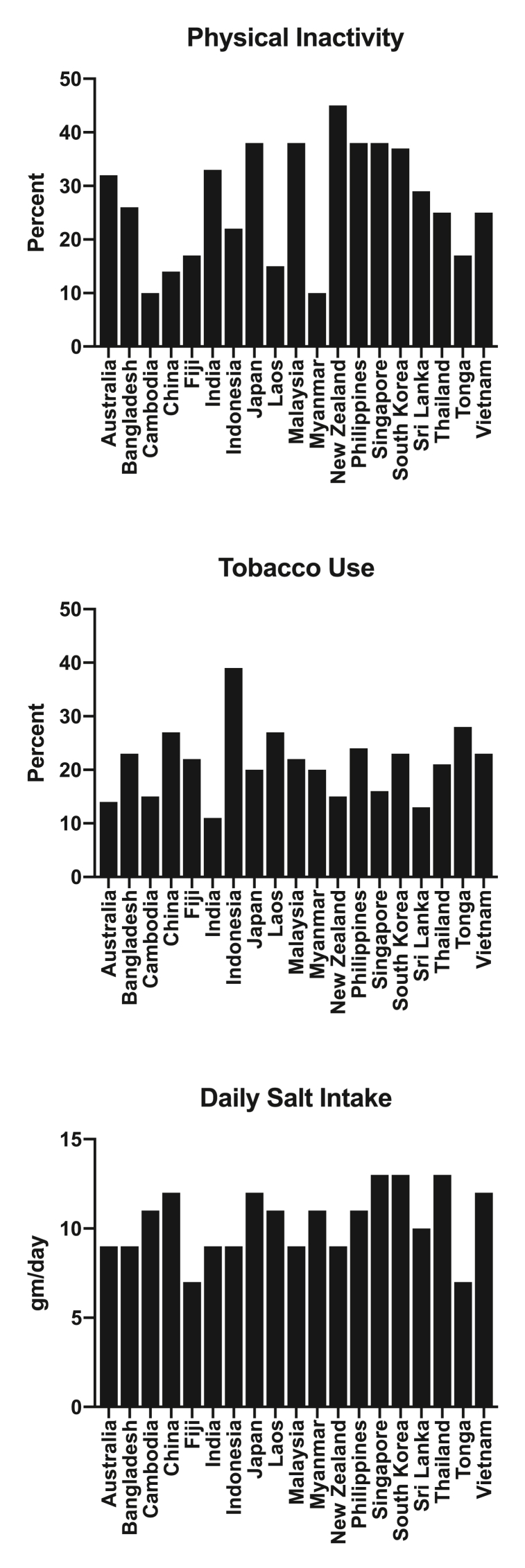
Fig. 2.
Proportion of adults with physical inactivity (top panel), tobacco use (middle panel) and average daily salt consumption (lower panel) in a range of countries across the Asia Pacific region [96].
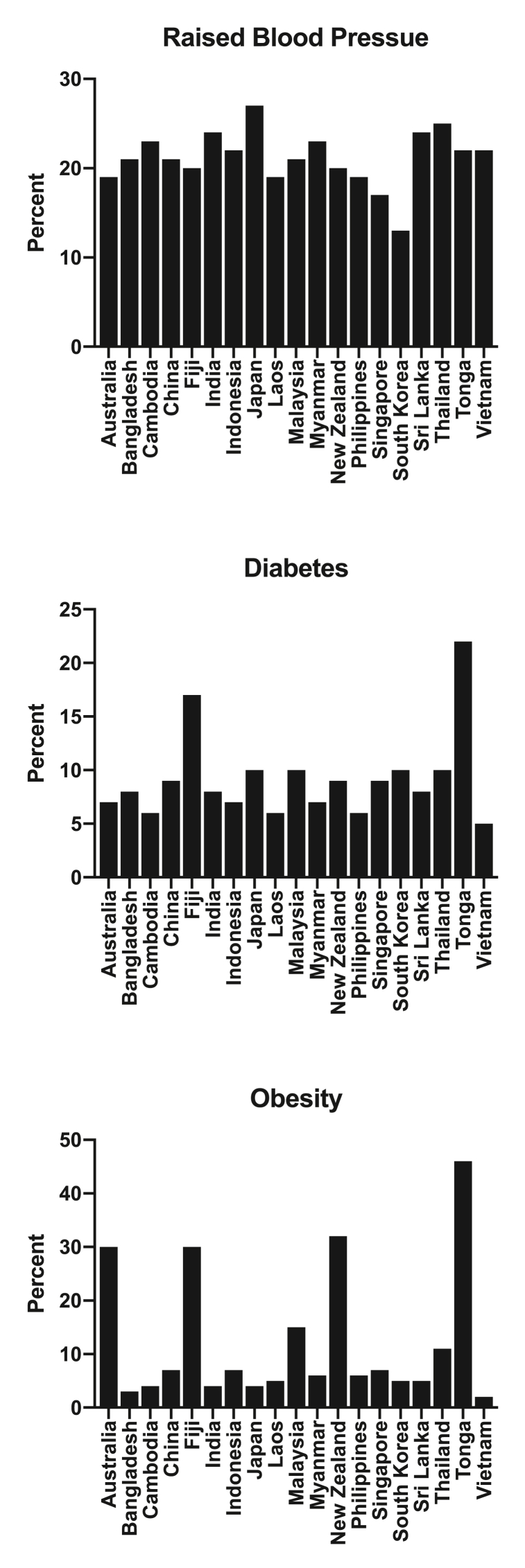
Fig. 3.
Proportion of adults with raised blood pressure (top panel), diabetes (middle panel) and obesity (lower panel) in a range of countries across the Asia Pacific region [96].
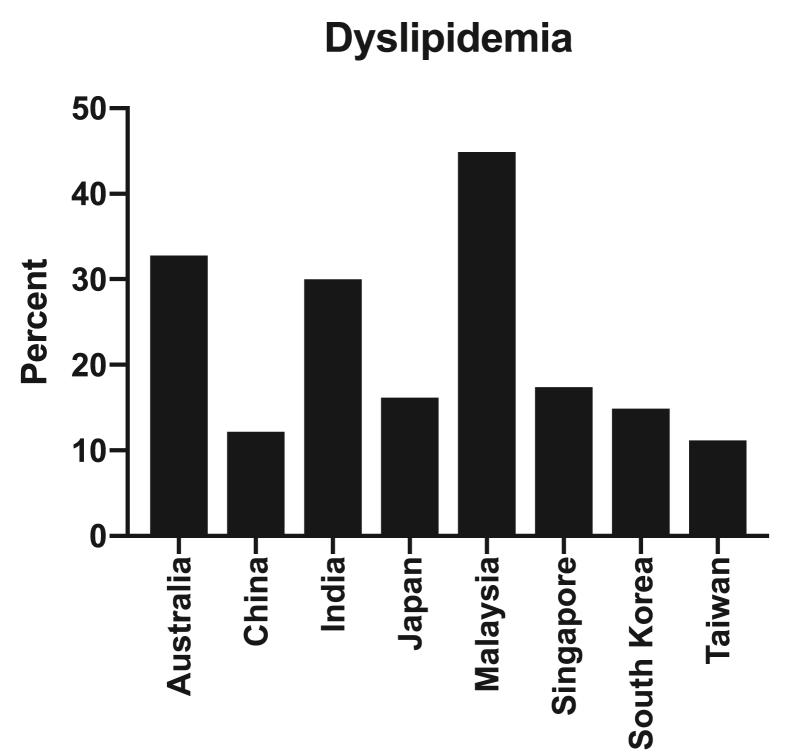
Fig. 4.
Proportion of adults with dyslipidemia (defined as either a total cholesterol ≥6.2 mmol/L or 240 mg/dL) in a range of countries across the Asia Pacific region.
1.1. Obesity
Increasing rates of adiposity has been attributed to a range of chronic disease complications worldwide. Global studies estimate a prevalence of overweight in 39% of adults and obesity in 13% [2]. This is likely to worsen with estimates that nearly one in five adolescents are overweight. Asia Pacific demonstrates marked heterogeneity across the region with regard to both the prevalence of obesity and its rate of consequent fatal complications, ranging from rates less than 5% in South Korea, Japan and Singapore through to more than 35% of individuals in the Middle East and Polynesia [2]. Since 1975 this has more than doubled in the majority of countries across the Asia Pacific region [2]. These changes have occurred as a consequence of a broad increase in daily caloric intake, not matched by an appropriate increase in energy expenditure. As obesity associates with the full complement of cardiometabolic risk factors, there remain major efforts to tackle adiposity from both a public health and interventional perspective. This has led to efforts across the region that attempt to promote both the availability and consumption of more healthy diets and looking to increasing daily exercise. At a medical level, a range of pharmacotherapies have been developed with the aim to both promote weight loss and reduce cardiovascular risk, although the later has proven difficult to establish in large clinical trials to date [3]. Contemporary trials of glucagon-like 1 peptide receptor agonists which have been demonstrated to promote weight loss, improve glycemic control and reduce cardiovascular risk in patients with type 2 diabetes [4] are now being investigated in the setting of overweight and obesity without diabetes [3]. For those patients with the most severe forms of obesity, bariatric surgery has now been demonstrated to not only result in weight loss, but also to improve the cardiometabolic risk profile [5]. How this translates to reductions in cardiovascular event rates remain to be determined.
1.2. Diabetes mellitus
Over the past three decades, diabetes mellitus and its associated complications have reached epidemic levels worldwide, particularly in developing countries. It has been estimated that there are 138.2 million people with diabetes living in the Western Pacific region, 90% of these being type 2 diabetes mellitus. Furthermore, the number of cases has been projected to rise to 201.8 million by 2035. Asia is one of the major epicentres of the type 2 diabetes global epidemic, with China and India having the largest number of diabetic individuals in the region. Of paramount concern is the increasing prevalence of diabetes in the region. The prevalence of diabetes in China was 0.67% in 1980, and the latest survey data from 2013 estimates that this figure has risen to 10.9%, and that for prediabetes was 35.7% [6]. Among individuals with diabetes, just 36.5% were aware of their diagnosis and only 49% of those treated had adequate glycemic control. In India, data from a population-based cross-sectional study reported in 2017 that the national prevalence of diabetes was 7.3% and prediabetes was 10.3%, and almost half of the individuals identified with diabetes in the study were previously undiagnosed [7]. Moreover, the rapid growth in the prevalence of type 2 diabetes among adolescents and young adults in South-East Asia and the Western Pacific regions is particularly concerning [8].
The rising prevalence of diabetes in Asia has been attributed to the changes in lifestyle associated with rapid industrialization and urbanization interacting with genetic and epigenetic factors. In comparison with Caucasians, Asians tend to develop diabetes at a younger age (5–10 years earlier) and at a lower body mass index (BMI). Despite being less obese, Asians are more insulin resistant as they have more visceral adiposity than Caucasians regardless of BMI. In addition, beta cell dysfunction also plays an important role in the pathogenesis of diabetes in Asians, particularly in those who are not obese or have a strong family history. There is also a predisposition to impaired insulin secretion, and beta cell exhaustion is likely to occur at a younger age. Hence, Asians tend to have poorer beta-cell reserve and greater insulin resistance compared to Caucasian populations [[8], [9], [10]].
1.3. Complications of diabetes
Most patients with diabetes have at least one or more complications, with cardiovascular complications being one of the major causes of morbidity and mortality. A recent post hoc analysis from the Action in Diabetes and Vascular Disease trial (ADVANCE) has shown that overall, type 2 diabetic patients from Asian countries have a lower risk of major coronary events and peripheral vascular disease than patients from eastern Europe or established market economies [11]. On the other hand, they have a higher incidence of stroke and renal complications. Heterogeneity has been demonstrated within different regions in Asia as the susceptibility to vascular complications varies across ethnicities. There are ethnic differences in the pattern of diabetes complications, and genetic background, lifestyle and patient awareness of complications might have contributed to these ethnic differences. In the WHO Multinational Study of Vascular Disease in Diabetes, the prevalence of cardiovascular complications was high in South Asia, but lower in China, Hong Kong, and Japan [12]. These reported variations may be partly attributed to differences in the detection of coronary artery disease and in the duration of diabetes mellitus of the recruited subjects. Recent studies in multiethnic populations have also confirmed the high prevalence of cardiovascular complications among South Asian populations, with the risk of cardiovascular mortality in South Asian men being twice that of European men [13]. Furthermore, the risk of diabetic kidney disease is much higher in Asian countries than in Western countries [9,11]. In China, the rates of proteinuria and retinopathy were significantly higher compared with other centres in the WHO Multinational Study of Vascular Disease in Diabetes [14].
Since the onset age of diabetes tends to be younger in Asians, there is a large proportion of individuals with young-onset diabetes (defined as diagnosis of diabetes before 40 years of age) in Asia. Earlier onset of disease is associated with a greater lifetime risk of diabetes-associated complications. It has been shown that many of the patients with young-onset diabetes have poor metabolic profiles. Coupled with long disease duration, individuals with young-onset diabetes had a 30%–50% higher age-adjusted incidence of cardiovascular and renal events compared with those with late onset diabetes at a similar age [15]. Overall, complications such as end-stage renal disease and cardiovascular disease are common in the Asian region. These complications are costly and have a major impact both on the individual as well as the healthcare system [16].
1.4. Treatment of diabetes in Asia Pacific
The current management of diabetes in most regions of Asia is suboptimal. In China, it has been estimated that only half of patients who received treatment had adequate glycemic control. A cross-sectional, multicenter observational study in China carried out to investigate the blood glucose, blood pressure, and blood lipid control among patients with type 2 diabetes demonstrated that the proportion of patients who achieved their individual target goals for the control of blood glucose, blood pressure and blood lipids were 47.7%, 28.4%, and 36.1%, respectively. Additionally, only 5.6% of patients achieved all three target goals [17]. Poor glycemic control (70–80% above target for HbA1C control) has also been reported in India and other South Asian countries, and control of cardiovascular risk factors is poor as well [18].
In a large longitudinal cohort of Chinese adults with type 2 diabetes in Hong Kong; improving the control of risk factors demonstrated a reduction in the incidence of complications due to diabetes and mortality [19]. Over a 13-year period, the proportion of patients achieving HbA1c<7.0% increased from 32.9 to 50.0%, blood pressure ≤130/80 mmHg from 24.7 to 30.7%, and LDL cholesterol <2.6 mmol/L from 25.8 to 38.1%. A corresponding reduction in the incidence of cardio-renal complications and mortality was observed. Among the high-risk individuals with long duration diabetes (>15 years), the crude incidence of acute myocardial infarction decreased from 8.7 to 5.8, stroke from 13.5 to 10.1, end stage renal disease from 25.8 to 22.5, and death from 29.0 to 26.6 per 1000 person-year. Despite these observed improvements, considerable gaps in patient care still remain because up to 50–70% of patients did not achieve treatment targets. Though the risks of complications have fallen, the disease burden is still substantial. The decrease in the risk of complications within the diabetic population is counter-balanced by the rapid increase in the prevalence of diabetes.
1.5. Challenges for treatment of diabetes
Sustainable strategies in the prevention of diabetes addressing obesity, physical inactivity, unhealthy dietary habits and smoking are urgently needed. Lifestyle intervention, smoking cessation, health education and patient empowerment will help to reduce the burden of diabetes. Management of risk factors from diagnosis is necessary to reduce the risk of microvascular and macrovascular complications. Greater efforts are therefore required to improve control of glycemia and CV risk factors as the beneficial effects of a multifactorial intervention approach has been demonstrated in clinical studies [19,20]. In addition to statin therapy, novel anti-diabetic agents like the sodium-glucose co-transporter 2 inhibitors and GLP-1 agonists have recently been shown to confer CV benefit in type 2 diabetic patients with cardiovascular disease. However, the much greater costs of these new agents present major access issues for the majority of patients with type 2 diabetes in the region. The development of national clinical management guidelines, taking into account both social, cultural and economic factors will help to improve diabetes care in the region.
1.6. Hypercholesterolemia
Hypercholesterolemia is a major risk factor for atherosclerotic disease [21] and may contribute to observations that CVD mortality is still disproportionally high in many Asia Pacific countries [22]. Data from several large registries has demonstrated that management of CV risk factors such as hypercholesterolemia, hypertension, and diabetes mellitus is both suboptimal in patients with documented CVD and patients with risk factors without clinically manifest CVD [[23], [24], [25]]. Although it has been demonstrated that the event rate in patients with good risk factor management is lower than those without [26], the overall need for aggressive risk factor management remains high. Moreover, the prevalence of CVD risk factors such as hypertension and diabetes mellitus is increasing, thus suggesting that CVD will become a bigger problem in Asia Pacific in the future.
Data from 120 studies during 2010–2014 on the prevalence and control rate of hypercholesterolemia demonstrated that the prevalence of hypercholesterolemia was 64–74% in patients with coronary artery disease (CAD), 40–70% in patients with stroke, 60–80% in patients with peripheral arterial disease (PAD), 50–84% in patients with diabetes, and 30–60% in patients with multiple risk factors [23]. Despite a high proportion of patients taking lipid lowering medication, there was still high variability in cholesterol levels, with overall levels varying from 15 to 65%. Among patients with heterozygous familial hypercholesterolemia, more than 90% were receiving pharmacological treatment, but less than half achieved therapeutic levels. Data on secondary prevention showed that if the therapeutic goal of LDL is less than 70 mg/dl, the control rate was 5.7–14.3%. However, if the goal is an LDL less than 100 mg/dl, the rate of control was 24.1–55.7%. For type 2 diabetes mellitus, if the goal is an LDL less than 70 mg/dl, the control rate is 7.0–26.6%. When the LDL goal is 100 mg/dl, the control rate was 22.7–68.5%. These findings suggest that in secondary prevention aiming for a lower LDL level are likely to have a beneficial effect on overall serum lipid levels. Similarly, data on primary prevention in populations with high CV risk indicates that if the LDL goal is 100 mg/dl the control rate is 10.6–65.3%. When the LDL goal is 115 mg/dl the control rate was 44.7%. The data on cholesterol control rate does not differ when comparing studies using Caucasian and Asian cohorts.
An analysis of cholesterol levels among patients with established CVD and multiple risk factors in REACH registry cohort was performed [24] and showed that 38% of patients followed up at the outpatient clinic had a cholesterol level above 200 mg/dl (5.18 mmol/L) with the variability of 9.3% at the country levels. This study also demonstrated that countries with a higher gross national income had a lower odds of elevated cholesterol and countries with a higher proportion of out-of-pocket payment had a higher odds of elevated cholesterol. This finding indicated that country economics and the healthcare system may influence the achievement rate of cholesterol treatment, warranting further investigation in future studies.
The prevalence of heterozygous familial hypercholesterolemia is approximately 0.4% or 1 in 250 individuals and 1 in 200,000 for homozygous familial hypercholesterolemia [27]. The prevalence is consistent among data from different regions around the globe. Unfortunately, considerable gaps exist across the region in terms of physician awareness of the prevalence, importance and approach to diagnosis of familial hypercholesterolemia, which has important implications with regard to both early initiation of preventive therapies and cascade screening of relatives [28]. Current guidelines for the treatment of patients with familial hypercholesterolemia recommends an LDL goal less than 100 mg/dl for adults, less than 70 for those with established CVD or diabetes, and less than 135 mg/dl for children [29]. Despite the use of statin and ezetimibe, the achievement rate of LDL cholesterol reduction is still poor in this population. The development of PCSK9 inhibitors which have a potent effect on LDL cholesterol reduction will hopefully shed some light on this therapeutic area and improve outcomes for patients with familial hypercholesterolemia [29]. Beyond simplifying treatment strategies based on proposed lipid targets, comprehensive investment in developing models of care for management of familial hypercholesterolemia are urgently needed, with greater gaps identified in less economically developed countries within the region [30].
One major factor underlying inability to achieve effective LDL cholesterol lowering in high risk patients involves problems with medication tolerance and adherence. Increasing attention has recognised that many patients will stop taking statin therapy within the first year of prescription [31]. In addition to general challenges with long term medication compliance, the incidence of side effects attributed by patients to statin therapy are likely to contribute to discontinuation [32]. While the true rate of muscle injury with statin therapy remains very low, cohort studies have reported that 20–30% of patients may experience muscle pains in association with statin treatment. While blinded cross-over studies have demonstrated a fairly significant nocebo effect [33], the reality in clinical practice is that these symptoms have the potential to promote either inappropriate dose reduction or elimination of statin therapy altogether in these patients. When combined with reports of a greater incidence of diagnosis of type 2 diabetes in statin treated patients, who are likely to have had pre-diabetes prior to adminstration [34], in addition to reports of a range of other potential symptoms, particularly those related to neurocognitive function [32], patients appear to be constantly exposed to reports of negative aspects of therapy, without any attention paid to the clinical benefits, which have been unequivocally demonstrated in multiple clinical trials. This creates a major problem for effective CV risk reduction across the world, despite unequivocal evidence of their macrovascular benefit on coronary and cerebrovascular events, even in the setting of diabetes [35]. While limited information on statin intolerance and adherence is known on patients across the Asia Pacific region [36], the clinical implications of not targeting this challenge are considerable.
1.7. Lipid factors beyond LDL cholesterol
The residual risk of CV events, despite widespread use of lipid lowering agents, has highlighted the ongoing need to target risk factors beyond LDL cholesterol. Atherogenic dyslipidemia is characterized by the presence of hypertriglyceridemia, low levels of high-density lipoprotein (HDL) cholesterol and elevated circulating concentrations of small, dense LDL particles [37]. This combination of lipid abnormalities is considered to be highly atherogenic, with evidence of heightened CV risk, even when LDL cholesterol levels are at current treatment goal levels [37]. Their close association with obesity, insulin resistance and type 2 diabetes further intensifies their impact on CV risk. With increasing rates of obesity in the Asia Pacific region, the prevalence of atherogenic dyslipidemia has increased [38]. This is further compounded by the appearance of atherogenic dyslipidemia at lower BMI levels than encountered in western nations [39,40].
Treatment of atherogenic dyslipidemia has proven to be challenging. Despite widespread interest in HDL cholesterol raising, clinical trials of several classes of agents (CETP inhibitors, niacin) have been disappointing [[41], [42], [43], [44], [45], [46]]. Infusion of HDL mimetics have had variable effects on atherosclerotic plaque in studies conducted outside of the region, although the impact of one agent on cardiovascular outcomes is currently being evaluated in a large clinical trial [[47], [48], [49], [50], [51], [52], [53], [54]]. Hypertriglyceridemia, in combination with low HDL cholesterol levels, has also been targeted by a range of therapies with variable effects. Fibrates lower triglycerides and raise HDL cholesterol, with evidence of cardiovascular benefit with gemfibrozil, but not with other agents [[55], [56], [57]]. Meta-analyses have demonstrated the greatest clinical benefit of fibrates in patients with baseline hypertriglyceridemia [55]. Pioglitazone has similar lipid effects, in addition to its benefit on insulin sensitivity. While a large outcome trial failed to demonstrate benefit with pioglitazone [58], subsequent analyses revealed a reduction in clinical risk in patients with prior infarction [59] and slowing of progression of coronary atherosclerosis [60]. In fact, lowering the triglyceride/HDL cholesterol ratio strongly associated with the slowing of plaque progression [61]. In insulin-resistant patients following a stroke or transient ischemic attack, pioglitazone treatment was demonstrated to reduce subsequent cardiovascular events [62]. Further developments with more potent or combination peroxisome proliferator-activated receptor (PPAR) agonists has also failed to be of clinical benefit.
More recent studies of a selective PPAR modulator, which brings the promise of lipid efficacy with a more favorable safety profile, in Japan have led to a large clinical trial to evaluate its impact on cardiovascular events in patients with atherogenic dyslipidemia [63]. In a similar fashion, administration of high doses of eicosapentanoic acid (EPA) have been demonstrated to reduce cardiovascular events in several large studies [64,65]. While this benefit does not necessarily correlate with changes in either triglycerides or HDL cholesterol, imaging studies have demonstrated that this strategy does exert favorable effects on coronary atherosclerosis [66,67]. This provides a novel approach to patients with high cardiovascular risk and has led to an increase use of EPA, particularly in Japanese clinics. Additional approaches involve directly targeting factors that influence metabolism of triglyceride rich lipoproteins [68,69]. However, these programs are at an early stage, with no real experience by patients in Asia Pacific.
Lp(a) has emerged as an additional lipid target for both risk prediction and therapeutic modification. This atherogenic lipoprotein has been demonstrated to exert proinflammatory, prothrombotic and procalcific effects in addition to its conventional impact on the artery wall as an apolipoprotein B containing particle [70]. While cohort studies have demonstrated the curvilinear relationship between Lp(a) levels and CV risk for decades [71], more recent reports from genomic analyses have implicated Lp(a) directly in the causal pathway for both atherosclerosis and calcific aortic stenosis [72]. A major challenge in the Lp(a) field has been the ability to develop effective Lp(a) lowering strategies. Statins are known to raise Lp(a) levels to a modest degree [70]. Niacin and estrogen both lower Lp(a) [70], yet both have failed to reduce CV events in contemporary trials. Apheresis can be used to lower Lp(a) levels in a small number of very high-risk patients [73]. While PCSK9 inhibitors lower Lp(a), with reports that this contributes to their clinical benefit [74,75], they will continue to be primarily used to lower LDL cholesterol levels. A number of important therapeutic advances have presented the opportunity to directly lower Lp(a) through the delivery of antisense or RNA inhibitory approaches [76]. While early studies have demonstrated favorable efficacy and safety, they will need to be further evaluated in larger trials. This attention to Lp(a) as a potentially modifiable risk factor will lead to further investigation to understand the patterns of Lp(a), both in terms of its overall quantity [77,78] and its specific isoforms [79,80], across the Asia Pacific region and expand a limited degree of literature further confirming it as an important driver of CV risk in these countries.
1.8. Hypertension
Hypertension is the leading preventable risk factor for premature death. Approximately 54% of stroke and 47% of ischemic heart disease are reported to be attributed to high blood pressure [81]. The global prevalence of hypertension ranges from 24.1% to 31.1% in men, and 20.1%–30.1% in women [[82], [83], [84]], which has been gradually increasing. In a report, 26.4% of the adult population worldwide had hypertension in 2000, and this number is predicted to increase to 29.2% by 2025 [85]. The prevalence of hypertension in the Asia Pacific region is relatively comparable to other parts of the world [[86], [87], [88], [89]]. A systematic analysis from 90 countries has shown that the prevalence of hypertension in East Asia and Pacific region (32.7% for men, 29.2% for women in 2010) was similar to other parts of the world (31.1% in men, 28.5% in women) [84]. The crude prevalence of hypertension in the Asia pacific region ranged from Fiji’s less than 10% to Mongolia’s over 40% [83]. Age-adjusted prevalence of hypertension, however, was approximately 30% in men and 25% in women, which is comparable to that in Western/developed countries [83]. While the percentage of the hypertensive patients is relatively similar, given the large populations in the region the numbers of hypertensive patients in East Asia and Pacific region in 2010 are reported as 232 million in men and 207 million in women [84], highlighting the tremendous public health burden in this region.
The impact of hypertension in Asians has been investigated in The Asia Pacific Cohort Studies Collaboration (APCSC) [90,91]. This study included 44 prospective cohort studies from the Asia Pacific region and found that the impact of risk factors including BMI, total cholesterol, smoking, HDL cholesterol, and triglycerides was similar between Australian/New Zealand cohorts and Asian cohorts. However, the hazard ratio for fatal coronary heart disease was 1.22 for each 10 mmHg increment in Australia/New Zealand cohorts, while the ratio was 1.31 in Asian population, suggesting an increased importance of controlling blood pressure in Asians, compared with Caucasians.
An extensive cohort study from Japan reports an interesting trend. The Hisayama study, a well-organized population-based prospective study in Japan, shows data from over half a century demonstrating secular trends in cardiovascular disease and risk factors [87]. In their study, the prevalence of hypertension did not show a dramatic change in the past half-century (men: 38.4% in 1961 and 41.3% in 2002). However, average blood pressure levels of hypertensive individuals showed a continuous decrease as a result of the hypertensive treatment. Marked reduction in salt intake (18.3g/day in 1965 to 9.8g/day in 2004) is also considered to have played a role. The study also demonstrated a significant reduction in hemorrhagic stroke over the study decades. However, there was not a clear trend in the incidence of myocardial infarction, which is presumably due to increased prevalence of other metabolic risk factors, possibly negating the benefit of improvement in hypertension control [87]. These findings suggest the importance of comprehensive treatment strategy in the prevention of atherosclerotic disease.
In a report from NCD Risk factor collaboration, the highest worldwide blood pressure levels have shifted from high-income countries to low-income countries in South Asia and sub-Saharan Africa [82]. In their report, East, Southeast Asia, and South Asia showed a trend towards higher mean systolic blood pressure, while high-income Asia Pacific countries including Singapore, South Korea, and Japan showed a declining mean systolic blood pressure. Although the prevalence of hypertension cannot be easily modified by lifestyle modifications, medical treatment is highly effective in controlling hypertension. The lowering trend of blood pressure in high-income Asia Pacific countries can be explained by the spreading use of antihypertensive treatments. Thus, disparities in the access to medical resources may pose an important challenge when trying to achieve optimal treatment goals in lower income regions.
Other challenges in treating hypertension may include suboptimal awareness and undertreatment. Several studies from China reported that the awareness of hypertension was as low as 30%–40% [88,89]. Since there is minimal to no symptoms related to elevated blood pressure, more awareness should be emphasized. Undertreatment is another issue. A prospective cohort study including 500,223 Chinese participants showed that only 29.6% among those treated for hypertension achieved blood pressure control [88]. These may not exactly be region-specific issues; however, the sizeable population in Asia warrants more efforts in public education, early diagnosis and rigorous blood pressure control in primary care.
1.9. Smoking
Tobacco smoking continues to present a major preventable cause of chronic disease worldwide, with increasing evidence of the geographic spread of its health burden from higher income to lower income countries [92]. Among a range of modifiable risk factors, smoking is only second to high blood pressure with regards to the total number of attributable deaths worldwide, accounting for more than 7 million deaths on an annual basis [92]. Mechanistic studies have implicated smoking as a major factor promoting inflammatory, oxidative and thrombotic pathways involved in the generation and progression of atherosclerotic cardiovascular disease. While increasing efforts in each country have attempted to reduce the amount of smoking, both the rate of smoking and attributable deaths remains high in many parts of the Asia Pacific region [92]. Ongoing strategies are required at both a population level and in the context of clinical consultation to reduce smoking rates to reduce cardiovascular risk.
1.10. Additional aspects for consideration
Beyond these conventional and modifiable risk factors, a number of additional points require ongoing attention with regard to attempts to achieve more effective prevention of CVD in the Asia Pacific region. As highlighted in this review, there remains a major evidence gap across the region with regard to the epidemiology of cardiometabolic risk. The paucity of research in this space is paralleled by a consistent under-representation of patients from the Asia Pacific region in clinical trials. When patients are recruited in such trials, background therapy can often be different, largely due to systematic approaches to under-dosing of medications in the region. This has major implications with regard to subsequent attempts to translate evidence-based therapies to the clinic.
Lifestyle guidelines can be challenging, given the heterogeneity of diet and exercise throughout the region. However, efforts to promote consumption of a more balanced diet, looking to reduce intake of saturated fat and sugar, with moderate consumption of alcohol and attention to regular exercise, should be encouraged. More recent focus on the importance of air and noise pollution as causal mediators of atherosclerotic disease will lead to new efforts to reduce their associated CVD risk.
Clinical trials have also highlighted the potential to target novel pathways implicated in CVD. The benefits of both the interleukin 1 antagonist, cannakinumab [93], and colchicine [94] in large clinical trials have suggested that targeting inflammation may provide an alternative approach to reducing residual risk. In a similar fashion, the rise in abdominal obesity throughout Asia Pacific presents the opportunity to directly target this factor to reduce CVD risk. While several anti-obesity agents have failed to demonstrate CV benefit, there is ongoing interest in the use of agents that more directly modulate the abnormal metabolic factors associated with obesity [95]. Given the large population of higher risk patients within Asia Pacific, a greater need is required to include them in future clinical trials.
In addition to an urgent need to increase the access of high CV risk patients within Asia Pacific to clinical trials of novel cardioprotective agents, we also need to develop more effective approaches to providing equitable access to proven therapies. This will require delivery of these agents to all parts of Asia Pacific, in an affordable manner, and for effective approaches to promote their implementation in health care systems. This will require thoughtful attempts to promote the evidence base across a diverse network of primary care physicians, specialists with a predominant interest in cardiovascular prevention and allied health care professionals throughout the region. Coordinated efforts to increase access and promote evidence-based prescription practices will be required, with sensitivity to local needs.
2. Conclusion
The large population combined with an increasing prevalence of cardiometabolic risk factors presents a formidable challenge to curb the risk of developing CVD in the Asia Pacific region. The cultural and economic diversity across the region highlights the need to develop coordinated approaches to research and implementation, while at the same time recognising local factors that may influence the ability to effectively implement new approaches to CVD prevention and treatment.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
KKY has received research grants from Amgen, Astra Zeneca, Bayer, Boston Scientific, Biofourmis, Holmusk, is a consultant for Abbott Vascular, Boston Scientific, Medtronic, Medopad, Bayer and speaker for Shockwave Medical, Abbott Vascular, Boston Scientific, Medtronic, Philips, Alvimedica, Bayer, Biotronik, Orbus Neich; JA has received honoraria from Sanofi and Astellas-Amgen; CSPL is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc., Eko.ai Pte Ltd, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Stealth BioTherapeutics, The Corpus, Vifor Pharma and WebMD Global LLC.; and serves as co-founder & non-executive director of EKo.ai Pte Ltd; AF is a speaker and consultant for AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Pfizer, Novartis, Roche Diagnostics, Siemens, Medtronic, Boston Scientific, and OrbusNeich Medical and received research grants from Boehringer Ingelheim, and Medtronic; RCO is a consultant or speaker for MSD, Mylan, Amgen and Sanofi; SJN has received research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Esperion, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron and LipoScience and is a consultant for AstraZeneca, Akcea, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, Boehringer Ingelheim. All other authors have no relationships to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajpc.2020.100096.
Contributor Information
Jian-Jun Li, Email: lijianjun938@126.com.
Khung Keong Yeo, Email: yeo.khung.keong@singhealth.com.sg.
Stephen J. Nicholls, Email: stephen.nicholls@monash.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kohro T., Furui Y., Mitsutake N., Fujii R., Morita H., Oku S., Ohe K., Nagai R. The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int Heart J. 2008;49:193–203. doi: 10.1536/ihj.49.193. [DOI] [PubMed] [Google Scholar]
- 2.2018. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- 3.Ryan D.H., Lingvay I., Colhoun H.M., Deanfield J., Emerson S.S., Kahn S.E., Kushner R.F., Marso S., Plutzky J., Brown-Frandsen K., Gronning M.O.L., Hovingh G.K., Holst A.G., Ravn H., Lincoff A.M. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–69. doi: 10.1016/j.ahj.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., Woo V., Hansen O., Holst A.G., Pettersson J., Vilsboll T., Investigators S.- Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 5.Schauer P.R., Bhatt D.L., Kirwan J.P., Wolski K., Brethauer S.A., Navaneethan S.D., Aminian A., Pothier C.E., Kim E.S., Nissen S.E., Kashyap S.R., Investigators S. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Gao P., Zhang M., Huang Z., Zhang D., Deng Q., Li Y., Zhao Z., Qin X., Jin D., Zhou M., Tang X., Hu Y., Wang L. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. J Am Med Assoc. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anjana R.M., Deepa M., Pradeepa R., Mahanta J., Narain K., Das H.K., Adhikari P., Rao P.V., Saboo B., Kumar A., Bhansali A., John M., Luaia R., Reang T., Ningombam S., Jampa L., Budnah R.O., Elangovan N., Subashini R., Venkatesan U., Unnikrishnan R., Das A.K., Madhu S.V., Ali M.K., Pandey A., Dhaliwal R.S., Kaur T., Swaminathan S., Mohan V., Group I.-I.C.S. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. The lancet Diabetes & endocrinology. 2017;5:585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 8.Lascar N., Brown J., Pattison H., Barnett A.H., Bailey C.J., Bellary S. Type 2 diabetes in adolescents and young adults. The lancet Diabetes & endocrinology. 2018;6:69–80. doi: 10.1016/S2213-8587(17)30186-9. [DOI] [PubMed] [Google Scholar]
- 9.Ma R.C., Chan J.C. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattar N., Gill J.M. Type 2 diabetes in migrant south Asians: mechanisms, mitigation, and management. The lancet Diabetes & endocrinology. 2015;3:1004–1016. doi: 10.1016/S2213-8587(15)00326-5. [DOI] [PubMed] [Google Scholar]
- 11.Clarke P.M., Glasziou P., Patel A., Chalmers J., Woodward M., Harrap S.B., Salomon J.A., Group A.C. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee E.T., Keen H., Bennett P.H., Fuller J.H., Lu M. Follow-up of the WHO multinational study of vascular disease in diabetes: general description and morbidity. Diabetologia. 2001;44(2):S3–S13. doi: 10.1007/pl00002936. [DOI] [PubMed] [Google Scholar]
- 13.Forouhi N.G., Sattar N., Tillin T., McKeigue P.M., Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia. 2006;49:2580–2588. doi: 10.1007/s00125-006-0393-2. [DOI] [PubMed] [Google Scholar]
- 14.Chi Z.S., Lee E.T., Lu M., Keen H., Bennett P.H. Vascular disease prevalence in diabetic patients in China: standardised comparison with the 14 centres in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S82–S86. doi: 10.1007/pl00002944. [DOI] [PubMed] [Google Scholar]
- 15.Luk A.O., Lau E.S., So W.Y., Ma R.C., Kong A.P., Ozaki R., Chow F.C., Chan J.C. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care. 2014;37:149–157. doi: 10.2337/dc13-1336. [DOI] [PubMed] [Google Scholar]
- 16.Jiao F., Wong C.K.H., Tang S.C.W., Fung C.S.C., Tan K.C.B., McGhee S., Gangwani R., Lam C.L.K. Annual direct medical costs associated with diabetes-related complications in the event year and in subsequent years in Hong Kong. Diabet Med. 2017;34:1276–1283. doi: 10.1111/dme.13416. [DOI] [PubMed] [Google Scholar]
- 17.Ji L., Hu D., Pan C., Weng J., Huo Y., Ma C., Mu Y., Hao C., Ji Q., Ran X., Su B., Zhuo H., Fox K.A., Weber M., Zhang D., Board C.A., Investigators C.-B.S. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126:925 e11–22. doi: 10.1016/j.amjmed.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Unnikrishnan R., Anjana R.M., Deepa M., Pradeepa R., Joshi S.R., Bhansali A., Dhandania V.K., Joshi P.P., Madhu S.V., Rao P.V., Lakshmy R., Jayashri R., Velmurugan K., Nirmal E., Subashini R., Vijayachandrika V., Kaur T., Shukla D.K., Das A.K., Mohan V., Group I.-I.C.S. Glycemic control among individuals with self-reported diabetes in India--the ICMR-INDIAB Study. Diabetes Technol Therapeut. 2014;16:596–603. doi: 10.1089/dia.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luk A.O.Y., Hui E.M.T., Sin M.C., Yeung C.Y., Chow W.S., Ho A.Y.Y., Hung H.F., Kan E., Ng C.M., So W.Y., Yeung C.K., Chan K.S., Chan K.W., Chan P.F., Siu S.C., Tiu S.C., Yeung V.T.F., Chan J.C.N., Chan F.W.K., Cheung C., Cheung N.T., Ho S.T., Lam K.S.L., Yu L.W.L., Chao D., Lau I.T. Declining trends of cardiovascular-renal complications and mortality in type 2 diabetes: the Hong Kong diabetes database. Diabetes Care. 2017;40:928–935. doi: 10.2337/dc16-2354. [DOI] [PubMed] [Google Scholar]
- 20.Gaede P., Lund-Andersen H., Parving H.H., Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 21.Soran H., Schofield J.D., Durrington P.N. Cholesterol, not just cardiovascular risk, is important in deciding who should receive statin treatment. Eur Heart J. 2015;36:2975–2983. doi: 10.1093/eurheartj/ehv340. [DOI] [PubMed] [Google Scholar]
- 22.Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Sierra A., Pinto X., Guijarro C., Miranda J.L., Callejo D., Cuervo J., Subira R., Rubio M. Prevalence, treatment, and control of hypercholesterolemia in high cardiovascular risk patients: evidences from a systematic literature review in Spain. Adv Ther. 2015;32:944–961. doi: 10.1007/s12325-015-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkitachalam L., Wang K., Porath A., Corbalan R., Hirsch A.T., Cohen D.J., Smith S.C., Jr., Ohman E.M., Steg P.G., Bhatt D.L., Magnuson E.A., Investigators R.R. Global variation in the prevalence of elevated cholesterol in outpatients with established vascular disease or 3 cardiovascular risk factors according to national indices of economic development and health system performance. Circulation. 2012;125:1858–1869. doi: 10.1161/CIRCULATIONAHA.111.064378. [DOI] [PubMed] [Google Scholar]
- 25.Phrommintikul A., Krittayaphong R., Wongcharoen W., Yamwong S., Boonyaratavej S., Kunjara-Na-Ayudhya R., Tatsanavivat P., Sritara P., Investigators C.O.-T. Management of atherosclerosis risk factors for patients at high cardiovascular risk in real-world practice: a multicentre study. Singapore Med J. 2017;58:535–542. doi: 10.11622/smedj.2017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Vallejo-Vaz A.J., Ray K.K. Epidemiology of familial hypercholesterolaemia: community and clinical. Atherosclerosis. 2018;277:289–297. doi: 10.1016/j.atherosclerosis.2018.06.855. [DOI] [PubMed] [Google Scholar]
- 28.Pang J., Hu M., Lin J., Miida T., Nawawi H.M., Park J.E., Wu X., Ramli A.S., Kim N.T., Kwok S., Gonzalez-Santos L.E., Su T.C., Truong T.H., Soran H., Yamashita S., Tomlinson B., Watts G.F. An enquiry based on a standardised questionnaire into knowledge, awareness and preferences concerning the care of familial hypercholesterolaemia among primary care physicians in the Asia-Pacific region: the "Ten Countries Study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raal F.J., Hovingh G.K., Catapano A.L. Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis. 2018;277:483–492. doi: 10.1016/j.atherosclerosis.2018.06.859. [DOI] [PubMed] [Google Scholar]
- 30.Pang J., Chan D.C., Hu M., Muir L.A., Kwok S., Charng M.J., Florkowski C.M., George P.M., Lin J., Loi D.D., Marais A.D., Nawawi H.M., Gonzalez-Santos L.E., Su T.C., Truong T.H., Santos R.D., Soran H., Tomlinson B., Yamashita S., Ademi Z., Watts G.F. Comparative aspects of the care of familial hypercholesterolemia in the "Ten Countries Study. Journal of clinical lipidology. 2019;13:287–300. doi: 10.1016/j.jacl.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Ofori-Asenso R., Zoungas S., Liew D. Reinitiation of statin therapy after discontinuation: a meta-analysis. Mayo Clin Proc. 2018;93:666–668. doi: 10.1016/j.mayocp.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Banach M., Rizzo M., Toth P.P., Farnier M., Davidson M.H., Al-Rasadi K., Aronow W.S., Athyros V., Djuric D.M., Ezhov M.V., Greenfield R.S., Hovingh G.K., Kostner K., Serban C., Lighezan D., Fras Z., Moriarty P.M., Muntner P., Goudev A., Ceska R., Nicholls S.J., Broncel M., Nikolic D., Pella D., Puri R., Rysz J., Wong N.D., Bajnok L., Jones S.R., Ray K.K., Mikhailidis D.P. Statin intolerance - an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci : AMS. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nissen S.E., Stroes E., Dent-Acosta R.E., Rosenson R.S., Lehman S.J., Sattar N., Preiss D., Bruckert E., Ceska R., Lepor N., Ballantyne C.M., Gouni-Berthold I., Elliott M., Brennan D.M., Wasserman S.M., Somaratne R., Scott R., Stein E.A., Investigators G.- Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. J Am Med Assoc. 2016;315:1580–1590. doi: 10.1001/jama.2016.3608. [DOI] [PubMed] [Google Scholar]
- 34.Preiss D., Seshasai S.R., Welsh P., Murphy S.A., Ho J.E., Waters D.D., DeMicco D.A., Barter P., Cannon C.P., Sabatine M.S., Braunwald E., Kastelein J.J., de Lemos J.A., Blazing M.A., Pedersen T.R., Tikkanen M.J., Sattar N., Ray K.K. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. J Am Med Assoc. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 35.Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C., Bhala N., Peto R., Barnes E.H., Keech A., Simes J., Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomlinson B., Chan P., Liu Z.M. Statin intolerance-an asian perspective. J Atherosclerosis Thromb. 2020;27(5):485–488. doi: 10.5551/jat.50435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halcox J.P., Banegas J.R., Roy C., Dallongeville J., De Backer G., Guallar E., Perk J., Hajage D., Henriksson K.M., Borghi C. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc Disord. 2017;17:160. doi: 10.1186/s12872-017-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel A., Barzi F., Jamrozik K., Lam T.H., Ueshima H., Whitlock G., Woodward M., Asia Pacific Cohort Studies C. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110:2678–2686. doi: 10.1161/01.CIR.0000145615.33955.83. [DOI] [PubMed] [Google Scholar]
- 39.Misra A., Shrivastava U. Obesity and dyslipidemia in South Asians. Nutrients. 2013;5:2708–2733. doi: 10.3390/nu5072708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirakawa Y., Lam T.H., Welborn T., Kim H.C., Ho S., Fang X., Ueshima H., Suh I., Giles G., Woodward M., Asia Pacific Cohort Studies C. The impact of body mass index on the associations of lipids with the risk of coronary heart disease in the Asia Pacific region. Prev Med Rep. 2016;3:79–82. doi: 10.1016/j.pmedr.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barter P.J., Caulfield M., Eriksson M., Grundy S.M., Kastelein J.J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J.C., Waters D.D., Shear C.L., Revkin J.H., Buhr K.A., Fisher M.R., Tall A.R., Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz G.G., Olsson A.G., Abt M., Ballantyne C.M., Barter P.J., Brumm J., Chaitman B.R., Holme I.M., Kallend D., Leiter L.A., Leitersdorf E., McMurray J.J., Mundl H., Nicholls S.J., Shah P.K., Tardif J.C., Wright R.S., dal O.I. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 43.Lincoff A.M., Nicholls S.J., Riesmeyer J.S., Barter P.J., Brewer H.B., Fox K.A.A., Gibson C.M., Granger C., Menon V., Montalescot G., Rader D., Tall A.R., McErlean E., Wolski K., Ruotolo G., Vangerow B., Weerakkody G., Goodman S.G., Conde D., McGuire D.K., Nicolau J.C., Leiva-Pons J.L., Pesant Y., Li W., Kandath D., Kouz S., Tahirkheli N., Mason D., Nissen S.E., Investigators A. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 44.Group H.T.R.C. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 45.Bloomfield H.E. ACP Journal Club: adding niacin plus laropiprant to statins did not reduce vascular events and increased serious adverse events. Ann Intern Med. 2014;161:JC8. doi: 10.7326/0003-4819-161-10-201411180-02008. [DOI] [PubMed] [Google Scholar]
- 46.Group H.T.C., Landray M.J., Haynes R., Hopewell J.C., Parish S., Aung T., Tomson J., Wallendszus K., Craig M., Jiang L., Collins R., Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 47.Nissen S.E., Tsunoda T., Tuzcu E.M., Schoenhagen P., Cooper C.J., Yasin M., Eaton G.M., Lauer M.A., Sheldon W.S., Grines C.L., Halpern S., Crowe T., Blankenship J.C., Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. J Am Med Assoc. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 48.Tardif J.C., Gregoire J., L’Allier P.L., Ibrahim R., Lesperance J., Heinonen T.M., Kouz S., Berry C., Basser R., Lavoie M.A., Guertin M.C., Rodes-Cabau J. Effect of r HDLoA-S and Efficacy I. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. J Am Med Assoc. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 49.Waksman R., Torguson R., Kent K.M., Pichard A.D., Suddath W.O., Satler L.F., Martin B.D., Perlman T.J., Maltais J.A., Weissman N.J., Fitzgerald P.J., Brewer H.B., Jr. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55:2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 50.Kallend D.G., Reijers J.A., Bellibas S.E., Bobillier A., Kempen H., Burggraaf J., Moerland M., Wijngaard P.L. A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur Heart J Cardiovasc Pharmacother. 2016;2:23–29. doi: 10.1093/ehjcvp/pvv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kempen H.J., Asztalos B.F., Moerland M., Jeyarajah E., Otvos J., Kallend D.G., Bellibas S.E., Wijngaard P.L. High-density lipoprotein subfractions and cholesterol efflux capacities after infusion of MDCO-216 (apolipoprotein A-IMilano/palmitoyl-oleoyl-phosphatidylcholine) in healthy volunteers and stable coronary artery disease patients. Arterioscler Thromb Vasc Biol. 2016;36:736–742. doi: 10.1161/ATVBAHA.115.307052. [DOI] [PubMed] [Google Scholar]
- 52.Reijers J.A.A., Kallend D.G., Malone K.E., Jukema J.W., Wijngaard P.L.J., Burggraaf J., Moerland M. MDCO-216 does not induce adverse immunostimulation, in contrast to its predecessor ETC-216. Cardiovasc Drugs Ther. 2017;31:381–389. doi: 10.1007/s10557-017-6746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews J., Janssan A., Nguyen T., Pisaniello A.D., Scherer D.J., Kastelein J.J., Merkely B., Nissen S.E., Ray K., Schwartz G.G., Worthley S.G., Keyserling C., Dasseux J.L., Butters J., Girardi J., Miller R., Nicholls S.J. Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: rationale and design of the CARAT study. Cardiovasc Diagn Ther. 2017;7:45–51. doi: 10.21037/cdt.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tardif J.C., Ballantyne C.M., Barter P., Dasseux J.L., Fayad Z.A., Guertin M.C., Kastelein J.J., Keyserling C., Klepp H., Koenig W., L’Allier P.L., Lesperance J., Luscher T.F., Paolini J.F., Tawakol A., Waters D.D., Can H.D. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J. 2014;35:3277–3286. doi: 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jun M., Foote C., Lv J., Neal B., Patel A., Nicholls S.J., Grobbee D.E., Cass A., Chalmers J., Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 56.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 57.Keech A., Simes R.J., Barter P., Best J., Scott R., Taskinen M.R., Forder P., Pillai A., Davis T., Glasziou P., Drury P., Kesaniemi Y.A., Sullivan D., Hunt D., Colman P., d’Emden M., Whiting M., Ehnholm C., Laakso M., Investigators Fs Effects of lIong-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 58.Dormandy J.A., Charbonnel B., Eckland D.J., Erdmann E., Massi-Benedetti M., Moules I.K., Skene A.M., Tan M.H., Lefebvre P.J., Murray G.D., Standl E., Wilcox R.G., Wilhelmsen L., Betteridge J., Birkeland K., Golay A., Heine R.J., Koranyi L., Laakso M., Mokan M., Norkus A., Pirags V., Podar T., Scheen A., Scherbaum W., Schernthaner G., Schmitz O., Skrha J., Smith U., Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 59.Erdmann E., Dormandy J.A., Charbonnel B., Massi-Benedetti M., Moules I.K., Skene A.M., Investigators P.R. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J Am Coll Cardiol. 2007;49:1772–1780. doi: 10.1016/j.jacc.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 60.Nissen S.E., Nicholls S.J., Wolski K., Nesto R., Kupfer S., Perez A., Jure H., De Larochelliere R., Staniloae C.S., Mavromatis K., Saw J., Hu B., Lincoff A.M., Tuzcu E.M. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. J Am Med Assoc. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 61.Nissen S.E., Nicholls S.J., Wolski K., Nesto R., Kupfer S., Perez A., Jure H., De Larochelliere R., Staniloae C.S., Mavromatis K., Saw J., Hu B., Lincoff A.M., Tuzcu E.M., Investigators P. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. J Am Med Assoc. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 62.Kernan W.N., Viscoli C.M., Furie K.L., Young L.H., Inzucchi S.E., Gorman M., Guarino P.D., Lovejoy A.M., Peduzzi P.N., Conwit R., Brass L.M., Schwartz G.G., Adams H.P., Jr., Berger L., Carolei A. Pioglitazone after ischemic stroke or transient. Ischemic Attack. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arai H., Yamashita S., Yokote K., Araki E., Suganami H., Ishibashi S., Group K.S. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), in combination with statin treatment: two randomised, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis. 2017;261:144–152. doi: 10.1016/j.atherosclerosis.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 64.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., Kita T., Kitabatake A., Nakaya N., Sakata T., Shimada K., Shirato K., Japan EPAlisI Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 65.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., Tardif J.C., Ballantyne C.M., Investigators R.-I. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe T., Ando K., Daidoji H., Otaki Y., Sugawara S., Matsui M., Ikeno E., Hirono O., Miyawaki H., Yashiro Y., Nishiyama S., Arimoto T., Takahashi H., Shishido T., Miyashita T., Miyamoto T., Kubota I., investigators Cs. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017;70:537–544. doi: 10.1016/j.jjcc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Budoff M.J., Bhatt D.L., Kinninger A., Lakshmanan S., Muhlestein J.B., Le V.T. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41(40):3925–3932. doi: 10.1093/eurheartj/ehaa652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaudet D., Brisson D., Tremblay K., Alexander V.J., Singleton W., Hughes S.G., Geary R.S., Baker B.F., Graham M.J., Crooke R.M., Witztum J.L. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 69.Gaudet D., Gipe D.A., Pordy R., Ahmad Z., Cuchel M., Shah P.K., Chyu K.Y., Sasiela W.J., Chan K.C., Brisson D., Khoury E., Banerjee P., Gusarova V., Gromada J., Stahl N., Yancopoulos G.D., Hovingh G.K. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med. 2017;377:296–297. doi: 10.1056/NEJMc1705994. [DOI] [PubMed] [Google Scholar]
- 70.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 71.Bennet A., Di Angelantonio E., Erqou S., Eiriksdottir G., Sigurdsson G., Woodward M., Rumley A., Lowe G.D., Danesh J., Gudnason V. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168:598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 72.Burgess S., Ference B.A., Staley J.R., Freitag D.F., Mason A.M., Nielsen S.F., Willeit P., Young R., Surendran P., Karthikeyan S., Bolton T.R., Peters J.E., Kamstrup P.R., Tybjaerg-Hansen A., Benn M., Langsted A., Schnohr P., Vedel-Krogh S., Kobylecki C.J., Ford I., Packard C., Trompet S., Jukema J.W., Sattar N., Di Angelantonio E., Saleheen D., Howson J.M.M., Nordestgaard B.G., Butterworth A.S., Danesh J. European prospective investigation into C and nutrition-cardiovascular disease C. Association of lpa variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3:619–627. doi: 10.1001/jamacardio.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson G., Parhofer K.G. Current role of lipoprotein apheresis. Curr Atherosclerosis Rep. 2019;21:26. doi: 10.1007/s11883-019-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Donoghue M.L., Fazio S., Giugliano R.P., Stroes E.S.G., Kanevsky E., Gouni-Berthold I., Im K., Lira Pineda A., Wasserman S.M., Ceska R., Ezhov M.V., Jukema J.W., Jensen H.K., Tokgozoglu S.L., Mach F., Huber K., Sever P.S., Keech A.C., Pedersen T.R., Sabatine M.S. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 75.Bittner V.A., Szarek M., Aylward P.E., Bhatt D.L., Diaz R., Edelberg J.M., Fras Z., Goodman S.G., Halvorsen S., Hanotin C., Harrington R.A., Jukema J.W., Loizeau V., Moriarty P.M., Moryusef A., Pordy R., Roe M.T., Sinnaeve P., Tsimikas S., Vogel R., White H.D., Zahger D., Zeiher A.M., Steg P.G., Schwartz G.G., Committees O.O., Investigators Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–144. doi: 10.1016/j.jacc.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 76.Tsimikas S., Karwatowska-Prokopczuk E., Gouni-Berthold I., Tardif J.C., Baum S.J., Steinhagen-Thiessen E., Shapiro M.D., Stroes E.S., Moriarty P.M., Nordestgaard B.G., Xia S., Guerriero J., Viney N.J., O’Dea L., Witztum J.L., Investigators A.K.-A.-L.S. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 77.Feng Z., Li H.L., Bei W.J., Guo X.S., Wang K., Yi S.X., Luo D.M., Li X.D., Chen S.Q., Ran P., Chen P.Y., Islam S.M.S., Chen J.Y., Liu Y., Zhou Y.L. Association of lipoprotein(a) with long-term mortality following coronary angiography or percutaneous coronary intervention. Clin Cardiol. 2017;40:674–678. doi: 10.1002/clc.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bilen O., Kamal A., Virani S.S. Lipoprotein abnormalities in South Asians and its association with cardiovascular disease: current state and future directions. World J Cardiol. 2016;8:247–257. doi: 10.4330/wjc.v8.i3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erqou S., Thompson A., Di Angelantonio E., Saleheen D., Kaptoge S., Marcovina S., Danesh J. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 80.Takayama S., Yasumuro Y., Kim J., Ishikawa M., Tsujino D., Matsuo S., Harada Y., Sugii S. An application of apo(a) isoforms for the clinical assessment of Lp(a) J Clin Lab Anal. 2000;14:53–58. doi: 10.1002/(SICI)1098-2825(2000)14:2<53::AID-JCLA3>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lawes C.M., Vander Hoorn S., Rodgers A., International Society of H. Global burden of blood-pressure-related disease. Lancet. 2001;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 82.Collaboration N.C.D.R.F. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389:37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martiniuk A.L., Lee C.M., Lawes C.M., Ueshima H., Suh I., Lam T.H., Gu D., Feigin V., Jamrozik K., Ohkubo T., Woodward M., Asia-Pacific Cohort Studies C. Hypertension: its prevalence and population-attributable fraction for mortality from cardiovascular disease in the Asia-Pacific region. J Hypertens. 2007;25:73–79. doi: 10.1097/HJH.0b013e328010775f. [DOI] [PubMed] [Google Scholar]
- 84.Mills K.T., Bundy J.D., Kelly T.N., Reed J.E., Kearney P.M., Reynolds K., Chen J., He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 86.Han K., Yao J., Yin X., Zhao M., Sun Q. Review on the prevalence of diabetes and risk factors and situation of disease management in floating population in China. Glob Health Res Policy. 2017;2:33. doi: 10.1186/s41256-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hata J., Ninomiya T., Hirakawa Y., Nagata M., Mukai N., Gotoh S., Fukuhara M., Ikeda F., Shikata K., Yoshida D., Yonemoto K., Kamouchi M., Kitazono T., Kiyohara Y. Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961-2009) Circulation. 2013;128:1198–1205. doi: 10.1161/CIRCULATIONAHA.113.002424. [DOI] [PubMed] [Google Scholar]
- 88.Lewington S., Lacey B., Clarke R., Guo Y., Kong X.L., Yang L., Chen Y., Bian Z., Chen J., Meng J., Xiong Y., He T., Pang Z., Zhang S., Collins R., Peto R., Li L., Chen Z., China Kadoorie Biobank C. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524–532. doi: 10.1001/jamainternmed.2016.0190. [DOI] [PubMed] [Google Scholar]
- 89.Lu J., Lu Y., Wang X., Li X., Linderman G.C., Wu C., Cheng X., Mu L., Zhang H., Liu J., Su M., Zhao H., Spatz E.S., Spertus J.A., Masoudi F.A., Krumholz H.M., Jiang L. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 90.Peters S.A.E., Wang X., Lam T.H., Kim H.C., Ho S., Ninomiya T., Knuiman M., Vaartjes I., Bots M.L., Woodward M., Asia Pacific Cohort Studies C. Clustering of risk factors and the risk of incident cardiovascular disease in Asian and Caucasian populations: results from the Asia Pacific Cohort Studies Collaboration. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woodward M., Huxley R., Ueshima H., Fang X., Kim H.C., Lam T.H. The Asia pacific cohort studies collaboration: a decade of achievements. Glob Heart. 2012;7:343–351. doi: 10.1016/j.gheart.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Collaborators G.B.D.R.F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., Kastelein J.J.P., Cornel J.H., Pais P., Pella D., Genest J., Cifkova R., Lorenzatti A., Forster T., Kobalava Z., Vida-Simiti L., Flather M., Shimokawa H., Ogawa H., Dellborg M., Rossi P.R.F., Troquay R.P.T., Libby P., Glynn R.J., Group C.T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 94.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., Pinto F.J., Ibrahim R., Gamra H., Kiwan G.S., Berry C., Lopez-Sendon J., Ostadal P., Koenig W., Angoulvant D., Gregoire J.C., Lavoie M.A., Dube M.P., Rhainds D., Provencher M., Blondeau L., Orfanos A., L’Allier P.L., Guertin M.C., Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 95.Andrew C.A., Saunders K.H., Shukla A.P., Aronne L.J. Treating obesity in patients with cardiovascular disease: the pharmacotherapeutic options. Expet Opin Pharmacother. 2019;20:585–593. doi: 10.1080/14656566.2018.1561867. [DOI] [PubMed] [Google Scholar]
- 96.https://www.who.int/nmh/countries/en/. 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.