Abstract
Background
Elevated triglycerides (TGs) are associated with atherosclerotic cardiovascular disease (ASCVD). Despite statin therapy, many US adults have borderline or elevated TG levels. Not characterized is the ASCVD risk associated with borderline TG levels in statin users, including the estimated number of adults who will sustain ASCVD events.
Methods
We studied 4986 US adults (weighted to 113 million) aged 40–74 from the National Health and Nutrition Examination Surveys 2007–2014. The proportion of persons at low (<5%), borderline (5-<7.5%), intermediate (7.5-<20%), and high (≥20%) 10-year ASCVD risk among those on statins was quantified for low (<70 mg/dL, 70-<100 mg/dL), borderline (100-<135 mg/dL and 135-<150 mg/dL), borderline high (150-<200 mg/dL), and elevated (≥200 mg/dL) TGs. Multiple logistic regression examined these TG categories in relation to high risk status.
Results
Overall, 18.6% of participants had TG < 70 mg/dL, 24.2% TG 70-<100 mg/dL, 22.0% TG 100-<135 mg/dL, 6.2% TG 135-<150 mg/dL, 15.0% TG 150-<200 mg/dL, and 14.0% TG ≥ 200 mg/dL. Mean 10-year ASCVD risk for these groups were 5.6%, 6.9%, 7.8%, 10.3%, 9.6% and 10.8%, respectively (p < 0.0001). One-fifth or more of statin users with TGs over 135 mg/dL were at ≥ 20% 10-year ASCVD risk and ≥60% of persons in all TG groups were at borderline or higher ASCVD risk. Compared to those with TGs <70 mg/dL, multiple logistic regression showed odds ratios of 3.1 to 4.6 (p < 0.05 to p < 0.01) for those in TG groups ≥135 mg/dL in the overall sample, but 3.4 to 8.1 (p < 0.05 to p < 0.01) for those in TG groups of ≥100 mg/dL in statin users, despite adjustment including HDL-C.
Conclusion
Many US adults with borderline levels of TGs are at elevated ASCVD risk despite statin therapy, suggesting the need first for greater lifestyle modification efforts, and when indicated, evidence-based therapies known to reduce this residual ASCVD risk.
Keywords: Triglycerides, Dyslipidemia, Cardiovascular disease, Risk estimation
Elevated triglycerides (TGs) are frequently encountered in clinical practice and many epidemiological studies have shown it is an independent risk factor for atherosclerotic cardiovascular disease (ASCVD) [1]. According to current guidelines, a TG level of 200 mg/dL or greater is defined as hypertriglyceridemia (HTG) while TGs between 150 and 199 mg/dL are defined as borderline HTG [2]. In the United States, the prevalence of borderline HTG and HTG is 12.8% and 11.9%, respectively [3].
There is evidence, however, that ASCVD risk may increase at levels far below 150 mg/dL, warranting a reconsideration of these definitions. Earlier results from the Baltimore Coronary Observational Long-Term study suggested that TGs ≥100 mg/dL are independently associated with a 50% higher risk of coronary artery disease (CAD), and thus a “normal” TG level is predictive of incident CAD events [4]. The PROVE IT-TIMI 22 trial observed a 1.6% lower risk of the composite end point including death, myocardial infarction (MI), and recurrent acute coronary syndrome (ACS) for each 10 mg/dL decline in on-treatment TG after adjusting for low-density lipoprotein cholesterol (LDL-C) and other covariates among people after an ACS [5]. In addition, results from the Prospective Copenhagen City Heart Study have shown that normal to borderline HTG (TG between 89 and 176 mg/dL) is associated with a 30% significantly higher risk of ischemic stroke in women as compared to those with TG below 89 mg/dL (p < 0.0001) [6]. Quantification of estimated ASCVD risk among people within categories of normal to borderline TG on a population level in the US has not yet been previously studied.
The objective of the current study was to estimate the 10-year ASCVD risk based on the Pooled Cohort Risk Calculator among people with normal to borderline TG levels, including estimating the number of US adults affected, and to determine whether normal and borderline TG levels are independently associated with high calculated ASCVD risk.
1. Research design and methods
1.1. Study sample
We studied US adults who were required to fast at least 8.5 h for laboratory tests from the 8-year combined National Health and Nutrition Examination Survey (NHANES) 2007–2014. The methodology of NHANES data collection has been previously described [7]. The inclusion criteria for our analysis were [1]: adults aged 40–74 years old (required age range for 10-year ASCVD risk calculation) [2]; with available TG data [3]; with available information to calculate pooled cohort 10-year ASCVD risk; and [4] without prior ASCVD defined as self-reported heart attack, stroke, coronary heart disease or angina. A total of 4986 participants (projected to 113.0 million US population) were included in our study. Of those, 1083 (projected to 24.3 million) were statin users while 3903 (88.7 M) were not on statin treatment. Our study utilizing publicly available NHANES data was exempt from Institutional Review Board review.
1.2. Measurements
We abstracted data from NHANES demographic, laboratory test, examination, and questionnaire files. TG was assayed using enzymatic reactions on a Roche/Hitachi Modular P Chemistry Analyzer and were categorized into the following groups: < 70 mg/dL, 70-<100 mg/dL, 100-<135 mg/dL, 135-<150 mg/dL, 150-<200 mg/dL, and TG ≥ 200 mg/dL. High density lipoprotein cholesterol (HDL-C) was analyzed through a modified traditional multistep precipitation reaction while LDL-C was computed using the Friedewald equation (defined as total cholesterol minus HDL-C and TG/5 in mg/dL) in those with TG < 400 mg/dL. Hypertension was defined (based on the accepted definition at the time of the survey years) as a systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or currently taking prescription medication for hypertension. Participants were defined as having diabetes if they met one or more of the following criteria: 1) fasting glucose ≥126 mg/dL; 2) non-fasting glucose ≥200 mg/dL; 3) taking medication to lower blood glucose; 4) taking insulin; or 5) HbA1c ≥ 6.5% (48 mmol/mol). Obesity was defined as a BMI ≥30 kg/m2. Information related to smoking status and family history of heart attack were based on self-report. The AHA/ACC pooled cohort 10-year ASCVD risk score (%) was calculated based on age, gender, ethnicity, systolic blood pressure (SBP), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), hypertension treatment, diabetes, and smoking status [2].
1.3. Statistical analysis
We first created an 8-year sample weighting variable to properly project our sample to the US population. The Student’s t-test and Chi-square test of proportions were used to compare whether the means (of continuous variables) or proportions (of categorical variables) of demographic characteristics were significantly different between statin users and non-users. We then calculated the weighted number and proportion of participants at different TG levels overall with further stratification by statin use. The average 10-year pooled cohort ASCVD risk score was determined in each TG group and then multiplied with the corresponding population size to estimate anticipated 10-year ASCVD events. Next, we categorized the 10-year pooled cohort ASCVD risk into low (<5%), borderline (5-<7.5%), intermediate (7.5-<20%) and high (≥20%) groups and determined the weighted prevalence of ASCVD risk categories by TG levels overall and among statin users and non-users, respectively. Finally, we performed multiple logistic regression analysis to explore the association between TG categories and being at high ASCVD risk (≥20%), initially adjusted for age, sex, and ethnicity (model 1), and then further by diabetes, smoking, systolic blood pressure, antihypertensive treatment (other variables in the ASCVD risk score), as well as LDL-C (model 2) (and in sensitivity analyses non-HDL-C). A final model further adjusted for HDL-C (model 3) and replace LDL-C with non-HDL-C (model 4). Analyses applied our NHANES 8-year sample weighting procedure to project to the U.S. population in millions. SAS version 9.3 was used for data analysis.
2. Results
For our sample of 4986 participants (weighted to 113.0 million) from NHANES 2007–2014, the average age was 55.0 ± 0.2 years old, with 53.3% female and 72.0% non-Hispanic Whites. Compared to non-statin users, statin users were significantly older (60.5 vs. 53.5 yrs, p < 0.0001), with a higher proportion of obesity (44.4% vs. 34.6%, p < 0.0001), diabetes (28.4% vs. 8.8%, p < 0.0001), hypertension (64.3% vs. 35.9%, p < 0.0001) and family history of myocardial infarction (15.8% vs. 12.5%, p = 0.0294). Statin users expectedly had significantly lower total and LDL-C but also higher HDL-C levels as compared to non-statin users (Table 1).
Table 1.
Demographic characteristics comparison between statin users and non-users, NHANES 2007–2014.
| Total | Statin Users | Non-Statin Users | p-value | |
|---|---|---|---|---|
| Age (years) | 55.0 ± 0.2 | 60.5 ± 0.4 | 53.5 ± 0.2 | <0.0001 |
| Female gender n (%) | 2631 (60.2 M, 53.3%) | 556 (12.2 M, 50.4%) | 2075 (47.8 M, 54.0%) | 0.0585 |
| Ethnicity, n (%) | ||||
| Mexican Americans | 764 (7.5 M, 6.6%) | 123 (1.0 M, 4.1%) | 641 (6.5 M, 7.3%) | <0.0001 |
| Other Hispanics | 582 (5.5 M, 4.9%) | 106 (0.8 M, 3.3%) | 476 (4.7 M, 5.3%) | |
| Non-Hispanic Whites | 2190 (81.3 M, 72.0%) | 527 (18.6 M, 76.8%) | 1663 (62.7 M, 70.6%) | |
| Non-Hispanic Blacks | 971 (11.6 M, 10.3%) | 222 (2.2 M, 9.0%) | 749 (9.4 M, 10.6%) | |
| Other races | 479 (7.1 M, 6.2%) | 105 (1.6 M, 6.8%) | 374 (5.5 M, 6.1%) | |
| BMI (kg/m2) | 29.1 ± 0.1 | 30.2 ± 0.2 | 28.9 ± 0.2 | <0.0001 |
| Obesity n(%) | 1901 (41.5 M, 36.7%) | 505 (10.8 M, 44.4%) | 1396 (30.7 M, 34.6%) | <0.0001 |
| Systolic BP (mmHg) | 123.0 ± 0.4 | 125.3 ± 0.6 | 122.3 ± 0.4 | <0.0001 |
| Diastolic BP (mmHg) | 71.2 ± 0.3 | 68.8 ± 0.4 | 71.8 ± 0.3 | <0.0001 |
| Total cholesterol (mg/dL) | 202.4 ± 0.9 | 182.8 ± 2.1 | 207.7 ± 0.9 | <0.0001 |
| LDL-C (mg/dL) | 120.8 ± 0.7 | 101.4 ± 1.7 | 126.2 ± 0.8 | <0.0001 |
| HDL-C (mg/dL) | 55.1 ± 0.3 | 53.6 ± 0.6 | 55.6 ± 0.3 | 0.0005 |
| Triglycerides (mg/dL) | 135.2 ± 2.3 | 141.0 ± 4.6 | 133.6 ± 2.6 | 0.1633 |
| Diabetes n(%) | 889 (14.7 M, 13.0%) | 414 (6.9 M, 28.4%) | 475 (7.8 M, 8.8%) | <0.0001 |
| Current cigarette smoking n(%) | 922 (19.7 M, 17.5%) | 151 (3.3 M, 13.7%) | 771 (16.4 M, 18.5%) | 0.0096 |
| Hypertension n(%) | 2302 (47.4 M, 42.0%) | 754 (15.6 M, 64.3%) | 1548 (31.8 M, 35.9%) | <0.0001 |
| Family history of heart attack n(%) | 617 (14.9 M, 13.2%) | 157 (3.8 M, 15.8%) | 460 (11.1 M,12.5%) | 0.0294 |
Numbers were displayed as weighted means ± SE for continuous variables and unweighted number (weighted number, weighted percentage) for categorical variables; BP = blood pressure, LDL-C = low density lipoprotein-cholesterol, HDL-C = high density lipoprotein-choleserol.
Overall, 18.6% (21.0 M) of the participants had TG < 70 mg/dL, 24.2% (27.4 M) of TG 70-<100 mg/dL, 22.0% (24.9 M) of TG 100-<135 mg/dL, 6.2% (7.0 M) of TG 135-<150 mg/dL, 15.0% (16.9 M) of TG 150-<200 mg/dL, and 14.0% (15.8 M) of TG ≥ 200 mg/dL, with the corresponding mean 10-year ASCVD risk of 5.6%, 6.9%, 7.8%, 10.3%, 9.6%, 10.8%, respectively. This translates to the number of predicted 10-year ASCVD events of 1.2 M, 1.9 M, 1.9 M, 0.7 M, 1.6 M, and 1.7 M, respectively. Of note, statin users had almost double the ASCVD risk compared to non-statin users especially among those with TG below 135 mg/dL (Table 2).
Table 2.
Weighted population size, mean 10-year ASCVD risk, and anticipated ASCVD events in 10 years stratified by triglyceride level, NHANES 2007–2014.
| TG Categories | Weighted Population (n = 4986, 113.0 M) | Mean 10-year ASCVD Risk | Anticipated 10-year ASCVD Events |
|---|---|---|---|
| Overall | |||
| <70 mg/dL | 902 (21.0 M, 18.6%) | 5.6 | 1.17 M |
| 70-<100 | 1198 (27.4 M, 24.2%) | 6.9 | 1.88 M |
| 100-<135 | 1128 (24.9 M, 22.0%) | 7.8 | 1.94 M |
| 135-<150 | 325 (7.0 M, 6.2%) | 10.3 | 0.71 M |
| 150-<200 | 732 (16.9 M, 15.0%) | 9.6 | 1.63 M |
| >=200 |
701 (15.8 M, 14.0%) |
10.8 |
1.71 M |
| Statin Users | |||
| <70 mg/dL | 161 (3.6 M, 14.7%) | 10.7 | 0.38 M |
| 70-<100 | 239 (5.5 M, 22.8%) | 10.8 | 0.59 M |
| 100-<135 | 258 (5.5 M, 22.8%) | 11.5 | 0.63 M |
| 135-<150 | 90 (1.7 M, 7.1%) | 14.2 | 0.24 M |
| 150-<200 | 174 (4.3 M, 17.7%) | 13.2 | 0.57 M |
| >=200 |
161 (3.6 M, 14.9%) |
14.6 |
0.53 M |
| Non-Statin Users | |||
| <70 mg/dL | 741 (17.4 M, 19.7%) | 4.6 | 0.79 M |
| 70-<100 | 959 (21.9 M, 24.7%) | 5.9 | 1.29 M |
| 100-<135 | 870 (19.3 M, 21.8%) | 6.8 | 1.31 M |
| 135-<150 | 235 (5.2 M, 5.9%) | 9.0 | 0.47 M |
| 150-<200 | 558 (12.6 M, 14.2%) | 8.4 | 1.06 M |
| >=200 | 540 (12.2 M, 13.7%) | 9.7 | 1.18 M |
Anticipated 10-year ASCVD events were calculated by multiplying weighted population and mean 10-years ASCVD risk in each TG group.
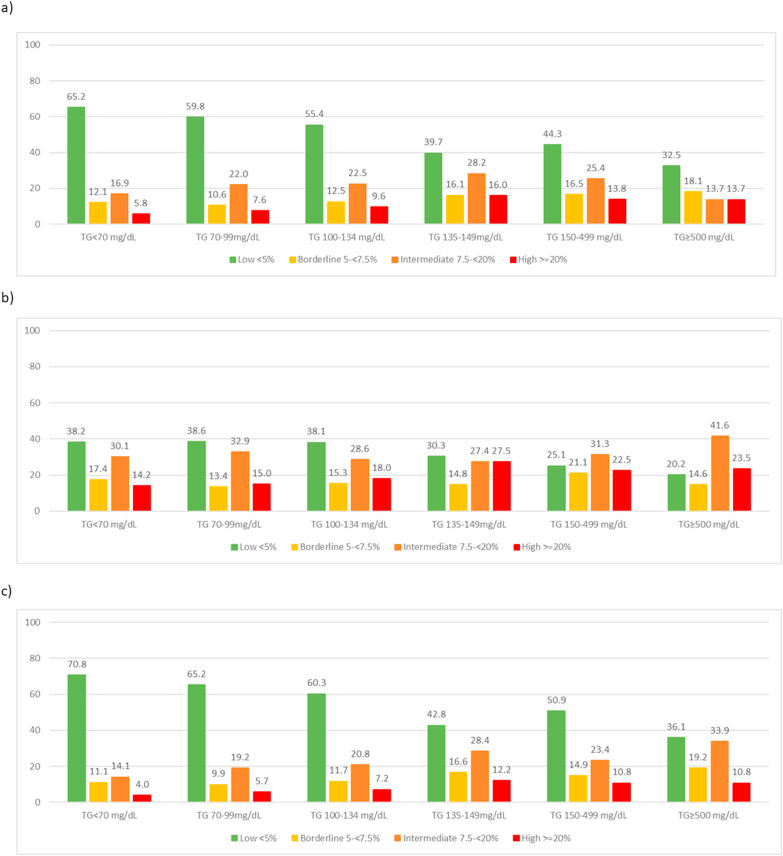
We also observed a trend for a greater proportion of persons in the high ASCVD risk category (≥20%) across increasing TG categories, regardless of statin use (Fig. 1). A similar trend was seen for borderline and intermediate ASCVD risk (5-<20%) among non-statin users while it remained relatively stable among statin users. Unexpectedly, individuals with TGs of 135–<150 mg/dL had the highest proportion at high ASCVD risk status (16.0%), including those both with or without statin treatment (27.5%, 12.2%, respectively). At least one-fifth or more of statin users with elevated TG over 135 mg/dL were at ≥ 20% 10-year ASCVD risk and >60% of persons in all TG groups were at borderline or higher ASCVD risk. A comparison of risk factors that were part of the Pooled Cohort risk score among TG categories (Appendix Table 1) shows that age and Black race may be in part responsible for the higher risk seen in those with TGs of 135–<150 mg/dL; however, adjustment for age, race and other factors largely diminishes this effect so that those with TGs in this range have similar adjusted risk to those in higher TG categories. Also of interest, LDL-C levels are quite similar for TG categories beginning at 100 mg/dL, so the increased risks at higher levels are not due to increases in LDL-C.
Fig. 1.
Prevalence (%) of 10-Year ASCVD Risk Categories by Triglyceride Category among a) Overall Population, b) Statin Users, c) No Statin Use.
Multiple logistic regression analyses show that compared to those with TGs under 70 mg/dL, those with TG levels of 100 mg/dL or greater have more than double the likelihood of being at high ASCVD risk after adjusting for age, gender, and ethnicity, overall and among statin users(Table 3). In the overall sample, after additional adjustment for LDL-C and other risk factors, these relationships remain significant at TG levels of 100 mg/dL or greater, and after further adjustment for HDL-C at TG levels of 135 mg/dL or greater. Of note, among statin users, even after adjustment for HDL-C, TG levels of 100-<135 mg/dL are associated with more than a 3-fold greater odds of high risk status, with levels of 135 mg/dL or higher shown to have more than a 6-fold greater odds. In those not on statins, however, relationships are attenuated after adjustment for HDL-C. In addition, replacing LDL-C with non-HDL-C largely attenuated relationships (Table 3 model 4).
Table 3.
Multiple logistic regression models of the association between triglyceride categories and likelihood of ≥20% ASCVD risk overall and by statin use.
| Overall Adjusted OR (95%) | Statin Users Adjusted OR (95%) | Non-statin Users Adjusted OR (95%) | |
|---|---|---|---|
| Model 1 - Adjusted for age, gender, ethnicity (n = 4986) | |||
| <70 mg/dL | Reference | ||
| 70-<100 | 1.64 (1.06–2.52)∗ | 1.76 (0.93–3.34) | 1.61 (0.94–2.78) |
| 100-<135 | 2.42 (1.51–3.88)∗∗ | 2.74 (1.45–5.19)∗∗ | 2.33 (1.29–4.23)∗∗ |
| 135-<150 | 5.00 (2.79–8.96)∗∗∗ | 5.25 (2.38–11.57)∗∗∗ | 4.95 (2.20–11.15)∗∗ |
| 150-<200 | 4.90 (2.66–9.02)∗∗∗ | 4.42 (2.05–9.54)∗∗ | 5.31 (2.72–10.36)∗∗∗ |
| >=200 | 9.47 (6.27–14.29)∗∗∗ | 8.83 (3.90–19.98)∗∗∗ | 10.00 (6.10–16.39)∗∗∗ |
| Model 2 - Adjusted for age, DM, SBP, gender, current smoking, hypertension medication, ethnicity, and LDL-C (n = 4889) | |||
| <70 mg/dL | Reference | ||
| 70-<100 | 1.69 (0.77–3.70) | 2.42 (0.64–9.20) | 1.35 (0.49–3.69) |
| 100-<135 | 2.19 (1.00–4.77)∗ | 3.82 (1.32–11.07)∗ | 1.68 (0.58–4.90) |
| 135-<150 | 6.69 (2.71–16.48)∗∗∗ | 8.94 (2.79–28.69)∗∗ | 5.08 (1.34–19.19)∗ |
| 150-<200 | 5.07 (2.14–12.01)∗∗ | 7.28 (2.14–24.70)∗∗ | 3.59 (1.12–11.53)∗ |
| >=200 | 7.48 (3.09–18.12)∗∗∗ | 8.50 (2.64–27.41)∗∗ | 6.44 (1.75–23.75)∗∗ |
| Model 3 - Adjusted for age, DM, SBP, gender, current smoking, hypertension medication, ethnicity, LDL-C and HDL-C (n = 4889) | |||
| <70 mg/dL | Reference | ||
| 70-<100 | 1.48 (0.66–3.33) | 2.24 (0.59–8.57) | 1.15 (0.41–3.25) |
| 100-<135 | 1.51 (0.67–3.41) | 3.40 (1.13–10.29)∗ | 0.95 (0.33–2.78) |
| 135-<150 | 4.57 (1.91–10.91)∗∗ | 8.07 (2.23–29.20)∗∗ | 2.79 (0.79–9.92) |
| 150-<200 | 3.12 (1.25–7.81)∗ | 6.32 (1.67–23.83)∗∗ | 1.69 (0.48–5.94) |
| >=200 | 3.68 (1.39–9.75)∗∗ | 6.80 (1.64–28.29)∗∗ | 2.18 (0.57–8.30) |
| Model 4 - Adjusted for age, DM, SBP, gender, current smoking, hypertension medication, ethnicity, non-HDL-C and HDL-C (n = 4889) | |||
| <70 mg/dL | Reference | Reference | Reference |
| 70-<100 | 1.22 (0.55–2.72) | 1.74 (0.44–6.87) | 0.96 (0.34–2.71) |
| 100-<135 | 0.97 (0.43–2.21) | 2.12 (0.67–6.74) | 0.60 (0.20–1.80) |
| 135-<150 | 2.38 (1.03–5.51) | 3.92 (1.01–15.14) | 1.46 (0.43–4.99) |
| 150-<200 | 1.38 (0.53–3.55) | 2.48 (0.63–9.76) | 0.77 (0.21–2.85) |
| >=200 | 1.30 (0.49–3.43) | 2.18 (0.51–9.43) | 0.75 (0.19–2.90) |
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.0001.97 subjects had missing values of LDL-C.
Given the premise that our calculated ASCVD risks from the pooled cohort risk score are likely to be underestimated due to not being able to account for pre-treatment cholesterol levels, we have done a sensitivity analysis involving recalculating our 10-year risk based on the assumption that pre-treatment total cholesterol levels would be 30% higher on average than our currently calculated levels. This results in 10-year risks that are on average only 1.8% higher.
Our logistic regression results showing those in the 100–<150 mg/dl TG ranges to be significantly associated with high risk status, moreover, still hold among statin users (HR’s of 3.4 and 8.1 for TGs of 100-<135 mg/dL and 135-<150 mg/dL, respectively, p < 0.05 to p < 0.01, in analyses adjusted for all factors except non-HDL-C). Of note, much of the difference in estimated risk between statin users and non-users are due to differences in age and other risk factors that are greater/more common in statin users, not surprising given the nature of persons prescribed statins. Between statin users and non-statin users, there is an unadjusted difference in 10-year ASCVD risk of 5.3%; after adjustment for age, gender, ethnicity, systolic blood pressure, HDL-C, hypertension treatment, diabetes, and smoking (but not total cholesterol), this adjusted risk difference diminishes to 0.03%, indicating factors other than differences in cholesterol account for nearly all of the difference in risk between statin users and non-users.
3. Discussion
Our study is unique in quantifying the prevalence of borderline TG levels in the US population and further estimating their 10-year ASCVD risk both in those on and not on statin treatment. We demonstrate that despite statin use, more than 60% of such persons were at borderline or higher (≥5%) ASCVD risk, with ≥20% at high ASCVD risk. Moreover, these relationships of borderline and borderline high TG with high 10-year ASCVD risk (≥20%) were independent of major ASCVD risk factors, including LDL-C and HDL-C. Among statin users, the likelihood of high risk status was 3-fold or more in those with TGs 100 mg/dL or greater compared to <70 mg/dL, suggesting that despite LDL-C reductions induced by statins, significant serum levels of atherogenic triglyceride-rich remnant lipoproteins may still circulate even at TG levels that have traditionally been classified in the normal range. For non-statin users, who are in general considered to be healthier people, the association between TG and ASCVD risk is mainly through HDL-C (odds ratios become non-significant after adjusting for HDL-C). However, for statin users who are significantly older and with greater risk factors than non-users, the potential effects of these risk factors might overwhelm HDL-C alone, and thus TG remains significantly associated with ASCVD risk.
There has been considerable debate on the role of TG in the development and progression of ASCVD, as patients with severely elevated TG (≥1000 mg/dL) may not be at high risk of ASCVD while other individuals with moderate elevated TG are at increased risk. One possible argument is that the predominant TG lipoproteins are chylomicrons in patients with severe hypertriglyceridemia, which are too large to penetrate the endothelial surface and promote atherosclerosis [8]. However, for patients with moderately elevated TG, the predominant TG-rich lipoproteins are very-low-density lipoproteins (VLDL), VLDL remnants, and intermediate-density lipoprotein (IDL) that are small enough to penetrate the intimal surface and lead to atherosclerosis. This might also explain why our subjects with TGs between 135 and 149 mg/dL (and as low as 100 mg/dL among statin users) are at increased likelihood of high ASCVD risk status, which remains significant even after adjustment for major ASCVD risk factors including LDL-C and HDL-C. However, adjustment for non-HDL-C attenuated findings, possibly due to “overadjustment” from the inclusion of some TG rich lipoproteins within non-HDL-C. There is variability in the definition of remnant cholesterol measurement, including estimation using Friedwald-calculated VLDL-C: TGs divided by 5 [9], total cholesterol minus HDL-C minus LDL-C (with LDL-C was estimated by the Friedewald equation) [10], or measured directly by immunoseparation assay [11]. These particles can hold more cholesterol than LDL particles and therefore may be particularly atherogenic. In addition, triglyceride-enriched lipoproteins have been shown to be proinflammatory, induce endothelial dysfunction, and stimulate a large number of proatherogenic phenomena [12,13]. Macrophages can scavenge TG-rich remnants directly, which potentiates foam cell formation and atherogenesis [14,15]. Low levels of HDL cholesterol are commonly associated with hypertriglyceridemia. While TG levels can vary widely on a daily basis, HDL-C levels are less variable and, therefore, low HDL-C may be a marker for the presence of TG-rich remnant lipoproteins [14]. It also explains why it is more strongly associated with ASCVD risk than TG in our analysis. Results from the Emerging Risk Factors Collaboration which assessed over 300,000 participants from 68 prospective studies found a CAD hazard ratio (HR) of 1.37 (95% confidence interval [95% CI]: 1.31–1.42) with increased TG, which attenuated to a nonsignificant hazard ratio of 0.99 (95% CI: 0.94–1.05) after adjustment for HDL-C and non-HDL-C. These investigators concluded that, when assessing vascular risk on a population level, TG measurement provides no additional information about vascular risk beyond HDL-C and total cholesterol levels [16]. Moreover, the disconnect of LDL-C/LDL particles with HTG, with elevated number of small LDL particles prominently associated with increased TG and decreased HDL-C levels, might also explain the high ASCVD risk among borderline and borderline high TG group [17]. In our study, among those factors used to calculate 10-year ASCVD risk, those with TG 135-<150 mg/dL had higher mean age and prevalence of Black ethnicity (compared to TG 150-<200 mg/dL and TG ≥ 200 mg/dL); however, in our multivariable analyses, after adjustment for these and other factors, those with TG 135-<150 mg/dL had similar likelihood of being at high risk status as those with higher TG levels.
Epidemiological studies have suggested a dose-dependent, causal relationship between TG and ASCVD. Results from a Mendelian randomization study of 73,513 participants from Copenhagen suggested that TG increases of 39 mg/dL were associated with a 2.8-fold increased risk for ischemic heart disease, although failure to adequately adjust for ApoB may render the results less reliable [18]. Meta-analysis of published data reported relative risks for incident CVD adjusting for age, HDL-C, total cholesterol, LDL-C, smoking, BMI and blood pressure of 1.14 (95% CI 1.05–1.28) in men and 1.37 (95% CI, 1.13–1.66) in women per 1-mmol/L (88.6 mg/dL) increase in triglycerides [19]. Among adults with metabolic syndrome, TGs of 139 mg/dL or higher were associated with a 40% higher risk of future CVD independent of age, diabetes, lipid-lowering medication, total cholesterol or HDL-C [20]. Finally, a recent analysis of average TGs over time in Framingham Heart Study participants notes an increasing risk of CVD events within the range previously considered normal, plateauing at a level of approximately 100 mg/dL in men and 200 mg/dL in women [21]. Thus, individuals with borderline TG are at substantial risk that needs to be addressed through prevention strategies. It also warrants further investigation on the appropriateness of using fixed cut-off point for TG analysis in other studies that have controversial conclusions, especially when examining the independent contribution from TG to the development of ASCVD. In addition, evidence from prospective cohort studies have also emphasized the role of non-fasting TG in predicting CVD with a suggested cut-off point of 175 mg/dL. It argues that non-fasting TG are at least equivalent to fasting TG in CVD risk evaluation [22]. Decreases in the prevalence of elevated TG might have been achieved through promoting lifestyle modification and lipid-lowering medication use, especially with statins. However, our results show that a substantial proportion of the US population remains at high ASCVD risk among normal to borderline TG despite statin use, warranting the need for further therapies that can reduce ASCVD risk in such patients. The REDUCE-IT trial has shown that 4 g/d of icosapent ethyl EPA reduces first ASCVD events by 25% in persons with known ASCVD or diabetes plus other risk factors who were on statin therapy and had TG levels of 135–499 mg/dL [23]. Together with the findings from our current study, identification of supra-optimal TG levels in patients already on statin therapy might further help reveal their unmanaged CVD risk, regardless of whether there is a direct casual relation, and lifestyle changes and any therapeutics shown to be effective in these patients should be considered.
There are several strengths and limitations in our study. Most importantly, our study is cross-sectional in design correlating TG levels with estimated ASCVD risk, and not actual subsequent ASCVD events. Of note, NHANES has a weighting procedure allowing estimates to be projected (in millions) to the US population, including the estimated ASCVD events in the US that could be expected within the next decade as we have shown according to TG categories and statin use. One limitation is that we only include those aged 40–79 based on the eligibility for ASCVD risk assessment, thus overall risk may be higher than if younger persons were included in the sample. In addition, the ASCVD event risks may also be underestimated among statin users because their risk is calculated based on their cholesterol level at the time of survey and may not reflect the duration of high cholesterol often present for decades before treatment. We show, however, that much of the difference in risk between statin users and non-users is not due to the difference in total cholesterol, but instead and other risk factors that are part of the risk equation. A regression analysis of each risk factor component in relation to the 10-year risk shows the relative contribution of these factors were as follows (from high to low): age, diabetes, systolic blood pressure, gender, smoking status, total cholesterol, HDL-C, ethnicity, hypertension treatment. Being as female and having higher HDL-C level was negatively associated with the increased of ASCVD risk, while all other factors in the model had positive association. Also, our study only compared the estimated ASCVD risk instead of the measurement of actual ASCVD risk in this population. NHANES also does not collect information regarding duration of statin treatment, statin intensity, and statin adherence which may also affect the ASCVD risk estimates. Lastly, NHANES relies on self-reported medical history information, but does have accurate and standardized measurements of many risk factors including glucose, lipids, and blood pressure.
Our study suggests that many individuals with borderline levels of, especially at or above 135 mg/dL are at high 10-year calculated ASCVD risk. Noteworthy, we show among statin users, the likelihood of high risk status was 3-fold or more in those with TGs 100 mg/dL or greater compared to <70 mg/dL, suggesting significant amounts of atherogenic lipoproteins and remnants may still circulate even at such low TG levels. Prospective studies stratified by statin use are needed to confirm our results. While such increased risks are often explained by low HDL-C and other risk factors that often accompanies even borderline TG levels, our findings show such risk in statin-treated persons may persist despite adjustment for HDL-C. The AHA suggests that the optimal TG levels may be less than 100 mg/dL [24]. Our study suggests greater attention should be given to those with TGs as low as 100 mg/dL, especially among those on statin therapy. Greater efforts first at lifestyle modification and consideration of evidence-based therapies, where indicated, can be suggested to further address this persistent residual ASCVD risk.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This study was supported by a contract from Amarin Pharma, Inc. to the University of California, Irvine. Dr. Wong reports research support through the University of California, Irvine from Amarin, Amgen, Novartis, Boehringer-Ingelheim, Gilead and Novo-Nordisk and is on the speakers bureau for Amarin, Sanofi, and Esperion. Dr. Toth is a consultant to Amarin, Amgen, and Kowa. He is a member of the speakers bureau for Amarin, Amgen, Esperion, and Novo-Nordisk. Drs. Philip and Granowitz are employees and stock shareholders of Amarin Pharma, Inc.
Acknowledgements and Disclosures
This study was presented in part at the American Heart Association Scientific Sessions, Dallas, TX, November 2018 and was supported by a contract from Amarin Pharma, Inc. to the University of California, Irvine. Dr. Wong reports research support through the University of California, Irvine from Amarin, Amgen, Novartis, Boehringer-Ingelheim, Gilead and Novo-Nordisk and is on the speakers bureau for Amarin, Sanofi, and Esperion. Dr. Toth is a consultant to Amarin, Amgen, and Kowa. He is a member of the speakers bureau for Amarin, Amgen, Esperion, and Novo-Nordisk. Drs. Philip and Granowitz are employees and stock shareholders of Amarin Pharma, Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajpc.2020.100087.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Xiao C., Dash S., Morgantini C., Hegele R.A., Lewis G.F. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes. 2016;65(7):1767–1778. doi: 10.2337/db16-0046. [DOI] [PubMed] [Google Scholar]
- 2.Grundy S.M., Stone N.J., Bailey A.L. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Fan W., Philip S., Granowitz C., Toth P.P., Wong N.D. Hypertriglyceridemia in statin-treated US adults: the national Health and nutrition examination survey. J Clin Lipidol. 2019;13(1):100–108. doi: 10.1016/j.jacl.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Miller M., Seidler A., Moalemi A., Pearson T.A. Normal triglyceride levels and coronary artery disease events: the Baltimore Coronary Observational Long-Term Study. J Am Coll Cardiol. 1998;31(6):1252–1257. doi: 10.1016/s0735-1097(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 5.Miller M., Cannon C.P., Murphy S.A., Qin J., Ray K.K., Braunwald E. PROVE IT-TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Freiberg J.J., Tybjaerg-Hansen A., Jensen J.S., Nordestgaard B.G. Nonfasting triglycerides and risk of ischemic stroke in the general population. J Am Med Assoc. 2008;300(18):2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 7.NHANES . US Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2007-2008. Laboratory procedures Manual. [Google Scholar]
- 8.Stalenhoef A.F., de Graaf J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr Opin Lipidol. 2008;19(4):355–361. doi: 10.1097/MOL.0b013e328304b63c. [DOI] [PubMed] [Google Scholar]
- 9.Jones S.R., Martin S.S., Brinton E.A. Letter by Jones et al regarding article,“Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation”. Circulation. 2014;129(24) doi: 10.1161/CIRCULATIONAHA.113.005954. e655-e655. [DOI] [PubMed] [Google Scholar]
- 10.Würtz P., Kangas A.J., Soininen P. Lipoprotein subclass profiling reveals pleiotropy in the genetic variants of lipid risk factors for coronary heart disease: a note on Mendelian randomization studies. J Am Coll Cardiol. 2013;62(20):1906–1908. doi: 10.1016/j.jacc.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 11.Brinton E.A., Ballantyne C.M., Bays H.E., Kastelein J.J., Braeckman R.A., Soni P.N. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200–500 mg/dL), and on statin therapy at LDL-C goal: the ANCHOR study. Cardiovasc Diabetol. 2013;12(1):100. doi: 10.1186/1475-2840-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varbo A., Benn M., Nordestgaard B.G. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141(3):358–367. doi: 10.1016/j.pharmthera.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Varbo A., Nordestgaard B.G. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2016;36(11):2133–2135. doi: 10.1161/ATVBAHA.116.308305. [DOI] [PubMed] [Google Scholar]
- 14.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 15.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S.M., Khaw K.T., Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 16.Emerging Risk Factors Collaboration, Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K.K., Thompson A., Wood A.M., Lewington S., Sattar N., Packard C.J., Collins R., Thompson S.G., Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. J Am Med Assoc. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kathiresan S., Otvos J.D., Sullivan L.M. Increased small low-density lipoprotein particle number. Circulation. 2006;113(1):20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 18.Sarwar N., Sandhu M.S., Ricketts S.L. Triglyceride coronary disease genetics consortium and emerging risk factors collaboration. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375(9726):1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hokanson J.E., Austin M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–219. [PubMed] [Google Scholar]
- 20.Onat A., Sarı İ., Yazıcı M., Can G., Hergenç G., Avcı G.Ş. Plasma triglycerides, an independent predictor of cardiovascular disease in men: a prospective study based on a population with prevalent metabolic syndrome. Int J Cardiol. 2006;108(1):89–95. doi: 10.1016/j.ijcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 21.Abberra T., Peterson E.D., Pagidipati N.J. The association between triglycerides and incident cardiovascular disease: what is ‘‘optimal’’? J Clin Lipidol. 2020 doi: 10.1016/j.jacl.2020.04.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White K.T., Moorthy M.V., Akinkuolie A.O. Identifying an optimal cutpoint for the diagnosis of hypertriglyceridemia in the nonfasting state. Clin Chem. 2015;61(9):1156–1163. doi: 10.1373/clinchem.2015.241752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatt D.L., Steg P.G., Miller M. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 24.Miller M., Stone N.J., Ballantyne C. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.