Abstract
Background
Doxycycline is often used for treating COVID-19 respiratory symptoms in the community despite an absence of evidence from clinical trials to support its use. We aimed to assess the efficacy of doxycycline to treat suspected COVID-19 in the community among people at high risk of adverse outcomes.
Methods
We did a national, open-label, multi-arm, adaptive platform randomised trial of interventions against COVID-19 in older people (PRINCIPLE) across primary care centres in the UK. We included people aged 65 years or older, or 50 years or older with comorbidities (weakened immune system, heart disease, hypertension, asthma or lung disease, diabetes, mild hepatic impairment, stroke or neurological problem, and self-reported obesity or body-mass index of 35 kg/m2 or greater), who had been unwell (for ≤14 days) with suspected COVID-19 or a positive PCR test for SARS-CoV-2 infection in the community. Participants were randomly assigned using response adaptive randomisation to usual care only, usual care plus oral doxycycline (200 mg on day 1, then 100 mg once daily for the following 6 days), or usual care plus other interventions. The interventions reported in this manuscript are usual care plus doxycycline and usual care only; evaluations of other interventions in this platform trial are ongoing. The coprimary endpoints were time to first self-reported recovery, and hospitalisation or death related to COVID-19, both measured over 28 days from randomisation and analysed by intention to treat. This trial is ongoing and is registered with ISRCTN, 86534580.
Findings
The trial opened on April 2, 2020. Randomisation to doxycycline began on July 24, 2020, and was stopped on Dec 14, 2020, because the prespecified futility criterion was met; 2689 participants were enrolled and randomised between these dates. Of these, 2508 (93·3%) participants contributed follow-up data and were included in the primary analysis: 780 (31·1%) in the usual care plus doxycycline group, 948 in the usual care only group (37·8%), and 780 (31·1%) in the usual care plus other interventions group. Among the 1792 participants randomly assigned to the usual care plus doxycycline and usual care only groups, the mean age was 61·1 years (SD 7·9); 999 (55·7%) participants were female and 790 (44·1%) were male. In the primary analysis model, there was little evidence of difference in median time to first self-reported recovery between the usual care plus doxycycline group and the usual care only group (9·6 [95% Bayesian Credible Interval [BCI] 8·3 to 11·0] days vs 10·1 [8·7 to 11·7] days, hazard ratio 1·04 [95% BCI 0·93 to 1·17]). The estimated benefit in median time to first self-reported recovery was 0·5 days [95% BCI −0·99 to 2·04] and the probability of a clinically meaningful benefit (defined as ≥1·5 days) was 0·10. Hospitalisation or death related to COVID-19 occurred in 41 (crude percentage 5·3%) participants in the usual care plus doxycycline group and 43 (4·5%) in the usual care only group (estimated absolute percentage difference −0·5% [95% BCI −2·6 to 1·4]); there were five deaths (0·6%) in the usual care plus doxycycline group and two (0·2%) in the usual care only group.
Interpretation
In patients with suspected COVID-19 in the community in the UK, who were at high risk of adverse outcomes, treatment with doxycycline was not associated with clinically meaningful reductions in time to recovery or hospital admissions or deaths related to COVID-19, and should not be used as a routine treatment for COVID-19.
Funding
UK Research and Innovation, Department of Health and Social Care, National Institute for Health Research.
Introduction
There is an urgent need to identify effective and safe treatments for COVID-19, especially for older people (age ≥50 years) and those with comorbidities who are at higher risk of hospitalisation and death compared with younger patients and those without comorbidities.1
Doxycycline is a licensed, widely available, inexpensive antibiotic with a favourable safety profile that has been proposed as a treatment for COVID-19,2, 3 due to its in-vitro activity against SARS-CoV-2, with a 50% effective concentration of 4·5 μM, which is consistent with lung doxycycline levels at standard oral doses of 100–200 mg daily.4 In addition, doxycycline has anti-inflammatory properties that might reduce adverse outcomes. It decreases nitrous oxide production5 and inhibits matrix metalloproteinase-9,6 which has a role in acute respiratory distress syndrome.7 Doxycycline can also treat bacterial super-infection, which is a potentially important pathway to severe COVID-19—particularly in older people or those with comorbidities.
Doxycycline has been used as a specific treatment for COVID-19 in India and Brazil;8, 9 whereas, in the UK, national guidelines recommend doxycycline for suspected COVID-19 pneumonia in patients at high risk of adverse outcomes in the community, or if bacterial infection is suspected.10 WHO and the US Centers for Disease Control and Prevention recommend antibiotics for suspected bacterial pneumonia in COVID-19, with doxycycline included in the treatment guidelines for community acquired pneumonia.11, 12, 13 Community prescribing data from the USA and the UK suggests there has been increased use of doxycycline for respiratory tract infections during the COVID-19 pandemic,14, 15, 16, 17 which could exacerbate antimicrobial resistance.18 Randomised trials evaluating doxycycline as a treatment for COVID-19 are therefore needed to either provide evidence for its effectiveness, or if it is shown to be ineffective, to prevent its unnecessary use.
We aimed to assess whether doxycycline effects self-reported recovery time or reduces hospital admissions or deaths related to COVID-19 in people at high risk of adverse outcomes in the community.
Methods
Study design
We did a national, open-label, multi-arm, adaptive platform randomised trial of interventions against COVID-19 in older people (PRINCIPLE) across primary care centres in the UK. A platform trial allows multiple treatments for the same disease to be trialled simultaneously. A master protocol defines prospective decision criteria for stopping interventions because of futility, declaring interventions superior, or adding new interventions.19 This design allows the rapid assessment of multiple interventions, with the aim of rapidly stopping interventions with little evidence of meaningful benefit, and thereby directing resources towards evaluation of new interventions, with the overarching aim in this case of identifying community-based treatments for COVID-19. Interventions evaluated in PRINCIPLE have included hydroxychloroquine (discontinued), azithromycin (discontinued), doxycycline, and inhaled budesonide (discontinued). Here, we report outcomes for doxycycline.
The UK Medicines and Healthcare products Regulatory Agency and the South Central-Berkshire Research Ethics Committee (20/SC/0158), recognised by the UK Ethics Committee Authority, approved the trial protocol version 6.3, and all trial recruitment processes. Online informed consent was obtained from all participants. An independent trial steering committee and data monitoring and safety committee provided trial oversight. The protocol is available online and in the appendix (pp 2–67).
Research in context.
Evidence before this study
We searched PubMed on Feb 24, 2021, using the search terms (“randomised” OR “trial”) AND (“doxycycline” OR “tetracycline”) AND (“COVID*” OR “SARS-CoV-2” OR “SARS-CoV”), with no language or date restrictions, and identified 21 papers, one of which reported findings from a randomised controlled trial that provided some data for the effectiveness of doxycycline as a treatment for COVID-19 compared with controls or usual care. In this double-blind trial from Bangladesh, the investigators compared oral doxycycline (200 mg on day 1, followed by 100 mg every 12 h for the next 4 days) plus oral ivermectin (12 mg once daily for 5 days), oral ivermectin alone, and a placebo control, in 72 adults (mean age 42 years) hospitalised with COVID-19 (n=24 per group). There was no evidence of difference in the primary outcome of mean time to viral clearance between the doxycycline plus ivermectin group (11·5 days [95% CI 9·8–13·2]) and the placebo group (12·7 days [11·3–14·2]; p=0·27), although time to viral clearance was shorter in the ivermectin alone group (9·7 days [7·8–11·8]) than in the placebo group. We searched ClinicalTrials.gov on Feb 24, 2021, with the same search strategy and identified 13 additional ongoing or completed randomised controlled trials assessing doxycycline as a treatment for COVID-19, none of which had yet reported results.
Added value of this study
To our knowledge, this is the first randomised controlled trial to report the efficacy of doxycycline as a standalone treatment for patients with COVID-19 in the community. We did not find evidence that doxycycline meaningfully improved recovery time or reduced hospitalisations or deaths when used in this setting.
Implications of all the available evidence
Our findings, from this study among older adults and those with comorbidities, do not support the routine use of doxycycline for suspected COVID-19 in the community in the absence of other indications such as bacterial pneumonia. Emerging evidence suggests that bacterial co-infection in COVID-19 is uncommon, therefore antibiotic treatment is unlikely to benefit most individuals with COVID-19 in the community in well resourced countries, and wider use without clear benefit could lead to public health harm through increased antibiotic resistance. Further research to identify strategies for diagnosing bacterial pneumonia in patients with COVID-19 in the community is needed.
Participants
We enrolled people in the community who were aged 65 years or older, or 50 years or older with comorbidities, who had ongoing symptoms (for ≤14 days) from PCR-confirmed SARS-CoV-2 infection or suspected COVID-19 (in accordance with the UK National Health Service [NHS] definition of high temperature, new continuous cough, or change in sense of smell or taste).20 Comorbidities required for eligibility in people aged 50–64 years were: weakened immune system, heart disease, hypertension, asthma or lung disease, diabetes, mild hepatic impairment, stroke or neurological problem, and self-reported obesity or body-mass index of 35 kg/m2 or greater. People were ineligible if: they were currently an inpatient in hospital, they had almost recovered (general condition much improved and COVID-19 symptoms now mild or almost absent), in the judgement of the recruiting clinician was deemed ineligible, or if they had previously been assigned to a group in the PRINCIPLE trial. People were ineligible to be assigned to doxycycline if they were already taking antibiotics for an acute condition or if doxycycline was contraindicated (appendix p 55). Initially, eligible people were recruited, screened, and enrolled through participating general medical practices, but from May 17, 2020, people across the UK could enrol online or by telephone. After each patient completed a baseline and screening questionnaire, a clinician or trained research nurse confirmed their eligibility using the patient's primary care medical record, accessed remotely where necessary, before randomisation.
Randomisation and masking
Participants were randomly assigned using a secure, web-based, in-house, randomisation system (Sortition version 2.3). When the doxycycline group opened, the azithromycin and usual care only groups were also active, and participants were randomly assigned (1:1:1) to one of the three groups, stratified by age and comorbidity. Subsequent randomisation probabilities were determined using response adaptive randomisation via regular interim analyses, which allowed allocation of more participants to interventions with better observed outcomes (appendix pp 68–167). The trial team was masked to randomisation probabilities.
Procedures
The interventions reported in this manuscript are usual care plus oral doxycycline (200 mg on day 1, followed by 100 mg once daily for the following 6 days) and usual care only. Usual care in the NHS for suspected uncomplicated COVID-19 in the community is largely supportive. Antibiotics are only recommended for suspected COVID-19 pneumonia if bacterial infection is suspected or if the patient is at high risk of adverse outcomes, in which case the guidelines recommend doxycycline.10 In this trial, doxycycline was either prescribed or issued directly by the participant's general medical practitioner, or issued centrally by the study team and delivered by urgent courier to the participant.
Participants were followed up through an online, daily symptom diary for 28 days after randomisation, supplemented with telephone calls on days 2, 14, and 28. Participants were encouraged to nominate a trial partner to help provide follow-up data. We obtained consent to ascertain health-care use outcome data from general practice and hospital records. We aimed to provide a SARS-CoV-2 self-swab for PCR testing promptly after randomisation, but capacity issues early in the COVID-19 pandemic meant swab testing was unavailable for some participants.
Outcomes
The trial commenced with the primary outcome of hospitalisation or death related to COVID-19 within 28 days of randomisation. However, the proportion of patients requiring admission to hospital in the UK21 was lower than initially expected.22 Therefore, the trial management group and steering committee recommended amending the primary outcome to include a measure of illness duration.23, 24 Duration of illness is an important outcome for patients and has important economic and social impacts. Furthermore, treatments that do not shorten illness duration are also unlikely to provide a benefit in COVID-19-related hospitalisations or deaths. This change received ethical approval on Sept 16, 2020, and was implemented before any interim analyses were done. Thus, the trial had two coprimary endpoints measured over 28 days from randomisation: time to first self-reported recovery (defined as the first instance that a participant reported feeling recovered), and hospitalisation or death related to COVID-19.
Secondary outcomes were a rating of how well participants feel (participants were asked “How well are you feeling today? Please rate how you are feeling now using a scale of 1–10, where 1 is the worst you can imagine, and 10 is feeling the best you can imagine”), time to sustained recovery (date the participant first reported feeling recovered and subsequently remained well until 28 days after randomisation), time to initial alleviation of symptoms (date participant first reported all symptoms as minor or none), time to sustained alleviation of symptoms, time to initial reduction of severity of symptoms, duration of hospital admission, contacts with health services, adherence to study treatment, the WHO-5 Well-Being Index,25 and treatment effects among SARS-CoV-2 PCR-positive participants. We included secondary outcomes capturing sustained recovery due to the recurrent nature of COVID-19 symptoms.
Statistical analysis
Sample size calculations are detailed in the appendix (p 79), where we justify sample sizes by simulating the operating characteristics of the adaptive design in multiple scenarios, which explicitly account for response adaptive randomisation, early stopping for futility or success, and multiple interventions. In brief, for the primary outcome analyses, assuming a median time to recovery of 9 days in the usual care only group, approximately 400 participants per group would provide 90% power to detect a 2-day difference in median recovery time. Assuming 5% hospitalisation in the usual care only group, approximately 1500 participants per group would provide 90% power to detect a 50% reduction in the relative risk of hospitalisation or death.
Statistical analyses are detailed in the master statistical analysis plan (appendix pp 168–207). The first primary outcome, time to first self-reported recovery, was analysed using a Bayesian piecewise exponential model regressed on treatment and stratification covariates, and included parameters for temporal drift. The second primary outcome, hospitalisation or death related to COVID-19, was analysed using a Bayesian logistic regression model regressed on treatment and stratification covariates. The primary outcomes were evaluated using a gate-keeping strategy to preserve the overall type I error of the primary endpoints without additional adjustments for multiple hypotheses. The hypothesis for the time to first self-reported recovery endpoint was evaluated first, and if the null hypothesis was rejected, the hypothesis for the second coprimary endpoint of hospitalisation or death was evaluated. In the context of multiple interim analyses, the master protocol specifies that each null hypothesis is rejected if the Bayesian posterior probability of superiority exceeded 0·99 for the time to recovery endpoint and 0·975 (via gate-keeping) for the hospitalisation or death endpoint. Based on trials of antibiotics for lower respiratory tract infection,26 a minimum of 1·5 days difference in median time to first report of recovery, and 2% difference in hospitalisation or mortality rate were prespecified as clinically meaningful. If there was insufficient evidence of a clinically meaningful benefit in time to recovery, futility was declared and randomisation to that intervention was stopped, meaning other interventions could be evaluated more rapidly in the trial.
Bayesian methods were specified for the primary analysis for multiple reasons, including: the ability to incorporate response adaptive randomisation based on a Bayesian posterior distribution of each intervention being the best intervention; the ability to update Bayesian posterior distributions via interim analyses and base decisions on probabilistic summaries; and the ability to account for temporal drift using Bayesian smoothing methodologies. Bayesian prior distributions were prespecified and were chosen to allow the data to dominate model estimation.
The prespecified primary analysis population included all eligible participants who were assigned to usual care plus doxycycline, usual care only, or usual care plus other interventions, from the start of the platform trial until randomisation to doxycycline was stopped, with data extracted after a further 28 days of follow-up. Because this population included participants who were assigned to usual care only before the usual care plus doxycycline group opened, the primary analysis models include parameters to adjust for temporal drift in the study population, which might have occurred due to changes in circulating SARS-CoV-2, usual care, or the pandemic situation, as well as changes in the inclusion or exclusion criteria over time. These parameters provide an estimated trajectory for the primary endpoint in the usual care only group over time via Bayesian hierarchical modelling; methodological details are provided in the appendix (pp 68–167). We did a sensitivity analysis that compared each intervention versus the concurrently randomised controls (participants who were randomly assigned to usual care only during the time period when the usual care plus doxycycline group was open to randomisation), which should be consistent with the primary analysis results. Although analyses for non-concurrent randomised controls are not typically implemented in traditional trials, they are becoming standard practice in many high-profile adaptive platform trials.19, 27, 28, 29, 30 In addition, we did a secondary analysis restricted to SARS-CoV-2 PCR-positive participants in the primary analysis population.
Analyses of the secondary outcomes, and prespecified subgroup analyses on age, comorbidity, swab results, duration of symptoms before randomisation, and severity of symptoms scores at baseline (appendix p 200), were done on the concurrent randomisation analysis population, defined as all participants who were randomly assigned to usual care plus doxycycline or usual care only during the time period when the usual care plus doxycycline group was open to randomisation. Secondary time-to-event outcomes were analysed using Cox proportional hazard models, and binary outcomes were analysed using logistic regression, adjusting for comorbidity status, age, duration of illness, and eligibility for doxycycline at baseline.
All statistical analyses were done using R (version 3.6.0) and Stata (version 16.1). This trial is registered with ISRCTN, 86534580.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
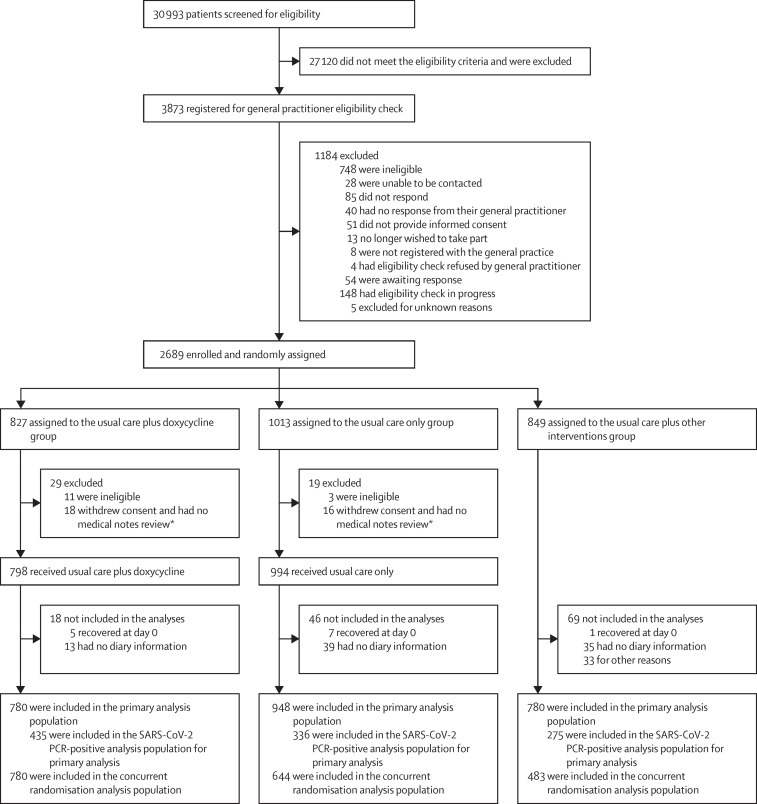
The trial opened on April 2, 2020, and randomisation to doxycycline began on July 24, 2020. On Dec 14, 2020, the trial steering committee, after review of planned interim analyses by the data monitoring and safety committee, advised the trial management group to stop randomisation to doxycycline because the prespecified futility criterion was met. By then, 2689 people had been enrolled and randomised. 712 (26·5%) participants were enrolled directly through 223 primary care practices and 1972 (73·5%) were enrolled via online or telephone contact with the study team. 827 (30·8%) participants were assigned to the usual care plus doxycycline group, 1013 (37·7%) to the usual care only group, and 849 (31·6%) to the usual care other interventions group (figure 1 ). 2508 participants contributed follow-up data and were included in the primary analysis: 780 (31·1%) in the usual care plus doxycycline group, 948 (37·8%) in the usual care only group, and 780 (31·1%) in the usual care plus other interventions group. To protect the integrity of the platform trial and other interventions, here we only provide descriptive summaries of participants assigned to usual care plus doxycycline and usual care only.
Figure 1.
Trial profile
Data for participants assigned to usual care plus other interventions are not presented in this report. *Participants provided no diary information.
The mean age of participants was 61 years (range 50–90); 999 (55·7%) participants were female and 790 (44·1%) were male. 1563 (87·2%) of 1792 participants in the usual care plus doxycycline and usual care only groups had comorbidities. The median duration of illness before randomisation was 6 days (IQR 4–9). 1544 (86·2%) participants had a SARS-CoV-2 PCR test result available, taken a median of 4 days (IQR 2–9) after symptom onset, of whom 791 (51·2%) had a positive result. Baseline characteristics were similar between the two groups (table 1 ), particularly in the concurrent analysis population (appendix pp 421–422).
Table 1.
Baseline characteristics of all eligible, randomly assigned participants by treatment group
| Usual care plus doxycycline group (n=798) | Usual care only group (n=994) | Total (n=1792) | ||
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 61·3 (7·7) | 60·9 (7·9) | 61·1 (7·9) | |
| ≥65 | 303 (38·0%) | 359 (36·1%) | 662 (36·9%) | |
| <65 | 495 (62·0%) | 635 (63·9%) | 1130 (63·1%) | |
| Sex | ||||
| Female | 439 (55·0%) | 560 (56·3%) | 999 (55·7%) | |
| Male | 358 (44·9%) | 432 (43·5%) | 790 (44·1%) | |
| Missing | 1 (0·1%) | 2 (0·2%) | 3 (0·2%) | |
| Ethnicity* | ||||
| White | 739 (92·6%) | 820 (82·5%) | 1559 (87·0%) | |
| Mixed background | 8 (1·0%) | 22 (2·2%) | 30 (1·7%) | |
| South Asian | 43 (5·4%) | 45 (4·5%) | 88 (4·9%) | |
| Black | 6 (0·8%) | 5 (0·5%) | 11 (0·6%) | |
| Other | 2 (0·3%) | 10 (1·0%) | 12 (0·7%) | |
| Missing | 0 | 92 (9·3%) | 92 (5·1%) | |
| Index of multiple deprivation quintile | ||||
| 1 (most deprived) | 183 (22·9%) | 241 (24·3%) | 424 (23·7%) | |
| 2 | 152 (19·1%) | 190 (19·1%) | 342 (19·1%) | |
| 3 | 159 (19·9%) | 189 (19·0%) | 348 (19·4%) | |
| 4 | 154 (19·3%) | 196 (19·7%) | 350 (19·5%) | |
| 5 (least deprived) | 149 (18·7%) | 176 (17·7%) | 325 (18·1%) | |
| Missing | 1 (0·1%) | 2 (0·2%) | 3 (0·2%) | |
| Duration of illness before randomisation in days, median (IQR) | 6 (4–9) | 6 (4–9) | 6 (4–9) | |
| Smoking status | ||||
| Current smoker | 74 (9·3%) | 125 (12·6%) | 199 (11·1%) | |
| Former smoker | 309 (38·7%) | 367 (36·9%) | 676 (37·7%) | |
| Never smoker | 404 (50·6%) | 476 (47·9%) | 880 (49·1%) | |
| Missing | 11 (1·4%) | 26 (2·6%) | 37 (2·1%) | |
| SARS-CoV-2 PCR result | ||||
| Negative | 293 (36·7%) | 460 (46·3%) | 753 (42·0%) | |
| Positive | 442 (55·4%) | 349 (35·1%) | 791 (44·1%) | |
| No result | 9 (1·1%) | 7 (0·7%) | 16 (0·9%) | |
| Not available | 54 (6·8%) | 178 (17·9%) | 232 (12·9%) | |
| Comorbidity | 697 (87·3%) | 866 (87·1%) | 1563 (87·2%) | |
| Comorbidities | ||||
| Asthma, COPD, or lung disease | 304 (38·1%) | 364 (36·6%) | 668 (37·3%) | |
| Diabetes | 134 (16·8%) | 188 (18·9%) | 322 (18·0%) | |
| Heart problems† | 107 (13·4%) | 148 (14·9%) | 255 (14·2%) | |
| High blood pressure requiring medication | 318 (39·8%) | 425 (42·8%) | 743 (41·5%) | |
| Liver disease | 18 (2·3%) | 24 (2·4%) | 42 (2·3%) | |
| Stroke or other neurological problem | 53 (6·6%) | 58 (5·8%) | 111 (6·2%) | |
| Taking ACE inhibitor‡ | 163 (20·4%) | 204 (20·5%) | 367 (20·5%) | |
| Fever at baseline | ||||
| No problem | 377 (47·2%) | 432 (43·5%) | 809 (45·1%) | |
| Minor problem | 247 (31·0%) | 339 (34·1%) | 586 (32·7%) | |
| Moderate problem | 156 (19·5%) | 198 (19·9%) | 354 (19·8%) | |
| Major problem | 18 (2·3%) | 25 (2·5%) | 43 (2·4%) | |
| Cough at baseline | ||||
| No problem | 162 (20·3%) | 170 (17·1%) | 332 (18·5%) | |
| Minor problem | 320 (40·1%) | 393 (39·5%) | 713 (39·8%) | |
| Moderate problem | 275 (34·5%) | 371 (37·3%) | 646 (36·0%) | |
| Major problem | 41 (5·1%) | 60 (6·0%) | 101 (5·6%) | |
| Shortness of breath at baseline | ||||
| No problem | 339 (42·5%) | 327 (32·9%) | 666 (37·2%) | |
| Minor problem | 303 (38·0%) | 431 (43·4%) | 734 (41·0%) | |
| Moderate problem | 134 (16·8%) | 213 (21·4%) | 347 (19·4%) | |
| Major problem | 22 (2·8%) | 23 (2·3%) | 45 (2·5%) | |
| Muscle ache at baseline | ||||
| No problem | 246 (30·8%) | 298 (30·0%) | 544 (30·4%) | |
| Minor problem | 294 (36·8%) | 376 (37·8%) | 670 (37·4%) | |
| Moderate problem | 203 (25·4%) | 238 (23·9%) | 441 (24·6%) | |
| Major problem | 55 (6·9%) | 82 (8·2%) | 137 (7·6%) | |
| Nausea at baseline | ||||
| No problem | 604 (75·7%) | 743 (74·7%) | 1347 (75·2%) | |
| Minor problem | 138 (17·3%) | 205 (20·6%) | 343 (19·1%) | |
| Moderate problem | 45 (5·6%) | 38 (3·8%) | 83 (4·6%) | |
| Major problem | 11 (1·4%) | 8 (0·8%) | 19 (1·1%) | |
| Feeling generally unwell or malaise at baseline | ||||
| No problem | 60 (7·5%) | 52 (5·2%) | 112 (6·3%) | |
| Minor problem | 357 (44·7%) | 333 (33·5%) | 690 (38·5%) | |
| Moderate problem | 322 (40·4%) | 321 (32·3%) | 643 (35·9%) | |
| Major problem | 59 (7·4%) | 61 (6·1%) | 120 (6·7%) | |
| Diarrhoea at baseline | ||||
| No problem | 598 (74·9%) | 577 (58·0%) | 1175 (65·6%) | |
| Minor problem | 150 (18·8%) | 134 (13·5%) | 284 (15·8%) | |
| Moderate problem | 39 (4·9%) | 44 (4·4%) | 83 (4·6%) | |
| Major problem | 11 (1·4%) | 12 (1·2%) | 23 (1·3%) | |
| Taken antibiotics since illness started | 14 (1·8%) | 41 (4·1%) | 55 (3·1%) | |
| Use of health-care services | ||||
| General practice | 185 (23·2%) | 279 (28·1%) | 464 (25·9%) | |
| Other primary care services | 35 (4·4%) | 66 (6·6%) | 101 (5·6%) | |
| NHS 111 | 106 (13·3%) | 179 (18·0%) | 285 (15·9%) | |
| Emergency department | 8 (1·0%) | 14 (1·4%) | 22 (1·2%) | |
| Other health-care services | 13 (1·6%) | 30 (3·0%) | 43 (2·4%) | |
| WHO-5 Well-Being Index score, mean (SD)§¶ | 53·3 (24·6) | 49·5 (24·5) | 51·2 (24·6) | |
Data are n (%) unless otherwise stated. Data are shown for all eligible, randomised participants, some of whom were excluded from the final analyses. COPD=chronic obstructive pulmonary disease. ACE=angiotensin-converting enzyme. NHS=National Health Service.
Data on ethnicity were collected retrospectively via notes review before July, 2020.
Heart problems included angina, heart attack, heart failure, atrial fibrillation, and valve problems.
Such as ramipril, lisinopril, perindopril, captopril, or enalapril.
Wellbeing was measured using the WHO-5 Well-Being Index, which includes five items relating to wellbeing measured on a 5-point scale; a total score is computed by summing the scores from the five individual questions to give a raw score ranging from 0 to 25 which is then multiplied by 4 to give the final score from 0 (representing the worst imaginable wellbeing) to 100 (representing the best imaginable wellbeing).
Data on WHO-5 Well-Being Index were missing for 24 participants in the usual care group.
Follow-up information was available for 1728 (96·4%) of 1792 participants. 656 (82·2%) of 798 participants in the usual care plus doxycycline group reported taking doxycycline for at least 6 days.
Of 780 participants who received doxycycline in the primary analysis population, 596 (76·4%) reported first feeling recovered within 28 days after randomisation, compared with 717 (75·6%) of 948 in the usual care only group. Based on the Bayesian primary analysis model, adjusted for temporal drift, the estimated median time to first recovery was 9·6 days in the usual care plus doxycycline group versus 10·1 days in the usual care only group (hazard ratio [HR] 1·04 [95% Bayesian Credible Interval (BCI) 0·93 to 1·17]; table 2 ), equating to an estimated median benefit of 0·5 days (95% BCI −0·99 to 2·04). The probability that median time to recovery was shorter in the usual care plus doxycycline group than in the usual care only group (ie, the probability of superiority) was 0·74 and did not meet the 0·99 threshold to declare superiority. The probability that there was a clinically meaningful benefit (≥1·5 days) in time to recovery was 0·10.
Table 2.
Primary outcomes
| Usual care plus doxycycline group | Usual care only group | Estimated benefit, median (95% BCI) | HR or OR (95% BCI) | Probability of clinically meaningful benefit | Probability of superiority | |
|---|---|---|---|---|---|---|
| Model-based estimates in the primary analysis population (n=780 in the usual care plus doxycycline group and n=948 in the usual care only group) | ||||||
| Time to first reported recovery in days | 9·6 (8·3 to 11·0)* | 10·1 (8·7 to 11·7)* | 0·50 (−0·99 to 2·04)† | 1·04 (0·93 to 1·17) | 0·10‡ | 0·74§ |
| Hospitalisation or death related to COVID-19 | 5·1% (3·6 to 6·8)¶ | 4·6% (3·4 to 6·1)¶ | −0·5% (−2·6 to 1·4)‖ | 1·13 (0·73 to 1·74) | 0·005** | 0·30†† |
| Model-based estimates in the SARS-CoV-2 PCR-positive population (n=435 in the usual care plus doxycycline group and n=336 in the usual care only group) | ||||||
| Time to first reported recovery in days | 11·8 (10·3 to 13·7)* | 12·5 (10·8 to 14·8)* | 0·70 (−1·45 to 3·03)† | 1·05 (0·90 to 1·24) | 0·24‡ | 0·74§ |
| Hospitalisation or death related to COVID-19 | 8·0% (5·7 to 10·8)¶ | 9·2% (6·6 to 12·6)¶ | 1·2% (−2·7 to 5·2)‖ | 0·85 (0·52 to 1·42) | 0·35** | 0·73†† |
BCI=Bayesian credible interval. HR=hazard ratio. OR=odds ratio.
Median time to first reported recovery (95% BCI).
Estimated benefit in median time to recovery derived from a Bayesian piecewise exponential model adjusted for age and comorbidity at baseline; a positive value in estimated benefit in median time to recovery (or HR >1) corresponds to a reduction in time to recovery in days with usual care plus doxycycline compared with usual care only.
Estimated probability that the benefit in median time to recovery in the usual care plus doxycycline group compared with the usual care only group is at least 1·5 days.
Estimated probability that usual care plus doxycycline is superior to usual care only (treatment superiority is declared if probability is ≥0·99).
Model-based estimated percentage of patients who had hospitalisation or death within 28 days after randomisation (95% BCI).
Estimated benefit expressed as percentage difference in hospitalisations or deaths, derived from a Bayesian logistic regression model adjusted for age and comorbidity at baseline; a positive value in the estimated percentage difference (or OR <1) favours usual care plus doxycycline.
Estimated probability that the benefit in hospitalisation or death rates in the usual care plus doxycycline group compared with the usual care only group is at least 2%.
Estimated probability that usual care plus doxycycline is superior to usual care only (treatment superiority is declared if probability is ≥0·975).
A slightly higher rate of hospitalisations or deaths related to COVID-19 within 28 days of follow-up was observed in the usual care plus doxycycline group than in the usual care only group (41 [crude percentage 5·3%] vs 43 [4·5%]; estimated absolute percentage difference −0·5% [95% BCI −2·6 to 1·4]; table 2). There were five deaths (0·6%) in the usual care plus doxycycline group and two (0·2%) in the usual care only group. The probability that rate of hospitalisations or deaths related to COVID-19 was lower in the usual care plus doxycycline group than in the usual care only group (ie, the probability of superiority) was 0·30, and was not formally analysed for significance due to the gate-keeping hypothesis structure. The probability that there was a reduction in rate of hospitalisations or deaths related to COVID-19 of at least 2% (the predefined threshold of a clinically meaningful benefit) was 0·005.
Results of both primary outcomes were consistent in the SARS-CoV-2 PCR-positive population (time to recovery HR 1·05 [95% BCI 0·90 to 1·24], estimated median benefit 0·70 days [–1·45 to 3·03], probability of meaningful benefit 0·24; rate of hospitalisations or deaths related to COVID-19 estimated absolute percentage difference 1·2% [–2·7% to 5·2%], probability of meaningful benefit 0·35; table 2).
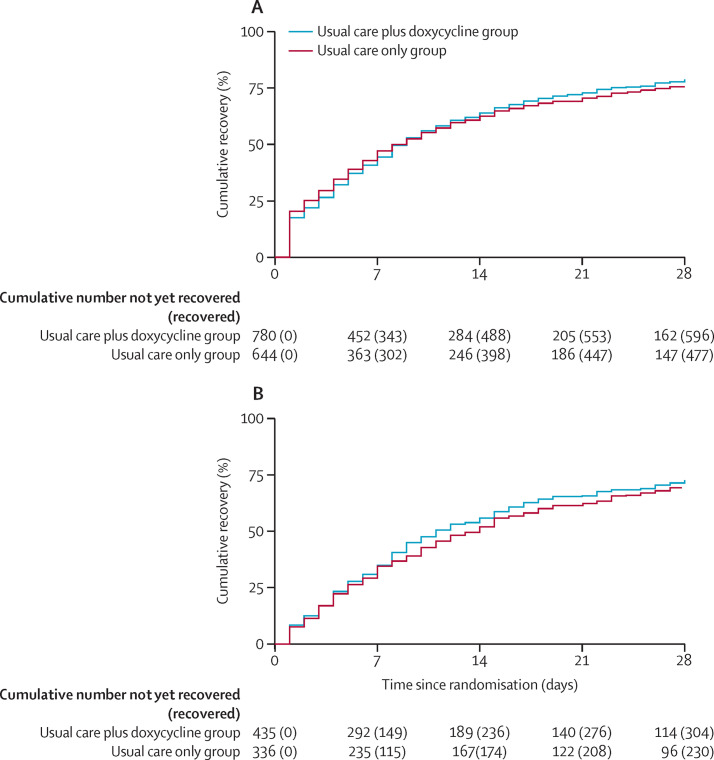
Similarly, results of both primary outcomes were consistent in the concurrent randomisation analysis population (time to recovery HR 1·04 [0·93 to 1·17], estimated median benefit 0·57 days [–0·95 to 2·13], probability of meaningful benefit 0·12; rate of hospitalisations or deaths related to COVID-19 estimated absolute percentage difference 0·2% [–2·1 to 2·5], probability of meaningful benefit 0·062; figure 2 ; appendix p 210).
Figure 2.
Time to first self-reported recovery
(A) Concurrent randomisation analysis population. (B) SARS-CoV-2 PCR-positive participants in the concurrent randomisation analysis population. The concurrent randomisation analysis population was defined as all participants who were randomly assigned to usual care plus doxycycline or usual care only during the time period when the usual care plus doxycycline group was open to randomisation.
Analyses of secondary outcomes showed little evidence of differences between the two groups in the daily score of how well participants felt over 28 days (appendix p 424), the WHO-5 Well-Being Index, or any of the hospitalisation secondary outcomes (table 3 ). Similarly, there was little evidence of treatment benefit in the usual care plus doxycycline group compared with the usual care only group in time to first alleviation of symptoms, time to sustained alleviation of symptoms, or time to initial reduction of severity of all symptoms and individual symptoms (appendix pp 212–215). Health-care service use was similar between the two groups, and the proportions of patients who were subsequently prescribed antibiotics were small in both the usual care plus doxycycline group (18 [5·3%] of 341) and the usual care only group (20 [6·5%] of 306), although data for this outcome were available for less than half of the participants (table 3).
Table 3.
Secondary outcomes
| Usual care plus doxycycline group | Usual care only group | Estimated treatment effect (95% CI) | p value | ||
|---|---|---|---|---|---|
| Sustained recovery | 502/780 (64·4%) | 396/644 (61·5%) | .. | .. | |
| Time to sustained recovery in days | 22 (9–not reached) | 22 (8–not reached) | 1·00 (0·88 to 1·14)* | 0·96 | |
| Alleviation of all symptoms | 618/671 (92%) | 522/551 (94·7%) | .. | .. | |
| Time to alleviation of all symptoms in days | 3 (2–7) | 3 (1–8) | 0·96 (0·86 to 1·09)* | 0·55 | |
| Sustained alleviation of all symptoms | 542/648 (83·6%) | 428/515 (83·1%) | .. | .. | |
| Time to sustained alleviation of all symptoms in days | 8 (3–23) | 10 (3–23) | 1·03 (0·90 to 1·17)* | 0·68 | |
| Initial reduction of severity of symptoms | 701/780 (89·9%) | 572/644 (88·8%) | .. | .. | |
| Time to initial reduction of severity of symptoms | 5 (1–12) | 4 (1–11) | 0·99 (0·88 to 1·11)* | 0·84 | |
| Rating of how well participant feels (1 worst, 10 best), mean (SD) [n] | |||||
| Day 7 | 7·1 (1·9) [757] | 7·0 (1·9) [636] | 0·05 (−0·16 to 0·25)† | 0·66 | |
| Day 14 | 7·8 (1·7) [752] | 7·7 (1·7) [632] | 0·06 (−0·16 to 0·28)† | 0·58 | |
| Day 21 | 8·1 (1·6) [689] | 8·0 (1·6) [566] | 0·00 (−0·25 to 0·25)† | 0·99 | |
| Day 28 | 8·3 (1·5) [754] | 8·3 (1·5) [629] | −0·06 (−0·34 to 0·22)† | 0·69 | |
| WHO-5 Well-Being Index score, mean (SD) [n] | |||||
| Day 14 | 45·4 (24·1) [738] | 44·3 (23·9) [616] | 0·20 (−2·06 to 2·45)† | 0·86 | |
| Day 28 | 54·5 (23·2) [737] | 53·8 (23·7) [605] | 0·01 (−2·25 to 2·28)† | 0·99 | |
| Self-reported contact with ≥1 health-care service | 381/773 (49·3%) | 314/642 (48·9%) | 1·04 (0·84 to 1·29)‡ | 0·72 | |
| General-practitioner reported contact with ≥1 health-care service | 203/381 (53·3%) | 181/345 (52·5%) | 0·99 (0·73 to 1·34)‡ | 0·96 | |
| Prescription of antibiotics | 18/341 (5·3%) | 20/306 (6·5%) | 0·81 (0·44 to 1·50)§ | 0·51 | |
| Hospital assessment without admission | 8/767 (1·0%) | 11/628 (1·8%) | 0·60 (0·24 to 1·47)§ | 0·35 | |
| Oxygen administration | 24/757 (3·2%) | 20/621 (3·2%) | 0·98 (0·55 to 1·76)§ | >0·99 | |
| Mechanical ventilation | 3/757 (0·4%) | 5/621 (0·8%) | 0·49 (0·12 to 2·05)§ | 0·48 | |
| Intensive care unit admission | 4/755 (0·5%) | 6/620 (1·0%) | 0·55 (0·16 to 1·93)§ | 0·36 | |
| Duration of hospital admission in days | 5 (3–8) | 7 (4–9) | −2·40 (−5·40 to 0·52)¶ | 0·10 | |
Data are n/N (%) or median (IQR) unless otherwise stated. All secondary outcome analyses were done on the concurrent randomisation analysis population, defined as all participants who were randomly assigned to usual care plus doxycycline or usual care only during the time period when the usual care plus doxycycline group was open to randomisation, and were restricted to these treatment groups only.
Estimated hazard ratio derived from a Cox proportional hazards model adjusted for age, comorbidity at baseline, duration of illness, and eligibility for doxycycline at baseline.
Mixed-effect model adjusted for age, comorbidity, duration of illness, eligibility for doxycycline at baseline, and time since randomisation; participants were fitted as a random effect; WHO-5 Well-Being Index score was also adjusted for score at baseline.
Relative risk adjusted for age, comorbidity at baseline, duration of illness, and eligibility for doxycycline at baseline.
Unadjusted relative risk due to low event rate.
Quantile regression adjusted for age, comorbidity, duration of illness, and eligibility for doxycycline at baseline.
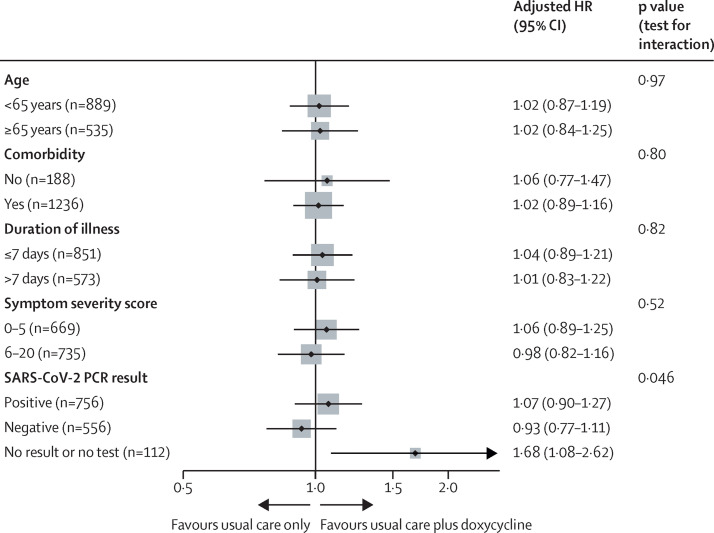
In the prespecified subgroup analyses, duration of illness before randomisation, baseline illness severity score, age, and comorbidity did not affect the efficacy of doxycycline on time to first self-reported recovery (figure 3 ). A treatment benefit in time to first self-reported recovery was observed in the 112 participants who had no SARS-CoV-2 PCR result available; but there was no effect in those with a positive or a negative SARS-CoV-2 PCR test. In terms of serious adverse events, five participants reported hospitalisations unrelated to COVID-19, all of whom were in the usual care only group.
Figure 3.
Forest plot of subgroup analyses on time-to-recovery outcome in the concurrent randomisation analysis population
HR=hazard ratio. The concurrent randomisation analysis population was defined as all participants who were randomly assigned to usual care plus doxycycline or usual care only during the time period when the usual care plus doxycycline group was open to randomisation.
Discussion
This adaptive platform randomised trial, in people in the community in the UK with suspected or PCR-confirmed COVID-19 who were at high risk of adverse outcomes, showed that doxycycline did not meaningfully shorten time to recovery or reduce hospitalisations or deaths related to COVID-19 compared with usual care only. Findings were unchanged in the secondary analysis restricted to participants with a positive PCR test for SARS-CoV-2 infection, and in subgroup analyses by age and presence of comorbidities.
We identified no published randomised controlled trials of doxycycline as a standalone treatment for COVID-19. A small randomised controlled trial in 72 adults hospitalised with COVID-19 in Bangladesh compared doxycycline for 5 days plus single-dose ivermectin, ivermectin alone for 5 days, and placebo.31 The primary outcome of mean time to negative SARS-CoV-2 PCR was 12·7 days (95% CI 11·3–14·2) in the placebo group and a similar 11·5 days (9·8–13·2; p=0·27) in the doxycycline plus ivermectin group, and was shorter in the ivermectin alone group (9·7 days [7·8–11·8]; p=0·02) than in the placebo group. There were no differences in hospitalisation duration. A prospective observational study of 315 patients hospitalised with COVID-19 pneumonia, of whom 148 (47%) received doxycycline, found no evidence that doxycycline was associated with decreased 30-day mortality (adjusted HR 0·92 [95% CI 0·49–1·69]; p=0·79).32
The strengths of our study include the evaluation of doxycycline as a standalone, early treatment, the focus on patients in the community at increased risk of complications, and the use of 28 days of patient-reported outcomes which, in the case of hospitalisations and deaths, were confirmed by medical record review. Only three-quarters of patients reported feeling fully recovered during follow-up, and the median time to sustained recovery was 22 days, reflecting the now well known potential for COVID-19 to cause recurrent and protracted symptoms, but we did not assess outcomes beyond 28 days.
A potential limitation of our study is the inclusion of patients without PCR-confirmed SARS-CoV-2 infection. However, the inclusion of these patients reflects the management of suspected COVID-19 early in the UK pandemic, and in many other community and low-resource hospital settings, where limited availability of SARS-CoV-2 testing might necessitate early empirical treatment. Given the variation in PCR testing sensitivity, particularly if self-administered by unwell older people in the community, some participants are likely to have had false-negative tests.33 SARS-CoV-2 PCR positivity within PRINCIPLE has increased as the pandemic has progressed, and our findings were unchanged when restricted to the 51·2% participants with PCR-confirmed infection. We did an open-label study as rapidly generating a placebo for multiple trial interventions was not feasible, and our study is a pragmatic trial which aims to determine whether the addition of doxycycline to usual care was effective, rather than to compare doxycycline with placebo. Although this could introduce potential bias, any possible placebo effect on time to self-reported recovery would most likely have biased results towards benefit from doxycycline. As we did not observe any clinically meaningful benefit, this is unlikely to have influenced our results.
There was a relatively higher proportion of individuals who reported recovery on day 1 among those without a positive SARS-CoV-2 test (figure 2). This finding might be an artifact of the recruitment and screening strategy that was implemented early on in the COVID-19 pandemic during 2020, when there were difficulties obtaining data to confirm eligibility from some general practices. Difficulties in obtaining this information resulted in delays between trial screening and randomisation for some participants, who are likely to have then reported recovery sooner after randomisation. Subsequent improved screening processes enabled assessment of eligibility for participation far more rapidly. The proportions of participants who had a SARS-CoV-2 PCR test result and those who did not differed over the duration of the study, due to the non-availability of testing in the early months of the trial, before screening processes were improved. These differences are taken account of in the primary analysis model, which adjusted for temporal trends in time to recovery.
We found a marginally higher HR for time to first self-reported recovery favouring doxycycline among those with PCR-confirmed SARS-CoV-2 infection and in the concurrent randomisation population, when compared with the primary analysis population which included people diagnosed on the basis of symptoms (figure 2). However, the estimated benefit in terms of time to recovery was around half a day for all study populations.
In the primary analysis, there were slightly more hospital admissions or deaths related to COVID-19 in the usual care plus doxycycline group than in the usual care only group; whereas in the SARS-CoV-2 PCR-positive population, there were 1·2% fewer hospitalisations or deaths in the usual care plus doxycycline group, with a low probability that doxycycline was superior on this outcome. However, the hospitalisation analysis did not account for temporal drift (in line with low event counts and the statistical analysis plan effective at time of the analysis), and the estimated difference of 1·2% might be overestimated, given increasing hospitalisation over the duration of the study. In the concurrent randomisation analysis, there was a 0·2% estimated difference in hospitalisations or deaths.
The challenge of designing trials with relatively little information early in a novel pandemic has meant that it is not unusual to update key outcomes as new information emerges.24 Due to lower than expected hospitalisations and mortality in PRINCIPLE, and to allow measurement of effects on illness duration, the primary outcome was changed to a coprimary outcome of self-reported time to recovery, and COVID-19-related hospitalisation or death, analysed using a gate-keeping approach. This change occurred before any interim analyses were done. This approach, in which interventions that meet prespecified futility criteria on time to recovery are stopped, assumes that interventions that did not show benefit on time to recovery are unlikely to show a benefit on reducing COVID-19-related hospitalisations and deaths. This design enables the platform trial to cycle through multiple interventions using response adaptive randomisation, and increases the probability of achieving the trial objectives of identifying effective community treatments for COVID-19.
Doxycycline has previously been recommended for COVID-19,8, 9, 34 particularly in patients with pneumonia and those who are at high risk of complications,10 and there is now evidence of increased use of respiratory antibiotics including doxycycline during the COVID-19 pandemic in both the UK and USA.14, 15, 16, 17 Our study, among older people and those with comorbidities, with two-thirds of participants reporting shortness of breath at baseline, does not support the routine use of doxycycline for suspected COVID-19 in the community in the absence of other indications such as bacterial pneumonia. However, emerging evidence suggests bacterial co-infection in COVID-19 is uncommon,35 therefore doxycycline is unlikely to benefit most individuals with COVID-19 in well resourced countries. Wider use of doxycycline could lead to public health harm through increased antimicrobial resistance.18 Further research into strategies to identify bacterial co-infection in the community are needed to allow targeted, appropriate use of antibiotics in patients with COVID-19.
Data sharing
Data can be shared with qualifying researchers who submit a proposal with a valuable research question as assessed by a committee formed from the Trial Management Group including senior statistical and clinical representation. A contract should be signed. Data sharing requests should be sent to the corresponding author.
Declaration of interests
BRS, MAD, CS, MF, and NB report grants from University of Oxford, for the sponsor's grant from the UK National Institute for Health Research (NIHR), for statistical design and analyses for the trial, during the conduct of the study. RD reports grants and personal fees from Synairgen, during the conduct of the study; personal fees from TEVA Pharmaceuticals, Sanofi, Boehringer, and Novartis, outside of the submitted work; and grants from the Innovative Medicines Initiative, the UK Medical Research Council, and Novartis, outside of the submitted work. FDRH reports grants from UK Research and Innovation (UKRI), during the conduct of the study. OVH reports grants from UKRI, outside of the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by UK Research and Innovation and the Department of Health and Social Care through the National Institute for Health Research Urgent Public Health Priority research funding (MC_PC_19079). CCB acknowledges part support as Senior Investigator of the National Institute of Health Research, the NIHR Community Healthcare Medtech and In-Vitro Diagnostics Co-operative (MIC), and the NIHR Health Protection Research Unit on Health Care Associated Infections and Antimicrobial Resistance. FDRH acknowledges his part-funding from the NIHR School for Primary Care Research, the NIHR Applied Research Collaboration Oxford, the NIHR Oxford Biomedical Research Centre, and the NIHR MIC. JD and OG are funded by the Wellcome Trust PhD Programme for Primary Care Clinicians (216421/Z/19/Z and 203921/Z/16/Z respectively). For the purpose of Open Access, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. GH is funded by an NIHR Advanced Fellowship and by the NIHR MIC. The authors vouch for the accuracy and completeness of the data and for fidelity to the protocol. We thank the patients who participated in this study. We also thank the many health and social-care professionals who contributed to the trial. The PRINCIPLE trial platform is led from the Primary Care and Vaccines Collaborative Clinical Trials Unit at the University of Oxford's Nuffield Department of Primary Care Health Sciences. PRINCIPLE is supported by a large network of care homes, pharmacies, NHS 111 Hubs, hospitals, and 1401 general practices across the UK. The trial is integrated with the Oxford Royal College of General Practitioners Research and Surveillance Centre ORCHID digital platform and works closely with the NIHR Clinical Research Network, NHS DigiTrials, Public Health England, Health and Care Research Wales, NHS Research Scotland, and the Health and Social Care Board in Northern Ireland.
Principle Trial Management Group
Julie Allen, Monique Andersson, Nick Berry, Emily Bongard, Aleksandra Borek, Christopher C Butler, Simon de Lusignan, Jienchi Dorward, Philip H Evans, Filipa Ferreira, Oghenekome Gbinigie, Jenna Grabey, Gail Hayward, F D Richard Hobbs, Susan Hopkins, David Judge, Mona Koshkouei, Martin J Llewelyn, Emma Ogburn, Mahendra G Patel, Dan Richards-Doran, Heather Rutter, Benjamin R Saville, Hannah Swayze, Nicholas P B Thomas, Manasa Tripathy, Sarah Tonkin-Crine, Sharon Tonner, Oliver Van Hecke, Ly-Mee Yu. A complete list of the PRINCIPLE Trial Collaborative Group members and their roles in the the trial is provided in the appendix (pp 216–217).
Contributors
CCB and FDRH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. BRS, NB, L-MY, CCB, FDRH, SG, RD, JK, GH, OVH, OG, JD, and MJL contributed to trial design. SdL, MA, MJL, PHE, NPBT, and SH helped plan the trial. EO, HS, EB, JA, ST, NPBT, PHE, HR, SdL, MGP, and JG were responsible for acquisition of data. CCB, FDRH, L-MY, BRS, JD, GH, OVH, and OG drafted the manuscript. BRS, NB, L-MY, MAD, MF, CS, and VH contributed to the statistical analysis. DJ designed the information systems. JG led the data management. All authors critically revised the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Kim PS, Read SW, Fauci AS. Therapy for early COVID-19: a critical need. JAMA. 2020;324:2149–2150. doi: 10.1001/jama.2020.22813. [DOI] [PubMed] [Google Scholar]
- 2.Yates PA, Newman SA, Oshry LJ, Glassman RH, Leone AM, Reichel E. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620951053. 1753466620951053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malek AE, Granwehr BP, Kontoyiannis DP. Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gendrot M, Andreani J, Jardot P, et al. In vitro antiviral activity of doxycycline against SARS-CoV-2. Molecules. 2020;25 doi: 10.3390/molecules25215064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyt JC, Ballering J, Numanami H, Hayden JM, Robbins RA. Doxycycline modulates nitric oxide production in murine lung epithelial cells. J Immunol. 2006;176:567–572. doi: 10.4049/jimmunol.176.1.567. [DOI] [PubMed] [Google Scholar]
- 6.Kim H-S, Luo L, Pflugfelder SC, Li D-Q. Doxycycline inhibits TGF-β1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 7.Hsu AT, Barrett CD, DeBusk GM, et al. Kinetics and role of plasma matrix metalloproteinase-9 expression in acute lung injury and the acute respiratory distress syndrome. Shock. 2015;44:128–136. doi: 10.1097/SHK.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Information and Publicity Government of Goa Home isolation monitoring kits for COVID-19 launched. Oct 9, 2020. https://www.goa.gov.in/wp-content/uploads/2020/10/Home-Isolation-Monitoring-Kits-For-COVID-19-Launched.pdf
- 9.Reuters Aplicativo do Ministério da Saúde recomenda medicamentos sem eficácia comprovada para tratar Covid [Ministry of Health app recommends medications without proven efficacy to treat COVID] Jan 20, 2021. https://www.reuters.com/article/saude-covid-app-cloroquina-idLTAKBN29P29Y
- 10.National Institute for Health and Care Excellence COVID-19 rapid guideline: managing suspected or confirmed pneumonia in adults in the community. April 23, 2020. https://www.nice.org.uk/guidance/ng165 [PubMed]
- 11.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. Feb 3, 2021. https://www.covid19treatmentguidelines.nih.gov [PubMed]
- 13.WHO Clinical management of COVID-19: interim guidance. 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 14.Buehrle DJ, Nguyen MH, Wagener MM, Clancy CJ. Impact of the coronavirus disease 2019 pandemic on outpatient antibiotic prescriptions in the United States. Open Forum Infect Dis. 2020;7:a575. doi: 10.1093/ofid/ofaa575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malcolm W, Seaton RA, Haddock G, et al. Impact of the COVID-19 pandemic on community antibiotic prescribing in Scotland. JAC Antimicrob Resist. 2020;2 doi: 10.1093/jacamr/dlaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lusignan S, Joy M, Sherlock J, et al. PRINCIPLE trial demonstrates scope for in-pandemic improvement in primary care antibiotic stewardship: retrospective sentinel network cohort study. BJGP Open (in press). [DOI] [PMC free article] [PubMed]
- 17.Zhu N, Aylin P, Rawson T, Gilchrist M, Majeed A, Holmes A. Investigating the impact of COVID-19 on primary care antibiotic prescribing in north west London across two epidemic waves. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.02.007. S1198-743X(21)00082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nature Microbiol Antimicrobial resistance in the age of COVID-19. Nat Microbiol. 2020;5:779. doi: 10.1038/s41564-020-0739-4. [DOI] [PubMed] [Google Scholar]
- 19.Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 20.National Health Service Symptoms of coronavirus. 2021. https://www.nhs.uk/conditions/coronavirus-covid-19/symptoms/
- 21.UK Government Coronavirus (COVID-19) in the UK. Feb 12, 2021. https://coronavirus.data.gov.uk/
- 22.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84:167–176. doi: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 26.Little P, Stuart B, Moore M, et al. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infect Dis. 2013;13:123–129. doi: 10.1016/S1473-3099(12)70300-6. [DOI] [PubMed] [Google Scholar]
- 27.Dreyer SB, Jamieson NB, Cooke SL, et al. PRECISION-Panc: the next generation therapeutic development platform for pancreatic cancer. Clin Oncol (R Coll Radiol) 2020;32:1–4. doi: 10.1016/j.clon.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buxton MB, Natsuhara K, DeMichele A, et al. Transforming the clinical trial process: the I-SPY 2 trial as a model for improving the efficiency of clinical trials and accelerating the drug-screening process. J Clin Oncol. 2014;32 [Google Scholar]
- 30.Angus DC, Alexander BM, Berry S, et al. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18:797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falcone M, Tiseo G, Barbieri G, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:a563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 34.McCullough PA, Alexander PE, Armstrong R, et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19) Rev Cardiovasc Med. 2020;21:517–530. doi: 10.31083/j.rcm.2020.04.264. [DOI] [PubMed] [Google Scholar]
- 35.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be shared with qualifying researchers who submit a proposal with a valuable research question as assessed by a committee formed from the Trial Management Group including senior statistical and clinical representation. A contract should be signed. Data sharing requests should be sent to the corresponding author.