Abstract
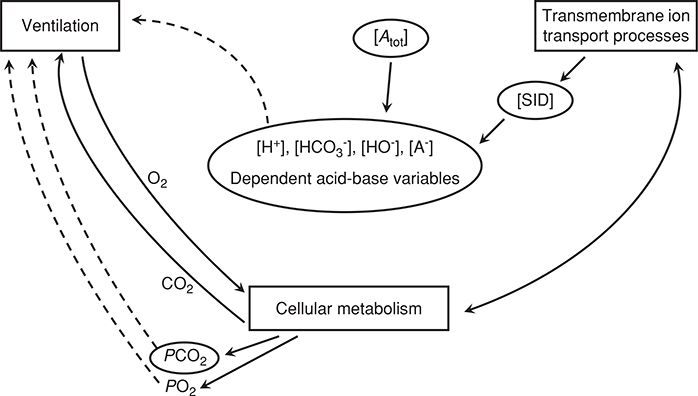
As the first step in the oxygen-transport chain, the lung has a critical task: optimizing the exchange of respiratory gases to maintain delivery of oxygen and the elimination of carbon dioxide. In healthy subjects, gas exchange, as evaluated by the alveolar-to-arterial PO2 difference (A-aDO2), worsens with incremental exercise, and typically reaches an A-aDO2 of approximately 25 mmHg at peak exercise. While there is great individual variability, A-aDO2 is generally largest at peak exercise in subjects with the highest peak oxygen consumption. Inert gas data has shown that the increase in A-aDO2 is explained by decreased ventilation-perfusion matching, and the development of a diffusion limitation for oxygen. Gas exchange data does not indicate the presence of right-to-left intrapulmonary shunt developing with exercise, despite recent data suggesting that large-diameter arteriovenous shunt vessels may be recruited with exercise. At the same time, multisystem mechanisms regulate systemic acid-base balance in integrative processes that involve gas exchange between tissues and the environment and simultaneous net changes in the concegovntrations of strong and weak ions within, and transfer between, extracellular and intracellular fluids. The physicochemical approach to acid-base balance is used to understand the contributions from independent acid-base variables to measured acid-base disturbances within contracting skeletal muscle, erythrocytes and noncontracting tissues. In muscle, the magnitude of the disturbance is proportional to the concentrations of dissociated weak acids, the rate at which acid equivalents (strong acid) accumulate and the rate at which strong base cations are added to or removed from muscle.
Introduction
At rest, pulmonary gas exchange is remarkably efficient. Fresh gas is delivered to the alveoli by the process of ventilation, and when alveoli are perfused by blood, gas exchange takes place by the passive diffusion of oxygen into, and carbon dioxide out of the blood. Several processes are involved: delivery of fresh gas by alveolar ventilation, matching of perfusion to ventilation such that well-ventilated lung regions are equally well-perfused, diffusion of gas across the alveolar wall into pulmonary capillary, diffusion of gas through the plasma and then into the red blood cell. Thus, the primary measure of the efficiency of the process of pulmonary gas exchange is given by the alveolar-arterial partial pressure difference for oxygen (A-aDO2) reflecting the difference between the partial pressure of oxygen in alveolar gas and what subsequently ends up in the arterial blood. Alveolar ventilation is critically important for the elimination of metabolically produced CO2, as alveolar ventilation that does not increase appropriately with the increased CO2 production during exercise will result in systemic acidosis secondary to CO2 accumulation. Thus, alveolar ventilation is an important mechanism for regulation of acid-base balance.
During exercise, the delivery of deoxygenated blood to the lung increases dramatically and flux of oxygen across the alveolar membrane must also increase to match tissue demands. As a first step to understanding the integrative processes outlined above, a brief overview of pulmonary gas exchange principles are outlined. Next, the normal pulmonary blood gas responses to exercise are detailed in healthy normal young subjects, and the mechanisms for the changes with exercise are discussed. Subsequent sections discuss how the gas exchange responses to exercise may differ between men and women, with normal aging, and at high altitude. Common techniques for assessment of these mechanisms are also discussed, since the reader will need a working knowledge of them in reading the literature. It is important to appreciate that for a variety of reasons, the amount of data collected in healthy normal exercising subjects is rather small compared to the clinical data collected in patients with disease. Many researchers are reluctant to obtain direct measures of arterial blood because of technical difficulties associated with data collection, or have not measured body temperature during exercise studies where arterial blood gas data is collected. Thus, the gas exchange section will finish with a review of methodological considerations in gas exchange, followed by a review of ventilation/acid-base interactions during exercise.
Background Pulmonary Physiology
Alveolar ventilation
The first step in delivery of oxygen to the tissues takes place in the process of delivery of fresh gas to the gas exchanging portions of the lung, a process termed alveolar ventilation. However, in the mammalian lung, the functions of ventilation and gas exchange are not anatomically separate as they are for some other species, notably birds, but are combined into an organ that does both. Consequently, not all inspired gas reaches the gas exchanging alveoli, but rather a portion remains in the conducting airways, and is known as anatomic dead space, reflecting the lack of participation in gas exchange. In addition, the fraction of alveoli that are either unperfused or are very poorly perfused relative to their ventilation are termed alveolar dead space. Combined, these are referred to as physiologic dead space . Thus, total expired ventilation consists of both alveolar ventilation and . Physiologic dead space can be calculated as a fraction of total ventilation using the Bohr (20) dead space equation as follows:
| (1) |
where FACO2, FECO2, and FICO2 are fractional concentrations of alveolar, expired, and inspired CO2 measured dry, and is expired minute ventilation. Equation (1) can also be expressed in pressure, and assuming that FICO2 is zero, results in the following equation:
| (2) |
Since there is difficulty estimating PACO2, Enghoff suggested substituting arterial PCO2 (PaCO2) for PACO2 in the Bohr equation (60). Assuming alveolar-capillary equilibrium for CO2, PaCO2 respresents a blood flow-weighted mean of the various PACO2 values in the lung, whereas true mean PACO2 represents a ventilation-weighted mean of PACO2 values (6) and thus the two are not identical. While the effect is relatively small, any ventilation/perfusion mismatch or diffusion limitation of CO2 transport or pulmonary shunt would increase PaCO2 relative to PACO2 (6), resulting in an increase in the calculated dead space fraction.
Once the dead space is accounted for, alveolar ventilation , the delivery of fresh gas to the alveoli is given by:
| (3) |
All of the carbon dioxide in the expired gas comes from alveolar ventilation, and the alveolar partial pressure of carbon dioxide, PACO2, is related to alveolar ventilation as (242):
| (4) |
PACO2 is reported in mmHg, while both the rate of CO2 elimination and are reported in liter/min. is always given at 0°C, 760 mmHg, Standard Temperature and Pressure, Dry (STPD); and PACO2 are reported under body temperature, ambient pressure, and Body Temperature ambient Pressure, Saturated (BTPS) with water vapor. The K is a conversion factor [(273 + t) × 760/273], where t = body temperature (273 is 0°C converted to °Kelvin). K is used to adjust to body temperature and pressure (and is equal to 863 mmHg at sea level and at normal body temperature of 37°C) (155, 242).
From Eq. (4), it can be appreciated that for a given rate of carbon dioxide production PACO2 is determined by alveolar ventilation. As above, the effect of mismatch, shunt and the diffusion limitation of CO2 transport in the lung is considered negligible, and therefore PACO2 ≈ PaCO2 (see later discussion regarding this during exercise).
Alveolar PO2
If the barometric pressure (PB) and the inspired fraction of oxygen (FIO2) and respiratory exchange ratio (R, the ratio of CO2 production to O2 consumption) are known, alveolar PO2 (PAO2) can be calculated from:
| (5) |
It is convention to use the directly measured arterial PCO2, PaCO2, substituted for PACO2 (255). PB is ambient barometric pressure, and PH2O is the partial pressure of water vapor at body temperature. This equation is frequently shortened as:
| (6) |
When R is not measured, it is either assumed to be 1, or 0.8. However, this is only a rough estimate and for research purposes, the full form of the equation should be used, along with the appropriate corrections for the saturation vapor pressure of water. At 37°C, PH2O is 47 mmHg, but PH2O increases with increasing body temperature and can be estimated by the formula, accurate to within 1% between 37 and 42°C (146) as:
| (7) |
Where t is body temperature (°C) and 2.718 is the Base of Natural Logarithms to 3 decimals (146). Although PAO2 can be calculated without correcting for the difference in saturation vapor pressure of water at different body temperatures, it should be appreciated that as body temperature rises with exercise, this correction becomes increasingly important.
Alveolar-arterial O2 tension difference
Using the PAO2 calculated from equation 5 and the arterial PO2 (PaO2) measured from a systemic arterial blood sample, corrected for body temperature, the alveolar to arterial pressure difference for O2 (A-aDO2 i.e. PAO2-PaO2) can be then be calculated. The A-aDO2 is the primary index of pulmonary gas exchange efficiency, and is discussed in detail below. It is important to recognize that in addition to individual variability in A-aDO2, at rest and during exercise, an accurate calculation of A-aDO2 requires accurate measurement of six variables: PaO2, body temperature, PB, PaCO2, , and , each with an associated measurement error. Thus, this calculation has variability introduced on this basis as well.
Pulmonary diffusion
As fresh inspired gas is being delivered to the alveolus by the process of ventilation, it becomes saturated with water vapor and mixed with gas resident in the alveoli. Then, oxygen must diffuse into, and CO2 out of the blood—a passive process. For a hypothetical homogeneous lung with no heterogeneity, the physiological definition of lung diffusion capacity for O2 (DLO2) is:
| (8) |
Where PcO2 is the mean PO2 passing through the pulmonary capillaries, which is typically estimated by arterial blood sampling (i.e., assuming that PcO2 = PaO2). This physiological definition provides that with the increased O2 consumption with exercise, the lung must increase its diffusive capacity to maintain PcO2 and thus PaO2. However, it is important to recognize that the lung is overbuilt for most of every day life, and it is generally exceptional for this to limit arterial oxygenation. For a more comprehensive review of the fundamentals in pulmonary diffusion, the reader is referred to the Handbook of Physiology—Exercise chapter on gas exchange (146); however, principles of diffusion will be briefly reviewed here for the sake of completeness.
Structural determinants of pulmonary diffusion
What components of lung structure determine pulmonary diffusion? The acinus is the largest structural unit of gas exchange, and consists of respiratory bronchioles, alveolar ducts, and alveoli. The structural components determining the overall resistance to alveolar gas diffusion can be conceptualized as an electrical analog model of series resistances:
| (9) |
DL is the overall rate of diffusion capacity of a given gas across the acini; DG is the rate of diffusion of gas through the alveolar air space from the acinar airways to the tissue membrane; DM is the rate of diffusion across tissue membrane and plasma barrier; DE is the combined rate of uptake across the erythrocyte membrane and reaction with hemoglobin. This conceptual relationship was well summarized previously (146), and therefore, we will briefly review these components in relation to exercise.
Structural basis of gas phase diffusion resistance (1/DG)
Convective flux of gas refers to the bulk movement of gas along the airways as generated by differences between alveolar and mouth pressures, while diffusive flux relates to the passive movement of gas due to the respective concentration gradients of each gas. At a given rate of inspiration, convective flux (flow/unit area) is inversely proportional to total airway cross-sectional area; therefore, convective flux will progressively decrease as the interface of inspired air moves peripherally even though total flow (flux × cross-sectional area) remains fixed. Importantly, diffusive flux is independent of total airway cross-sectional area; therefore, diffusive flux will increase with respect to the concentration gradient as the convective interface moves peripherally while total diffusive transport (flux × cross-sectional area) will progressively increase. A peripheral location within the small airways will be reached where the rate of diffusive transport exceeds the rate of convective transport. Modeling studies have shown that at this point, airflow almost ceases because of the reduction in convection transport (221). Mixing between inspired and alveolar gases beyond this gas phase interface would thus be achieved almost entirely by diffusion, which could theoretically cause a gas-phase diffusion limitation (146). As the depth of inspiration increases, the gas-phase resistance (i.e., the point within the acinar airways where convective flux has stopped and gas exchange is only occurring with diffusion) is reduced (146). This reduction in gas phase resistance would theoretically improve overall gas exchange, and may contribute to the increase in diffusion observed with exercise (70, 95, 120, 130, 133, 134, 148, 305). The importance of the gas phase resistance at rest and during exercise in humans remains uncertain (146), but there is evidence for it in reptiles with non-alveolar lungs [and thus large diffusion distances (123)] as well as in horses (119).
Structural basis of pulmonary membrane resistance (1/DM)
The alveolar capillary membrane resistance is related directly to the available alveolar-capillary surface area and inversely to the mean harmonic thickness of the tissue-plasma barrier as illustrated by the equation:
| (10) |
Where k is the diffusion constant, a function of gas permeability in lung tissue and plasma (130). The membrane thickness capacity accounts for the conductance of the air-blood barrier, defined functionally as the tissue (alveolar surface lining layer, epithelium, interstitial space, and endothelium) as well as plasma regions interposed between alveolar space and erythrocytes (340). Of note, the mean length of the molecular diffusion path will vary with spacing between red cells and red cell shape (68, 132). Further, the erythrocyte deforms into a parachute-like shape as it passes through the capillary (130, 335), which has the effect of minimizing blood flow resistance (16), but may also reduce diffusion (132), presumably by shielding portions of the erythrocyte membrane from the capillary surface and causing greater heterogeneity in the distribution of gas exchange over the surface of the red blood cell (130, 132).
The functionally effective membrane surface area for diffusion depends on the matching of alveolar surface area and capillary surface area. Alveolar surface area may be expanded with exercise by capillary recruitment as well by membrane unfolding and/or stretching associated with lung inflation (10, 80, 82, 146, 312). As will be discussed later within this article, there are also data suggesting that exercise may cause interstitial pulmonary edema. This has the potential to increase barrier thickness, affecting DM, although it is unclear that this is a significant contribution to diffusion limitation during exercise (see Section “Mechanisms of diffusion limitation with exercise”).
Structural basis of erythrocyte resistance (1/DE)
The resistance to gas uptake within a volume of blood (1/DE) is determined by the combined resistances of the red cell membrane and reaction rate with hemoglobin (1/θ) and by the total volume of capillary blood (1/Vc).
| (11) |
Capillary blood volume can be expanded by distention and/or recruitment of pulmonary capillaries secondary to increased microvascular perfusion or pressure. Anatomical capillary recruitment may occur via augmentation of either plasma or red cell volume; however, only augmentation of red cell volume contributes to increased gas exchange (147). With incremental upright exercise, there is a central shift of blood volume into the thorax (71), increasing pulmonary arterial, and venous pressures (17, 56, 57, 110, 245, 246, 300 28, 311), increasing capillary recruitment and capillary distention, and as a result, both pulmonary capillary blood volume (Vc) (120, 148, 338) and surface area for gas exchange are increased. This increase in capillary recruitment and distention results in reduced pulmonary vascular resistance with incremental exercise (110, 300, 328) and but also helps to maintain the red blood cell capillary transit time with increasing cardiac output.
Because of their high binding affinities for Hb, carbon monoxide (DLCO) and nitric oxide (DLNO) uptake can be used as an estimate of DLO2 based on the ratio of Krogh diffusion constant for the two gases. Alternately, in the presence of a diffusion limitation DLO2 can be calculated from data using the multiple inert-gas-elimination technique (MIGET). From rest to peak exercise, DLO2, DLCO, DLNO, DMCO, and Vc all increase linearly with respect to cardiac output (70, 95, 120, 130, 133, 134, 148, 282, 305). The increase in DL with exercise may come from several sources including: (i) reduction in the gas-phase resistance (i.e., the point within the acinar airways where convective flux has stopped and gas exchange is only occurring with diffusion), (ii) unfolding and distension of alveolar septa as the lung expands, (iii) opening and/distension of capillaries secondary to increased capillary blood volume, (iv) increased capillary hematocrit, and (v) more homogeneous distribution of erythrocytes within and among capillaries (130). Studies that have measured either DLCO or DLNO have failed to show a plateau with incremental exercise. It may be that there is no true plateau in DL with incremental exercise; however, most studies using DLCO/DLNO techniques have not measured diffusion above 80% of , that is, at the exercise intensity above which it is most expected to occur (70, 133, 134, 148, 282, 305). Indeed, MIGET data have demonstrated the development of a diffusion limitation at high exercise intensities (93, 251, 316), typically in endurance-trained subjects (117), indicating that at high workloads the increase in DL may not be sufficient to maintain adequate O2 exchange.
Diffusion between gas and liquid phases
In describing diffusive transfer between gas and liquid phases, in this case alveolar gas and the blood, (or for CO2 from the blood into alveolar gas) differential solubilities must be taken into account. The Krogh-modified Fick equation to describe diffusion of a specific gas between two phases is:
| (12) |
where dV/dt is the volume transferred per unit time (cm3/min) in the x direction; A is the area in square centimeters through which gas exchange is occurring; α is the Bunsen solubility coefficient at body temperature in milliliters gas per milliliter of blood per atmosphere of pressure (i.e., atm−1); d is the diffusion coefficient of the gas in square centimeters per second,; ∂P/∂x is the partial pressure gradient of the gas in mmHg per centimeter in the x direction; 60 is s/min and 760 is mmHg/atm; [60 × αd/760] is known as the Krogh diffusion constant (146).
Rates of diffusion are dependent upon the velocity of random motion particles. Average kinetic energy (EK) of different molecules is the same at a given temperature. Since EK = mass × velocity2/2, heavier molecules move more slowly and have lower diffusion coefficients. Thus, diffusion coefficients are directly proportional to mean velocity and inversely proportional to the square root of molecular weight (Grahams’s law). However, in liquid or tissue phase, the solubility of a gas must also be taken into account when examining diffusive gas transport that is highlighted by the diffusion of CO2. CO2 is a larger molecule compared to O2, and therefore, based on Graham’s law CO2 would diffuse slower for given concentration gradient; however, CO2 has a 20 times greater diffusivity than O2. The reason for this is related to the greater solubility in the alveolar wall for CO2 than O2. In addition, this greater diffusitivity does not necessarily translate into a faster rate of diffusion equilibrium for CO2, as the diffusivity only refers to transport across the blood-gas barrier and does not account for CO2 transport in blood. The chemical reaction kinetics for CO2 are complex: CO2 is carried in the blood predominately as HCO3− after diffusing into the erythrocyte, undergoing carbonic anhydrase-mediated conversion to HCO3− and transport back into the plasma in exchange for chloride (Cl−). In the lung, this process is reversed, and the time constants for HCO3− Cl− exchange are relatively slow, and thus might contribute to a diffusion limitation. However, there is now a substantial body of literature that carbonic anhydrase is also present in the pulmonary capillaries and thus greatly speeds the conversion of HCO3− to CO2, overcoming this limitation (41, 72, 73, 159, 240). Thus, we generally assume that the diffusion limitation of CO2 transport in the lung is negligible, and therefore, that PACO2 ≈ PaCO2. Experimentally, data are sparse, but this has been suggested to be true even during very heavy exercise, in the vast majority of individuals (129).
Equilibration index
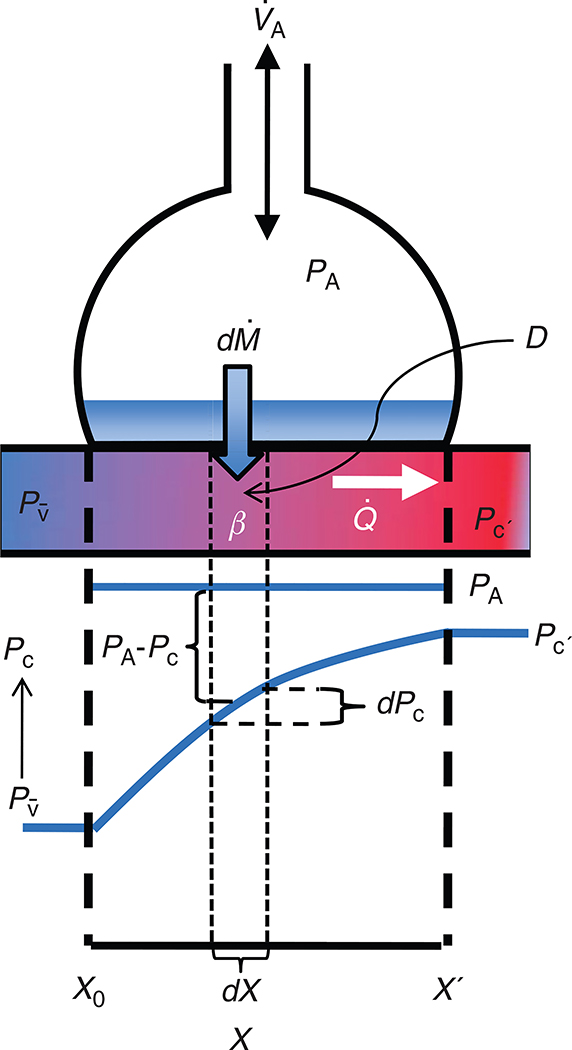
Given the factors affecting diffusing capacity of the lung discussed above, what is the net effect on the equilibration of gas across the alveolar-capillary membrane? The equilibration of a gas with blood in the pulmonary capillary has been modeled on the balance between diffusing capacity (DL) and pulmonary capillary blood flow as described by Piiper and Scheid (269). Consider a hypothetically uniform alveolus perfused under steady-state conditions by a capillary containing a uniform, perfectly mixed volume of blood (the reasons for these simplifying assumptions are discussed in (228, 324)). A model of this is given in Figure 1. The rate of transfer of a gas into the blood contained in a tiny cross-sectional segment of capillary (defined by the length dx) is given by Fick’s law.
| (13) |
Where Dx is the diffusing capacity for the gas at location dx, PA is its alveolar partial pressure and Pcx the partial pressure in the capillary blood at location dx [note that equation 13 is a modified form of Eq. (12)]. This flux of gas creates a change in the gas content of the blood dConx along this segment as:
| (14) |
where is steady state perfusion [note that Eq. (14) is just a restatement of the Fick equation, seen in its more familiar form in Eq. (22) below]. To determine equilibration between alveolar and end-capillary gas these two equations can be combined, rearranged, and integrated along the length of the capillary to give:
| (15) |
the coefficient β is the conversion of blood gas content to partial pressure, and is the effective solubility of the gas in blood (in units of ml gas/100 mL blood per mmHg) representing the slope of dissociation curve between gas and blood (269). Thus, β can be calculated as:
| (16) |
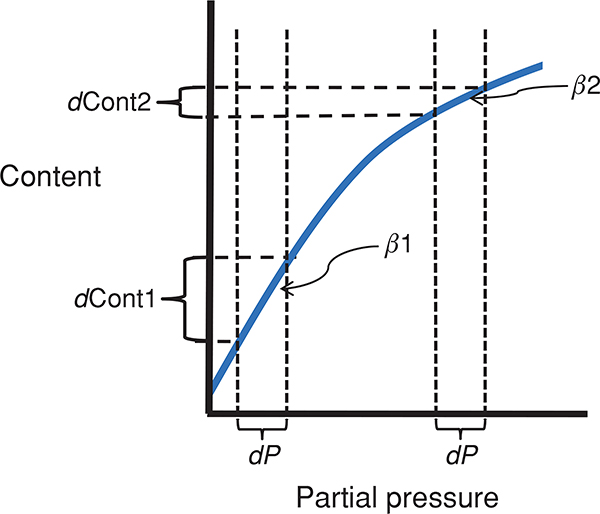
For inert gases (such as used in the multiple inert gas elimination technique) and to an approximation for CO2, there is a linear relationship, between solubility (i.e., β) and partial pressure and thus β is a constant. It is evident from Eq. (15) that either an increase in diffusion capacity or a decrease in the rate of blood flow would allow more complete equilibration of blood with alveolar gas (113, 269) at any time point along the pulmonary capillary. In the situation with a minimal extent of diffusion limitation, the amount of gas exchanged per minute depends on content of incoming venous blood and the rate of gas equilibration in the blood, times the amount of blood flow per minute (113). When is high for a given gas, gas exchange is said to be “perfusion limited”. For example, nitrous oxide (N2O) is readily soluble in lung tissue (high DL) and thus the partial pressure rises extremely rapidly in the blood. Consequently the rate of N2O gas transfer through the lung is dependent on blood flow, and therefore perfusion limited. In contrast, CO has a β that is very high (due to the high affinity and avidity of CO for hemoglobin), and hemoglobin acts as an almost “infinite sink” for CO. Therefore, the partial pressure of CO in blood rises very slowly, resulting in very low (i.e.,), and thus CO gas exchange is “diffusion limited.” This makes CO an ideal gas to study lung diffusion (113). Importantly, oxygen is unique as the oxyhemoglobin dissociation curve (i.e., content/pressure ratio) is not linear, and therefore, β for O2 would vary depending on the location of mixed venous blood on the oxyhemoglobin dissociation curve (conceptualized in Fig. 2). While in normoxia, the curvilinear nature of the oxygen hemoglobin dissociation curve means that this approach is not quantitatively accurate, it is still helpful from a conceptual standpoint. At rest in normoxia, is approximately 10 for O2, resulting in O2 equilibrating within the first 1/3 segment of pulmonary capillary transit (113). As capillary blood equilibrates with PAO2, O2 exchange is, therefore, perfusion limited in this condition. In hypoxia, both PAO2 and mixed venous O2 are reduced. As gas exchange shifts to the steeper part of the O2 dissociation curve in hypoxia, β is increased, causing a reduction in and a prolonged equilibration time.
Figure 1.
Schematic diagram showing equilibration of a gas in the pulmonary capillary of a homogeneous lung. The top half of this figure represents a schematic alveolus and pulmonary capillary, the bottom half the corresponding changes of gas partial pressure in the pulmonary capillary. X represents the distance along the pulmonary capillary from X0, the start of the contact point with the diffusion barrier to X’, the end of the point of contact with the diffusion barrier. Fresh gas is delivered to the alveolus by the process of alveolar ventilation . The alveolus is perfused by a pulmonary capillary, with a partial pressure of mixed venous gas at X0 which rises to a maximum end-capillary partial pressure (Pc’) at X’. Consider a tiny increment of distance along the pulmonary capillary (dX). The flux of gas across the alveolar wall into the blood is described by Fick’s law of diffusion and is given by the product of the diffusing capacity (D) of the element of the barrier corresponding to dX and difference between the partial pressure in alveolar air (PA) and the partial pressure of the gas in capillary blood (Pc), PA–Pc. The uptake of gas into the blood at point dX results in a change in the content of gas in capillary blood (dPc). Under steady-state conditions, this is also equal to and is described by the Fick equation and is calculated as the product of the steady-state perfusion and dPc. The content of gas in blood is related to the partial pressure in blood by β the effective solubility of the gas (the slope of the dissociation curve, i.e., dcontent/dPc).
Figure 2.
The effect of a nonlinear dissociation curve on the effective solubility (β) and diffusion equilibrium. Here dP is considered to be the change in partial pressure in the blood of a gas required for diffusion equilibrium. When β is relatively large (the steep slope seen in β1) there must be a large change in content (dcont1) for dP whereas when β is relatively small (β2) the same partial pressure change is accomplished with a much smaller change in content (dcont2). Thus, a large β (i.e., β1) means that there is a large sink for a gas, more molecules must be transferred before the partial pressure rises, compared to the situation where β is relatively small (i.e., β2).
From the relationship it should be evident that a diffusion limitation for a gas can develop when DL is reduced (i.e., with lung disease), β is increased (i.e., for oxygen under conditions of hypoxia) or is increased (such as with exercise). Exercise is, therefore, a particular strain on O2 equilibration because of the unique combination of an increase in combined with a reduced β secondarily to the reduction in mixed venous O2. When these changes are large, they may be sufficient to overwhelm any increases in DL that are occurring due to increasing pulmonary blood volume with exercise and result in the net development of a diffusion limitation. There is considerable evidence to show that this is one of the causes of impaired pulmonary gas exchange. Somewhat paradoxically, in healthy subjects, impaired pulmonary gas exchange and an increased A-aDO2 during exercise occur most often in endurance athletes. In these individuals, very high cardiac output may impair diffusion in the lung despite normal (or even supranormal) healthy lungs (see Section “Pulmonary gas exchange and exercise”).
For oxygen the relationship can be further modeled as (225):
| (17) |
This formula integrates pulmonary perfusion, diffusion, the oxyhemoglobin dissociation curve, alveolar, and mixed venous PO2 to predict the PO2 difference between the alveoli and the end capillary (i.e., A-aDO2) for a given mixed venous PO2. Within this formula, overall gas exchange efficiency between the alveoli and end capillary (i.e., PAO2-Pc′O2) would firstly be improved as mixed venous PO2 increases, as this narrows the entrance difference (i.e.,) (269). In addition, as gas exchange efficiency (i.e., PAO2-Pc′O2) is determined by the entrance difference to the negative exponent of , as becomes larger the difference between PAO2 and Pc′O2 is reduced, and therefore, gas exchange improved.
Ventilation-perfusion matching
Up until this point, we have been concerned with the delivery of oxygen to the alveolus and diffusion into the blood, and the same process in the opposite direction for CO2, but the process does not stop there. It is important to recognize that the lung is not a uniform organ. Thus, on the airway side, there is heterogeneity in ventilation of the millions of alveoli within the lung and consequently regional PO2 and PCO2 varies in the alveolus. Similarly, on the pulmonary vascular side, pulmonary perfusion is also heterogeneous, and so efficient gas exchange depends on the optimal matching of ventilation to perfusion such that well ventilated areas of the lung are also well perfused.
Mass balance of O2 and CO2 and ventilation-perfusion matching
The basic mass balance equations for O2 and CO2 provide a basis for quantitatively understanding ventilation-perfusion matching. Under steady-state conditions, pulmonary gas exchange obeys the principals of mass balance: this means that the loss of CO2 from the capillary blood is balanced by entry of CO2 into the alveolar gas, and similarly for O2, the gain into the capillary blood is balanced by the loss from alveolar gas. We exploited this principle in the previous section in discussing diffusion equilibrium across the alveolar wall. Assuming inspiratory minute ventilation, , and expiratory ventilation, are converted to standard temperature and pressure, oxygen uptake, and carbon dioxide production on the alveolar gas side can be calculated as:
| (18) |
and
| (19) |
Consider the case of a single gas-exchanging unit without dead space. The assumption is made in this case that there is diffusion equilibrium. For the purposes of this explanation, the further simplifying assumption is made that the respiratory quotient is 1, and thus the volume of inspired and expired air are identical. In the alveolus, the volume of oxygen taken up from the alveolus can be calculated as:
| (20) |
and the corresponding volume of CO2 evolving into the alveolus is:
| (21) |
When combined with the Fick equations for O2 and CO2 and solved for , this allows the effects of ventilation perfusion inequality on pulmonary gas exchange in a single gas exchange unit to be described. The Fick equation describes the uptake of oxygen into the blood:
| (22) |
Under steady-state conditions, the equations describing blood and gas oxygen exchange can be combined using K as a conversion factor from fractional concentrations to partial pressures (and to allow for the fact that gas concentrations are STPD and ventilation volumes are BTPS) and solved for as
| (23) |
A similar equation can be written for CO2 and takes the form:
| (24) |
Since the inspired partial pressure of CO2 is very low, this can be simplified to
| (25) |
Equations 23 to 25 point out the intimate relationship between oxygen and carbon dioxide that are inextricably linked by the ratio in a gas-exchanging unit. Note that these relationships can also be derived for the whole lung without the simplifying assumptions above. For CO2 in the whole lung, since PACO2 is dependent on alveolar ventilation, the equation is the same. However, the derivation for oxygen is much more complicated and the reader is referred to (241, 242, 254, 255), for further explanation.
Ventilation-perfusion matching; effect on gas exchange
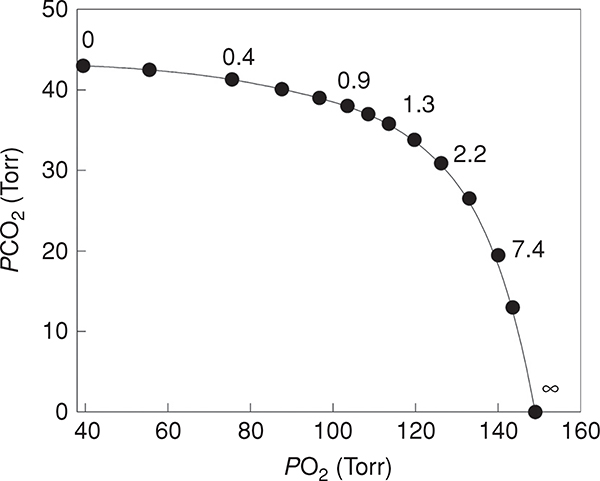
The precise matching of ventilation to perfusion is one of the primary determinants of the efficiency of pulmonary gas exchange. The relationship between ratio and alveolar gas concentrations can be visualized graphically in the O2-CO2 diagram [Fig. 3 from Farhi (67)]. From this, it can be appreciated that in a given alveolus, as ratio falls, the PO2 and PCO2 in the blood leaving that alveolus approaches pulmonary mixed venous blood. In the extreme case, ventilation is absent and pulmonary mixed venous (deoxygenated) blood enters the systemic circulation. This region of the lung that is perfused but not ventilated is termed a shunt, and when present results in a significant increase in A-aDO2 and a corresponding reduction in arterial oxygenation. On the other hand, as ratio rises, the PO2 and PCO2 in the blood leaving the alveolus approaches the inspired concentrations. Portions of the lung with extremely high ratios are termed dead space ventilation—areas of the lung that are ventilated but not perfused. This is in essence “wasted” ventilation, lowering the local PACO2, but doing little to change PaO2.
Figure 3.
The relationship between oxygen and carbon dioxide as a function of differing ratios [adapted, with permission, from Fahri (67)]. When the ratio is low, the composition of alveolar gas approaches that of mixed venous blood. When the ratio is high the PO2 and PCO2 of alveolar gas approaches that of the inspired gas.
The reason for this is because of the alinearity of the oxygen-hemoglobin dissociation curve, in contrast to a nearly linear CO2 dissociation curve (342, 345). mismatch affects both O2 and CO2 leading to an increase in PaCO2 and a decrease in PaO2. The high ventilation does little to increase PaO2 because the blood leaving the well-ventilated units of the lung are already on the flat part of the oxygen-hemoglobin dissociation curve and almost fully saturated with O2. This effect is commonly seen in patients with lung disease such as chronic obstructive pulmonary disease, where mismatch results in hypoxemia but near normal arterial CO2. As a result of the reduced PaO2, the peripheral chemoreceptors are stimulated, increasing alveolar ventilation, reducing PACO2 and thus PaCO2. However, increased ventilation of the high sections cannot “make up” PO2 for the low sections because in high regions large changes in partial pressure are associated with only minimal changes in content, and therefore hypoxemia is maintained. In contrast, because the dissociation curve for CO2 is much more linear, the PCO2 of the blood leaving the high units is lowered as compared to the low regions, and the net effect is that PaCO2 will be maintained at nearly normal levels. This can be seen in Figure 3, where an increase in the ratio from 1.3 to 7.4 halves PCO2 but PO2 increases by less than 20%.
At rest, in healthy normal people, the distribution of ventilation-perfusion ratios amongst the millions of gas-exchanging units in the lung is centered at approximately 1, consistent with the ratio of overall resting alveolar ventilation (~5 liter/min) to overall perfusion (cardiac output also ~5 liter/min). However, it would be a mistake to think of the effects of inequality on gas exchange efficiency only in terms of overall and overall . This is because the heterogeneity about these overall means, and how well regions of relatively high are matched by relatively high is a major determinant of the effect on gas exchange. In resting normal subjects, the distribution of and are relatively uniform, although there is a well-described gravitational gradient in ratio in addition to other factors that contribute to heterogeneity [see reference (83) for review]. However, during exercise the distribution of widens and becomes less uniform, and may form a significant contribution to gas exchange impairment during exercise as discussed below in the Section “Pulmonary gas exchange and exercise”. Note the assumption that there is diffusion equilibrium may not always be the case for oxygen during exercise, particularly during hypoxic exercise or at very high cardiac outputs, as discussed in the Section “Pulmonary gas exchange in hypoxia”.
Pulmonary gas exchange and exercise
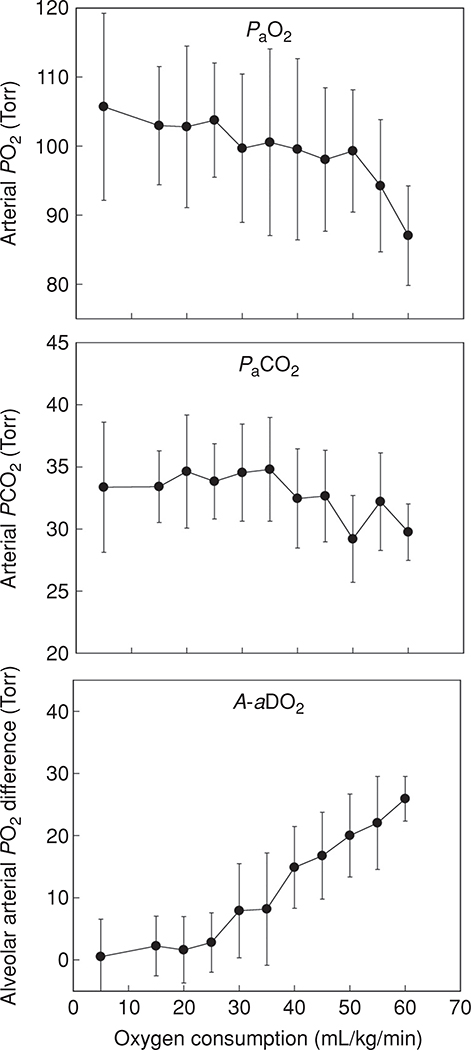
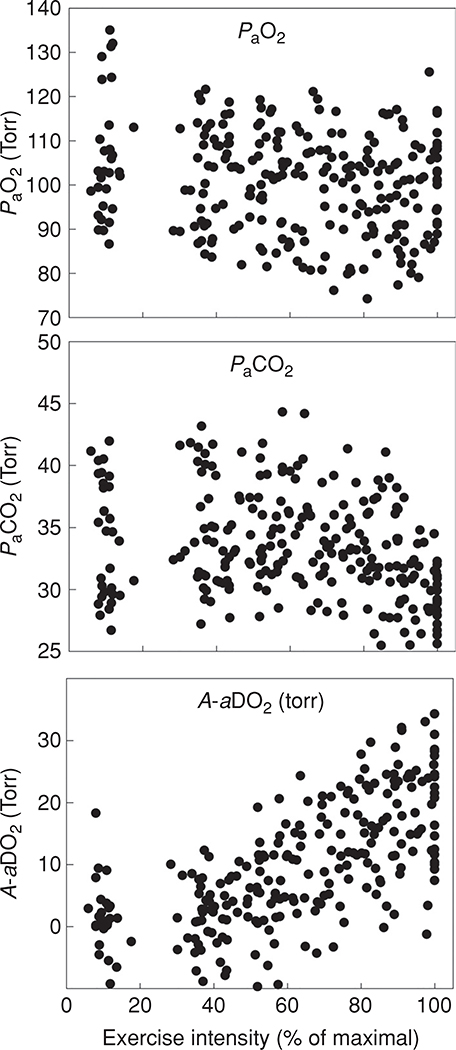
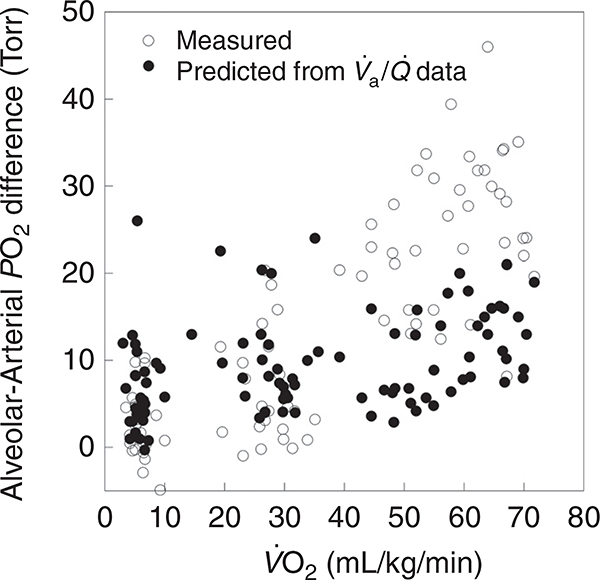
Now that the basic components of pulmonary gas exchange have been introduced, pulmonary gas exchange during exercise with respect to these components can be evaluated. Figures 4 and 5 show temperature-corrected arterial blood gas data obtained from 32 healthy normal athletic subjects (15 males and 17 females) during progressive cycle exercise from pre-exercising rest to , documenting the changes in the A-aDO2, PaCO2 and PaO2. It should be noted that pre-exercise does not represent a true resting condition as the subjects are on the cycle ergometer, breathing through a mouth piece, anticipating exercise, and therefore, are hyperventilating compared to a true resting baseline. In addition, some of the A-aDO2 values are calculated as negative, which likely represents measurement error, reflecting the inherent lack of steady state conditions of these pre-exercise data. The general patterns of gas exchange responses are largely similar when the data are plotted as a percentage of for the same group of subjects, as in Figure 5. It can be appreciated that although the efficiency of gas exchange as measured by the A-aDO2 deteriorates with increasing exercise intensity, PaO2 is largely maintained until the oxygen consumption is greater than 50 mL/kg/min. However, during very heavy exercise approaching , in some individuals the PaO2 may fall. This is secondary to a substantial increase in A-aDO2, in combination with a lack of increase in PAO2 because of a blunted hyperventilatory response to heavy exercise. Although most of the subjects who have an A-aDO2 greater than 25 mmHg are exercising at a above 60 mL/kg min, there are still some who experience an A-aDO2 greater than 25 mmHg at a of less than 50 mL/kg/min. A significant fall in PaO2 (> 10 mmHg) and hemoglobin saturation (SaO2, > 5%) from rest can be sufficient to compromise oxygen transport and is termed exercise induced arterial hypoxemia (EIAH-discussed below) (47).
Figure 4.
Temperature corrected arterial blood gas data and calculated A-aDO2 obtained from 32 healthy normal subjects (15 male, 17 female) during progressive cycle exercise to . Arterial PO2 falls and the A-aDO2 increases with increasing exercise intensity. In this data set, the samples at “rest” are obtained with the subject sitting upright on the cycle ergometer, breathing through a mouthpiece and anticipating maximal exercise. Thus, the PaO2 is somewhat elevated and PaCO2 reduced over true resting values.
Figure 5.
Individual subject PaO2, PaCO2, and A-aDO2 for the same 32 subjects whose data appears in Figure 2, plotted as a percentage of . Here, the wide variation in the blood gas responses to exercise can be appreciated.
Pulmonary gas exchange during submaximal exercise
From rest to moderate-intensity exercise, there is a proportional increase in alveolar ventilation, and as a result there is little change in PaO2 from the pre-exercise values. As seen in Figures 4 and 5, many subjects hyperventilate, as indicated by a reduction in PaCO2 pre-exercise and at low exercise intensities, but this is largely an artifact of the laboratory setting. Once exercise intensity increases beyond approximately 60% to 80% of maximal oxygen uptake, ventilation increases disproportionately to metabolic demand, resulting in a reduced PaCO2 and an increased PAO2. This effect is beneficial for the regulation of acid-base balance because the acidifying effects of CO2 are more than completely eliminated, thus providing some compensation for the metabolic acidosis associated with increased arterial [lactate] and [protein]. Pre-exercise there is minimal gas exchange impairment observed in young healthy subjects (i.e., A-aDO2 is < 5 mmHg), and A-aDO2 tends to increase linearly with incremental exercise reaching a peak at (Fig. 4). As PaCO2 and PAO2 are typically well maintained from pre-exercise to moderate exercise, and A-aDO2 is low, PaO2 is usually maintained close to pre-exercise levels up to moderate intensity exercise (see Figs. 4 and 5). A reduction in PaO2 during submaximal exercise has been noted in some highly trained subjects (250) as well in some less well-trained women (100), and has been ascribed (particularly in women) to an inadequate ventilatory response to exercise (250) (see Section “Sex and pulmonary gas exchange”).
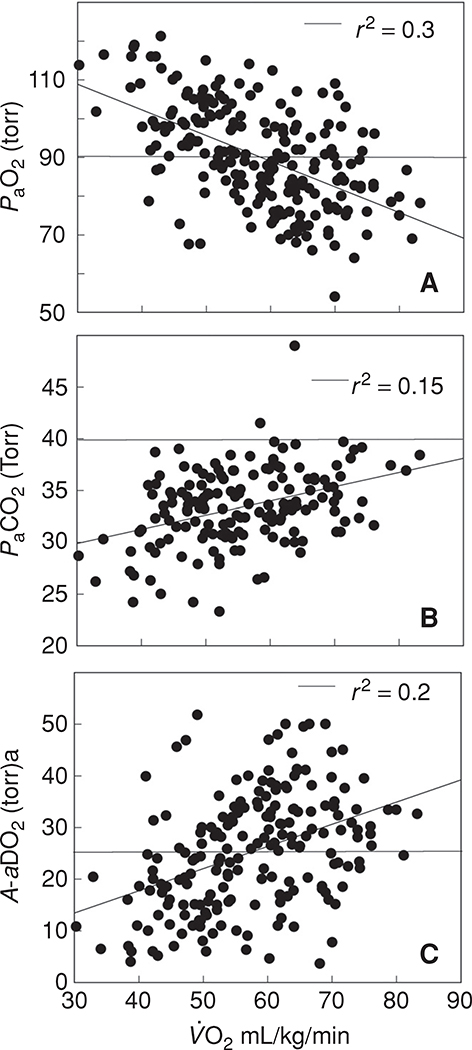
Pulmonary gas exchange during maximal exercise
Although the time spent in maximal exercise is limited in duration for any one individual, it is of great interest from a physiological standpoint because it represents the greatest stress to the pulmonary system. The blood gas responses to exercise near are shown in Figure 6, which shows the PaO2, PaCO2, and A-aDO2, from cycle and treadmill exercise tests in 198 healthy normal subjects exercising at 90% to 100% of . The of these subjects spans the physiological range for healthy normal subjects varying from 30 to approximately 80 mL/kg/min. Of note, the pulmonary gas exchange response to exercise varies with the mode of exercise even among the same subjects exercising at the same absolute and relative oxygen consumption (78, 118). Some of these differences relate in part, to differences in alveolar ventilation, as PaCO2 is greater during running than cycling exercise; nonetheless the A-aDO2 is greater during running (118) than during cycle exercise for reasons, that are obscure. However, irrespective of exercise modality, a common pattern emerges: there is an inverse relationship between and PaO2 at such that, paradoxically, individuals capable of extraordinary levels of aerobic work have on average a lower PaO2. However, as can be seen from Figure 6A, the PaO2 during very heavy exercise is highly variable even among populations of similar aerobic fitness. For example, at a of 70 mL/kg/min the PaO2 varies from 110 to 53 mmHg and even at a more modest level of , there is a similar range of responses. Also notable is the large variability in the PaCO2 during maximal exercise. As seen in Figure 6B, the majority of subjects show a hyperventilatory response to maximal exercise and with few exceptions PaCO2 is less than 40 mmHg. On average, there is a weak positive association with such that subjects with a higher have a greater PaCO2.
Figure 6.
Temperature corrected arterial blood gases obtained at near maximal and maximal exercise (cycle ergometer or treadmill running) in normal subjects [(A) n = 198; (B) and (C) n = 175)]. Data are, with permission, from references (15, 45, 76, 93, 94, 118, 121, 124, 219, 231, 251, 328, 357). The horizontal line in A and B defines the normal value and in C the limits of the expected increase in A-aDO2 with exercise as defined by Dempsey and Wagner (47). The arterial PO2 is lower and the PaCO2 and A-aDO2 higher with increasing aerobic capacity. Above a of 65 to 70 mL/kg/min the majority of individuals have significant gas exchange impairment although it is uncommon in individuals with a .
Exercise-induced arterial hypoxemia
Exercise-induced arterial hypoxemia (EIAH) refers to a decrease in PaO2 and/or the saturation of hemoglobin in arterial blood with exercise. EIAH has been has been comprehensively reviewed (47,117) and defined in several ways depending on the research question at hand. For questions related to systemic oxygen transport and delivery, arterial oxygen saturation (SaO2) is the best indicator of the consequences of EIAH, whereas for questions concerned with the efficiency of gas exchange, then the extent of the increase of the A-aDO2 with exercise is more appropriate. Similarly, when the ventilatory response to exercise is under consideration, then the arterial PCO2 can be used. Within these guidelines, mild EIAH is defined as a SaO2 of 93% to 95% (or a decrease of 3–4% from rest), moderate EIAH as a SaO2 of 88% to 93%, and severe EIAH as a SaO2 of less than 88%. Similarly, an A-aDO2 greater than 25 mmHg is consistent with a mild gas exchange inefficiency, and an A-aDO2 greater than 35 to 40 mmHg consistent with a severe gas exchange impairment (47). It is important to consider PaCO2, because many subjects will maintain their arterial PCO2 to within a few mmHg of resting values until close to on a graded exercise test as seen in Figure 5, and thus especially during submaximal exercise, the hyperventilatory response to exercise may be inadequate. A PaCO2 at in the 35 to 38 mmHg range represents a borderline hyperventilatory response and PaCO2 greater than 38 mmHg, an absent hyperventilatory response (47). The use of these different criteria allows for identification of the key components of EIAH, which individually may not result in a decrement in PaO2 or SaO2. It should be noted that EIAH is not confined to humans and is especially notable in the horse which develops a large A-aDO2 during maximal exercise associated with a considerable decline in PaO2 (to ~70 mmHg) and SaO2 (~88%) (272, 329). Although EIAH is often most pronounced at maximal exercise, some subjects develop a reduction in PaO2 even during moderate exercise (100,250) and this is suggested by some authors to be exaggerated in women (see Section “Sex and pulmonary gas exchange”).
While most exercising humans show an increase in AaDO2 with exercise, when arterial blood gas data are directly acquired and corrected for body temperature, EIAH as indicated by decreased PaO2 affects a minority of healthy individuals. As seen in Figure 6, it is only above an oxygen consumption of 55 to 60 mL/kg/min that the average A-aDO2 is greater than 25 mmHg, and the average PaO2 less than 90 mmHg—the boundaries of the normal response (47). Thus, it is important when using this information in the clinical evaluation of patients that despite the attention given to pulmonary gas exchange and EIAH in the research literature, that EIAH is uncommon in all but the most highly aerobic athletes. Other explanations should be sought for the increased A-aDO2 and the decreased PaO2 in an individual who presents with cardiorespiratory symptoms. In addition, it is essential that the blood gas data be corrected for temperature before conclusions are drawn, as failure to do this will lead to an underestimation of PaO2 and PaCO2, and as a result an overestimation of the calculated PAO2 and A-aDO2 (see Section “Temperature correction of arterial blood samples” for further discussion) (278). Also, it is essential that blood gases and oxygen content are directly measured and that EIAH is not inferred from measurements made with pulse oximetry because of the very limited validity of these devices during exercise (see Section “Pulse oximetry”).
Mechanisms of exercise-induced alterations in pulmonary gas exchange
The effect of exercise on pulmonary gas exchange is perhaps best understood when consideration is given to the contributions of the four causes of hypoxemia as classically defined by: hypoventilation (i.e., inadequate alveolar ventilation), diffusion limitation, ventilation-perfusion mismatch, and shunt.
Alveolar ventilation and pulmonary gas exchange
Assessment of alveolar ventilation
The alveolar PCO2 is a balance between the rate of replenishment of CO2 diffusing out of blood perfusing the pulmonary capillaries and the rate of removal by the process of alveolar ventilation as seen in Eq. 3. PaCO2 is used as an indicator of the adequacy of alveolar ventilation, which assumes that that the alveolar-arterial difference for CO2 is negligible. If steady-state conditions can be assumed, and arterial blood gas and expired gas data are available, then fractional physiological dead space ventilation can be calculated during exercise using Enghoff’s modification (60) of the Bohr equation (20).
| (26) |
If the tidal volume is known, then can be quantified. The multiple inert gas elimination technique (64, 326, 331) (MIGET), which is described in more detail below, also allows for the calculation of dead space in a similar manner using gases of high solubility such as acetone and ether.
Alterations in alveolar ventilation with exercise
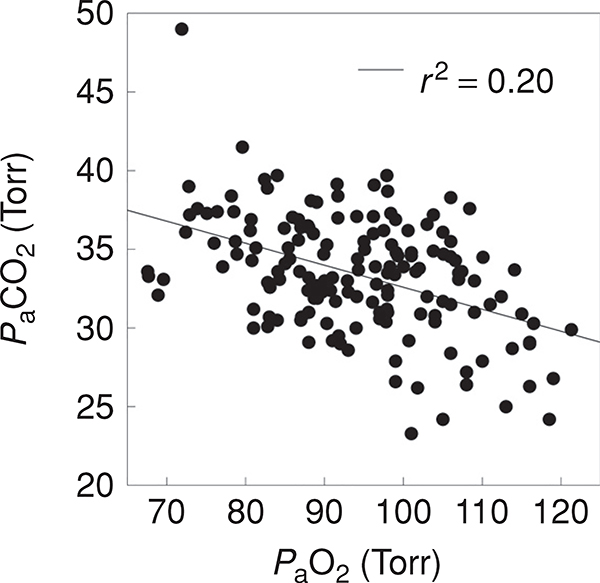
Figure 6B shows the relationship between and PaCO2 during exercise near in 175 of the subjects from Figure 6A and Figure 7 shows the relationship between PaCO2 and PaO2 in these same individuals. It can be appreciated that very few of these subjects have overt hypoventilation and the PaCO2 is almost always less than 40 mmHg at . The vast majority of subjects (114, ~70%) have an adequate hyperventilatory response as defined as by Dempsey and Wagner (47) although a minority (n = 12, ~7%) exhibit a minimal hyperventilatory response. This minimal hyperventilatory response is implicated as a contributor to EIAH (45, 103, 203, 235), as the failure to lower PACO2 limits the increase in PAO2 with exercise. However, even in the subjects with an absent hyperventilatory response, the variation in PaO2 is substantial and across all subjects PaO2 is only loosely associated with PaCO2 across all subjects (Fig. 7).
Figure 7.
The Relationship between PaO2 and PaCO2 during maximal exercise for the subjects from Figure 6. PaCO2 is an index of alveolar ventilation and it can be seen that limited hyperventilation explains only approximately 20% of the variance in PaO2.
Mechanisms for limitation of alveolar ventilation with exercise
A number of mechanisms for inadequate hyperventilation are possible including: a decreased peripheral chemoreceptor function (103), respiratory muscle fatigue (142), and mechanical constraints imposed on inspiratory and expiratory flow (145, 203), with the last explanation the most likely. In young fit subjects, almost all of the maximal expiratory flow-volume (MEFV) curve may be approached during exercise (145) and even when helium-oxygen mixtures are used to remove expiratory flow limitation EIAH is not completely abolished (203). Also, although ventilatory muscle fatigue may not play a major role during short-term maximal and very low intensity exercise, respiratory muscle fatigue has been demonstrated during high intensity exercise (142), where it may further contribute to relative hypoventilation.
Ventilation-perfusion matching and exercise
Assessment of matching during exercise
Most of the quantitative information on matching has come from a technique known as the multiple inert gas elimination technique (64, 326, 331), MIGET, which can be used to study resting gas exchange in both health and disease and also can be applied during exercise. Using MIGET, the relative contributions of inequality, diffusion limitation of oxygen transport, and intrapulmonary shunt to the A-aDO2 can be estimated (93, 95). To do this, relationships between arterial, expired and mixed venous concentrations of trace amounts of marker gases dissolved in saline and infused intravenously, are used to solve for the distribution of ratio in multiple gas exchange units. These marker gases are inert, meaning that they do not participate in chemical reactions in the blood, and obey Henry’s law; that is, they have linear relationships between solubility and partial pressure. After approximately 20 min to allow for equilibration at rest, or 2 to 3 min during exercise, arterial and pulmonary mixed venous blood samples are obtained from indwelling catheters. In addition, mixed-expired air samples are obtained by expired gas sampling from a heated mixing chamber. With an independent measure of cardiac output, the pulmonary mixed venous concentrations can be calculated by the Fick principle (333), eliminating the need for pulmonary arterial sampling. After the blood samples undergo equilibration with nitrogen and extraction, the concentrations of the inert gases are measured in all samples using gas chromatography. For a homogenous lung at a specified VA/Q, the values for PA and Pa are described by the equation:
| (27) |
where Pa, , and PA are the arterial, mixed venous and alveolar concentrations of each gas respectively, and λ is the blood gas partition coefficient. The concentrations of the exhaled gases are diluted by the anatomic dead space, so that PA is obtained by dividing the measured by (1 − VD/VT) gases, where VD/VT is the fractional Bohr dead space. Six gases, each separated by a decade in λ, gives sufficient resolution to evaluate lung function, with low-solubility gases such as SF6 (λ ~ 0.0005) sensitive to shunt and ether (λ ~ 10) and acetone (λ ~ 300) sensitive to dead space (114, 332).
Because a normal lung is a mixture of units with different ratios, for each of the six gases, the measured value for Pa will be greater than the homogenous value, and the value for PA will be less than the homogenous value. The MIGET model chooses the distribution of units that best fits with the measured Pa and PA values obtained for each of the six inert gases. Thus, in a hypothetical homogeneous lung without dead space (i.e., matching is perfectly uniform), there is no alveolar-arterial difference for the inert gases (i.e., the inert gases are fully in equilibrium with alveolar gas) and thus However, in the presence of mismatch, inert gas Pa becomes greater than PA as elimination of the inert gas is impaired (114). Thus, the effect of inequality is to increase the retention of any gas (R, the ratio of ) and decrease the excretion (E, the ratio of ). The effect of dead space ventilation is to decrease the excretion of the gas, but has no effect on the retention since dead space ventilation does not come in contact with the blood perfusing the alveoli.
MIGET is quantitative, and there are several ways of describing these data (114). For example, the alveolar-arterial difference for each gas can be plotted as a function of λ (on a log scale), and the area under this curve calculated. A larger number implies a greater A-a difference and more inequality (114, 346). Alternately, the distribution of ventilation and perfusion can be expressed as a function of ratio. MIGET modeling of the distribution considers the lung “as if” it were comprised of fifty individual gas exchange units with different ratios equally spaced on a logarithmic scale. Total ventilation and cardiac output are partitioned between the 50 compartments to minimize error between data predicted from the modeled distributions and experimentally measured data (63, 139, 332). The and (the standard deviation of the ventilation and perfusion distributions derived using the 50-compartment model, respectively) are used to express the global extent of inequality with larger number representing more inequality (330) (Fig. 8).
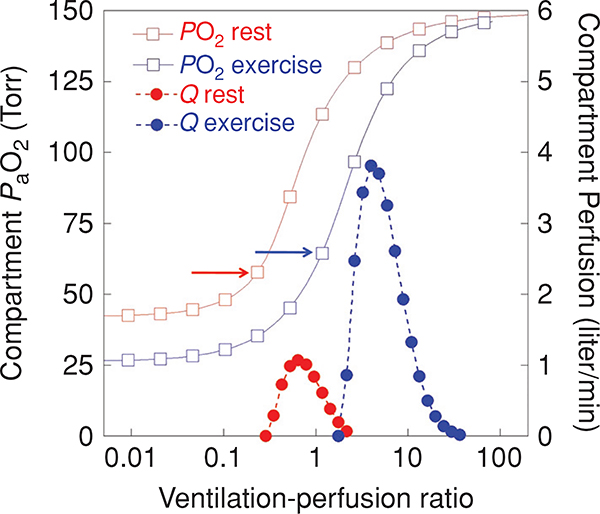
Figure 8.
The effect of exercise on the distribution and PaO2. Data are shown for a healthy normal subject at rest and during heavy near-maximal exercise ( liter/min). The closed circles represent perfusion (plotted on the right-hand y-axis in liter/min) and the open squares represent the arterial PO2 (plotted on the left-hand y-axis in mmHg) from a lung unit with the ratio given on the x-axis. Data in red are resting data; blue are exercising data. Exercise results in an alteration in the PO2 versus relationship because of the lower mixed venous PO2 entering the lung and also because of changes in the oxygen-hemoglobin dissociation curve in the blood. There is an increase in cardiac output in this subject from 6.2 liter/min at rest to 25.0 liter/min during heavy exercise, and alveolar ventilation is increased from 6.2 liter/min to 170 liter/min, thus the plot of versus ratio moves to the right with exercise. inequality is also increased during exercise and the blood flow distribution is broader ( rest, 0.53 exercise). Despite these changes the increased inequality with exercise does little to lower PaO2. This is because the lowest perfused units occur at a higher ratio with exercise (arrows).
The MIGET technique also quantifies intrapulmonary shunt and dead space ventilation—the extremes of ventilation-perfusion inequality, where ratio is zero or infinite. Since in terms of gas exchange, blood flow to regions with a ratio less than 0.005 is indistinguishable from shunted blood it is characterized as shunt, and similarly ventilation to regions with a ratio greater than 100 is characterized as dead space ventilation. Importantly, for the application of MIGET to the study of exercise gas exchange, once the distribution of units is obtained from the inert gas data, then a predicted value for PaO2 and PaCO2 can be calculated, using the relationship illustrated by the O2/CO2 diagram in Figure 3, where each value of corresponds to a unique pair of predicted values for PaO2 and PaCO2. This is important, as it allows quantification of the contribution of mismatch and shunt (with the exception of that from the bronchial circulation and thebesian veins) to the A-aDO2. The contribution of diffusion limitation is thus obtained indirectly as the amount of A-aDO2 not accounted for by mismatch and shunt.
Alterations in ventilation-perfusion matching with exercise
inequality accounts for almost all of the A-aDO2 (35, 76, 93, 124, 251, 316) at rest (Fig. 9). There is a small contribution to A-aDO2 from venous admixture (i.e., physiological shunting) as a result of the bronchial and thebesian circulation returning deoxygenated blood to the left ventricle (see discussion below). As exercise intensity increases, the extent of inequality increases, as does the A-aDO2. As can be seen from Figure 9, inequality accounts for the majority of the A-aDO2 until is greater than approximately 40 mL/kg/min. Then the observed A-aDO2 cannot be accounted for by inequality and diffusion limitation of oxygen transport likely contributes to A-aDO2. In some individuals, inequality may comprise up to 60% of the A-aDO2 at (124). However, the effects of inequality on gas exchange are mitigated in part by the overall increase in ratio of the lung as a whole, consistent with an approximately 20-fold increase in ventilation from rest to , compared to a fivefold increase in cardiac output. Thus, in Figure 9 the A-aDO2 accounted for by mismatch increases with a lower slope than the overall A-aDO2. This effect can be conceptualized in Figure 8 which shows a graphical representation of PaO2 versus ratio with the distribution of perfusion to different ratios overlaid. It can be appreciated that compared to rest, inequality increases slightly with exercise, as demonstrated by a broadening of the distribution of ratios. Importantly, however, the overall ratio (i.e., peak or mean ratio) increases with exercise as demonstrated by a rightward shift in the distribution. Because of this rightward shift, the well-documented broadening of the distribution with exercise has a limited effect on the units of relatively low ratio that will cause a widening of the A-aDO2.
Figure 9.
Estimation of pulmonary diffusion limitation during exercise using the multiple inert gas elimination technique. The measured ventilation-perfusion inequality and shunt are used to calculate the expected A-aDO2 under the measurement conditions. The results are compared to the measured A-aDO2. At rest, the two sets of data overlie one another but as exercise intensity increases, the measured A-aDO2 exceeds that expected from the amount of inequality and shunting. This indirect index is a measure of pulmonary diffusion limitation although a contribution from the bronchial circulation and thebesian veins cannot be excluded. Data, with permission, from references (121, 124, 251)
Mechanisms of increased inequality with exercise –exercise-induced pulmonary edema
With incremental upright exercise, there is a central shift of blood volume into the thorax (71), increasing both right and left ventricular filling pressures (245). Reeves and Taylor (247) have shown that approximately 80% of the variance in mean pulmonary artery pressure is due to pulmonary arterial wedge pressure (i.e., left-ventricular end-diastolic pressure) (311), and therefore pulmonary arterial and pulmonary arterial wedge pressures both increase with exercise (17, 56, 57, 110, 245, 246, 300, 311, 328). The rise in pulmonary vascular pressures distends and recruits pulmonary capillaries, increasing capillary blood volume, the area for gas diffusion, and diffusion capacity (148). The distension of pulmonary vessels, combined with capillary recruitment, results in reduced pulmonary vascular resistance with incremental exercise (300, 328). In addition, capillary recruitment helps to offset the increase in cardiac output and maintain red blood cell capillary transit time.
Importantly, the pulmonary microcirculation has evolved to maximize gas exchange efficiency, and as a result, vessel walls are very thin and distensible. West has calculated that there is a considerable increase in pulmonary capillary wall stress with exercise which greatly increases the risk for exercise-induced hydrostatic pulmonary edema, or even stress failure (343, 344). Indeed, there is evidence suggesting that pulmonary edema, or vascular damage, does develop with exercise (31, 98, 127, 206, 355).
Exercise-induced pulmonary edema, if present, could be a cause of mismatch. Interstitial edema fluid would be expected to distort the surrounding architecture of the alveoli and capillary network. Altered airway and blood vessel diameter resulting from the presence of cuffing would affect distribution of air flow and blood flow in the lung (121). In addition, alveolar interstitial fluid may alter regional lung compliance with further impairment of airflow distribution in the lung (29, 121). The pattern of the increased mismatch with exercise is consistent with interstitial edema being the mechanism (76, 93, 94, 121, 124, 219, 231). Although this has not been conclusively established, there is considerable indirect evidence: when comparing those who increase mismatch during exercise to those who do not show an increase with exercise, there are differences in mismatch in recovery. Those who increase mismatch during exercise also have greater mismatch in recovery which persists beyond the point at which ventilation and cardiac output normalize to pre-exercise levels (268). Hypoxia and hyperoxia alter pulmonary arterial pressures and thus capillary filtration, and therefore, administration of different inspired oxygen concentrations would show a predictable pattern on mismatch. Consistent with the theory that interstitial edema causes mismatch, exercise in normobaric hypoxia causes a greater increase in mismatch than in normoxia, and this increase is relieved by breathing 100% oxygen (93). Additionally, the extent of mismatch increases with exercise duration even at relatively low exercise intensities (121). Finally, prolonged exercise makes the distribution of pulmonary blood flow less spatially uniform (29), as would be expected from compression of small blood vessels, and the extent of this derangement is correlated with the degree of mismatch previously measured by MIGET. Possible alternate mechanisms for the increased mismatch with exercise include heterogeneity of hypoxic pulmonary vasoconstriction (137) reduction of gas mixing in large airways (319) or heterogeneity because of increased ventilation alone.
The occurrence of exercise-induced pulmonary edema is still debated (128), and there is evidence against exercise-induced pulmonary edema playing a significant role in pulmonary gas exchange. Not all studies have been able to document evidence of pulmonary edema following exercise using various techniques (55, 90, 115, 195), which may be due to some of the basic limitations of these techniques as well as the difficulties in quantifying subclinical pulmonary edema during/following exercise which typically involve a delay between termination of exercise and assessment. Repeated exercise does not further impair gas exchange (292, 356). In addition, no study has successfully linked anatomical evidence of edema with impairments in pulmonary gas exchange as assessed by MIGET or the A-aDO2. Interventions that acutely increase pulmonary arterial pressure, pulmonary arterial wedge pressure, and therefore, pulmonary capillary wall stress during exercise have not been shown to affect gas exchange efficiency as measured by the A-aDO2 (259, 301). Finally, if pulmonary edema were to develop regularly with exercise, it could be argued that significant edema would be more frequently observed, such as in the thoroughbred horse, which develops pulmonary capillary stress failure but not typically pulmonary edema (222, 350).
Diffusion limitation
Assessment of diffusion limitation of oxygen transport during exercise
Diffusion limitation of gas transport is comprised of two components, (i) gas-phase diffusion limitation where alveolar air is not perfectly mixed and (ii) alveolar-capillary membrane diffusion limitation representing a failure of complete equilibrium from across the alveolar wall. While both have the potential to affect gas exchange, the effect of gas-phase diffusion limitation is thought to be small, particularly during exercise where high flow rates are expected to improve gas mixing. Many of the techniques used to measure diffusing capacity of the lung can also be used during exercise (see Section “Measuring pulmonary diffusing capacity”). However, perhaps the most information has come from MIGET studies, which indirectly assessed the contribution of diffusion limitation to the A-aDO2 and pulmonary gas exchange.
Calculating diffusion limitation with MIGET
In contrast to the respiratory gases, the inert gases that are infused during MIGET studies do not demonstrate measurable diffusion limitation in their elimination from the lung. An important factor determining diffusion equilibrium is the ratio of solubilities of a gas in the alveolar wall (α) to solubility in blood (β). In the previous discussion of diffusion equilibrium, α is a component of diffusing capacity for a gas (D). Unlike oxygen, the solubility of an inert gas in the alveolar wall is similar to its solubility in blood and thus the ratio of α/β is approximately 1. However, for respiratory gases β is much higher than α. For oxygen, the ratio of α/β = is 1/27 and for CO2 1/11, thus they are more vulnerable to diffusion limitation than inert gases. If lung structure showed a significant diffusion limitation for inert gases, then the limitation of O2 exchange would be so severe as to not be compatible with life.
The extent of pulmonary diffusion limitation is inferred by estimating the contribution of inequality and intrapulmonary shunt, as measured by inert gas data to the A-aDO2 and attributing the remainder to diffusion limitation. The problem with this approach is that it is an indirect estimate of diffusion limitation, and cannot distinguish the contributions of pulmonary O2 diffusion limitation from postpulmonary shunting (e.g., from bronchial and thebesian veins) toward the overall A-aDO2. Pulmonary shunting at rest via the bronchial and thebesian veins is believed to comprise less than 2% of total cardiac output (93, 316, 328) and it is argued that it is unlikely to change proportionally with exercise. Studies conducted during hypoxia, indicate that such shunts would have to increase to 10% to 20% of cardiac output to explain the contribution to the A-aDO2 (251, 316). It has been suggested that since such shunts are anatomic, they will not change with FIO2 however as mentioned in the section on shunt, the agitated saline contrast data suggest that this may not be strictly true for pulmonary arterio-venous pathways. It is generally thought that extrapulmonary shunting contributes a very small amount to the overall A-aDO2, as extrapulmonary shunt likely comprises a relatively small amount of cardiac output.
Effect of exercise on diffusion equilibrium
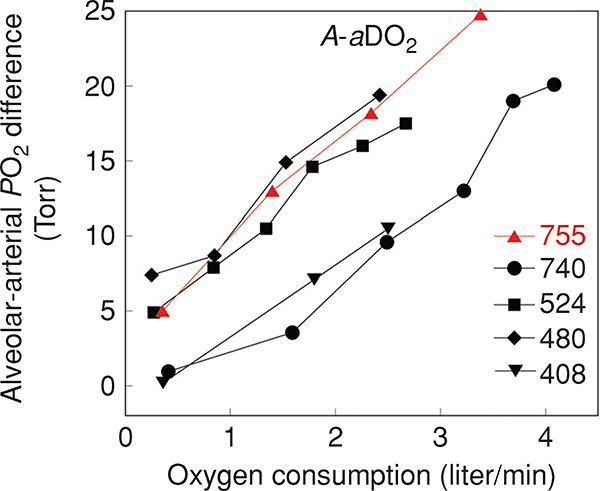
As described earlier (in Section “Structural determinants”), there are several factors that act to optimize the diffusion of oxygen across the alveolar wall during exercise, and reduce the extent of diffusion limitation. These changes include reduced gas-phase diffusion resistance, distension, and recruitment of capillaries secondary to increased capillary blood volume and increased perfusion pressures and an increase in hematocrit and optimized red cell spacing (130). Nonetheless, there is considerable evidence that diffusion equilibrium fails during heavy exercise in some cases. The vast majority of data comes from studies using MIGET with the contribution of diffusion limitation estimated as described above. In healthy normal subjects, diffusion limitation is evident in subjects exercising at oxygen consumptions above approximately 3 liter/min or 40 mL/kg/min (Fig. 9) (93, 316) and is increased by increasing exercise intensity and moderate hypoxia (15, 94, 251). In highly trained athletic subjects, the extent of diffusion limitation has been suggested to distinguish those who develop EIAH from those who do not develop EIAH (117, 251).
Mechanisms of diffusion limitation with exercise
Dempsey et al. (45) proposed that a diffusion limitation may develop during high-intensity exercise due to an inadequate time for oxygen equilibration, across the blood-gas barrier. As noted above, capillary recruitment during exercise helps maintain capillary transit time. One study estimated that the capillary blood volume plateaus at an oxygen uptake of approximately 3.5 liter/min (338); however, there were two important limitations to this study: first, the study used DLCO and DLNO to estimate capillary blood volume. Since NO is a pulmonary vasodilator this could affect the measurement. Second, the difficulty of the measurements meant that the highest exercise intensity evaluated was 80% of max, and thus the physiological range of exercise was not evaluated. In athletes who can exercise above 4.5 liter/min, and with a cardiac output more than 32 liter/min, mean pulmonary capillary transit time is expected to drop below 0.33 s, and thus infringe upon equilibration time. Importantly, capillary transit time is likely to vary throughout the lung, and therefore, it may not be mean capillary transit time that is the critical factor. Rather, the distribution of transit times through the pulmonary capillaries, and the volume of blood exposed to very low transit times may result in a diffusion limitation and increase in the A-aDO2. Studies measuring whole lung red cell transit times (i.e., from right ventricle to left atrium) demonstrate a marked heterogeneity in transit times that if present to a similar extent in the pulmonary capillaries would support this idea (120). Also, data obtained from the multiple inert gas elimination technique would tend to support the reduced transit time hypothesis, as a diffusion limitation as measured by MIGET typically develops at oxygen uptake more than 2.5 liter/min (93, 124, 251, 328).
Both red cell capillary transit time and whole lung pulmonary transit times fall with exercise (120, 338, 357) but unequivocal evidence to show that transit times approach the hypothetical minimum time (120, 338) for oxygen equilibration is lacking. In part, this is because the techniques used to measure pulmonary transit time either measured whole lung pulmonary transit of red blood cells, (however, capillary transit time is the important variable for gas exchange), or used inspired gases that can alter the physiological conditions of measurement.
Also arguing against reduced transit time as the likely mechanism for diffusion limitation during exercise is the observation that subjects who develop MIGET evidence of diffusion impairment often do so at submaximal levels of exercise (251), where presumably capillary recruitment is not maximal and transit time is not minimal. However it is the ratio of diffusional (DL) to perfusional conductance that determines the completeness of end capillary equilibration (226, 227) as previously discussed. Subjects with EIAH have a lower compared to those who do not develop EIAH (251). Thus, it is possible that the perfusional conductance (i.e., cardiac output) is recruited more quickly than diffusional conductance (i.e., DM and Vc) causing diffusion impairment even at relatively low levels of exercise. Isolated lung studies that have attempted to replicate exercise by reducing mean capillary transit time to 0.18 s, while maintaining a near homogenous ventilation-perfusion distribution and a mixed venous oxygen tension of approximately 22 mmHg, have not shown any affect of red cell transit time on the A-aDO2 (8) and thus this issue is largely unresolved.
Decreases in DLCO, and DM have been observed following exercise (97, 197, 205, 209, 251, 275, 299). The development of pulmonary edema with exercise has been suggested to explain postexercise reductions in DLCO and DM (97, 209). However, researchers have failed to find a relationship between postexercise changes in pulmonary diffusion and pulmonary gas exchange during exercise (251), or indices of blood-gas barrier injury (217). During recovery from exercise there is a redistribution of blood to the periphery, and this redistribution of blood from the thorax would lower surface area for gas exchange secondary to a decrease in pulmonary capillary blood volume (96, 205). Indeed, DM is related to surface area (130), and any change in capillary blood volume (i.e., Vc) would affect surface area for gas exchange and thus DM. However, DM has been shown to decrease despite Vc being elevated after exercise in some studies (197, 209). Combined, these findings indicate that the functional significance of reductions in diffusion capacity following exercise in unclear. Diffusion limitation from thickening of the blood-gas barrier is unlikely, but cannot be definitively ruled out. After prolonged, heavy exercise in humans, the diffusing capacity for CO decreases but there is no decrease in the O2 diffusing capacity calculated from the inert gases (8). This is best explained by a decrease in pulmonary capillary volume with a redistribution of central blood flow (96); however, there may also be a change in the matching of blood flow and diffusing capacity, which can impact gas exchange similarly to inequality (3).
Diffusion limitation—CO2 exchange
It has been suggested that much like for O2, a diffusion limitation for CO2 may also develop with exercise. Calculations based on suggests a slight diffusion limitation for CO2 at rest resulting in an arterial-alveolar PCO2 difference of approximately 0.2 mmHg which may reach 7 mmHg at peak exercise (269). Importantly, impaired CO2 equilibration (and thus the retention of CO2) will impair O2 exchange (324). The rate of rise in PO2 is delayed a small amount by the presence of simultaneous CO2 exchange (324). This can be explained by the progressive leftward shift in the oxyhemoglobin curve as capillary PCO2 falls, allowing more O2 to be taken up by hemoglobin (324). Simulation experiments indicated that CO2 exchange enhanced O2 transfer by approximately 2% (111), and may become more important when alveolar PO2 is low and CO2 equilibration is incomplete (112). Thus, while a typical assumption in pulmonary physiology is that PACO2 = PaCO2, this may not be appropriate during some conditions of exercise, although definitive evidence for this is lacking.
Pulmonary shunt
In the classic Riley three-compartment model of the lung, a pulmonary shunt is defined as blood that passes from the venous system to the arterial system without passing through ventilated areas of the lung (255). In some circumstances, blood from the left side of the heart may be shunted to the right side of the heart but we are not concerned with these here because they do not decrease arterial PO2. Venous-arterial shunts can take several forms: (i) outside the lungs, that is, extrapulmonary such as in the heart between atria or ventricles (intracardiac), (ii) within the lungs (intrapulmonary), or (iii) postpulmonary either arising from blood in the bronchial circulation that is returned to the left atrium after perfusing the bronchi, or from the coronary circulation which drains blood into the left ventricle through the thebesian veins. All of these shunts will act to depress the oxygen concentration of the blood leaving the left ventricle and perfusing the body, and increase A-aDO2. In addition, lung units of very low ratio that are very poorly ventilated essentially deliver mixed venous blood into the systemic circulation. Determining which of these alone or in combination are responsible for any increase in A-aDO2 with exercise is difficult, particularly when the shunted blood does not contain pure mixed venous blood. Most information on the contribution of shunts to gas exchange during exercise has come from using MIGET or the 100% O2 breathing technique.
Assessment of shunts during exercise
Gas exchange techniques
Oxygen
The impact of venous admixture/shunt fraction on arterial oxygenation can be appreciated using the classic shunt equation:
| (28) |
Where Qs/Qt is the ratio of blood flow through a right-to-left shunt relative to total flow (i.e., cardiac output), is pulmonary end-capillary O2 content (i.e., O2 content based on the calculated alveolar PO2), CaO2 is measured arterial O2 content, and is mixed venous O2 content. The formula can be rearranged to
| (29) |
From this equation, it is important to note that shunting of mixed venous blood will have a greater effect on CaO2 during heavy exercise than rest, as the O2 content of the shunted blood (i.e.,) is lower during exercise as more oxygen is extracted and the declines (158). In addition, the oxygen hemoglobin curve is right shifted from the effect of increased mixed venous CO2, decreased pH and increased temperature. The effect of a fixed degree of shunt on the A-aDO2 at rest and during heavy exercise can be calculated as follows: Assuming:, oxyhemoglobin saturation of end-capillary blood (ScO2) = 100%, and [Hb] = 15 g/dL, then is 20.4 mL O2/100 ml.
At rest, assuming a cardiac output of approximately 5 liter/min and mixed venous O2 content of 15 mL O2/100 mL, the effect of a 3% shunt on CaO2 is minimal relative to (156).
In contrast, if the same end-capillary CcO2 of 20.4 mL O2/100 mL is assumed, but with a peak cardiac output of 25 liter/min and mixed venous O2 content of 5 mL O2/100 mL, the effect of a 3% shunt on O2 content during exercise is much greater (156).
From this, it is evident that a 2% to 3% shunt of true mixed-venous blood, if present, would account for all of the increased A-aDO2 during exercise. Breathing 100% O2 and examining the change in arterial PO2 compared to room air breathing provides an estimate of combined intra- and extrapulmonary shunt. Using the 100% O2 technique, venous admixture/shunt fraction has been calculated to be minimal (i.e., <1% of cardiac output) during exercise (45, 93, 316, 323, 328). However, recent imaging studies (discussed below) have raised the possibility that anatomic arteriovenous conduits may respond to altered inspired oxygen concentrations differently than the rest of the pulmonary circulation and constrict in hyperoxia, thus potentially affecting venous admixture/shunt fraction calculation (59, 191) when measured using the oxygen technique. It should be noted at the time of writing of this manuscript this issue is controversial and unresolved.
Measuring shunt during exercise with MIGET
MIGET has the ability to detect venous admixture/shunt fraction, and can quantify its effect on the A-aDO2. In simplistic terms, MIGET detects venous admixture/shunt because gases of very low solubility are not retained in the blood after passing through the pulmonary capillaries, and thus retention of these gases only occur as a result of right to left shunting of blood. In fact gases of all solubilities are retained in the presence of shunt, however only gases of low solubility (such as SF6, λ ~ 0.0005) give sufficient resolution for accurate quantification; gases such as acetone (λ ~ 300) are so soluble that they are essentially fully retained except in the presence of units with a ratio more than 1. This can be verified by solving Eq. (27) for SF6 and acetone and assuming . It should be noted that MIGET is insensitive to postpulmonary shunting (e.g., bronchial circulation or thebesian veins). This is because oxygen will be consumed in these circulations, thus reducing the oxygen content of blood returning via these pathways to the left heart, but inert gas exchange does not take place. Of note, none of the previous studies using MIGET have documented any significant intrapulmonary shunt during exercise in healthy humans (93, 121, 124, 153, 219, 231, 251, 328).
Anatomical evidence for intrapulmonary shunting
In the isolated lung perfused with microspheres larger than a typical pulmonary capillary, microspheres have been detected in the fluid leaving the lung, indicating the existence of anatomical arteriovenous anastomoses in the lung. Vascular anastomoses between pulmonary arteries and veins have been demonstrated in many mammals (193, 238, 298, 315, 352), and the diameter of these conduits in humans has been shown to be up to 55 μm in infants (352), and 200 μm in adults (315); roughly 5 to 20 times the diameter of a typical pulmonary capillary. A criticism of previous work has been that injection pressures used to examine arteriovenous conduits may have been nonphysiologic; however, recent work has shown that arteriovenous anastomoses are present in isolated human and dog lungs when similar perfusion pressure observed during exercise are used (193, 298). Thus, despite the gas exchange findings of no significant right-to-left shunt, considerable anatomical evidence supports the existence of large-diameter arteriovenous anastomoses within the lung. We refer to them subsequently as arteriovenous anatomic shunts to distinguish them from a right-to-left shunt affecting gas exchange by increasing calculated venous admixture/shunt fraction.
Recent work using agitated saline contrast echocardiography (58, 192, 300, 301), Technetium-99m-labeled albumin microspheres (351) and macroaggregated albumin (190) suggests that exercise recruits arteriovenous anatomic shunts in most subjects. These arteriovenous anatomic shunts have been shown to be correlated with cardiac output, mean pulmonary artery pressure and A-aDO2 in exercising humans (300). Recently it was demonstrated that those subjects who recruited arteriovenous anatomic shunts had higher peak cardiac output and , while having lower pulmonary artery systolic pressures and lower pulmonary vascular resistance compared to those who did not recruit shunts (167). Consistent with the human studies, intrapulmonary arteriovenous anatomic shunts have been observed in dogs during exercise (298). Intravenously injected 25 μm microspheres were found in the tissue and arterial blood of the systemic circulation during exercise but not at rest. In these animals, the absence of intracardiac shunts was confirmed and based on the size of the microspheres, the size of these arteriovenous anatomic shunts were in vessels of at least 25 μm in diameter.
It is important to note that the methodologies used to study arteriovenous anatomic shunts in humans have significant limitations. In studies using albumin microspheres and macroaggregated albumin (190, 351), the lower range of the particles injected were within the diameter of a typical pulmonary capillary. Therefore, it is possible that the greater arteriovenous anatomic shunts fraction with exercise is the result of capillary recruitment and distention. The passing of these smaller particles through capillaries would also explain the larger than expected resting shunt fraction of 2.9% seen in healthy humans (351). Agitated saline contrast echocardiography as currently implemented is qualitative and does not allow for determination of the magnitude of arteriovenous anatomic shunts. Although there have been recent attempts to assign a qualitative scoring system (191) this approach is invalidated. More importantly, the exact size of the contrast bubbles that traverse the pulmonary circulation is unknown. Furthermore, while unlikely, the appearance of saline contrast bubbles in the left ventricle could be due to other factors including: (i) small diameter (<11μm) bubbles which are able to pass through normal capillaries, (ii) deformation of larger bubbles and their subsequent transit through the pulmonary capillaries, and/or (iii) capillary distension for consistency. The estimated survival time for a microbubble small enough to travel through a typical pulmonary capillary is 190 to 550 ms in static fluid (208); however, microbubble dissolution is rapidly accelerated with increased fluid velocity and hydrostatic pressure (318, 354). The fourfold to fivefold increase in cardiac output combined with the onefold to twofold increase in pulmonary vascular pressures typically observed with incremental exercise, as well as the time delay of appearance of contrast bubbles in the left ventricle, suggests that the contrast bubbles observed in the left ventricle are unlikely to be contrasts bubbles less than 11 μm that have gone through pulmonary capillaries. Importantly studies confirming arteriovenous anatomic shunts during exercise by using microspheres of a known diameter greater than a typical pulmonary capillary are lacking in humans.
Resolving discrepant information between functional gas exchange-dependent data and anatomical data
Gas exchange-dependent and anatomical shunt exercise data evaluate the lung from two different viewpoints (functional vs. anatomical). The disparity between shunt calculated with functional gas exchange-dependent techniques (i.e., venous admixture/shunt fraction) versus anatomical techniques has been a topic of recent debate (125, 126, 188, 189). As stated above, none of the previous work using MIGET has documented significant intrapulmonary shunt during exercise (93, 121, 124, 153, 219, 231, 251, 328), and studies using the 100% oxygen technique have similarly confirmed a venous admixture/shunt fraction of less than 1% of cardiac output during exercise (45, 93, 316, 323, 328). Thus, the contribution of shunts to the increased A-aDO2 during exercise has been argued to be minimal (47, 125, 126, 323). Conversely, anatomic methods are suggestive that anatomic pathways large enough to allow red blood cells to bypass gas-exchanging regions are present in the majority of healthy subjects. One possible explanation for this discrepancy may be due to gas exchange occurring proximal to or within arteriovenous anastomoses.
Our classic understanding of the lungs is that gas exchange occurs only between alveoli and pulmonary capillaries. However, precapillary gas exchange of both O2 and N2 has been demonstrated in humans (140, 283) and animals (39). These studies demonstrated that precapillary O2 exchange occurs in smaller vessles in normoxia, with a greater O2 exchange occurring in larger vessels when the fraction of inspired oxygen is increased. Accordingly, in subjects breathing 100% O2 during exercise, some O2 exchange may occur proximal to the intrapulmonary arteriovenous pathways (39, 140, 283), and thus these vessels would not be recorded as true shunt, as the calculated venous admixture (Qs/Qt) would be reduced. In addition, intrapulmonary arteriovenous pathways themselves may participate in limited gas exchange restricted to their perimeter blood, which would allow some deoxygenated core blood to bypass the pulmonary capillary bed in normoxia, but not be recorded as true mixed venous shunt (81, 297). Recent work has shown that the exercise-induced intrapulmonary arteriovenous anastomoses as assessed by contrast echocardiography can be eliminated in subjects by breathing 100% O2 (191). This observation could explain why previous work does not show significant gas exchange shunt when using 100% O2 (45, 93, 316, 323, 328). The lack of intrapulmonary arteriovenous anastomoses when breathing 100% O2 may be due to actual changes within the pulmonary vasculature, or alternately, the increased dissolved PO2 from breathing 100% O2 may be affecting contrast bubble survival, resulting in the apparent reduction in intrapulmonary arteriovenous anastomoses. The latter explanation appears less likely based on work showing that contrast echo detection is not affected by gas bubble composition (59). Importantly, the effect of the recruitment of these arteriovenous anatomic anastomoses on pulmonary gas exchange during exercise remains to be determined. Since significant shunt is not detected using gas exchange techniques (such as MIGET), it is highly unlikely that these vessels act as true shunt vessels from a gas exchange perspective.
Pulmonary gas exchange in hypoxia
For a more thorough detail of pulmonary gas exchange in hypoxia the reader is referred to additional resources (325). The primary physiological challenge at high altitude is the reduction in oxygen availability resulting from reduced barometric pressure (PB) (24). Although the fractional concentration of oxygen remains constant at 0.21, with increasing elevation (and corresponding decline in barometric pressure), PO2 falls, and importantly, at extremely high altitudes small fluctuations are of particular importance.
Hypoxia gas exchange efficiency: rest
Resting A-aDO2 decreases with increasing altitude, and thus the fall in PaO2 with increasing elevation is somewhat buffered. The reasons for this decrease in A-aDO2 are related to the relative contributions from pulmonary diffusion limitation of oxygen transport, ventilation-perfusion heterogeneity and shunt (341). The interested reader is referred to other extensive reviews (325); however, determinants of pulmonary gas exchange at rest in hypoxia will be briefly reviewed.
While ventilation-perfusion inequality may be increased at high altitude, A-aDO2 may not be affected. Modeling studies (325) have shown that the effect of a given (unchanging) amount of ventilation-perfusion inequality on the A-aDO2 is reduced at altitude. The reason for this is twofold: in hypoxia, more of the ventilation-perfusion ratios of the individual lung subunits fall on the steep part of the oxygen hemoglobin dissociation curve, and consequently the range of PO2 values from low to high ventilation-perfusion ratio parts of the lung is less, reducing A-aDO2. Secondly, the overall ventilation of the lung is increased relative to cardiac output (325), shifting the overall ventilation-perfusion ratio of the lung rightward toward higher ratios, in a similar fashion to that depicted for normoxic exercise in Figure 8. Gas density is also reduced with increasing altitude, resulting in a more uniform distribution of inspired gas in the lung. Similarly, cardiac output and pulmonary artery pressure are increased with hypoxia, which would cause recruitment and distention of the capillary bed (33, 34) increasing the uniformity of the distribution of perfusion. Thus, since the distribution of both ventilation and perfusion are expected to be more uniform at high altitude, one might similarly expect ventilation-perfusion matching to be more uniform.
Since the increase in pulmonary arterial pressure with hypoxia will increase the driving pressure for fluid efflux, pulmonary edema may develop, compressing small airways and blood vessels which can disrupt ventilation-perfusion matching (268). Thus, the effect of edema can complicate the matching at altitude, and counterbalance the other factors increasing uniformity detailed above. In keeping with this idea, hypobaric chamber studies have shown that the resting inequality is increased with increasing altitude, consistent with the development of interstitial edema (76, 328). However, as mentioned above, A-aDO2 may actually be reduced at altitude in comparison to sea level because of the relative position of the subunits on the oxyhemoglobin dissociation curve.
In healthy normal subjects, diffusion limitation generally does not occur at rest. While the reduction in driving pressure with altitude would be expected to contribute to a diffusion limitation, there is an increased diffusing capacity in hypoxia (15, 322, 334) that helps to prevent a diffusion limitation. As previously stated, A-aDO2 falls with increasing altitude, which is consistent with inequality providing a greater contribution to resting A-aDO2 in hypoxia than diffusion limitation (15, 334). Of note, there is some evidence that at extremely high altitudes, diffusion limitation may develop at rest in a small number of individuals (334), although this requires further investigation.
Similar to that for ventilation-perfusion inequality, the contribution of a fixed level of shunt to pulmonary gas exchange decreases with altitude (323). This is because in hypoxia the lung enters the steep part of the oxygen-hemoglobin dissociation curve: for a constant content difference between pulmonary mixed venous and arterial blood (i.e., a fixed shunt) there is a numerically smaller difference between the two partial pressures. MIGET studies have shown that the effect of intrapulmonary shunting on gas exchange is negligible at altitude (15, 334), the extreme exception being the development of pulmonary edema and alveolar flooding, which would lead to an intrapulmonary shunt (249).
Pulmonary gas exchange during hypoxic exercise
At sea level, with the exception of highly trained athletes, PaO2 is typically maintained near resting levels with incremental exercise. However, during exercise at altitude in acclimatized subjects, virtually all individuals experience a fall in PaO2 from resting values secondary to a greater increase in A-aDO2. The preceding discussion on the relative contributions of ventilation-perfusion heterogeneity and diffusion limitation to the A-aDO2 might lead one to think that since both ventilation-perfusion matching (76) and pulmonary diffusion limitation (316) are expected to increase with increasing exercise intensity, that the A-aDO2 might similarly be expected to further increase with when the altitude is also increased. While this is true for subjects exercising in acute hypoxia (94, 316), this is not the case for acclimatized subjects.
Figure 10, shows the remarkable variability in the A-aDO2 between groups of subjects exercising at differing altitudes. While at each barometric pressure the A-aDO2 increases with increasing exercise intensity, there is no apparent additional effect of altitude. The same is true even in chamber studies when the same group of subjects is repeatedly studied at different barometric pressures under standardized conditions (334). In addition to intersubject variability, this finding can likely be explained by the relative contributions of pulmonary diffusion limitation and ventilation-perfusion inequality to the A-aDO2 during exercise, and the differing effects that altitude has on each of them, discussed further below.
Figure 10.
The alveolar-arterial difference at rest and during exercise to near maximal at different barometric pressures. There is no systematic relationship between A-aDO2 and barometric pressure. This is likely due to a combination of factors including individual subject variability and the relative contributions of diffusion limitation, inequality and shunt to the A-aDO2 within a subject at different elevations. See text for details. Data are, with permission, from references (15, 46, 300, 302, 327, 334).
Ventilation-perfusion inequality during hypoxic exercise
As previously mentioned, resting ventilation-perfusion inequality increases with increasing hypobaric hypoxia, although the magnitude of the increase varies between subjects and depends on ascent profile, with faster ascent associated with greater ventilation-perfusion inequality. Ventilation-perfusion inequality increases with increasing exercise intensity in normoxia (124) and is also further increased in hypoxic exercise (76, 328). As an example, at a barometric pressure equivalent to the summit of Mt. Everest, significant resting and exercising ventilation-perfusion inequality is present (334). Data from a chamber study by Wagner et al. show that at both 348 mmHg (~6100 m) and 253 mmHg (~8850 m, Everest Summit) ventilation-perfusion inequality is elevated above that seen at other barometric pressures, possibly reflecting the rapid ascent profile and the development of pulmonary edema (see below) before these data were collected.
As described in an earlier section, there is considerable evidence that increased inequality during exercise results from the development of interstitial pulmonary edema. There are additional data suggesting that hypoxic exercise also results in edema and mismatch: (i) subjects who have a history of high-altitude pulmonary edema (HAPE) also show larger increases in exercise induced ventilation-perfusion inequality compared to subjects without a history of HAPE (231); (ii) in a large scale study, climbers at 1200 and 4559 m had an increase in closing volume (44) suggesting interstitial edema, because when present, excess peribronchial fluid forms around and compresses small airways, leading to air trapping.
The extent of inequality during exercise may also be altered by drugs commonly taken for altitude illness. In humans exercising in normoxia and acute hypoxia, acetazolamide reduces inequality, decreases the A-aDO2 and increases the PaO2 compared to placebo (153). The mechanism for this is speculated to be release of hypoxic pulmonary vasoconstriction and reductions in pulmonary arterial pressure, although other effects of acetazolamide on the pulmonary circulation cannot be eliminated. Similarly, sildenafil, a phosphodiesterase inhibitor that causes pulmonary vasodilation, reduces pulmonary arterial pressure and the A-aDO2 with an increase in PaO2 in acclimatized subjects exercising at 4350 m (252), although inequality has not been directly measured in this context.
Thus, inequality as a contributor to inefficient gas exchange is likely present in most individuals exercising at altitude. However, as mentioned earlier, at altitude more of the ventilation-perfusion ratios of the individual lung subunits fall on the steep part of the oxygen hemoglobin dissociation curve, and the overall ventilation of the lung is increased relative to cardiac output (325), shifting the overall ventilation-perfusion ratio of the lung even further rightward toward higher ratios. Although the extent of inequality increases markedly, the effect on A-aDO2 decreases to an even greater extent as more of the lung moves on to the steepest part of the dissociation curve. The combined result is that inequality contributes relatively little to the exercising A-aDO2 at the most extreme altitudes. Thus in contrast to the resting data, inequality is a relatively minor contributor to gas exchange inefficiency during exercise at altitude, where diffusion limitation becomes the major contributor.
Diffusion limitation during hypoxic exercise
With hypoxic exercise, the DLO2 is increased above resting values as pulmonary capillaries become maximally recruited; however, this not sufficient to prevent a diffusion limitation. Thus, it can be appreciated that, in contrast to ventilation-perfusion inequality, diffusion limitation becomes an increasingly larger contributor to the A-aDO2 during hypoxic exercise, despite an overall increase in DLO2. The reason for the increased diffusion limitation with hypoxic exercise can again be explained by . As elevation increases, the ratio of falls since the slope of the O2-hemoglobin dissociation curve (i.e., β) increases in hypoxia. Thus, diffusion limitation becomes a progressively more important contributor to the A-aDO2 as the altitude increases. The contribution of diffusion limitation (as measured indirectly by MIGET) to the A-aDO2 during exercise has been shown to decrease with acclimatization (15) compared to acute hypoxia, presumably on the basis of reduced and a consequent increase in . Interestingly, in addition to the effects on ventilation-perfusion matching described above, acetazolamide has also been shown to reduce diffusion limitation during acute hypoxic exercise, likely by reducing β and improving diffusion equilibrium (153), although it is unknown if the effect is sustained with chronic ingestion at altitude.
Sex and pulmonary gas exchange
Sex differences in pulmonary structure and function have been well documented. For example, in comparison to males of the same height, females have smaller lung volumes (42, 270, 313, 336), small airway diameter (202, 207), lower expiratory flow rates (270, 336), and lower lung diffusing capacity (43). Differences in pulmonary function appear early in childhood, where forced vital capacity (FVC) is greater in boys than girls starting around approximately 8 years of age, but the maximal expiratory flow volume (MEFV) curve is greater in girls than boys (when matched for lung size) until about 13 years of age (84, 170, 271, 307, 308). Airway resistances have also been shown to be higher in young girls compared to young boys (48, 84). These data suggest that lung parenchyma and airways grow at different rates (termed dysanapsis) between the sexes (86, 207). In the mature lung, males exceed (age- and height-matched) females in all measures of pulmonary function (3). While the consequences of exercise on pulmonary gas exchange have been well studied, a comprehensive understanding of sex-based differences in pulmonary gas exchange (particularly during exercise) is still lacking. This is due to the fact that the majority of the classical studies examining pulmonary gas exchange during exercise used almost exclusively male subjects. During the last decade interest is sex-related differences in exercise and performance has increased (88–91,100,101,118,219,234,253,356), leading to the publication of several reviews on this topic (102, 122, 277).
At present, however, only a handful of studies have attempted to examine exercise differences in pulmonary gas exchange between the sexes. Harms et al. (100) were first to report that females may experience greater fall in PaO2, SaO2, and a wider gap in A-aDO2 compared to male counter-parts, suggesting that prevalence of EIAH may be greater in women. In contrast, Hopkins et al. (118) report the PaO2, at , was not significantly different in women compared to men. Likewise, in a study where men and women were matched according to age, lung size, and aerobic capacity (, mL/kg/min), there was no significant difference in PaO2, SaO2, or A-aDO2 in response to normoxic or hypoxic (FIO2 = 0.12) exercise (219) between sexes. This study also showed that, even though women had a lower DLO2, the ratio of during hypoxic exercise was similar between the sexes, and that inequality (i.e., ) was slightly lower in women compared to men during all levels of exercise in normoxia or hypoxia (219). These data suggest that lung size, and not sex per se, may be important in determining susceptibility to EIAH, and that in females there may be factors that are beneficial for gas exchange that may compensate for lung size.
Differences in ventilation are known to exist between men and women. In children and adolescents, sex reportedly accounts for very little (<2%) of the differences in ventilatory drive at rest or during exercise (220); however, it appears that females around midpuberty become more sensitive to CO2 compare to males (2, 220). While it is currently debatable if the chemical control of ventilation (chemosensitivity) in response to CO2 or O2 is different in men or women (2, 88, 187, 303, 348, 349), it is clear the hormonal influences and timing of the menstrual cycle can influence alveolar ventilation (61, 237, 279, 303, 304) and DLCO (11, 65, 66). Healthy women have on average a slightly lower arterial PCO2 than men, reflecting progesterone-related increased chemosensitivity. In principle, these effects could alter gas exchange during exercise; however, there is evidence that menstrual cycle has no significant effect on exercise performance, , respiratory exchange ratio and/or blood lactate accumulation (280), suggesting that any potential effect on gas exchange is minimal or of little functional consequence. It is known that women, by virtue of their smaller lungs and airways, experience greater mechanical constraints in expiratory airflow compared to men (91, 203). This is evident as tidal flow-volume loops begin to impinge upon the maximal flow-volume envelope during periods of high ventilatory demand as occurs during exercise (see Fig. 11) (276) and results in an inadequate hyperventilation (relative hypoventilation) response to exercise which likely contributes to EIAH. Supporting this, Dempsey et al. (45) and McClaran et al. (203) reported that replacing air with lower density gas mixtures (20% O2/80% Helium) reduces expiratory flow limitation and improves PaO2 during exercise. These data support the notion that individuals with small lung volumes experience greater mechanical constraints, and thus it has been speculated that any individual with small lungs, regardless of sex, is likely to be more prone to pulmonary gas exchange impairment compared to those with larger lungs. However, additional studies that have a wider range of subjects with respect to age, body/lung size, and aerobic capacities, are warranted before a clear understanding of sex differences in pulmonary gas exchange are known.
Figure 11.
Response to progressive exercise in age- and height-matched men and women. Based on prediction equations, women have a smaller forced vital capacity (FVC), and peak expiratory flow (PEF). With incremental exercise there is expiratory flow-limitation (EFL) observed in the woman, and hyperinflation as demonstrated by an increase in end-expiratory lung volume (EELV). Figure, with permission, from Sheel and Guenette (276).
Ageing and pulmonary gas exchange
The normal aging process produces a number of anatomical and functional changes within the lung. For example, changes in lung shape (4), greater number and size of pores of Kohn (239), decreased alveolar surface-to-volume ratio (107, 312), decreased vital capacity (30, 42), increased FRC and RV (42, 152, 172), increased dead space ventilation (196), increased chest wall (74, 213, 257) and pulmonary arterial stiffness (85, 194), loss of elastic recoil (160, 320), and decreased respiratory muscle strength (40) occur with aging. These changes have been extensively reviewed and the interested reader is referred to Handbook of Physiology: The Respiratory System (25), Handbook of Physiology: Aging (286), and The Textbook of Respiratory Medicine (229), for a comprehensive review. This summary will focus principally on the overall consequences of aging on pulmonary gas exchange during exercise; however, it is worth noting that there are relatively few studies that have specifically examined pulmonary gas exchange efficiency as measured by the A-aDO2 during exercise in relation to aging, and therefore other variables relating to gas exchange will be discussed.
A decline in resting PaO2 with advancing age clearly points to an age-associated alteration in gas exchange (9, 154, 200, 215, 216, 243, 285). Although the rate of decline is somewhat variable, ranging from less than 1 mmHg to 5 mmHg every 10 years, it is estimated that the average decrease in resting PaO2 is 3 to 4 mmHg per decade after 20 years of age (201, 218). However, it should be noted that most of the previous studies have included very few elderly subjects beyond 70 years of age, and therefore, rely extensively on the extrapolation of PaO2 data from young and middle aged subjects. More recent studies, specifically examining PaO2 in elderly subjects ranging between 69 to 104 years old, find similar overall reduction in PaO2 with elderly subjects more than 70 years of age as with the previous studies, but report no further decline of PaO2 beyond the seventh decade of life (38, 87, 99). It is tempting to speculate that the processes responsible for the declining PaO2 may have stabilized after the seventh decade, and/or, perhaps, the loss of PaO2 decline beyond the seventh decade is a potential predictor of lung health and longevity (i.e., those who continue to decline do not survive as long). In contrast to arterial PO2, alveolar PO2 (PAO2) remains stable or even increases with age, and consequently an increase in resting A-aDO2 (>30 mmHg) is often observed with advancing age. Classically, the fall in PaO2 with age has been attributed to an increase inequality occurring due to losses in lung elastic recoil that leads to early airway closure (i.e., greater lung closing volume) in the dependent lung regions of aged individuals. This, in turn, alters the distribution of ventilation (which in the “healthy lung” is already topographically distributed) and serves to reduce alveolar ventilation within the dependent lung regions (5, 53, 169, 210). Although pulmonary blood flow to the apices of the lung increases with age, total blood flow still remains greater in the dependent lung regions (116). Thus the increasing heterogeneity of ventilation (reduced alveolar ventilation in the dependent lung region) leads to greater mismatch, increasing A-aDO2. Supporting this, data from one study using MIGET has reported significantly greater, albeit still normal, inequality in healthy 39- to 60-year-old subjects compare to younger 21- to 24-year-old subjects (330). Likewise, evaluation of phase III and phase IV alveolar slopes obtained from single breath N2 washout maneuvers, which are independent measures of ventilation distribution and lung closing volume, respectively, find greater heterogeneity in ventilation and increased lung closing volumes with advancing age (26–28, 161, 258). Other factors also capable of contributing to greater inequality with senescence include a reduction in cardiac output and/or decreased distensibility of the pulmonary vasculature.
Children and adolescents (ages 8–18 years old) demonstrate age-related decreases in mass-specific ventilation and ventilation efficiency (i.e.,) at rest and during exercise (77, 220). In adults, however, ventilatory efficiency at rest appears to be largely independent of age, but is found to be less in men compared to women (92). During exercise, ventilatory efficiency declines with age in both men and women, as indicated by elevated and relationships [reviewed by reference (144)]. Losses in lung recoil and increased chest wall stiffness are thought to underlie the decline in ventilatory efficiency and contribute to declines in vital capacity, expiratory flow rates, and maximal ventilatory response to exercise, resulting in greater O2 cost of breathing and expiratory flow limitation during exercise (143). In some very fit elderly subjects, EIAH has also been reported due inadequate hyperventilation and expiratory flow limitation (143, 236). In addition, the decreases in alveolar surface-to-volume ratio and decreases in lung diffusing capacity (as measured by DLCO) that occur with age would also contribute gas exchange impairment (43, 49, 310, 312). The average decline in DLCO is approximately 0.15 mL/min/mmHg per year after 20 to 25 years of age, with greater rates of decline in males (0.2–0.32 mL/min/mmHg per year) compared to females (0.06–0.18 mL/min/mmHg per year) (214, 309). While it is clear that age effects on gas exchange can undoubtedly reduce ventilatory efficiency, particularly in response to exercise, it should also be noted that changes in respiratory sensitivity (or decreases in baseline PaCO2) may also increase the or relationship without representing a loss of ventilation efficiency per se. Thus, age-associated increases in or cannot be assumed to be solely from increased heterogeneity.
Theoretically, age-associated increases in A-aDO2 could, at least in part, contribute to the overall decline in exercise performance seen with age. However, it must be emphasized that the transfer of O2 across the healthy lung is not normally limited (93,118,219,251,316,328) and typically provides approximately four to six times the total O2 content needed for basal metabolism. Thus, under basal conditions, the normal age-associated reductions in PaO2 would not be expected to significantly impact the healthy lung. But, given the anatomical and functional changes occurring in the lung with age, it is theoretically possible that the respiratory system (particularly in the elderly athletic subjects capable of high pulmonary blood flow, i.e., cardiac output) may play some role in limiting maximal exercise capacity. However, evidence supporting pulmonary-related exercise limitation in healthy elderly subjects is generally lacking. Rather, it must be remembered the determinants of maximal aerobic performance (i.e.,) are multifactorial, and it is more likely that age-related declines in cardiovascular function (i.e., lower maximum heart rate and stroke volume) (287, 306), reduced skeletal muscle capillarity, oxidative capacity, and mass (36,166,173); and decline in neuromuscular function (168, 288), collectively serve to lower exercise tolerance (and limit ) in the elderly.
Methodological issues for evaluating pulmonary gas exchange during exercise
Although all measurements have their limitations, some common issues arise when measuring pulmonary gas exchange, particularly during exercise. Generally speaking, these issues are most problematic when values are indirectly evaluated or estimated rather than measured directly, such as with the use of pulse oximeters to indirectly estimate hemoglobin saturation. A few of the more common issues are discussed below.
Estimating alveolar gases
Alveolar PO2 can be estimated from end-tidal oxygen tension, or calculated using Eq. 5 substituting arterial PCO2 for alveolar PCO2 (255). End-tidal PO2 is mean weighted for regional alveolar ventilation, while PAO2 calculated from Eq. 5 is weighted for regional blood flow, as the CO2 dissociation curve is essentially linear over the physiological range. PETCO2 can similarly be used to estimate PACO2/PaCO2. Importantly, at rest PETCO2 is less than PACO2 (and correspondingly PETO2 more than PAO2) due to dilution of gas from poorly perfused alveoli (i.e., dead space). Using end-tidal values to predict alveolar pressures has the potential of underestimating PACO2, resulting in an overestimation of PAO2; however, in the healthy lung at rest, alveolar dead space is extremely low, and PETCO2 is a good approximation of PACO2. With exercise, there is an increase in VT, VCO2, and mixed venous CO2, such that the within-breath fluctuations of alveolar gas composition are greater (50). With the rapid increase in alveolar volume on inspiration during exercise, end-inspiratory PACO2 is well below the mean PACO2, whereas during expiration, PACO2 increases toward more rapidly than at rest as the increased CO2 production of exercise is evolved into a lung volume becoming smaller as expiration continues (151). The latter factor results in PETCO2 being higher than mean PACO2/PaCO2 during exercise (150), and thus PETCO2 has the potential to overestimate PACO2/PaCO2 at peak exercise. Jones et al. developed a prediction equation to calculate PaCO2 from PETCO2 during exercise (151); however, it is worth noting that this equation was developed with subjects exercising up to 50% (peak VCO2 production = 2.219 liter/min). Further, the equation should not be used in patients with abnormal pulmonary function as areas of high time constants would lead to PETCO2 rising further above PaCO2, and the equation should not be used in children as they typically use much higher ventilatory rates than what was studied by Jones et al. (151).
Pulse oximetry
Pulse oximeters use a light source and photodiode light detector to measure the amount of light passing through an arteriolar bed. SaO2 can be estimated noninvasively because the light absorbing characteristics of hemoglobin differs between oxyhemoglobin and deoxyhemoglobin. However, pulse oximeters are notoriously inaccurate during exercise. This is, in part, because of motion resulting in signal corruption (230), or loss of signal in digital probes where handgrip is used such as in cycling exercise (317, 339). There have been many validation studies of pulse oximetry during exercise over the last 20 years, with widely varying conclusions with standard error of estimates (see numerically similar to precision) ranging from approximately 2% (233, 353), to ~5% (281). New generation pulse oximeters incorporate advanced signal processing to deal with nonstandard signal detection (12,14,289); however, this is not entirely satisfactory. Importantly, significant measurement errors occur even when adequate pulse rate signal detection is evident (353), yet many data points with poor pulse rate signal detection may still provide reliable data. Thus, this criterion alone cannot be used to judge the quality of the data obtained using these devices. An estimate of the extent that a particular device underestimates SaO2 may be obtained by the administration of several breaths of a hyperoxic gas mixtures (FIO2 0.5–1.0) in the final few seconds of an exercise test. This will elevate the SaO2 to approximately 100% and any systematic bias by the device will become apparent. Many modern devices come equipped with a variety of sensor probes allowing for monitoring of SaO2 in the finger, ear lobe, or forehead. In some studies (353), forehead sensors have been suggested to be more accurate. However, if central venous pressure is raised, such as in patients with heart failure, contamination from venous blood may affect the signal obtained from forehead sensors biasing the measurements toward low readings (267, 293). Overall the nature of the measurement bias of pulse oximeters is such that when significant errors in measurement are present the tendency is toward underestimating the true SaO2.
Aside from their use in monitoring, pulse oximeters have significant limitations that preclude their widespread use for research. When answering research questions, precise measurements of oxygen transport are important, and monitoring SaO2 alone is probably not adequate. This is particularly true during normoxic exercise, where SaO2 values are on the flat part of the oxygen hemoglobin dissociation curve. In this range, relatively small changes in SaO2 are associated with large differences in PaO2 and even small uncertainties in SaO2 would have a big effect on estimated PaO2 [discussed in reference (353)]. Also SaO2 is affected by the temperature and pH changes during exercise that result in a decrement in SaO2 of 4% to 5% in the absence of any change in PaO2. Dempsey and Wagner (47) have offered a standardized definition of mild exercise induced arterial hypoxemia as a fall in SaO2 to below 95% from a normal resting value of 98%. A change of 4% to 5% in SaO2 is very close to the precision of many pulse oximeters and data points could be expected to fit this definition on the basis of nonrandom measurement error alone. Thus, the use of pulse oximeters should be confined to a few very limited circumstances such as in pediatric populations. If no alternative is available, the investigator should validate the device in use in their laboratory against direct measures of SaO2 in a small number of subjects.
Temperature correction of arterial blood samples
With exercise, there is an increase in body (and therefore arterial blood) temperature. However, the electrodes within a standard blood gas analyzer are maintained at 37°C in a thermostatically regulated chamber, and therefore, all blood gas measurements are made at 37°C regardless of subject temperature. When body temperature is higher than 37°C, the reported PaO2 and PaCO2 measured at 37°C are lower than the actual value within the subject. Failure to correct arterial blood gases will result in a lower PaO2 and PaCO2, and correspondingly a higher calculated PAO2 and A-aDO2. Thus gas exchange will appear to be worse than is actually present. A variety of equations have been developed to correct PaO2 and PaCO2, and the reader is directed to reviews that summarize many of these formulas (7, 75). For PaCO2, a common equation used is (157, 273)
| (30) |
where measured is the value given by the blood gas analyzer, and T is the body temperature.
The temperature correction of PaO2 is more difficult, as the change in PO2 with respect to temperature depends on the degree that hemoglobin is saturated with oxygen. As an example, the effect of changing temperature on PaO2 varies from 7.4%/°C at low hemoglobin saturation, to 1.3/°C when hemoglobin is fully saturated with oxygen (274). Again, the reader is directed to other resources for further discussion of temperature correction formulas (7, 75, 274); however, a relatively simple formula from Severinghaus (273) provides a correction equation with reasonable accuracy within the physiological range during normoxic exercise:
| (31) |
Of note, when temperature correction formulas (75, 273) are compared using representative data which spans the typical range observed during exercise (temperature = 38–40°C, PaO2 = 100–70 mmHg, PaCO2 = 30–40 mmHg) the difference in adjusted PaO2 values between the formulas is less than 4 mmHg (i.e., <6%), while the difference in adjusted PaCO2 values is less than 0.5 mmHg (i.e., <2%).
Measuring diffusing capacity
Carbon monoxide
As described earlier MIGET calculates a DLO2 by calculating the diffusing capacity for oxygen that accounts for the amount of the A-aDO2 that is not ascribed to inequality and shunt. The reader is referred to other work for a more complete explanation (95). Of note, a limitation of using MIGET to calculate DLO2 is that a calculation of DLO2 is only possible in the presence of diffusion limitation.
It is also possible to estimate DLO2 by measuring diffusion of CO. For a more complete description of DLCO, see the Handbook of Physiology chapter on gas exchange in Comprehensive Physiology (146). It is recognized that the binding affinity of Hb for CO is over 200 times greater than that for O2, which can be expressed in the following equation:
| (32) |
Where SCO and SO2 are carboxy and oxyhemoglobin saturation. CO and O2 saturations are typically 1% and 97%, respectively, at a PO2 of 100 mmHg, and therefore, CO tension would be only (1.00/97)/2.15 = 0.005 mmHg. Even at an SCO = 10%, the PCO would be only 0.05 mmHg and as a result can be neglected. Thus, it was reasoned that if CO uptake could be measured using brief breath holds, then diffusing capacity for CO could be determined based on the following equation:
| (33) |
DLCO can thus be used to provide an estimate of DLO2 based on the ratio of Krogh diffusion constant for the two gases:
| (34) |
α is the respective Bunsen solubility coefficient at body temperature in milliliters gas per milliliter of blood per atmosphere of pressure, d is the diffusion coefficient of the gas in square centimeters per second, and DK is the respective Krogh diffusion constant (60× αd/760) (146). Using this equation, it is conventionally assumed that DLO2 = 1.23/DLCO, although empirical data in exercising foxhounds indicates that this ratio is approximately 1.61 (134). Importantly, the reaction of CO and O2 with hemoglobin is not instantaneous (105, 106). It has now become apparent that the reaction kinetics of gases with hemoglobin can result in significant resistance to alveolar/capillary gas transfer and that measurements of DLO2 and DLCO may not accurately reflect true membrane diffusing capacity (DM) for these gases.
Nitric oxide
Inhaled NO can also be used to evaluate diffusing capacity. The affinity of NO to hemoglobin and the reaction velocity of NO with hemoglobin is substantially greater that than of CO, and due to the marked differences in reaction velocities relative to O2, DLCO, and DLNO can provide different but complementary information about the capillary bed. It was generally assumed that the erythrocyte resistance is virtually zero for NO (130); however, recent work indicated that the resistance to alveolar-capillary NO uptake is not zero (22). Importantly, NO is a pulmonary vasodilator, and it is unclear how using NO to evaluate diffusion may itself alter perfusion/capillary blood volume and ultimately diffusing capacity. As mentioned previously, DLO2, DLCO, DLNO, and DM increase linearly with exercise (70, 95, 120, 130, 133, 134, 148, 282,305), and while there is no evidence of a plateau near peak exercise in DLCO, DLNO, or DM, MIGET data demonstrate the development of a diffusion limitation in some subjects at high exercise intensities (93, 251, 316).
Relative importance of diffusion and reaction kinetics in gas exchange
Roughton and Foster (263) separated diffusion into the membrane and red cell resistances as expressed in the following equation:
| (35) |
Where DL is the diffusing capacity of the lung in mL/min/mmHg, DM is the membrane diffusing capacity incorporating resistance from both alveolar capillary membrane and plasma barriers, θ represents both diffusion and reaction kinetics within the red cell contained in a milliliter of blood with a normal hematocrit and O2 capacity, and Vc is capillary blood volume. The reciprocal of DL is thus the total resistance of all the diffusion barriers and reaction kinetics for gas exchange. Roughton et al. (264) found that 1/θCO increases as PO2 increases, indicating that DLCO will decrease as PO2 increases. Using this relationship, it is possible to measure DLCO at more than one PAO2, resulting in a linear relationship between 1/DLCO and PAO2 and from this, DMCO and Vc can be calculated. Assumptions of this equation include: (i) diffusive resistance in the alveolar gas phase is negligible, (ii) there is no interaction between septal tissue and erythrocyte membrane, (iii) PAO2 itself does not affect DMCO or Vc (131), and (iv) that θ measured in vitro can accurately reflect the in vivo values (discussed below).
Breath holding to determine diffusing capacity
The traditional single-breath diffusing capacity method requires a breath hold of up to 10 s (70, 148), which may be impractical during high-intensity exercise, while the fixed intrathoracic pressure may alter cardiac output, pulmonary artery pressure, or capillary blood volume as compared to steady-state breathing. Rebreathe (13, 131, 305), open-circuit (282), and single-breath techniques that use a prolonged expiration (135, 136, 244) appear to be suitable alternatives to evaluate exercise DLCO/DLNO. The classic method by Roughton and Foster to partition DM and Vc requires DLCO during steady state at at least two different PAO2 levels (typically room air and FIO2 = 1.0). This technique would be difficult during exercise, and further, continuously breathing 100% O2 during exercise may affect cardiac output/pulmonary artery pressure and thus DLCO. As a result, this technique has typically been performed at rest before and after exercise. However, combined measurements of DLNO and DLCO can allow for simultaneous calculations of DM and Vc during exercise in a single maneuver (130, 133).
Estimation of θ during exercise
With the method developed by Roughton and Forster, there is concern regarding the value given for θ for CO, and the reader is referred to the Handbook of Physiology gas exchange chapter in Comprehensive Physiology for a more thorough review (146). Importantly, θ (i.e., resistance of gas uptake by the red cell) declines as the fraction of unbound heme sites decreases (291), while θ can also be modulated by changes in blood temperature. These two factors may make valid calculations of DM and Vc using a fixed value for θ problematic during incremental exercise, because of the drop in mixed venous PO2 and increase in body temperature with exercise. Indeed recent work has shown that the value given for θ can significantly affect the values derived for DM and Vc at rest and during exercise (37). As mentioned above, it was previously assumed that the erythrocyte resistance for NO is virtually zero (130); however recent work indicates that θ for NO uptake is around 4.5 mL NO/(min/mmHg/mL blood) (22).
Basic concepts of acid-base physiology
It should be evident that disturbances or inadequacies of gas exchange that affect delivery of oxygen to the tissues, or removal of CO2 from the tissues, will result in a change in blood and tissue acid-base balance. Inadequate O2 delivery and uptake by muscles, particularly during exercise, will result in an increased reliance on non-aerobic sources of ATP production, that is, phosphocreatine (PCr2−) degradation and glycolytic pyruvate−/lactate− production. In this section of the article, we will consider the physicochemical basis for these changes and the contributions of these, and associated changes in acid-base variables in determining acid-base state.
The traditional (descriptive) approach to acid-base balance recognizes respiratory and metabolic origins of acid-base disturbances, and various approaches have been developed to determine their contributions. It is now recognized that acid-base disturbances do not simply have respiratory and metabolic origins. The “metabolic” origins may result from high rates of acid/alkali production such as occurs within the digestive tract and skeletal muscle, from ingesting weak-acid salts (e.g., sodium bicarbonate), dehydration or overhydration, imbalances in plasma and lymph protein concentrations, altered cellular metabolism, and rapid fluid/electrolyte shifts between fluid compartments.
The physicochemical approach to understanding acid-base balance described here has its historical basis in the physiological chemistry research of Henderson (109), and van Slyke (321) nearly 100 years ago. In addition, the approach embraces the known physicochemical reactions between water, strong and weak ions and CO2 that occur in aqueous physiological solutions, as described in detail in early texts on physical chemistry, in particular those by Edsall and Wyman (54), and Harned and Owen (104). In 1981, Peter Stewart (295) published a detailed monograph that integrated the physiology of acid-base balance within the context of the underlying physical chemistry of aqueous (physiological) solutions. This is now referred to as the physicochemical approach or the Stewart approach to acid-base balance, although Lawrence Henderson first described blood as a physicochemical system in the 1920s (109). A detailed introduction to the physicochemical approach to acid-base balance is provided in another article of this series (176).
Overview of Physicochemical determinants of acid-base balance
The physicochemical approach is founded on four underlying physical premises:
Protons are a main constituent of water, the most prevalent molecule within the body. Water thus provides an almost limitless source of H+ for biochemical and physicochemical reactions. Protons are part of the solvent that comprises the milieu of the body. It is because of the ability of water to so rapidly dissociate (H+ and −OH) and reassociate that makes water the “universal” solvent.
A dissociated proton molecule (H+) is only in physical existence for a fleeting instance of time, approximately 10−5 second. The proton is highly reactive, associating briefly with negative charges on proteins, −OH molecules, HCO3− molecules, and amino acids to name a few. The proton is therefore very unlike inorganic electrolytes such as Na+, K+, and Cl− that are relatively unreactive.
The positive charge in pure water is physically represented by oxonium or hydronium ion (H3O+) and it is conventional for physical chemists and physiologists to ignore the water to which the proton is attached, hence giving reference to H+ (55).
The addition of strong or weak acids to an aqueous solution affects the association between protons and water. For example hydrochloric acid (HCl) exists only in aqueous form and is characterized by a very high [Cl−] while [H+] is relatively low. Although H+ is an integral part of the aqueous system, it is the strong acid anion, Cl−, that makes this solution so acidic. The strong acid anion Cl− can be neutralized by the addition of an equivalent amount of the strong base cation Na+ to the solution, but without an accompanying acid anion such as Cl−, HCO3−, or H2PO4−. Thus, if NaOH is added—the strong anions Cl− and Na+ will remain fully dissociated in solution while a rapid reaction between H+, −OH, and water results in decreased [H+]. One might think that the resultant solution is saline with a neutral pH. However, following the addition of the NaOH, a very small number of Na+ bind with water, such that the anionic charge due to Cl− exceeds the resultant cationic charge of Na+, thus the resultant solution is saline with a slightly acidic pH.
Strong ions and strong ion difference
In this section, the activity of ions in solution, as well as their total molal concentrations, are considered. Because plasma proteins, both globulin and albumin, loosely bind cations (69, 171), the measured ion activities are somewhat less than their molal concentration. Van Leeuwen (171) has termed this the net cation equivalency (or “base-binding power”) of plasma proteins.
The values for the key variables used in the physicochemical assessment of acid-base balance, for resting humans, are provided in Table 1. The concentrations of strong acid anions and strong base cations within a fluid compartment are summed, with consideration of the charge, to yield the strong ion difference, or [SID]. The [SID] represents the sum (charge considered) of the strong acid anions and strong base cations, where the term “strong” refers to the fact that the ion will be fully, or nearly so, dissociated in aqueous solutions. The strong ions are important determinants of the concentrations of [H+] and [HCO3−] because they directly affect the associated state of H2O, and thereby determine the concentrations of H+ and −OH.
Table 1.
Physiologically Important Acid-Base Variables, and Their Concentrations, in Arterial Plasma and Skeletal Muscle of Humas at Rest
|
Plasma |
Skeletal muscle |
|||
| Dependent variables | Normal value | Normal range | Normal value | Normal rangec |
| [H+] nanoEq/L | 40 | 33–45 | 100 | 71–126 |
| pH | 7.40 | 7.35–7.48 | 7.0 | 6.90–7.15 |
| [HCO3−] mEq/L | 28 | 22–34 | 10 | 8–12 |
| pCO2 mmHg | 40 | 35–45 | 45 | 40–50 |
| [total CO2] mmol/L | 30 | 23–36 | 10a | 8–12a |
| [SID] mEq/L | 40 | 37–43 | 110c | 60–130 |
| Strong ions | ||||
| [Na+] mEq/L | 140 | 132–146 | 15 | 8–30 |
| [K+] mEq/L | 3.7 | 2.7–4.7 | 140 | 110–165 |
| [Ca2+] mEq/L | 2.5 | 2–3 | 0.4 | 0.2–1.0 |
| [Mg2+] mEq/L | 1.0 | 0.5–2.0 | 5 | 3–7 |
| [Cl−] mEq/L | 105 | 99–109 | 10 | 5–25 |
| [lactate−] mEq/L | 1.0 | 0.5–1.5 | 1.5 | 1.0–2.0 |
| [PCr2−] mEq/L | Na | Na | 50 | 45–60 |
| [SO42−] mEq/L | 0.5 | 0.3–0.7 | – | – |
| Plasma |
Skeletal muscle |
|||
| Weak ions | Normal value | Normal range | Normal value | Normal rangec |
| [Atot] mEq/L | 12d | 11–13 | 140 | 120–170 |
| [plasma protein] g/L | 70 | 60–85 | Na | Na |
| [albumin] g/L | 45 | 30–50 | Na | Na |
| [globulins] g/dL | 25 | 20–35 | Na | Na |
| [HPO42−] + [H2PO4−] mmol/L | 2.7 | 2.0–3.5 | 8 | 7–9 |
| Carnosine mmol/L | Na | Na | 6 | 3–9 |
| Protein histidine concentration mmol/L | – | – | 46 | 30–60 |
Within plasma and the extracellular fluid compartment the [SID] may be calculated as:
| (36) |
In practice, the free concentrations of the divalent cations and anions are approximately equivalent and can be ignored, leaving:
| (37) |
In some treatments of acid-base balance using the physicochemical approach, [lactate−] is also ignored. However [lactate−] cannot be ignored with exercise, recovery and in many clinical conditions.
When assessing the acid-base state of skeletal muscle, PCr2− and Mg2+ must be used within the equation because their free concentrations are large and change substantially during exercise:
| (38) |
A decrease in [SID] (without concurrent change in PCO2 or Atot), can be due to either a decrease in strong cation concentration OR an increase in strong anion concentration. Either of these changes will increase [H+] and decrease [HCO3−]—and acidification of the solution occurs (Fig. 12). Conversely, an increase in [SID] has an alkalinizing effect and decreases [H+] and increases [HCO3−].
Figure 12.
Relationships showing the dependence of plasma pH, [H+], and [HCO3−] on plasma [SID] when PCO2 is held constant at 40 mmHg and [Atot] is held constant at 16 mEq/L. Note the decreasing slope in the [SID]: [H+] relationship with increasing [SID], indicating increased ability to remove (buffer) H+ from solution. For comparison, the dashed—dotted line extrapolates a linear decrease in [H+] with increasing [SID]; increasing [SID] from 39 to 45 mEq/L has the effect of buffering approximately 3 mEq/L of H+.
Weak acids and bases, and [Atot]
The total weak acid concentration (Atot) represents the sum (charge considered) of the weak acids and bases such as plasma proteins, inorganic phosphate and some of the amino acids. The dependence of [H+] on [Atot] in the plasma system is shown in Figure 13. The term ‘weak’ refers to those anion acids and cation bases that are not fully dissociated in solution. This physical attribute of many weak acid anions such as phosphate, bicarbonate, and albumin are what makes these ions good proton “buffers.” These molecules are weak acids because they have one or more proton dissociation constant (pK′) that is less than 7.4. In the absence of weak acids, the acidification resulting from decreased [SID] or increased PCO2 is more profound (compare Figs. 12, 13, and 14).
Figure 13.
The dependence of plasma [H+] on [Atot] and [SID] when PCO2 is constant at 40 mmHg.
Figure 14.
The dependence of plasma [H+] on PCO2 and [SID] when [Atot] is constant at 16 mEq/L.
The main weak acids and bases within the extracellular fluid compartment are albumin, globulin, phosphate, and bicarbonate. Bicarbonate, however, is part of the CO2 system and thus is not used in the calculation, or estimation, of [Atot]. Similar to the strong ions, the weak ions also directly affect the concentrations of H+ and HCO3− in solution. Within skeletal muscle, it is primarily the histidine moieties on proteins that contribute to [Atot], with creatine, Pi, ATP, ADP, and other molecules also contributing (174, 248). The main contributors to [Atot] in resting skeletal muscle are the histidine groups of proteins (60%), creatine (15%), Pi (8%), and ATP (7%), with other compounds accounting for about 10%. It is primarily the histidine moieties on structural proteins that provide most (55–65%) of the capacity for nonbarbonate proton binding, with creatine (15%), inorganic phosphates (8%), organic phasophates (7%; ATP and ADP), and carnosine (7%) playing lesser roles (184, 198, 199). There is a fiber-type distribution in carnosine content and nonbicarbonate buffering, with fast type II fibers having a greater ability to both generate and buffer protons (199). The non-bicarbonate buffering capacity of mixed human muscle has been determined by acid titration to be 160 mmol H+/kg dry muscle mass/pH (176, 177), which equates to approximately 42 mmol H+/L intracellular fluid/pH.
The carbon dioxide system
The PCO2 is one of the main determinants of acid-base balance and the third independent variable so long as the body behaves as an open system with respect to CO2 (Fig. 14). The production of CO2 by most cells of the body requires that CO2 be transported from production sites and dissipated to the ambient environment by the lungs (146, 149), hence the requirement for gas exchange to maintain acid-base balance. By its physicochemical nature CO2 is essentially a strong acid and, because it is a major end product of cellular respiration, its accumulation in the body will result in what has traditionally been termed a “respiratory” acidosis manifested as an elevated PaCO2 and [H+] (and a depressed pHa). Conversely, excessive exchange of CO2 with the environment (induced or voluntary hyperventilation) will lower CO2 and can result in a respiratory alkalosis manifest by decreased PaCO2 and [H+]a (increased pHa). This, in fact, typically occurs in the arterial circulation during exercise. The majority of acid-base disturbances usually have complex origins, comprising a mix of respiratory, and nonrespiratory origins.
Carbon dioxide is a strong acid by virtue of its ability to combine with water to increase the concentration of H+ while at the same time increasing the weak acid [HCO3−]. This reaction effectively acidifies the solution to which CO2 has been added. The majority (about 95%) of the total CO2 within the body is in the form of HCO3−, with much smaller concentrations of H2CO3, CO32−, dissolved CO2 [CO2(d)], and carbamino compounds (CO2 bound to amino groups on protein). The chemical reactions involved in the hydration and dehydration of CO2 are
| (39) |
With carbonic anhydrase catalyzing the hydration/dehydration of CO2 there is a near equilibrium of these CO2 compounds at the various CO2 exchange sites in various tissues and at the lungs. The inhibition of carbonic anhydrase, using acetazolamide, for example, impairs the ability to regulate acid-base state at tissue production sites and at the lungs (164, 165). These reactions are of fundamental importance in the control of ventilation because two of the variables are detected by peripheral and central chemoreceptors, PCO2 and H+. Here, the physical chemistry of CO2 within aqueous solutions is merged with applied physiology. Furthermore, in normal people under normal situations the CO2 system is an open system because CO2 arising from cellular metabolism or titration of CO2 stores is passed into the ambient environment. Operationally, the open CO2 system facilities the ability to get rid of CO2 from production sites and provides a direct link between cellular metabolism and ventilation. Many factors can contribute to an increase in PaCO2, and hence drive an increase in , including an increase in cellular CO2 production, acidification, and titration of body CO2 stores, impaired cardiac output, impaired pulmonary perfusion, impaired pulmonary airway conductance, and impaired erythrocyte CO2/HCO3− release.
Solving equations to determine acid-base balance
The physicochemical approach explicitly acknowledges that the physical and chemical laws governing the reactions between molecules within aqueous solutions must be obeyed (54, 104, 295). Accordingly, the following five mass action equations, and one equation expressing electrical neutrality of solutions, describe the physicochemical characteristics of any aqueous, physiological solution (295, 296):
Water dissociation
| (40) |
Weak electrolyte system
| (41) |
| (42) |
Carbon dioxide system
| (43) |
Electrical neutrality
| (44) |
It is noteworthy that [H+] appears in each of these equations and its dependence on the concentrations of strong and weak acids/base and CO2 is evident. These six equations can be combined into a single equation that may then be solved for [H+] when the three independent variables and the constants (Table 2) are known (295, 296)
| (45) |
Table 2.
Values of the Constants Used Within the Acid-Base Equations
This equation, and variations of it, can be solved using available software (http://ppn.med.sc.edu/watson/Acidbase/Acidbase.htm; www.acid-base.org; www.acidbase.org).
Determinants of [H+] in different body compartments
Interactions between systems
The body is capable of tolerating relatively large physiological disturbances of muscle, plasma, and erythrocyte acid-base balance. For example, during high-intensity exercise the pH of intensely contracting type II fibers can be estimated to decrease from approximately 7.0 to approximately 6.0 [based on data of reference (284)]; this is equivalent to an increase in [H+] from 100 to 1000 nmol/L. Similarly, femoral venous plasma [H+] as high as 100 nmol/L has been reported during very high intensity exercise (180). At the same time, arterial plasma [H+] is only 64 nmol/L, indicating important roles of the lungs, erythrocytes, and other noncontracting tissues in reducing the magnitude of the acidosis (162, 179, 204). The source of the acidosis in the femoral vein during high-intensity leg-cycling exercise is clearly the leg muscles, with the circulation operating to remove metabolic products and heat from, and deliver nutrients and oxygen to, contracting muscle. The circulatory system thus plays an important role in distributing, throughout the entire body, products generated by contracting muscle, necessitating reactions by other tissues and organ systems to try to restore their own balance, as well as balance within the whole body. Accordingly, when a disturbance to acid-base balance occurs, the processes involved in maintaining or recovering acid-base balance are multifactorial, with a level of complexity that reflects the necessary integration of responses amongst tissues and environments. One of the best ways that we have for understanding these processes is to perform a quantitative analysis of each of the factors that can contribute to the acid-base state within each tissue compartment of interest, arterial plasma, or skeletal muscle, for example.
The multisystem mechanisms that regulate systemic acid-base balance are integrative processes that involve gas exchange and the simultaneous net changes in the concentrations of strong and weak ions within and between extracellular and intracellular fluids (Fig. 15). Acid-base balance is, therefore, the net effect of gas and ion transfer processes across cell membranes and amongst tissues. Skeletal muscle is the pre-dominant tissue in the body and plays important roles in both generating and ameliorating acid-base disturbances. Renal and gastrointestinal water and electrolyte transport mechanisms are also involved in the regulation of acid-base balance, but will not be considered within this paper. For the sake of simplicity and clarification, we will now examine mechanisms for dealing with acid-base disturbances within contracting muscle, venous blood, and arterial blood as independent systems. This will be followed by a quantitative analysis of the contributions of independent variables to an exercise-induced acid-base disturbance within these key compartments.
Figure 15.
Diagram showing interrelationships between ventilation, acid-base variables, and cellular processes. The dashed arrows indicate pathways through peripheral and central chemoreceptors that drive ventilation according to the acid-base status of the individual.
Contributors to [H+] in muscle at rest and exercise [Atot]
Both [Atot] and its apparent dissociation constant (KA) increase with moderate to high-intensity exercise (177, 184), mainly due to increases in creatine and inorganic phosphate concentrations, and thus this increases the capacity for proton buffering. The relationship between the dependent acid-base variables and changes in muscle [Atot] are shown in Figure 16.
Figure 16.
(A) Relationships between the dependent acid-base variables and [Atot] in resting muscle. The hatched bar indicates a normal range for resting muscle. (B) Relationships between the dependent acid-base variables and [Atot] in muscle with high intensity exercise. The hatched bar indicates a normal range for very high intensity exercise.
Carbon dioxide system
Within skeletal muscle, CO2 exists primarily in the form of HCO3− (88%), with minor contributions by CO32− (8%), carbamates other compounds, and dissolved CO2. Dissolved CO2, HCO3−, and CO32− are in rapid equilibrium due to the ubiquitous presence of various carbonic anhydrase isoforms within most intracellular and extracellular compartments and membranes. The PCO2 in resting muscle is 45 to 50 mmHg, with [HCO3−] 10 to 15 mEq/L (Fig. 17). Increased rates of aerobic metabolism, such as occurs with very high intensity exercise, can raise femoral venous PCO2 to over 100 mmHg (163, 180), therefore, intramuscular PCO2 can exceed 100 mmHg (Fig. 17B). In adequately perfused muscle, with the action of carbonic anhydrases, metabolically produced CO2 is rapidly removed from muscle. Another contributor to increased PCO2 in muscle during exercise results from protons reacting with HCO3−, which contributes to the reduction in [HCO3−].
Figure 17.
(A) Relationships between the dependent acid-base variables and PCO2 in resting muscle. The hatched bar indicates a normal range for resting muscle. (B) Relationships between the dependent acid-base variables and PCO2 in muscle with high intensity exercise. The hatched bar indicates a normal range for very high intensity exercise.
Strong ion systems
The concentrations of strong ions within the intracellular fluid compartments are determined by the simultaneous and ever-changing activities of numerous ion channels and ion transport systems (32,183) and metabolism. The influence of metabolic activity on muscle [SID] needs to be included for three reasons. First, PCr2− is a divalent strong acid anion with a pK′ = 4.5, and its concentration decreases during moderate to high-intensity muscle contractions, which contributes to both the decrease in muscle [SID] and increase in muscle [Atot] (increased phosphate and creatine) with exercise. Second, lactate− is also a strong acid anion (pK′ 3.9), and its accumulation contributes nearly 50% to the decrease in muscle [SID] during exercise (177). Third, metabolic activity (ATP production) is ultimately required to maintain or restore ion concentrations within cellular compartments. The main ATP-dependent ion transport systems are the sarcolemmal and T-system Na,K ATPase and the Ca ATPase of the sarcoplasmic reticulum. The major ions involved in the strong ion determination of muscle [SID] are: (i) Cl− (typically in electrochemical equilibrium across the sarcolemma due to the high Cl− conductance of this membrane); (ii) Na+, and (iii) K+ whose concentrations are determined mainly by the Na+-K+ ATPase and secondarily by the Na+-K+-2Cl− co-transporter and other cation channels (iv) PCr2− which is determined by its simultaneous and ongoing rates of degradation and resynthesis; and (v) lactate− whose concentration is determined by the rate of glycolytic pyruvate− production, pyruvate− conversion to lactate− (in part determined by pyruvate− conversion to acetyl CoA and the aerobic metabolic rate), and the net effects of monocarboxylate transporter (MCT) facilitated uptake and efflux. The relationships between the dependent variables and [SID] in resting and exercised muscle are shown in Figure 18.
Figure 18.
(A) Relationships between the dependent acid-base variables and [Atot] in resting muscle. The hatched bar indicates a normal range for resting muscle. (B) Relationships between the dependent acid-base variables and [Atot] in muscle with high intensity exercise. The hatched bar indicates a normal range for very high intensity exercise.
Production of the acid-base disturbance within contracting muscle
At any point in time, muscle [H+] can be determined by the measuring the concentrations of the main strong and weak ions and the PCO2. This does not imply that protons are not involved in biochemical and chemical reactions, merely that there always exists a physicochemical balance between protons and other molecules.
Acid-base effects of cellular metabolism in normal individuals: High-intensity exercise
Alterations in cellular metabolism, and particularly skeletal muscle metabolism during the performance of activities, may have a profound effect on acid-base balance within contracting muscle and in blood (108, 138, 174, 180). These changes and interactions are summarized in Figure 19. It is important to remember that the water content of the cells increase during exercise, due mainly to the rapid accumulation of osmotically active molecules (creatine, Pi, and lactate−) during the rest-to-work transition (177,185). Within contracting skeletal muscle, increased glycolytic activity results in the net accumulation of strong acid anions (mainly lactate− with a small amount of pyruvate−), and weak acid anions (most other glycolytic intemediates). By definition, the maintenance of electroneutrality requires that the net increase in the concentrations of acid anions will affect the balance between the two most abundant weak ions in solution, namely, HO− and H+. An increase in the concentration of acid anion molecules must be accompanied by an equal increase in the concentration of net positive charge, and this positive charge is provided in large part by the dissociation of water. For example, in an aqueous solution that contains only strong ions:
| (46) |
| (47) |
which is the same as:
| (48) |
From this equation, it is evident that an increase in the concentration of strong acid anions, which lowers [SID], directly contributes to an increase in [H+].
Figure 19.
Schematic of whole body acid-base interactions during high intensity exercise.
We will now consider the contributions of changes in the independent acid-base variables to muscle [H+] in a study in which men performed very high intensity leg bicycling exercise for 30 s followed by 90 min of recovery (162, 163). The effects of changes in the independent acid-base variables PCO2, [Atot], and [SID] are shown in Figure 20. In normal, healthy individuals the provision of ATP within contracting skeletal muscle during transition from rest to exercise, during step increases in work intensity and during performance of high-intensity exercise, relies heavily on the degradation of PCr2− and on glycolysis with attendant production of lactate−. The degradation of PCr2− results in an increased intracellular [SID] (174), and therefore, has an initial alkalinizing effect on intracellular acid-base balance (51, 186, 266). The PCr2− store is very limited and net degradation slows rapidly (within 2 min) when the ATP demand is maximal (290), so the alkalinization that occurs is brief. It is brief because glycolysis, supplied by stores of glycogen within muscle and the muscle’s ability to extract glucose from the circulation, is rapidly and maximally activated. Increased glycolytic flux results in the production of pyruvate− that is rapidly converted to lactate−, both of which are strong acid anions. The production and accumulation of lactate− within muscle can be rapid (180, 184), with production rates of approximately 60 mEq/L/min and intracellular concentrations exceeding 45 mEq/L (184). Simultaneous to the increase in intracellular [lactate−], there occurs a rapid and considerable loss of intracellular K+ to the extracellular fluids and plasma as a result of repeated depolarizations and repolarizations of the sarcolemma. The decrease in [K+] contributes approximately 50% to the decrease in intracellular [SID]. The decrease in [SID] can occur very quickly and be very large, and the associated increase in intracellular [H+] is indicative of the pronounced intracellular acidification (177).
Figure 20.
The relationships between muscle [H+] and each of the independent variables PCO2, [Atot], and [SID] in response to 30 s of very high intensity exercise and subsequent 0.5 min of recovery. The time course of change is indicated by the arrows. R = resting; 0.5 = 0.5 min of recovery; 3.5 = 3.5 min of recovery; 9.5 = 9.5 min of recovery. The solid lines indicate [H+] isopleths at resting [SID] of 100 mEq/L (with KA of 1.64 × 10−7 Eq/L) and [SID] of 60 mEq/L which occurred at 3.5 min of recovery (with Ka of 1.98 × 10−7 Eq/L. The dashed line indicates the resting [H+] isopleth but using the exercise KA of 1.98 × 10−7 Eq/L.
There is evidence that the increases in [K+], [H+], and arachidonic acid within the interstitium of contracting muscle stimulate CIII and CIV afferent nerves (260–262, 314) which have inputs to the respiratory control center (19) and thus drive ventilation (224). It is likely that a peripheral driver of ventilation is very important at this time because arterial, and therefore, CSF, [H+] initially falls which removes the central chemoreceptor drive to ventilate.
[H+] in contracting tissues and the venous circulation will increase during the period of high-intensity exercise and produce a modest increase in arterial [H+] by the end of exercise and into post-exercise recovery (162, 163). Within contracting muscle, the increase in [H+] is very rapid, resulting in the titration of CO2 stores which, together with increasing mitochondrial CO2 production, results in a pronounced increase (up to 100 mmHg) in muscle and venous PCO2 (163, 180). By this time, the is already so high that arterial PCO2 falls below (to 30 mmHg) resting values.
Even at lower exercise intensities, skeletal muscle contraction results in the progressive increase in activities of TCA cycle, beta-oxidation, and respiratory chain enzymes such that increased flux of carbohydrate and fatty acid carbon through the oxidative systems results in activation of pyruvate dehydrogenase (337) and a consequent increase in mitochondrial CO2 production. This necessitates the rapid removal of CO2 from cells to minimize cellular acidification. To accomplish this, there are a number of carbonic anhydrase isoforms present on mitochondrial membranes (62), sarcolemma, T-system membranes, within the cytoplasm and in the interstitial fluid and blood plasma (347). Carbonic anhydrase isoforms are also abundant on erythrocyte plasma membrane and within erythrocytes and the lungs (23, 79). Erythrocytes play a profound role in buffering the increases in [H+], [lactate−], and pCO2 (18, 141, 180, 204) such that in the absence of erythrocytes the concentrations of these metabolites in plasma would likely be toxic (184). The body is thus endowed with a catalyzed, physicochemical system for rapid CO2 transfer from cells to lungs to ambient environment (atmosphere).
The relationship between muscle [H+] and PCO2 during 30 s of very high intensity exercise and 9.5 min of recovery is shown in Figure 20A. Because of the high [Atot] (strong nonbicarbonate proton buffering) within skeletal muscle ([Atot] = 140 mEq/L) large changes in PCO2 are required to produce significant changes in [H+] and [HCO3−]. In addition to these nonaerobic mechanisms, there is also a rapid simultaneous increase in muscle CO2 release due to both acid titration of CO2 “stores” [bicarbonate, carbonate, carbamino compounds within tissues (163, 179, 204, 223) and increased aerobic metabolism (180, 223)]. This release of stored CO2 is also readily evident from noncontracting tissues during and following periods of high-intensity exercise (162, 179, 204). An indication of the rate of total CO2 production and release by contracting muscle and noncontracting tissues can be obtained from the venous-arterial PCO2 difference (Fig. 21). As exercise intensity increases from low to high, there will also be an increasing contribution from the dehydration of HCO3− resulting from acidification such that titration of CO2 stores (primarily bicarbonate) within noncontracting tissues and within contracting muscle generates CO2 that contributes to elevated plasma PCO2 and [H+] during exercise, and that both of these contribute to the ventilatory drive to increase .
Figure 21.
Arterial—antecubital venous (∎) and arterial—femoral venous (•) PCO2 (top panel) and [H+] (bottom) differences during four repeated, 30 s bouts of very high-intensity exercise interspersed with 4 min rest periods, followed by 90 min of recovery. Data, with permission, from Lindinger et al. (179, 180).
The relationship between muscle [H+] and [Atot] during 30 s of very high intensity exercise and 9.5 min of recovery is shown in Figure 20B. The increase in [Atot] from 140 to 170 mmol/L represents the increase in total weak acid concentration that occurs, so this has a modest acidifying effect. These increases are due to the increased [Pi] resulting from PCr2− hydrolysis (from 26 to 98 mmol/kg dry wt), increased glycolytic phosphates from 3 to 22 mmol/kg dry wt (174, 176, 177) and increases in [ADP] and [AMP]. The increase in [Atot] is accompanied by an increase in the apparent dissociation constant for [Atot], the KA, from 1.64 × to 1.98 × l0−7 eq/L (174, 176, 177). It is estimated that the increases in [Atot] and KA contribute 19% and 7%, respectively, to the increase in [H+]i during 30 s of very high intensity exercise (Fig. 20B).
The relationship between muscle [H+] and [SID] during 30 s of very high intensity exercise and 9.5 min of recovery is shown in Figure 20C. Compared to changes in PCO2 and [Atot], physiological changes in [SID] have rather pronounced effects on cellular acid-base state. Changes in intracellular [SID] are affected by changes in the concentrations of strong ions by means of biochemical reactions (PCr2− and Lac−), transmembrane ion transport processes (Na+, K+, Ca2+, Mg2+, Cl−, and Lac−), and transmembrane water movement. After the initial alkalinizing effect of PCr2− hydrolysis, which raises [SID], there occurs an increase in [Lac−] and a decrease in [K+], both of which contribute to decreased [SID] and hence cellular acidification. A 40 mEq/L increase in [Lac−] such as occurs with 30 s of very high intensity exercise has the effect of increasing [H+]i by 158 nEq/L, and accounts for about 50% of the total increase in [H+], with the decrease in cellular [K+] accounting for the remainder. Thus, decreases in intramuscular [SID] result in a pronounced intra- and extracellular acidification associated with increased CO2 release (metabolic CO2 production as well as titration of intracellular CO2 stores).
Exercise and acid-base summary
The primary effect of changes in the ion concentrations on muscle [H+]i during contraction is a decrease in intracellular [K+] that reduces intracellular [SID] (since [Na+] and [Cl−] accumulate to similar degrees). With high-intensity exercise, the net reduction in [K+] occurs rapidly and, through its effect on the intracellular [SID], contributes to the increases in [H+]. Coincident with this change, however, is the concurrent hydrolysis of PCr2−. The rapid hydrolysis of PCr2− reduces its concentration which has a direct effect on increasing intracellular [SID], and thus contributes to a decrease in [H+]. In the first seconds of the rest to work transition, the decrease in [PCr2−] effectively raises [SID] and thus [H+] must decrease. It should also be recognized that PCr2− hydrolysis results in the production of creatine, which is electroneutral, and that the subsequent hydrolysis of ATP from the creatine kinase reaction results in the production of the weak acid inorganic phosphate. PCr2 hydrolysis is thus a potent means for increasing [SID] and for reducing intracellular [H+], albeit while modestly increasing [Atot]. With increasing glycogenolytic and glycolytic activity, the strong acid anion lactate− progressively increases in concentration and its net accumulation is not effectively balanced by the simultaneous changes in other intracellular strong ions. Thus, concurrent decreases in intracellular [K+] and increases in [lactate−] result in progressive decreases in intracellular [SID] because [PCr2−] either does not change further or may increase if ATP demand is reduced. The unequal accumulation and/or removal of cations and anions during exercise leads to a decrease in intracellular [SID] (altered balance of strong and weak ions in solution) which directly and physicochemically contributes to the increase in [H+] during moderate to high-intensity exercise. The magnitude of this increase is proportional to: (i) the concentrations of dissociated weak acids (i.e., structural nonbicarbonate proton buffering capacity of muscle); (ii) the rate at which acid equivalents (strong acid anions such as PCr2−, pyruvate−, and lactate−) accumulate or are removed from muscle or consumed within muscle; and (iii) the rate at which strong base cations are added to or removed from muscle.
Arterial and venous plasma
With moderate- to high-intensity exercise there is an integrated set of responses within plasma and erythrocytes, with each of these two “compartments” necessarily reflecting the changes imposed by the tissues that were just perfused by the vascular system. Venous blood draining contracting muscle thus reflects metabolic and ionic events occurring within contracting muscle cells. Within venous plasma and erythrocytes draining contracting muscle there are pronounced increases in [K+], [lactate−] (with consequent decrease in [SID]), PCO2, osmolarity (increased [Na+] and [Cl−], and protein (and hence [Atot]). Arterial blood reflects the major effects of ventilation on the removal of CO2 and the replenishment of O2, including redistribution of Cl− and HCO3− between erythrocytes and plasma. Venous blood draining noncontracting tissues reflects the effects of those tissues on their ability to further correct some of the exercise-induced changes that occurred in venous plasma draining contracting muscles. Noncontracting skeletal muscle also participates in these “corrections” and includes extraction of lactate− and K+ from, and net addition of water to, plasma (162, 176).
An example of how one determines contributions to the changes in acid-base state of plasma during and following high-intensity exercise
Here, we will again use the example of 30 s of high-intensity leg bicycling exercise with 10 min of recovery [Table 3; (162, 163)]. A similar analysis has been performed with four repeated bouts of high-intensity exercise with 90 min of recovery (176, 180, 204). It should be remembered that the net shift of relatively (compared to plasma) dilute fluid into contracting muscles decreases plasma and extracellular water content of many tissues, and contributes to increases the concentrations of most molecules present within plasma.
Table 3.
Arterial and Femoral Venous Plasma Independent and Dependent Acid-Base Variables at Rest and After 30 s of Very High Intensity Leg Bicycling Exercise [From Kowalchuk et al. (162, 163)]
| Rest | 30 s recovery | 3.5 min recovery | 9.5 min recovery | ||
|---|---|---|---|---|---|
| PCO2 (mmHg) | Arterial | 41 | 37 | 30 | 32 |
| FV | 46 | 106 | 48 | 40 | |
| [SID] (mEq/L) | Arterial | 37 | 34 | 29 | 31 |
| FV | 42 | 39 | 29 | 30 | |
| [Atot] (mEq/L) | Arterial | 17 | 18.8 | 18.4 | 18.4 |
| FV | 17 | 18.6 | 18.5 | 17.4 | |
| [H+] (nEq/L) | Arterial | 38 | 49 | 54 | 54 |
| FV | 41 | 95 | 76 | 64 | |
| [HCO3−] (mEq/L) | Arterial | 26 | 19 | 14 | 14 |
| FV | 27 | 28 | 15 | 15 |
The effects of changes in plasma PCO2 and [SID] on [H+] and [HCO3−], at a constant [Atot] of 17 mEq/L are shown in Figure 22. At a normal plasma [SID] of 40 mEq/L, changes in PCO2 have a profound, nearly linear, effect on [H+]. An important point to make is that as [SID] increases, the relationship between PCO2 and [H+] becomes less steep—thus increases in [SID] have the effect of acting as a nonbicarbonate proton buffer. Note that the relationship between [PCO2] and [H+] becomes steeper as [SID] decreases. The relationship is such that an increase in FV PCO2 from 46 (point R on Fig. 22, top) to 106 mmHg (at 30 s of recovery) increases [H+] by 51 nEq/L if [SID] stays at 42 mEq/L. However, FV [SID] is also decreased by 3 mEq/L with exercise due to increased [Lac−], which has the effect of increasing [H+] by a further 8 nEq/L at a PCO2 of 106 mmHg, giving a combined increase in [H+] of 63 nEq/L and an [H+] of 103 nEq/L (point 0.5 on Fig. 22, top). In addition, high-intensity exercise is associated with a decrease in plasma fluid volume which has the effect of raising [Atot]—this relationship is shown in Figure 22, bottom. The increase in [Atot] from 17 to 18.6 mEq/L has the effect of increasing [H+] by 5 nEq/L at a PCO2 of 106 mmHg. Using this example, therefore, the separate effects of increased PCO2, decreased [SID] and increased [Atot] on [H+] have been quantified: the effect of increased PCO2 alone raised [H+] by 51 nEq/L; the effect of decreased [SID] alone, in the presence of increased PCO2, raised [H+] by 8 nEq/L; and the effect of increased [Atot] alone, in the presence of raised PCO2, raised [H+] by 5 nEq/L. The final FV plasma [H+] estimated in this manner is 108 nEq/L, which is equivalent to a pH of 6.97. Compensating factors limited the actual measured increase in FV [H+] to 95 nEq/L (pH 7.02; Table 3). It is, therefore, clear that approximately 80% of the increase in [H+] 30 s after the end of exercise was due to the increased PCO2.
Figure 22.
Plasma [Atot] as functions of PCO2 and [SID] (top) and [Atot] and [SID] bottom. The arrows show the time course of change going from rest (R) to 0.5 min after 30 s of very high intensity exercise, then to 3.5 and 9.5 min of recovery. Experimental data from Kowalchuk et al. (163).
At 3.5 min of recovery, as Lac− continues to flux from muscle into blood and as ventilation continues at a high rate, there is a marked change in the contributions of the independent variables to [H+] (point 3.5 on Fig. 22, bottom). FV [SID] decreased a further 10 to 29 mEq/L, while PCO2 decreased by 58 to 48 mmHg. Thus, while PCO2 returned to preexercise values, effectively removing the CO2 contribution to the acidosis, the albeit reduced acidosis is sustained by the decreased [SID]. The contribution from increased [Atot] remains minor. At 9.5 min of recovery, FV PCO2 has decreased further to 40 mmHg, with no further change in [SID] and [Atot], indicative of a ventilatory compensation for the current metabolic acidosis. A similar analysis of the effects of changes in the independent variables on [HCO3−], and on arterial variables, can be performed in the same fashion.
Imbalance between lactate− and total acid appearance in plasma/blood
There continues to be some debate surrounding the stoichiometry of the molar increase in plasma (or blood) [lactate−] and the molar increase in plasma (or blood) total acidity (21,178). The crux of the debate is that some researchers argue that, during exercise and recovery, there is a 1:1 stoichiometry between the appearance of metabolic acid in blood and the appearance of lactate−, while others have evidence that the stoichiometry can far from 1:1. The main issues related to this problem will be noted here, and the interested reader is referred to a debate of the problem for more information [56, 57]. The first problem is that in many studies researchers are comparing a [HCO3−] measured on only the plasma portion of blood, and comparing this value to a [lactate−] measured on whole blood (plasma and lysed cells). This is not a valid comparison because different compartments are compared, and because of the disequilibrium that frequently exists between the intracellular fluid of erythrocytes and plasma (see Section “The role of erythrocytes”). This problem can simply be rectified by measuring plasma and not whole blood [lactate−], because whole blood measures of [HCO3−] are difficult to obtain and will not accurately represent the in vivo condition. Furthermore, if one could measure the change in whole blood acidity and compare it to the change in lactate−, there would rarely be a 1:1 stoichiometry. When the change in whole blood acidity is calculated (182), a 1:1 stoichiometry with whole blood lactate in humans is not found, nor is it observed in perfused rodent hindlimbs. With moderate to high-intensity exercise, the increase in blood acidity consistently exceeds the increase in [lactate−] (162, 181, 183). The second problem relates to the intensity and duration of exercise; low- and moderate-intensity exercise tend to generate data that agree with a 1:1 stoichiometry, albeit still a plasma [HCO2−] versus whole blood [lacatate−] comparison. The third is that lactate− is not the only contributor to the plasma acidosis during exercise, as has been detailed above. The other main contributors include PCO2 and [Atot] changes and, also as detailed above, each of these exert independent effects on the measured concentrations of [HCO3− and [H+]. We can illustrate these points by again using the data of (163), and there are many other studies using human and animal models that support a changing stoichiometry between lactate− and acid appearance in blood.
In Figure 23, we have shown using blood from three different sites of blood sampling, the time course of plasma [lactate−] and plasma [HCO3−] where, to facilitate a direct comparison, the [HCO3−] at time t has been subtracted from the resting preexercise values. In arterial plasma, there is a 1:1 stoichiometry between changes in the two variables. However, it is typically antecubital venous blood that is sampled and analyzed in the exercise studies cited above. There are clear and large discrepancies in the magnitude and time course of these two variables in antecubital venous plasma and in femoral venous plasma.
Figure 23.
The time course of plasma [lactate−] and negative change in plasma [HCO3−] from preexercise (time = −1 min) at the end of 30 s of very high intensity exercise (time 0) and during 9.5 min of resting recovery. Using data, with permission, from Kowalchuk et al. (162, 163).
The role of erythrocytes
Erythrocytes play an active role in regulating plasma and body acid-base state through the clearance of metabolic products, mainly CO2 and lactate−, from contracting skeletal muscle. The role of erythrocytes is far greater than the delivery of O2 to, and removal of CO2 from, working muscles. From the acid-base perspective erythrocytes play important roles with both the CO2 and SID systems. First, and relatively well-studied, transport of CO2 within erythrocytes in the form of HCO3− greatly reduces the magnitude of the plasma acidosis and likely helps maintain plasma [HCO3−] within tolerable limits. Second, and less well known, is that erythrocytes transport considerable amounts of lactate− from one body compartment to another (180, 184, 204). Much of the lactate− that enters into the venous circulation by sarcolemmal MCT-facilitated diffusion from contracting skeletal muscle rapidly enters the erythrocytes through MCTs in the erythrocyte plasma membrane. This has the effect of reducing plasma [lactate−] from what would be toxic levels (184), increasing plasma [SID] and thereby reducing the plasma acidosis.
An example of the time course of changes in erythrocyte [lactate−] in blood sampled from the femoral vein, brachial artery, and antecubital vein in a study of high-intensity leg cycling exercise is provided in Figure 24. In this study, subjects performed four 30 s bouts of exercise, interspersed with 4 min rest periods, followed by 90 min of recovery (180, 204). Peak plasma [lactate−] occurred at 5 min of recovery and averaged 25.9 and 25.7 mEq/L in arterial and antecubital venous plasma (204) and 21.0 and 21.3 mEq/L in arterial and femoral venous plasma (180). The arterial erythrocytes [lactate−] were similar in both of these studies, allowing the following analysis. In femoral vein and artery, at the end of the first 30 s bout (last 10 s of exercise + first 10 s of rest), erythrocyte [lactate−] had increased by more than 3 mEq/L, and this further increased to 12 to 13 mEq/L at the end of the fourth exercise bout (Fig. 24). What is noteworthy is that there was no difference between femoral venous and arterial erythrocyte [lactate−] during the period of repeated exercise, while erythrocyte [lactate−] in antecubital venous blood was greatly reduced. These results show that lactate− originating within contracting skeletal muscle is transported in not only plasma, but also erythrocytes. The differing dynamics of the arterial and femoral venous erythrocyte [lactate−] during the 90 min recovery period is also very interesting.
Figure 24.
Time course of erythrocyte [lactate−] in blood sampled from two sets of subjects performing four 30 s bouts of high intensity exercise interspersed with 4 min rest periods (shaded area), and subsequent recovery. Data are a composite from the arterial and femoral venous data of Lindinger et al. (180) and the arterial and antecubital venous data of McKelvie et al. (204).
The time course of recovery of erythrocyte [lactate−] in the arterial circulation is markedly slower than in femoral venous erythrocytes; the difference in [lactate−] was not due to differences in mean corpuscular volume of cells between the two sampling sites. This indicates a pronounced and prolonged reuptake of lactate− by skeletal muscles recovering from high-intensity exercise, consistent with the view that lactate− is a highly preferred fuel source by skeletal muscle (211). The first 60 min of recovery is also characterized by a continued extraction of lactate− by noncontracting tissues, as evident in the differences between arterial and antecubital venous erythrocyte [lactate−]. During recovery, the markedly greater arterial (than either vein) erythrocyte [lactate−] is due to the equilibration of lactate− between plasma (5–13 mEq/L greater than in erythrocytes) and erythrocytes while in the arterial portion of the circulation. As blood perfuses the tissues, there appears to be a selective offloading of lactate− from erythrocytes because erythrocyte [lactate−] recovered at a faster rate than plasma [lactate−] within both venous plasma “compartments.” The a-v differences for [lactate−] across inactive tissues and across previously contracting skeletal muscle recovering from exercise were greater for erythrocytes than for plasma. This apparent preferential loss of lactate− from erythrocytes will be facilitated by efflux through MCTs, whereas diffusion of lactate− into the extracellular compartment of muscles occurs passively from the capillary plasma into the extracellular solution.
Lactate oxidation and roles of noncontracting muscle/tissues
Noncontracting muscle, similar to many other noncontracting tissues, serves to regulate plasma and whole body acid-base, ion, and fluid balance during the period of exercise and initial recovery (162, 179, 204) and in response to experimentally induced increases in plasma [lactate−] (211, 212) or [K+] (175). The arterial blood that perfuses noncontracting muscle is higher in [lactate−], [K+], [protein] ([Atot]) osmolarity, and [H+] and lower in PCO2 and [HCO3−] than the intracellular fluid of muscles. As a result, there are net movements of these molecules, as well as water, between erythrocytes, plasma, and muscle as blood perfuses these muscles—these reactions help to restore plasma ion concentrations, acid-base state, osmolarity, and blood volume.
When lactate− is presented to noncontracting muscle, it is a preferred metabolic substrate, where by facilitated diffusion through monocarboxylate transporters, it is extracted by muscle at high rates and subsequently oxidized (211). Using the 30 s high-intensity exercise study of Kowalchuk et al. (162, 163), the time course of change in muscle and plasma [lactate−] within each of the three main plasma compartments is shown in Figure 25. It is very evident from this figure that during this brief 10 min recovery period following very high intensity exercise there was a persistent gradient from contracting quadriceps muscle, to femoral venous plasma, to arterial plasma and ultimately to noncontracting muscle (and other tissues) where initial [lactate−] would have been about 5 mEq/L. The effects of this lactate− removal on plasma acid-base state are pronounced because the increase in [lactate−] is the primary contributor to the decreases in plasma [SID] and hence the decrease in [HCO3−] and increase in [H+] seen with exercise (Figs. 26 and 27). As described above, lactate− removal from plasma is a relatively slow process because there are substantial amounts of lactate− transported by erythroyctes, and erythrocytes recover their [lactate−] faster than plasma, which has the effect of prolonging the plasma [lactate−] recovery. Thus, as lactate− is removed from the plasma compartment there is an initially slow (32, 162, 163) though continuing (179,180) recovery of plasma [H+] and [HCO3−].
Figure 25.
Time course of muscle and plasma [lactate−] in humans that performed 30 s of very high intensity leg bicycling exercise. Data, with permission, from Kowalchuk et al. (162, 163).
Figure 26.
Time course of plasma [H+] in humans that performed 30 s of very high intensity leg bicycling exercise. Data, with permission, from Kowalchuk et al. (162, 163).
Figure 27.
Time course of plasma PCO2 and [HCO3−] in humans that performed 30 s of very high intensity leg bicycling exercise. Data, with permission, from Kowalchuk et al. (162, 163).
Another noteworthy effect of noncontracting muscle is its response to the increased arterial plasma osmolarity and [protein] that occurs with high-intensity exercise. The increased [protein], resulting from the net flux of fluid into contracting muscle, is the main contributor to the increased [Atot] which is the second most important contributor to the plasma acidosis in arterial plasma (because CO2 has been eliminated at the lungs, the greatly reduced PCO2 has an alkalinizing effect on arterial plasma). In a study of four repeated bouts of high-intensity exercise, there was no difference between arterial and antecubital venous plasma [protein] as blood perfused noncontracting muscles, while there was a significant decrease in the antecubital venous plasma total ion concentration of 18.4 mEq/L at the end of the second exercise bout 2 (204). This suggests a net addition of water to the plasma, matched with a net gain of protein by the vascular compartment, during perfusion of noncontracting tissues. The net effect was a prolonged, albeit minor, contribution by [Atot] to the acidosis during the recovery period.
Integration of mechanisms in acid-base balance and pulmonary CO2 excretion
There is a very intimate relationship between ventilation and acid-base balance, because ventilation responds to changes in acid-base balance, and alterations in ventilation also alter acid-base balance. Accordingly, the reactions and responses of the CO2 system are of fundamental importance in the control of ventilation because two of the variables, PCO2 and [H+], directly impact the intracellular [H+] that is sensed by metaboreceptors, and peripheral and central chemoreceptors. In addition to a close, regulatory coupling between arterial plasma/CSF [H+] and control of ventilation (1, 52), there is also significant input from the periphery in ventilatory control (232). Also, because CO2 responses are not confined to a compartment, but are systemic, an acid-base perturbation in any system will affect peripheral and arterial [H+] and thus have effects on the control of ventilation.
To exemplify this interaction, the , , and acid-base data collected in response to high-intensity leg cycling exercise (162, 163) will continue to be used as depicted in Figures 26–28. The ventilatory data show very high and at the end of the 30 s period of exercise and first 5 min of recovery (Fig. 28), at a time when arterial [H+] and PCO2 are low (Fig. 26 and 27) and not sufficiently elevated to stimulate an increase in ventilation. The increased , then, must occur in response to other signals, signals that likely arise within contracting muscle and stimulate CIII and CIV sympathetic nerve activity within muscle (232). Evidence to support this lies in the greatly elevated femoral venous plasma PCO2 and [H+] (Figs. 26, 27), reflecting concentrations within contracting muscle, when and are maximal. Also, the time course of decline in and closely follow the time course of decline in femoral venous [H+] and PCO2. Together, these data demonstrate a close coupling of the ventilatory response to acid-base changes within working muscle during the first 3.5 min of recovery, and not to the acid-base state of the arterial plasma/CSF compartments. After 3.5 min of recovery, however, there is minimal further decrease in femoral venous [H+] and PCO2, yet and continue to fall, perhaps reflecting an increasing contribution from low arterial plasma/CSF PCO2 to suppress the ventilatory stimuli occurring in the periphery (recovering skeletal muscle). At 9.5 min of recovery, ventilatory variables are still elevated above pre-exercise (Fig. 28), consistent with elevations in arterial and venous plasma [H+] (Fig. 26), and despite low or normal arterial and plasma PCO2 (Fig. 27).
Figure 28.
Time course of and in humans that performed 30 s of very high intensity leg bicycling exercise. Data, with permission, from Kowalchuk et al. (162, 163).
When the effect of ventilation on acid-base state is considered, then, already at 30 s of recovery, alveolar ventilation was fully responsible for decreasing arterial plasma PCO2 by 71 mmHg and [H+] by 46 neq/L (compare Fig. 22 with Fig. 27). It is also noteworthy that plasma [HCO3−] only loosely follows the time course of change in plasma PCO2 (Fig. 27). The end-exercise femoral venous [HCO3−] increased by only 2 mEq/L despite a nearly 60 mmHg increase in PCO2 and 30 nEq/L increase in [H+]. This demonstrates that femoral venous plasma [HCO3−] is more sensitive to nonrespiratory factors that contribute to the acidosis (decreased [SID] and increased [Atot]) than to increased PCO2. The subsequent sustained decrease in arterial and femoral venous [HCO3−] reflects the loss of CO2 stores due to the high pulmonary excretion of CO2.
Acknowledgements
This work was funded by M. K. Stickland: Canadian Institutes of Health Research Operating & New Investigator Awards, Natural Science and Engineering Research Council of Canada, Heart and Stroke Foundation of Canada New Investigator Salary Award, M. I. Lindinger: Natural Science and Engineering Reseach Council of Canada, I. M. Olfert: AHA 10BGIA3630002, and S. R. Hopkins: NIH R01-HL081171.
References
- 1.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Aitken ML, Franklin JL, Pierson DJ, Schoene RB. Influence of body size and gender on control of ventilation. J Appl Physiol 60: 1894–1899, 1986. [DOI] [PubMed] [Google Scholar]
- 3.AmericanThoracicSociety. Lung function testing: Selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis 144: 1202–1218, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Anderson WF, Anderson AE Jr, Hernandez JA, Foraker AG. Topography of aging and emphysematous lungs. Am Rev Respir Dis 90: 411–423, 1964. [DOI] [PubMed] [Google Scholar]
- 5.Anthonisen NR, Danson J, Robertson PC, Ross WR. Airway closure as a function of age. Respir Physiol 8: 58–65, 1969. [DOI] [PubMed] [Google Scholar]
- 6.Anthonisen NR, Fleetman JA. Ventilation: Total, alveolar, and dead space. In: Fishman AP, Farhi LE, Tenney SM, Geiger SR, editors. Handbook of Physiology: The Respiratory System, Gas Exchange, 1987, pp. 113–129, Williams and Wilkins, Baltimore, MA. [Google Scholar]
- 7.Ashwood ER, Kost G, Kenny M. Temperature correction of blood-gas and pH measurements. Clin Chem 29: 1877–1885, 1983. [PubMed] [Google Scholar]
- 8.Ayappa I, Brown LV, Wang PM, Katzman N, Houtz P, Bruce EN, LaiFook SJ. Effect of blood flow on capillary transit time and oxygenation in excised rabbit lung. Respir Physiol 105: 203–216, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Bachofen H, Hobi HJ, Scherrer M. Alveolar-arterial N 2 gradients at rest and during exercise in healthy men of different ages. J Appl Physiol 34: 137–142, 1973. [DOI] [PubMed] [Google Scholar]
- 10.Bachofen H, Schurch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol 62: 1878–1887, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Bacon CJ, Prior JC, Abboud RT, Oldham AR, McKenzie DC. Changes in pulmonary transfer factor with menstrual cycle phase. Respir Physiol Neurobiol 146: 195–203, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Baker C, Yorkey T. Method and apparatus for estimating physiological parameters using model based adaptive filtering. United States Patent 5853364. USA: Nellcor Puritan Bennett Inc., 1998. [Google Scholar]
- 13.Barazanji KW, Ramanathan M, Johnson RL Jr, Hsia CC. A modified rebreathing technique using an infrared gas analyzer. J Appl Physiol 80: 1258–1262, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Barker SJ, Shah NK. The effects of motion on the performance of pulse oximeters in volunteers. Anesthesiology 86: 101–108, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Bebout DE, Storey D, Roca J, Hogan MC, Poole DC, Gonzales-Camerena R, Ueno O, Habb P, Wagner PD. Effects of altitude acclimatization on pulmonary gas exchange during exercise. J Appl Physiol 67: 2286–2295, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Betticher DC, Reinhart WH, Geiser J. Effect of RBC shape and deformability on pulmonary O2 diffusing capacity and resistance to flow in rabbit lungs. J Appl Physiol 78: 778–783, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Bevegard S, Holmgren A, Jonsson B. Circulatory studies in well trained athletes at rest and during heavy exercise, with special reference to stroke volume and the influence of body position. Acta Physiol Scand 57: 26–50, 1963. [DOI] [PubMed] [Google Scholar]
- 18.Bidani A Analysis of abnormalities of capillary CO2 exchange in vivo. J Appl Physiol 70: 1686–1699, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2). J Physiol 588: 2455–2471, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohr C Ueber die lungenatmung. Scand Arch Physiol 2: 236–268, 1891. [Google Scholar]
- 21.Boning D, Maassen N. Point: Lactic acid is the only physicochemical contributor to the acidosis of exercise. J Appl Physiol 105: 358–359, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Borland CD, Dunningham H, Bottrill F, Vuylsteke A, Yilmaz C, Dane DM, Hsia CC. Significant blood resistance to nitric oxide transfer in the lung. J Appl Physiol 108: 1052–1060, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3–related species. Biochim Biophys Acta 1804: 410–421, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouverot P, Farner DS, Heinrich B, Johansen K, Langer H, Neuweiler G, Randall DJ. Adaptation to Altitude-Hypoxia in Vertebrates. Berlin: Springer-Verlag, 1985. [Google Scholar]
- 25.Brody JS, Thurlbeck WM. Development, growth and aging of the lung. In: Geiger SR, Fishman AP, Macklem PT, Mead J, editors. Handbook of Physiology: The Respiratory System, : American Physiological Society, Williams and Wilkins, Baltimore, MA, 1986. [Google Scholar]
- 26.Buist AS, Ghezzo H, Anthonisen NR, Cherniack RM, Ducic S, Macklem PT, Manfreda J, Martin RR, McCarthy D, Ross BB. Relationship between the single-breath N test and age, sex, and smoking habit in three North American cities. Am Rev Respir Dis 120: 305–318, 1979. [DOI] [PubMed] [Google Scholar]
- 27.Buist AS, Ross BB. Predicted values for closing volumes using a modified single breath nitrogen test. Am Rev Respir Dis 107: 744–752, 1973. [DOI] [PubMed] [Google Scholar]
- 28.Buist AS, Ross BB. Quantitative analysis of the alveolar plateau in the diagnosis of early airway obstruction. Am Rev Respir Dis 108: 1078–1087, 1973. [DOI] [PubMed] [Google Scholar]
- 29.Burnham KJ, Arai TJ, Dubowitz DJ, Henderson AC, Holverda S, Buxton RB, Prisk GK, Hopkins SR. Pulmonary perfusion heterogeneity is increased by sustained, heavy exercise in humans. J Appl Physiol 107: 1559–1568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burrows B, Lebowitz MD, Camilli AE, Knudson RJ. Longitudinal changes in forced expiratory volume in one second in adults. Methodologic considerations and findings in healthy nonsmokers. Am Rev Respir Dis 133: 974–980, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Caillaud C, Serre-Cousine O, Anselme F, Capdevilla X, Prefaut C. Computerized tomography and pulmonary diffusing capacity in highly trained athletes after performing a triathlon. J Appl Physiol 79: 1226–1232, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Cairns SP, Lindinger MI. Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol 586: 4039–4054, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capen R, Latham L, Wagner WJ. Diffusing capacity of the lung during hypoxia: Role of capillary recruitment. J Appl Physiol 50: 165–171, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Capen R, Wagner WJ. Intrapulmonary blood flow redistribution during hypoxia increases gas exchange surface area. J Appl Physiol 52: 1575–1580, 1982. [DOI] [PubMed] [Google Scholar]
- 35.Cardus J, Burgos F, Diaz O, Roca J, Barbera JA, Marrades RM, Rodriguez-Roisin R, Wagner PD. Increase in pulmonary ventilation-perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med 156: 648–653, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Cartee GD. Aging skeletal muscle: Response to exercise. Exerc Sport Sci Rev 22: 91–120, 1994. [PubMed] [Google Scholar]
- 37.Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: Comparing the multiple- and single-inspired oxygen tension methods. J Appl Physiol 109: 643–653, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerveri I, Zoia MC, Fanfulla F, Spagnolatti L, Berrayah L, Grassi M, Tinelli C. Reference values of arterial oxygen tension in the middleaged and elderly. Am J Respir Crit Care Med 152: 934–941, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Conhaim RL, Staub NC. Reflection spectrophotometric measurement of O2 uptake in pulmonary arterioles of cats. J Appl Physiol 48: 848–856, 1980. [DOI] [PubMed] [Google Scholar]
- 40.Cook CD, Mead J, Orzalesi MM. Static volume-pressure characteristics of the respiratory system during maximal efforts. J Appl Physiol 19: 1016–1022, 1964. [DOI] [PubMed] [Google Scholar]
- 41.Crandall ED, O’Brasky JE. Direct evidence of participation of rat lung carbonic anhydrase in CO2 reactions. J Clin Invest 62: 618–622, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir 18: 419–425, 1982. [PubMed] [Google Scholar]
- 43.Crapo RO, Morris AH, Gardner RM. Reference values for pulmonary tissue volume, membrane diffusing capacity, and pulmonary capillary blood volume. Bull Eur Physiopathol Respir 18: 893–899, 1982. [PubMed] [Google Scholar]
- 44.Cremona G, Asnaghi R, Baderna P, Brunetto A, Brutsaert T, Cavallaro C, Clark TM, Cogo A, Donis R, Lanfranchi P, Luks A, Novello N, Panzetta S, Perini L, Putnam M, Spagnolatti L, Wagner H, Wagner PD. Pulmonary extravascular fluid accumulation in recreational climbers: A prospective study. Lancet 359: 303–309, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 355: 161–175, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dempsey JA, Reddan WG, Birnbaum ML, Forster HV, Thoden JS, Grover RF, Rankin J. Effects of acute through life-long hypoxic exposure on exercise pulmonary gas exchange. Respir Physiol 13: 62–89, 1971. [DOI] [PubMed] [Google Scholar]
- 47.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol 87: 1997–2006, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Doershuk CF, Fisher BJ, Matthews LW. Specific airway resistance from the perinatal period into adulthood. Alterations in childhood pulmonary disease. Am Rev Respir Dis 109: 452–457, 1974. [DOI] [PubMed] [Google Scholar]
- 49.Donevan RE, Palmer WH, Varvis CJ, Bates DV. Influence of age on pulmonary diffusing capacity. J Appl Physiol 14: 483–492, 1959. [Google Scholar]
- 50.Dubois AB, Britt AG, Fenn WO. Alveolar CO2 during the respiratory cycle. J Appl Physiol 4: 535–548, 1952. [DOI] [PubMed] [Google Scholar]
- 51.Dubuisson M Studies on the chemical processes which occur in muscle before, during and after contraction. J Physiol 94: 461–482, 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffin J Role of acid-base balance in the chemoreflex control of breathing. J Appl Physiol 99: 2255–2265, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Edelman NH, Mittman C, Norris AH, Shock NW. Effects of respiratory pattern on age differences in ventilation uniformity. J Appl Physiol 24: 49–53, 1968. [DOI] [PubMed] [Google Scholar]
- 54.Edsall J, Wyman H. Biophysical Chemistry. Academic Press Inc. New York, NY: 1958, p. 699. [Google Scholar]
- 55.Edwards MR, Hunte GS, Belzberg AS, Sheel AW, Worsley DF, McKenzie DC. Alveolar epithelial integrity in athletes with exercise-induced hypoxemia. J Appl Physiol 89: 1537–1542, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Ehrsam RE, Perruchoud A, Oberholzer M, Burkart F, Herzog H. Influence of age on pulmonary haemodynamics at rest and during supine exercise. Clin Sci (Lond) 65: 653–660, 1983. [DOI] [PubMed] [Google Scholar]
- 57.Ekelund LG, Holmgren A Central hemodynamics during exercise. Circ Res 20: I33–I43, 1967. [Google Scholar]
- 58.Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol 97: 797–805, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Elliott JE, Choi Y, Laurie SS, Yang X, Gladstone IM, Lovering AT. Effect of initial gas bubble composition on detection of inducible intrapulmonary arteriovenous shunt during exercise in normoxia, hypoxia, or hyperoxia. J Appl Physiol 110: 35–45, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Enghoff H Volumen inefficax. Bemerkungen zur frage des schadlichen raumes. Uppsala Lakarefoeren Fohr 44: 191–218, 1938. [Google Scholar]
- 61.England SJ, Farhi LE. Fluctuations in alveolar CO2 and in base excess during the menstrual cycle. Respir Physiol 26: 157–161, 1976. [DOI] [PubMed] [Google Scholar]
- 62.Esbaugh AJ, Tufts BL. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir Physiol Neurobiol 154: 185–198, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Evans JW, Wagner PD. Limits on VA/Q distributions from analysis of experimental inert gas elimination. J Appl Physiol 42: 889–898, 1977. [DOI] [PubMed] [Google Scholar]
- 64.Evans JW, Wagner PD, West JB. Conditions for reduction of pulmonary gas transfer by ventilation-perfusion inequality. J Appl Physiol 36: 533–537, 1974. [DOI] [PubMed] [Google Scholar]
- 65.Farha S, Asosingh K, Laskowski D, Hammel J, Dweik RA, Wiedemann HP, Erzurum SC. Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med 180: 304–310, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farha S, Asosingh K, Laskowski D, Licina L, Sekiguchi H, Losordo DW, Dweik RA, Wiedemann HP, Erzurum SC. Pulmonary gas transfer related to markers of angiogenesis during the menstrual cycle. J Appl Physiol 103: 1789–1795, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farhi LE. Ventilation-perfusion relationship and its role in alveolar gas exchange. In: Caro CG, editor. Advances in Respiratory Physiology, E. Arnold, London, 1966. [Google Scholar]
- 68.Federspiel WJ. Pulmonary diffusing capacity: Implications of two-phase blood flow in capillaries. Respir Physiol 77: 119–134, 1989. [DOI] [PubMed] [Google Scholar]
- 69.Figge J, Mydosh T, Fencl V. Serum proteins and acid-base equilibria: A follow-up. J Lab Clin Med 120: 713–719, 1992. [PubMed] [Google Scholar]
- 70.Fisher JT, Cerny FJ. Characteristics of adjustment of lung diffusing capacity to work. J Appl Physiol 52: 1124–1127, 1982. [DOI] [PubMed] [Google Scholar]
- 71.Flamm SD, Taki J, Moore R, Lewis SF, Keech F, Maltais F, Ahmad M, Callahan R, Dragotakes S, Alpert N. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 81: 1550–1559, 1990. [DOI] [PubMed] [Google Scholar]
- 72.Fleming RE, Crouch EC, Ruzicka CA, Sly WS. Pulmonary carbonic anhydrase IV: Developmental regulation and cell-specific expression in the capillary endothelium. Am J Physiol 265: L627–L635, 1993. [DOI] [PubMed] [Google Scholar]
- 73.Fleming RE, Moxley MA, Waheed A, Crouch EC, Sly WS, Longmore WJ. Carbonic anhydrase II expression in rat type II pneumocytes. Am J Respir Cell Mol Biol 10: 499–505, 1994. [DOI] [PubMed] [Google Scholar]
- 74.Frank NR, Mead J, Ferris BG Jr. The mechanical behavior of the lungs in healthy elderly persons. J Clin Invest 36: 1680–1687, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabel RA. Algorithms for calculating and correcting blood-gas and acid-base variables. Respir Physiol 42: 211–232, 1980. [DOI] [PubMed] [Google Scholar]
- 76.Gale GE, Torre-Bueno JR, Moon RE, Saltzman HA, Wagner PD. Ventilation-perfusion inequality in normal humans during exercise at sea level and simulated altitude. J Appl Physiol 58: 978–988, 1985. [DOI] [PubMed] [Google Scholar]
- 77.Gaultier C, Perret L, Boule M, Buvry A, Girard F. Occlusion pressure and breathing pattern in healthy children. Respir Physiol 46: 71–80, 1981. [DOI] [PubMed] [Google Scholar]
- 78.Gavin TP, Stager JM. The effect of exercise modality on exercise-induced hypoxemia. Respir Physiol 115: 317–323, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80: 681–715, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Gehr P, Bachofen M, Weibel ER. The normal human lung: Ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol 32: 121–140, 1978. [DOI] [PubMed] [Google Scholar]
- 81.Genovesi MG, Tierney DF, Taplin GV, Eisenberg H. An intravenous radionuclide method to evaluate hypoxemia caused by abnormal alveolar vessels. Limitation of conventional techniques. Am Rev Respir Dis 114: 59–65, 1976. [DOI] [PubMed] [Google Scholar]
- 82.Gil J, Bachofen H, Gehr P, Weibel ER. Alveolar volume-surface area relation in air- and saline-filled lungs fixed by vascular perfusion. J Appl Physiol 47: 990–1001, 1979. [DOI] [PubMed] [Google Scholar]
- 83.Glenny RW, Robertson HT. Spatial Distribution of Ventilation and Perfusion: Mechanisms and Regulation. Compr Physiol 1:373–395, 2011. [DOI] [PubMed] [Google Scholar]
- 84.Godfrey S, Kamburoff PL, Nairn JR. Spirometry, lung volumes and airway resistance in normal children aged 5 to 18 years. Br J Dis Chest 64: 15–24, 1970. [DOI] [PubMed] [Google Scholar]
- 85.Gozna ER, Marble AE, Shaw A, Holland JG. Age-related changes in the mechanics of the aorta and pulmonary artery of man. J Appl Physiol 36: 407–411, 1974. [DOI] [PubMed] [Google Scholar]
- 86.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. J Appl Physiol 37: 67–74, 1974. [DOI] [PubMed] [Google Scholar]
- 87.Guenard H, Marthan R. Pulmonary gas exchange in elderly subjects. Eur Respir J 9: 2573–2577, 1996. [DOI] [PubMed] [Google Scholar]
- 88.Guenette JA, Diep TT, Koehle MS, Foster GE, Richards JC, Sheel AW. Acute hypoxic ventilatory response and exercise-induced arterial hypoxemia in men and women. Respir Physiol Neurobiol 143: 37–48, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Guenette JA, Martens AM, Lee AL, Tyler GD, Richards JC, Foster GE, Warburton DE, Sheel AW. Variable effects of respiratory muscle training on cycle exercise performance in men and women. Appl Physiol Nutr Metab 31: 159–166, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Guenette JA, Sporer BC, Macnutt MJ, Coxson HO, Sheel AW, Mayo JR, McKenzie DC. Lung density is not altered following intense normobaric hypoxic interval training in competitive female cyclists. J Appl Physiol 103: 875–882, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581: 1309–1322, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Habedank D, Reindl I, Vietzke G, Bauer U, Sperfeld A, Glaser S, Wernecke KD, Kleber FX. Ventilatory efficiency and exercise tolerance in 101 healthy volunteers. Eur J Appl Physiol Occup Physiol 77: 421–426, 1998. [DOI] [PubMed] [Google Scholar]
- 93.Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during exercise at sea level. J Appl Physiol 60: 1590–1598, 1986. [DOI] [PubMed] [Google Scholar]
- 94.Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during normobaric hypoxic exercise. J Appl Physiol 61: 1749–1757, 1986. [DOI] [PubMed] [Google Scholar]
- 95.Hammond MD, Hempleman SC. Oxygen diffusing capacity estimates derived from measured VA/Q distributions in man. Respir Physiol 69: 129–147, 1987. [DOI] [PubMed] [Google Scholar]
- 96.Hanel B Pulmonary function after exercise with special emphasis on diffusion capacity. Dan Med Bull 47: 196–217, 2000. [PubMed] [Google Scholar]
- 97.Hanel B, Clifford PS, Secher NH. Restricted postexercise pulmonary diffusion capacity does not impair maximal transport for O2. J Appl Physiol 77: 2408–2412, 1994. [DOI] [PubMed] [Google Scholar]
- 98.Hanel B, Law I, Mortensen J. Maximal rowing has an acute effect on the blood-gas barrier in elite athletes. J Appl Physiol 25: 25, 2003. [DOI] [PubMed] [Google Scholar]
- 99.Hardie JA, Vollmer WM, Buist AS, Ellingsen I, Morkve O. Reference values for arterial blood gases in the elderly. Chest 125: 2053–2060, 2004. [DOI] [PubMed] [Google Scholar]
- 100.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol 507(Pt 2): 619–628, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Effect of exercise-induced arterial O2 desaturation on VO2max in women. Med Sci Sports Exerc 32: 1101–1108, 2000. [DOI] [PubMed] [Google Scholar]
- 102.Harms CA, Rosenkranz S. Sex differences in pulmonary function during exercise. Med Sci Sports Exerc 40: 664–668, 2008. [DOI] [PubMed] [Google Scholar]
- 103.Harms CA and Stager JM. Low chemoresponsiveness and inadequate hyperventilation contribute to exercise-induced hypoxemia. J Appl Physiol 79: 575–580, 1995. [DOI] [PubMed] [Google Scholar]
- 104.Harned H, Owen B. The Physical Chemistry of Electrolytic Solutions. New York: Reinhold, 1958. [Google Scholar]
- 105.Hartridge H, Roughton FJW. The velocity with which carbon monoxide displaces oxygen from combination with hemoglobin Part I. Proc R Soc Lond 94: 336–367, 1923. [Google Scholar]
- 106.Hartridge H, Roughton FJW. The velocity with which carbon monoxide displaces oxygen from combination with hemoglobine Part III. Proc R Soc Lond 107: 654–683, 1925. [Google Scholar]
- 107.Hasleton PS. The internal surface area of the adult human lung. J Anat 112: 391–400, 1972. [PMC free article] [PubMed] [Google Scholar]
- 108.Heisler N Buffering and H+ ion dynamics in muscle tissues. Respir Physiol Neurobiol 144: 161–172, 2004. [DOI] [PubMed] [Google Scholar]
- 109.Henderson L, Boc KA, Field H, Stoddard J. Blood as a physicochemical system. J Biol Chem 379–431, 1924. [Google Scholar]
- 110.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res 58: 281–291, 1986. [DOI] [PubMed] [Google Scholar]
- 111.Hill EP, Power GG, Longo LD. Mathematical simulation of pulmonary O2 and CO2 exchange. Am J Physiol 224: 904–917, 1973. [DOI] [PubMed] [Google Scholar]
- 112.Hlastala MP. Significance of the Bohr and Haldane effects in the pulmonary capillary. Respir Physiol 17: 81–92, 1973. [DOI] [PubMed] [Google Scholar]
- 113.Hlastala MP. Ventilation/perfusion: From the bench to the patient. Cardiologia 41: 405–415, 1996. [PubMed] [Google Scholar]
- 114.Hlastala MP, Robertson HT. Inert gas elimination characteristics of the normal and abnormal lung. J Appl Physiol 44: 258–266, 1978. [DOI] [PubMed] [Google Scholar]
- 115.Hodges AN, Sheel AW, Mayo JR, McKenzie DC. Human lung density is not altered following normoxic and hypoxic moderate-intensity exercise: Implications for transient edema. J Appl Physiol 103: 111–118, 2007. [DOI] [PubMed] [Google Scholar]
- 116.Holland J, Milic-Emili J, Macklem PT, Bates DV. Regional distribution of pulmonary ventilation and perfusion in elderly subjects. J Clin Invest 47: 81–92, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hopkins SR. Exercise induced arterial hypoxemia: The role of ventilation-perfusion inequality and pulmonary diffusion limitation. Adv Exp Med Biol 588: 17–30, 2006. [DOI] [PubMed] [Google Scholar]
- 118.Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S, Wagner PD. Pulmonary gas exchange during exercise in women: Effects of exercise type and work increment. J Appl Physiol 89: 721–730, 2000. [DOI] [PubMed] [Google Scholar]
- 119.Hopkins SR, Bayly WM, Slocombe RF, Wagner H, Wagner PD. Effect of prolonged heavy exercise on pulmonary gas exchange in horses. J Appl Physiol 84: 1723–1730, 1998. [DOI] [PubMed] [Google Scholar]
- 120.Hopkins SR, Belzberg AS, Wiggs BR, McKenzie DC. Pulmonary transit time and diffusion limitation during heavy exercise in athletes. Respir Physiol 103: 67–73, 1996. [DOI] [PubMed] [Google Scholar]
- 121.Hopkins SR, Gavin TP, Siafakas NM, Haseler LJ, Olfert IM, Wagner H, Wagner PD. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J Appl Physiol 85: 1523–1532, 1998. [DOI] [PubMed] [Google Scholar]
- 122.Hopkins SR, Harms CA. Gender and pulmonary gas exchange during exercise. Exerc Sport Sci Rev 32: 50–56, 2004. [DOI] [PubMed] [Google Scholar]
- 123.Hopkins SR, Hicks JW, Cooper TK, Powell FL. Ventilation and pulmonary gas exchange during exercise in the savannah monitor lizard (Varanus exanthematicus). J Exp Biol 198: 1783–1789, 1995. [DOI] [PubMed] [Google Scholar]
- 124.Hopkins SR, McKenzie DC, Schoene RB, Glenny RW, Robertson HT. Pulmonary gas exchange during exercise in athletes. I. Ventilation-perfusion mismatch and diffusion limitation. J Appl Physiol 77: 912–917, 1994. [DOI] [PubMed] [Google Scholar]
- 125.Hopkins SR, Olfert IM, Wagner PD. Last Word on Point:Counterpoint: Exercise-induced intrapulmonary shunting is imaginary vs. real. J Appl Physiol 107: 1002, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hopkins SR, Olfert IM, Wagner PD. Point: Exercise-induced intrapulmonary shunting is imaginary. J Appl Physiol 107: 993–994, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hopkins SR, Schoene RB, Henderson WR, Spragg RG, Martin TR, West JB. Intense exercise impairs the integrity of the pulmonary blood-gas barrier in elite athletes. Am J Respir Crit Care Med 155: 1090–1094, 1997. [DOI] [PubMed] [Google Scholar]
- 128.Hopkins SR, Sheel AW, McKenzie DC. Point: Counterpoint “Pulmonary edema does/does not occur in human athletes performing heavy sea-level exercise.”. J Appl Physiol. [DOI] [PubMed] [Google Scholar]
- 129.Hopkins SR, Wagner PD. No evidence for pulmonary diffusion limitation of carbon dioxide exchange during exercise at sea level and extreme altitude. In: Houston CS, Coates G, editors. International Hypoxia Symposium, Burlington: Vermont, 1997. [Google Scholar]
- 130.Hsia CC. Recruitment of lung diffusing capacity: Update of concept and application. Chest 122: 1774–1783, 2002. [DOI] [PubMed] [Google Scholar]
- 131.Hsia CC, Chuong CJ, Johnson RL Jr. Critique of conceptual basis of diffusing capacity estimates: A finite element analysis. J Appl Physiol 79: 1039–1047, 1995. [DOI] [PubMed] [Google Scholar]
- 132.Hsia CC, Chuong CJ, Johnson RL Jr. Red cell distortion and conceptual basis of diffusing capacity estimates: Finite element analysis. J Appl Physiol 83: 1397–1404, 1997. [DOI] [PubMed] [Google Scholar]
- 133.Hsia CC, McBrayer DG, Ramanathan M. Reference values of pulmonary diffusing capacity during exercise by a rebreathing technique. Am J Respir Crit Care Med 152: 658–665, 1995. [DOI] [PubMed] [Google Scholar]
- 134.Hsia CC, Wagner PD, Dane DM, Wagner HE, Johnson RL, Jr. Predicting diffusive alveolar oxygen transfer from carbon monoxide-diffusing capacity in exercising foxhounds. J Appl Physiol 105: 1441–1447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang YC, Helms MJ, MacIntyre NR. Normal values for single exhalation diffusing capacity and pulmonary capillary blood flow in sitting, supine positions, and during mild exercise. Chest 105: 501–508, 1994. [DOI] [PubMed] [Google Scholar]
- 136.Huang YC, O’Brien SR, MacIntyre NR. Intrabreath diffusing capacity of the lung in healthy individuals at rest and during exercise. Chest 122: 177–185, 2002. [DOI] [PubMed] [Google Scholar]
- 137.Hultgren HN. Pulmonary hypertension and pulmonary edema. In: Loeppky JA, Riedesel ML, eds. Oxygen Transport to Human Tissue. New York, Elsevier/North Holland, 243–254, 1982. [Google Scholar]
- 138.Hultman E, Sahlin K. Acid-base balance during exercise. Exerc Sport Sci Rev 8: 41–128, 1980. [PubMed] [Google Scholar]
- 139.Jaliwala SA, Mates RE, Klocke FJ. An efficient optimization technique for recovering ventilation-perfusion distributions from inert gas data. Effects of random experimental error. J Clin Invest 55: 188–192, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jameson AG. Diffusion of gases from alveolus to precapillary arteries. Science 139: 826–828, 1963. [DOI] [PubMed] [Google Scholar]
- 141.Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand 182: 215–227, 2004. [DOI] [PubMed] [Google Scholar]
- 142.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol 460: 385–405, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnson BD, Badr MS, Dempsey JA. Impact of the aging pulmonary system on the response to exercise. Clin Chest Med 15: 229–246, 1994. [PubMed] [Google Scholar]
- 144.Johnson BD, Dempsey JA Demand vs. capacity in the aging pulmonary system. Exerc Sport Sci Rev 19: 171–210, 1991. [PubMed] [Google Scholar]
- 145.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 73: 874–886, 1992. [DOI] [PubMed] [Google Scholar]
- 146.Johnson RL Jr, Heigenhauser GF, Hsia CCW, Jones NL, Wagner PD. Determinants of gas exchange and acid-base balance during exercise. In: Rowell L, Shepard J, editors. Handbood of Physiology: Exercise: Regulation and Integration of Multiple Systems, New York: Oxford University Press, 1996, sect. 12, p. 515–584. [Google Scholar]
- 147.Johnson RL Jr, Hsia CC. Functional recruitment of pulmonary capillaries. J Appl Physiol 76: 1405–1407, 1994. [DOI] [PubMed] [Google Scholar]
- 148.Johnson RL Jr, Spicer WS, Bishop JM, Forster RE. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol 15: 893–902, 1960. [DOI] [PubMed] [Google Scholar]
- 149.Jones NL, Heigenhauser GJ. Getting rid of carbon dioxide during exercise. Clin Sci (Lond) 90: 323–335, 1996. [DOI] [PubMed] [Google Scholar]
- 150.Jones NL, McHardy GJ, Naimark A, Campbell EJ. Physiological dead space and alveolar-arterial gas pressure differences during exercise. Clin Sci 31: 19–29, 1966. [PubMed] [Google Scholar]
- 151.Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol 47: 954–960, 1979. [DOI] [PubMed] [Google Scholar]
- 152.Jones RL, Overton TR, Hammerlindl DM, Srpoule BJ. Effects of age on regional residual volume. J Appl Physiol 44: 195–199, 1978. [DOI] [PubMed] [Google Scholar]
- 153.Jonk AM, van den Berg IP, Olfert IM, Wray DW, Arai T, Hopkins SR, Wagner PD. Effect of acetazolamide on pulmonary and muscle gas exchange during normoxic and hypoxic exercise. J Physiol 579: 909–921, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kanber GJ, King FW, Eshchar YR, Sharp JT. The alveolar-arterial oxygen gradient in young and elderly men during air and oxygen breathing. Am Rev Respir Dis 97: 376–381, 1968. [DOI] [PubMed] [Google Scholar]
- 155.Kellogg RH. Laws of physics pertaining to gas exchange. In: Fishman AP, Farhi LE, Tenney SM, Geiger SR, editors. Handbook of Physiology: The Respiratory System, Gas Exchange, 1987, pp. 13–30. [Google Scholar]
- 156.Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol 21: 1375–1376, 1966. [DOI] [PubMed] [Google Scholar]
- 157.Kelman GR, Nunn JF. Nomograms for correction of blood Po2, Pco2, pH, and base excess for time and temperature. J Appl Physiol 21: 1484–1490, 1966. [DOI] [PubMed] [Google Scholar]
- 158.Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol 32: 516–522, 1972. [DOI] [PubMed] [Google Scholar]
- 159.Klocke RA. Catalysis of CO2 reactions by lung carbonic anhydrase. J Appl Physiol 44: 882–888, 1978. [DOI] [PubMed] [Google Scholar]
- 160.Knudson RJ, Clark DF, Kennedy TC, Knudson DE. Effect of aging alone on mechanical properties of the normal adult human lung. J Appl Physiol 43: 1054–1062, 1977. [DOI] [PubMed] [Google Scholar]
- 161.Knudson RJ, Lebowitz MD, Burton AP, Knudson DE. The closing volume test: Evaluation of nitrogen and bolus methods in a random population. Am Rev Respir Dis 115: 423–434, 1977. [DOI] [PubMed] [Google Scholar]
- 162.Kowalchuk JM, Heigenhauser GJ, Lindinger MI, Obminski G, Sutton JR, Jones NL. Role of lungs and inactive muscle in acid-base control after maximal exercise. J Appl Physiol 65: 2090–2096, 1988. [DOI] [PubMed] [Google Scholar]
- 163.Kowalchuk JM, Heigenhauser GJ, Lindinger MI, Sutton JR, Jones NL. Factors influencing hydrogen ion concentration in muscle after intense exercise. J Appl Physiol 65: 2080–2089, 1988. [DOI] [PubMed] [Google Scholar]
- 164.Kowalchuk JM, Heigenhauser GJ, Sutton JR, Jones NL. Effect of acetazolamide on gas exchange and acid-base control after maximal exercise. J Appl Physiol 72: 278–287, 1992. [DOI] [PubMed] [Google Scholar]
- 165.Kowalchuk JM, Heigenhauser GJ, Sutton JR, Jones NL. Effect of chronic acetazolamide administration on gas exchange and acid-base control after maximal exercise. J Appl Physiol 76: 1211–1219, 1994. [DOI] [PubMed] [Google Scholar]
- 166.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005. [DOI] [PubMed] [Google Scholar]
- 167.La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, Heidbuchel H, Prior DL. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol 109: 1307–1317, 2010. [DOI] [PubMed] [Google Scholar]
- 168.Laroche DP, Knight CA, Dickie JL, Lussier M, Roy SJ. Explosive force and fractionated reaction time in elderly low- and high-active women. Med Sci Sports Exerc 39: 1659–1665, 2007. [DOI] [PubMed] [Google Scholar]
- 169.Leblanc P, Ruff F, Milic-Emili J. Effects of age and body position on “airway closure” in man. J Appl Physiol 28: 448–451, 1970. [DOI] [PubMed] [Google Scholar]
- 170.Leeder SR, Swan AV, Peat JK, Woolcook AJ, Blackburn CR. Maximum expiratory flow-volume curves in children: Changes with growth and individual variability. Bull Eur Physiopathol Respir 13: 249–260, 1977. [PubMed] [Google Scholar]
- 171.Leeuwe AM. Net cation equivalency (‘base binding power’) of the plasma proteins. Acta Med Scand 176(Suppl 422): 421+, 1964. [PubMed] [Google Scholar]
- 172.Leith DE, Mead J. Mechanisms determining residual volume of the lungs in normal subjects. J Appl Physiol 23: 221–227, 1967. [DOI] [PubMed] [Google Scholar]
- 173.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. [DOI] [PubMed] [Google Scholar]
- 174.Lindinge MI. Origins of [H+] changes in exercising skeletal muscle. Can J Appl Physiol 20: 357–368, 1995. [DOI] [PubMed] [Google Scholar]
- 175.Lindinger MI, Franklin TW, Lands LC, Pedersen PK, Welsh DG, Heigenhauser GJ. Role of skeletal muscle in plasma ion and acid-base regulation after NaHCO3 and KHCO3 loading in humans. Am J Physiol 276: R32–R43, 1999. [DOI] [PubMed] [Google Scholar]
- 176.Lindinger MI, Heigenhauser GJ. Acid Base Systems in Skeletal Muscle and Their Response to Exercise. Champaign, IL: Human Kinetics, 1990. [Google Scholar]
- 177.Lindinger MI, Heigenhauser GJ. The roles of ion fluxes in skeletal muscle fatigue. Can J Physiol Pharmacol 69: 246–253, 1991. [DOI] [PubMed] [Google Scholar]
- 178.Lindinger MI, Heigenhauser GJ. Counterpoint: Lactic acid is not the only physicochemical contributor to the acidosis of exercise. J Appl Physiol 105: 359–361; discussion 361–352, 2008. [DOI] [PubMed] [Google Scholar]
- 179.Lindinger MI, Heigenhauser GJ, McKelvie RS, Jones NL. Role of non-working muscle on blood metabolites and ions with intense intermittent exercise. Am J Physiol 258: R1486–R1494, 1990. [DOI] [PubMed] [Google Scholar]
- 180.Lindinger MI, Heigenhauser GJ, McKelvie RS, Jones NL. Blood ion regulation during repeated maximal exercise and recovery in humans. Am J Physiol 262: R126–R136, 1992. [DOI] [PubMed] [Google Scholar]
- 181.Lindinger MI, Heigenhauser GJ, Spriet LL. Effects of alkalosis on muscle ions at rest and with intense exercise. Can J Physiol Pharmacol 68: 820–829, 1990. [DOI] [PubMed] [Google Scholar]
- 182.Lindinger MI, Heigenhauser GJF. Effects of gas exchange on acid-base balance. Comp Physiol 2: 1–52, 2012. [DOI] [PubMed] [Google Scholar]
- 183.Lindinger MI, Kowalchuk JM, Heigenhauser GJ. Applying physicochemical principles to skeletal muscle acid-base status. Am J Physiol Regul Integr Comp Physiol 289: R891–R894; author reply R904-R910, 2005. [DOI] [PubMed] [Google Scholar]
- 184.Lindinger MI, McKelvie RS, Heigenhauser GJ. K+ and Lac-distribution in humans during and after high-intensity exercise: Role in muscle fatigue attenuation? J Appl Physiol 78: 765–777, 1995. [DOI] [PubMed] [Google Scholar]
- 185.Lindinger MI, Spriet LL, Hultman E, Putman T, McKelvie RS, Lands LC, Jones NL, Heigenhauser GJ. Plasma volume and ion regulation during exercise after low- and high-carbohydrate diets. Am J Physiol 266: R1896–R1906, 1994. [DOI] [PubMed] [Google Scholar]
- 186.Lipmann F, and Meyerhof O. Uber die enzymaticshe unwaldung van phosphoglycerinsaure in brentzraubensaure und phosphosaure. Biochem 60–72, 1934. [Google Scholar]
- 187.Loeppky JA, Scotto P, Charlton GC, Gates L, Icenogle M, Roach RC. Ventilation is greater in women than men, but the increase during acute altitude hypoxia is the same. Respir Physiol 125: 225–237, 2001. [DOI] [PubMed] [Google Scholar]
- 188.Lovering AT, Eldridge MW, Stickland MK. Counterpoint: Exercise-induced intrapulmonary shunting is real. J Appl Physiol 107: 994–997, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lovering AT, Eldridge MW, Stickland MK. Last word on point: Counterpoint: Exercise-induced intrapulmonary shunting is imaginary vs. real. J Appl Physiol 107: 1003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lovering AT, Haverkamp HC, Romer LM, Hokanson JS, Eldridge MW. Transpulmonary passage of 99mTc macroaggregated albumin in healthy humans at rest and during maximal exercise. J Appl Physiol 106: 1986–1992, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lovering AT, Stickland MK, Amann M, Murphy JC, O’Brien MJ, Hokanson JS, Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol 586: 4559–4565, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lovering AT, Stickland MK, Eldridge MW. Intrapulmonary shunt during normoxic and hypoxic exercise in healthy humans. Adv Exp Med Biol 588: 31–45, 2006. [DOI] [PubMed] [Google Scholar]
- 193.Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25- and 50-{micro}m arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol 292: H1777–H1781, 2007. [DOI] [PubMed] [Google Scholar]
- 194.Mackay EH, Banks J, Sykes B, Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax 33: 335–344, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.MacNutt MJ, Guenette JA, Witt JD, Yuan R, Mayo JR, McKenzie DC. Intense hypoxic cycle exercise does not alter lung density in competitive male cyclists. Eur J Appl Physiol 99: 623–631, 2007. [DOI] [PubMed] [Google Scholar]
- 196.Malmberg P, Hedenstrom H, Fridriksson HV. Reference values for gas exchange during exercise in healthy nonsmoking and smoking men. Bull Eur Physiopathol Respir 23: 131–138, 1987. [PubMed] [Google Scholar]
- 197.Manier G, Moinard J, Techoueyres P, Varene N, Guenard H. Pulmonary diffusion limitation after prolonged strenuous exercise. Respir Physiol 83: 143–153, 1991. [DOI] [PubMed] [Google Scholar]
- 198.Mannion AF, Jakeman PM, Dunnett M, Harris RC, Willan PL. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur J Appl Physiol Occup Physiol 64: 47–50, 1992. [DOI] [PubMed] [Google Scholar]
- 199.Mannion AF, Jakeman PM, Willan PL. Skeletal muscle buffer value, fibre type distribution and high intensity exercise performance in man. Exp Physiol 80: 89–101, 1995. [DOI] [PubMed] [Google Scholar]
- 200.Mansell A, Bryan C, Levison H. Airway closure in children. J Appl Physiol 33: 711–714, 1972. [DOI] [PubMed] [Google Scholar]
- 201.Marshall BE, Wyche MQ Jr. Hypoxemia during and after anesthesia. Anesthesiology 37: 178–209, 1972. [DOI] [PubMed] [Google Scholar]
- 202.Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol 63: 2042–2047, 1987. [DOI] [PubMed] [Google Scholar]
- 203.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol 84: 1872–1881, 1998. [DOI] [PubMed] [Google Scholar]
- 204.McKelvie RS, Lindinger MI, Jones NL, Heigenhauser GJ. Erythrocyte ion regulation across inactive muscle during leg exercise. Can J Physiol Pharmacol 70: 1625–1633, 1992. [DOI] [PubMed] [Google Scholar]
- 205.McKenzie DC, Lama IL, Potts JE, Sheel AW, Coutts KD. The effect of repeat exercise on pulmonary diffusing capacity and EIH in trained athletes. Med Sci Sports Exerc 31: 99–104, 1999. [DOI] [PubMed] [Google Scholar]
- 206.McKenzie DC, O’Hare T J, Mayo J. The effect of sustained heavy exercise on the development of pulmonary edema in trained male cyclists. Respir Physiol Neurobiol 145: 209–218, 2005. [DOI] [PubMed] [Google Scholar]
- 207.Mead J Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. [DOI] [PubMed] [Google Scholar]
- 208.Meltzer RS, Tickner EG, Popp RL. Why do the lungs clear ultrasonic contrast? Ultrasound Med Biol 6: 263–269, 1980. [DOI] [PubMed] [Google Scholar]
- 209.Miles DS, Doerr CE, Schonfeld SA, Sinks DE, Gotshall RW. Changes in pulmonary diffusing capacity and closing volume after running a marathon. Respir Physiol 52: 349–359, 1983. [DOI] [PubMed] [Google Scholar]
- 210.Milic-Emili J, Leblanc P, Ruff F. Effects of age and body composition on airway closure in man. Br J Anaesth 42: 86–87, 1970. [PubMed] [Google Scholar]
- 211.Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI, Brooks GA. Lactate and glucose interactions during rest and exercise in men: Effect of exogenous lactate infusion. J Physiol 544: 963–975, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Miller BF, Lindinger MI, Fattor JA, Jacobs KA, Leblanc PJ, Duong M, Heigenhauser GJ, Brooks GA. Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J Appl Physiol 98: 856–865, 2005. [DOI] [PubMed] [Google Scholar]
- 213.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 103: 57–67, 1971. [DOI] [PubMed] [Google Scholar]
- 214.Murray JD. Aging: The Normal Lung. Philadelphia, PA: WB Saunders, 1986. [Google Scholar]
- 215.Nelson NM. Neonatal pulmonary function. Pediatr Clin North Am 13: 769–799, 1966. [DOI] [PubMed] [Google Scholar]
- 216.Neufeld O, Smith JR, Goldman SL. Arterial oxygen tension in relation to age in hospital subjects. J Am Geriatr Soc 21: 4–9, 1973. [DOI] [PubMed] [Google Scholar]
- 217.Nielsen HB, Hanel B, Loft S, Poulsen HE, Pedersen BK, Diamant M, Vistisen K, Secher NH. Restricted pulmonary diffusion capacity after exercise is not an ARDS-like injury. J Sports Sci 13: 109–113, 1995. [DOI] [PubMed] [Google Scholar]
- 218.Nunn JF. Applied Respiratory Physiology. London: Butterworths, 1987. [Google Scholar]
- 219.Olfert IM, Balouch J, Kleinsasser A, Knapp A, Wagner H, Wagner PD, Hopkins SR. Does gender affect human pulmonary gas exchange during exercise? J Physiol 557: 529–541, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ondrak KS, McMurray RG. Exercise-induced breathing patterns of youth are related to age and intensity. Eur J Appl Physiol 98: 88–96, 2006. [DOI] [PubMed] [Google Scholar]
- 221.Paiva M Gas transport in the human lung. J Appl Physiol 35: 401–410, 1973. [DOI] [PubMed] [Google Scholar]
- 222.Pascoe JR, Ferraro GL, Cannon JH, Arthur RM, Wheat JD. Exercise-induced pulmonary hemorrhage in racing thoroughbreds: A preliminary study. Am J Vet Res 42: 703–707, 1981. [PubMed] [Google Scholar]
- 223.Peronnet F, Aguilaniu B. Lactic acid buffering, nonmetabolic CO2 and exercise hyperventilation: A critical reappraisal. Respir Physiol Neurobiol 150: 4–18, 2006. [DOI] [PubMed] [Google Scholar]
- 224.Piepoli M, Clark AL, Coats AJ. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol 269: H1428–H1436, 1995. [DOI] [PubMed] [Google Scholar]
- 225.Piiper J Apparent increase of the O2 diffusing capacity with increased O2 uptake in inhomogeneous lungs: Theory. Respir Physiol 6: 209–218, 1969. [DOI] [PubMed] [Google Scholar]
- 226.Piiper J, Scheid P. Gas transport efficacy of gills, lungs and skin: Theory and experimental data. Respir Physiol 23: 209–221, 1975. [DOI] [PubMed] [Google Scholar]
- 227.Piiper J, Scheid P. Models for a comparative functional analysis of gas exchange organs in vertebrates. J Appl Physiol 53: 1321–1329, 1982. [DOI] [PubMed] [Google Scholar]
- 228.Piiper J, Scheid P. Comparison of diffusion and perfusion limitations in alveolar gas exchange. Respir Physiol 51: 287–290, 1983. [DOI] [PubMed] [Google Scholar]
- 229.Plopper CG, Thurlbeck WM. Growth, aging and adaptation. In: Murray JF, Nadel JA, editors. Textbook of Respiratory Medicine (2nd ed). Philadelphia: W.B. Saunders Co., 1994, pp. 36–49. [Google Scholar]
- 230.Plummer JL, Zakaria AZ, Ilsley AH, Fronsko RR, Owen H. Evaluation of the influence of movement on saturation readings from pulse oximeters. Anaesthesia 50: 423–426, 1995. [DOI] [PubMed] [Google Scholar]
- 231.Podolsky A, Eldridge MW, Richardson RS, Knight DR, Johnson EC, Hopkins SR, Johnson DH, Michimata H, Grassi B, Feiner J, Kurdak SS, Bickler PE, Severinghaus JW, Wagner PD. Exercise-induced VA/Q inequality in subjects with prior high-altitude pulmonary edema. J Appl Physiol 81: 922–932, 1996. [DOI] [PubMed] [Google Scholar]
- 232.Poon CS, Tin C, Yu Y. Homeostasis of exercise hyperpnea and optimal sensorimotor integration: The internal model paradigm. Respir Physiol Neurobiol 159: 1–13; discussion 14–20, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Powers SK, Dodd S, Freeman J, Ayers GD, Samson H, McKnight T. Accuracy of pulse oximetry to estimate HbO2 fraction of total Hb during exercise. J Appl Physiol 67: 300–304, 1989. [DOI] [PubMed] [Google Scholar]
- 234.Powers SK, Lawler J, Dempsey JA, Dodd S, Landry G. Effects of incomplete pulmonary gas exchange on VO2 max. J Appl Physiol 66: 2491–2495, 1989. [DOI] [PubMed] [Google Scholar]
- 235.Powers SK, Martin D, Cicale M, Collop N, Huang D, Criswell D. Exercise-induced hypoxemia in athletes: Role of inadequate hyperventilation. Eur J Appl Physiol Occup Physiol 65: 37–42, 1992. [DOI] [PubMed] [Google Scholar]
- 236.Prefaut C, Anselme F, Caillaud C, Masse-Biron J. Exercise-induced hypoxemia in older athletes. J Appl Physiol 76: 120–126, 1994. [DOI] [PubMed] [Google Scholar]
- 237.Preston ME, Jensen D, Janssen I, Fisher JT. Effect of menopause on the chemical control of breathing and its relationship with acid-base status. Am J Physiol Regul Integr Comp Physiol 296: R722–R727, 2009. [DOI] [PubMed] [Google Scholar]
- 238.Prinzmetal M, Ornitz ME, Simkin B, Bergman HC. Arterio-venous anastomoses in liver, spleen, and lungs. Am J Physiol 152: 48–52, 1948. [DOI] [PubMed] [Google Scholar]
- 239.Pump KK. Emphysema and its relation to age. Am Rev Respir Dis 114: 5–13, 1976. [DOI] [PubMed] [Google Scholar]
- 240.Purkerson JM, Schwartz GJ. Expression of membrane-associated carbonic anhydrase isoforms IV, IX, XII, and XIV in the rabbit: Induction of CA IV and IX during maturation. Am J Physiol Regul Integr Comp Physiol 288: R1256–R1263, 2005. [DOI] [PubMed] [Google Scholar]
- 241.Rahn H A concept of mean alveolar air and the ventilation-blood flow relationships during pulmonary gas exchange. Am J Physiol 158: 21–30, 1949. [DOI] [PubMed] [Google Scholar]
- 242.Rahn H, Fenn WO. A Graphical Analysis of the Respiratory Exchange: The O2-CO2 Diagram. Washington, DC: Am Physiol Soc, 1955. [Google Scholar]
- 243.Raine JM, Bishop JM. A-a difference in O2 tension and physiological dead space in normal man. J Appl Physiol 18: 284–288, 1963. [DOI] [PubMed] [Google Scholar]
- 244.Ramage JE Jr, Coleman RE, MacIntyre NR. Rest and exercise cardiac output and diffusing capacity assessed by a single slow exhalation of methane, acetylene, and carbon monoxide. Chest 92: 44–50, 1987. [DOI] [PubMed] [Google Scholar]
- 245.Reeves JT, Groves BM, Cymerman A, Sutton JR, Wagner PD, Turkevich D, Houston CS. Operation Everest II: Cardiac filling pressures during cycle exercise at sea level. Respir Physiol 80: 147–154, 1990. [DOI] [PubMed] [Google Scholar]
- 246.Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: Preservation of cardiac function at extreme altitude. J Appl Physiol 63: 531–539, 1987. [DOI] [PubMed] [Google Scholar]
- 247.Reeves JT, Taylor AE. Pulmonary hemodynamics and fluid exchange in lungs during exercise. In: Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press, 1996, p. 585–613. [Google Scholar]
- 248.Reeves RB. An imidazole alphastat hypothesis for vertebrate acid-base regulation: Tissue carbon dioxide content and body temperature in bullfrogs. Respir Physiol 14: 219–236, 1972. [DOI] [PubMed] [Google Scholar]
- 249.Reyes A, Roca J, Rodriguez-Roisin R, Torres A, Ussetti P, Wagner PD. Effect of almitrine on ventilation-perfusion distribution in adult respiratory distress syndrome. Am Rev Respir Dis 137: 1062–1067, 1988. [DOI] [PubMed] [Google Scholar]
- 250.Rice AJ, Scroop GC, Gore CJ, Thornton AT, Chapman MA, Greville HW, Holmes MD, Scicchitano R. Exercise-induced hypoxaemia in highly trained cyclists at 40% peak oxygen uptake. Eur J Appl Physiol Occup Physiol 79: 353–359, 1999. [DOI] [PubMed] [Google Scholar]
- 251.Rice AJ, Thornton AT, Gore CJ, Scroop GC, Greville HW, Wagner H, Wagner PD, Hopkins SR. Pulmonary gas exchange during exercise in highly trained cyclists with arterial hypoxemia. J Appl Physiol 87: 1802–1812, 1999. [DOI] [PubMed] [Google Scholar]
- 252.Richalet JP, Gratadour P, Robach P, Pham I, Dechaux M, Joncquiert-Latarjet A, Mollard P, Brugniaux J, Cornolo J. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am J Respir Crit Care Med 171: 275–281, 2005. [DOI] [PubMed] [Google Scholar]
- 253.Richards JC, McKenzie DC, Warburton DE, Road JD, Sheel AW. Prevalence of exercise-induced arterial hypoxemia in healthy women. Med Sci Sports Exerc 36: 1514–1521, 2004. [DOI] [PubMed] [Google Scholar]
- 254.Riley RL. Development of the three-compartment model for dealing with uneven distribution. In: West JB, editor. Pulmonary Gas Exchange, Vol. 1. Ventilation, Blood Flow, and Diffusion. New York: Academic Press, 67–85, 1980. [Google Scholar]
- 255.Riley RL, Cournand A. Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol 1: 825–847, 1949. [DOI] [PubMed] [Google Scholar]
- 256.Riley RL, Cournand A. Analysis of factors affecting partial pressures of oxygen and carbon dioxide in gas and blood of lungs: Theory. J Appl Physiol 4: 77–101, 1951. [DOI] [PubMed] [Google Scholar]
- 257.Rizzato G, Marazzini L. Thoracoabdominal mechanics in elderly men. J Appl Physiol 28: 457–460, 1970. [DOI] [PubMed] [Google Scholar]
- 258.Roberts CM, MacRae KD, Winning AJ, Adams L, Seed WA. Reference values and prediction equations for normal lung function in a nonsmoking white urban population. Thorax 46: 643–650, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Robertson HT, Pellegrino R, Pini D, Oreglia J, DeVita S, Brusasco V, Agostoni P. Exercise response after rapid intravenous infusion of saline in healthy humans. J Appl Physiol 97: 697–703, 2004. [DOI] [PubMed] [Google Scholar]
- 260.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- 261.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol 68: 861–867, 1990. [DOI] [PubMed] [Google Scholar]
- 262.Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67: 256–263, 1989. [DOI] [PubMed] [Google Scholar]
- 263.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 11: 290–302, 1957. [DOI] [PubMed] [Google Scholar]
- 264.Roughton FJ, Forster RE, Cander L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol 11: 269–276, 1957. [DOI] [PubMed] [Google Scholar]
- 265.Sahlin K Intracellular pH and energy metabolism in skeletal muscle of man. With special reference to exercise. Acta Physiol Scand Suppl 455: 1–56, 1978. [PubMed] [Google Scholar]
- 266.Sahlin K, Alvestrand A, Brandt R, Hultman E. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J Appl Physiol 45: 474–480, 1978. [DOI] [PubMed] [Google Scholar]
- 267.Sami HM, Kleinman BS, Lonchyna VA. Central venous pulsations associated with a falsely low oxygen saturation measured by pulse oximetry. J Clin Monit 7: 309–312, 1991. [DOI] [PubMed] [Google Scholar]
- 268.Schaffartzik W, Poole DC, Derion T, Tsukimoto K, Hogan MC, Arcos JP, Bebout DE, Wagner PD. VA/Q distribution during heavy exercise and recovery in humans: Implications for pulmonary edema. J Appl Physiol 72: 1657–1667, 1992. [DOI] [PubMed] [Google Scholar]
- 269.Scheid P, Piiper J. Diffusion. In: Crystal RG, West JB, Barnes PJ, Weibel ER, editors. The Lung: Scientific Foundations (2nd ed). Philadelphia, PA: Lippincott-Raven, 1997, p. 1681–1690. [Google Scholar]
- 270.Schwartz J, Katz SA, Fegley RW, Tockman MS. Sex and race differences in the development of lung function. Am Rev Respir Dis 138: 1415–1421, 1988. [DOI] [PubMed] [Google Scholar]
- 271.Schwartz JD, Katz SA, Fegley RW, Tockman MS. Analysis of spirometric data from a national sample of healthy 6- to 24-year-olds (NHANES II). Am Rev Respir Dis 138: 1405–1414, 1988. [DOI] [PubMed] [Google Scholar]
- 272.Seaman J, Erickson BK, Kubo K, Hiraga A, Kai M, Yamaya Y, Wagner PD. Exercise induced ventilation/perfusion inequality in the horse. Equine Vet J 27: 104–109, 1995. [DOI] [PubMed] [Google Scholar]
- 273.Severinghaus JW. Blood gas calculator. J Appl Physiol 21: 1108–1116, 1966. [DOI] [PubMed] [Google Scholar]
- 274.Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol 46: 599–602, 1979. [DOI] [PubMed] [Google Scholar]
- 275.Sheel AW, Coutts KD, Potts JE, McKenzie DC. The time course of pulmonary diffusing capacity for carbon monoxide following short duration high intensity exercise. Respir Physiol 111: 271–281, 1998. [DOI] [PubMed] [Google Scholar]
- 276.Sheel AW, Guenette JA. Mechanics of breathing during exercise in men and women: Sex versus body size differences? Exerc Sport Sci Rev 36: 128–134, 2008. [DOI] [PubMed] [Google Scholar]
- 277.Sheel AW, Richards JC, Foster GE, Guenette JA. Sex differences in respiratory exercise physiology. Sports Med 34: 567–579, 2004. [DOI] [PubMed] [Google Scholar]
- 278.Shipp NJ, Scroop GC, Jackson SC, Holmes MD, Thornton AT, Gore CJ. Rectal temperature correction overestimates the frequency of exercise-induced hypoxemia. Med Sci Sports Exerc 36: 1111–1116, 2004. [DOI] [PubMed] [Google Scholar]
- 279.Slatkovska L, Jensen D, Davies GA, Wolfe LA. Phasic menstrual cycle effects on the control of breathing in healthy women. Respir Physiol Neurobiol 154: 379–388, 2006. [DOI] [PubMed] [Google Scholar]
- 280.Smekal G, von Duvillard SP, Frigo P, Tegelhofer T, Pokan R, Hofmann P, Tschan H, Baron R, Wonisch M, Renezeder K, Bachl N. Menstrual cycle: No effect on exercise cardiorespiratory variables or blood lactate concentration. Med Sci Sports Exerc 39: 1098–1106, 2007. [DOI] [PubMed] [Google Scholar]
- 281.Smyth RJ, D’Urzo AD, Slutsky AS, Galko BM, Rebuck AS. Ear oximetry during combined hypoxia and exercise. J Appl Physiol 60: 716–719, 1986. [DOI] [PubMed] [Google Scholar]
- 282.Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: Comparison to rebreathe. J Appl Physiol 99: 1985–1991, 2005. [DOI] [PubMed] [Google Scholar]
- 283.Sobol BJ, Bottex G, Emirgil C, Gissen H. Gaseous Diffusion from Alveoli to Pulmonary Vessels of Considerable Size. Circ Res 13: 71–79, 1963. [DOI] [PubMed] [Google Scholar]
- 284.Soderlund K, Hultman E. ATP and phosphocreatine changes in single human muscle fibers after intense electrical stimulation. Am J Physiol 261: E737–E741, 1991. [DOI] [PubMed] [Google Scholar]
- 285.Sorbini CA, Grassi V, Solinas E, Muiesan G. Arterial oxygen tension in relation to age in healthy subjects. Respiration 25: 3–13, 1968. [DOI] [PubMed] [Google Scholar]
- 286.Sparrow D, Weiss ST. Respiratory System. In: Masoro EJ, editor. Handbook of Physiology: Aging, New York: Oxford University Press, 1995, sect. 11, pp. 475–483. [Google Scholar]
- 287.Spina RJ. Cardiovascular adaptations to endurance exercise training in older men and women. Exerc Sport Sci Rev 27: 317–332, 1999. [PubMed] [Google Scholar]
- 288.Spirduso WW. Reaction and movement time as a function of age and physical activity level. J Gerontol 30: 435–440, 1975. [DOI] [PubMed] [Google Scholar]
- 289.Sprague D, Richardson MS, Baish JW, Kemp JS. A new system to record reliable pulse oximetry data from the Nellcor N-200 and its applications in studies of variability in infant oxygenation. J Clin Monit 12: 17–25, 1996. [DOI] [PubMed] [Google Scholar]
- 290.Spriet LL, Soderlund K, Thomson JA, Hultman E. pH measurement in human skeletal muscle samples: Effect of phosphagen hydrolysis. J Appl Physiol 61: 1949–1954, 1986. [DOI] [PubMed] [Google Scholar]
- 291.Staub NC, Bishop JM, Forster RE. Velocity of O2 uptake by human red blood cells. J Appl Physiol 16: 511–516, 1961. [Google Scholar]
- 292.St Croix CM, Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Effects of prior exercise on exercise-induced arterial hypoxemia in young women. J Appl Physiol 85: 1556–1563, 1998. [DOI] [PubMed] [Google Scholar]
- 293.Stewart KG, Rowbottom SJ. Inaccuracy of pulse oximetry in patients with severe tricuspid regurgitation. Anaesthesia 46: 668–670, 1991. [DOI] [PubMed] [Google Scholar]
- 294.Stewart PA. Independent and dependent variables of acid-base control. Respir Physiol 33: 9–26, 1978. [DOI] [PubMed] [Google Scholar]
- 295.Stewart PA. How to Understand Acid-Base: A Quantitative Acid-Base Primer for Biology and Medicine. New York: Elsevier/North Holland, 1981. [Google Scholar]
- 296.Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol 61: 1444–1461, 1983. [DOI] [PubMed] [Google Scholar]
- 297.Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev 34: 99–106, 2006. [DOI] [PubMed] [Google Scholar]
- 298.Stickland MK, Lovering AT, Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med 176: 300–305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Stickland MK, Petersen SR, Haykowsky MJ, Taylor DA, Jones RL. The effects of cycle racing on pulmonary diffusion capacity and left ventricular systolic function. Respir Physiol Neurobiol 138: 291–299, 2003. [DOI] [PubMed] [Google Scholar]
- 300.Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol 561: 321–329, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Effect of acute increases in pulmonary vascular pressures on exercise pulmonary gas exchange. J Appl Physiol 100: 1910–1917, 2006. [DOI] [PubMed] [Google Scholar]
- 302.Sutton JR, Reeves JT, Wagner PD, Groves BM, Cymerman A, Malconian MK, Rock PB, Young PM, Walter SD, Houston CS. Operation Everest II: Oxygen transport during exercise at extreme simulated altitude. J Appl Physiol 64: 1309–1321, 1988. [DOI] [PubMed] [Google Scholar]
- 303.Takano N Reflex hypoxic drive to respiration during the menstrual cycle. Respir Physiol 56: 229–235, 1984. [DOI] [PubMed] [Google Scholar]
- 304.Takano N, Sakai A, Iida Y. Analysis of alveolar PCO2 control during the menstrual cycle. Pflugers Arch 390: 56–62, 1981. [DOI] [PubMed] [Google Scholar]
- 305.Tamhane RM, Johnson RL Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest 120: 1850–1856, 2001. [DOI] [PubMed] [Google Scholar]
- 306.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37: 153–156, 2001. [DOI] [PubMed] [Google Scholar]
- 307.Taussig LM. Maximal expiratory flows at functional residual capacity: A test of lung function for young children. Am Rev Respir Dis 116: 1031–1038, 1977. [DOI] [PubMed] [Google Scholar]
- 308.Taussig LM, Cota K, Kaltenborn W. Different mechanical properties of the lung in boys and girls. Am Rev Respir Dis 123: 640–643, 1981. [DOI] [PubMed] [Google Scholar]
- 309.Taylor BJ, Johnson BD. The pulmonary circulation and exercise responses in the elderly. Semin Respir Crit Care Med 31: 528–538, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Terry PB, Traystman RJ, Newball HH, Batra G, Menkes HA. Collateral ventilation in man. N Engl J Med 298: 10–15, 1978. [DOI] [PubMed] [Google Scholar]
- 311.Thadani U, Parker JO. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Cardiol 41: 52–59, 1978. [DOI] [PubMed] [Google Scholar]
- 312.Thurlbeck WM. The internal surface area of nonemphysematous lungs. Am Rev Respir Dis 95: 765–773, 1967. [DOI] [PubMed] [Google Scholar]
- 313.Thurlbeck WM. Postnatal human lung growth. Thorax 37: 564–571, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tibes U Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles. Some evidence for involvement of group III or IV nerve fibers. Circ Res 41: 332–341, 1977. [DOI] [PubMed] [Google Scholar]
- 315.Tobin CE. Arteriovenous shunts in the peripheral pulmonary circulation in the human lung. Thorax 21: 197–204, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE, Moon RE. Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J Appl Physiol 58: 989–995, 1985. [DOI] [PubMed] [Google Scholar]
- 317.Trivedi NS, Ghouri AF, Shah NK, Lai E, Barker SJ. Effects of motion, ambient light, and hypoperfusion on pulse oximeter function. J Clin Anesth 9: 179–183, 1997. [DOI] [PubMed] [Google Scholar]
- 318.Tsujino T, Shima A. The behaviour of gas bubbles in blood subjected to an oscillating pressure. J Biomech 13: 407–416, 1980. [DOI] [PubMed] [Google Scholar]
- 319.Tsukimoto K, Arcos JP, Schaffartzik W, Wagner PD, West JB. Effect of common dead space on VA/Q distribution in the dog. J Appl Physiol 68: 2488–2493, 1990. [DOI] [PubMed] [Google Scholar]
- 320.Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol 25: 664–671, 1968. [DOI] [PubMed] [Google Scholar]
- 321.Van Slyke DD, Hastings AB, Hiller A, Sendroy J. Studies of gas and electrolyte equilibria in blood; the amounts of alkali bound by serum albumin and globulin. J Biol Chem: 769–780, 1928. [Google Scholar]
- 322.Vincent J, Hellot MF, Vargas E, Gautier H, Pasquis P, Lefrancois R. Pulmonary gas exchange, diffusing capacity in natives and newcomers at high altitude. Respir Physiol 34: 219–231, 1978. [DOI] [PubMed] [Google Scholar]
- 323.Vogiatzis I, Zakynthinos S, Boushel R, Athanasopoulos D, Guenette JA, Wagner H, Roussos C, Wagner PD. The contribution of intrapulmonary shunts to the alveolar-to-arterial oxygen difference during exercise is very small. J Physiol 586: 2381–2391, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wagner PD. Diffusion and chemical reaction in pulmonary gas exchange. Physiol Rev 57: 257–312, 1977. [DOI] [PubMed] [Google Scholar]
- 325.Wagner PD. Gas Exchange. In: Hornbein TF, Schoene R, editors. High Altitude. An Exploration of Human Adaptation, New York: Marcel Dekker Inc., 2001, pp. 199–234. [Google Scholar]
- 326.Wagner PD. The multiple inert gas elimination technique (MIGET). Intensive Care Med 34: 994–1001, 2008. [DOI] [PubMed] [Google Scholar]
- 327.Wagner PD, Araoz M, Boushel R, Calbet JA, Jessen B, Radegran G, Spielvogel H, Sondegaard H, Wagner H, Saltin B. Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J Appl Physiol 92: 1393–1400, 2002. [DOI] [PubMed] [Google Scholar]
- 328.Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol 61: 260–270, 1986. [DOI] [PubMed] [Google Scholar]
- 329.Wagner PD, Gillespie JR, Landgren GL, Fedde MR, Jones BW, De-Bowes RM, Pieschl RL, Erickson HH. Mechanism of exercise-induced hypoxemia in horses. J Appl Physiol 66: 1227–1233, 1989. [DOI] [PubMed] [Google Scholar]
- 330.Wagner PD, Laravuso RB, Uhl RR, West JB. Continuous distributions of ventilation-perfusion ratios in normal subjects breathing air and 100 per cent O2. J Clin Invest 54: 54–68, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wagner PD, Naumann PF, Laravuso RB. Simultaneous measurement of eight foreign gases in blood by gas chromatography. J Appl Physiol 36: 600–605, 1974. [DOI] [PubMed] [Google Scholar]
- 332.Wagner PD, Saltzman HA, West JB. Measurement of continuous distributions of ventilation-perfusion ratios: Theory. J Appl Physiol 36: 588–599, 1974. [DOI] [PubMed] [Google Scholar]
- 333.Wagner PD, Smith CM, Davies NJH, McEvoy RD, Gale GE. Estimation of ventilation-perfusion inequality by inert gas elimination without arterial sampling. J Appl Physiol 59: 376–383, 1985. [DOI] [PubMed] [Google Scholar]
- 334.Wagner PD, Sutton JR, Reeves JT, Cymerman A, Groves BM, Malconian MK. Operation Everest II: Pulmonary gas exchange during a simulated ascent of Mt. Everest. J Appl Physiol 63: 2348–2359, 1987. [DOI] [PubMed] [Google Scholar]
- 335.Wang CH, Popel AS. Effect of red blood cell shape on oxygen transport in capillaries. Math Biosci 116: 89–110, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 15: 75–88, 1993. [DOI] [PubMed] [Google Scholar]
- 337.Ward GR, Sutton JR, Jones NL, Toews CJ. Activation by exercise of human skeletal muscle pyruvate dehydrogenase in vivo. Clin Sci (Lond) 63: 87–92, 1982. [DOI] [PubMed] [Google Scholar]
- 338.Warren GL, Cureton KJ, Middendorf WF, Ray CA, Warren JA. Red blood cell pulmonary capillary transit time during exercise in athletes. Med Sci Sports Exerc 23: 1353–1361, 1991. [PubMed] [Google Scholar]
- 339.Webb RK, Ralston AC, Runciman WB. Potential errors in pulse oximetry. II. Effects of changes in saturation and signal quality. Anaesthesia 46: 207–212, 1991. [DOI] [PubMed] [Google Scholar]
- 340.Weibel ER. The lung as gas exchanger. The Pathway for Oxygen. Cimbridge, MA: Harvard University Press, 1984, pp. 339–377. [Google Scholar]
- 341.West J Respiratory Physiology: The Essentials (5th ed). Baltimore: Williams & Wilkins, 1995, pp. 39–43. [Google Scholar]
- 342.West JB. Effect of slope and shape of dissociation curve on pulmonary gas exchange. Respir Physiol 8: 66–85, 1969. [DOI] [PubMed] [Google Scholar]
- 343.West JB. Invited review: Pulmonary capillary stress failure. J Appl Physiol 89: 2483–2489;discussion 2497, 2000. [DOI] [PubMed] [Google Scholar]
- 344.West JB, Mathieu-Costello O. Stress failure of pulmonary capillaries as a limiting factor for maximal exercise. Eur J Appl Physiol Occup Physiol 70: 99–108, 1995. [DOI] [PubMed] [Google Scholar]
- 345.West JB, Wagner PD. Pulmonary gas exchange. In: West JB, editor. Bioengineering Aspects of the Lung, New York: Marcel Dekker, 1997, pp. 267–360. [Google Scholar]
- 346.West JB, Wagner PD, Derks CM. Gas exchange in distributions of VA-Q ratios: Partial pressure-solubility diagram. J Appl Physiol 37: 533–540, 1974. [DOI] [PubMed] [Google Scholar]
- 347.Wetzel P, Gros G. Carbonic anhydrases in striated muscle. Exs 375–399, 2000. [DOI] [PubMed] [Google Scholar]
- 348.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis 126: 530–533, 1982. [DOI] [PubMed] [Google Scholar]
- 349.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol 54: 874–879, 1983. [DOI] [PubMed] [Google Scholar]
- 350.Whitwell KE, Greet TR. Collection and evaluation of tracheobronchial washes in the horse. Equine Vet J 16: 499–508, 1984. [DOI] [PubMed] [Google Scholar]
- 351.Whyte MK, Peters AM, Hughes JM, Henderson BL, Bellingan GJ, Jackson JE, Chilvers ER. Quantification of right to left shunt at rest and during exercise in patients with pulmonary arteriovenous malformations. Thorax 47: 790–796, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wilkinson MJ, Fagan DG. Postmortem demonstration of intrapulmonary arteriovenous shunting. Arch Dis Child 65: 435–437, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yamaya Y, Bogaard HJ, Wagner PD, Niizeki K, Hopkins SR. Validity of pulse oximetry during maximal exercise in normoxia, hypoxia, and hyperoxia. J Appl Physiol 92: 162–168, 2002. [DOI] [PubMed] [Google Scholar]
- 354.Yang WJ, Echigo R, Wotton DR, Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. II. Moving bubbles or liquids. J Biomech 4: 283–288, 1971. [DOI] [PubMed] [Google Scholar]
- 355.Young M, Sciurba F, Rinaldo J. Delirium and pulmonary edema after completing a marathon. Am Rev Respir Dis 136: 737–739, 1987. [DOI] [PubMed] [Google Scholar]
- 356.Zavorsky GS, Saul L, Murias JM, Ruiz P. Pulmonary gas exchange does not worsen during repeat exercise in women. Respir Physiol Neurobiol 153: 226–236, 2006. [DOI] [PubMed] [Google Scholar]
- 357.Zavorsky GS, Walley KR, Hunte GS, McKenzie DC, Sexsmith GP, Russell JA. Acute hypervolemia lengthens red cell pulmonary transit time during exercise in endurance athletes. Respir Physiol Neurobiol 131: 255–268, 2002. [DOI] [PubMed] [Google Scholar]