Abstract
Background:
Financial payments from the drug industry to US physicians are common. Payments may influence physicians’ clinical decision-making and drug prescribing.
Purpose:
To evaluate whether receipt of payments from the drug industry is associated with physician prescribing practices.
Data Sources:
We searched Medline (Ovid), Embase, the Cochrane Library, Web of Science, and EconLit without language restrictions. The search had no limiting start date and concluded on September 16, 2020.
Study Selection:
We included studies that estimated the association between receipt of industry payments (exposure) and prescribing (outcome).
Data Extraction:
Pairs of reviewers extracted the primary analysis or analyses from each study and evaluated risk of bias.
Data Synthesis:
36 studies comprising 101 analyses were included. The majority of studies (30) identified a positive association between payments and prescribing in all analyses; the remainder (6) had a mix of positive and null findings; no study had only null findings. 89 of 101 individual analyses identified a positive association. Payments were associated with increased prescribing of the paying company’s drug, prescribing costs, and increased prescribing of branded drugs. 9 studies assessed and found evidence of a temporal association; 25 studies assessed and found evidence of a dose-response relationship.
Limitations:
Observational design; 21 of 36 studies had serious ROB; potential publication bias.
Conclusions:
The association between industry payments and physician prescribing was consistent across all studies that have evaluated this association. Findings regarding a temporal association and dose-response suggest a causal relationship.
Introduction
Personal financial payments to physicians are a common marketing strategy used by the pharmaceutical industry. These payments include both cash (typically for consulting services or invited lectures) and in-kind gifts such as meals. From 2015-2017, 67% of all US physicians received payments (1). This proportion exceeded 80% in many specialties (medical oncology, orthopedic surgery, urology, and others), and in many specialties the dollar value of personal payments has increased in recent years (2–5). The value of industry payments to US physicians is substantial, totaling $2.18 billion in 2018 alone (6,7).
Concern has been raised that industry payments may result in the inappropriate influence of commercial interests on medical practice (8,9). A small portion of physicians receive payments substantial enough to constitute the majority of their income (3,10–12). Smaller payments are more common, but even small payments may influence physician behavior by triggering a sense of mutual obligation, or reciprocity, to persuade physicians to increase prescribing (15,16).
Previous work has found an association between physician prescribing and contact with the drug industry. Physicians who receive industry information on pharmaceutical products, direct contact with industry salespersons (“detailing”), or free drug samples increase their prescribing of the paying company’s drugs (17–21). However, these types of interactions frequently also involve financial payments to physicians, and until recently the opacity of these payments limited investigation into their relative importance as a component of industry marketing.
Beginning in 2013, the Open Payments reporting system created by the “Sunshine Act” has made public all financial transfers greater than $10 in value from drug and device manufacturers to US physicians and several other provider groups including chiropractors and dentists. Open Payments has enabled large-scale, quantitative investigation into the financial component of physician-industry interactions. Since then, numerous studies have used Open Payments data to assess whether industry payments influence physician prescribing. Given the rapid, recent emergence of the literature on industry payments, the totality of this body of work has not yet been examined.
These recent studies have assessed industry payments across a broad range of medical specialties and drug classes, with heterogeneous results. As a result, there has not been consensus on the overall association of industry payments with physician prescribing, or whether such an association is causal. Therefore, we conducted a systematic review of the association between industry payments and physician prescribing. The goal of this review was to understand: 1) the association between payments and prescribing across the spectrum of medical practice, and 2) whether there is sufficient evidence to conclude that payments cause physicians to change prescribing.
Methods
The review protocol was submitted to PROSPERO on September 20, 2019 but was not eligible for registration in accordance with PROSPERO policies to include only studies of direct measures of human health. The submitted protocol is available in the Appendix.
Data Source and Searches
Our search included 5 databases: Medline (Ovid), Embase.com, the Cochrane Library (Wiley), Web of Science Core Collection (Clarivate), and EconLit (EBSCO). The search strategy was designed in Medline and translated to the remaining databases. It combined two main concepts, represented with keywords and subject headers, linked using the AND operator: prescribing (e.g., prescriptions, practice patterns, inappropriate prescribing) and pharmaceutical relationships (e.g., conflict of interest, Sunshine Act, Open Payments). In Medline and Embase, the Cochrane Handbook filter was used to exclude animal-only studies (22). A second librarian performed a Peer Review of Electronic Search Strategies (PRESS) review of the search (23). We did not apply language restrictions or a beginning date cutoff. Duplicates studies were removed using the Bramer method (24). Databases were searched on September 23, 2019, with an update on September 16, 2020. Separately, on September 16, 2020 we searched MEDLINE to identify previously published reviews on this topic. We used the database Scopus to compile references cited by the included studies to identify additional relevant studies. See the Appendix for the full search strategy.
Study Selection
After deduplication, search results were imported into a reference management tool (Covidence, Veritas Health Innovation Ltd). Titles and abstracts were screened independently by 2 reviewers (A.M., N.T., S.C., S.T., or D.K.). Disagreements were resolved through group consensus. All studies deemed eligible during screening underwent full-text review by 2 reviewers independently (A.M., N.T., S.C., S.T., or D.K). Disagreements were resolved through group consensus. Studies were eligible for inclusion if they (1) had full text available, (2) were empirical, peer-reviewed experimental or observational studies (e.g. excluding guidelines, opinion pieces, and reviews), (3) focused on physicians (although other independent clinical practitioners could be included, as well), (4) studied financial payments from pharmaceutical companies as an exposure, and (5) studied prescribing of pharmaceutical products as an outcome.
Data Extraction and Quality Assessment
We used a standardized template to extract study characteristics, analytic design (eg., independent and dependent variables, statistical tests applied), results, and risk of bias (additional details below). Data were independently extracted from eligible studies by one reviewer (A.M., N.T., S.C., S.T., D.K. or R.G.) and checked for accuracy by a second reviewer, with any disagreements resolved through group consensus.
Risk of Bias Assessment
We applied the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool to assess risk of bias (ROB) (25). With ROBINS-I, each analysis is assessed for ROB on several individual domains, and then assigned an overall ROB that must be at least as high as the highest-risk individual domain. The possible levels of ROB in ROBINS-I are: unclear, low, moderate, serious, and critical. Because none of the included studies compared prospective interventions, the ROBINS-I domain of risk of bias due to deviations from intended interventions was not applicable and was omitted. Because some studies comprised multiple analyses which used different methods, ROB was assessed for each analysis separately. Analyses assessed as having critical ROB were determined not to make meaningful contributions to the overall assessment of the payment-prescribing association and therefore were excluded from the evidence synthesis.
Data Synthesis and Analysis
For each study, we abstracted results of the primary analysis. In cases where studies had performed several different analyses to address the same underlying question, we selected the outcome that was the broadest (in cases where both overall and subgroup results were presented), used continuous measures of industry payments and prescribing (vs. binary or categorical measures), and measured the prescribing of drugs manufactured by the specific company making payments (vs. measured prescribing of multiple drugs). We selected results that accounted for physicians’ overall prescribing volume or prescribing volume within the same drug class (vs. those that measured prescribing of the drug[s] of interest without accounting for prescribing volume of other drugs), and results that adjusted for physician characteristics including gender, specialty, and others (vs. unadjusted analyses) when available. In cases where industry payments were measured on a categorical scale, we chose the analysis with the greatest contrast (e.g., $1,000 vs. $0 was preferred over $100 vs. $0). In cases where the study presented several co-equal primary analyses, we abstracted all of them.
The analytic approaches and characteristics of included studies were sufficiently heterogeneous that quantitative meta-analysis was not feasible. We therefore performed a qualitative synthesis of the results.
For each analysis, we assessed whether the association between payments and prescribing was positive (industry payments had a statistically significant association with increased prescribing), inverse (industry payments had a statistically significant association with reduced prescribing), or null (no statistically significant association). For each study, we assessed whether all constituent analyses identified a positive association between payments and prescribing: yes (all analyses had a positive association), no (all analyses had either a null or inverse association), or mixed (some analyses had a positive association, while some were null or inverse). We also characterized whether each analysis assessed temporal or dose-response relationships between payments and prescribing.
This review conforms to the Meta-analysis of Observational Studies in Epidemiology reporting guidelines. This was supported by the NCI MSK Cancer Center Support Grant, P30 CA008748.
Results
Figure 1 provides details on study selection in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A total of 3,460 unique studies were identified in database searches. Of these, 103 were accepted for full-text review, of which 37 were eligible for inclusion. The list of 66 excluded studies and reasons for exclusion are provided in Appendix Table 1. Review of references of included studies did not identify any new studies eligible for inclusion.
Figure 1.
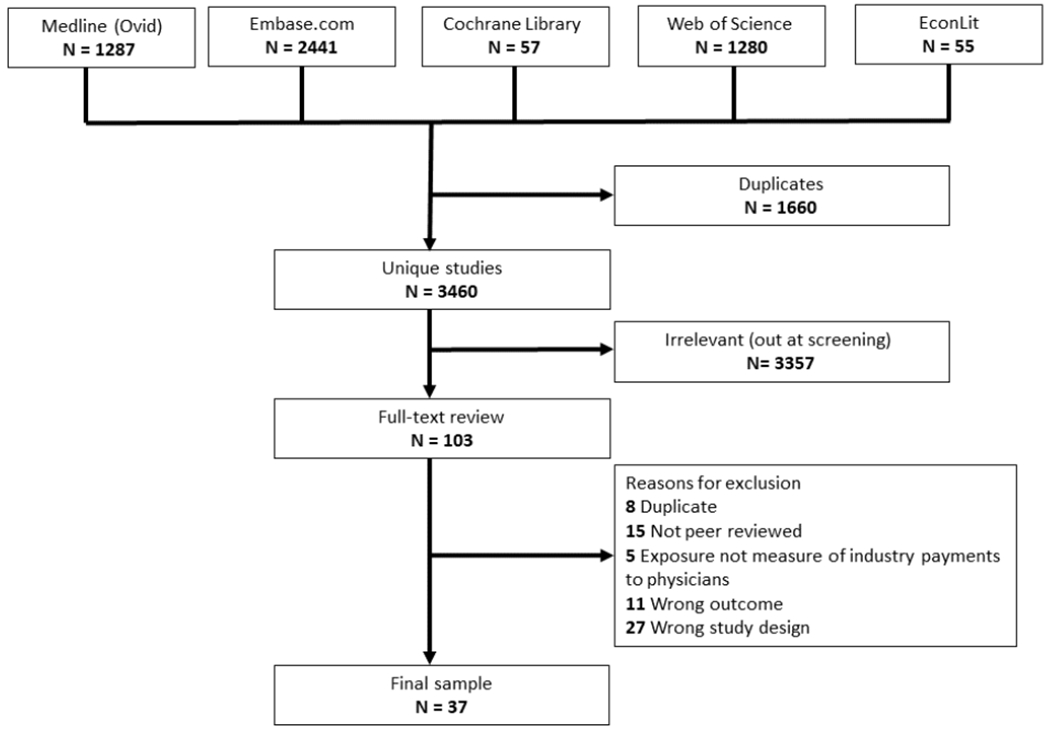
Study Selection.
Risk of Bias
The primary analyses for 15 of the 37 studies had moderate ROB, 21 studies had serious ROB, and one study had critical ROB and was excluded (26) (Appendix Figure 1). This left 36 studies for inclusion in the evidence synthesis. For one study, some analyses had serious ROB and others had critical ROB; the analyses with critical ROB were excluded (27). All studies had at least moderate ROB due to confounding, as the ROBINS-I tool specifies that low ROB due to confounding is typically achieved only in prospective, randomized trials. We determined that any study using Open Payments data to measure industry payment would have at least moderate ROB due to classification of interventions, because of concerns that Open Payments data contain inaccuracies (28,29); this was the primary reason that 33 studies had at least moderate risk of bias in this domain. Studies commonly had low ROB due to missing data (35 studies) and measurement of outcomes (26 studies), resulting from use of broad government datasets to measure physician characteristics and prescribing behavior.
Study Characteristics
Characteristics of the included studies are summarized in Table 1 (individual study characteristics are in Appendix Table 2). The majority of studies (35) were published in 2016 or later. Medicare data was the most commonly used source to measure physician prescribing (34 studies), and Open Payments was used most commonly (32 studies) to measure industry payments. All studies but 1 were US-based, and most (32) were nationwide, while the remainder (3) focused on smaller regions.
Table 1:
Study characteristics. For Type[s] of prescribing outcome assessed, “prescribing volume” indicates that the measured outcome was the number of prescriptions or claims for the drug[s] of interest; “prescribing costs” indicates the financial cost (eg., Medicare reimbursement) of prescriptions for the drug[s] of interest; “fraction of prescribing for drug of interest” indicates the proportion of prescriptions or claims for the drug[s] of interest within the drug class or within an identified set of substitutable medications; “fraction of prescribing for branded drugs” indicates the proportion of prescriptions or claims for the drug[s] of interest that were for branded versions rather than generics.
| Characteristic | Number of studies (%) |
|---|---|
| Year of publication | |
| 1992 | 1 (2.8) |
| 2016 | 4 (11.1) |
| 2017 | 6 (16.7) |
| 2018 | 9 (25.0) |
| 2019 | 11(30.6) |
| 2020 | 5 (13.9) |
| Route of administration of drugs studied | |
| Oral | 28 (77.8) |
| Subcutaneous | 3 (8.3) |
| Intravitreal | 3 (8.3) |
| Intravenous | 1 (2.8) |
| Intranasal | 1 (2.8) |
| Class of drugs studied | |
| Multiple drugs from different classes | 11 (30.6) |
| Opioids | 7 (19.4) |
| Antineoplastic | 3 (8.3) |
| Anti-VEGF | 3 (8.3) |
| Biologics for inflammatory bowel disease | 1 (2.8) |
| Erectile dysfunction | 1 (2.8) |
| Gabapentinoids | 1 (2.8) |
| Intranasal corticosteroids | 1 (2.8) |
| Multiple sclerosis drugs | 1 (2.8) |
| Alpha blockers and overactive bladder drugs | 1 (2.8) |
| Proton-pump inhibitors | 1 (2.8) |
| Statins | 1 (2.8) |
| Tumor necrosis factor inhibitors | 1 (2.8) |
| Anticoagulant | 1 (2.8) |
| Antipsychotic | 1 (2.8) |
| NMDA receptor antagonist | 1 (2.8) |
| Data source for industry payments | |
| Open Payments | 32 (88.9) |
| Any other source | 4 (11.1) |
| Data source for physician prescribing | |
| Medicare (Public Use File) | 29 (80.6) |
| Medicare (Opioid Supplement) | 2 (5.6) |
| Medicare (Claims) | 2 (5.6) |
| Medicare (Freedom of Information Act request) | 1 (2.8) |
| Hospital inventory | 1 (2.8) |
| French National Health Data System | 1 (2.8) |
| Type[s] of prescribing outcome assessed § | |
| Prescribing volume for the drug of interest | 15 |
| Prescribing costs for the drug of interest | 10 |
| Fraction of prescribing for drug of interest | 8 |
| Fraction of prescribing for branded drugs | 6 |
| Other* | 1 |
| Physician Specialty § | |
| All Physicians†‡ | 22 |
| Cardiology | 2 |
| Gastroenterology | 2 |
| Hematology-Oncology | 5 |
| Nephrology | 2 |
| Neurology | 3 |
| Ophthalmology | 3 |
| Primary Care | 5 |
| Psychiatry | 2 |
| Rheumatology | 2 |
| Urology | 4 |
| Other (Chiropractic, Dentistry, Dermatology, Endocrinology, General Surgery, Optometry, Otolaryngology) | 7 |
| Categories of Industry Payments Assessed § | |
| All general payments | 30 |
| Subsets of general payments | 9 |
| All general and research payments combined | 3 |
| All research payments | 1 |
| Temporal Relationship Evaluated | |
| Yes | 9 (25.0) |
| No | 27 (75.0) |
| Dose-Response Relationship Evaluated | |
| Yes | 25 (69.4) |
| No | 11 (30.6) |
| Geographic region | |
| Entire US | 32 (88.9) |
| US state, municipality, or hospital | 3 (8.3) |
| France | 1 (2.8) |
Outcome was a range of prescribing quality measures.
Two papers included all clinicians (Physicians, Nurse Practitioners, etc.).
One paper included all physicians, except gastroenterologists.
This category sums to >36 because some studies contained multiple categories
11 studies analyzed multiple drug classes. Studies analyzing specific drug classes focused on opioids (7 studies), antineoplastics (3 studies), anti-VEGF agents (3 studies), and (1 study each) biologics for inflammatory bowel disease, erectile dysfunction drugs, gabapentinoids, intranasal corticosteroids, multiple sclerosis drugs, alpha blockers and overactive bladder drugs, proton pump inhibitors, statins, tumor necrosis factor inhibitors, anticoagulants, antipsychotics, and NMDA receptor antagonists.
22 studies analyzed all physicians in aggregate. Specialties that were analyzed separately within one or more studies (some studies analyzed multiple specialties) included: primary care (5 studies), hematology-oncology (5), urology (4), neurology (3), ophthalmology (3), cardiology (2), gastroenterology (2), nephrology (2), psychiatry (2), rheumatology (2), and (1 study each) chiropractic, dentistry, dermatology, endocrinology, general surgery, optometry, otolaryngology.
Payment-prescribing associations
Of the 36 studies, 30 found only positive associations between industry payments and prescribing in all constituent analyses (Figure 2A, Appendix Table 3). 6 studies had mixed results, reporting one or more positive associations and one or more null associations among their analyses; no study reported only null results. By ROB, 11 of 15 studies with moderate ROB and 19 of 21 studies with serious ROB identified a positive association in all analyses.
Figure 2:
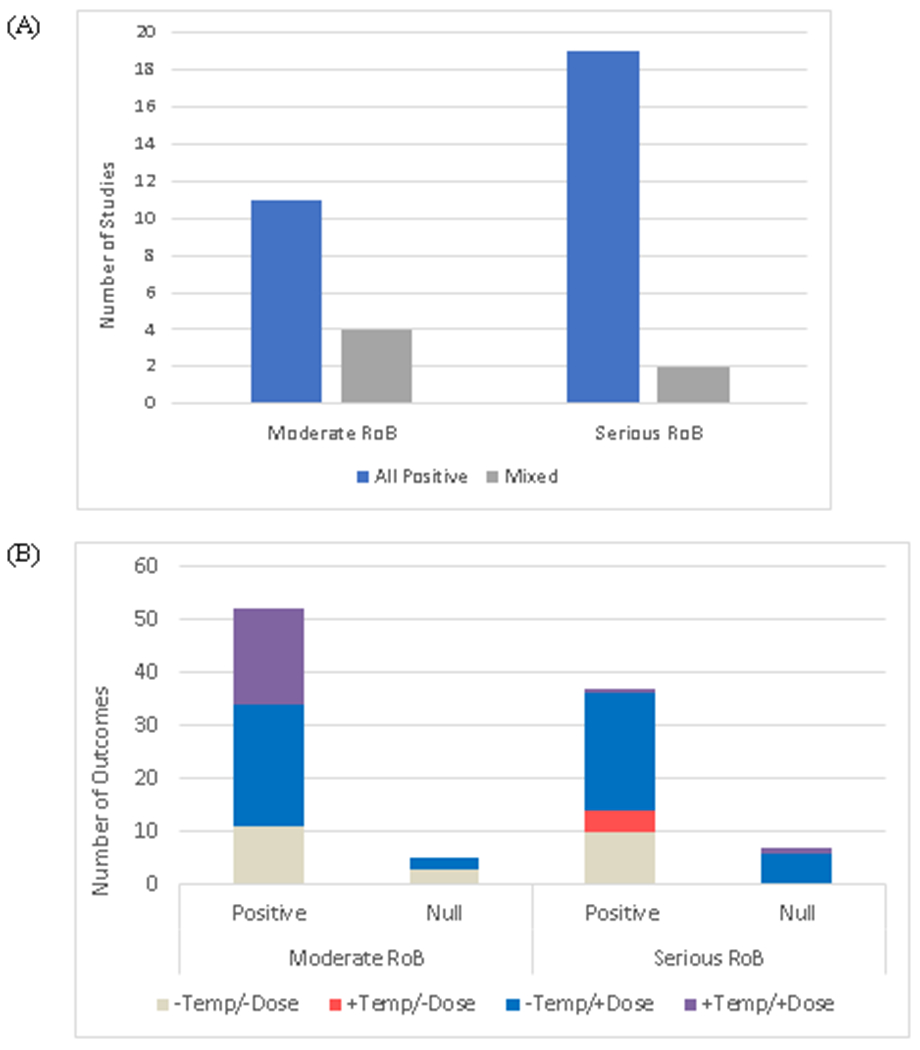
Study results regarding the presence of a positive association between industry payments and prescribing. Results are shown on the level of the overall study (2A) and the individual analyses (2B). For studies, results were characterized as “all positive” (all analyses within that study had a positive association) or “mixed” (some analyses within that study had a positive association and some were null). For individual analyses, results were characterized as “positive” (increased industry payments associated with increased prescribing), “inverse” (increased industry payments associated with reduced prescribing), or “null” (no statistically significant association). Individual outcomes were also characterized as having assessed for a temporal association (+Temp/−Dose), a dose-response association (−Temp/+Dose), neither (−Temp/−Dose), or both (+Temp/+Dose).
The 36 studies contained 101 individual analyses. 89 of the 101 analyses (88%) identified a positive association between industry payments and prescribing; 12 analyses were null, and none identified an inverse association (Figure 2B). Of the 12 null analyses, 5 had moderate ROB and 7 had serious ROB.
Types of payments
Studies reporting on the distribution of industry payments consistently found that food and beverage payments were the most common, and payments for compensation, honoraria, and consulting were less common but of greater dollar value (30–33). Regarding the association of different payment types with physician prescribing, 30 studies analyzed all personal payments to physicians (“general payments” under Open Payments terminology) in aggregate, including payment types such as consulting fees, speaker fees, and food/beverage together (Table 1, Appendix Table 2). 1 study analyzed industry payments supporting research (“research payments” under Open Payments terminology) (34), and 3 studies analyzed the sum of general payments and research payments (35–37). 9 studies presented analyses of the payment-prescribing association across different categories of general payments (31,32,38–44). Though there was heterogeneity regarding how general payments categories were grouped, 6 of the 9 studies compared food & beverage payments to other payment categories. Of these, 5 studies found that the magnitude of the payment-prescribing association was greater among food & beverage payments (31,32,40,41,44), while 1 study found a stronger association with other payment types such as consulting and compensation (42). 2 studies analyzed food & beverage payments only and found a positive association (38,39), and 1 study found a positive association for travel & lodging payments (43).
Types of prescribing outcomes
The most common prescribing outcome was prescribing volume for the drug of interest (15 studies) (Table 1, Appendix Table 2). 8 studies measured the fraction of prescriptions for the drug of interest, taking into account prescribing volume for other drugs within the same class or substitutable alternatives. 6 studies measured the fraction of prescriptions for branded drugs (either within class of overall). 10 studies measured prescribing in terms of the prescribing costs for the drug of interest. 1 study assessed a range of prescribing quality measures.
Temporal association
A total of 9 studies included analyses which assessed the temporal relationship between payments and prescribing (Table 1, Appendix Table 2). 6 of these assessed the temporal relationship by analyzing the receipt of payments with respect drug prescribing in subsequent calendar years (34,39,42,45–47). One also applied propensity score matching to control for prior payments and prescribing patterns in order to estimate the association between incident payments and future prescribing.
3 studies assessed temporality by conducting time series analyses. One of these used data from a single hospital pharmacy to assess the utilization of two different drugs before and after physicians at that hospital were exposed to payments from those drugs’ manufacturers (43). The other 2 studies constructed within-physician time series, comparing prescribing immediately after receipt of a payment to each physician’s past prescribing history (31,32).
Dose-response relationship
Of the 36 studies, 25 assessed for a dose-response relationship between payments and prescribing (Table 1, Appendix Table 2). These studies analyzed payments as either a continuous or categorical variable, assessing whether receipt of more payments (in terms of payment count or dollar value) was associated with greater changes in prescribing compared to receipt of less payments. All 25 studies found evidence of a dose-response relationship in one or more of their primary analyses.
Discussion
Prior work has established that interaction with the drug industry influences physician formulary recommendations (48), clinical research (49–52), and clinical practice guideline recommendations (53). Published reviews (identified by MEDLINE search) have also found that physician-industry interactions influence prescribing (17–21). However, the older studies included in these reviews measured interactions in non-financial terms, such as frequency of sales representative office visits or physician participation in sponsored education events. More recently, enabled by the availability of Open Payments data, direct measures of financial COI have become possible.
In this review, we therefore focused on studies that measured physician-industry interactions in solely financial terms. Each included study found a positive association between payments and prescribing in one or more of its constituent analyses. These analyses included several types of prescribing decisions, finding that physicians who received industry payments were more likely to prescribe drugs made by the companies that had paid them over alternatives, had higher prescribing costs, and prescribed relatively more brand-name products over generic alternatives. The positive results of these studies spanned a broad range of physician specialties and drug classes. The consistency of the payment-prescribing association across the type of prescribing decision, physician specialty, and drug class suggests that financial payments are an important mechanism by which physician-industry interactions influence prescribing.
Several mechanisms may explain the observed association. First, payments may cause prescribing: receiving payments from a drug company may lead a physician to prescribe more of that company’s drug in the future. Second, prescribing may cause payments: drug companies may target payments to physicians who are already high prescribers of their drugs. Both mechanisms are plausible. To shape policy around acceptance of payments, it is critical to understand the relative importance of these mechanisms.
9 of the studies included in this review evaluated the temporal relationship between payments and prescribing to approach the question of causality. Analyzing prescribing with respect to payments received in previous years – an approach used by six included studies – supports the mechanism of payments causing prescribing. However, the strongest evidence for a causal relationship comes from the three studies that conducted time series analyses (31,32,43). Each of these studies reported substantial increases in prescribing occurring immediately after receipt of each industry payment. Though these results do not exclude the possibility that manufacturers may also target payments to physicians who are already high prescribers, these findings strongly suggest that industry payments cause physicians to change their prescribing practices. A causal relationship is further supported by the dose-response analyses conducted by the majority of included studies, which consistently found that increasing numbers or dollar value of payments is associated with greater differences in prescribing.
25 of the studies conducted analyses that measured prescribing (either volume or cost) of only the drug[s] of interest. These outcomes have the potential for confounding by factors that may influence “opportunities to prescribe,” such as case volume and physician specialty. Though some studies controlled for physician specialty, there may be residual confounding; even within a specialty, physicians may focus on different diseases and therefore have differential opportunities to prescribe the drugs that treat them. Measuring prescribing in terms of the fraction of relevant prescriptions may be a better measure of physician prescribing preferences, because it incorporates the denominator of patients who were indicated for treatment. The positive associations reported by the 14 studies that measured fractional prescribing (of either a specific drug of interest, or of branded drugs in general) suggest that payments do shift physicians’ prescribing towards promoted drugs, and that the payment-prescribing association is not fully explained by “opportunities to prescribe.”
The cross-sectional design of most included studies allows for the possibility that some of the observed payment-prescribing association may be accounted for by manufacturers targeting payments to physicians who are already high prescribers, or potentially by other mechanisms. More research is needed to better understand the factors contributing to the overall association between payments and prescribing. While the association is likely multifactorial, our findings suggest that a causal relationship of payments on prescribing is an important contributing factor to the overall association.
The influence of industry payments on prescribing raises questions regarding the quality of care. In cases where patients would benefit from increased utilization of a high-value drug, it is very plausible that industry payments might lead to improved patient outcomes. However, the distribution of industry payments makes it unlikely that patients would achieve improved outcomes in aggregate: industry spending on drug promotion disproportionately targets drugs that are less effective or offer little therapeutic advancement (54,55). This may be because physicians are more inclined to use effective, innovative drugs regardless of promotion, while marginally-effective drugs require more intensive promotion to increase prescribing (54,55).
Empiric findings further suggest that industry drug promotion may lead to lower-quality prescribing. A systematic review of primarily non-financial physician-industry interactions found them to be consistently associated with inappropriate and lower-quality prescribing (17). In another review, a majority of studies found an association between the receipt of industry information and lower-quality prescribing (21). Results from studies in the current review also support this conclusion. Several studies reported associations between industry payments and increased prescribing of low-value drugs – including both less-effective drugs and those that are similarly effective but more expensive that competitors – over higher-value alternatives such as less expensive generics (38,56–63). One study stratified patients by CHADS2 and HAS-BLED scores and found that payments were associated with similar increases in low-risk and high-risk anticoagulant prescribing (32). Another found that payments were associated with a range of adverse prescribing quality measures, such as benzodiazepine prescriptions >12 weeks duration and vasodilator prescriptions for patients aged >65 years (64). Another study found evidence of “product hopping,” the promotion of a newer product in anticipation of patent expiration on an older product (65). Specifically, industry payments were associated with increased use of nilotinib for treatment of chronic myeloid leukemia, ahead of market entry of generic imatinib (34). As imatinib has better real-world safety compared to nilotinib (66), payments promoting nilotinib may result in both increased costs and worse outcomes. Further research is needed to understand more fully the potential for industry payments to affect care quality and patient outcomes.
Despite longstanding concerns regarding the potential for industry influence on medical practice, industry payments to physicians remain common. Federal regulation – the Sunshine Act – has been limited to ensuring transparent disclosure of payments and has not directly attempted to reduce them. An important barrier to reform has been physician opposition (67). Historically, the majority of physicians has believed that receiving industry gifts is appropriate and that this practice should continue (68–70). Physicians’ opposition to ending financial payments from the drug industry may be rooted in the belief that accepting such payments will not affect their practice (16,68,71,72). Our findings suggest that this belief is not well-founded.
This analysis has limitations related to characteristics of the included studies. Most studies relied on Open Payments data, which is known to contain some errors (28). However, both random errors and attribution of payments to physicians when no true COI exists (eg., funding of academic grants awarded by third party organizations) (29) would be expected to bias results towards the null. Open Payments does not include nurse practitioners or physician assistants; these provider groups will be added in the 2021 reporting year. Most studies focused on prescribing within Medicare, which may limit generalizability to other patient groups, such as the commercially insured or the uninsured. Few studies examined industry research funding; more work is needed to understand whether this form of payment is also associated with physician prescribing. Because of the observational design of the included studies, the causality of the payment-prescribing association cannot be determined. However, the temporal association between payments and prescribing observed in several studies strongly suggests a causal relationship. Results of the included studies may be subject to publication bias. However, in the context of studies on industry payments, publication bias against studies reporting null results may be less likely; all of the studies we identified found a positive association between payments and prescribing in some of their analyses, and a study finding a robust, null association would therefore have been a novel contribution to the literature and of high interest to journals.
The strength of evidence is reduced by the fact that the majority of studies had serious ROB, and no study had low ROB. However, some of the studies with moderate ROB received this assessment only because of 1) observational design and 2) use of Open Payments data, and were unlikely to have other sources of bias. That these studies also identified a payment-prescribing association increases confidence in the overall results.
We present evidence that receipt of financial payments from industry is consistently associated with increased prescribing. This association has been identified across a broad range of physician specialties, drug classes, and prescribing decisions. Additionally, evidence of a temporal association and dose-responsiveness strongly suggests a causal relationship. We also find evidence, consistent with prior studies, that industry payments are associated with increased use of lower-value drugs. Taken together, our results support the conclusion that personal payments from industry reduce the ability of physicians to make independent therapeutic decisions and that they may be harmful to patients. The medical community must change its historical opposition to reform and call for an end to such payments.
Supplementary Material
Acknowledgements:
Grant Support: NCI MSK Cancer Center Support Grant, P30 CA008748
Funding Source:
National Cancer Institute / MSK Cancer Center Support Grant, P30-CA008748
References
- 1.Inoue K, Blumenthal DM, Elashoff D, Tsugawa Y. Association between physician characteristics and payments from industry in 2015-2017: observational study. BMJ Open. 2019. September 20;9(9):e031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell AP, Mishra AA, Dey P, Curry MA, Trivedi NA, Haddadin M, et al. The association between drug industry payments and NCCN guideline panel membership. J Clin Oncol. 2020. May 20;38(15_suppl):2068–2068. [Google Scholar]
- 3.Robbins NM, Meyer MJ, Bernat JL. Scope and nature of financial conflicts of interest between neurologists and industry: 2013-2016. Neurology. 2019. September 3;93(10):438–49. [DOI] [PubMed] [Google Scholar]
- 4.Schlager E, Flaten H, St Claire C, Maxim E, Dunnick C, Dellavalle RP. Industry payments to dermatologists: updates from the 2016 open payment data. Dermatol Online J. 2018. 13;24(4). [PubMed] [Google Scholar]
- 5.Elsamadicy AA, Freedman IG, Koo AB, Reeves BC, Havlik J, David WB, et al. Characteristics of Reported Industry Payments to Neurosurgeons: A 5-Year Open Payments Database Study. World Neurosurg. 2020. October 1; [DOI] [PubMed] [Google Scholar]
- 6.The Facts About Open Payments Data: 2018 Totals [Internet]. CMS; [cited 2020 Mar 28]. Available from: https://openpaymentsdata.cms.gov/summary
- 7.Schwartz LM, Woloshin S. Medical Marketing in the United States, 1997-2016. JAMA. 2019. January 1;321(1):80–96. [DOI] [PubMed] [Google Scholar]
- 8.Brennan TA, Rothman DJ, Blank L, Blumenthal D, Chimonas SC, Cohen JJ, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006. January 25;295(4):429–33. [DOI] [PubMed] [Google Scholar]
- 9.Lichter AS. Conflict of Interest and the Integrity of the Medical Profession. JAMA. 2017. May 2;317(17):1725–6. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell AP, Basch EM, Dusetzina SB. Financial Relationships With Industry Among National Comprehensive Cancer Network Guideline Authors. JAMA Oncol. 2016. August 25;2(12):1628–31. [DOI] [PubMed] [Google Scholar]
- 11.Carr D, Welch HG. Industry Payments to Physician Directors of National Cancer Institute-Designated Cancer Centers, 2015-2017. JAMA Intern Med. 2019. August 5;179(11):1595–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill J, Haslam A, Crain T, Herrera-Perez D, Prasad V. Comparison of Industry Payments in 2017 With Annual Salary in a Cohort of Academic Oncologists. JAMA Intern Med. 2020. March 23;180(5):797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel AM, Webb ML, Lukasiewicz AM, Bohl DD, Basques BA, Russo GS, et al. Orthopaedic Surgeons Receive the Most Industry Payments to Physicians but Large Disparities are Seen in Sunshine Act Data. Clin Orthop. 2015. October;473(10):3297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tringale KR, Marshall D, Mackey TK, Connor M, Murphy JD, Hattangadi-Gluth JA. Types and Distribution of Payments From Industry to Physicians in 2015. JAMA. 2017. May 2;317(17):1774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oldani MJ. Thick prescriptions: toward an interpretation of pharmaceutical sales practices. Med Anthropol Q. 2004. September;18(3):325–56. [DOI] [PubMed] [Google Scholar]
- 16.Sah S, Fugh-Berman A. Physicians under the influence: social psychology and industry marketing strategies. J Law Med Ethics J Am Soc Law Med Ethics. 2013;41(3):665–72. [DOI] [PubMed] [Google Scholar]
- 17.Brax H, Fadlallah R, Al-Khaled L, Kahale LA, Nas H, El-Jardali F, et al. Association between physicians’ interaction with pharmaceutical companies and their clinical practices: A systematic review and meta-analysis. PloS One. 2017;12(4):e0175493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davari M, Khorasani E, Tigabu BM. Factors Influencing Prescribing Decisions of Physicians: A Review. Ethiop J Health Sci. 2018. November;28(6):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017. September 27;7(9):e016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lexchin J Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ Can Med Assoc J J Assoc Medicale Can. 1993. November 15;149(10):1401–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Spurling GK, Mansfield PR, Montgomery BD, Lexchin J, Doust J, Othman N, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010. October;7(10):e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. 6th ed. Cochrane; 2019. [cited 2020 Jun 23]. Available from: www.training.cochrane.org/handbook [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 24.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc JMLA. 2016. July;104(3):240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. October 12;i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker-Lue S The impact of reducing pharmaceutical industry payments on physician prescribing. Health Econ. 2020. March;29(3):382–90. [DOI] [PubMed] [Google Scholar]
- 27.Bandari J, Turner RM, Jacobs BL, Canes D, Moinzadeh A, Davies BJ. The Relationship of Industry Payments to Prescribing Behavior: A Study of Degarelix and Denosumab. Urol Pract. 2017. January;4(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murrin S OPEN PAYMENTS DATA: REVIEW OF ACCURACY, PRECISION, AND CONSISTENCYIN REPORTING [Internet]. Office of the Inspector General; 2018. August [cited 2019 Sep 26]. Report No.: OEI-03-15-00220. Available from: https://oig.hhs.gov/oei/reports/oei-03-15-00220.pdf
- 29.Ratain MJ. Forecasting unanticipated consequences of “The Sunshine Act”: mostly cloudy. J Clin Oncol Off J Am Soc Clin Oncol. 2014. August 1;32(22):2293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleischman W, Agrawal S, Gross CP, Ross JS. Association of Pharmaceutical Manufacturer Payments to Physicians and Prescribing Dosage of Opioids. J Gen Intern Med. 2019;34(7):1074–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey C, Lieber EMJ, Miller S. Drug Firms’ Payments and Physicians’ Prescribing Behavior in Medicare Part D [Internet]. Cambridge, MA: National Bureau of Economic Research; 2020. February [cited 2020 Mar 27]. Report No.: w26751. Available from: http://www.nber.org/papers/w26751.pdf [Google Scholar]
- 32.Agha L, Zeltzer D. DRUG DIFFUSION THROUGH PEER NETWORKS: THE INFLUENCE OF INDUSTRY PAYMENTS. NBER Work Pap Ser [Internet]. 2019. October [cited 2020 Sep 28]; Available from: http://www.nber.org/papers/w26338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunt CS. Physician characteristics, industry transfers, and pharmaceutical prescribing: Empirical evidence from medicare and the physician payment sunshine act. Health Serv Res. 2019;54(3):636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell AP, Winn AN, Dusetzina SB. Pharmaceutical Industry Payments and Oncologists’ Selection of Targeted Cancer Therapies in Medicare Beneficiaries. JAMA Intern Med. 2018. April 9;178(6):854–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartung DM, Johnston K, Cohen DM, Nguyen T, Deodhar A, Bourdette DN. Industry Payments to Physician Specialists Who Prescribe Repository Corticotropin. JAMA Netw Open. 2018. June 1;1(2):e180482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modi PK, Wang Y, Kirk PS, Dupree JM, Singer EA, Chang SL. The Receipt of Industry Payments is Associated With Prescribing Promoted Alpha-blockers and Overactive Bladder Medications. Urology. 2018. July;117:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian J, Hansen RA, Surry D, Howard J, Kiptanui Z, Harris I. Disclosure of industry payments to prescribers: industry payments might be a factor impacting generic drug prescribing. Pharmacoepidemiol Drug Saf. 2017. July;26(7):819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeJong C, Aguilar T, Tseng C-W, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical Industry-Sponsored Meals and Physician Prescribing Patterns for Medicare Beneficiaries. JAMA Intern Med. 2016. June 20;176(8):1114–22. [DOI] [PubMed] [Google Scholar]
- 39.Hadland SE, Cerdá M, Li Y, Krieger MS, Marshall BDL. Association of Pharmaceutical Industry Marketing of Opioid Products to Physicians With Subsequent Opioid Prescribing. JAMA Intern Med. 2018. 01;178(6):861–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan R, Nugent CM, Scaffidi MA, Gimpaya N, Grover SC. Association of Biologic Prescribing for Inflammatory Bowel Disease With Industry Payments to Physicians. JAMA Intern Med. 2019. July 8; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta HB, Moore TJ, Alexander GC. Association of Pharmaceutical Industry Payments to Physicians With Prescription and Medicare Expenditures for Pimavanserin. Psychiatr Serv Wash DC. 2020. August 25;appips202000251. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell AP, Winn AN, Lund JL, Dusetzina SB. Evaluating the Strength of the Association Between Industry Payments and Prescribing Practices in Oncology. The Oncologist. 2019. February 6;24(5):632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orlowski JP, Wateska L. The effects of pharmaceutical firm enticements on physician prescribing patterns. There’s no such thing as a free lunch. Chest. 1992. July;102(1):270–3. [DOI] [PubMed] [Google Scholar]
- 44.Morse E, Hanna J, Mehra S. The Association between Industry Payments and Brand-Name Prescriptions in Otolaryngologists. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2019;161(4):605–12. [DOI] [PubMed] [Google Scholar]
- 45.Zezza MA, Bachhuber MA. Payments from drug companies to physicians are associated with higher volume and more expensive opioid analgesic prescribing. PloS One. 2018;13(12):e0209383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollander MAG, Donohue JM, Stein BD, Krans EE, Jarlenski MP. Association between Opioid Prescribing in Medicare and Pharmaceutical Company Gifts by Physician Specialty. J Gen Intern Med. 2020. August;35(8):2451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue K, Figueroa JF, Orav EJ, Tsugawa Y. Association between industry payments for opioid products and physicians’ prescription of opioids: observational study with propensity-score matching. J Epidemiol Community Health. 2020. April 29; [DOI] [PubMed] [Google Scholar]
- 48.Chren MM, Landefeld CS. Physicians’ behavior and their interactions with drug companies. A controlled study of physicians who requested additions to a hospital drug formulary. JAMA. 1994. March 2;271(9):684–9. [PubMed] [Google Scholar]
- 49.Hayes MJ, Prasad V. Association between conflict of interest and published position on tumor-treating fields for the treatment of glioblastoma. J Cancer Policy. 2019. September;21:100189. [Google Scholar]
- 50.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017. 16;2:MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peppercorn J, Blood E, Winer E, Partridge A. Association between pharmaceutical involvement and outcomes in breast cancer clinical trials. Cancer. 2007. April 1;109(7):1239–46. [DOI] [PubMed] [Google Scholar]
- 52.Liang F, Zhu J, Mo M, Zhou CM, Jia HX, Xie L, et al. Role of industry funders in oncology RCTs published in high-impact journals and its association with trial conclusions and time to publication. Ann Oncol Off J Eur Soc Med Oncol. 2018. August 2;29(10):2129–34. [DOI] [PubMed] [Google Scholar]
- 53.Tibau A, Bedard PL, Srikanthan A, Ethier J-L, Vera-Badillo FE, Templeton AJ, et al. Author financial conflicts of interest, industry funding, and clinical practice guidelines for anticancer drugs. J Clin Oncol Off J Am Soc Clin Oncol. 2015. January 1;33(1):100–6. [DOI] [PubMed] [Google Scholar]
- 54.Greenway T, Ross JS. US drug marketing: how does promotion correspond with health value? BMJ. 2017. May 2;357:j1855. [DOI] [PubMed] [Google Scholar]
- 55.Lexchin J The relation between promotional spending on drugs and their therapeutic gain: a cohort analysis. CMAJ Open. 2017. September 13;5(3):E724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahr MA, Hodge DO, Erie JC. Association between Industry Payments and Anti-vascular Endothelial Growth Factor Use in Medicare Beneficiaries. Ophthalmol Retina. 2017. February;1(1):19–24. [DOI] [PubMed] [Google Scholar]
- 57.Morse E, Fujiwara RJT, Mehra S. The Association of Industry Payments to Physicians with Prescription of Brand-Name Intranasal Corticosteroids. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2018. September;159(3):442–8. [DOI] [PubMed] [Google Scholar]
- 58.Morse E, Fujiwara RJT, Mehra S. Industry Payments to Physicians and Prescriptions of Brand-Name Proton-Pump Inhibitors. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2018. October 16;194599818803337. [DOI] [PubMed] [Google Scholar]
- 59.Rhee TG, Ross JS. Association Between Industry Payments to Physicians and Gabapentinoid Prescribing. JAMA Intern Med. 2019. July 8;179(10):1425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma M, Vadhariya A, Johnson ML, Marcum ZA, Holmes HM. Association between industry payments and prescribing costly medications: an observational study using open payments and medicare part D data. BMC Health Serv Res. 2018. April 2;18(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh P, Forman H, Adamson AS, Mostaghimi A, Ogdie AR, Oganisian A, et al. Impact of Industry Payments on Prescribing Patterns for Tumor Necrosis Factor Inhibitors Among Medicare Beneficiaries. J Gen Intern Med. 2018. October 15;34(2):176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh N, Chang JS, Rachitskaya AV. Open Payments Database: Anti-Vascular Endothelial Growth Factor Agent Payments to Ophthalmologists. Am J Ophthalmol. 2017. January;173:91–7. [DOI] [PubMed] [Google Scholar]
- 63.Taylor SC, Huecker JB, Gordon MO, Vollman DE, Apte RS. Physician-Industry Interactions and Anti-Vascular Endothelial Growth Factor Use Among US Ophthalmologists. JAMA Ophthalmol. 2016. 01;134(8):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goupil B, Balusson F, Naudet F, Esvan M, Bastian B, Chapron A, et al. Association between gifts from pharmaceutical companies to French general practitioners and their drug prescribing patterns in 2016: retrospective study using the French Transparency in Healthcare and National Health Data System databases. BMJ. 2019. November 5;367:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrier M, Shadowen S. Pharmaceutical Product Hopping: A Proposed Framework For Antitrust Analysis [Internet]. Health Affairs Blog. 2017. [cited 2020 Jun 30]. Available from: https://www.healthaffairs.org/do/10.1377/hblog20170601.060360/full/
- 66.Cole AL, Wood WA, Muluneh B, Lund JL, Elston Lafata J, Dusetzina SB. Comparative Safety and Health Care Expenditures Among Patients With Chronic Myeloid Leukemia Initiating First-Line Imatinib, Dasatinib, or Nilotinib. JCO Oncol Pract. 2020. March 20;JOP1900301. [DOI] [PubMed] [Google Scholar]
- 67.Paid to Prescribe? Exploring the Relationship Between Doctors and the Drug Industry. Sect. Special Committee on Aging, United States Senate, Serial No. 110-10 Washington, D.C; June 7, 2007. p. 128–31. [Google Scholar]
- 68.Korenstein D, Keyhani S, Ross JS. Physician attitudes toward industry: a view across the specialties. Arch Surg Chic Ill 1960. 2010. June;145(6):570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer MA, Keough ME, Baril JL, Saccoccio L, Mazor KM, Ladd E, et al. Prescribers and Pharmaceutical Representatives: Why Are We Still Meeting? J Gen Intern Med. 2009. July;24(7):795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brett AS, Burr W, Moloo J. Are gifts from pharmaceutical companies ethically problematic? A survey of physicians. Arch Intern Med. 2003. October 13;163(18):2213–8. [DOI] [PubMed] [Google Scholar]
- 71.Halperin EC, Hutchison P, Barrier RC. A population-based study of the prevalence and influence of gifts to radiation oncologists from pharmaceutical companies and medical equipment manufacturers. Int J Radiat Oncol Biol Phys. 2004. August 1;59(5):1477–83. [DOI] [PubMed] [Google Scholar]
- 72.Cain DM, Detsky AS. Everyone’s a little bit biased (even physicians). JAMA. 2008. June 25;299(24):2893–5. [DOI] [PubMed] [Google Scholar]
- 73.Altawalbeh SM, Ibrahim IA, Al-Shatnawi SF. Influence of pharmaceutical promotion on prescribers in Jordan. Int J Clin Pharm. 2020;42(2):744–55. [DOI] [PubMed] [Google Scholar]
- 74.Annapureddy A, Minges KE, Henien S, Wang Y, Ross JS, Spatz ES, et al. Association between industry payments to physicians and device selection: A report from the NCDR ICD registry. Circulation [Internet]. 2018;138. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L627103946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ayyash OM, Bandari J, Turner RM, Jacobs BL, Davies BJ. The relationship of physician payments from drug manufacturers to Medicare claims for abiraterone and enzalutamide. Can Urol Assoc J. 2016;10(9–10):S183. [DOI] [PubMed] [Google Scholar]
- 76.Ayyash O, Bandari J, Turner R, Jacobs B, Davies B. Small effect of pharmaceutical industry payments to physicians on medicare prescription habits: Using abiraterone and enzalutamide. J Urol. 2017;197(4):e1013. [Google Scholar]
- 77.Bandari J, Turner RM, Jacobs BL, Davies BJ. An analysis of industry effects on prescriber behavior: Degarelix and denosumab. Can Urol Assoc J. 2016;10(9–10):S179–80. [Google Scholar]
- 78.Berger JT. Pharmaceutical industry influences on physician prescribing: gifts, quasi-gifts, and patient-directed gifts. Am J Bioeth. 2003;3(3):56–7. [DOI] [PubMed] [Google Scholar]
- 79.Bourdette D, Van Leuvin S, Johnston K, Lei M, Hartung D. Industry payments to neurologists who commonly prescribe repository corticotropin gel (H.P. Acthar). Neurology [Internet]. 2017;88(16). Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L616550658 [Google Scholar]
- 80.Carlat D DOCTORS AND INDUSTRY Exploring the link between industry payments to doctors and prescribing habits. Bmj-Br Med J. 2014;349:3. [DOI] [PubMed] [Google Scholar]
- 81.Catricalà A International non-proprietary name (INN) prescribing and conflict of interest. Ric E Prat. 2007;23(1):37–8. [Google Scholar]
- 82.Chua K, Li G, Stahl P, Hyams E. Are Industry Payments for Tadalafil Associated with Prescribing Habits Among Urologists and Primary Care Physicians? J Urol. 2019;201(4):E383–E383. [Google Scholar]
- 83.Duarte-Garcia A, Crowson CS, McCoy R, Ross J, Matteson EL, Shah N. Association between payments by pharmaceutical manufacturers and prescribing behavior in rheumatology. Arthritis Rheumatol. 2018;70:3379–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dyer O Industry payments to doctors drive surge in gabapentinoid prescribing, study finds. BMJ. 2019;366:14672. [DOI] [PubMed] [Google Scholar]
- 85.Eisenberg MD, Stone EM, Pittell H, McGinty EE. The Impact Of Academic Medical Center Policies Restricting Direct-To-Physician Marketing On Opioid Prescribing. Health Aff (Millwood). 2020;39(6):1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eloy JA, Svider PF, Bobian M, Harvey RJ, Gray ST, Baredes S, et al. Industry relationships are associated with performing a greater number of sinus balloon dilation procedures. Int Forum Allergy Rhinol. 2017;7(9):878–83. [DOI] [PubMed] [Google Scholar]
- 87.Fleischman W, Agrawal S, King M, Venkatesh AK, Krumholz HM, McKee D, et al. Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study. BMJ. 2016;354:i4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freemantle N, Johnson R, Dennis J, Kennedy A, Marchment M. Sleeping with the enemy? A randomized controlled trial of a collaborative health authority/industry intervention to influence prescribing practice. Br J Clin Pharmacol. 2000;49(2):174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujiwara RJT, Shih AF, Mehra S. Cross-sectional Analysis of the Relationship between Paranasal Sinus Balloon Catheter Dilations and Industry Payments among Otolaryngologists. Otolaryngol Head Neck Surg. 2017;157(5):880–6. [DOI] [PubMed] [Google Scholar]
- 90.Glass HE. Do clinical grant payment practices in phase 3 clinical trials influence subsequent clinical investigator prescribing behavior? Dis Manag. 2004;7(1):77–87. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez-Campoy JM. The physician-industry relation ship: lessons from the minesota experience. Endocr Pract. 2009;15(4):292–7. [DOI] [PubMed] [Google Scholar]
- 92.Guo T, Sriram S, Manchanda P. “Let the Sunshine In”: The Impact of Industry Payment Disclosure on Physician Prescription Behavior. Mark Sci. 2020;39(3):516–39. [Google Scholar]
- 93.Hadland SE, Rivera-Aguirre A, Marshall BDL, Cerda M. Association of Pharmaceutical Industry Marketing of Opioid Products With Mortality From Opioid-Related Overdoses. Jama Netw Open. 2019;2(1):e186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hadland SE, Cerdá M, Earlywine JJ, Krieger MS, Anderson TS, Marshall BDL. Analysis of Pharmaceutical Industry Marketing of Stimulants, 2014 Through 2018. JAMA Pediatr. 2020;174(4):385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffman MN. Pharmaceutical Detailing Is Not for Everyone: Side Effects May Include Sub-Optimal Prescribing Decisions, Compromised Patient Health, and Increased Prescription Drug Spending. J Leg Med. 2012;33(3):381–97. [DOI] [PubMed] [Google Scholar]
- 96.Humphreys H Conflicts of interest for medical practitioners. J R Coll Physicians Edinb. 2020;50(1):92–3. [DOI] [PubMed] [Google Scholar]
- 97.Ichikawa I, Clayton EW. Doping Doctors: The Influence of the Marketing Departments of Pharmaceutical Companies on Physician and Researcher Behavior in Japan. Account Res. 2016;23(4):245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan R, Nugent CM, Scaffidi MA, Grover SC. Association of Biologic Prescribing for Inflammatory Bowel Disease with Industry Payments to Physicians. Gastroenterology. 2019;156(6):S–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee AJ, Bandari J, Macleod LC, Davies BJ, Jacobs BL. Concentration of Opioid-Related Industry Payments in Opioid Crisis Areas. J Gen Intern Med. 2019;34(2):187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee A, Ayyash O, Maganty A, Macleod L, Bandari J, Jacobs B, et al. Pharmaceutical Payments for Opioids Significantly Favor Extended-Release Medication and Correlate with Physician Prescriptions. J Urol. 2019;201(4):E420–1. [Google Scholar]
- 101.Lee A, Ayyash O, Maganty A, Macleod L, Bandari J, Davies B, et al. Key opinion leaders motivate increased prescriptions among their local physicians. J Urol. 2019;201(4):e628. [Google Scholar]
- 102.Lichter PR. Physician-industry interactions and anti-vascular endothelial growth factor use among US ophthalmologists. Jama Ophthalmol. 2016;134(8):903–4. [DOI] [PubMed] [Google Scholar]
- 103.Lo B, Grady D. Payments to physicians: Does the amount of money make a difference? JAMA - J Am Med Assoc. 2017;317(17):1719–20. [DOI] [PubMed] [Google Scholar]
- 104.Marcum ZA, Chang CY, Barthold D, Holmes HM, Lo-Ciganic W. ASSOCIATION BETWEEN PHARMACEUTICAL INDUSTRY PAYMENTS TO PHYSICIANS AND PRESCRIBING OF BRANDED MEMANTINE AND DONEPEZIL COMBINATION. Value Health. 2020;23:S272–S272. [Google Scholar]
- 105.Maruf M, Sidana A, Purnell S, Fleischman W, Brancato SJ, Agrawal S, et al. Medications for urologic malignancies in the open payments data: Financial relationships between industry and urologists. J Clin Oncol [Internet]. 2017;35(6). Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L618006597 [Google Scholar]
- 106.Maruf M, Sidana A, Fleischman W, Brancato SJ, Purnell S, Agrawal S, et al. Financial Relationships between Urologists and Industry: An Analysis of Open Payments Data. Urol Pract. 2018;5(3):180–6. [DOI] [PubMed] [Google Scholar]
- 107.McCarthy M Doctors who take company cash are more likely to prescribe brand name drugs, analysis finds. BMJ. 2016;352:i1645. [DOI] [PubMed] [Google Scholar]
- 108.Mitchell AP, Winn A, Dusetzina S. Pharmaceutical industry payments and oncologist drug selection. J Clin Oncol [Internet]. 2017;35(15). Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L617388512 [Google Scholar]
- 109.Mitchell AP, Winn A, Lund JL, Dusetzina S. Duration of physician-industry relationships and prescribing changes in oncology. J Clin Oncol [Internet]. 2018;36(15). Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L625971791 [Google Scholar]
- 110.Modi P, Ingham M, Singer E, Chang S. Pharmaceutical industry payments and physician prescribing of urologic drugs. J Urol. 2017;197(4):e929. [Google Scholar]
- 111.Nalleballe K, Veerapaneni KD, Harada Y, Veerapaneni P, Arulprakash N, Lopez-Castellanos JR, et al. Trends of Industry Payments in Neurology Subspecialties. Cureus. 2020;12(7):e9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olch DI. Conflict of interest and physician dispensing. Internist. 1987;28(9):13–6, 24. [PubMed] [Google Scholar]
- 113.Pham-Kanter G, Alexander GC, Nair K. Effect of physician payment disclosure laws on prescribing. Arch Intern Med. 2012;172(10):819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.PharmacoEconomics & Outcomes News. Drug Company Gifts to GPs May Influence Prescribing. Pharm Med. 2020;34(1):66–7. [Google Scholar]
- 115.Prescrire International. Gifts to doctors wield undue influence in France. Prescrire Int. 2020;29(215):135. [Google Scholar]
- 116.Rampulla C Relationships between physicians and industry. Rassegna Patol DellApparato Respir. 2007;22(4–5):253–7. [Google Scholar]
- 117.Rodwin MA. Medical commerce, physician entrepreneurialism, and conflicts of interest. Camb Q Healthc Ethics. 2007;16(4):387–97; discussion 439. [DOI] [PubMed] [Google Scholar]
- 118.Roehr B Pharma gifts associated with higher number and cost of prescriptions written. BMJ. 2017;359:j4979. [DOI] [PubMed] [Google Scholar]
- 119.Schofferman J, Banja J. Conflicts of interest in pain medicine: Practice patterns and relationships with industry. Pain. 2008;139(3):494–7. [DOI] [PubMed] [Google Scholar]
- 120.Serhiyenko V, Ravishanker N, Venkatesan R. Multi-stage multivariate modeling of temporal patterns in prescription counts for competing drugs in a therapeutic category. Appl Stoch Models Bus Ind. 2018;34(1):61–78. [Google Scholar]
- 121.Seto B, Juarez D, Singh D. The relationship between pharmaceutical manufacturer funding and prescribing patterns for anticoagulants in the United States. J Manag Care Spec Pharm. 2015;21:S55. [Google Scholar]
- 122.Sharma M, Johnson ML, Vadhariya A, Marcum ZA, Holmes HM. The association of prescriber characteristics with prescriptions for proton pump inhibitors in the medicare part D beneficiaries. Value Health. 2016;19(3):A317. [Google Scholar]
- 123.Singh P, Adamson A, Mostaghimi A, Foreman H, Barbieri J. Impact of industry payments on prescribing patterns for TNF-alpha inhibitors among Medicare beneficiaries. J Invest Dermatol. 2018;138(9):B6–B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Somai D, van Dijk L, Verheij R, de Bakker D. The effect of pharmaceutical marketing on the prescription of rofecoxib in Dutch general practice. Eur J Public Health. 2004;14(4):70–70. [Google Scholar]
- 125.Steinbrook R Industry payments to physicians: Lessons from orthopedic surgery. Arch Intern Med. 2011;171(19):1765–6. [DOI] [PubMed] [Google Scholar]
- 126.Steinbrook R Industry Payments to Physicians and Prescribing of Brand-name Drugs. Jama Intern Med. 2016;176(8):1123. [DOI] [PubMed] [Google Scholar]
- 127.Steinbrook R. Physicians, Industry Payments for Food and Beverages, and Drug Prescribing. JAMA. 2017;317(17):1753–4. [DOI] [PubMed] [Google Scholar]
- 128.Steinbrook R Industry Payments and Physician Prescribing. Jama Intern Med. 2019;08:08. [DOI] [PubMed] [Google Scholar]
- 129.Taylor R, Giles J. Cash interests taint drug advice. Nature. 2005;437(7062):1070–1. [DOI] [PubMed] [Google Scholar]
- 130.Tsai HJ. Physician-industry interactions: There is no such thing as a free lunch. Taiwan J Obstet Gynecol. 2008;47(2):252–5. [DOI] [PubMed] [Google Scholar]
- 131.Vogel L Pharma freebies for doctors linked to opioidprescribing habits. CMAJ Can Med Assoc J. 2019;191(7):E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim A. Association of physicians’ financial relationships with pharmaceutical companies and their lipid-lowering medication prescribing patterns. J Gen Intern Med. 2015;30:S106. [Google Scholar]
- 133.Bandari J, Ayyash OM, Turner RM 2nd, Jacobs BL, Davies BJ. The lack of a relationship between physician payments from drug manufacturers and Medicare claims for abiraterone and enzalutamide. Cancer. 2017;123(22):4356–62. [DOI] [PubMed] [Google Scholar]
- 134.Chua Kevin J, Li Gen, Stahl Peter J, Hyams Elias S. Receiving Industry Payments is Associated with Prescribing Habits of Tadalafil. Urol Pract. 2019;6(5):282–8. [DOI] [PubMed] [Google Scholar]
- 135.Nguyen T, Andraka-Christou B, Simon K, Bradford WD. Provider-directed marketing may increase prescribing of medications for opioid use disorder. J Subst Abuse Treat. 2019;104:104–15. [DOI] [PubMed] [Google Scholar]
- 136.Nguyen TD, Bradford WD, Simon KI. Pharmaceutical payments to physicians may increase prescribing for opioids. Addiction. 2019;114(6):1051–9. [DOI] [PubMed] [Google Scholar]
- 137.Perlis RH, Perlis CS. Physician Payments from Industry Are Associated with Greater Medicare Part D Prescribing Costs. PLoS ONE Electron Resour. 2016;11(5):e0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wood SF, Podrasky J, McMonagle MA, Raveendran J, Bysshe T, Hogenmiller A, et al. Influence of pharmaceutical marketing on Medicare prescriptions in the District of Columbia. PLoS ONE Electron Resour. 2017;12(10):e0186060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim AS. Association of Industry Payments to Physicians With the Prescribing of Brand-name Statins in Massachusetts. Jama Intern Med. 2016;176(6):763–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


