ABSTRACT
Metallo-β-lactamases (MBLs) result in resistance to nearly all β-lactam antimicrobial agents, as determined by currently employed susceptibility testing methods. However, recently reported data demonstrate that variable and supraphysiologic zinc concentrations in conventional susceptibility testing media compared with physiologic (bioactive) zinc concentrations may be mediating discordant in vitro-in vivo MBL resistance. While treatment outcomes in patients appear suggestive of this discordance, these limited data are confounded by comorbidities and combination therapy. To that end, the goal of this review is to evaluate the extent of β-lactam activity against MBL-harboring Enterobacterales in published animal infection model studies and provide contemporary considerations to facilitate the optimization of current antimicrobials and development of novel therapeutics.
KEYWORDS: β-lactams, animal models, metallo-β-lactamase
INTRODUCTION
The emergence and rapid spread of bacterial infections due to carbapenemase-producing organisms pose a global health challenge (1, 2). Using conventional antimicrobial susceptibility testing (AST) methods, metallo-β-lactamases (MBLs) confer high-level β-lactam resistance which severely limits therapeutic options (2–7). In clinical practice, phenotypic profiling as characterized by the MIC is used to guide therapeutic decision-making via susceptibility classification and breakpoints. In addition, MIC values are pivotal in generating pharmacokinetic/pharmacodynamic (PK/PD) indices in both preclinical and clinical studies for optimizing dosing regimens (8, 9). Finally, in vitro susceptibility data are also used for epidemiological purposes to track changing resistance patterns within geographic regions or institutions (10–12).
An important step in developing effective therapeutic strategies is to understand the dynamics of microbial resistance in the context of in vitro susceptibility, host factors, and clinically relevant antibiotic exposure (13–15). Indeed, based on host factors, modifications to traditional susceptibility testing criteria are required for certain agents, including daptomycin and cefiderocol, to derive clinically meaningful MICs representative of therapeutic success or failure (16–18). Recently, we demonstrated that in vitro resistance of MBL-harboring Enterobacterales did not correlate with in vivo resistance or therapeutic failure after carbapenem treatment. Therein, clinically achievable exposures of meropenem resulted in bacterial reduction among a variety of clinical MBL-harboring Enterobacterales in a murine lung and thigh infection model (19). Of note, we also showed that the in vitro-in vivo discordance observed with MBLs and meropenem extends to noncarbapenem β-lactams, such as cefepime (20). As such, we concluded that differences between physiologic and supraphysiologic zinc concentrations in humans and Mueller-Hinton broth, respectively, could be mediating discordant zinc-dependent MBL resistance.
Further insights can be gained from human studies. However, interpretation of current outcome data among patients infected with MBL-producing organisms is challenging, as most studies have small sample sizes and are retrospective in design with investigators having no control over patient characteristics and treatment (21–23). Furthermore, infected patients are critically ill at baseline and typically receive combination antimicrobial therapy or ineffective empiric therapy. In the preclinical research arena, animal infection models have been instrumental in bridging in vitro data to human studies. These experimental animal models provide a reliable means of confirming or establishing PK/PD relationships and evaluating antimicrobial efficacy against isolates typically not represented in clinical trials (9, 24). Furthermore, specific magnitudes of bacterial reduction, such as 1-log kill, have been adopted as preclinical surrogates for clinical efficacy (25–27).
As a result of an apparent high level of in vitro resistance, clinical use of β-lactam antibiotics and subsequent efficacy data against MBL-harboring organisms are limited. To address this knowledge gap and ascertain the effect, if any, of β-lactam monotherapy, this review leverages data from MBL-infected animal studies, of which the majority were designed to evaluate investigational metallo-β-lactamase inhibitors in vivo.
LITERATURE SEARCH
A literature search for studies evaluating antimicrobial efficacy against metallo-β-lactamase-producing isolates using PubMed from January 2000 to December 2020 was conducted. A 2-step search strategy was developed, as follows: one identifying articles on “metallo-β-lactamase,” and a second limiting the search to mentions of “in vivo,” “murine,” or “mouse.” In addition, a manual search of reference lists from relevant articles was performed.
DATA ABSTRACTION AND ANALYSIS
Study data were entered into a Microsoft Excel spreadsheet, and accuracy was verified by all authors. Type of animal infection model, isolate genotype, in vitro susceptibility, dose, and administration route of the β-lactam, β-lactamase-inhibitor (BL-I), metallo-β-lactamase-inhibitor (MBL-I), and endpoint measured (i.e., change in bacterial burden) were extracted and collated. Extracted data were limited to Enterobacterales and studies in which bacterial density in the control (vehicle) arm at study endpoint was reported. Given that the primary objective of many studies meeting inclusion criteria was to assess the potential of the BL/MBL-I combination to reverse β-lactam-resistance, bacterial density (i.e., CFU count) results after treatment with the β-lactam monotherapy were rarely reported as a numerical value. In those instances, we abstracted data from graphs.
In studies that reported bacterial density at the time of treatment (0-h control), we determined if microbiological activity of β-lactam monotherapy or BL/MBL-I combination therapy at the study endpoint met the following criteria: (i) ≥1-log10 bacterial growth relative to 0-h control, (ii) bacteriostatic (stasis) relative to 0-h control, or (iii) ≥1-log10 bacterial reduction relative to 0-h control. In studies that did not report 0-h control bacterial density, we defined microbiological activity at study endpoint as (i) “no difference” if no change in bacterial density relative to the control group at the study endpoint was observed or (ii) “↓CFU” if a reduction in bacterial density relative to the control group at the study endpoint was observed.
ANIMAL MODELS AND STUDY ENDPOINTS
We identified 27 articles that reported in vivo studies evaluating β-lactam activity against MBL-producing Enterobacterales. Key characteristics of the animal infection model, β-lactam doses administered, and change in bacterial burden are summarized in Table 1 and 2 (19, 20, 28–52). Table 1 highlights studies (n = 14) that reported bacterial density counts for 0-h control animals, allowing for a ≥1-log10 bacterial reduction assessment (preclinical surrogate for clinical efficacy) (25–27), while Table 2 describes characteristics and outcomes of all other studies.
TABLE 1.
Activity of β-lactam monotherapy or combination therapy on MBL-bacterial burden in animal studies reporting 0-h control bacterial density required for bacteriostatic and 1-log10 bacterial reduction assessmenta
| Reference | Animal infection model | Isolate (genotype) | Inoculum (CFU/ml) | BL MIC (mg/liter) | BL dose and frequencyb | MBL-I or BL-I dose and frequency | Dose initiation | Measured endpoint | BL alone activity | BL/MBL-I or BL/BL-I activity |
|---|---|---|---|---|---|---|---|---|---|---|
| Ooi et al., 2020 (38) | Murine neutropenic thigh model | E. coli IR3 (NDM-1) | 1.95 × 106 | MEM: 16 | MEM 50 mg/kg q2h s.c. | Compound-272 100 mg/kg q2h s.c. | 1 h postinoculation | 9 h thigh | ∼0.5-log kill | ≥1-log kill |
| MEM 250 mg/kg q2h s.c. | Compound-272 100 mg/kg q2h s.c. | 1 h postinoculation | 9 h thigh | ≥1-log kill | ≥1-log kill | |||||
| Abdelraouf et al., 2020 (20) | Murine neutropenic thigh model | 21 Isolates (NDM, VIM, IMP) | 107 | FEP MIC range: 64 to >256; FEP MIC rangeb: ≤0.03 to 8 | FEP 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (19/21 isolates) | ||
| 5 Isolates (wild-type, isogenic-derived and clinical NDM, VIM, IMP) | FEP MIC range: ≤0.25 to >256; FEP MIC rangec: ≤0.03 to 32 | FEP 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (4/5 isolates) | |||||
| 3 Isolates (KPC-2, KPC-3) | FEP MIC: >256; FEP MIC rangec: >32 | FEP 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | Stasis (2/3 isolates) | |||||
| Das et al., 2020 (46) | Murine neutropenic thigh model | E. coli NTBC121 (NDM-1) | NA | MEM: 32 | MEM 50 mg/kg q4h s.c. | ANT2681 ∼10 mg/kg q4h i.v. | 2 h postinoculation | 24 h thigh | Stasis | Stasis |
| MEM 50 mg/kg q4h s.c. | ANT2681 200 mg/kg q4h i.v. | 2 h postinoculation | 24 h thigh | Stasis | ≥1-log kill | |||||
| MEM 75 mg/kg q4h s.c. | 2 h postinoculation | 24 h thigh | Stasis | |||||||
| MEM 100 mg/kg q4h s.c. | 2 h postinoculation | 24 h thigh | ≥1-log kill | |||||||
| MEM 200 mg/kg q4h s.c. | 2 h postinoculation | 24 h thigh | ≥1-log kill | |||||||
| Asempa et al., 2020 (19) | Murine neutropenic lung model | 10 Isolates (NDM, VIM, IMP) | 107 | MEM MIC range: 16 to >64; MEM MIC rangec: ≤0.06 to 0.5 | MEM 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h lung | ≥1-log kill (10/10 isolates) | ||
| Murine neutropenic thigh model | 24 Isolates (NDM, VIM, IMP) | 107 | MEM MIC range: 8 to >64; MEM MIC rangec: ≤0.06 to 1 | MEM 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (21/24 isolates) | |||
| Moya et al., 2019 (47) | Murine neutropenic thigh model | 4 Isolates (NDM, CMY, SHV, TEM, CTX-M-15) | 5 × 106 | FEP MIC range: 128 to >256 | FEP 1,200 mg/kg/day fractionated q2h s.c. | ZID 900 mg/kg/day fractionated q2h s.c. | 2 h postinoculation | 24 h thigh | ≥1-log growth (4/4 isolates) | ≥0.5-log kill (4/4 isolates) |
| FEP 1,200 mg/kg/day fractionated q2h s.c. | WCK 5153 900 mg/kg/day fractionated q2h s.c. | 2 h postinoculation | 24 h thigh | ≥1-log growth (4/4 isolates) | ≥0.5-log kill (4/4 isolates) | |||||
| ATM MIC range: >256 | ATM 900 mg/kg/day fractionated q2h s.c. | ZID 900 mg/kg/day fractionated q2h s.c. | 2 h postinoculation | 24 h thigh | ≥1-log growth (4/4 isolates) | ≥0.5-log kill (4/4 isolates) | ||||
| ATM 900 mg/kg/day fractionated q2h s.c. | WCK 5153 900 mg/kg/day fractionated q2h s.c. | 2 h postinoculation | 24 h thigh | ≥1-log growth (4/4 isolates) | ≥0.5-log kill (4/4 isolates) | |||||
| 4 Isolates (NDM, CMY, SHV, TEM, CTX-M-15) | 5 × 106 | MEM MIC range: ≥128 | MEM-cilastatin 225 mg/kg/day fractionated q4h s.c. (HSR) | 2 h postinoculation | 24 h thigh | ≥1-log growth (4/4 isolates) | ||||
| Weiss et al., 2019 (48) | Murine neutropenic thigh model | K. pneumoniae UNT170-1 (KPC-2) | ∼105 to 106 | MEM: ≥16 | MEM 300 mg/kg/day fractionated q4h s.c.d | 2 h postinoculation | 24 h thigh | ≥1-log growth | ||
| LYS228: 0.5 to 1 | LYS228 60 mg/kg/day fractionated q4h s.c.d | 2 h postinoculation | 24 h thigh | Stasis | ||||||
| LYS228 1,620 mg/kg/day fractionated q4h s.c.d | 2 h postinoculation | 24 h thigh | ≥1-log kill | |||||||
| K. pneumoniae UNT184-1 (NDM-1) | ∼105 to 106 | MEM: ≥16 | MEM 300 mg/kg/day fractionated q4h s.c.d | 2 h postinoculation | 24 h thigh | ≥1-log kill | ||||
| LYS228: 8 to 16 | LYS228 60 mg/kg/day fractionated q4h s.c.d | 2 h postinoculation | 24 h thigh | ∼0.5-log kill | ||||||
| LYS228 1620 mg/kg/day fractionated q4h s.c.d | 2 h postinoculation | 24 h thigh | ≥1-log kill | |||||||
| Everett et al., 2018 (49) | Murine neutropenic thigh model | E. coli IR3 (NDM-1) | 1.5 × 106 | MEM: 32 | MEM 50 mg/kg i.v. | ANT431 300 mg/kg i.v. | 1, 3, 5, and 7 h postinoculation | 9 h thigh | ∼1-log growth | ∼0.5-log kill |
| MEM 250 mg/kg i.v. | ANT431 300 mg/kg i.v. | 1, 3, 5, and 7 h postinoculation | 9 h thigh | ∼0.5-log kill | ≥1-log kill | |||||
| Monogue et al., 2017 (50) | Murine neutropenic lung model | K. pneumoniae (NDM, OXA-48, CTX-M) | 107 | CAZ MIC: >64 | CAZ 2 g q8h (HSR, s.c.)d | AVI 500 mg q8h (HSR, s.c.)d | 2 h postinoculation | 24 h lung | ≥1-log kill | |
| ATM MIC: 64 | ATM 2 g q6h (HSR, s.c.)d | 2 h postinoculation | 24 h lung | ≥1-log growth | ||||||
| CAZ 2 g q8h (HSR, s.c.)d + ATM 2 g q6h (HSR, s.c.)d | AVI 500 mg q8h (HSR, s.c.)d | 2 h postinoculation | 24 h lung | ≥1-log kill | ||||||
| Marshall et al., 2017 (51) | Murine neutropenic thigh model | K. pneumoniae 1.41 (NDM-1, CTX-M-15, DHA, SHV, TEM) | 5.01 log10 | CAZ: >512 | CAZ 256 mg/kg q8h s.c. | AVI 64 mg/kg q8h s.c. | 2 h postinoculation | 24 h thigh | Stasis | ≥1-log kill |
| CAZ 128 mg/kg q8h s.c. | AVI 32 mg/kg q8h s.c. | ≥1-log growth | Stasis | |||||||
| CAZ 64 mg/kg q8h s.c. | AVI 16 mg/kg q8h s.c. | ≥1-log growth | ≥1-log growth | |||||||
| CAZ 32 mg/kg q8h s.c. | AVI 8 mg/kg q8h s.c. | ≥1-log growth | ||||||||
| ATM: 128 | ATM 256 mg/kg q8h s.c. | 2 h postinoculation | 24 h thigh | ≥1-log growth | ||||||
| ATM 128 mg/kg q8h s.c. | ≥1-log growth | |||||||||
| ATM 64 mg/kg q8h s.c. | ≥1-log growth | |||||||||
| CAZ/AVI: 64 | CAZ 32 mg/kg q8h s.c. + ATM 32 mg/kg q8h s.c. | AVI 8 mg/kg q8h s.c. | 2 h postinoculation | 24 h thigh | ∼0.5-log kill | |||||
| CAZ 16 mg/kg q8h s.c. + ATM 16 mg/kg q8h s.c. | AVI 4 mg/kg q8h s.c. | ≥1-log growth | ||||||||
| CAZ 8 mg/kg q8h s.c. + ATM 8 mg/kg q8h s.c. | AVI 2 mg/kg q8h s.c. | ≥1-log growth | ||||||||
| CAZ 4 mg/kg q8h s.c. + ATM 4 mg/kg q8h s.c. | AVI 1 mg/kg q8h s.c. | ≥1-log growth | ||||||||
| Ghazi et al., 2015 (52) | Murine neutropenic thigh model | 9 Isolates (wild-type, isogenic-derived and clinical VIM) | 107 | MEM MIC range: 0.06 to >512 | MEM 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (9/9 isolates) | ||
| FEP MIC range: 0.06 to >512 | FEP 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (3/9 isolates) | ||||||
| MacVane et al., 2014 (28) | Murine neutropenic thigh model | 6 Isolates (wild-type, isogenic-derived and clinical NDM) | 107 | CAZ MIC range: 0.25 to >128 | CAZ 2 g q8h (HSR, s.c.)d | AVI 500 mg q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (2/6 isolates) | ≥1-log kill (5/6 isolates) |
| Crandon et al., 2013 (29) | Murine neutropenic thigh model | 14 Isolates (NDM) | 107 | ATM MIC range: 16 to >256 | ATM 2 g q6h (HSR, s.c.)d | AVI 600 mg q6h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (2/14 isolates) | ≥1-log kill (12/14 isolates) |
| Wiskirchen et al., 2014 (43) | Murine neutropenic thigh model | 4 Isolates (wild-type, isogenic-derived and clinical NDM) | 107 | DOR MIC range: 0.03 to 32 | DOR 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (4/4 isolates) | ||
| ETP MIC range: 0.012 to >32 | ETP 1 g q24h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (4/4 isolates) | ||||||
| CAZ MIC range: 0.25 to >128 | CAZ 2 g q8h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (2/4 isolates) | ||||||
| ATM MIC range: ≤0.25 to >256 | ATM 2 g q6h (HSR, s.c.)d | 2 h postinoculation | 24 h thigh | ≥1-log kill (2/4 isolates) | ||||||
| Daikos et al., 2007 (31) | Murine neutropenic thigh model | K. pneumoniae-S (wild-type) | 107 | IMI: 0.125 | IMI 30 mg/kg q2h i.p. | 2 h postinoculation | 24 h thigh | ≥1-log kill | ||
| IMI 60 mg/kg q2h i.p. | ≥1-log kill | |||||||||
| K. pneumoniae-Ia (VIM-1) | IMI: 2 | IMI 30 mg/kg q2h i.p. | 2 h postinoculation | 24 h thigh | Stasis | |||||
| IMI 60 mg/kg q2h i.p. | ≥1-log kill | |||||||||
| K. pneumoniae-Ib (VIM-1) | IMI: 4 | IMI 30 mg/kg q2h i.p. | 2 h postinoculation | 24 h thigh | Stasis | |||||
| IMI 60 mg/kg q2h i.p. | Stasis | |||||||||
| K. pneumoniae-R (VIM-1) | IMI: 32 | IMI 30 mg/kg q2h i.p. | 2 h postinoculation | 24 h thigh | Stasis | |||||
| IMI 60 mg/kg q2h i.p. | Stasis |
BL, β-lactam; MBL-I, metallo-β-lactamase inhibitor; MEM, meropenem; IMI, imipenem; DOR, doripenem; ETP, ertapenem; FEP, cefepime; CAZ, ceftazidime; AZT, aztreonam; AVI, avibactam; ZID, zidebactam; NAC, nacubactam; HSR, human simulated regimen; cUTI, complicated urinary tract infection; s.c., subcutaneous; i.p., intraperitoneal; i.v., intravenous; NA, not available.
q2h, every 2 hours, q4h, every 4 hours; q6h, every 6 hours; q8h, every 8 hours; q24h, every 24 hours.
MIC determined in zinc-depleted media.
Pretreatment with uranyl nitrate.
TABLE 2.
Activity of β-lactam monotherapy or combination therapy on MBL-bacterial burden in animal studiesa
| Reference | Animal infection model | Isolate (genotype) | Inoculum (CFU/ml) | BL MIC (mg/liter) | BL dose and frequency | MBL-I or BL-I dose and frequency | Dose initiation | Measured endpoint | Activity, BL alone | Activity, BL/MBL-I or BL/BL-I |
|---|---|---|---|---|---|---|---|---|---|---|
| Samuelsen et al., 2020 (32) | Murine neutropenic peritonitis model | K. pneumoniae 50752501 (NDM-1) | 5 × 106 | MEM: 64 | MEM 33 mg/kg single dose s.c. | ZN148 10 mg/kg single dose s.c. | MEM: 1.5 h postinoculation; ZN148: 1 h postinoculation | 5 h blood | ↓CFU | ↓CFU |
| 5 h peritoneal fluid | ↓CFU | ↓CFU | ||||||||
| MEM 33 mg/kg single dose s.c. | ZN148 33 mg/kg single dose s.c. | MEM: 1.5 h postinoculation; ZN148: 1 h postinoculation | 5 h blood | ↓CFU | ↓CFU | |||||
| 5 h peritoneal fluid | ↓CFU | ↓CFU | ||||||||
| MEM 33 mg/kg single dose s.c. | ZN148 100 mg/kg single dose s.c. | MEM: 1.5 h postinoculation; ZN148: 1 h postinoculation | 5 h blood | ↓CFU | ↓CFU | |||||
| 5 h peritoneal fluid | ↓CFU | ↓CFU | ||||||||
| Chen et al., 2020 (33) | Murine peritonitis model | E. coli EC10 (NDM-1) | 2 × 107 | MEM: 128 | MEM 10 mg/kg single dose i.p. | Cisplatin 5 mg/kg single dose i.p. | 0.5 h postinoculation | 48 h liver | No difference | ↓CFU |
| 48 h spleen | No difference | ↓CFU | ||||||||
| Liu et al., 2019 (34) | Murine neutropenic thigh model | E. coli B2 (NDM-5) | 1.5 × 106 | MEM: 32 | MEM 16 mg/kg single dose i.p. | PEP4 16 mg/kg single dose i.p. | 2 h postinoculation | 24 h thigh | No difference | ↓CFU |
| Liu et al., 2019 (35) | Murine thigh model | E. coli ZC-YN3 (NDM-1) | 5 × 106 | MEM: 16 | MEM 10 mg/kg s.c. | Pterostilbene 80 mg/kg s.c. | NA postinoculation, 3 doses at q8h for 24 hours | 72 h thigh | No difference | ↓CFU |
| Ma et al., 2019 (36) | Murine peritonitis model | E. coli XJ141026 (NDM-1) | 8 × 107 | MEM: 64 | MEM 10 mg/kg single dose i.p. | Thanatin 0.1 mg/kg single dose i.p. | 1 h postinoculation | 24 h spleen | ↓CFU | ↓CFU |
| 24 h liver | ↓CFU | ↓CFU | ||||||||
| Yarlagadda et al., 2018 (37) | Murine peritonitis model | K. pneumoniae R3934 (NDM-1) | ∼106 | MEM: >100 | MEM 10 mg/kg i.p. | Dipi-van 10 mg/kg i.p. | 2 and 24 h postinoculation | 48 h liver | ↓CFU | ↓CFU |
| 48 h kidney | ↓CFU | ↓CFU | ||||||||
| 48 h lungs | No difference | ↓CFU | ||||||||
| 48 h spleen | No difference | ↓CFU | ||||||||
| Monogue et al., 2018 (39) | Murine neutropenic cUTI model | 3 Isolates (NDM-1) | 105.5 | MEM: 64 to 256 | MEM 1 g q8h (HSR, s.c.) | NAC 1.5 g q8h (HSR, s.c.) | 3 h postinoculation | 48 h kidney | ↓CFU (3/3 isolates) | ↓CFU (3/3 isolates) |
| CAZ 25 mg/kg s.c. | AVI 6.25 mg/kg s.c. | 3, 7, 23, and 31 h postinoculation | 48 h kidney | No difference (3/3 isolates) | ||||||
| 7 Isolates (KPC, OXA-48) | 105.5 | MEM: 16 to 512 | MEM 1 g q8h (HSR, s.c.) | NAC 1.5 g q8h (HSR, s.c.) | 2 h postinoculation | 48 h kidney | No difference (5/7 isolates) | ↓CFU (7/7 isolates) | ||
| CAZ 25 mg/kg s.c. | AVI 6.25 mg/kg s.c. | 3, 7, 23, and 31 h postinoculation | 48 h kidney | ↓CFU (7/7 isolates) | ||||||
| Sully et al., 2017 (40) | Murine peritonitis model | E. coli CVB-1 (NDM-1) | 3 × 106 (5% mucin) | MEM: 16 | MEM 50 mg/kg s.c. | PPMO 5 mg/kg i.p. with inoculum | 0 h postinoculation | 6 h blood | No difference | ↓CFU |
| 0 and 6 h postinoculation | 10 h blood | ↓CFU | ↓CFU | |||||||
| 0 and 6 h postinoculation | 10 h spleen | ↓CFU | ↓CFU | |||||||
| Falconer et al., 2015 (41) | Murine peritonitis model | K. pneumoniae (NDM-1) | 2 × 106 | MEM: >2 µM | MEM 10 mg/kg single dose s.c. | SIT-Z5 10 mg/kg single dose s.c. | 0.5 h postinoculation | 48 h liver | No difference | ↓CFU |
| 48 h spleen | No difference | ↓CFU | ||||||||
| King et al., 2014 (42) | Murine peritonitis model | K. pneumoniae N11-2218 (NDM-1) | 2 × 106 | MEM: 32 | MEM 10 mg/kg single dose s.c. | AMA 10 mg/kg single dose s.c. | 0.5 h postinoculation | 48 h spleen | No difference | ↓CFU |
| 48 h liver | No difference | ↓CFU | ||||||||
| Wiskirchen et al., 2014 (43) | Murine thigh model | 10 Isolates (wild-type, isogenic-derived and clinical NDM) | 108 | DOR MIC range: 0.03 to >64 | DOR 2 g q8h (HSR, s.c.)b | 2 h postinoculation | 24 h thigh | ↓CFU (10/10 isolates) | ||
| ETP MIC range: 0.012 to 128 | ETP 1 g q24h (HSR, s.c.)b | 2 h postinoculation | 24 h thigh | ↓CFU (8/10 isolates) | ||||||
| CAZ MIC range: 0.25 to >128 | CAZ 2 g q8h (HSR, s.c.)b | 2 h postinoculation | 24 h thigh | ↓CFU (3/10 isolates) | ||||||
| ATM MIC range: ≤0.25 to >256 | ATM 2 g q6h (HSR, s.c.)b | 2 h postinoculation | 24 h thigh | ↓CFU (4/10 isolates) | ||||||
| Wiskirchen et al., 2013 (30) | Murine thigh model | 6 Isolates (wild-type, isogenic-derived and clinical NDM) | 108 | ETP MIC range: 0.012 to >32 | ETP 1 g q24h (HSR, s.c.)b | 2 h postinoculation | 24 h thigh | ↓CFU (5/6 isolates) | ||
| DOR MIC range: 0.03 to >32 | DOR 2 g q8h (HSR, s.c.)b | ↓CFU (6/6 isolates) | ||||||||
| Isogenic-derived KPC-2 | 108 | ETP: 0.012 to 4 | ETP 1 g q24h (HSR, s.c.)b | 2 h postinoculation | 24 h thigh | No difference | ||||
| DOR: 0.03 to 1 | DOR 2 g q8h (HSR, s.c.)b | No difference | ||||||||
| Murine thigh model | E. coli 389 (NDM-1, TEM-1, CTX-M-15) | 108 | ETP: >32 | ETP 1 g q24h (HSR, s.c.)b | 2 h postinoculation | 72 h thigh | ↓CFU | |||
| DOR: 8 | DOR 2 g q8h (HSR, s.c.)b | ↓CFU | ||||||||
| Yoshizumi et al., 2012 (44) | Murine peritonitis model | E. coli TUM10702 (NDM-1) | 2.7 × 107 | IMI: 512 | IMI/cilastatin 25 mg/kg single dose s.c. | Ca-EDTA 200 mg/kg single dose s.c. | 2 h postinoculation | 4 h liver | No difference | ↓CFU |
| 4 h blood | ↓CFU | |||||||||
| Murine neutropenic peritonitis model | E. coli TUM10702 (NDM-1) | 6 × 107 | IMI: 512 | IMI/cilastatin 10 mg/kg single dose s.c. | Ca-EDTA 100 mg/kg single dose s.c. | 2 h postinoculation | 4 h liver | No difference | ↓CFU | |
| 4 h blood | No difference | ↓CFU | ||||||||
| Souli et al., 2011 (45) | Rabbit intra-abdominal abscess model | E. coli (VIM-1) | 108 | IMI: 1 | IMI 70 mg/kg q12h, 30 min i.v. infusion | 4 h postinoculation | 96 h bacterial abscess | ↓CFU | ||
| MEM: ≤0.25 | MEM 125 mg/kg q12h, 10 min i.v. infusion | 4 h postinoculation | 96 h bacterial abscess | ↓CFU | ||||||
| ERT: 1.5 | ERT 60 mg/kg q12h, i.v. bolus | 4 h postinoculation | 96 h bacterial abscess | ↓CFU | ||||||
| ATM: ≤0.25 | ATM 70 mg/kg q12h, i.v. bolus | 4 h postinoculation | 96 h bacterial abscess | ↓CFU |
BL, β-lactam; MBL-I, metallo-β-lactamase inhibitor; MEM, meropenem; IMI, imipenem; DOR, doripenem; ETP or ERT, ertapenem; FEP, cefepime; CAZ, ceftazidime; AZT, aztreonam; AVI, avibactam; ZID, zidebactam; NAC, nacubactam; HSR, human simulated regimen; cUTI, complicated urinary tract infection; s.c., subcutaneous; i.p., intraperitoneal; i.v., intravenous; NA, not available; q12h, every 12 hours.
Pretreatment with uranyl nitrate.
With the exception of the rabbit intra-abdominal abscess study by Souli et al. (45), all studies were conducted in mice. The most common infection model was the thigh model in 18 studies, followed by peritonitis model (9 studies). In the peritonitis model, CFU was enumerated from a variety of sites, including liver (7 studies), spleen (6 studies), blood (6 studies), kidney (5 studies), and peritoneal fluid (3 studies). Change in bacterial density was assessed at a variety of time points, ranging from 4 to 96 hours postinoculation, with the majority at 24 and 48 hours. A slightly larger proportion of studies utilized the neutropenic model than the immunocompetent model (19 versus 13 studies). Historically, in vivo efficacy or PK/PD assessments have utilized the neutropenic model for several reasons. First, immunosuppression allows for the evaluation of antimicrobial activity against the infecting pathogen without the contribution and confounding effect of the immune system (27). Consequently, a larger dose of drug is typically required to achieve a similar change in bacterial burden (i.e., bacteriostasis or 1-log kill) in neutropenic than in immunocompetent infection models (53). Second, inhibition of the immune system is sometimes necessary to enable bacteria to establish an infection in vivo (27).
ISOLATE CHARACTERISTICS
Isolate selection in the studies was consistent with real-world prevalence and distribution (54, 55). Indeed, the vast majority of MBL-harboring Enterobacterales evaluated in these animal models were clinical Klebsiella pneumoniae or Escherichia coli isolates harboring NDM-1. Only 5 studies included either a VIM- or IMP-harboring isolate (19, 20, 31, 45, 56). Current surveillance data suggest that among MBLs, NDMs are the most frequently identified worldwide, with NDM-1 being the most detected NDM variant (6, 57, 58). For example, in a multiyear, global distribution study, Kazmierczak et al. reported that among MBL-positive Enterobacteriaceae, 44% of isolates carried blaNDM, 39% carried blaVIM, and 17% carried blaIMP (58). However, sporadic outbreaks due to Enterobacterales isolates harboring VIM and IMP in parts of Europe as well as an increasing global prevalence suggest preclinical animal studies evaluating novel therapies should not be limited only to NDM producers (59, 60). Furthermore, evidence of different zinc sensitivities between genotypes and the evolution of MBL variants also warrants inclusion of a genotypically diverse isolate collection when evaluating β-lactam and BL/MBL-I efficacy (42, 61–64).
In select studies, investigators utilized wild-type (WT) strains and their isogenic derivatives with inserted plasmids encoding an NDM, VIM, IMP, or KPC as experimental positive and negative controls (Table 1 and 2).
The rabbit model utilized a VIM-producing E. coli isolate with a carbapenem-susceptible phenotypic profile determined by broth microdilution (meropenem and imipenem) and Etest (ertapenem). Notably, this isolate was shown to be positive for MBL production with the imipenem-EDTA double-disc synergy test and negative for extended-spectrum β-lactamases (ESBLs) by the isoelectric focusing test. Despite the presence of a MBL (VIM-1 by genotypic confirmation), the isolate was susceptible to imipenem (MIC, 1 mg/liter), meropenem (MIC, ≤0.25 mg/liter), ertapenem (MIC, 1.5 mg/liter), and aztreonam (MIC, ≤0.25 mg/liter) (45). In contrast, all murine studies in this literature review utilized carbapenem nonsusceptible isolates, with susceptibility routinely determined by conventional broth microdilution. Additional in vitro susceptibility testing with zinc-depleted media was reported in 2 studies (19, 20).
Besides reporting the MBL genotype, only a few studies provided details on ESBLs, which are typically coproduced by these clinical strains. Similarly, few studies utilized publicly available or clinical reference strains such as ATCC or NCTC isolates. In the future, reporting of both target (i.e., NDM-1) and coharbored enzymes as well as inclusion of reference strains will enable valuable drug-bug comparisons by other laboratories as drug development advances.
A range of bacterial inoculums (105 to 108 CFU/ml) were used to establish infections in the studies evaluated, reflecting typical animal inoculation practices observed in the literature.
CHOICE OF β-LACTAM AND DOSING REGIMEN
Meropenem was the preferred β-lactam backbone to pair with MBL-Is in all but one study (imipenem) (44). In contrast, a variety of β-lactam agents were administered in the studies designed to evaluate β-lactam agents alone (i.e., no MBL-I combination therapy), namely, meropenem, doripenem, imipenem, ertapenem, cefepime, ceftazidime, and aztreonam. In a small number of these studies, a serine-based β-lactamase inhibitor such as avibactam was also used in combination (Table 1 and 2).
The β-lactam dose and frequency of administration differed greatly between studies with no clear dose justification in the majority of studies for the MBL-I activity assessed. Notably, 10 mg/kg of body weight meropenem was the most common dose administered and was typically given as a single dose. In comparison, studies designed to evaluate β-lactam agents alone tended to utilize murine doses that resulted in drug exposures similar to those achieved in humans receiving clinical doses.
Understandably, the majority of MBL-I studies are in the early preclinical phase and utilized carbapenem/MBL-I doses sufficient enough to demonstrate proof of concept. Nonetheless, the importance of dosage and dosing frequency selection in preclinical in vivo research cannot be understated. Indeed, for studies utilizing approved and existing β-lactams such as meropenem as the backbone therapy, consideration of the well-established pharmacokinetics of meropenem in early studies will provide more accurate assessments and a higher degree of confidence in human translatability upon addition of the investigational inhibitor. For instance, recognizing meropenem’s rapid clearance after pharmacokinetic analysis of the β-lactam backbone (meropenem) and MBL-I (compound-272), Ooi et al. utilized a frequent dosing interval (every 2 hour) to compensate for clearance. The half-life of meropenem was determined to be 0.3 hours (18 minutes) compared with 1 hour for compound-272, demonstrating that a single dose or infrequent dosing would have been significantly inadequate (38). With regard to the rabbit experimental model, doses were selected to achieve concentrations comparable to those achievable in humans, with >50% time of a 24-h period that the drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (T>MIC) for the respective isolates for the dosing interval (45).
ACTIVITY OF β-LACTAMS ON BACTERIAL BURDEN
Observations of reductions in bacterial density after administration of β-lactam monotherapy were markedly different between studies (Table 1 and 2). In studies that utilized nonoptimized β-lactam exposures (e.g., single-dose frequency) and demonstrated treatment benefit, this evidence of bacterial killing was not limited to any one infection model, immune status, isolate genotype/phenotype, or site of infection. Notably, the administration route (subcutaneous [s.c.] and intravenous [i.v.]) of identical meropenem doses appeared to result in different magnitudes of efficacy for 2 studies with similar infection model designs (E. coli IR3; NDM-1) (38, 49). The lack of reported CFU counts from 0-h controls in several studies reviewed precludes a more robust comparison of β-lactam efficacy to established bacteriostatic or 1-log bacterial density reduction endpoints indicative of clinical efficacy. As a result, bacterial density of treatment groups could be compared only with control groups enumerated at the end of study (Table 2). So, while a β-lactam treatment effect relative to endpoint controls may have been observed, this approach does not account for the possibility that net bacterial growth relative to bacterial density at the onset of infection (0-h control) may have still occurred with the treatment regimen. Furthermore, the lack of CFU data from 0-h control animals hinders any efforts to evaluate if an in vitro-in vivo inoculum effect exists with MBL-producing bacteria (65).
Several antimicrobial agents, such as meropenem, doripenem, imipenem, ertapenem, ceftazidime-avibactam, aztreonam-avibactam, and aztreonam-ceftazidime-avibactam, demonstrated microbiological activity when administered at clinically achievable exposures (Table 1). The majority of these studies demonstrated bacterial activity ranging from bacteriostasis to 2-log10 bacterial reduction in the thigh, lung, and kidney infection models relative to bacterial density at onset of infection (0-h controls). Of these agents, the activity of aztreonam in combination with avibactam is not unexpected given aztreonam’s stability against MBLs and avibactam’s role in protecting aztreonam from hydrolysis by coharbored ESBLs. In isolates lacking clinically relevant ESBLs, cefepime alone also demonstrated microbiological in vivo activity despite high-level in vitro resistance (20).
Notably, a humanized meropenem regimen in the murine thigh study by Moya et al. was reported to be inefficacious against 4 NDM-harboring isolates (47). Unfortunately, the human meropenem dose on which the murine PK profile was based on was not provided, but a 42% free T>MIC (fT>MIC) at a MIC of 1 mg/liter and 26% fT>MIC at an MIC of 4 mg/liter were reported (47). For comparison, the percent free time above MIC achieved in humans receiving either 2 g meropenem every 8 hours (q8h) as a 3-h intravenous infusion is 100% fT>MIC, at an MIC of 1 mg/liter, and 85% fT>MIC, at an MIC of 4 mg/liter, or 1 g meropenem q8h (0.5-h intravenous infusion) is 86% fT>MIC, at an MIC of 1 mg/liter, and 50% fT>MIC, at an MIC of 4 mg/liter, making interpretation of their results challenging (39, 66–68). In this regard, reporting the human β-lactam dose that is humanized in the animal model and comparison of relevant T>MIC indices is imperative for providing robust translatable data.
In the five studies that included wild-type (WT) and isogenically derived isolates with inserted plasmids, clinically relevant carbapenem and ceftazidime-avibactam exposures resulted in bacterial killing in the WT, WT-blaNDM, and WT-blaVIM strains, while growth was observed with the WT-blaKPC strain (20, 28, 30, 43, 52). Similarly, a select number of studies included clinical KPC- and OXA-48-harboring isolates as controls and demonstrated inefficacy with carbapenems (Table 1 and 2).
In the rabbit model, investigators created an intra-abdominal abscess that was inoculated with a carbapenem-susceptible VIM-1-producing E. coli isolate. Concordant with in vitro susceptibility and an ESBL-negative profile, treatment with meropenem, imipenem, ertapenem, and aztreonam resulted in significant bacterial reduction (45).
DISCUSSION
Over the last 2 decades, there has been a warranted and growing interest in the discovery and development of MBL-Is (5, 69, 70). As detailed in this review, several investigational MBL-Is with mechanisms of action ranging from zinc chelation to zinc-independent enzyme inhibition and novel gene silencing are in the preclinical pipeline and are being studied in combination with existing β-lactam backbones. In tandem, evidence of unexpected β-lactam efficacy against MBL-producing Enterobacterales in animal models has been growing (Fig. 1), but the extent and range of available data have not been comprehensively reviewed until now (19, 46, 51, 71–73). In addition, we recently detailed how currently utilized susceptibility testing media (i.e., Mueller-Hinton broth) may be inappropriate for characterizing MBL resistance due to variable and supraphysiologic zinc cation concentrations (63). When these zinc cations are reduced to mimic physiologic bioactive concentrations elicited by nutritional immunity during an infection, β-lactam MICs are reduced severalfold (19, 20, 63, 74).
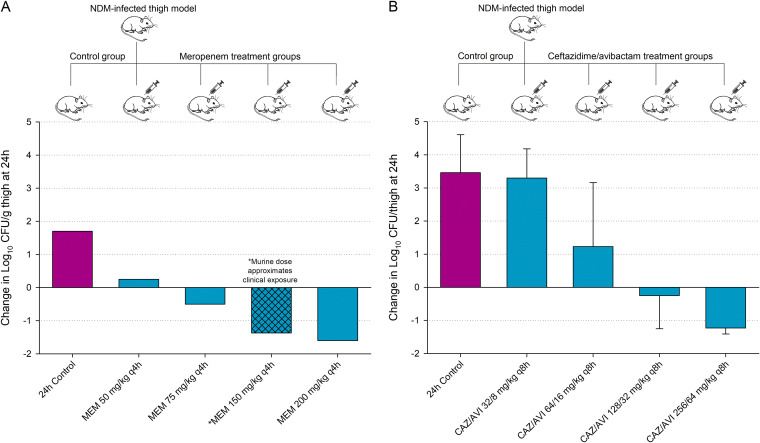
FIG 1.
Mean change in bacterial density at 24 hours relative to 0-h controls after administration of meropenem (A) and ceftazidime-avibactam (B) against an NDM-harboring E. coli and K. pneumoniae, respectively. Data adapted from 2 contemporary β-lactam dose-ranging studies (46, 51).
Within the limitations of this review, abstracted animal data suggest that (i) β-lactams with intrinsic stability against ESBLs/cephalosporinases (i.e., carbapenems) and (ii) ceftazidime, aztreonam, and cefepime in combination with a broad-spectrum BL-I (i.e., avibactam) result in substantial bacterial reduction among clinical and engineered MBL-harboring Enterobacterales when administered at clinically relevant exposures. While these results are potentially promising, it remains to be determined if the addition of a MBL-I to optimized carbapenem therapy results in enhanced efficacy (i.e., further reduction in bacterial burden compared with the carbapenem alone) in the animal model. It is also worth noting that animal studies that utilize subtherapeutic backbone exposures continue to have a critical role in drug development as a means to dose fractionate or to demonstrate additional or enhanced efficacy upon addition of the BL-I or adjunctive agent. However, key to the design of these studies is the reporting on the efficacy of the full therapeutic exposure of the backbone agent alone (75, 76).
Observed trends in microbiological activity among studies in this review also suggest that despite an apparent in vitro resistant profile, inclusion of the β-lactam backbone as a monotherapy arm in future β-lactam/MBL-I animal studies will yield valuable insights and opportunities to further our understanding of MBL in vitro-in vivo discordance (77, 78). In addition, a recently published study demonstrating unexpected in vivo activity with imipenem and ertapenem against an isogenic derivative E. coli with inserted NDM-1 plasmid was not included in this literature review due to a lack of 24-h control groups, which is critical for appropriately evaluating the impact of treatment versus placebo/vehicle over the course of the study period and as an indicator of bacterial fitness (71).
This review highlights the diversity in animal infection models and study endpoints employed within MBL-I development studies. Similar heterogeneity was observed in a recent study aimed at better understanding the range and scientific use of animal lung infection models, resulting in the authors advocating for the need for harmonized consensus models in drug development (79). Nonetheless, animal infection models continue to be an essential translational tool for assessing the toxicity and efficacy of antimicrobial agents. Data generated in these models have been valuable components of regulatory-approval applications (79, 80). However, there is an awareness to remain cautious when drawing conclusions from experimental animal studies especially in regard to MBL-harboring organisms, as our knowledge of zinc variability, MBL-variant evolution, bacterial species differences, and most importantly host factors (mouse versus human) appears to be the tip of the iceberg (7, 19, 20, 62, 81–84). Indeed, our current understanding of the impact of zinc concentration on MBL-mediated resistance is limited to murine models but provides noteworthy insights. For example, in a murine study by Corbin et al., imaging of metal distribution in a staphylococcal liver abscess revealed a cation-starved environment (i.e., devoid of zinc and manganese) (85). This host versus pathogen interaction raises important clinical and microbiological questions. Similar to the importance of characterizing free antibiotic concentrations at various infection sites, how does bioactive zinc availability compare between infection sites, e.g., soft tissue, blood, epithelial lining fluid, peritoneal fluid, and urine; and importantly, what is the temporal profile of zinc over the course of an infection at each site? Furthermore, how does the ability of the pathogen to acquire zinc from its environment (host or susceptibility media) impact MBL resistance and ultimately β-lactam efficacy? Also, what physiochemicals in the pathogen’s environment are required to facilitate this zinc uptake? Clearly, the manifestation of MBL resistance (in vitro and in vivo) is complex and presents a unique opportunity for multiple disciplines to begin to systematically reassess our current microbiological, PK/PD, and clinical knowledge.
STUDY DESIGN CONSIDERATIONS
The drug pipeline does demonstrate innovative efforts to increase the throughput of lead MBL-I candidates, and their subsequent integration with robust classic and translational PK/PD studies will go a long way to derisk clinical development programs. Previous PK/PD reviews have described general study considerations one may take with respect to the design of animal infection models, drug pharmacokinetics, and study endpoints (27, 66, 80, 86, 87), and we echo those considerations and summarize additional recommendations relevant to this current topic:
Antimicrobial susceptibility testing.
Underpinning MBL in vitro-in vivo discordance is the MIC. The challenge, therefore, is to elucidate the relationship between MIC and likelihood of outcome (clinical or animal). While there is insufficient data to propose a change in current susceptibility testing methodology, efforts to create a host-mimicking media (zinc-limited) through addition of EDTA or zinc chelation/removal and resupplementation have provided a physiologically plausible means with which to describe in vivo data. Studies should consider reporting MICs from conventional (Mueller-Hinton broth) and zinc-limited media to aid in a more complete understanding of exposure-response relationship.
Isolate selection.
A sufficient number of clinical and engineered strains (if available) that harbor a variety of MBLs should be evaluated in the animal model to enable robust microbiological activity profiling across genotypes and variants.
One should consider an inclusion of reference strains (i.e., ATCC, NCTC, and CDC AR Bank) to enable drug-bug comparisons across different laboratories.
One should report both target (i.e., NDM-1) and coharbored enzymes (i.e., CTX-M) to discern the activity of the β-lactam backbone and the contribution of MBL-I.
Exposure-response assessment.
Due to a lack of clinical data with β-lactams and evidence of MBL in vitro-in vivo discordance, (i) a number of efficacy studies in small and large mammals may be required to elucidate β-lactam monotherapy activity against MBLs before being relied upon as a model to bridge to humans, and (ii) each of these animal models should include a β-lactam monotherapy arm in addition to the β-lactam/MBL-I arm as a means with which to interrogate MBL in vitro-in vivo resistance correlation.
Faster drug elimination in animal models requires careful design of the dosing strategy. A PK profile at the site of infection is important for the backbone alone, inhibitor alone, and in combination to assess drug exposure and any drug interaction effects.
A variety of dosing strategies can be administered to elucidate a relevant PD index. However, for efficacy studies, one should consider a therapeutic exposure of the backbone if utilizing a clinically approved agent in an effort to derisk the drug development program.
One should report 0-h bacterial density to allow for appropriate preclinical endpoint assessment of the treatment arm, i.e., bacteriostasis, 1-log kill, and 2-log kill.
CONCLUSIONS
Developing effective therapeutic agents against Gram-negative bacteria has always been a challenge in the antimicrobial arena and is more so true for MBL-harboring organisms for which no β-lactamase inhibitor has yet made it into clinical practice. This review highlights animal studies, albeit heterogeneous in design, in which select β-lactam agents demonstrate activity (with and without a MBL-I) against MBL-harboring Enterobacterales, suggesting an urgent need for robust preclinical studies in small and large mammals to both optimize current antimicrobials and advance the translational development of MBL-Is. As more robust data are generated, we can collectively refine our approach to the selection of animal species and model, infection site, dosing strategy, and in vitro susceptibility tests to improve data interpretation and our ability to correlate preclinical exposure-response relationships to clinical efficacy.
ACKNOWLEDGMENTS
This work was performed as part of our regular professional activities, and we received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We have no conflicts of interest to declare.
REFERENCES
- 1.Queenan AM, Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. 2018. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 66:1290–1297. doi: 10.1093/cid/cix893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. 2014. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58:654–663. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somboro AM, Sekyere JO, Amoako DG, Essack SY, Bester LA. 2018. Diversity and proliferation of metallo-lactamases: a clarion call for clinically effective metallo-lactamase inhibitors. 84:e00698-18. doi: 10.1128/AEM.00698-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. 2019. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115-18. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd SE, Livermore DM, Hooper DC, Hope WW. 2020. Metallo-β-lactamases: structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob Agents Chemother 64:e00397-20. doi: 10.1128/AAC.00397-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillespie EL, Kuti JL, Nicolau DP. 2005. Pharmacodynamics of antimicrobials: treatment optimisation. Expert Opin Drug Metab Toxicol 1:351–361. doi: 10.1517/17425255.1.3.351. [DOI] [PubMed] [Google Scholar]
- 9.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. [9455502] [DOI] [PubMed] [Google Scholar]
- 10.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doern GV, Brecher SM. 2011. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J Clin Microbiol 49:S11–S14. doi: 10.1128/JCM.00580-11. [DOI] [Google Scholar]
- 12.Masterton R. 2008. The importance and future of antimicrobial surveillance studies. Clin Infect Dis 47:S21–S31. doi: 10.1086/590063. [DOI] [PubMed] [Google Scholar]
- 13.Aminov RI. 2010. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould IM. 1999. A review of the role of antibiotic policies in the control of antibiotic resistance. J Antimicrob Chemother 43:459–465. doi: 10.1093/jac/43.4.459. [DOI] [PubMed] [Google Scholar]
- 15.Allen R, Waclaw B. 2016. Antibiotic resistance: a physicist’s view. Phys Biol 13:e045001. doi: 10.1088/1478-3975/13/4/045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry AL, Reller LB, Miller GH, Washington JA, Schoenknect FD, Peterson LR, Hare RS, Knapp C. 1992. Revision of standards for adjusting the cation content of Mueller-Hinton broth for testing susceptibility of Pseudomonas aeruginosa to aminoglycosides. J Clin Microbiol 30:585–589. doi: 10.1128/JCM.30.3.585-589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs PC, Barry AL, Brown SD. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn Microbiol Infect Dis 38:51–58. doi: 10.1016/S0732-8893(00)00164-4. [DOI] [PubMed] [Google Scholar]
- 18.Huband MD, Ito A, Tsuji M, Sader HS, Fedler KA, Flamm RK. 2017. Cefiderocol MIC quality control ranges in iron-depleted cation-adjusted Mueller–Hinton broth using a CLSI M23-A4 multi-laboratory study design. Diagn Microbiol Infect Dis 88:198–200. doi: 10.1016/j.diagmicrobio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Asempa TE, Abdelraouf K, Nicolau DP. 2020. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 75:997–1005. doi: 10.1093/jac/dkz532. [DOI] [PubMed] [Google Scholar]
- 20.Abdelraouf K, Reyes S, Nicolau DP. 2021. The paradoxical in vivo activity of β-lactams against metallo-β-lactamase-producing Enterobacterales is not restricted to carbapenems. J Antimicrob Chemother 76:684–691. doi: 10.1093/jac/dkaa467. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher M, Allignol A, Beyersmann J, Binder N, Wolkewitz M. 2013. Hospital-acquired infections—appropriate statistical treatment is urgently needed! Int J Epidemiol 42:1502–1508. doi: 10.1093/ije/dyt111. [DOI] [PubMed] [Google Scholar]
- 22.Chibabhai V, Nana T, Bosman N, Thomas T, Lowman W. 2018. Were all carbapenemases created equal? Treatment of NDM-producing extensively drug-resistant Enterobacteriaceae: a case report and literature review. Infection 46:1–13. doi: 10.1007/s15010-017-1070-8. [DOI] [PubMed] [Google Scholar]
- 23.Schmid A, Wolfensberger A, Nemeth J, Schreiber PW, Sax H, Kuster SP. 2019. Monotherapy versus combination therapy for multidrug-resistant Gram-negative infections: systematic review and meta-analysis. Sci Rep 9:15290. doi: 10.1038/s41598-019-51711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos FL, Kuti JL, Nicolau DP. 2007. Employing pharmacokinetic and pharmacodynamic principles to optimize antimicrobial treatment in the face of emerging resistance. Braz J Microbiol 38:183–193. doi: 10.1590/S1517-83822007000200001. [DOI] [Google Scholar]
- 25.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 26.Housman ST, Crandon JL, Nichols WW, Nicolau DP. 2014. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother 58:1365–1371. doi: 10.1128/AAC.02161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andes DR, Lepak AJ. 2017. In vivo infection models in the pre-clinical pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Curr Opin Pharmacol 36:94–99. doi: 10.1016/j.coph.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 28.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging Gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2013. Efficacy of humanized carbapenem exposures against New Delhi metallo-β-lactamase (NDM-1)-producing Enterobacteriaceae in a murine infection model. Antimicrob Agents Chemother 57:3936–3940. doi: 10.1128/AAC.00708-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daikos GL, Panagiotakopoulou A, Tzelepi E, Loli A, Tzouvelekis LS, Miriagou V. 2007. Activity of imipenem against VIM-1 metallo-β-lactamase-producing Klebsiella pneumoniae in the murine thigh infection model. Clin Microbiol Infect 13:202–205. doi: 10.1111/j.1469-0691.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- 32.Samuelsen Ø, Åstrand OAH, Fröhlich C, Heikal A, Skagseth S, Carlsen TJO, Leiros HS, Bayer A, Schnaars C, Kildahl-Andersen G, Lauksund S, Finke S, Huber S, Gjøen T, Andresen AMS, Økstad OA, Rongved P. 2020. ZN148 is a modular synthetic metallo-β-lactamase inhibitor that reverses carbapenem resistance in Gram-negative pathogens in vivo. Antimicrob Agents Chemother 64:e02415-19. doi: 10.1128/AAC.02415-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C, Sun L-Y, Gao H, Kang P-W, Li L-Q, Zhen J-B, Yang K-W. 2020. Identification of cisplatin and palladium(II) complexes as potent metallo-β-lactamase inhibitors for targeting carbapenem-resistant Enterobacteriaceae. ACS Infect Dis 6:975–985. doi: 10.1021/acsinfecdis.9b00385. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Yang K, Jia Y, Wang Z. 2019. Repurposing peptidomimetic as potential inhibitor of New Delhi metallo-β-lactamases in Gram-negative bacteria. ACS Infect Dis 5:2061–2066. doi: 10.1021/acsinfecdis.9b00364. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Zhang J, Zhou Y, Hu N, Li J, Wang Y, Niu X, Deng X, Wang J. 2019. Pterostilbene restores carbapenem susceptibility in New Delhi metallo‐β‐lactamase‐producing isolates by inhibiting the activity of New Delhi metallo‐β‐lactamases. Br J Pharmacol 176:4548–4557. doi: 10.1111/bph.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma B, Fang C, Lu L, Wang M, Xue X, Zhou Y, Li M, Hu Y, Luo X, Hou Z. 2019. The antimicrobial peptide thanatin disrupts the bacterial outer membrane and inactivates the NDM-1 metallo-β-lactamase. Nat Commun 10:3517. doi: 10.1038/s41467-019-11503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarlagadda V, Sarkar P, Samaddar S, Manjunath GB, Mitra SD, Paramanandham K, Shome BR, Haldar J. 2018. Vancomycin analogue restores meropenem activity against NDM-1 Gram-negative pathogens. ACS Infect Dis 4:1093–1101. doi: 10.1021/acsinfecdis.8b00011. [DOI] [PubMed] [Google Scholar]
- 38.Ooi N, Lee VE, Chalam-Judge N, Newman R, Wilkinson AJ, Cooper IR, Orr D, Lee S, Savage VJ. 2021. Restoring carbapenem efficacy: a novel carbapenem companion targeting metallo-β-lactamases in carbapenem-resistant Enterobacterales. J Antimicrob Chemother 76:460–466. doi: 10.1093/jac/dkaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monogue ML, Giovagnoli S, Bissantz C, Zampaloni C, Nicolau DP. 2018. In vivo efficacy of meropenem with a novel non-β-lactam-β-lactamase inhibitor, nacubactam, against Gram-negative organisms exhibiting various resistance mechanisms in a murine complicated urinary tract infection model. Antimicrob Agents Chemother 62:e02596-17. doi: 10.1128/AAC.02596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sully EK, Geller BL, Li L, Moody CM, Bailey SM, Moore AL, Wong M, Nordmann P, Daly SM, Sturge CR, Greenberg DE. 2017. Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) restores carbapenem susceptibility to NDM-1-positive pathogens in vitro and in vivo. J Antimicrob Chemother 72:782–790. doi: 10.1093/jac/dkw476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falconer SB, Reid-Yu SA, King AM, Gehrke SS, Wang W, Britten JF, Coombes BK, Wright GD, Brown ED. 2015. Zinc chelation by a small-molecule adjuvant potentiates meropenem activity in vivo against NDM-1-producing Klebsiella pneumoniae. ACS Infect Dis 1:533–543. doi: 10.1021/acsinfecdis.5b00033. [DOI] [PubMed] [Google Scholar]
- 42.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, Walsh TR, Coombes BK, Wright GD. 2014. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. In vivo efficacy of human simulated regimens of carbapenems and comparator agents against NDM-1-producing Enterobacteriaceae. Antimicrob Agents Chemother 58:1671–1677. doi: 10.1128/AAC.01946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshizumi A, Ishii Y, Livermore DM, Woodford N, Kimura S, Saga T, Harada S, Yamaguchi K, Tateda K. 2013. Efficacies of calcium-EDTA in combination with imipenem in a murine model of sepsis caused by Escherichia coli with NDM-1 β-lactamase. J Infect Chemother 19:992–995. doi: 10.1007/s10156-012-0528-y. [DOI] [PubMed] [Google Scholar]
- 45.Souli M, Konstantinidou E, Tzepi I, Tsaganos T, Pefanis A, Chryssouli Z, Galani I, Giamarellos-Bourboulis E, Giamarellou H. 2011. Efficacy of carbapenems against a metallo-β-lactamase-producing Escherichia coli clinical isolate in a rabbit intra-abdominal abscess model. J Antimicrob Chemother 66:611–617. doi: 10.1093/jac/dkq470. [DOI] [PubMed] [Google Scholar]
- 46.Das S, Johnson A, McEntee L, Farrington N, Kirby A, Unsworth J, Jimenez-Valverde A, Kolamunnage-Dona R, Bousquet J, Alibaud L, Sable C, Zalacain M, Everett M, Hope W. 2020. Pharmacodynamics of the novel metallo-β-lactamase inhibitor ANT2681 in combination with meropenem for the treatment of NDM-producing Enterobacteriaceae. Antimicrob Agents Chemother 64:e01076-20. doi: 10.1128/AAC.01076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moya B, Barcelo IM, Cabot G, Torrens G, Palwe S, Joshi P, Umarkar K, Takalkar S, Periasamy H, Bhagwat S, Patel M, Bou G, Oliver A. 2019. In vitro and in vivo activities of β-lactams in combination with the novel -lactam enhancers zidebactam and WCK 5153 against multidrug-resistant metallo-lactamase-producing klebsiella pneumoniae. Antimicrob Agents Chemother 63:e00128-19. doi: 10.1128/AAC.00128-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss WJ, Pulse ME, Nguyen P, Growcott EJ. 2019. In vivo efficacy of novel monobactam LYS228 in murine models of carbapenemase-producing Klebsiella pneumoniae infection. Antimicrob Agents Chemother 63:e02214-18. doi: 10.1128/AAC.02214-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Everett M, Sprynski N, Coelho A, Castandet J, Bayet M, Bougnon J, Lozano C, Davies DT, Leiris S, Zalacain M, Morrissey I, Magnet S, Holden K, Warn P, De Luca F, Docquier J-D, Lemonnier M. 2018. Discovery of a novel metallo-β-lactamase inhibitor that potentiates meropenem activity against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 62:e00074-18. doi: 10.1128/AAC.00074-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monogue ML, Abbo LM, Rosa R, Camargo JF, Martinez O, Bonomo RA, Nicolau DP. 2017. In vitro discordance with in vivo activity: humanized exposures of ceftazidime-avibactam, aztreonam, and tigecycline alone and in combination against New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 61:e00486-17. doi: 10.1128/AAC.00486-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghazi IM, Crandon JL, Lesho EP, McGann P, Nicolau DP. 2015. Efficacy of humanized high-dose meropenem, cefepime, and levofloxacin against enterobacteriaceae isolates producing verona integron-encoded metallo-β-lactamase (VIM) in a murine thigh infection model. Antimicrob Agents Chemother 59:7145–7147. doi: 10.1128/AAC.00794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdelraouf K, Nicolau DP. 2017. Comparative in vivo efficacies of tedizolid in neutropenic versus immunocompetent murine Streptococcus pneumoniae lung infection models. Antimicrob Agents Chemother 61:e01957-16. doi: 10.1128/AAC.01957-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, Cantón R, Carmeli Y, Friedrich AW, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Nordmann P, Poirel L, Rossolini GM, Seifert H, Vatopoulos A, Walsh T, Woodford N, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. 2017. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 17:153–163. [DOI] [PubMed] [Google Scholar]
- 55.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 56.Ghazi IM, Crandon JL, Lesho EP, McGann P, Nicolau DP. 2016. In vivo efficacy of humanized high dose meropenem and comparators against Pseudomonas aeruginosa isolates producing verona integron-encoded metallo-β-lactamase (VIM). Heliyon 2:e00121. doi: 10.1016/j.heliyon.2016.e00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dadashi M, Yaslianifard S, Hajikhani B, Kabir K, Owlia P, Goudarzi M, Hakemivala M, Darban-Sarokhalil D. 2019. Frequency distribution, genotypes and prevalent sequence types of New Delhi metallo-β-lactamase-producing Escherichia coli among clinical isolates around the world: a review. J Glob Antimicrob Resist 19:284–293. doi: 10.1016/j.jgar.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bush K, Bradford PA. 2020. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev 33:e00047-19. doi: 10.1128/CMR.00047-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahr G, Vitor-Horen L, Bethel CR, Bonomo RA, González LJ, Vila AJ. 2017. Clinical evolution of New Delhi metallo-β-lactamase (NDM) optimizes resistance under Zn(II) deprivation. Antimicrob Agents Chemother 62:e01849-17. doi: 10.1128/AAC.01849-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng Z, Thomas PW, Ju L. 2019. Evolution of New Delhi metallo-β-lactamase (NDM) in the clinic: effects of NDM mutations on stability, zinc affinity, mono-zinc activity. J Biol Chem 293:12606–12618. doi: 10.1074/jbc.RA118.003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bilinskaya A, Buckheit DJ, Gnoinski M, Asempa TE, Nicolau DP. 2020. Variability in zinc concentration among Mueller-Hinton broth brands: impact on antimicrobial susceptibility testing of metallo-β-lactamase-producing Enterobacteriaceae. J Clin Microbiol 58:e02019-20. doi: 10.1128/JCM.02019-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wade N, Tehrani KHME, Brüchle NC, van Haren MJ, Mashayekhi V, Martin NI. 2021. Mechanistic investigations of metallo-β-lactamase inhibitors: strong zinc binding is not required for potent enzyme inhibition. ChemMedChem doi: 10.1002/cmdc.202100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenhard JR, Bulman ZP. 2019. Inoculum effect of β-lactam antibiotics. J Antimicrob Chemother 74:2825–2843. doi: 10.1093/jac/dkz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gill CM, Asempa TE, Nicolau DP. 2020. Human-simulated antimicrobial regimens in animal models: transparency and validation are imperative. Antimicrob Agents Chemother 64:e00594-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wenzler E, Gotfried MH, Loutit JS, Durso S, Griffith DC, Dudley MN, Rodvold KA. 2015. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother 59:7232–7239. doi: 10.1128/AAC.01713-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C, Kuti JL, Nightingale CH, Nicolau DP. 2006. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol 46:1171–1178. doi: 10.1177/0091270006291035. [DOI] [PubMed] [Google Scholar]
- 69.Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VHA, Takebayashi Y, Spencer J. 2019. β-Lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol 431:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi C, Chen J, Kang X, Shen X, Lao X, Zheng H. 2019. Approaches for the discovery of metallo-β-lactamase inhibitors: a review. Chem Biol Drug Des 94:1427–1440. doi: 10.1111/cbdd.13526. [DOI] [PubMed] [Google Scholar]
- 71.Roujansky A, de Lastours V, Guérin F, Chau F, Cheminet G, Massias L, Cattoir V, Fantin B. 2020. Analysis of paradoxical efficacy of carbapenems against carbapenemase-producing Escherichia coli in a murine model of lethal peritonitis. Antimicrob Agents Chemother 64:e00853-20. doi: 10.1128/AAC.00853-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheminet G, de Lastours V, Poirel L, Chau F, Poec’h K, Massias L, Fantin B, Nordmann P. 2020. Dimercaptosuccinic acid in combination with carbapenems against isogenic strains of Escherichia coli producing or not producing a metallo-β-lactamase in vitro and in murine peritonitis. J Antimicrob Chemother 75:3593–3600. doi: 10.1093/jac/dkaa347. [DOI] [PubMed] [Google Scholar]
- 73.Asempa TE, Abdelraouf K, Nicolau DP. 2020. The ongoing challenge with NDM-harboring Enterobacteriaceae in murine infection models. Antimicrob Agents Chemother 65:e02243-20. doi: 10.1128/AAC.02243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asempa TE, Motos A, Abdelraouf K, Bissantz C, Zampaloni C, Nicolau DP. 2020. Meropenem-nacubactam activity against AmpC-overproducing and KPC-expressing Pseudomonas aeruginosa in a neutropenic murine lung infection model. Int J Antimicrob Agents 55:105838. doi: 10.1016/j.ijantimicag.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 76.Asempa TE, Abdelraouf K, Carabeo T, Schuch R, Nicolau DP. 2020. Synergistic activity of exebacase (CF-301) in addition to daptomycin against Staphylococcus aureus in a neutropenic murine thigh infection model. Antimicrob Agents Chemother 64:e02176-19. doi: 10.1128/AAC.02176-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Growcott EJ, Cariaga TA, Morris L, Zang X, Lopez S, Ansaldi DA, Gold J, Gamboa L, Roth T, Simmons RL, Osborne CS. 2019. Pharmacokinetics and pharmacodynamics of the novel monobactam LYS228 in a neutropenic murine thigh model of infection. J Antimicrob Chemother 74:108–116. doi: 10.1093/jac/dky404. [DOI] [PubMed] [Google Scholar]
- 78.Lepak AJ, Zhao M, Andes DR. 2019. WCK 5222 (cefepime-zidebactam) pharmacodynamic target analysis against metallo-β-lactamase-producing Enterobacteriaceae in the neutropenic mouse pneumonia model. Antimicrob Agents Chemother 63:e01648-19. doi: 10.1128/AAC.01648-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waack U, Weinstein EA, Farley JJ. 2020. Assessing animal models of bacterial pneumonia used in investigational new drug applications for the treatment of bacterial pneumonia. Antimicrob Agents Chemother 64:e02242-19. doi: 10.1128/AAC.02242-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL, Hoover JL. 2019. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. 63:e02307-18. doi: 10.1128/AAC.02309-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS. 2007. Comparison of treatment effects between animal experiments and clinical trials: systematic review. Br Med J 334:197–200. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rahman MT, Karim MM. 2018. Metallothionein: a potential link in the regulation of zinc in nutritional immunity. Biol Trace Elem Res 182:1–13. doi: 10.1007/s12011-017-1061-8. [DOI] [PubMed] [Google Scholar]
- 83.Berti A, Rose W, Nizet V, Sakoulas G. 2020. Antibiotics and innate immunity: a cooperative effort toward the successful treatment of infections. Open Forum Infect Dis 7:ofaa302. doi: 10.1093/ofid/ofaa302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hobson CA, Cointe A, Bidet P, Poupon J, Bonacorsi S, Birgy A. 2020. Urine zinc concentrations allow proper expression of metallo-β-lactamases in Enterobacteriaceae. J Antimicrob Chemother 75:3077–3079. doi: 10.1093/jac/dkaa295. [DOI] [PubMed] [Google Scholar]
- 85.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 86.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19:261–268. doi: 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 87.Zhao M, Lepak AJ, Andes DR. 2016. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg Med Chem 24:6390–6400. doi: 10.1016/j.bmc.2016.11.008. [DOI] [PubMed] [Google Scholar]