Abstract
Eastern Hermann’s tortoise (Testudo hermanni boettgeri) is a subspecies of Hermann’s tortoise (Testudo hermanni) found in Albania. Gender determination is one of the crucial elements in determining the population dynamics in all species. Female and male adult tortoises look different, but these differences can be difficult to distinguish in captivity or when their sex indicators are deformed or injured. Therefore, the aim of this study was to use indirect and non-invasive methods such as geometric morphometric analysis to determine the sex of adult tortoises. For the geometric morphometry, 17 female and 23 male Hermann’s tortoises were collected and photographed from the hills and mountains around the Tirana district of Albania between August and October 2019. Sexes were discriminated based on geometric morphometry, and plastron shape was a better indicator than carapace shape. In addition, abdominal, femoral and anal scutes on the plastron and the ratio of femoral to pectoral suture lengths were important for the sex distinction. Females had a larger plastron than males; this may have been supported by fecundity selection, because a large plastron suggests more volume in which to store eggs. The femoral and anal scutes were larger in male tortoises, and serve as a stronger base during mating. This study was conducted for adults only, and future studies are needed to determine if these indicators also apply to hatchlings and juveniles.
Keywords: Testudo hermanni boettgeri, Sex discriminate, Geometric morphometry, Abdominal scutes, Albania
BACKGROUND
Hermann’s tortoise (Testudo hermanni) is one of the many members of Albania’s fauna (Perälä 2004; Djordjević et al. 2013; Kicaj et al. 2016), and is mostly found in the country’s agricultural lands, canals and pastures in the hills with sparse vegetation and near forest areas. Many factors—e.g., rapid urbanization, habitat loss, climate change, extremely high tempera-tures, summer fires, prolonged drought or floods and increased human activity—threaten this tortoise in rapidly development countries (Kicaj et al. 2016), and the species has been classified as threatened by some (Cheylan 2001; Böhm et al. 2013) and in danger of extinction by others (Cox and Temple 2009).
Excessive use of reptiles in the pet trade can cause serious harm to their populations (Auliya et al. 2016). Hermann’s tortoise covers 13% of the world’s Testudo trade (Türkozan et al. 2008), and is listed as ‘‘near threatened’’ at the global scale according to the International Union for Conservation of Nature (IUCN 2018). Moreover, T. hermanni is listed in the Bern Convention and European Habitat Directive, and international trade of the species is regulated by Convention for International Trade of Wildlife Fauna and Flora (Türkozan et al. 2019).
The most visible structure of the tortoise, as in turtles in general, is its shell, which is composed of two halves—a dorsal part (carapace) and a flatter ventral part (plastron), which are fused together on either sides between the fore and hind limbs by the bridge (Girling 2003). The middle line of the carapace contains five vertebral scutes; more laterally on either side are four pleural scutes, and 12 marginal scutes surround the first ones. The scutes in the plastron are arranged in the following order, starting from the cranial end: gular, humeral, pectoral, abdominal, femoral and anal (Evans 1986; Girling 2003; O ´Malley 2005) (Fig. 1).
Fig. 1.
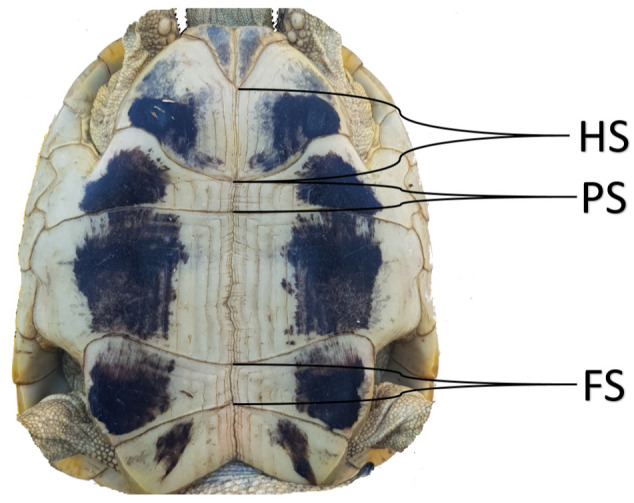
HS: Humeral suture, PS: Pectoral suture, FS: Femoral suture.
Hermann’s tortoises—which are found in Europe, the Balkans and Turkish Thrace—comprise two recognized subspecies: Testudo hermanni hermanni and Testudo hermanni boettgeri (Fritz et al. 2006). Testudo h. hermanni inhabits the western part of the Po Valley river and T. h. boettgeri inhabits the Balkans and Turkey Thrace (Türkozan et al. 2019).
Albanian contains the Eastern Hermann’s tortoise subspecies (T. h. boettgeri), which is distinguishable by several external morphological features: 1) the exterior color of the carapace is not a strong yellow, and 2) the plastron’s black pigment is not like that of the bands, but less defined and discontinued, and in some cases discolored. Also, the length of the inter-pectoral suture is greater than or equal to the inter-femoral suture, and inguinal scutes are present on either side of the shell in almost all individuals (Soler et al. 2012).
The sex ratio in hatchlings, juveniles and adults may differ, and therefore comparing them may provide information about differences in mortality, migration and dispersal between the sexes (Bulmer 1994). This makes knowing an individual’s sex one of the crucial elements for determining the population dynamics of Testudo hermanni (Djordjević et al. 2011). Although adults show morphological differences in many turtle species, hatchlings and juveniles typically exhibit little or no pronounced dimorphism, which allows sex to be determined directly by observation (Ernst and Barbour 1989). Therefore, researchers use different techniques to assess the sex of hatchlings, such as gonadal histology (Godfrey and Mrosovsky 2006), radioimmunoassays to measure testosterone levels in blood or chorioallantois fluid (Owens et al. 1978; Gross et al. 1995), laparoscopy on live post-hatchlings (Wyneken et al. 2007), direct observations of the gonads in situ (McCoy et al. 1983) and clearing of the gonads in toto (Van der Heiden et al. 1985). Another approach is to reveal the morphological differences between the sexes using geometric morphometry and linear morphology. Some researchers that used this approach could successfully distinguished between the sexes (Michel-Morfin et al. 2001; Valenzuela et al. 2004), while others could not (Lubiana and Ferreira-Junior 2009; Kircher and Wyneken 2017; Sönmez et al. 2016 2019).
Adult male Hermann’s tortoises are clearly differentiated from females: the plastron is more concave, caudal parts are wider and their tails are longer and more prominent (Girling 2003; Ljubisavljević et al. 2012). However, these indicators often do not work in captive adults (Willemsen and Hailey 2003; Soler et al. 2012; Djurakic et al. 2011; Djordjević et al. 2013), in juvenile, in hatchlings or in individuals with deformed or injured sex indicators like the tail or plastron. Although plastron and carapace deformation and injured rates differ in natural populations (4.2% for Turkey Thrace, Türkozan et al. 2019; 90% for Italy, Biaggini and Corti 2018), they make it very difficult to distinguish the sexes during studies of wild or natural population, making it difficult to describe the populations and therefore to implement protection and breeding programs.
Therefore, it is important to use inexpensive, easily applicable and non-invasive methods for individuals whose sexes cannot be determined under the above-mentioned circumstances. The ideal method for distinguishing the sexes in this scenario is the landmark-based geometric morphometric analysis (Valenzuela et al. 2004; Sönmez et al. 2019). Geometric analysis has been used in taxonomy studies in recent years (Bernal 2007). In addition, this method reveals the differences between the genders (Veeramani et al. 2010). This working principle is based on shape differences instead of size measurements (Corrucini 1987). Two-dimensional photographs are marked using landmarks, and these points are displayed on the coordinate plane in the computer environment, at which point statistical analysis of these reference points can be made. In geometric morphometry, principal components are used to analyze nonlinear shapes obtained with photographs (Wold et al. 1987). These principal components can also demonstrate inter-group shape variation and statistical differences.
Studies of phenotypic variation using geometric morphometry has been increased over the last two decades in amphibians and reptiles (Kaliontzopoulou 2011). Examples of these studies include a phylogenetic analysis of Phytosauria (Jones and Butler 2018), evolutionary divergence in the skull of turtles (Claude et al. 2004), evolution of the turtle shell (Claude et al. 2003) and sex differences in sea turtles (Sönmez et al. 2019) and non-sea turtles (Valenzuela et al. 2004). This analysis allows researchers to show the distance between the samples on a coordinate system and establish a relationship between the groups (Wold et al. 1987; Hotelling 1933), which can be visualized with the help of software (Klingenberg 2011). The first report on geometric morphometry for Hermann’s tortoises was published for populations with different haplotypes in Greek populations (Djurakic and Milankov 2020). However, there is no report of this method being used for sex discrimination in adult, juvenile or hatchling individuals. In the present study, we tested the hypothesis that shape differences in the carapace and plastron, visualized using geometric morphometry, can distinguish between adult Hermann’s tortoise males and females.
MATERIALS AND METHODS
Study sites and sampling
We conducted this study in the hills and mountains around the Tirana district of Albania, from August to October 2019. Ethical approval for the study was obtained from the Ethic Commission of the Veterinary Faculty of Tirana (Decision Number 143/15.04.2020). All Hermann’s tortoises were found in the wild and were evaluated carefully for all external morphologic characteristics to determine the correct sex and subspecies. Sex was determined based on the concavity of the plastron, tail length, and curve of the supracaudal scutes in male tortoises, which are more prominent than that in female tortoises (Girling 2003; O ´Malley 2005; Ljubisavljević et al. 2012). We evaluated a total of 44 adult Hermann’s tortoises that were healthy and lacked any damage or deformity to their shells. After evaluating and photographing each tortoise, we carefully released them back in the same place where we found them.
Photographing and marking of landmarks
Photographs of the carapace and plastron were taken from the same distance for each sample. The photos obtained were uploaded to the same file in the computer environment. During the first stage, all the photos were converted to TPS format (TpsUtil, Version 1.74). Then, the tpsDig (Version 2) program was used to place landmarks onto the TPS photos. The landmark process was saved and converted into a Text file for statistical analysis, and the numerical coordinates in the text were transferred to the MorphoJ (Version 1.06d) and Past (Version 2.17c) package software (Klingenberg 2011) for statistical analyses. The landmarks registered were superimposed on the coordinate system to minimize statistical errors caused by differences such as size and position in the samples used.
We also evaluated the ratios of the length among three sutures on the plastron. These are the ratio of inter-pectoral to inter-femoral sutures (FS/PS) and the ratio between inter-humeral and inter-femoral sutures (HS/FS) (Fig. 1) (Soler et al. 2012).
Statistical analysis
In the analysis of geometric morphometry, the Generalized Procrustes Analysis (GPA), “Procrustes (2D + 3D)” process and generation covariance matrix were applied. Procrustes ANOVA test was used to compare the differences in shape between the sexes, and discriminant function analysis (DFA) was used to test whether these differences were statistically significant (Valenzuela et al. 2004; Kircher and Wyneken 2017; Sönmez et al. 2019). In addition, principal component analysis (PCA) was applied for both shell regions and a 95% confidence ellipse graph was used to visualize the differences between sexes (Sönmez et al. 2019). Ratios of the distances among three sutures on the plastron was tested with the Independent-Samples t-test.
RESULTS
Twenty-three of the 44 (57.5%) adult Hermann’s tortoises examined were identified as male. Four samples (9.1%) were excluded from the analysis because they had character anomalies, such as carapace or plastron deformations. In total, 18 scute intersection landmarks were recorded on the carapace; the distribution of landmarks is presented in figure 2. We only used carapace scute intersection landmarks from the second vertebral scutes. The carapace shape analyses indicated significant differences in the Procrustes shape ANOVA (F = 18.6, d.f. = 32, p < 0.001); this was further supported by DFA (p < 0.05). In the cross-validation classification, females and males had a mean similarity of 76% and 82% to their own groups, respectively.
Fig. 2.
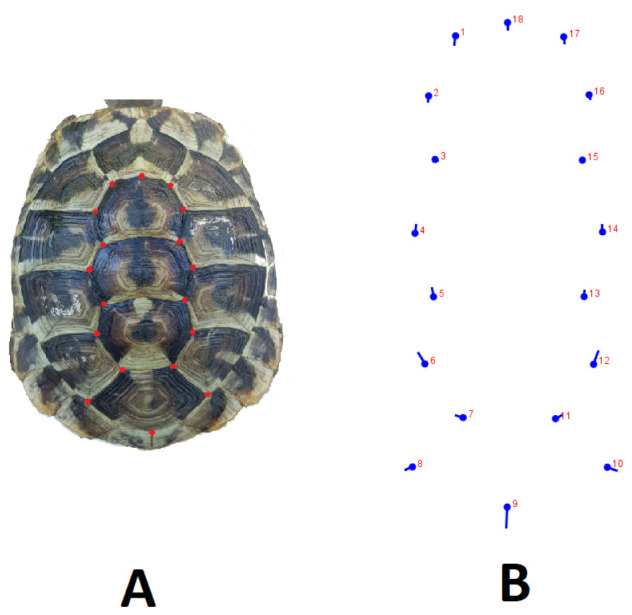
Dorsal view of landmarks used for the carapace. A, Landmark points used on the photo. B, The differences between females and males are indicated by landmarks (MorphoJ). The round marks represent the female, and extensions from those marks indicate the direction and changes in the male turtles.
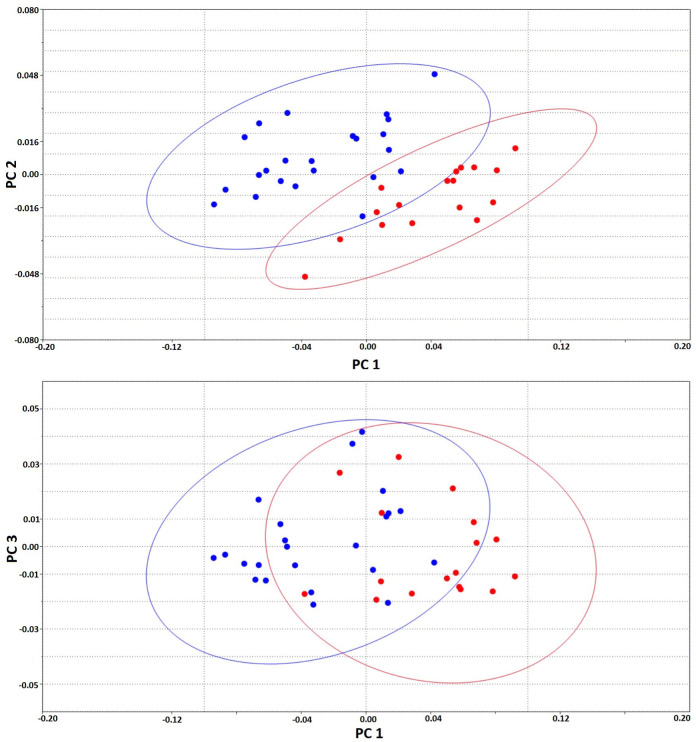
A total of 36 principal components were produced in PCA. The first three (PC1, PC2 and PC3) described 64.7%, 8.9% and 6.7% of the total variance, respectively. However, although the total variance was high and DFA showed significance, sexes were not separated, but were clustered over the elliptical figure with a 95% confidence interval in PCs (Fig. 3).
Fig. 3.
95% confidence ellipses for carapace landmarks (used past, Version 2.17c). PC1-PC2 and PC1-PC3. Blue dots are male; red dots are female.
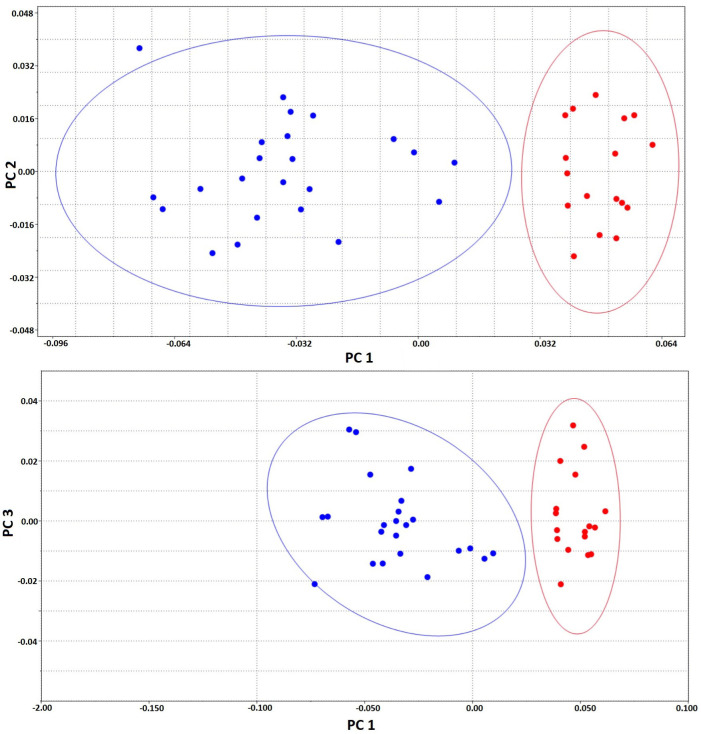
In total, 19 landmarks were recorded on the plastron; the distribution of landmarks is presented in figure 4. The plastron shape analyses indicated significant differences in the Procrustes shape ANOVA (F = 34.6, d.f. =34, p < 0.001). This was further supported by DFA (p < 0.05), and in the cross-validation classification, female had a mean of 65% and male 57% similarity to their groups. A total of 38 principal components were produced in the PCA. The first three principal components (PC1, PC2 and PC3) described 64.1%, 7.1% and 5.9% of the total variance, respectively. The sexes were separated over the elliptical figure, with a 95% confidence interval in PCs (Fig. 5). The difference was obtained between males and females in the middle and posterior region of plastron. In particular, landmarks 17 and 18 were larger in females, and landmarks 9, 10 and 15 were longer in males.
Fig. 4.
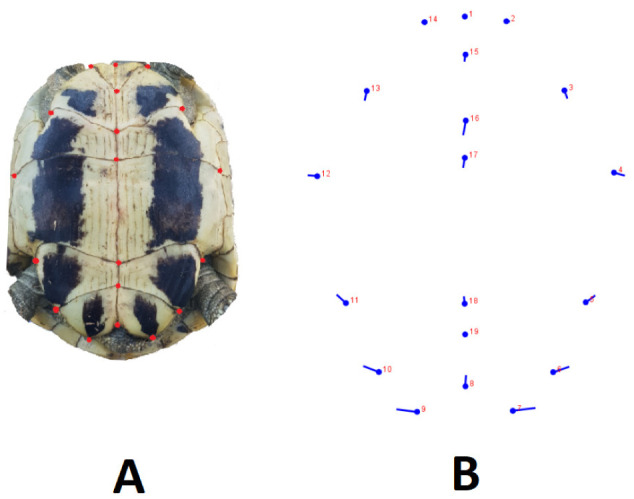
Dorsal view of landmarks used for the plastron. A, Landmark points used on the photo. B, The differences between females and males are indicated by landmarks (MorphoJ). The round marks represent the female, and extensions from those marks indicate the direction and changes in the male turtles.
Fig. 5.
95% confidence ellipses for plastron landmarks (used past, Version 2.17c). PC1-PC2 and PC1-PC3. Blue dots are male; red dots are female.
General descriptive statistics of the ratios among inter-pectoral, inter-humeral and inter-femoral suture lengths on the plastron are given in table 1. While the FS/PS ratio showed a significant difference between females and males, the HS/FS ratio did not differ. The FS/PS ratio in females was one-fifth (1/5) shorter than that in males.
Table 1.
The length ratios of three interscute sutures in the midline of the Hermann’s tortoise plastron
| Measurement | Sex | N | Mean | SD | Significance |
| FS/PS | Female | 17 | 0.868 | 0.216 | P < 0.01 |
| Male | 23 | 1.116 | 0.242 | ||
| HS/FS | Female | 17 | 2.237 | 0.451 | NS |
| Male | 23 | 2.094 | 0.467 |
HS: Humeral sture, PS: Pectoral sture, FS: Femoral sture, SD: Standard Deviation, NS: Non-Significant.
DISCUSSION
Geometric morphometry analysis is a useful way to discriminate between sexes in adults Hermann’s tortoise, and plastron shape is a better indicator than carapace shape. Similarly, Ceballos and Valenzuela (2011) also analyzed geometric morphometry and found that hatchlings of the snapping turtle (Chelydra serpentina) were sexually dimorphic in their plastron shape, but not in their carapace shape. In contrast, Valenzuela et al. (2004) stated that carapace shapes differed between sexes of hatchlings of the freshwater turtles Podocnemis expansa and Chrysemys picta. The same authors noted that, although the differences are in the hatchlings, adults can also display significant sexual dimorphism. For example, in adult C. serpentina, precloacal length—a feature associated with reproduction—can act as an indicator of sexual dimorphism as early as the hatching stage (de Solla et al. 2002). The sexual shape dimorphism found in adults is consistent with that detected in hatchlings, and may be present in hatchlings as a precursor to adult dimorphism (Ceballos and Valenzuela 2011). In adult Hermann’s tortoises, sexual shape dimorphism (SShD) studies found that both carapace and plastron shapes differ between the sexes (Willemsen and Hailey 2003; Djurakic et al. 2011; Djordjević et al. 2013). We, on the other hand, found significant differences just in plastron shape. These discrepancies may be due to the differences in the methods chosen (geometric morphometry versus classical linear morphometry) or the number or locality of the landmark that we chose for the carapace.
Size and shape analyses are widely used to determine sexual dimorphism in tortoises. In sexual size dimorphism (SSD), linear dimensions are typically measured and differences between the sexes are tested with classic statistics (i.e., ANOVA), whereas in SShD, the same or similar linear dimensions are tested with covariance analysis (i.e., ANCOVA with straight carapace length (SCL) or log SCL as the covariate). Several sexual dimorphism studies also found that SShD is more stable than SSD (Kaddour et al. 2008; Djurakic et al. 2011). It was reported that female Hermann’s tortoises are longer than the males according to linear measurements of the body, e.g., SCL (Willemsen and Hailey 2003; Djurakic et al. 2011; Djordjević et al. 2013). In contrast, males have greater plastron concavity, tail length, anal notch width, supracaudal curve and horny claw length in the tail (Willemsen and Hailey 2003; Djurakic et al. 2011).
Our geometric morphometry results showed that landmarks 17 and 18 on plastron were larger in females, whereas landmarks 9 and 10 were larger and landmark 15 was longer in males. Landmarks 9 and 10 were more extended sideways, making the shell widest in the caudal part, which serves as a stronger base during mating. On the other hand, it makes the tortoise’s body more aerodynamic: the cranial part is narrowest and the caudal part wider, allowing the male tortoises to move faster than females.
Landmark 15 is the termination of the gular scute, and this thickened gular area of the plastron in males may help hit the butting of the female carapace during courtship (Willemsen and Hailey 2003).
Landmarks 17 and 18 are the outset and termination of the abdominal scutes. These larger landmarks in females may be related to the larger volume of the abdominal cavity, which provides more space for the eggs (Willemsen and Hailey 2003; Djurakic et al. 2011). Similarly, it was reported in Hermann’s tortoise that the abdominal suture length of the plastron plate was longer in females (Djurakic et al. 2011; Djordjević et al. 2013). Females have a more voluminous shell than do the males, giving them a greater internal volume (Kaddour et al. 2008). Greater volume for eggs in females could be the result of natural selection for fecundity (fecundity selection) (Willemsen and Hailey 2003; Djurakic et al. 2011).
Although geometric morphometry analysis proved to be a suitable technique to determine the sex of adult Hermann’s tortoises, it might fail in the field for tortoises with damaged plastrons due to change the ratio between landmarks. There has not been any study on plastron damage or injury rate in natural populations. However, a study of T. hermani in Italy found that most injuries were concentrated on the rear of the carapace (Biaggini and Corti 2018). Moreover, 4.2% of T. h. boettgeri had damage to their carapace, plastron, head or tail in Thrace of Turkey (Türkozan et al. 2019). However, although the success rate of our geometric morphometry technique weakens in for tortoises that have suffered excessive plastron injury or damage, it is successful when analyzing individuals in captivity and those with damage to the carapace. Since the landmarks that play a role in gender discrimination are on the abdominal, femoral and anal scutes, we can say that only very extensive or bilateral damages and injuries that occur on these scutes may prevent gender discrimination in our technique, because they may alter the ratios between landmarks. However small damages or unilateral damages should not be an obstacle.
Our study showed that the ratio of humeral to femoral sutures (HS/FS) is not useful for sex discrimination, but the ratio of the femoral to pectoral sutures (FS/PS) is. FS/PS in females is less than one, which means that PS is relatively longer. FS/PS and HS/FS values are similar to those of previously studies (Willemsen and Hailey 2003; Djurakic et al. 2011; Djordjevic et al. 2013; Soler et al. 2012).
However, none of these previous studies compared FS/PS or HS/FS ratios between the sexes. The comparisons carried out in the present study measured the differences in suture lengths between the sexes. Comparing PS and HS lengths between sexes is more convenient (both are longer in females), but FS length is not useful because it does not differ between sexes (Willemsen and Hailey 2003; Djurakic et al. 2011; Djordjevic et al. 2013). Willemsen and Hailey (2003) stated that PSL and FSL may have no functional explanation in sexual shape differences such as plastral pigmentation. However, longer PS may support a larger plastron, and therefore may offer a clue about more space for the eggs in fecundity selection.
CONCLUSIONS
In conclusion, this paper presents the first data supporting the use of geometric morphometry to discriminate between the sexes in T. h. boettgeri. Plastron shape and the FS/PS ratio are convenient for sex discrimination using geometric morphometry. The outset and termination of abdominal scutes on the plastron plate were larger in females, while the femoral and anal scutes were larger in males. Geometric morphometric analysis was shown to be highly accurate in determining the sex of the adult Hermann's tortoise. This offers researchers a non-invasive and inexpensive alternative for injured or deformed individuals in captivity whose sex cannot be determined with existing sex determination techniques. However, this technique may be unsuitable for the field studies, especially for individuals with very large deformations on the plastron. We suggest that individuals with plastron damage or deformation be investigated in future studies. However, individuals in captivity with carapace damage can still be analyzed with this technique. Thus, conclusions can be obtained about sex ratios, which are part of the life story of populations. This study was conducted for adults only, and future studies are needed to determine if these indicators also apply to hatchlings and juveniles. We also recommend that future studies test this geometric morphometric technique in different regions with more samples. In addition, the geometric morphometry can also use in other testudines species.
Acknowledgments
We as authors are grateful to Oltion Selimja, Laurent Bardhaj and Marjol Shehaj for their help with fieldwork.
Footnotes
Authors’ contributions: SD designed the study and prepared the manuscript, OG prepared the geometry analysis, TJ and TS took measurements and prepared parts of the manuscript, BS prepared and wrote the manuscript, GP and AK prepared the statistical analysis. All authors approved the final manuscript.
Competing interests: The authors declare that they have no conflict of interests.
Availability of data and materials: The data that support the findings in this study are available from the corresponding author upon reasonable request.
Consent for publication: Not applicable.
Ethic approval consent to participate: Our study used non-lethal invasive methods; nevertheless, we still received permission from the ethics commission of the Veterinary Faculty of Tirana (No. 143/15.04.2020).
References
- Auliya M, Altherr S, Ariano-Sanchez D, Baard EH, Brown C, Brown RM, Hintzmann J. 2016. Trade in live reptiles, its impact on wild populations, and the role of the European market. Bio Conserv 204:103–119. doi:10.1016/j.biocon.2016.05.017.
- Bernal V. 2007. Size and shape analysis of human molars: comparing traditional and geometric morphometric techniques. Homo 58(4):279–296. doi:10.1016/j.jchb.2006.11.003. [DOI] [PubMed]
- Biaggini M, Corti C. 2018. Facing Habitat Reduction in Your Own Shell: Patterns of Non-lethal Injuries in the Endangered Tortoise Testudo hermanni in Italy. Herpetol Conserv Bio 13:539–550.
- Böhm M, Collen B, Baillie JE, Bowles P, Chanson J, Cox N, Rhodin AG. 2013. The conservation status of the world’s reptiles. Bio Conserv 157:372–385. doi:10.1016/j.biocon.2012.07.015.
- Bulmer M. 1994. Theoretical evolutionary ecology. Sinauer Associates, Inc., Sunderland, MA, USA.
- Ceballos CP, Valenzuela N. 2011. The role of sex-specific plasticity in shaping sexual dimorphism in a long-lived vertebrate, the snapping turtle Chelydra serpentina. Evol Biol 38:163. doi:10.1007/s11692-011-9117-8.
- Cheylan M. 2001. Testudo hermanni Gmelin, 1789-Griechische Landschildkröte. In: Schildkröten (Testudines) I (Bataguridae, Testudinidae, Emydidae). (Fritz, U. ed.), ser. Handbuch der Reptilien und Amphibien Europas., vol 3/IIIA, Aula-Verlag, Wiesbaden, pp. 179–289.
- Claude J, Paradis E, Tong H, Auffray JC. 2003. A geometric morphometric assessment of the effects of environment and cladogenesis on the evolution of the turtle shell. Biol J Linn 79:485–501. doi:10.1046/j.1095-8312.2003.00198.x.
- Claude J, Pritchard PC, Tong H, Paradis E, Auffray JC. 2004. Ecological correlates and evolutionary divergence in the skull of turtles: a geometric morphometric assessment. Syst Biol 53:933–948. doi:10.1080/10635150490889498. [DOI] [PubMed]
- Corrucini RS. 1987. Shape in Morphometrics: Comparative Analyses. Am J Phys Anthropol 73:289–303. doi:10.1002/ajpa.1330730303.
- Cox NA, Temple HJ. 2009. European Red List of Reptiles. Luxembourg: Office for Official Publications of the European Communities, Cambridge Publishers.
- de Solla SR, Bishop CA, Brooks RJ. 2002. Sexually dimorphic morphology of hatchling snapping turtles (Chelydra serpentina) from contaminated and reference sites in the Great Lakes and St. Lawrence River basin, North America. Environ Toxicol Chem 21:922–929. doi:10.1002/etc.5620210506. [PubMed]
- Djordjević S, Tomović L, Golubović A, Simović A, Sterijovski B, Djurakić M, Bonnet X. 2013. Geographic (in-)variability of Gender-specific traits in Hermann’s tortoise. Herpetol J 23:67–74.
- Djurakic M, Djordjevic S, Bonnet X, Tomovic L, Ajtic R, Golubovic A. 2011. Sexual body size and body shape dimorphism of Testudo hermanni in central and eastern Serbia. Amphibia-Reptilia 32:445–458. doi:10.1163/156853811X598479.
- Djurakic MR, Milankov VR. 2020. The utility of plastron shape for uncovering cryptic diversity in Hermann’s tortoise. J Zool 310:145–157. doi:10.1111/jzo.12736.
- Ernst CH, Barbour RW. 1989. Turtles of the world. Smithsonian Institution Press, Washington, DC, USA.
- Evans HE. 1986. Reptiles –Introduction and anatomy. In: M. E. Fowler (ed.), Zoo and wild animal medicine, 2nd edn. Philadelphia: WB Saunders, pp. 108–132.
- Fritz U, Auer M, Bertolero A, Cheylan M, Fattizzo T, Hundsdörfer AK, Wink M. 2006. A rangewidephylogeography of Hermann’s tortoise, Testudo hermanni (Reptilia: Testudines: Testudinidae): implications for taxonomy. Zool Scr 35:531–543. doi:10.1111/j.1463-6409.2006.00242.x.
- Girling S. 2003. Veterinary nursing of exotic pets. Blackwell Publishing Ltd. Editorial Offices. Oxford OX4 2DQ, UK.
- Godfrey MH, Mrosovsky N. 2006. Pivotal temperature for green sea turtles, Chelonia mydas, nesting in Suriname. Herpetol J 16:55–61.
- Gross TS, Crain DA, Bjorndal KA, Bolten AB, Carthy RR. 1995. Identification of sex in hatchling loggerhead turtles (Caretta caretta) by analysis of steroid concentrations in chorioallantoic/amniotic fluid. Gen Comp Endocrin 99:204–210. doi:10.1006/gcen.1995.1103. [DOI] [PubMed]
- Hotelling H. 1933. Analysis of a complex of statistical variables into principal components. J Educ Psychol 24:417. doi:10.1037/h0071325.
- International Centre (IUCN). 2018. The IUCN Red List of Threatened Species. Version 2018-1. http://www.iucnredlist.org. Accessed 15 Apr. 2020.
- Jones AS, Butler RJ. 2018. A new phylogenetic analysis of Phytosauria (Archosauria: Pseudosuchia) with the application of continuous and geometric morphometric character coding. Peer J 6:e5901. doi:10.7717/peerj.5901. [DOI] [PMC free article] [PubMed]
- Kaddour KB, Mouden EHE, Slimani T, Bonnet X, Lagarde F. 2008. Sexual dimorphism in the Greek tortoise: a test of the body shape hypothesis. Chel Conserv Biol 7:21–27. doi:10.2744/CCB-0649.1.
- Kaliontzopoulou A. 2011. Geometric morphometrics in herpetology: modern tools for enhancing the study of morphological variation in amphibians and reptiles. Basic Appl Herpetol 25:5–32. doi:10.11160/bah.11016.
- Kicaj H, Saçdanaku E, Shkurtaj B. 2016. Morphometric Data of Testudo hermanni in the Vlora Area. J Life Sci 10:307–311. doi:10.17265/1934-7391/2016.06.006.
- Kircher L, Wyneken J. 2017. Sex Estimation by Geometric Morphometric Analysis of Loggerhead (Caretta caretta) Sea Turtle Hatchlings. Mar TurtNewsl 154:12–15.
- Klingenberg CP. 2011. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecolresour 11:353–357. doi:10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed]
- Ljubisavljević K, Džukić G, Vukov T, Kalezić M. 2012. Morphological variability of the Hermann’s tortoise (Testudo hermanni) in the Central Balkans. Acta Herpetol 7:253–262. doi:10.13128/Acta_Herpetol-10930.
- Lubiana A, Ferreira-Junior PD. 2009. Pivotal temperature and sexual dimorphism of Podocnemis expansa hatchlings (Testudines: Podocnemididae) from Banana Island, Brazil. Zoologia 26:527–533. doi:10.1590/S1984-46702009000300016.
- McCoy CJ, Vogt RC, Censky EJ. 1983. Temperature-controlled sex determination in the sea turtle Lepidochelys olivacea. J Herpetol 17:404–406. doi:10.2307/1563594.
- Michel-Morfin JE, Gomez Munoz V, Navarro Rodriguez C. 2001. Morphometric model for sex assessment in hatchling olive ridley sea turtles. Chel Conserv Biol 4:53–58.
- O ´Malley B. 2005. Clinical anatomy and physiology of exotic species. Structure and function of mammals, birds, reptiles and amphibians. Elsevier Limited Saunders. Philadelphia, USA.
- Owens DW, Hendrickson JR, Lance V, Callard IP. 1978. A technique for determining sex of immature Chelonia mydas using a radioimmunoassay. Herpetologica 34:270–273.
- Perälä J. 2004. Testudo hercegovinensis Werner, 1899. Manouria 7:19–20.
- Soler J, Pfau B, Martínez-Silvestre A. 2012. Detecting intraspecific hybrids in Testudo hermanni (Gmelin 1789). Radiata 21:4–29.
- Sönmez B, Bağda E, Candan O, Yilmaz HE. 2019. Sex determination in green turtle hatchlings: Geometric morphometry and molecular sex markers. NESciences 4:42–54. doi:10.28978/nesciences.522623.
- Sönmez B, Turan C, Özdilek ŞY, Turan F. 2016. Sex determination of green sea turtle (Chelonia mydas) hatchlings on the bases of morphological characters. J Black Sea/Mediterranean Environment 22:93–102.
- Türkozan O, Özdemir A, Kiremit F. 2008. International Testudo trade. Chel Conserv Biol 7(2):269–274. doi:10.2744/CCB-0724.1.
- Türkozan O, Yilmaz C, Karakaya Ş, Karaman S, Ulger C. 2019. Distribution, Size, and Demographics of Eastern Hermann’s Tortoise, Testudo hermanni boettgeri, in Turkey. Chel Conserv Biol 18(2):210–216. doi:10.2744/CCB-1329.1.
- Valenzuela N, Adams DC, Bowden RM, Gauger AC. 2004. Geometric morphometric sex estimation for hatchling turtles: a powerful alternative for detecting subtle sexual shape dimorphism. Copeia 4:735–742. doi:10.1643/CH-03-248R1.
- Van der Heiden AM, Briseno R, Rios-Olmeda D. 1985. A simplified method for determining sex in hatchling sea turtles. Copeia 1985:779–782.
- Veeramani R, Shankar N, Narayanan S, Ranganath P, Rajagopalan R. 2010. Gender differences in the medio lateral placement of the patella and tibial tuberosity: a geometric analysis. Anatomy 4:45–50. doi:10.2399/ana.09.039.
- Willemsen RE, Hailey A. 2003. Sexual dimorphism of body size and shell shape in European tortoises. J Zool 260:353–365. doi:10.1017/S0952836903003820.
- Wold S, Esbensen K, Geladi P. 1987. Principal component analysis, Chemometr. Intell Lab 2:37–52. doi:10.1016/0169-7439(87)80084-9.
- Wyneken J, Epperly SP, Crowder LB, Vaughan J, Esper KB. 2007. Determining sex in posthatchling loggerhead sea turtles using multiple gonadal and accessory duct characteristics. Herpetologica 63:19–30. doi:10.1655/0018-0831(2007)63[19:DSIPLS]2.0.CO;2.