Abstract
The outbreak of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, in December 2019, and its global dissemination became the coronavirus disease 2019 (COVID-19) pandemic declared by the World Health Organization (WHO) on 11 March 2020. In patients undergoing immunotherapy, the effect and path of viral infection remain uncertain. In addition, viral-infected mice and humans show T-cell exhaustion, which is identified after infection with SARS-CoV-2. Notably, they regain their T-cell competence and effectively prevent viral infection when treated with anti-PD-1 antibodies. Four clinical trials are officially open to evaluate anti-PD-1 antibody administration's effectiveness for cancer and non-cancer individuals influenced by COVID-19 based on these findings. The findings may demonstrate the hypothesis that a winning strategy to combat SARS-CoV-2 infection could be the restoration of exhausted T-cells. In this review, we outline the potential protective function of the anti-PD-1 blockade against SARS-CoV-2 infection with the aim to develop SARS-CoV-2 therapy.
Keywords: SARS-CoV-2, COVID-19, Anti-PD-1, T-cells, Immunotherapy
Graphical Abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) is an emerging pandemic disease triggered by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1], [2], [3], [4]. SARS-CoV-2 affected more than 188,655,968 people worldwide as of 7:21 pm CEST, 16 July 2021, contributing to more than 4,067,517 deaths, with a fatality rate of 2.15% [5]. COVID-19 is described by a wide range of clinical symptoms, varying from asymptomatic or mild disease to chronic disease involving hospitalization and oxygen therapy [6]. Immunological responses to SARS-CoV-2 remain critically important for developing effective vaccines and appropriate treatments for patients with COVID-19. Because patients are immunosuppressed owing to their treatments, such as chemotherapy and radiation, and because of the tumor itself, those who acquire COVID-19 will likely have a severe infection [7]. The manner in which the pathway of viral infection is altered in people receiving immunotherapy remains unclear.
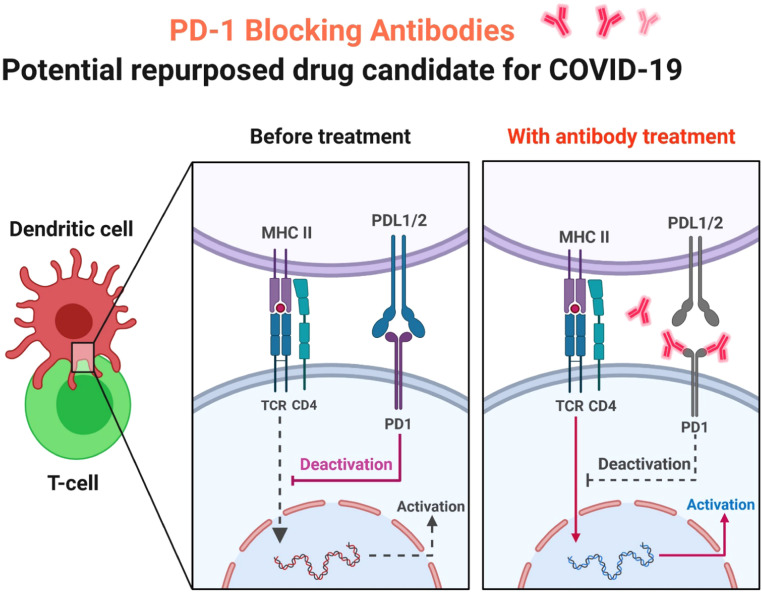
Laura Pala et al. documented the case of a patient with metastatic melanoma, who had been diagnosed with SARS-CoV-2 and healed without side effects in a long-term response to the anti-PD-1 blockade ( Fig. 1) [8]. This study and limited findings endorse the likelihood of continuity of immunotherapy in patients with and without comorbidity-free disease and make a conjecture on the defensive function of PD-1 blockade against COVID-19 [8]. Since the United States Food and Drug Administration (US FDA) approval of the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) ipilimumab inhibitor in 2011, indications for immune checkpoint inhibitors (ICIs) have significantly increased [9]. They have been an important therapeutic choice for a broad spectrum of cancers, such as melanoma, renal carcinoma, lung cancer, urothelial cancer, and head and neck carcinoma [9]. Because of the suspected warning of severe pneumonia in the case of COVID-19, it is also uncertain how to manage checkpoint inhibitor medication in the sense of the present emergency [10].
Fig. 1.
PD-1 Blocking Antibodies. In patients with severe pneumonia caused by COVID-19, monoclonal antibodies that block PD-1 activity will increase T cell proliferation, cytokine production and reduce death.
Cancer immunotherapy succeeds by decreasing the effector T cells' inhibition, respiratory epithelial cells, particularly CD8+, through preserving cellular immunocompetence and enhancing tumor-specific immune responses [9]. One of the major drawbacks of the immune checkpoint inhibitor treatments is that they result in many inflammatory cytokines, such as IL-6 and TNF-α [9]. In around 3–5% of patients taking ICI, immune-mediated lung damage, named checkpoint inhibitor pneumonitis, happen; nevertheless, real-world occurrence could be higher, particularly now that ICIs are utilized outside clinical trials [11]. Therefore, in patients undergoing anti-cancer immunotherapy, a possible synergy between ICI lung toxicity and SARS-CoV-2 associated interstitial pneumonia has been speculated [10]. Patients undergoing ICI are in a hyperimmune situation and may develop unusual reactions in the event of COVID-19 infection because of the similarities between the two pathogenetic pathways of lung damage [9]. Compared to patients provided with chemotherapy and the general population, should the likelihood of developing diseases of cancer patients receiving immunotherapy be estimated?
Min-Seok Rha et al. characterized the immunological properties of SARS-CoV-2 related CD8+ T cells in acute and healing COVID-19 patients. In the acute and healing periods of COVID-19, their research offers insights into SARS-CoV-2-specific CD8+ T cell responses and CD8+ T cell memory [12]. These findings can fill gaps in current knowledge to improve prophylactics and therapeutics toward SARS-CoV-2 infection and to monitor the current COVID-19 pandemic, provided that CD8+ T cells serve as cardinal sentinels toward viral diseases [12]. It must, however, be validated by conclusive stem-like memory cell markers, like CD95. In addition, a stronger contrast between SARS-CoV-2-related memory T cells and memory T cells specific to other viruses will entail future experiments of long-term follow-up of COVID-19 convalescents. Min-Seok Rha et al. research focuses on the major histocompatibility complex (MHC) class I multimers specific to HLA-A*02-restricted epitopes in S protein. However, in phenotypes and function, CD8+ T cells specific to other SARS-CoV-2 proteins and epitopes limited by another human leukocyte antigen (HLA) class I allotypes may differ, which should be discussed in further research.
Up-to-date awareness of SARS-CoV-2 and COVID-19 is stated in the latest review articles. Special attention is given to new study methods to discover old and novel compounds for COVID-19 therapy and all currently studied therapeutic alternatives. Amidst these, the significance of modifying the host immune system is commonly debated, focusing on the clinical trial of the ICI anti-programmed death-1 (PD-1) blocking antibody and treatment currently utilized in oncology [13]. A study conducted by Laura Pala et al., which illustrated the persistence of anti-PD-1 therapy even after successful remission of underlying cancer, and minimal evidence reported in the literature, shows a preventative effect on COVID-19 and PD-1 blockade in cancer patients with and without chronic disease remission [8]. Future studies are required on the interplay of PD-1/PD-L1 inhibition in patients with cancer and COVID-19.
2. Immune checkpoint inhibitors' role in the fight against viral infections and SARS-CoV-2
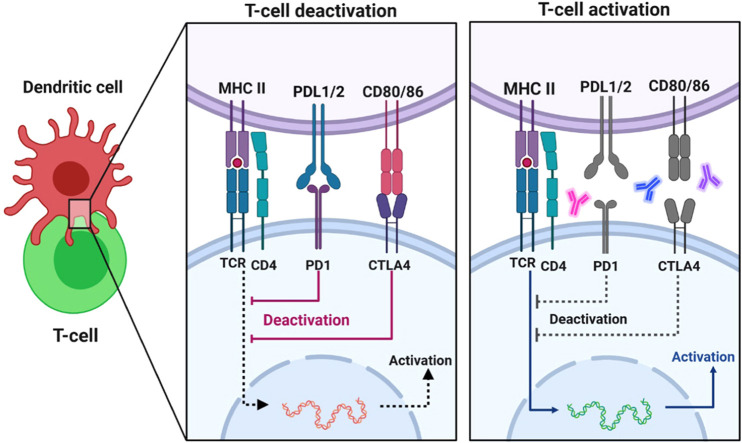
Due to drastic and long-lasting responses in several tumor types, ICIs are commonly frequently used in oncology. Still, there is little knowledge on their future application in the treatment of human infectious diseases. Aside from cancer immunosurveillance, the immune system's most crucial role is protection against pathogens, bacteria, viruses, and fungi [9]. T cells are continually subjected to antigens, including recurrent viral infections like human immunodeficiency virus (HIV) and Hepatitis B virus (HBV), contributing to T-cell exhaustion. The overexpression of inhibitory receptors CTLA4 and PD-1/PD-L1 is a typical feature of exhausted CD8 T cells, which results in decreased effector activity and low proliferative ability ( Fig. 2) [14]. The effectiveness of monoclonal antibodies against PD-1 and PD-L1 (Programmed death-ligand 1), which have revolutionized cancer therapy, means that therapeutically attacking these pathways may help prevent and manage various infectious diseases [9]. ICIs have been shown in many studies to boost T-cell responses and provide immune defenses against a variety of persistent infectious, bacterial, and parasitic infections, including malaria, HIV, HBV, and tuberculosis [9]. PD-1-targeted treatment inhibits tumor growth and lowers viral load in various cancer and persistent infection mouse models. In primates, identical observations were made. Many clinical trials, including the use of immune checkpoint blockade in managing recurrent viral infections, are planned and administered in cancer patients [15].
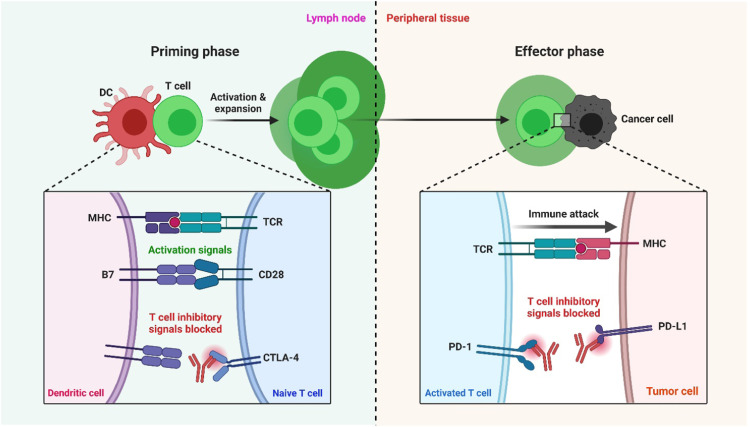
Fig. 2.
Blockade of PD-1 or CTLA-4 signaling in tumor immunotherapy. T cells use their T-cell receptor (TCR) to detect antigens provided by the MHC on cancer cells' surfaces. This first signal is insufficient to activate T cells; therefore, the B7 costimulatory molecules B7–1 (or CD80) and B7–2 (or CD86) must send a second signal. CTLA-4 (cytotoxic T-lymphocyte–associated antigen 4) is upregulated shortly after T-cell activation and initiates negative control signaling on T cells throughout ligation with antigen-presenting cells' B7 costimulatory molecules. These molecules have activation signals when they bind to CD28 and inhibitory signals when they bind to CTLA-4. CTLA-4 interacts with costimulatory molecules mainly during the priming process of a T-cell response within lymph nodes. T cells release the programmed death 1 (PD-1) inhibitory receptor during long-term antigen exposure, and ligation with PD-L1 and PD-L2, which are mainly expressed in inflamed tissues and the tumor microenvironment, results in the negative control of T cells. In peripheral tissues, the PD-1 association occurs during the effector process of a T-cell response. When antibodies to PD-1 or PD-L1 are used to inhibit it, T cells with cancer specificity are preferentially activated [19].
Edward Gane et al. recently conducted a Phase Ib analysis in persistent HBV patients contrasting Nivolumab (anti-PD-1) with and without GS-477, an HBV therapeutic vaccine. They discovered that Nivolumab was well-tolerated, with most patients seeing a decrease in hepatitis B surface antigen (HBsAg) levels. They achieved full HBsAg seroconversion and HBsAg loss in one patient [16]. In a Phase II trial, Cynthia L. Gay et al. documented the function of an anti-PD-L1 antibody (BMS-936559) in HIV-infected patients, receiving an enhancement in HIV-1-specific immunity in the majority of participants. This is the first ICI study in HIV patients who do not have cancer [17]. What's more, the inhibition of the immune checkpoint mechanism CD200-CD200R1 has been found to have beneficial effects on coronaviruses, preserving Interferon (IFN) output and rising viral clearance [18].
In COVID-19 patients, a study with another ICI anti-PD1 drug, Camrelizumab, is also underway. Checkpoint inhibitors show tremendous promise in managing viral infections and reducing viral load, considering the absence of evidence from clinical trials of infectious diseases. Though checkpoint blockade can lead to the ‘fueling' of the cytokine storm in the presence of COVID-19, the recovery of immunocompetence may perform a defensive or even therapeutic function in the face of viral infection [9]. We may thus hypothesize two strategies; The first ‘pre-infectious state’: the people treated with ICI is more ‘resistant’ to the attack of COVID-19; The other ‘infectious state’: patients taking immunotherapy, when getting the COVID-19, might develop more respiratory severe symptoms and complications, sustained concurrently by the ‘cytokine storm,’ and the ICI-immune mediated injury.
Since ICIs have only been investigated in a limited number of human infectious diseases, it is possible that they could quickly revolutionize the treatment of infectious agents, like COVID-19. Regrettably, in the era of ICIs, the clinical progress in the oncology field is yet to be matched with those in the infectious. Still, there are broad viewpoints for studies and development in the future [9].
3. Clinical result of cancer patients with SARS-CoV-2 Infection treated by immune checkpoint inhibitors
A lack of up-to-date reporting exists on the clinical effects of anti-cancer treatment on SARS-CoV2 infection [20], [21], [22], [23], [24], [25]. Compared to patients without cancer, patients with cancer and COVID-19 are at higher risk of severe illness and death. Both treatments, such as chemotherapy and radiotherapy, and the tumor itself, can contribute to overall immunosuppressive status [7]. Of the cancer patients who were given ICIs, especially anti-PD1 and anti-CTLA-4 treatments, nothing is known about the spread of SARS-CoV-2 infection. It is unclear whether PD-1/CTLA-4 suppression improves or worsens the effectiveness of COVID-19 in cancer patients. Immunogenicity has been shown to be increased when ICIs are administered [26]. ICIs, on the other hand, may adversely interact with virus pathogenesis, contributing to a systemic inflammatory disorder correlated with hyperactivation of the immune system identified as cytokine release syndrome (CRS), recorded as an undesirable effect of anti-PD-1 and other immunotherapy dependent on T cells, also worsening the impact of viral infection [27]. Pending further information, this immunization would pose an additional risk of developing immune-related pneumonitis in patients who have received anti-PD-1 [28].
The deterioration of immunotherapy-related lung function may correlate with infection-related symptoms and thus exacerbate the outcome. We can't say with certainty that the outcome of infection will be different for other forms of cancer because of varying risk factors or abnormal conditions connected with a specific kind of cancer, such as lung cancer associated with smoking [8]. Previously released results indicate that those with hypertension (Odds Ratio, OR: 2.29, 95% Confidence Interval: 1.69–3.10) and diabetes (OR: 2.47, 95% Confidence Interval: 1.67–3.66) had a higher likelihood of developing severe COVID-19 disease [29]. The absence of lung disease or other risk factors and the full therapeutic response of cancer, and no indication of radiological disorder correlated with a weak or even missing immunosuppressive condition, may have led to the infection's positive path [8]. Additionally, an anti-PD-1 blocking function may act as a virus defense mechanism in the early stages of infection.
The number of exhausted CD8+ T cells is substantially higher in patients with SARS-CoV-2 severe infection than in patients with moderate symptoms. The exhausted CD8+ T phenotype could be reversed by functional blockade of PD-1 and CTLA-4, thereby enhancing antigen-specific immunity and antiviral activity toward SARS-CoV-2 ( Fig. 3) [30]. In addition, in vivo studies have demonstrated rapid upregulation of PD-1 during lymphocytic choriomeningitis viral (LCMV) infection after activating specific virus-naïve CD8 T cells. During the early phase of acute illness, blocking of the PD-1 pathway utilizing anti-PD-L1 or anti-PD-1 antibodies contributes to activation of the response of CD8 T cells specific to the virus due to accelerated clearance of the infection [31]. No reliable evidence was found to indicate whether PD-1 inhibition would have a protective effect against SARS-CoV-2 infection in cancer patients. Most of the studies on oncology patients were done on patients who had received chemotherapy. Many case reports on patients with solid tumors treated with anti-PD-1 have yet to be published [8]. Da Costa et al. treated a Merkel cell carcinoma patient with Pembrolizumab who experienced a severe infection and respiratory distress syndrome that was complicated by acute renal damage, all of which led to his admission to intensive care (ICU). The patient was released after three months with the aid of extended recovery [32].
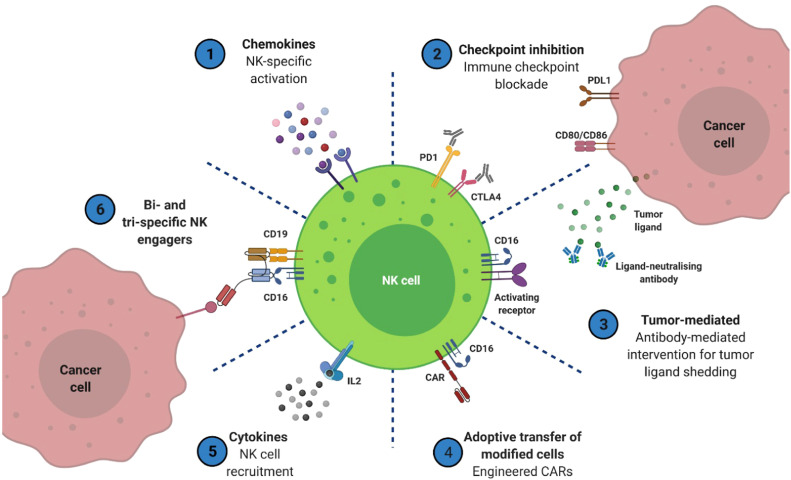
Fig. 3.
Using the innate effector lymphocyte natural killer (NK) cell, potential immunotherapeutic techniques for cancer treatment have been identified. 6 major strategies are highlighted to increase the effector activity of NK cells and assist in the targeting of cancer cells. Some are clinically licensed, such as cytokines and immune checkpoint inhibitors, whereas others are still in the pre-clinical stage. To improve the effectiveness of these therapies in solid tumors, further research is required [39].
Two newly released case reports identified two patients with anti-PD-1-treated melanoma who had fully healed from COVID-19 after exhibiting mild infection symptoms [33]. Recently, the biggest study on patients with cancer and COVID-19 has been completed. The research team examined results from the COVID-19 and Cancer Consortium (CCC19) databases on more than 900 patients with current cancer or previous malignancies in order to better understand the results and make predictions [34]. Less than 4% of patients were offered any treatment that included immunotherapy (including checkpoint inhibitors and allogeneic hemopoietic stem-cell transplant and adoptive cellular therapy). Of the 928 patients seen, 38 were diagnosed with melanoma [34]. Prognostic factors that were significantly associated with a 30-day mortality risk of two times higher were: age older. For males, the chance ratio is 1.63 (CI: 1.07–2.48). Also, the odds ratio per 10-year age increment is 1.84 (95% CI: 1.53–2.21) [34]. Regrettably, there was no study of the relationship between various kinds of therapies obtained (such as immunotherapy) and the incidence of COVID-19 [34].
One of the most comprehensive reported series recorded the results of 41 of the 69 consecutive lung cancer patients who received anti-PD-1 treatment [35]. According to the TERAVOLT database, there was no connection between the kind of anti-cancer treatment obtained and COVID-19-induced death in 147 patients with thoracic cancer. Conventional treatments include, but are not limited to, chemotherapy, tyrosine kinase inhibitors (TKIs), chemotherapy coupled with ICIs, and ICIs used alone as monotherapy [36]. Mengyuan Dai et al. found that a restricted number of patients in a series of 105 cancer patients who were administered immunotherapy had significant COVID-19 severity and death rates [37]. These contradictory observations from the data available to date illustrate the critical need to collect further data in order to draw accurate conclusions. In a comprehensive prospective study to monitor tumor immune response to SARS-CoV-2, the CAPTURE-COVID-19 antiviral response was measured across all forms of cancer. It was shown that in many cancers, the immune response is dramatically impaired, and new infections are more common [38].
Several clinical studies have begun recruiting patients who have been diagnosed with COVID-19 because of the anti-PD-1 drug's potential to restore cellular immunocompetence. In patients with COVID-19 sepsis, the clinical efficacy of anti-PD-1 Camrelizumab will be examined in combination with thymosin, an immunomodulatory medication, rather than using the standard therapy (NCT04268537) [8]. A phase-II randomized trial named IMMUNONCOVID (NCT04333914) is conducted in patients with cancer and SARS-CoV-2 illness. It aims to examine how well chloroquine analog (GNS561), an anti-PD-1 (Nivolumab), and an IL-6 receptor (Tocilizumab) perform versus the accepted standard of care. The standard of treatment of patients with COVID-19 who require hospitalization is being studied in a phase-II clinical trial (NCT04343144) [8]. Nivolumab's effectiveness in clearing SARS-CoV-2 in patients with moderate or asymptomatic disease is tested in a pilot study (NCT04356508). The COPERNICO study is a multicenter, two-arm phase-II trial evaluating Pembrolizumab plus Tocilizumab against the standard of treatment (NCT04335305) in patients with COVID-19-related pneumonia who have not adapted to frontline medication [8].
4. PD-1 and CTLA-4
PD-1, or CD279, is a protein that is present on the surface of cells and influences T-cell immunological functions, decreasing immune system responses while increasing self-tolerance [40]. PD-1 (Programmed cell death), or apoptosis, is triggered in the lymph nodes of patients treated with PD-1, an immune checkpoint inhibitor while restraining the impact on regulatory T-cells (anti-inflammatory, suppressive T-cells) [41]. The PD-1 receptor is located on the cell membranes of CD8+ and CD4+ T-cells. In the case of PD-1 and PD-L1/PDL2, the PD-1/PDL1 pathway prevents T cells from responding to the threat by tying PD-1 to its ligands (PD-L1 and PD-L2), which are found on both the tumor and peripheral tissues. The PD-L1 expression is also associated with the induction of IFN-secreting T-cells [42]. Moreover, significant increases in the levels of GITR, sTim-3, CD27, sPD-1, and sLAG-3 on the surface of CD4 and CD8 T-cells were seen in severe cases of the disease, as opposed to milder cases [43].
When the PD-1/PD-L1 axis is inhibited, the theory is that it will enable the T cells to identify better and destroy tumor cells. It is suggested that PD-1, which regulates T-cell responses, is more prevalent on CD8+ T-cells in chronic infections or cancers [44]. T-cell exhaustion has been demonstrated to result from continuous PD-1 expression, and it impairs the ability to combat infectious cells [45]. Because CD8+ T-cells produce IFN-γ and CD4+ T-cells secrete Th-1 and Th-2 cytokines, in addition to cytotoxic T-cells, the CD8+ and CD4+ effector T-cells must be known if COVID-19 patients are to eliminate and remove viral particles effectively [46], [47], [48]. COVID-19 patients may show a decrease in lymphocyte percentage, although it is correlated with an equivalent percentage of CD4+ and CD8+ T-cells. There is an abundance of CD8+ T-cells, with high levels of the late activity marker CD25, as well as an increase in PD-1 levels when T-cell exhaustion occurs. Since SARS-CoV-2 has the ability to affect the acquired immune system, including B and T cells, the virus may affect these cells [49]. Monocytes express PD-1 on their surface, and when PD-L1 binds to it, which happens as a result of the PD-L1/PD-1 complex, they release IL-10, which limits the ability of CD4+ T cells to respond [50]. Despite the presence of the PD-1/PD-L1 axis, which is often implicated in chronic viral infections, no studies have yet examined the impact of this axis on acute viral infections. It is uncertain since checkpoint molecules have not been well controlled in T-cell dysfunctions [44], [51].
CD95 (Fas) and PD-1 expression have also been seen in COVID-19 patients with increased CD4+ and CD8+ T-cells. This connection further indicates that regulatory molecules during COVID-19 infection, which causes an increase in the percentage of naïve T-cells, are associated with the cell death of antigen-activated T-cells, which in turn reduces the number of CD4+ T-cells [52]. Furthermore, patients diagnosed with COVID-19 exhibit lymphocytopenia and low lymphocytes, B cells, and T cells. Despite this, they contain high IL-6, IL-10, and TNF-α and increase exhaustion markers such as PD-1 and Tim-3, which reduce T-cell activity and subsequently memory T-cell function [53], [54]. While in COVID-19 patients, a rise in the percentage of monocytes, neutrophils, and natural killer cells (NK cells) that are essential for cytokine storm (CS) has been observed [55]. In chronic viral infections, such as HIV, it has been suggested that changes in the PD-1/PD-L1 pathway are an indication of monocytes reconfiguring and dendritic cells (DCs) responding to viral infection via STAT3-dependent IL-10 production [56]. More importantly, the PD-1/PD-L1 axis may be implicated in causing severe viral infections and monocyte reconfiguration in COVID-19 patients with elevated IL-10 levels [57].
In contrast to a healthy human, COVID-19 patients' neutrophils cannot control the release of PD-L1 on the surface of immune cells, resulting in low PD-L1 expression. On all monocytes and DCs, patients with more extreme conditions had higher PD-L1 expression ( Fig. 4) [57]. A considerable rise in PD-1, perforin, and granzyme B expression was seen in CD4+ or CD8+ T cells after three weeks of initiating treatments in those who had the most severe clinical symptoms [58]. A strategy that can reduce and inhibit both the inflammatory cascade and the generation of fatigued T-cells is the best way to treat COVID-19 and target PD-1 [31]. It is possible to reach this goal by using both anti-PD-1 and anti-IL-6R simultaneously.
Fig. 4.
A strategy for producing personalized viral-specific T cells as a possible therapeutic for preventing and/or treating SARS-CoV-2 infections in susceptible communities, including cancer patients. SARS-CoV-2 peptides are pulsed into an individual's monocytic-DCs and are then used to prime the same individual's T cells, resulting in SARS-CoV-2-specific T cells. These T cells may be cryopreserved or infused into the vulnerable individual as prevention or treatment against COVID-19 [66], [67], [68].
CTLA-4 has been identified as an inhibitory immune checkpoint molecule, but the mechanisms underlying its function remain unclear and may include many overlapping mechanisms [59]. This molecule prevents CD28's excitatory response by linking to CD86 and CD80 ligands, which are both active in the initial excitatory phases of immature and memory T cells [59]. CTLA-4 is capable of forming stronger bonds with these other ligands when ligand density is low. In contrast to CD28's monovalent association with its associated ligands, this attachment is improved by utilizing the B7 ligand to create network structures [59]. As a consequence, CTLA-4 casts CD28 out of the immunological synapse [59]. This molecule often sends inhibitory messages to T-cells, preventing them from activating; this, in turn, causes the ligands to be lost by endocytosis on antigen-presenting cells [60], [61]. To create CTLA-4 signaling, which could then activate ligand-expressing cells and block both regulatory and effector T-cells, the CTLA-4 proteins were fused to B-7 ligands [62], [63].
Dendritic cells activate T cells via promoting T-cell activation through CTLA-4. When antigen-presenting cells are present, T-cells are prompted to act. When two cell types, such as the MHC class I antigen complex on dendritic cells and CD28 on T cells, are joined, this prompts the T-cell activity to begin [64]. The T cell surface protein CTLA-4 has a higher affinity for B7 than CD28 and is associated with suppressing T cell responses and stimulating T cell activity [65]. As a result, preventing the pathways correlated with CTLA-4 enhances T cell activity by allowing B-7 to become available [59]. Ligands are only seen on T-cells expressing the CTLA-4 protein, which is found only on T-cells [59].
5. Conclusions
Concerning the identified cancer entities, our current understanding of the mutual effect of cancer and COVID-19 is minimal and inconsistent. ICI was first developed and is now widely utilized as a first-line treatment for skin cancer; research on the results of melanoma patients treated with ICI and COVID-19 is now rising [8], [69], [70], [71], [72]. As most frequently reported experiences, people encounter various kinds of cancer, including the aforementioned hematological malignancies and gastrointestinal, bladder, and lung cancers. COVID-19 tends to be more severe in lung cancer cases and is possibly attributed to preexisting lung injury from previous surgical treatments and a lengthy smoking background [36], [37], [73]. According to our in-depth examination of the most recent review, cancer therapies that target PD-1, PD-L1, and/or CTLA-4 aren't likely to help patients become infected with SARS-CoV-2. Though ICI use in cancer patients with COVID-19 has not yet been shown to cause mortality or morbidity with SARS-CoV-2 infection, it is unclear whether the ICI will raise these issues. From a practical standpoint, and in light of ICI's mode of action, this strategy tends to be safe for cancer patients who already have SARS-CoV-2 infection [22], [74], [75], [76], [77]. Consequently, it may be contraindicated for patients with malignancies, particularly those with distant metastases undergoing ICI cancer treatment [72], [78]. A newly released study on the NHS England website updated the recommended treatments for patients receiving care during the COVID-19 epidemic. According to their recommendations, chemotherapy should only be used for first-line malignancies if ICI monotherapy has already been started [79].
ICI may be administered for longer durations if in-label and recommended because the chance of harmful effects on healthcare workers and other patients is lower in this case [79], [80]. Home-based infusion could be an alternative for managing cancer patients throughout this pandemic in rare cases. Those who want to go off of ICI treatment for an extended period or discontinue ICI care in regions more susceptible to COVID-19 may also consider doing so [80]. The European Society for Medical Oncology (ESMO), the National Comprehensive Cancer Network (NCCN), S.H. Nahm et al., and Brian C. Baumann et al., both with an emphasis on melanoma treatment throughout the pandemic, have released guidance and suggestions, which were recently outlined by Ömer Faruk Elmas et al. [81], [82], [83], [84], [85]. The alternative approach to ICI care may include supplementary immunosuppressive therapies, which may help control the ICI-related immune-related adverse events (irAEs), including SARS-CoV-2 infection. Furthermore, asymptomatic COVID-19 patients may need immunosuppressive therapies. While some immunosuppressive drugs (e.g., corticosteroids, TNF-blockers, and IL-6-blockers) may cause severe lymphopenia in this instance, there are alternatives to these immunosuppressive medicines that don't negatively impact white blood cell counts. Still, because ICI-associated irAEs patients are taking immunosuppressants to treat SARS-CoV-2 infections, doctors who care for these patients should be on the lookout for symptoms or indications of a SARS-CoV-2 infection or a worsening of underlying COVID-19 [86].
Even though ICI is not known to cause immunosuppression in and of itself, excluding it from cancer patients' regimens in order to prevent SARS-CoV-2 infection is a missed opportunity [22], [23]. If the SARS-CoV-2 infection spreads further, the consequences of a lack of high-level oncology treatment would undoubtedly outweigh a COVID-19 disease in cancer patients [87]. Indeed, epidemiological extrapolations expect a 20% rise in cancer mortality due to the COVID-19 pandemic, owing mainly to delays in detection and care [88]. As a result, the possibility of developing COVID-19 or triggering an existing infection must be weighed against the risk of cancer progression [10], [22], [69], [89], [90], [91]. Finally, there is growing evidence that ICI may help fight tumors and SARS-CoV-2 infections. At the moment, the clinical trial activity includes those mentioned above. Cases of T-cell exhaustion, such as those seen by cancer and SARS-CoV-2 patients, are said to be quite common.
CRediT authorship contribution statement
Annoor Awadasseid and Qiang Yin. designed research, wrote the manuscript, and revised the manuscript. Yanling Wu conceived of the study. Wen Zhang designed the study, revised the manuscript, and provided funding support. All authors have read and approved the final manuscript.
Conflict of interest statement
The authors have declared that no competing interest exists.
Acknowledgments
We gratefully acknowledge the support by the National Natural Science Foundation of China (No. 21877101), the Zhejiang Leading Innovation and Entrepreneurship Team (2018R01015), and the Emergency Project of Key Research and Development Plan of Zhejiang Province (2020C03124).
References
- 1.Awadasseid A., Wu Y., Tanaka Y., Zhang W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. Int. J. Biol. Sci. 2020;16:1846–1860. doi: 10.7150/ijbs.45018. http://www.ijbs.com/v1816p1846.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awadasseid A., Wu Y., Tanaka Y., Zhang W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021;17:8–19. doi: 10.7150/ijbs.52569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awadasseid A., Wu Y., Tanaka Y., Zhang W. SARS-CoV-2 variants evolved during the early stage of the pandemic and effects of mutations on adaptation in Wuhan populations. Int. J. Biol. Sci. 2021;17:97–106. doi: 10.7150/ijbs.47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awadasseid A., Wu Y., Tanaka Y., Zhang W. Effective drugs used to combat SARS-CoV-2 infection and the current status of vaccines. Biomed. Pharmacother.. 2021;137 doi: 10.1016/j.biopha.2021.111330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://covid19.who.int/.
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pala L., Conforti F., Cocorocchio E., Ferrucci P., De Pas M.T., Stucchi S., Repetto M., Saponara M., Queirolo P. Course of Sars-CoV2 infection in patients with cancer treated with anti-PD-1: A case presentation and review of the literature. Cancer Investig. 2021;39:9–14. doi: 10.1080/07357907.2020.1844893. [DOI] [PubMed] [Google Scholar]
- 9.Gatto L., Franceschi E., Nunno V.D., Brandes A.A. Potential protective and therapeutic role of immune checkpoint inhibitors against viral infections and COVID-19, Future. Medicine. 2020 doi: 10.2217/imt-2020-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–273. doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suresh K., Naidoo J., Lin C.T., Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154:1416–1423. doi: 10.1016/j.chest.2018.08.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rha M.-S., Jeong H.W., Ko J.-H., Choi S.J., Seo I.-H., Lee J.S., Sa M., Kim A.R., Joo E.-J., Ahn J.Y. PD-1-expressing SARS-CoV-2-specific CD8+ T cells are not exhausted, but functional in patients with COVID-19. Immunity. 2021;54:44–52. e43. doi: 10.1016/j.immuni.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivarelli S., Falzone L., Torino F., Scandurra G., Russo G., Bordonaro R., Pappalardo F., Spandidos D.A., Raciti G., Libra M. Immune-checkpoint inhibitors from cancer to COVID‑19: a promising avenue for the treatment of patients with COVID‑19. Int. J. Oncol. 2021;58:145–157. doi: 10.3892/ijo.2020.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wykes M.N., Lewin S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018;18:91–104. doi: 10.1038/nri.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyck L., Mills K.H. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 16.Gane E., Verdon D.J., Brooks A.E., Gaggar A., Nguyen A.H., Subramanian G.M., Schwabe C., Dunbar P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J. Hepatol. 2019;71:900–907. doi: 10.1016/j.jhep.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Gay C.L., Bosch R.J., Ritz J., Hataye J.M., Aga E., Tressler R.L., Mason S.W., Hwang C.K., Grasela D.M., Ray N. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J. Infect. Dis. 2017;215:1725–1733. doi: 10.1093/infdis/jix191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaine C.A., Soberman R.J. The CD200–CD200R1 inhibitory signaling pathway: immune regulation and host–pathogen interactions. Adv. Immunol. 2014;121:191–211. doi: 10.1016/B978-0-12-800100-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas A. Tumor immunotherapy directed at PD-1. New Engl. J. Med. 2012;366:2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 20.Bonomi L., Ghilardi L., Arnoldi E., Tondini C.A., Bettini A.C. A rapid fatal evolution of coronavirus disease-19 in a patient with advanced lung cancer with a long-time response to nivolumab. J. Thorac. Oncol. 2020;15:e83–e85. doi: 10.1016/j.jtho.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bersanelli M., Buti S., De Giorgi U., Di Maio M., Giannarelli D., Pignata S., Banna G.L. State of the art about influenza vaccination for advanced cancer patients receiving immune checkpoint inhibitors: when common sense is not enough. Crit. Rev. Oncol. Hematol. 2019;139:87–90. doi: 10.1016/j.critrevonc.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Kattan J., Kattan C., Assi T. Do checkpoint inhibitors compromise the cancer patients’ immunity and increase the vulnerability to COVID-19 infection? Immunotherapy. 2020 doi: 10.2217/imt-2020-0077. imt-2020-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rassy E., Khoury-Abboud R.-M., Ibrahim N., Kattan C., Assi T., Kattan J. What the oncologist needs to know about COVID-19 infection in cancer patients. Future Med. 2020 doi: 10.2217/fon-2020-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Citarella F., Russano M., Pantano F., Dell’Aquila E., Vincenzi B., Tonini G., Santini D. Facing SARS-CoV-2 outbreak in immunotherapy era. Future Oncol. 2020;16:1475–1485. doi: 10.2217/fon-2020-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bamber L.S., Christensen T.E., Gaver K.M. Do we really ‘know’what we think we know? A case study of seminal research and its subsequent overgeneralization. Account., Organ. Soc. 2000;25:103–129. [Google Scholar]
- 26.Koralnik I.J. Can immune checkpoint inhibitors keep JC virus in check? New Engl. J. Med. 2019;380:1667–1668. doi: 10.1056/NEJMe1904140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceschi A., Noseda R., Palin K., Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front. Pharmacol. 2020;11:557. doi: 10.3389/fphar.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Q., Zhu E.C., Wu J.-b, Li T., Hou Y.-l, Wang D.-y, Gao Z.-h. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front. Immunol. 2019;10:108. doi: 10.3389/fimmu.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., Dong X.-Q., Zheng Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn E., Araki K., Hashimoto M., Li W., Riley J.L., Cheung J., Sharpe A.H., Freeman G.J., Irving B.A., Ahmed R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 2018;115:4749–4754. doi: 10.1073/pnas.1718217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Costa C.M., de Souza Z.S., Real Salgues A.C., Harada G., Marino Rodrigues Ayres P.P., Vieira Nunes D.B., Katz A., Munhoz R.R. COVID-19 in a patient with advanced Merkel cell carcinoma receiving immunotherapy. Immunotherapy. 2020;12:1133–1138. doi: 10.2217/imt-2020-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Giacomo A.M., Gambale E., Monterisi S., Valente M., Maio M. SARS-COV-2 infection in patients with cancer undergoing checkpoint blockade: Clinical course and outcome. Eur. J. Cancer. 2020;133:1–3. doi: 10.1016/j.ejca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.-Y., Desai A., de Lima G. Lopes Jr, Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garassino M.C., Whisenant J.G., Huang L.-C., Trama A., Torri V., Agustoni F., Baena J., Banna G., Berardi R., Bettini A.C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., Zhang Z., You H., Wu M., Zheng Q. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Au L., Boos L.A., Swerdlow A., Byrne F., Shepherd S.T., Fendler A., Turajlic S. Cancer, COVID-19, and antiviral immunity: the CAPTURE study. Cell. 2020;183:4–10. doi: 10.1016/j.cell.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nayyar G., Chu Y., Cairo M.S. Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front. Oncol. 2019;9:51. doi: 10.3389/fonc.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 41.Francisco L.M., Sage P.T., Sharpe A.H. The PD‐1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong Y., Wang Y., Wu X., Han J., Li G., Hua M., Han K., Zhang H., Li A., Zeng H. Storm of soluble immune checkpoints associated with disease severity of COVID-19. Signal Transduct. Target. Ther. 2020;5:1–3. doi: 10.1038/s41392-020-00308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schönrich G., Raftery M.J. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front. Cell. Infect. Microbiol. 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jubel J.M., Barbati Z.R., Burger C., Wirtz D.C., Schildberg F.A. The role of PD-1 in acute and chronic infection. Front. Immunol. 2020;11:487. doi: 10.3389/fimmu.2020.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M., Endeman H., van den Akker J.P., Molenkamp R., Koopmans M.P., van Gorp E.C. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88:11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor P., Askonas B. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58:417–420. [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X., Dai T., Zhou X., Qian H., Guo R., Lei L., Zhang X., Zhang D., Shi L., Cheng Y. Naturally activated adaptive immunity in COVID‐19 patients. J. Cell. Mol. Med. 2020;24:12457–12463. doi: 10.1111/jcmm.15771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Said E.A., Dupuy F.P., Trautmann L., Zhang Y., Shi Y., El-Far M., Hill B.J., Noto A., Ancuta P., Peretz Y. Programmed death-1–induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Alwis R., Bangs D.J., Angelo M.A., Cerpas C., Fernando A., Sidney J., Peters B., Gresh L., Balmaseda A., De A.D. Silva, Immunodominant dengue virus-specific CD8+ T cell responses are associated with a memory PD-1+ phenotype. J. Virol. 2016;90:4771–4779. doi: 10.1128/JVI.02892-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellesi S., Metafuni E., Hohaus S., Maiolo E., Marchionni F., D’Innocenzo S., La Sorda M., Ferraironi M., Ramundo F., Fantoni M. Increased CD95 (Fas) and PD‐1 expression in peripheral blood T lymphocytes in COVID‐19 patients. Br. J. Haematol. 2020;191:207–211. doi: 10.1111/bjh.17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Y. Zheng , Z. Huang , G. Yin , X. Zhang , W. Ye , Z. Hu , C. Hu , H. Wei , Y. Zeng , Y. Chi , Study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi-organ injury, 2020.
- 56.Planès R., BenMohamed L., Leghmari K., Delobel P., Izopet J., Bahraoui E., Tat H.I.V.-1. protein induces PD-L1 (B7-H1) expression on dendritic cells through tumor necrosis factor alpha-and toll-like receptor 4-mediated mechanisms. J. Virol. 2014;88:6672–6689. doi: 10.1128/JVI.00825-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parackova Z., Zentsova I., Bloomfield M., Vrabcova P., Smetanova J., Klocperk A., Mesežnikov G., Casas Mendez L.F., Vymazal T., Sediva A. Disharmonic inflammatory signatures in COVID-19: augmented Neutrophils’ but impaired monocytes’ and dendritic cells’ responsiveness. Cells. 2020;9:2206. doi: 10.3390/cells9102206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang C.K., Han G.-C., Kim M., Kim G., Shin H.M., Song K.-H., Choe P.G., Park W.B., Kim E.S., Kim H.B. Aberrant hyperactivation of cytotoxic T-cell as a potential determinant of COVID-19 severity. Int. J. Infect. Dis. 2020;97:313–321. doi: 10.1016/j.ijid.2020.05.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quezada S., Peggs K. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br. J. Cancer. 2013;108:1560–1565. doi: 10.1038/bjc.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider H., Downey J., Smith A., Zinselmeyer B.H., Rush C., Brewer J.M., Wei B., Hogg N., Garside P., Rudd C.E. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 61.Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 63.Corse E., Allison J.P. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J. Immunol. 2012;189:1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 64.Sansom D. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101:169–177. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rowshanravan B., Halliday N., Sansom D.M. CTLA-4: a moving target in immunotherapy. Blood J. Am. Soc. Hematol. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keller M.D., Bollard C.M. Virus-specific T-cell therapies for patients with primary immune deficiency. Blood J. Am. Soc. Hematol. 2020;135:620–628. doi: 10.1182/blood.2019000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chernykh E., Leplina O., Oleynik E., Tikhonova M., Tyrinova T., Starostina N., Ostanin A. Immunotherapy with interferon-α-induced dendritic cells for chronic HCV infection (the results of pilot clinical trial) Immunol. Res. 2018;66:31–43. doi: 10.1007/s12026-017-8967-2. [DOI] [PubMed] [Google Scholar]
- 68.Ko E.-J., Robert-Guroff M. Dendritic cells in HIV/SIV prophylactic and therapeutic vaccination. Viruses. 2020;12:24. doi: 10.3390/v12010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quaglino P., Fava P., Brizio M., Marra E., Rubatto M., Agostini A., Tonella L., Ribero S., Fierro M.T. Metastatic melanoma treatment with checkpoint inhibitors in the COVID‐19 era: experience from an Italian skin cancer unit. J. Eur. Acad. Dermatol. Venereol. 2020;34:1395–1396. doi: 10.1111/jdv.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidle P., Biedermann T., Posch C. COVID‐19 in a melanoma patient under treatment with checkpoint inhibition. J. Eur. Acad. Dermatol. Venereol. 2020;34:e465–e466. doi: 10.1111/jdv.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez-Cao M., Basa M.A., Puertolas T., Muñoz E., Manzano J.L., Carrera C., Marquez-Rodas I., Criado P.L., Moreno J.F.R., Castaño A.G. Cancer immunotherapy does not increase the risk of death by COVID-19 in melanoma patients. medRxiv. 2020 [Google Scholar]
- 72.Maio M., Hamid O., Larkin J., Covre A., Altomonte M., Calabrò L., Vardhana S.A., Robert C., Ibrahim R., Anichini A. Immune checkpoint inhibitors for cancer therapy in the COVID-19 era. Clin. Cancer Res. 2020;26:4201–4205. doi: 10.1158/1078-0432.CCR-20-1657. [DOI] [PubMed] [Google Scholar]
- 73.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A., Pradhan K., Thota R., Reissman S., Sparano J.A. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Redelman-Sidi G., Michielin O., Cervera C., Ribi C., Aguado J.M., Fernández-Ruiz M., Manuel O. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors) Clin. Microbiol. Infect. 2018;24:S95–S107. doi: 10.1016/j.cmi.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vivarelli S., Falzone L., Grillo C.M., Scandurra G., Torino F., Libra M. Cancer management during covid-19 pandemic: is immune checkpoint inhibitors-based immunotherapy harmful or beneficial? Cancers. 2020;12:2237. doi: 10.3390/cancers12082237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Odabasi Z., Cinel I. Consideration of severe coronavirus disease 2019 as viral sepsis and potential use of immune checkpoint inhibitors. Crit. Care Explor. 2020;(2) doi: 10.1097/CCE.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Cosimo S., Malfettone A., Pérez-García J.M., Llombart-Cussac A., Miceli R., Curigliano G., Cortés J. Immune checkpoint inhibitors: a physiology-driven approach to the treatment of coronavirus disease 2019. Eur. J. Cancer. 2020;135:62–65. doi: 10.1016/j.ejca.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan R.J., Johnson D.B., Rini B.I., Neilan T.G., Lovly C.M., Moslehi J.J., Reynolds K.L. COVID-19 and immune checkpoint inhibitors: initial considerations. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.National Institute for Health and Care Excellence . COVID-19 Rapid Guideline: Delivery of Systemic Anticancer Treatments. NICE Guideline [NG161]. https://www.nice.org.uk/guidance/ng161 (Accessed 1 November 2020). [PubMed]
- 80.J.R. Patrinely , D.B. Johnson , Pandemic medicine: the management of advanced melanoma during COVID-19, Future Medicine, 2020. [DOI] [PMC free article] [PubMed]
- 81.Elmas Ö.F., Demirbaş A., Düzayak S., Atasoy M., Türsen Ü., Lotti T. Melanoma and COVID‐19: a narrative review focused on treatment. Dermatol. Ther. 2020;33:14101. doi: 10.1111/dth.14101. [DOI] [PubMed] [Google Scholar]
- 82.Melanoma in the COVID-19 era. ESMO. https://www.esmo.org/guidelines/cancer-patientmanagement-during-the-covid-19-pandemic/melanoma-in-the-covid-19-era (Accessed 1 October 2020).
- 83.Short-term Recommendations for Cutaneous Melanoma Management during COVID-19 Pandemic. NCCN. https://www.bsmo.be/wp-content/uploads/guidelines-for-melanoma-treatment-during-COVID19-pandemic.pdf (Accessed 1 November 2020).
- 84.Nahm S., Rembielak A., Peach H., Lorigan P.C. Consensus guidelines for the management of melanoma during the COVID-19 pandemic: surgery, systemic anti-cancer therapy, radiotherapy and follow-up. Clin. Oncol. R. Coll. Radiol. 2021;33:54. doi: 10.1016/j.clon.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baumann B.C., MacArthur K.M., Brewer J.D., Mendenhall W.M., Barker C.A., Etzkorn J.R., Jellinek N.J., Scott J.F., Gay H.A., Baumann J.C. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900–3906. doi: 10.1002/cncr.32969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K., Hodeib S., Korol C. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moujaess E., Kourie H.R., Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit. Rev. Oncol. Hematol. 2020;150 doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vici P., Pizzuti L., Krasniqi E., Botticelli A., Ciliberto G., Barba M. Risk of SARS-CoV-2 infection and disease in metastatic triple-negative breast cancer patients treated with immune checkpoint inhibitors. Future Med. 2020 doi: 10.2217/imt-2020-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.A.P. Davis , M. Boyer , J.H. Lee , S.C. Kao , COVID-19: the use of immunotherapy in metastatic lung cancer, Future Medicine, 2020. [DOI] [PMC free article] [PubMed]
- 90.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abid M.B., Mughal M., Abid M.A. Coronavirus disease 2019 (COVID-19) and immune-engaging cancer treatment. JAMA Oncol. 2020;6:1529–1530. doi: 10.1001/jamaoncol.2020.2367. [DOI] [PubMed] [Google Scholar]