ABSTRACT
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has initiated a global pandemic, and several vaccines have now received emergency use authorization. Using the reference strain SARS-CoV-2 USA-WA1/2020, we evaluated modes of transmission and the ability of prior infection or vaccine-induced immunity to protect against infection in ferrets. Ferrets were semipermissive to infection with the USA-WA1/2020 isolate. When transmission was assessed via the detection of viral RNA (vRNA) at multiple time points, direct contact transmission was efficient to 3/3 and 3/4 contact animals in 2 respective studies, while respiratory droplet transmission was poor to only 1/4 contact animals. To determine if previously infected ferrets were protected against reinfection, ferrets were rechallenged 28 or 56 days postinfection. Following viral challenge, no infectious virus was recovered in nasal wash samples. In addition, levels of vRNA in the nasal wash were several orders of magnitude lower than during primary infection, and vRNA was rapidly cleared. To determine if intramuscular vaccination protected ferrets, ferrets were vaccinated using a prime-boost strategy with the S protein receptor-binding domain formulated with an oil-in-water adjuvant. Upon viral challenge, none of the mock or vaccinated animals were protected against infection, and there were no significant differences in vRNA or infectious virus titers in the nasal wash. Combined, these studies demonstrate direct contact is the predominant mode of transmission of the USA-WA1/2020 isolate in ferrets and that immunity to SARS-CoV-2 is maintained for at least 56 days. Our studies also indicate protection of the upper respiratory tract against SARS-CoV-2 will require vaccine strategies that mimic natural infection or induce site-specific immunity.
IMPORTANCE The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) USA-WA1/2020 strain is a CDC reference strain used by multiple research laboratories. Here, we show that the predominant mode of transmission of this isolate in ferrets is by direct contact. We further demonstrate ferrets are protected against reinfection for at least 56 days even when levels of neutralizing antibodies are low or undetectable. Last, we show that when ferrets were vaccinated by the intramuscular route to induce antibodies against SARS-CoV-2, ferrets remain susceptible to infection of the upper respiratory tract. Collectively, these studies suggest that protection of the upper respiratory tract will require vaccine approaches that mimic natural infection.
KEYWORDS: COVID-19, SARS-CoV-2, ferret, immune protection, transmission
INTRODUCTION
In late 2019, a novel coronavirus, designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), crossed the species barrier and began to cause human infections in Wuhan, China (1). The virus was readily able to transmit from person to person and subsequently spread around the globe, initiating a pandemic (2). SARS-CoV-2 infection of humans causes a range of clinical illness from asymptomatic infection to fever, shortness of breath, and cough, and the infection can progress to severe pneumonia with associated mortality (3, 4). The clinical manifestations of SARS-CoV-2 are referred to as coronavirus disease (COVID-19), and at the time of writing, SARS-CoV-2 has infected over 120 million people worldwide, causing more than a 2.5 million deaths (5). Early during the pandemic, the mode of viral transmission was unclear; however, the growing consensus is that large respiratory droplets are the predominant mode of transmission, with small droplet (sometimes referred to as “aerosol”) transmission also playing a role under certain environmental conditions (i.e., indoors with poor ventilation) (6).
To characterize SARS-CoV-2 and develop countermeasures, numerous virus isolates have been obtained from infected patients. One isolate, SARS-CoV-2 USA-WA1/2020, is widely used by the research community and was recovered from a patient that returned to Washington from China in January 2020 (7). In parallel, several animal models of SARS-CoV-2 have been developed. These models include transgenic mice expressing the human ACE2 receptor (8, 9), hamsters (10, 11), cats (12–14), ferrets (14–16), and nonhuman primates (17–21). The ferret is a well-established model of influenza transmission and immunity, and influenza vaccine efficacy in ferrets correlates with efficacy in humans (22–26). Importantly, several intramuscularly (i.m.) administered SARS-CoV-2 vaccine candidates have been shown to reduce the incidence of severe disease by up to 94% and have received emergency use authorization (27–31).
In the ferret model of respiratory virus transmission, direct contact transmission is assessed by housing an infected donor animal with a naive cage mate, while respiratory transmission involves housing an infected donor adjacent to a naive animal (designated respiratory contact) in cages that permit airflow between the animals but prevent direct contact. Importantly, in this system, large (>5 μm) and small droplet (<5 μm) transmission cannot be distinguished, and the term respiratory transmission encompasses transmission by airborne particles of both sizes. Since the emergence of SARS-CoV-2, several research groups characterized the transmission of different viral isolates in ferrets (14–16, 32, 33). These groups have reported that transmission was efficient (i.e., to 100%) to direct contacts, with variable transmission efficiency to respiratory contacts (i.e., to 33% to 75% of contacts) (15, 16, 32). As transmission efficiency in ferrets can vary with different experimental systems, to accurately assess transmission phenotypes, it is important for different research groups to assess the transmission of closely related viruses (23, 34). Therefore, we sought to evaluate direct contact and respiratory transmission for the reference strain USA-WA1/2020 in ferrets. Moreover, given that ferrets are a highly suitable model of immune-mediated and vaccine-mediated protection against influenza, we evaluated if prior SARS-CoV-2 infection or intramuscular vaccination against the S protein receptor-binding domain (RBD) would protect against infection. Collectively, we demonstrate that the USA-WA1/2020 isolate transmitted efficiently by direct contact in ferrets and that prior infection conferred protection against reinfection, while intramuscular vaccination did not protect against infection.
RESULTS
Evaluation of contact and respiratory transmission of SARS-CoV-2.
To determine the predominant mode of transmission of the SARS-CoV-2 USA-WA1/2020 isolate in ferrets, direct contact and respiratory droplet transmission were evaluated separately. Animals (n = 8) were inoculated with 105 50% tissue culture infective dose (TCID50) of SARS-CoV-2 in a 1-ml volume. This volume permits infection of both the upper and lower respiratory tract (35). Six hours after virus inoculation, one-half of the animals were cohoused with a naive cage mate, while the remaining animals were introduced into respiratory transmission cages and paired with a naive respiratory contact. The respiratory transmission cages are designed such that the ferrets are separated by 2 offset perforated barriers that are 2.5 cm apart. Air flows from the side of the infected animal toward the naive contact. After evaluating direct contact and respiratory transmission, a second confirmatory direct contact transmission experiment (n = 4 donors and n = 4 direct contacts) was performed. For all experiments, nasal wash samples were collected every other day from both the infected donor and contact animals, and viral RNA (vRNA) and infectious titers were determined by reverse transcription-quantitative PCR (qRT-PCR) and TCID50, respectively. All experimental animals were monitored daily for clinical signs, and body weight and temperatures were taken every other day.
For all experimental animals, no overt clinical signs, body weight loss, or elevated temperatures were observed (data not shown). Analyses of levels of vRNA and infectious virus in donor animals are shown in Fig. 1. Figure 1A to C and Fig. 1G to I display the initial direct contact and respiratory transmission experiment, respectively. Results for the confirmatory direct contact study are shown in Fig. 1D to F. In the initial direct contact and the respiratory transmission study, donor ferrets had high levels of vRNA in the nasal wash from days 1 to 7 postinfection, with levels declining at later time points; however, recovery of infectious virus was variable. Infectious virus could not be recovered from all animals between days 1 and 7 postinfection, and viral titers were low, between 101 and 103 TCID50/ml at most time points. When viral shedding in direct contact ferrets was evaluated (Fig. 1B), vRNA was detected in all of the contact ferrets at 2 or more time points. Infectious virus was recovered from 1 animal on day 3 and a second animal on days 7 to 11. One contact ferret was excluded from the study due to fighting with its cage mate. Thus, of the 3 direct contact ferrets, all 3 animals had vRNA in the nasal wash, and infectious virus was recovered from 2 of these animals. Analysis of the vRNA and infectious virus from the respiratory contact ferrets (Fig. 1H) showed that vRNA could be detected at 1 or more time points in 3 of the 4 contacts; however, only 1 animal had vRNA present in the nasal wash at 2 consecutive time points. None of the animals displayed an increase in vRNA levels over time, and infectious virus could not be recovered from any of the respiratory contacts.
FIG 1.
Direct contact and respiratory transmission of the SARS-CoV-2 USA-WA1/2020 isolate in ferrets. (A to C, D to F, and G to I) Display 3 separate transmission studies. (A to C) and (D to F) each represent a contact transmission study. (G to I) Display data from a respiratory transmission study. (A, D, G) Display nasal wash titers determined by qRT-PCR (left y axis) and TCID50 (right y axis) for the SARS-CoV-2-inoculated donor animals in each experiment. Line graphs indicate levels of vRNA, and bar graphs indicate infectious titers. (B, E, and H) Similarly display nasal wash titers for contact animals. In a given panel, each shaded bar or symbol represents the same animal sampled over multiple time points. Paired donor and contact animals have the same shaded bar or symbol between panels. (C, F, and I) Neutralizing antibody titers for each donor and contact animal. For all experiments, 4 pairs of ferrets (2 pairs of males and 2 pairs of females) were used, and nasal wash samples were collected every other day. Blood was collected on day 21 postcontact. In the first direct contact transmission study (A to C), 1 direct contact animal was removed due to fighting with its cage mate. Horizontal dashed lines indicate limit of detection.
To verify our findings on contact transmission, we repeated the contact transmission experiment (Fig. 1D to F). Four donor animals were inoculated with the same dose of virus, and each animal was housed with a naive cage mate. As shown in Fig. 1D, the donor animals had high levels of vRNA in their nasal wash, but again, recovery of infectious virus was variable, and titers were low. When levels of vRNA were evaluated in the contact animals (Fig. 1E), vRNA could be recovered from all 4 contacts at 1 or more time point, and 3 of the contacts had vRNA in the nasal wash at 3 or more time points. Infectious virus was recovered only from a single ferret on day 7 and 9 postcontact.
To determine if ferrets were seroconverting upon virus challenge and if any of the contact ferrets developed SARS-CoV-2 antibodies, microneutralization (MN) assays were performed on serum collected on day 21 postcontact. The levels of neutralizing antibodies are shown in Fig. 1C, F, and I. In all 3 transmission experiments, 2 or 3 of the 4 donor animals and 0 of the direct contact or respiratory contact animals developed neutralizing antibodies. Combined, these results indicate that ferrets are semipermissive to infection with the SARS-CoV-2 USA-WA1/2020 isolate; however, assessment of transmission efficiency depends upon which assay is used to assess viral infection. If the presence of vRNA at any time point is used to assess infection, transmission is highly efficient to 7 of 7 direct contacts and 3 of 4 respiratory contacts. Alternatively, if the isolation of infectious virus or seroconversion are used to assess transmission, direct contact transmission is moderate or poor to 3 of 7 or 0 of 7 direct contacts, respectively. For respiratory transmission, assessment by detection of infectious virus or seroconversion is also poor to 0 of 4 respiratory contacts. Given that neutralizing antibody titers were low or undetectable for donor animals and recovery of infectious virus was highly variable, neutralizing titers and the recovery of infectious virus likely underestimate infection. Thus, to assess transmission efficiency, a suitable approach may be to use the detection of vRNA at 2 or more consecutive time points, as this would indicate the persistence of vRNA in the nasal cavity for at least 3 days. Using these criteria, direct contact transmission is efficient to 6 of 7 contacts, and respiratory droplet transmission is inefficient to 1 of 4 animals.
Protection against reinfection.
To determine if prior infection and/or a neutralizing antibody response was protective against reinfection, donor ferrets from the initial respiratory and direct contact transmission studies were rechallenged at day 28 and 56 postprimary infection, respectively (Table 1). After virus challenge, animals were monitored for clinical illness, and nasal wash samples were collected every other day for 9 days to assay for levels of vRNA and infectious virus.
TABLE 1.
Summary of viral and antibody titers for reinfection experimentsa
| Animal no. by rechallenge time point | Primary infection |
Secondary infection |
||||||
|---|---|---|---|---|---|---|---|---|
| Peak viral titers |
Neutralizing antibody titere |
Peak viral titers |
Neutralizing antibody titer | |||||
| Infectious virus in nasal wash (log10 TCID50/ml [day p.i.]) | vRNA copies/ml in nasal wash (day p.i.) | Day 21 | Day 28 | Day 56 | Infectious virus in nasal wash (log10 TCID50/ml [day postreinfection]) | vRNA copies/ml in nasal wash (day postreinfection) | Day 14 postreinfectionb | |
| Day 28c | ||||||||
| F2846 | 2.95 (7) | 7.26E + 06 (5) | 13 | 14 | n/a | n.d. | 1.49E + 03 (1) | 28 |
| F0584 | 1.0 (3) | 7.99E + 05 (5) | n.d. | n.d. | n/a | n.d. | 5.21E + 04 (1) | 28 |
| F2850 | 1.0 (3) | 9.72E + 04 (5) | n.d. | n.d. | n/a | n.d. | 9.82E + 03 (1) | n.d. |
| F0591 | 1.7 (3) | 3.11E + 06 (1) | 10 | n.d. | n/a | n.d. | 1.60E + 04 (1) | 25 |
| Day 56d | ||||||||
| F2848 | 2.45 (3 and 5) | 2.65E + 08 (5) | n.d. | n.d. | n.d. | n.d. | 8.09E + 03 (1) | 16 |
| F0587 | 2.7 (3) | 3.56E + 08 (5) | 8 | 25 | 14 | n.d. | 4.36E + 03 (1) | 80 |
| F0132 | 3.45 (3) | 4.66E + 08 (3) | 10 | 25 | 25 | n.d. | 2.15E + 03 (1) | 101 |
| F0581 | n.d. | 9.99E + 05 (5) | n.d. | n.d. | n.d. | n.d. | 6.98E + 04 (3) | 20 |
p.i., postinfection; n/a, not applicable; n.d., none detected with the following limits of detection: log10 TCID50 = 0.5, vRNA = 10, and neutralizing antibody titer = 5.
Day 14 postreinfection for animals challenged on day 28 and 56 corresponds to days 42 and 70, postprimary infection, respectively.
Animals rechallenged on day 28 were the donor animals for the respiratory droplet transmission study shown in Fig. 1G.
Animals rechallenged on day 56 were the donor animals for the direct contact transmission study shown in Fig. 1A.
Results are expressed as the reciprocal serum dilution that neutralized 100 TCID50s of SARS-CoV-2.
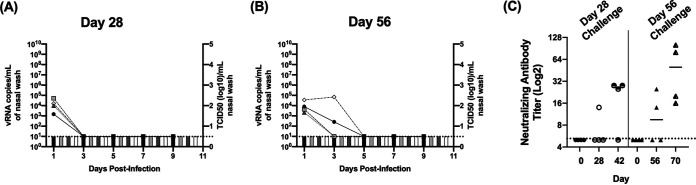
Following secondary virus challenge, none of the animals displayed clinical signs, weight loss, or elevated temperatures (data not shown). As shown in Fig. 2C and Table 1, 1 of 4 animals had neutralizing antibody titers at the time of challenge on day 28, and 2 of 4 animals had titers on day 56. The animals challenged on day 28 were the donors from the respiratory transmission study (Fig. 1G; Table 1), and neutralizing titers declined to below the limit of detection in 1 animal between day 21 and 28. Upon virus challenge at day 28, all 4 animals had moderate levels of vRNA on day 1, and this was cleared by day 3 (Fig. 2A and Table 1). For animals challenged on day 56 (Fig. 2B; Table 1), all 4 animals similarly had vRNA in the nasal wash on day 1, and 2 animals had vRNA in the nasal wash on day 3. No vRNA was detected at time points later than day 3, and for both the day 28 and day 56 challenge, no infectious virus was recovered at any time points postinfection. Importantly, of the animals challenged on day 56, the 2 animals that had vRNA in the nasal wash on day 3 did not have neutralizing antibody titers at the time of challenge. The presence of vRNA at 2 consecutive time points indicates these animals may have been infected and maintained low levels of replicating virus for several days. However, a comparison of the levels of vRNA during primary (Table 1; Fig. 1A and G) and secondary challenge (Table 1; Fig. 2) shows that upon rechallenge, vRNA levels were several logs lower and vRNA was rapidly cleared. Collectively, these results indicate ferrets are protected against rechallenge for at least 56 days postprimary infection.
FIG 2.
Viral and antibody titers in ferrets rechallenged with SARS-CoV-2 on day 28 and 56 postprimary infection. (A and B) Display nasal wash titers in ferrets rechallenged with SARS-CoV-2 on days 28 and 56 postprimary infection, respectively. Line graphs indicate levels of vRNA determined via N2 gene qRT-PCR (left y axis), and bar graphs indicate infectious titers (right y axis) determined via TCID50 on Vero cells. (C) Displays neutralizing antibody titers prior to primary infection (day 0), at the time of rechallenge (day 28 or 56), and 14 days post rechallenge (days 42 and 70). Horizontal dashed lines indicate limit of detection.
Intramuscular vaccination against the SARS-CoV-2 S protein RBD does not protect against infection.
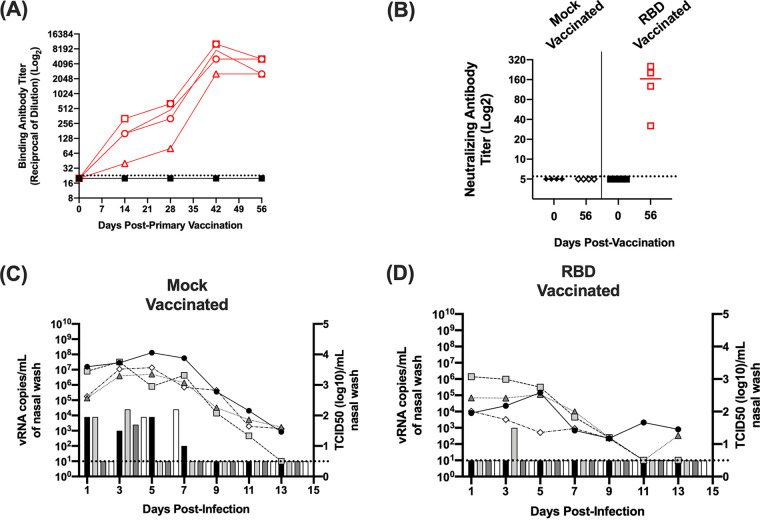
Multiple intramuscular SARS-CoV-2 vaccine candidates are being advanced to clinical trials or have received emergency use authorization (27, 29–31). While the authorized vaccines have been shown to induce neutralizing antibodies, it remains unclear if the induction of serum antibodies will reduce viral replication and/or prevent infection of the upper respiratory tract. Therefore, to determine if vaccine induced antibodies protect ferrets from SARS-CoV-2 infection, ferrets (n = 4) received an intramuscular vaccine consisting of a potent oil-in-water adjuvant (Sigma adjuvant system adjuvant [SAS]) alone (designated mock group) or the RBD of the SARS-CoV-2 S protein mixed with adjuvant (designated RBD vaccine group) (36–38). Animals were given a primary vaccination and boosted on day 28. Every 2 weeks during the course of vaccination, blood samples were collected to assay for the presence of antibodies. On day 56 (i.e., 28 days after boost vaccination), animals were challenged with SARS-CoV-2 and nasal wash samples were collected every other day for 13 days. As shown in Fig. 3A and B, prior to virus challenge, none of the mock-vaccinated animals developed binding or neutralizing antibodies against the RBD protein. In contrast, the vaccinated animals displayed increasing RBD binding antibodies over time with a boost in antibody titers following the secondary vaccination. At the time of virus challenge on day 56, the neutralizing activity of antibodies induced by vaccination was verified by microneutralization assay (Fig. 3B), and titers ranged from 1:32 to 1:320.
FIG 3.
Antibody and viral titers in SARS-CoV-2-infected mock- and RBD-vaccinated ferrets. (A) Displays binding antibody titers against the S protein RBD determined by ELISA on days 0, 14, 28, 42, and 56 postprimary vaccination. Red open symbols represent RBD-vaccinated ferrets. Closed black symbols represent mock-vaccinated animals. Animals were given a secondary vaccination on day 28. (B) Displays neutralizing antibody titers on day 56. (C and D) Display nasal wash titers in mock- and RBD-vaccinated animals challenged with SARS-CoV-2, respectively. Line graphs indicate levels of vRNA determined via N2 gene qRT-PCR (left y axis), and bar graphs indicate infectious titers (right y axis) determined via TCID50 on Vero cells. Horizontal dashed lines indicate limit of detection.
On day 56, 28 days postsecondary vaccination, ferrets were challenged with 106 TCID50 of SARS-CoV-2. For these studies, a 10-fold higher challenge dose was utilized to enhance the recovery of infectious virus from the mock-vaccinated animals. As shown in Fig. 3C, following virus challenge, vRNA was detected in all of the mock-vaccinated animals for at least 9 days. Levels of vRNA ranged from 103 to 108 copies per ml of nasal wash. As observed for the transmission studies, recovery of infectious virus was variable; however, all of the mock-vaccinated animals shed infectious virus at 1 or more time points with titers between 102 and 104.5 TCID50/ml. In the RBD-vaccinated animals, vRNA was similarly detected in the nasal wash samples for several days postinfection. Compared with the mock-vaccinated animals, RBD-vaccinated animals had ∼10- to 100-fold lower titers of vRNA in the nasal wash between 102 and 106 copies per ml, and infectious virus was only recovered from a single animal on day 3. While both measures indicated a trend toward reduced viral load, there were no significant differences between the levels of vRNA or infectious virus between the mock- and RBD-vaccinated animals (P ≥ 0.18). Combined, these findings indicate that vaccination against the RBD did not significantly reduce viral load in the nasal cavity or protect ferrets from infection with SARS-CoV-2.
DISCUSSION
Given the wide use of the SARS-CoV-2 USA-WA1/2020 strain in the research community, we evaluated transmission of this isolate in the ferret model and the role of pre-existing immunity induced via infection or vaccination on SARS-CoV-2 infection. We chose to use the ferret model because ferrets are recognized as the most suitable animal model with which to assess influenza transmission phenotypes and vaccine efficacy (22–25, 34). When we evaluated transmission by direct and respiratory contact in separate studies, the assessment of transmission efficiency depended on the stringency of the criteria used to evaluate infection in the contact ferrets. If we applied the human diagnostic standard of the presence of vRNA at any time point as evidence of infection, transmission was efficient to direct contact animals, with 3 of 3 and 4 of 4 contacts having vRNA in the nasal wash in separate studies, or a combined total of 7 of 7 contact animals being infected. If the recovery of infectious virus was used to assess transmission, transmission efficiency was reduced to 2 of 3 or 1 of 4 direct contacts in separate studies, or a total of 3 of 7 contacts. When seroconversion was evaluated, only a proportion of donor animals developed neutralizing antibodies, and no contact animals seroconverted. In our respiratory transmission studies, the presence of vRNA was detected in 3 of 4 contacts, but infectious virus could not be recovered from any of the animals. When evaluated for seroconversion, 2 of 4 donor animals seroconverted, while none of the respiratory contacts developed SARS-CoV-2 antibodies.
Evaluation of the vRNA kinetics and recovery of infectious virus in the donor ferrets (Fig. 1A, D, and G) demonstrates that despite high levels of vRNA, only low levels of infectious virus were recovered. Moreover, only a proportion of donor animals developed low titers of neutralizing antibodies, and none of the infected animals developed clinical signs or lost weight. Thus, the combination of poor virus recovery, limited seroconversion, and the absence of clinical signs indicate that ferrets are semipermissive to the SARS-CoV-2 USA-WA1/2020 isolate. Our findings are consistent with those of several research groups that similarly show ferrets are semipermissive to multiple SARS-CoV-2 isolates (Table 2). This contrasts with studies on the susceptibility of pandemic influenza viruses in ferrets (34, 39, 40). For example, in ferrets, pandemic H1N1 influenza readily transmits to 100% of direct and respiratory droplet contacts, with all animals shedding high titers of infectious virus and showing evidence of seroconversion (39). The differences in the susceptibility of ferrets to these viruses most likely reflects greater species restriction of the ACE2 receptor for SARS-CoV-2 compared with sialic acids, which are the receptor for influenza viruses (41–43).
TABLE 2.
Summary of SARS-CoV-2 transmission experiments in ferretsa
| SARS-CoV-2 strain | Ferret transmission model: ratio of donors to contacts | Direct contact transmission % efficiency (proportion positive contacts) according to: |
Respiratory droplet transmission % efficiency (proportion positive contacts) according to: |
Reference | ||||
|---|---|---|---|---|---|---|---|---|
| vRNA | Infectious virus | Seroconversion [assay] | vRNA | Infectious virus | Seroconversion [assay] | |||
| NMC-nCoV02 | 1:2 (1 donor paired with 2 contacts, both DC and RC transmission evaluated, 3 replicates per mode of transmission) | 100% (6/6) | 100% (6/6) | 100% (6/6) [VNT] | 33% (2/6) | 0% (0/6) | 17% (1/6) [MN] | 15 |
| Muc-IMB-1 | 9:3 (1 large study, DC transmission only) | 100% (3/3) | n.p. | 100% (1/3) [IIFA] | n.p. | n.p. | n.p. | 16 |
| BetaCov/Munich/BavPat1/2020 | 1:1:1 (1 donor paired with 1 DC and 1 RC, RC was housed 10 cm from other animals, 4 replicates) | 100% (4/4) | 100% (4/4) | 100% (4/4) [ELISA and VNT] | 75% (3/4) | 75% (3/4) | 75% (3/4) [ELISA and VNT] | 32 |
| BetaCov/Munich/BavPat1/2020 | 1:1 (1 donor paired with 1 RC, animals separated by 1 m, 4 replicates) | n.p. | n.p. | n.p. | 50% (2/4) | 50% (2/4) | 50% (2/4) [ELISA] | 45 |
| USA-WA1/2020 | 1:2 (1 donor paired with 2 DC, 3 replicates) | 100% (6/6) | 100% (6/6) | n.p. | n.p. | n.p. | n.p. | 44 |
n.p., not performed; VNT, virus neutralization assay; ELISA, enzyme-linked immunosorbent assay; IIFA, indirect immunofluorescence assay; DC, direct contact; RC, respiratory contact.
Several other groups have reported on the transmission of different SARS-CoV-2 isolates in ferrets using different experimental systems (15, 16, 32, 44, 45) (Table 2). In our studies, upon evaluation of the vRNA levels in direct contact animals (Fig. 1B, E, and H), we noted that some animals had SARS-CoV-2 vRNA present at a single time point, while others had vRNA detected over multiple consecutive time points. Using the criteria of detection of vRNA at multiple consecutive time points as evidence of infection, 6 of 7 direct contacts and 1 of 4 respiratory contacts became infected. Our findings for the SARS-CoV-2 USA-WA1/2020 strain are most consistent with studies on the NMC-nCoV02 and Muc-IMB-1 strains (15, 16). When these isolates were evaluated for direct contact transmission in ferrets, the recovery of infectious virus was variable from both donors and contact animals; however, when the detection of vRNA alone was used to assay for transmission to direct contacts, both viruses were detected in 100% of contact animals (15, 16). Similar to our findings, when the NMC-nCoV02 strain was evaluated for airborne transmission, only viral RNA was detected in contact animals, and transmission occurred to 2/6 animals (15). Evaluation of neutralizing antibody titers in donor and direct contact animals for the Muc-IMB-1 isolate indicated that all donors and 1 of 3 infected contacts seroconverted, while for the NMC-nCoV02 isolate, all donors and direct contacts seroconverted, but only 1 of 2 positive respiratory contacts developed antibodies (16) (Table 2).
Both direct contact and respiratory transmission have also been evaluated for the BetaCov/Munich/BavPat1/2020 isolate (32, 45). For this virus, transmission was observed to 100% of direct contact animals, which was determined by the presence of vRNA, recovery of infectious virus, and seroconversion. When respiratory transmission was evaluated, depending on the study design, transmission occurred to 2 of 4 or 3 of 4 contacts, and this was confirmed by all 3 measures of transmission, namely, presence of vRNA, recovery of infectious virus, and seroconversion. Importantly, for all infected donor animals, high levels of vRNA combined with low infectious titers were reported, while infected direct or respiratory contacts had further reduced levels of vRNA and infectious titers (32, 45) (Table 2). A recent report has also evaluated direct contact transmission of the SARS-CoV-2 USA-WA1/2020 isolate (44). This study evaluated the efficacy of a nucleoside analog inhibitor (MK-4482) to inhibit SARS-CoV-2 transmission. In untreated animals, the SARS-CoV-2 USA-WA1/2020 isolate transmitted to all cohoused direct contacts, and both vRNA and infectious virus were recovered from the direct contacts. When donor ferrets were treated with the MK-4482, vRNA but no infectious virus was detected in the nasal wash, and none of the direct contact animals became infected (44). In these studies, all animals were euthanized 72 h postcontact; therefore, seroconversion could not be evaluated. In contrast to our findings, it is unclear why infectious virus could be consistently recovered in this study, while recovery of infectious virus was variable in both our inoculated donors and direct contacts. Interestingly, limited recovery of infectious virus does not appear to be a feature of only our studies. Several other groups have reported recovery of low and variable virus titers from ferrets (14–16). One possible explanation for these differences is passage history of the viruses used in multiple studies. Importantly, next-generation sequence analysis of the viruses used in our studies confirmed that the virus matched the published consensus sequence and had a limited number of low-frequency variants. In addition, the virus maintained the RBD and furin cleavage site (Table 3 and Materials and Methods). However, our SARS-CoV-2 USA-WA1/2020 isolate was obtained at passage 4 and passaged once in Vero E6 cells. As repetitive passaging of SARS-CoV-2 has been shown to alter the quasispecies (46, 47), it is possible that our passage 5 virus had limited quasispecies diversity, while lower passage isolates used by other groups may have maintained more diversity, thus facilitating infection and/or recovery from ferrets.
TABLE 3.
Viral variants and frequency in SARS-CoV-2 USA-WA1/2020 virus stock
| Genomic position | Original nucleotide | Polymorphic nucleotide | Polymorphism frequency (%) | Gene | Amino acid polymorphism |
|---|---|---|---|---|---|
| 26538 | C | T | 8.95 | M | T7I |
| 28849 | T | A | 8.16 | N | S194T |
| 22339 | G | C | 6.60 | S | G261R |
| 22113 | T | G | 3.80 | S | N185K |
After completing the transmission studies, we rechallenged subsets of the donor animals on day 28 and 56 and demonstrated protection against reinfection. Following virus challenge on days 28 and 56, infectious virus was not recovered from any animals. vRNA was present in the nasal wash of all animals on day 1 in both groups. In the animals challenged on day 28, vRNA was cleared by day 3, while in 2 of 4 animals challenged on day 56, vRNA was also present on day 3 and was cleared by day 5. While vRNA was detected at 2 time points in the animals challenged on day 56, the levels of vRNA in the nasal washes were several orders of magnitude lower than during the primary infection, indicating immune-mediated control of the virus. Our findings are consistent with other groups that show protection against reinfection 28 days postinfection in ferrets, hamsters, and nonhuman primates (11, 17, 33); however, we extend the window of protection to at least 56 days. Moreover, the observation that most animals had low or undetectable neutralizing antibodies titers prior to challenge indicates that cellular immunity plays a significant role in protection against reinfection.
Last, we evaluated if the induction of high levels of serum antibodies via intramuscular vaccination would protect ferrets from infection. Ferrets were given a prime and boost vaccination 28 days apart consisting of the RBD protein formulated with an oil-in-water adjuvant. We specifically chose to vaccinate against the RBD because some vaccine approaches using the full-length SARS-CoV-1 S protein potentiated vaccine-enhanced disease (48–51), while this did not occur when the RBD alone was used as a vaccine (52–57). Using this vaccine strategy, we induced high levels of antibodies (Fig. 3C) measured by enzyme-linked immunosorbent assay (ELISA) and confirmed by neutralization assay. Upon viral challenge, vRNA was detected in nasal wash samples from both the mock- and RBD-vaccinated animals for at least 9 days postinfection. Infectious virus was recovered from all of the mock-vaccinated animals at 1 or more time points, while only a single RBD-vaccinated animal shed virus; however, these differences were not significant, indicating that high levels of serum antibodies did not prevent infection of the upper respiratory tract in ferrets. Our findings are consistent with SARS-CoV-2 vaccine studies in nonhuman primates (58–60) and a more recent study using an adenovirus-vectored vaccine in ferrets (61). In ferrets, intramuscular vaccination with an adenovirus-vectored vaccine did not confer protection of the upper respiratory tract, while in nonhuman primates, regardless of the vaccine formulation, the induction of high levels of neutralizing antibodies by intramuscular vaccination conferred protection of the lower respiratory tract but did not protect against infection of the upper airways. It is important to note that ferrets did not display clinical disease, and replication in the lower respiratory tract is not a feature of the ferret model of SARS-CoV-2 (14, 15). Therefore, our results do not suggest that current vaccines would not be effective at reducing severe disease. Indeed, licensed influenza vaccines which induce neutralizing antibodies in the blood often reduce disease severity but do not necessarily block infection (62, 63). Importantly, this observation is also consistent with greater than 90% reduction in the incidence of severe disease for the emergency use authorized intramuscular mRNA vaccines (64, 65).
Collectively, we demonstrate ferrets are semipermissive to infection with the SARS-CoV-2 USA-WA1/2020 isolate and that transmission of this isolate is efficient via direct contact and poor by respiratory droplet. We further demonstrate that ferrets are protected against reinfection for at least 56 days. Using a vaccination strategy to induce serum neutralizing antibodies, ferrets were not protected against SARS-CoV-2 challenge. Thus, while initial human vaccines will likely reduce disease burden, to completely prevent SARS-CoV-2 infections, vaccine strategies that induce immunity in the upper respiratory tract comparable to natural infection will need to be developed.
MATERIALS AND METHODS
Viruses and cells.
The CDC reference strain SARS-CoV-2 USA-WA1/2020 was used for all studies. The strain was deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH, SARS-related coronavirus 2, isolate USA-WA1/2020, NR-52281. The virus was obtained from BEI at passage 4 and cultured once in Vero E6 cells (ATCC) to generate a virus stock. Vero E6 cells were cultured in 10% fetal bovine serum-Dulbecco’s modified Eagle’s medium (FBS-DMEM; HyClone) supplemented with nonessential amino acids (Corning), 2 mM l-glutamine, 1 mM sodium pyruvate, 1,500 mg/liter sodium bicarbonate, and antibiotics and antimycotics (Life Technologies) at 37°C in 5% CO2. SARS-CoV-2 was cultured in the equivalent media with the serum component reduced to 2% FBS. The virus stock was aliquoted and stored at −80°C until use. The titer of propagated virus stock was determined on Vero E6 cells in 24-well plates. The titers of nasal wash samples were determined using both 24- and 96-well plates. To enhance the detection of infectious virus, 100 μl of a nasal wash sample was incubated on 2 wells of a 24-well plate for 1 hour. The nasal wash sample was removed and replaced with virus culture media. To assess peak titers in the nasal wash samples, titers of 10-fold serial dilutions of virus were determined in 96-well plates of Vero E6 cells. After inoculation onto Vero E6 cells, plates were incubated and scored 96 hours later for the presence of cytopathic effect (CPE). The 50% tissue culture infective dose was calculated using the method of Reed and Muench (66).
To confirm the virus maintained the furin cleavage site and receptor-binding domain, viral RNA was extracted using a QIAmp vRNA mini kit (Qiagen) and reverse transcribed using Superscript III reverse transcriptase with random hexamers (Life Technologies). Second-strand synthesis was performed with the Ultra II nondirectional RNA second strand synthesis module (New England BioLabs [NEB]). Sequencing libraries were made using the Nextera XT DNA library prep kit. Libraries were sequenced on the Illumina MiSeq platform in single-end 100-base pair mode. For sequence analysis, reads were trimmed and sieved for quality using Trimmomatic version 0.36 and then aligned to the SARS-CoV-2 USA-WA1/2020 (GenBank accession no. MT246667.1) sequence using blat. Polymorphisms, insertions, and deletions were quantified using a custom Python script. We report polymorphisms occurring at a frequency greater than 3 standard deviations from the mean mutation frequency (2.89%) of all reads from this sequencing run.
The sequence of the virus stock closely matches the published consensus sequence (GenBank accession no. MT246667.1). Four variants were identified at frequencies significantly higher than the deep-sequencing error rates for these runs (Table 3). This analysis confirms that the virus inoculum maintained both the receptor-binding domain (aa 319 to 541) and the furin cleavage site (amino acids [aa] 682 to 685). Of note, a small fraction of reads (<0.05%) exhibited a 15-nucleotide deletion (SΔ675 to 680) just upstream of the furin cleavage site.
Biocontainment and animal care and use.
All experiments with SARS-CoV-2 were conducted in the Eva Pell Laboratory for Advanced Biological Research, which is The Pennsylvania State University (PSU) biosafety level 3 enhanced laboratory. This facility is approved and inspected by the US Department of Agriculture (USDA) and Centers for Disease Control (CDC). All animal studies and procedures were conducted in compliance with relevant regulations and guidelines. All animal studies were approved by the PSU Animal Care and Use Committee under protocol no. 202001440. An equal number of male and female ferrets, 24 to 30 weeks old (Triple F Farms, Sayre, PA), were used for all studies. All animals were screened by hemagglutination inhibition assay and determined to be seronegative for currently circulating human influenza A viruses.
Transmission experiments.
Ferret transmission experiments were performed using large stainless steel ventilated ferret cages (Allentown, LLC, Allentown, NJ) modified to permit 2 ferrets to be separated by an offset perforated divider as described previously (67). For direct contact transmission experiments, ferrets were housed in the same caging system, except the perforated divider was removed to allow the animals to share the same cage. To evaluate direct contact or respiratory transmission, donor ferrets (n = 4) were sedated by intramuscular injection with a mixture of ketamine (30 mg/kg of body weight), xylazine (2 mg/kg), and atropine (0.05 mg/kg). Animals were then intranasally inoculated with 1 ml of virus culture media containing 105 TCID50 of SARS-CoV-2. After virus inoculation, animals were given the reversal agent atipamezole (0.5 mg/kg) via intramuscular injection and were housed separately in individual biocontainment ferret cages (Allentown, NJ). Six hours after virus inoculation, depending on the mode of transmission, each ferret was paired with either a naive direct contact or respiratory contact. The direct contact experiment was repeated for a total of 8 direct contact transmission pairs, while respiratory transmission was evaluated in 4 pairs of animals.
Starting 1-day postviral inoculation and every other day for 11 to 13 days, animals were sedated by i.m. injection with a mixture of ketamine (20 mg/kg), xylazine (2 mg/kg), and atropine (0.05 mg/kg), and nasal wash samples were collected by instilling a 1-ml volume of phosphate-buffered saline (PBS) into the nostrils and inducing sneezing on a petri dish. An additional 1-ml volume of PBS was used to rinse the dish, and the nasal wash samples were aliquoted and frozen at −80°C. All animals were monitored daily for clinical signs, and body weight and temperatures were collected at the time of nasal wash sample collection.
On day 21 postviral inoculation and pair housing, the animals were deeply sedated, and blood was collected via cardiac puncture prior to euthanasia with an overdose of sodium pentobarbital. Eight of the donor animals were maintained for rechallenge studies. Blood was collected via the anterior vena cava from these animals, and the animals were given atipamezole and monitored until recovery. Blood samples were processed to recover serum, and serum was stored at −80°C for evaluation of antibody titers.
Rechallenge experiments.
To evaluate protection against reinfection, 8 donor ferrets from the transmission studies were maintained, and blood samples were collected every 2 weeks. Blood samples were collected from the anterior vena cava under sedation with ketamine, xylazine, and atropine. On day 28 and day 56, 4 ferrets were rechallenged with 105 TCID50 as described above. Following viral challenge, nasal wash samples were collected every other day for 9 days. On day 14 postchallenge, animals were sedated, blood was collected, and the animals were humanely euthanized.
Vaccination and challenge studies.
Ferrets were mock vaccinated (n = 4) with Sigma adjuvant system adjuvant (SAS) alone or receptor-binding domain vaccinated (n = 4) with 50 μg of recombinant SARS-CoV-2 S-protein RBD (SinoBiological) mixed with SAS. RBD was purchased as a lyophilized powder and resuspended in sterile PBS. Adjuvant was mixed 1:1 with the PBS for mock-vaccinated animals or RBD protein solution, and at the time of vaccination, each animal received 500 μl of the vaccine given as 2 250-μl injections, 1 per hind leg. Animals were given a primary and secondary vaccination 28 days apart. On day 56 (i.e., 28 days postboost), animals were sedated and intranasally inoculated with 106 TCID50 of SARS-CoV-2 in a 1-ml volume. After viral challenge, nasal wash samples were collected every other day for 9 days, and animals were monitored for clinical signs and weight loss for 14 days.
Neutralization assay.
The titers of neutralizing antibodies in ferret sera were evaluated by a microneutralization (MN) assay as previously described (68). Briefly, serial 2-fold dilutions of heat-inactivated sera were prepared and mixed with 100 TCID50 of SARS-CoV-2 in an equal volume. The virus-serum mixture was incubated for 1 hour at room temperature and then overlaid in quadruplicate on Vero E6 cell monolayers in 96-well plates. Plates were incubated at 37°C for 4 days and scored for cytopathic effect. The neutralization titer was determined as the reciprocal of the serum dilution that neutralized virus, as evidenced by the absence of CPE.
S protein RBD ELISA.
To quantify anti-S protein RBD antibodies from ferret serum, 96-well ELISA plates (Nunc) were coated overnight with 2.0 μg/ml (100 ng/well) of SARS-CoV-2 RBD S protein (Sino Biological) in sodium bicarbonate buffer. Subsequently, plates were blocked for 2 hours at room temperature with PBS with Tween 20 (PBST; 0.05% Tween) with 3% goat serum and 5% skim milk powder. Plates were washed with wash buffer (i.e., PBST) and incubated for 2 hours at room temperature with serial 2-fold dilutions of heat-inactivated serum diluted in PBST with 1% milk powder. Plates were washed again and incubated for 2 hours at room temperature with horseradish peroxidase (HRP)-conjugated anti-ferret goat antibody (Rockland) diluted 1:10,000 in PBST and 1% skim milk powder. Following washes, OPD substrate solution (Sigma) was added to all wells and 3 M HCl was added after 10 minutes to stop the reaction. The optical density was measured at 490 nm on SpectraMax iD3 plate reader (Molecular Devices), and an absorbance reading greater than 3 standard deviations above the mean day 0 value was considered positive.
SARS-CoV-2 qRT-PCR.
To assess levels of vRNA in nasal wash samples, RNA was extracted from 140 μl of nasal wash using the QIAmp viral RNA mini kit (Qiagen) according to the manufacture’s recommendations. RNA was eluted in 40 μl of buffer AVE. Quantification of levels of vRNA was performed according to the CDC protocol approved for emergency use authorization (69). Purified RNA (5 μl) was analyzed with the 2019-nCoV CDC quantitative PCR (qPCR) N2 assay (IDT) using qScript one-step qRT-PCR, low ROX (QuantaBio) master mix on an ABI 7500 Fast real-time PCR system (ABI).
Statistical analyses.
For statistical analysis, nasal wash titers and levels of vRNA were compared between the mock- and RBD-vaccinated animals using a Mann-Whitney U test. Statistical analysis was performed with Prism GraphPad (version 8.4.3), and a P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
D.R.P., C.J.F., K.M.S., and T.C.S. planned and performed all experiments with assistance from T.A.H. and D.G.S. M.J.J. performed the qRT-PCR analysis and T.H.V. performed next-generation sequencing of viruses. D.R.P., C.J.F., K.M.S., and T.C.S. drafted the manuscript. T.C.S., T.H.V., and E.A.M. revised and edited the manuscript.
We declare that we have no competing interests.
We acknowledge the Animal Resource Program and the Eva Pell Laboratory for Advanced Biological Research facility managers for assistance and support with animal studies.
This research was supported by a seed grant from The Huck Institutes of Life Sciences at The Pennsylvania State University, by NIAID contract HHSN272201400004C (NIAID Centers of Excellence for Influenza Research and Surveillance [CEIRS]), by USDA National Institute of Food and Agriculture Hatch project 4605, and by Yerkes National Primate Research Center base grant P51 OD011132.
This article is dedicated to the memory of Talia A. Heinly. Talia’s spirit, positive attitude, and passion for research will never be forgotten.
Contributor Information
Troy C. Sutton, Email: tcs38@psu.edu.
Tom Gallagher, Loyola University Chicago.
REFERENCES
- 1.Hu B, Guo H, Zhou P, Shi ZL. 2021. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19:141–154. 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. 2020. WHO director-general’s opening remarks at the media briefing on COVID-19—11 March 2020. WHO, Geneva, Switzerland. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 . 2020. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. 2020. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323:1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Johns Hopkins Coronavirus Resource Center. 2020. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Johns Hopkins University, Baltimore, MD. https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 6.Zhang XS, Duchaine C. 2020. SARS-CoV-2 and health care worker protection in low-risk settings: a review of modes of transmission and a novel airborne model involving inhalable particles. Clin Microbiol Rev 34:e00184-20. 10.1128/CMR.00184-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harcourt J, Tamin A, Lu X, Kamili S, Sakthivel SK, Murray J, Queen K, Tao Y, Paden CR, Zhang J, Li Y, Uehara A, Wang H, Goldsmith C, Bullock HA, Wang L, Whitaker B, Lynch B, Gautam R, Schindewolf C, Lokugamage KG, Scharton D, Plante JA, Mirchandani D, Widen SG, Narayanan K, Makino S, Ksiazek TG, Plante KS, Weaver SC, Lindstrom S, Tong S, Menachery VD, Thornburg NJ. 2020. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg Infect Dis 26:1266–1273. 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, Qu Y, Li F, Lv Q, Wang W, Xue J, Gong S, Liu M, Wang G, Wang S, Song Z, Zhao L, Liu P, Zhao L, Ye F, Wang H, Zhou W, Zhu N, Zhen W, Yu H, Zhang X, Guo L, Chen L, Wang C, Wang Y, Wang X, Xiao Y, Sun Q, Liu H, Zhu F, Ma C, Yan L, Yang M, Han J, Xu W, Tan W, Peng X, Jin Q, Wu G, Qin C. 2020. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583:830–833. 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 9.Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, Li Q, Zhang L, Zhu Y, Si HR, Wang Q, Min J, Wang X, Zhang W, Li B, Zhang HJ, Baric RS, Zhou P, Yang XL, Shi ZL. 2020. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182:50–58.e8. 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai JP, Kok KH, Chu H, Chan KH, Sridhar S, Chen Z, Chen H, To KK, Yuen KY. 2020. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis 71, 2428–2446. 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, Watanabe T, Ujie M, Takahashi K, Ito M, Yamada S, Fan S, Chiba S, Kuroda M, Guan L, Takada K, Armbrust T, Balogh A, Furusawa Y, Okuda M, Ueki H, Yasuhara A, Sakai-Tagawa Y, Lopes TJS, Kiso M, Yamayoshi S, Kinoshita N, Ohmagari N, Hattori SI, Takeda M, Mitsuya H, Krammer F, Suzuki T, Kawaoka Y. 2020. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A 117:16587–16595. 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudreault NN, Trujillo JD, Carossino M, Meekins DA, Morozov I, Madden DW, Indran SV, Bold D, Balaraman V, Kwon T, Artiaga BL, Cool K, Garcia-Sastre A, Ma W, Wilson WC, Henningson J, Balasuriya UBR, Richt JA. 2020. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect 9:2322–2332. 10.1080/22221751.2020.1833687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halfmann PJ, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, Kinoshita N, Hattori SI, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Kawaoka Y. 2020. Transmission of SARS-CoV-2 in domestic cats. N Engl J Med 383:592–594. 10.1056/NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368:1016–1020. 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, Um J, Song MS, Jeong HW, Lai VD, Kim Y, Chin BS, Park JS, Chung KH, Foo SS, Poo H, Mo IP, Lee OJ, Webby RJ, Jung JU, Choi YK. 2020. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27:704–709.e2. 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlottau K, Rissmann M, Graaf A, Schon J, Sehl J, Wylezich C, Hoper D, Mettenleiter TC, Balkema-Buschmann A, Harder T, Grund C, Hoffmann D, Breithaupt A, Beer M. 2020. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe 1:e218–e225. 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, Tostanoski LH, Yu J, Maliga Z, Nekorchuk M, Busman-Sahay K, Terry M, Wrijil LM, Ducat S, Martinez DR, Atyeo C, Fischinger S, Burke JS, Slein MD, Pessaint L, Van Ry A, Greenhouse J, Taylor T, Blade K, Cook A, Finneyfrock B, Brown R, Teow E, Velasco J, Zahn R, Wegmann F, Abbink P, Bondzie EA, Dagotto G, Gebre MS, He X, Jacob-Dolan C, Kordana N, Li Z, Lifton MA, Mahrokhian SH, Maxfield LF, Nityanandam R, Nkolola JP, Schmidt AG, Miller AD, Baric RS, Alter G, Sorger PK, Estes JD, Andersen H, Lewis MG, Barouch DH. 2020. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369:812–817. 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Perez-Perez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, Avanzato VA, Rosenke R, Hanley PW, Saturday G, Scott D, Fischer ER, de Wit E. 2020. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585:268–272. 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NMA, Schipper D, van Run P, Leijten L, Sikkema R, Verschoor E, Verstrepen B, Bogers W, Langermans J, Drosten C, Fentener van Vlissingen M, Fouchier R, de Swart R, Koopmans M, Haagmans BL. 2020. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368:1012–1015. 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, Gumber S, Nekorchuk M, Busman-Sahay K, Strongin Z, Harper JL, Tharp GK, Pellegrini KL, Kirejczyk S, Zandi K, Tao S, Horton TR, Beagle EN, Mahar EA, Lee MYH, Cohen J, Jean SM, Wood JS, Connor-Stroud F, Stammen RL, Delmas OM, Wang S, Cooney KA, Sayegh MN, Wang L, Filev PD, Weiskopf D, Silvestri G, Waggoner J, Piantadosi A, Kasturi SP, Al-Shakhshir H, Ribeiro SP, Sekaly RP, Levit RD, Estes JD, Vanderford TH, Schinazi RF, Bosinger SE, Paiardini M. 2021. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 184:460–475.e21. 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Routhu NK, Cheedarla N, Gangadhara S, Bollimpelli VS, Boddapati AK, Shiferaw A, Rahman SA, Sahoo A, Edara VV, Lai L, Floyd K, Wang S, Fischinger S, Atyeo C, Shin SA, Gumber S, Kirejczyk S, Cohen J, Jean SM, Wood JS, Connor-Stroud F, Stammen RL, Upadhyay AA, Pellegrini K, Montefiori D, Shi PY, Menachery VD, Alter G, Vanderford TH, Bosinger SE, Suthar MS, Amara RR. 2021. A modified vaccinia Ankara vector-based vaccine protects macaques from SARS-CoV-2 infection, immune pathology, and dysfunction in the lungs. Immunity 54:542–556.e9. 10.1016/j.immuni.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belser JA, Barclay W, Barr I, Fouchier RAM, Matsuyama R, Nishiura H, Peiris M, Russell CJ, Subbarao K, Zhu H, Yen HL. 2018. Ferrets as models for influenza virus transmission studies and pandemic risk assessments. Emerg Infect Dis 24:965–971. 10.3201/eid2406.172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belser JA, Eckert AM, Tumpey TM, Maines TR. 2016. Complexities in ferret influenza virus pathogenesis and transmission models. Microbiol Mol Biol Rev 80:733–744. 10.1128/MMBR.00022-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belser JA, Katz JM, Tumpey TM. 2011. The ferret as a model organism to study influenza A virus infection. Dis Model Mech 4:575–579. 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodewes R, Rimmelzwaan GF, Osterhaus AD. 2010. Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev Vaccines 9:59–72. 10.1586/erv.09.148. [DOI] [PubMed] [Google Scholar]
- 26.Wong J, Layton D, Wheatley AK, Kent SJ. 2019. Improving immunological insights into the ferret model of human viral infectious disease. Influenza Other Respir Viruses 13:535–546. 10.1111/irv.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Aboagye J, Adams K, Ali A, Allen E, Allison JL, Anslow R, Arbe-Barnes EH, Babbage G, Baillie K, Baker M, Baker N, Baker P, Baleanu I, Ballaminut J, Barnes E, Barrett J, Bates L, Batten A, Oxford COVID vaccine trial group . 2020. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396:467–478. 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH, mRNA-1273 study group . 2020. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med 383:1920–1931. 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amanat F, Krammer F. 2020. SARS-CoV-2 vaccines: status report. Immunity 52:583–589. 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP, Dooling K. 2020. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 69:1922–1924. 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP, Dooling K. 2021. The advisory committee on immunization practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 69:1653–1656. 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard M, Kok A, de Meulder D, Bestebroer TM, Lamers MM, Okba NMA, Fentener van Vlissingen M, Rockx B, Haagmans BL, Koopmans MPG, Fouchier RAM, Herfst S. 2020. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun 11:3496. 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan KAea. 2020. Dose-dependent response to infection with SARS-CoV-2 in the ferret model: evidence of protection to re-challenge. bioRxiv, 10.1101/2020.05.29.123810. [DOI] [PMC free article] [PubMed]
- 34.Buhnerkempe MG, Gostic K, Park M, Ahsan P, Belser JA, Lloyd-Smith JO. 2015. Mapping influenza transmission in the ferret model to transmission in humans. eLife 4:e07969. 10.7554/eLife.07969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore IN, Lamirande EW, Paskel M, Donahue D, Kenney H, Qin J, Subbarao K. 2014. Severity of clinical disease and pathology in ferrets experimentally infected with influenza viruses is influenced by inoculum volume. J Virol 88:13879–13891. 10.1128/JVI.02341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Shi W, Joyce MG, Modjarrad K, Zhang Y, Leung K, Lees CR, Zhou T, Yassine HM, Kanekiyo M, Yang ZY, Chen X, Becker MM, Freeman M, Vogel L, Johnson JC, Olinger G, Todd JP, Bagci U, Solomon J, Mollura DJ, Hensley L, Jahrling P, Denison MR, Rao SS, Subbarao K, Kwong PD, Mascola JR, Kong WP, Graham BS. 2015. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun 6:7712. 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang L, Zhang Y, Joyce MG, Lingwood D, Moin SM, Andersen H, Okuno Y, Rao SS, Harris AK, Kwong PD, Mascola JR, Nabel GJ, Graham BS. 2015. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 21:1065–1070. 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 38.Sutton TC, Chakraborty S, Mallajosyula VVA, Lamirande EW, Ganti K, Bock KW, Moore IN, Varadarajan R, Subbarao K. 2017. Protective efficacy of influenza group 2 hemagglutinin stem-fragment immunogen vaccines. NPJ Vaccines 2:35. 10.1038/s41541-017-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481–483. 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakdawala SS, Jayaraman A, Halpin RA, Lamirande EW, Shih AR, Stockwell TB, Lin X, Simenauer A, Hanson CT, Vogel L, Paskel M, Minai M, Moore I, Orandle M, Das SR, Wentworth DE, Sasisekharan R, Subbarao K. 2015. The soft palate is an important site of adaptation for transmissible influenza viruses. Nature 526:122–125. 10.1038/nature15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Hu G, Wang Y, Ren W, Zhao X, Ji F, Zhu Y, Feng F, Gong M, Ju X, Zhu Y, Cai X, Lan J, Guo J, Xie M, Dong L, Zhu Z, Na J, Wu J, Lan X, Xie Y, Wang X, Yuan Z, Zhang R, Ding Q. 2021. Functional and genetic analysis of viral receptor ACE2 orthologs reveals a broad potential host range of SARS-CoV-2. Proc Natl Acad Sci U S A 118:e2025373118. 10.1073/pnas.2025373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Graaf M, Fouchier RA. 2014. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J 33:823–841. 10.1002/embj.201387442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plemper R, Cox R, Wolf J. 2020. Therapeutic MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Res Sq 10.21203/rs.3.rs-89433/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kutter JS, de Meudler D, Bestebroer TM, Lexmond P, Mulders A, Fouchier RA, Herfst S. 2020. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. bioRxiv, 10.1101/2020.10.19.345363. [DOI] [PMC free article] [PubMed]
- 46.Davidson AD, Williamson MK, Lewis S, Shoemark D, Carroll MW, Heesom KJ, Zambon M, Ellis J, Lewis PA, Hiscox JA, Matthews DA. 2020. Characterisation of the transcriptome and proteome of SARS-CoV-2 reveals a cell passage induced in-frame deletion of the furin-like cleavage site from the spike glycoprotein. Genome Med 12:68. 10.1186/s13073-020-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klimstra WB, Tilston-Lunel NL, Nambulli S, Boslett J, McMillen CM, Gilliland T, Dunn MD, Sun C, Wheeler SE, Wells A, Hartman AL, McElroy AK, Reed DS, Rennick LJ, Duprex WP. 2020. SARS-CoV-2 growth, furin-cleavage-site adaptation and neutralization using serum from acutely infected hospitalized COVID-19 patients. J Gen Virol 101:1156–1169. 10.1099/jgv.0.001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deming D, Sheahan T, Heise M, Yount B, Davis N, Sims A, Suthar M, Harkema J, Whitmore A, Pickles R, West A, Donaldson E, Curtis K, Johnston R, Baric R. 2006. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med 3:e525. 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lokugamage KG, Yoshikawa-Iwata N, Ito N, Watts DM, Wyde PR, Wang N, Newman P, Kent Tseng CT, Peters CJ, Makino S. 2008. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine 26:797–808. 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, Peters CJ, Couch RB. 2012. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One 7:e35421. 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasui F, Kai C, Kitabatake M, Inoue S, Yoneda M, Yokochi S, Kase R, Sekiguchi S, Morita K, Hishima T, Suzuki H, Karamatsu K, Yasutomi Y, Shida H, Kidokoro M, Mizuno K, Matsushima K, Kohara M. 2008. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol 181:6337–6348. 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z, Zhang L, Qin C, Ba L, Yi CE, Zhang F, Wei Q, He T, Yu W, Yu J, Gao H, Tu X, Gettie A, Farzan M, Yuen KY, Ho DD. 2005. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J Virol 79:2678–2688. 10.1128/JVI.79.5.2678-2688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y, Lu H, Siddiqui P, Zhou Y, Jiang S. 2005. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J Immunol 174:4908–4915. 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- 54.He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M, Jiang S. 2004. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun 324:773–781. 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Y, Zhu Q, Liu S, Zhou Y, Yang B, Li J, Jiang S. 2005. Identification of a critical neutralization determinant of severe acute respiratory syndrome (SARS)-associated coronavirus: importance for designing SARS vaccines. Virology 334:74–82. 10.1016/j.virol.2005.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang S, He Y, Liu S. 2005. SARS vaccine development. Emerg Infect Dis 11:1016–1020. 10.3201/1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S, Chou TH, Sakhatskyy PV, Huang S, Lawrence JM, Cao H, Huang X, Lu S. 2005. Identification of two neutralizing regions on the severe acute respiratory syndrome coronavirus spike glycoprotein produced from the mammalian expression system. J Virol 79:1906–1910. 10.1128/JVI.79.3.1906-1910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, Gao H, Ge X, Kan B, Hu Y, Liu J, Cai F, Jiang D, Yin Y, Qin C, Li J, Gong X, Lou X, Shi W, Wu D, Zhang H, Zhu L, Deng W, Li Y, Lu J, Li C, Wang X, Yin W, Zhang Y, Qin C. 2020. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 369:77–81. 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, Nkolola JP, Liu J, Li Z, Chandrashekar A, Martinez DR, Loos C, Atyeo C, Fischinger S, Burke JS, Slein MD, Chen Y, Zuiani A, Lelis FJN, Travers M, Habibi S, Pessaint L, Van Ry A, Blade K, Brown R, Cook A, Finneyfrock B, Dodson A, Teow E, Velasco J, Zahn R, Wegmann F, Bondzie EA, Dagotto G, Gebre MS, He X, Jacob-Dolan C, Kirilova M, Kordana N, Lin Z, Maxfield LF, Nampanya F, Nityanandam R, Ventura JD, Wan H, Cai Y, Chen B, Schmidt AG, Wesemann DR, Baric RS, Alter G, Andersen H, Lewis MG, Barouch DH. 2020. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369:806–811. 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, Bao L, Mo F, Li X, Huang Y, Hong W, Yang Y, Zhao Y, Ye F, Lin S, Deng W, Chen H, Lei H, Zhang Z, Luo M, Gao H, Zheng Y, Gong Y, Jiang X, Xu Y, Lv Q, Li D, Wang M, Li F, Wang S, Wang G, Yu P, Qu Y, Yang L, Deng H, Tong A, Li J, Wang Z, Yang J, Shen G, Zhao Z, Li Y, Luo J, Liu H, Yu W, Yang M, Xu J, Wang J, Li H, Wang H, et al. 2020. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 586:572–577. 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 61.Wu S, Zhong G, Zhang J, Shuai L, Zhang Z, Wen Z, Wang B, Zhao Z, Song X, Chen Y, Liu R, Fu L, Zhang J, Guo Q, Wang C, Yang Y, Fang T, Lv P, Wang J, Xu J, Li J, Yu C, Hou L, Bu Z, Chen W. 2020. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun 11:4081. 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arriola C, Garg S, Anderson EJ, Ryan PA, George A, Zansky SM, Bennett N, Reingold A, Bargsten M, Miller L, Yousey-Hindes K, Tatham L, Bohm SR, Lynfield R, Thomas A, Lindegren ML, Schaffner W, Fry AM, Chaves SS. 2017. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis 65:1289–1297. 10.1093/cid/cix468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson MG, Pierse N, Sue Huang Q, Prasad N, Duque J, Claire Newbern E, Baker MG, Turner N, McArthur C, SHIVERS investigation team . 2018. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 36:5916–5925. 10.1016/j.vaccine.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 64.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, Clinical trial group . 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Group CS. 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 67.Lakdawala SS, Lamirande EW, Suguitan AL, Jr., Wang W, Santos CP, Vogel L, Matsuoka Y, Lindsley WG, Jin H, Subbarao K. 2011. Eurasian-origin gene segments contribute to the transmissibility, aerosol release, and morphology of the 2009 pandemic H1N1 influenza virus. PLoS Pathog 7:e1002443. 10.1371/journal.ppat.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Houser KV, Broadbent AJ, Gretebeck L, Vogel L, Lamirande EW, Sutton T, Bock KW, Minai M, Orandle M, Moore IN, Subbarao K. 2017. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog 13:e1006565. 10.1371/journal.ppat.1006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.CDC. 2020. Lab advisory: FDA amends instructions for use of CDC 2019-nCoV real-time RT-PCR diagnostic panel. CDC, Atlanta, GA. https://www.cdc.gov/csels/dls/locs/2020/fda-amends-instructions-for-use-of-cdc-1029-ncov-real-time-rt-pcr-07-18-2020.html. [Google Scholar]