Abstract
The development of autoimmune diseases has been reported after SARS-CoV-2 infection. Vaccination against SARS-CoV-2 could also trigger auto-immunity, as it has been described with other vaccines. An aberrant immune response induced by molecular mimicry and bystander activation, especially in predisposed individuals, is a potential mechanism. We report the case of a 76-year-old woman with Hashimoto thyroiditis and prior COVID-19 infection who developed severe autoimmune hepatitis (with typical features including strongly positive anti-smooth muscle antibody and markedly elevated immunoglobulins G, as well as typical histological findings) following SARS-CoV-2 vaccination (mRNA-1273 SARS-CoV-2 vaccine, Moderna®). The link between SARS-CoV-2 vaccination and the development of autoimmune diseases needs to be further investigated. Although a causality relationship cannot be proven, caution may be warranted when vaccinating individuals with known autoimmune diseases.
Keywords: Autoimmune hepatitis, SARS-CoV-2, COVID-19, Vaccination
Article
A 76-year-old woman was referred to our Hepatology outpatient clinic with a 5-week history of dark urine, weight loss and fatigue, associated with elevated liver enzymes. Symptoms began two to three days after receiving the first dose of the mRNA-1273 SARS-CoV-2 vaccine. The patient had a medical history of COVID-19 infection 3 months prior (mild disease not requiring hospitalization), Hashimoto thyroiditis and prior urothelial carcinoma. Liver enzymes were completely normal in a routine check-up three years prior, as well as two months after the COVID-19 infection. Drug history included levothyroxine, midodrine (for a history of low blood pressure) and zolpidem, which are infrequently associated with drug-induced liver injury The patient did not take herbal remedies.
On examination, the patient had discrete scleral and sublingual icterus. Abdominal examination was remarkable for tender hepatomegaly.
Laboratory investigations found a total bilirubin of 65 μmol/L, elevated aspartate transaminase (811 U/L), alanine transaminase (579 U/L), alkaline phosphatase (124 U/L) and gamma-glutamyltransferase (361 U/L). INR was 1.23 and albumin was 28 g/L. Immunoglobulins G (IgG) were significantly increased (39.4 g/L). Anti-nuclear antibody (titer 1:1280, homogeneous, fine granular), anti-smooth muscle antibody (titer 1:1280, against F-Actin, no specific immunofluorescence pattern observed), anti-actin antibody 84 U (negativity cut-off <20 U) and anti-neutrophil cytoplasmic antibodies (titer >1:1280, perinuclear, MPO and PR3 negative) were strongly positive. Anti-M2 antibody, liver kidney microsome type 1 antibody and anti-soluble liver antigen antibody were negative. Complete blood count and creatinine were normal. Serologies for hepatitis A, B and C viruses were negative. PCR for hepatitis B and E viruses and for cytomegalovirus were negative.
Abdominal ultrasound showed a slightly enlarged and hyperechogenic liver without biliary duct dilatation.
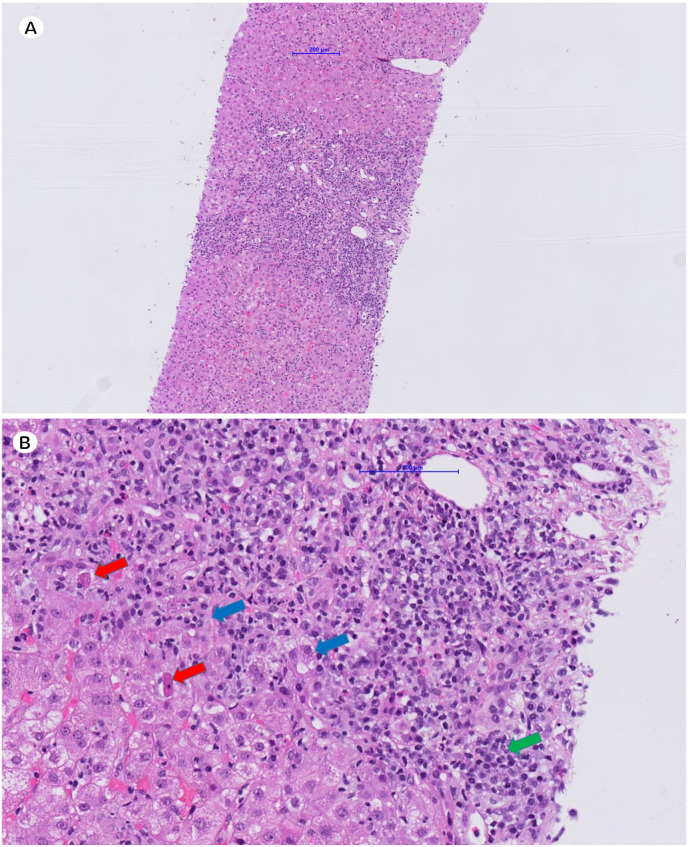
A liver biopsy showed a chronic markedly active hepatitis with interface hepatitis, plasma cells, feathery degeneration and pseudorosettes (Fig. 1 ), typical findings of autoimmune hepatitis (AIH). The degree of fibrosis could not be assessed given the significant parenchymal collapse. Simplified AIH Score was 8 (definite diagnosis of AIH).
Fig. 1.
Histological findings of autoimmune hepatitis after SARS-CoV-2 vaccination. Panel A: Medium‐magnification image (hematoxylin‐eosin, x5.88) showing a chronic, markedly active hepatitis including interface hepatitis. Panel B: High‐magnification image (hematoxylin‐eosin, x23.8) showing plasma cells (green arrow), apoptotic bodies (red arrows) and pseudorosettes (blue arrows).
Treatment with oral prednisolone 40 mg daily was started. Azathioprine was added two weeks later as maintenance therapy. After four weeks of treatment, the liver enzymes had completely normalized and the patient's symptoms had significantly improved. A decision was made to not administer the second dose of the vaccine. Prednisolone was tapered over a total of eight weeks and then stopped. The patient discontinued azathioprine on her own after two months of therapy due to drug intolerance and development of a nasal herpes simplex virus infection. Six weeks after stopping therapy there were however no biochemical signs of an AIH relapse.
Infections, including with SARS-CoV-2, may induce autoimmunity through an aberrant immune response triggered by molecular mimicry and bystander activation, especially in predisposed individuals [[1], [2], [3]]. Vaccination could, through similar mechanisms, lead to the development of autoimmune diseases, but proving such a relationship is challenging [3,4].
Given the close temporal relationship between the vaccination and onset of symptoms, we hypothesize that vaccination against COVID-19 could have triggered the development of AIH in our patient, with Hashimoto thyroiditis and prior COVID-19 infection acting as predisposing factors.
This is, to our knowledge, the second report of autoimmune hepatitis developing post SARS-CoV-2 vaccination [5], but the first with typical serological findings (strongly positive anti-smooth muscle antibody and markedly elevated IgG). In the case recently described by Bril et al., only anti-nuclear antibody and double-stranded DNA antibodies were positive and IgG levels were in the normal range.
While a massive, worldwide vaccination campaign against SARS-CoV-2 is occurring, the link between SARS-CoV-2 vaccination and the development of autoimmune diseases needs to be further investigated. Moreover, the question of whether vaccination should be avoided in individuals with severe autoimmune diseases deserves consideration [6].
Disclosures
There are no funding sources associated with the writing of this manuscript. Written consent for publication was obtained from the patient.
Contributors
Élise Vuille-Lessard: Conceptualization, Investigation, Writing - Original Draft and Review & Editing, Visualization. Matteo Montani: Formal analysis, Writing - Review & Editing. Jaume Bosch: Writing - Review & Editing, Supervision. Nasser Semmo: Writing - Review & Editing, Supervision.
Referees
Benedetta Terziroli Beretta-Piccoli (benedetta.terziroli@hin.ch), Fernando Bril (fbril@uab.edu), Aristo Vojdani (drari@msn.com), Michael Ehrenfeld (), Francesco Caso (francesco.caso@unina.it).
Declaration of competing interest
None.
Acknowledgments
Dr. Stefan Diem, Dr. Barbara Lobsiger
References
- 1.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R., et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;19:102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19:102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wraith D.C., Goldman M., Lambert P.H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362:1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 5.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to "potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2021;224:108665. doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]