LETTER
Staphylococcal osteomyelitis is a complex, costly disease that is difficult to treat, with high failure rates and often poor outcomes (1, 2). Staphylococci form biofilms and survive intracellularly, compromising the activity of traditional antibiotics, often requiring debridement/resection of infected and necrotic tissue (2–7). There is an unmet need for more effective, rapidly bactericidal treatments, including those that can be used with standard of care (SOC) therapeutics.
Lysins are recombinantly expressed and purified cell wall hydrolytic enzymes under development as novel antimicrobial agents. We previously showed that the lysin exebacase, targeting Staphylococcus aureus, has rapid bactericidal and antibiofilm activity in vitro (8) and, when given in a single dose with daptomycin, reduced methicillin-resistant S. aureus (MRSA) in a rat model of acute osteomyelitis (9). Lysin CF-296 is an engineered variant of exebacase, developed to maintain activity against biofilms and to be administered in repeated and/or higher doses than exebacase. Due to the complexity of staphylococcal osteomyelitis and poor activity of SOC antibiotics, addition of CF-296 to treatment regimens may offer a more robust antimicrobial response, potentially decreasing morbidity and time to recovery. Here, we tested the activity of CF-296 in vitro and in a rat model of MRSA acute osteomyelitis.
S. aureus MICs and minimum biofilm eradication concentrations (MBECs) of CF-296 were 0.5 to 1 μg/ml, including IDRL-6169, the strain studied in vivo (Table 1) (10, 11). The daptomycin MIC of IDRL-6169 was 0.5 μg/ml.
Table 1.
In vitro antistaphylococcal activity of CF-296
| Straina | Typeb | MIC (μg/ml) | MBEC (μg/ml) |
|---|---|---|---|
| IDRL-6169 | MRSA | 0.5 | 1 |
| MW2 | MRSA | 1 | 1 |
| ATCC BAA-42 | MRSA | 1 | 1 |
| JMI 227 | MRSA | 0.5 | 1 |
| JMI 21489 | MRSA | 1 | 1 |
| ATCC 29213 | MSSA | 1 | 0.5 |
| JMI 7141 | MSSA | 1 | 1 |
| JMI 3830 | MSSA | 1 | 1 |
| JMI 7140 | MSSA | 1 | 1 |
| JMI 7441 | MSSA | 1 | 1 |
Strain MW2 (NRS 123) was obtained from BEI Resources (NIAID, NIH); ATCC strains were obtained from the American Type Culture Collection (Manassas, VA); JMI strains were obtained from JMI Laboratories (North Liberty, IA) and are described in Watson et al. (13).
MSSA, methicillin-susceptible S. aureus.
A previously described model of rat acute osteomyelitis of the left tibiae used to test the in vivo activity of exebacase was used to evaluate CF-296 (9). A week after infection was established, rats were assigned to one of six groups: no treatment, 60 mg/kg daptomycin subcutaneously twice daily (12), 40 mg/kg CF-296 intravenously daily, 40 mg/kg CF-296 daily plus daptomycin, 100 mg/kg CF-296 intravenously once, or 100 mg/kg CF-296 once plus daptomycin. After 4 days, rats were euthanized and the left tibiae collected and analyzed, as previously described (9).
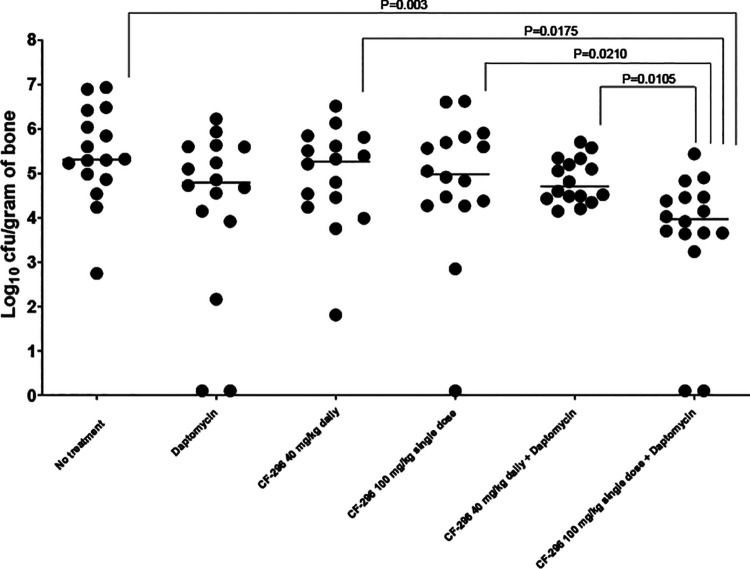
Results of bone cultures are shown in Fig. 1. Compared with no treatment, there were median 0.52-, 0.04-, and 0.33-log10 CFU/g reductions in MRSA for the daptomycin, CF-296 daily, and CF-296 single-dose animals, respectively, and 0.60- and 1.34-log10 CFU/g reductions in animals dosed with CF-296 daily or single-dose plus daptomycin, respectively. A CF-296 single 100 mg/kg dose plus daily daptomycin provided the most activity compared to no treatment (P = 0.003) and CF-296 single and daily doses alone (P = 0.0210 and 0.0175, respectively). Although not statistically significant, there was a trend toward the single 100 mg/kg dose of CF-296 administered plus daptomycin having more activity than daptomycin (additional 0.82 log10 CFU/g reduction). Despite reductions in CFUs, there was no cure of infection in the model studied.
FIG. 1.
Results of quantitative cultures (n = 16/group) of the tibiae after treatment (log10 CFU MRSA/g of bone). Bar represents median values.
Previously, we showed exebacase activity in our osteomyelitis model when given with daptomycin (9), and we now show similar activity using CF-296. Although different concentrations of exebacase and CF-296 were tested, the current and previous study showed a single dose of lysin administered in conjunction with daptomycin to result in a reduction in MRSA compared with untreated animals. Whether lysins might prevent emergence of daptomycin (or other antibiotic) resistance and possibly vice versa remains to be determined. In conclusion, CF-296 has in vitro antistaphylococcal activity and, when used with daptomycin, is active and well tolerated in rat MRSA acute osteomyelitis.
ACKNOWLEDGMENTS
This work was funded by ContraFect Corp.
R.P. reports grants from ContraFect, TenNor Therapeutics Limited, Hylomorph, and Shionogi; is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, and Qvella (monies are paid to Mayo Clinic); receives an editor’s stipend from Infectious Diseases Society of America and honoraria from the NBME, Up-to-Date and the Infectious Diseases Board Review Course; and is a consultant to Netflix. In addition, R.P. has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an antibiofilm substance issued. R.S. and C.C. are employees of ContraFect Corp, and D.L. is a consultant to ContraFect Corp.
REFERENCES
- 1.Dombrowski JC, Winston LG. 2008. Clinical failures of appropriately-treated methicillin-resistant Staphylococcus aureus infections. J Infect 57:110–115. doi: 10.1016/j.jinf.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J, O'Rourke S, Cahill KC, Kearney CJ, O'Brien FJ, Kerrigan SW. 2018. Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev 31:e00084-17. doi: 10.1128/CMR.00084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muthukrishnan G, Masters EA, Daiss JL, Schwarz EM. 2019. Mechanisms of immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr Osteoporos Rep 17:395–404. doi: 10.1007/s11914-019-00548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Chambers HF, Kaplan SL, Karchmer AW, Levine DP, Rybak MJ, Murray BE, Bayer A, Talan DA, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 5.Beck-Broichsitter BE, Smeets R, Heiland M. 2015. Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis 28:240–245. doi: 10.1097/QCO.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 6.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 7.Ellington JK, Harris M, Hudson MC, Vishin S, Webb LX, Sherertz R. 2006. Intracellular Staphylococcus aureus and antibiotic resistance: implications for treatment of staphylococcal osteomyelitis. J Orthop Res 24:87–93. doi: 10.1002/jor.20003. [DOI] [PubMed] [Google Scholar]
- 8.Karau MJ, Schmidt-Malan S, Mandrekar J, Lehoux D, Schuch R, Cassino C, Patel R. 2019. Activity of exebacase (CF-301) against methicillin-resistant Staphylococcus aureus (MRSA) biofilms on orthopedic Kirschner wires. Open Forum Infect Dis 6:S320. doi: 10.1093/ofid/ofz360.780. [DOI] [Google Scholar]
- 9.Karau MJ, Schmidt-Malan SM, Yan Q, Greenwood-Quaintance KE, Mandrekar J, Lehoux D, Schuch R, Cassino C, Patel R. 2019. Exebacase in addition to daptomycin is more active than daptomycin or exebacase alone in methicillin-resistant Staphylococcus aureus osteomyelitis in rats. Antimicrob Agents Chemother 63:e01235-19. doi: 10.1128/AAC.01235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—11th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing—29th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Rouse MS, Piper KE, Jacobson M, Jacofsky DJ, Steckelberg JM, Patel R. 2006. Daptomycin treatment of Staphylococcus aureus experimental chronic osteomyelitis. J Antimicrob Chemother 57:301–305. doi: 10.1093/jac/dki435. [DOI] [PubMed] [Google Scholar]
- 13.Watson A, Sauve K, Cassino C, Schuch R. 2020. Exebacase demonstrates in vitro synergy with a broad range of antibiotics against both methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 64:e01885-19. doi: 10.1128/AAC.01885-19. [DOI] [PMC free article] [PubMed] [Google Scholar]