ABSTRACT
Antifungal activity of AmBisome against Candida auris was determined in vitro and in vivo. AmBisome showed MIC50 and MIC90 values of 1 and 2 μg/ml, respectively. Unlike conventional amphotericin B, significant in vivo efficacy was observed in the AmBisome 7.5 mg/kg treatment group in survival and reduction of kidney tissue fungal burden compared to the untreated group. Our data show that AmBisome has significant antifungal activity against C. auris infection in vitro and in vivo.
KEYWORDS: Candida auris, multidrug resistant, AmBisome, conventional amphotericin B, fluconazole, susceptibility testing, mouse models
INTRODUCTION
Candida auris is an emerging multidrug-resistant yeast that has been associated with nosocomial infection worldwide (1), with a high infection rate in elderly patients and those with underlying illnesses (2–7). Critically, most C. auris isolates are resistant to at least two of the three main antifungal classes (azoles, polyenes, echinocandins), with some strains of C. auris exhibiting pan resistance (8–10). Although resistance of C. auris strains to conventional amphotericin B (CAmphoB) has been reported, activity of the liposomal preparation (AmBisome) against this yeast was not reported. Here, the in vitro and in vivo activity of AmBisome compared with CAmphoB and fluconazole against C. auris was investigated.
Clinical C. auris isolates (n = 35) were used in this study. Susceptibility testing was performed according to Clinical and Laboratory Standards Institute (CLSI) document M27-A4 (11). AmBisome was highly active against most of the tested strains (29/35, 83%), with an MIC range between 0.25 and 2 μg/ml and MIC50 and MIC90 values of 1 and 2 μg/ml (Table 1). In contrast, CAmphoB showed an MIC range of 1 to 4 μg/ml and MIC50 and MIC90 values of 4 μg/ml for both. Fluconazole was the least active, with most C. auris isolates demonstrating high resistance to this antifungal (12). Fluconazole showed MIC ranges of 0.25 to >64 μg/ml, with MIC50 and MIC90 values of 64 μg/ml.
TABLE 1.
MICs for AmBisome, amphotericin B, and fluconazole against C. auris
| Parameter for C. auris (n = 35) | MIC (μg/ml) for: |
||
|---|---|---|---|
| AmBisome | Amphotericin B | Fluconazole | |
| Range | 0.25–2 | 1–4 | 0.25 to >64 |
| MIC50 | 1 | 4 | 64 |
| MIC90 | 2 | 4 | >64 |
Using tentative breakpoints suggested by the CDC, we showed that 77% of the isolates tested were resistant to CAmphoB, with MIC values of ≥2 μg/ml, whereas only 17% of the examined isolates showed resistance to AmBisome.
In vivo testing was performed using a previously described disseminated C. auris infection model (13). All procedures were performed in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and approval of the Case Western Reserve University Institutional Animal Care and Use Committee (IACUC). Female BALB/c mice (weighing ∼20 g; Charles River Laboratories, Wilmington, MA) were used in the study.
C. auris (MRL 35368) with in vitro MICs for AmBisome, CAmphoB, and fluconazole of 1, 4, and >64 μg/ml, respectively, was used to challenge the mice. Treatment was initiated 2 h postchallenge. Treatment groups consisted of once-daily dosing of AmBisome at 3.5, 5, and 7.5 mg/kg; CAmphoB at 0.75 mg/kg administered intraperitoneally; and fluconazole at 25 mg/kg administered orally. Untreated control animals were included. Efficacy endpoints were animal survival and kidney fungal load.
Survival was monitored for 14 days postinoculation. The AmBisome 7.5 mg/kg treatment group showed 90% survival at 14 days postinoculation (Fig. 1), whereas CAmphoB- and fluconazole-treated groups showed 20% and 50% survival, respectively. The AmBisome 7.5 mg/kg group demonstrated significant survival compared with the untreated control group (P < 0.001) and had significantly higher survival rates than either the CAmphoB or fluconazole group (P = 0.003 and 0.025, respectively). The AmBisome formulation allows the drug to remain within the liposomes until it adheres to the fungal cell wall, where it enters the fungus intact, preventing the drug from interacting with mammalian cells to exert its toxic effects (14, 15).
FIG 1.
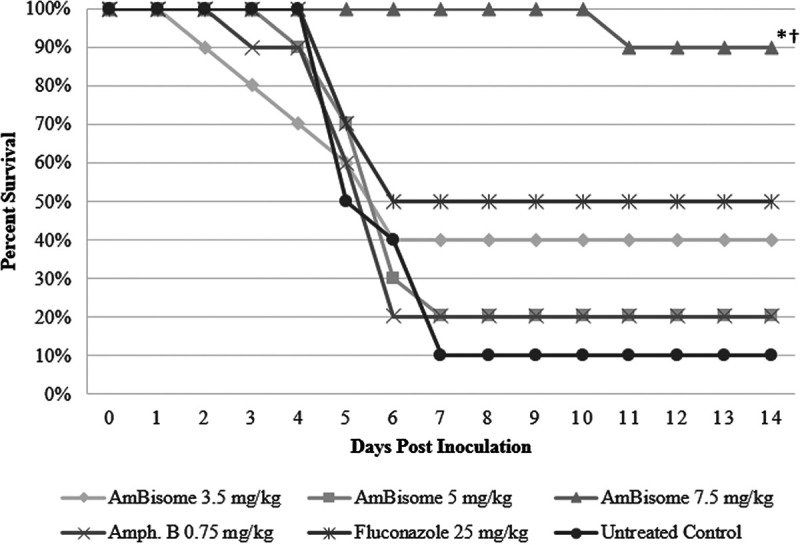
Survival curves in BALB/c mice infected with C. auris (3 × 107). Treatments were administered by intraperitoneal injection for AmBisome and conventional amphotericin B and orally for fluconazole (n = 10/group). Treatment consisted of once-daily dosing starting 2 h postchallenge and continuing for 7 days. Survival was monitored until day 14. Survival was plotted by Kaplan-Meier analysis, and differences in the percent survival among groups were analyzed by log-rank test and Fisher exact test. *, P ≤ 0.001 versus untreated control; †, P < 0.05 versus conventional amphotericin B and fluconazole, respectively.
Five mice from each group were euthanized 1 day after treatment (day 8) to determine kidney fungal burden. Kidneys were removed aseptically and weighed, homogenized in 1 ml of phosphate-buffered saline, serially diluted, plated onto potato dextrose agar (Becton, Dickinson and Company, Sparks, MD), and cultured at 37°C for 48 h to determine CFU per gram. The tissue fungal burden was expressed as CFU per gram of tissue. Efficacy of AmBisome was evaluated as a reduction in log10 CFU compared with other tested groups.
Table 2 shows the kidney tissue fungal burden of mice challenged with C. auris. As expected, the mice in the untreated control group showed the highest kidney tissue fungal burden (8.64 ± 0.7 log CFU/g ± SD). The AmBisome 7.5 mg/kg group demonstrated a tissue fungal burden of 5.55 ± 0.6 log CFU/g. The AmBisome 5 and 3.5 mg/kg treatment groups showed tissue fungal burdens of 5.88 ± 2.2 and 6.54 ± 1.2 log CFU/g, respectively. These data show that AmBisome reduced tissue burden in a dose-dependent manner, with AmBisome 7.5 mg/kg reaching a significant reduction in tissue fungal burden (P = 0.028). The 5- and 3.5-mg/kg AmBisome arms showed a trend toward reducing kidney tissue fungal burden compared with the untreated control group, which did not reach statistical significance (P = 0.069 and 0.387, respectively). In contrast to AmBisome 7.5 mg/kg, CAmphoB 0.75 mg/kg did not demonstrate significant efficacy against C. auris (6.18 ± 1.6 CFU/g; P = 0.356). However, fluconazole 25 mg/kg treatment showed significant reduction in fungal tissue burden (5.46 ± 1.4 CFU/g) compared with the untreated control (P = 0.022). No statistical difference in fungal tissue burden was noted between the AmBisome 7.5 mg/kg and fluconazole treatment groups (P > 0.05).
TABLE 2.
Average kidney tissue fungal burden compared with untreated control
| Treatment group | Kidney tissue fungal burden (avg log CFU/g ± SD) | P value |
|---|---|---|
| Untreated control | 8.64 ± 0.7 | |
| AmBisome (mg/kg) | ||
| 3.5 | 6.54 ± 1.2 | 0.387 |
| 5 | 5.88 ± 2.2 | 0.069 |
| 7.5 | 5.55 ± 0.6 | 0.028 |
| Amphotericin B 0.75 mg/kg | 6.18 ± 1.6 | 0.356 |
| Fluconazole 25 mg/kg | 5.46 ± 1.4 | 0.022 |
AmBisome 7.5 mg/kg demonstrated a significant reduction in CFU in the kidneys, with a better survival rate than CAmphoB and fluconazole. This could be explained by the ability of AmBisome to distribute to infected tissues at levels above the MIC values. AmBisome also demonstrated slow tissue clearance rates (16).
Interestingly, the isolate used in our animal model demonstrated resistance to fluconazole when tested in vitro, whereas fluconazole in vivo showed a significant reduction in CFU in the kidneys. This difference could be because susceptibility tests are designed to test the activity of antifungals in a static test situation. These conditions do not mimic the in vivo situation in that many factors may influence the intrinsic antifungal properties, including the underlying condition and immunological status of the animal model (17). Although fluconazole demonstrated activity in reducing the kidney tissue burden, it was not as effective as AmBisome in enhancing animal survival rates (P = 0.025 versus AmBisome 7.5 mg/kg).
The underlying mechanisms for AmBisome’s higher in vitro and in vivo activity against C. auris compared with CAmphoB are unknown. Recently, Walker et al. (14) reported that the viscoelastic properties of the Candida albicans and Cryptococcus neoformans cell wall allowed for travel of AmBisome as intact liposome vesicles. At the target site, the higher affinity of amphotericin B for fungal ergosterol over the lipid carrier and the availability of extracellular yeast and host lipases facilitated the release of amphotericin B from the lipid complex, where it binds to the cell membrane of the fungal pathogen (16). Additional evidence differentiating liposomal amphotericin B formulations from CAmphoB can be gleaned from the findings that lipid formulations are more efficient at inhibiting Candida biofilms than CAmphoB in vitro (18, 19) and in a rabbit model of catheter-associated C. albicans biofilm infection (20, 21).
Taken together our findings show that AmBisome possesses significant antifungal activity against C. auris in vitro and in vivo compared with CAmphoB and fluconazole.
ACKNOWLEDGMENTS
This work was supported by a grant from Gilead Sciences, Inc., and the National Institutes of Health (grant R21 AI143305-01).
M.G. has received funding from, Pfizer, Scynexis, Inc., Cidara Therapeutics, and Amplyx Pharmaceuticals. S.S. is an employee of Gilead Sciences, Inc.
REFERENCES
- 1.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 2.Adams E, Quinn M, Tsay S, Poirot E, Chaturvedi S, Southwick K, Greenko J, Fernandez R, Kallen A, Vallabhaneni S, Haley V, Hutton B, Blog D, Lutterloh E, Zucker H, Candida auris Investigation Workgroup . 2018. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis 24:1816–1824. doi: 10.3201/eid2410.180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emara M, Ahmad S, Khan Z, Joseph L, Al-Obaid I, Purohit P, Bafna R. 2015. Candida auris candidemia in Kuwait, 2014. Emerg Infect Dis 21:1091–1092. doi: 10.3201/eid2106.150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudramurthy SM, Chakrabarti A, Paul RA, Sood P, Kaur H, Capoor MR, Kindo AJ, Marak RSK, Arora A, Sardana R, Das S, Chhina D, Patel A, Xess I, Tarai B, Singh P, Ghosh A. 2017. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J Antimicrob Chemother 72:1794–1801. doi: 10.1093/jac/dkx034. [DOI] [PubMed] [Google Scholar]
- 5.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang HC. 2011. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magobo RE, Corcoran C, Seetharam S, Govender NP. 2014. Candida auris-associated candidemia, South Africa. Emerg Infect Dis 20:1250–1251. doi: 10.3201/eid2007.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, Jain S, Kathuria S, Randhawa HS, Hagen F, Meis JF. 2013. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis 19:1670–1673. doi: 10.3201/eid1910.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrowsky B, Greenko J, Adams E, Quinn M, O'Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S, Lutterloh E, Blog D, Group CIW, C. auris Investigation Work Group . 2020. Candida auris isolates resistant to three classes of antifungal medications - New York, 2019. MMWR Morb Mortal Wkly Rep 69:6–9. doi: 10.15585/mmwr.mm6901a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, Dinesh KR, Karim S, Singh SK, Hagen F, Meis JF. 2014. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis 33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg K, Woodworth K, Walters M, Berkow EL, Jackson B, Chiller T, Vallabhaneni S. 2019. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol 57:1–12. doi: 10.1093/mmy/myy054. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—4th ed. CLSI document M27-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Osei Sekyere J. 2018. Candida auris: a systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 7:e00578. doi: 10.1002/mbo3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hager CL, Larkin EL, Long LA, Ghannoum MA. 2018. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother 73:2085–2088. doi: 10.1093/jac/dky153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker L, Sood P, Lenardon MD, Milne G, Olson J, Jensen G, Wolf J, Casadevall A, Adler-Moore J, Gow NAR. 2018. The viscoelastic properties of the fungal cell wall allow traffic of AmBisome as intact liposome vesicles. mBio 9:e02383-17. doi: 10.1128/mBio.02383-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamill RJ. 2013. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 73:919–934. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 16.Adler-Moore J, Lewis RE, Bruggemann RJM, Rijnders BJA, Groll AH, Walsh TJ. 2019. Preclinical safety, tolerability, pharmacokinetics, pharmacodynamics, and antifungal activity of liposomal amphotericin B. Clin Infect Dis 68:S244–S259. doi: 10.1093/cid/ciz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex JH, Pfaller MA, Barry AL, Nelson PW, Webb CD. 1995. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob Agents Chemother 39:40–44. doi: 10.1128/AAC.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimoto K, Takemoto K. 2018. Efficacy of liposomal amphotericin B against four species of Candida biofilms in an experimental mouse model of intravascular catheter infection. J Infect Chemother 24:958–964. doi: 10.1016/j.jiac.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, Ghannoum MA. 2004. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother 48:1727–1732. doi: 10.1128/AAC.48.5.1727-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faustino C, Pinheiro L. 2020. Lipid systems for the delivery of amphotericin B in antifungal therapy. Pharmaceutics 12:29. doi: 10.3390/pharmaceutics12010029. [DOI] [PMC free article] [PubMed] [Google Scholar]


