ABSTRACT
Pseudomonas aeruginosa induces pathways indicative of low zinc availability in the cystic fibrosis (CF) lung environment. To learn more about P. aeruginosa zinc access in CF, we grew P. aeruginosa strain PAO1 directly in expectorated CF sputum. The P. aeruginosa Zur transcriptional repressor controls the response to low intracellular zinc, and we used the NanoString methodology to monitor levels of Zur-regulated transcripts, including those encoding a zincophore system, a zinc importer, and paralogs of zinc containing proteins that do not require zinc for activity. Zur-controlled transcripts were induced in sputum-grown P. aeruginosa compared to those grown in control cultures but not if the sputum was amended with zinc. Amendment of sputum with ferrous iron did not reduce expression of Zur-regulated genes. A reporter fusion to a Zur-regulated promoter had variable activity in P. aeruginosa grown in sputum from different donors, and this variation inversely correlated with sputum zinc concentrations. Recombinant human calprotectin (CP), a divalent-metal binding protein released by neutrophils, was sufficient to induce a zinc starvation response in P. aeruginosa grown in laboratory medium or zinc-amended CF sputum, indicating that CP is functional in the sputum environment. Zinc metalloproteases comprise a large fraction of secreted zinc-binding P. aeruginosa proteins. Here, we show that recombinant CP inhibited both LasB-mediated casein degradation and LasA-mediated lysis of Staphylococcus aureus, which was reversible with added zinc. These studies reveal the potential for CP-mediated zinc chelation to posttranslationally inhibit zinc metalloprotease activity and thereby affect the protease-dependent physiology and/or virulence of P. aeruginosa in the CF lung environment.
IMPORTANCE The factors that contribute to worse outcomes in individuals with cystic fibrosis (CF) with chronic Pseudomonas aeruginosa infections are not well understood. Therefore, there is a need to understand environmental factors within the CF airway that contribute to P. aeruginosa colonization and infection. We demonstrate that growing bacteria in CF sputum induces a zinc starvation response that inversely correlates with sputum zinc levels. Additionally, both calprotectin and a chemical zinc chelator inhibit the proteolytic activities of LasA and LasB proteases, suggesting that extracellular zinc chelators can influence proteolytic activity and thus P. aeruginosa virulence and nutrient acquisition in vivo.
KEYWORDS: Pseudomonas aeruginosa, calprotectin, cystic fibrosis, proteases, zinc
INTRODUCTION
In cystic fibrosis (CF), microbes such as Pseudomonas aeruginosa colonize airway mucus where they then compete with host cells and other microbes for nutrients, including metals. Divalent metal ions (e.g., Zn2+, Fe2+, Mn2+, etc.) are essential micronutrients for host and microbe alike, in part because they act as cofactors in enzymes important for a variety of cellular functions. While the concentration of metals, such as zinc, in CF sputum can vary, the concentration of zinc in expectorated sputum from CF patients is elevated, on average, compared to levels in samples from healthy controls (1–3). However, studies investigating the transcriptional response of P. aeruginosa in CF sputum show that a common gene expression pattern is the increased expression of zinc uptake and transport genes (4–9), which are normally expressed when zinc is limited. The P. aeruginosa zinc starvation response is regulated by the zinc uptake regulator (Zur), which is a transcriptional repressor (10). When intracellular zinc is high, Zur monomers bind zinc, dimerize, and bind DNA to repress gene expression of zinc uptake pathways. When intracellular zinc becomes low, the dimeric, zinc-bound fraction of Zur decreases, which leads to derepression of genes involved in zinc uptake and the expression of zinc-free paralogs of essential proteins (zinc-sparing response). The P. aeruginosa Zur regulon (11, 12) includes genes involved in zinc uptake, i.e., the zinc transporter-encoding operon znuABCD (10, 13, 14) and the zincophore-encoding operon cntILMO (15, 16). In addition, Zur regulates expression of zinc-free paralogs of ribosomal proteins (PA3600 and PA3601) (13, 17) and a transcriptional regulator (dksA2) (18) so as to reduce the requirement for zinc or to make the zinc stored in these proteins available for other cellular processes (19).
The host, on the other hand, utilizes nutritional immunity to sequester metal ions away from pathogens to reduce bacterial growth and control infection (20). One of the most abundant zinc-binding host proteins in CF is calprotectin (CP), which was previously named “the cystic fibrosis antigen” because of its abundance in the serum, sputum, and bronchoalveolar lavage fluid (BALF) of individuals with CF (2, 21–24). Neutrophils recruited to sites of inflammation release CP as S100A8/A9 heterodimers (25, 26), which then form tetramers in environments with sufficient levels of calcium (27, 28). Each heterodimer has two divalent-metal binding sites: the His3-Asp site has high affinity for zinc, while the His6 site is capable of binding divalent zinc, iron, manganese, or nickel (29). CP is thought to induce zinc limitation as a means to control infections caused by Staphylococcus aureus, Acinetobacter baumannii in tissues, and Salmonella enterica serovar Typhimurium in the gastrointestinal tract (30–32). However, little is known about the effect of CP-mediated zinc sequestration on P. aeruginosa growth and physiology.
Additionally, CP has been shown to inhibit the activity of metalloproteases such as host matrix metalloproteinases via zinc chelation (33). P. aeruginosa regulates expression of several metalloenzymes, including zinc metalloproteases, by quorum sensing (QS), which is a mechanism that regulates gene expression in accordance with cell density through the secretion of signal molecules. The secretion of zinc metalloproteases LasB (PA3724), LasA (PA1871), AprA (PA1249), ImpA (PA0572), and PepB (PA2939) (Table 1) are regulated by transcriptional regulators LasR and RhlR involved in QS (34, 35). This coordinated expression may be of particular importance for optimal protease activity given recent findings showing that zinc metalloproteases LasB and LasA in addition to the serine protease protease IV are activated, after being secreted, by a QS-induced proteolytic cascade, in which LasB activates protease IV and then protease IV, in turn, activates LasA (36, 37). Expression of these zinc metalloproteases is important for P. aeruginosa colonization and virulence because they play key roles in processes such as degrading host proteins (e.g., elastin) (38), invading host cells (39), evading host immune responses (40–42), and lysing other bacteria (e.g., S. aureus) (43, 44). While incubation of P. aeruginosa zinc metalloproteases with chemical zinc chelators inhibits their activity (45, 46), the effect of physiologically relevant zinc chelators such as CP on the activity of P. aeruginosa zinc metalloproteases remains unclear.
TABLE 1.
Zinc metalloproteases secreted by P. aeruginosa
| Gene no.a | PDB entryb | Protein name and description |
|---|---|---|
| PA0572 | 5KDW | ImpA, immunomodulating metalloprotease of P. aeruginosa |
| PA1249 | 1KAP | AprA, alkaline metalloprotease or aeruginolysin |
| PA1871 | 3IT5 | LasA, staphylolytic protease |
| PA2939 | NA | PepB or PaAP, aminopeptidase |
| PA3724 | 1EZM | LasB, elastase or pseudolysin |
From P. aeruginosa genome website, https://www.pseudomonas.com/.
From Protein Data Bank (PDB) website, https://www.rcsb.org/.
To test these hypotheses, we used a novel method in which P. aeruginosa strain PAO1 was grown directly in unamended expectorated CF sputum and matched sputum samples treated with divalent metals (e.g., Zn2+ and Fe2+) and zinc chelators (e.g., TPEN [N,N,N′,N′-tetrakis-2-pyridylmethyl-ethylenediamine] and CP). The effect of zinc chelators on P. aeruginosa zinc metalloprotease activity was further assessed using protease-specific assays. Overall, our findings support a model in which zinc chelation by CP in the mucus of the CF lung may affect the ecology of colonizing P. aeruginosa by inhibiting the activity of proteases involved in processes such as nutrient acquisition and interspecies competition.
RESULTS
P. aeruginosa exhibits a Zur-regulated zinc starvation response when grown in CF sputum samples from different donors.
Given that recent studies have shown that P. aeruginosa increases expression of Zur-regulated genes in CF sputum (4–6), we first aimed to construct a lacZ fusion to the promoter of a Zur-regulated gene on the chromosome of P. aeruginosa to act as a tool to explore factors that influence the activation of the Zur regulon. While the P. aeruginosa Zur regulon contains several candidates, we selected ribosomal protein PA3600 given the abundance of ribosomes in bacterial cells (10, 47) and the high Zur responsiveness of zinc-independent ribosomal proteins relative to that of other Zur-regulated genes (48). P. aeruginosa encodes two forms of the 50s ribosomal protein L36. PA4242/RpmJ is the zinc-dependent isoform that contains the zinc ribbon motif CXXC…CXXXXXH, while Zur-regulated PA3600 is the zinc-independent isoform that lacks the zinc ribbon motif (see Fig. S1A in the supplemental material) (11–13, 17). PA4242 is highly similar (87% identity) to RpmJ, the zinc-dependent isoform of L36 encoded by Escherichia coli strain K-12 (Fig. S1), and as such the gene is commonly annotated as rpmJ (48). PA3600 is highly similar (80% identity) to RpmJ2, the zinc-independent isoform of L36 encoded by E. coli strain K-12 (Fig. S1), and thus we will refer to PA3600 as RpmJ2 for the remainder of this study.
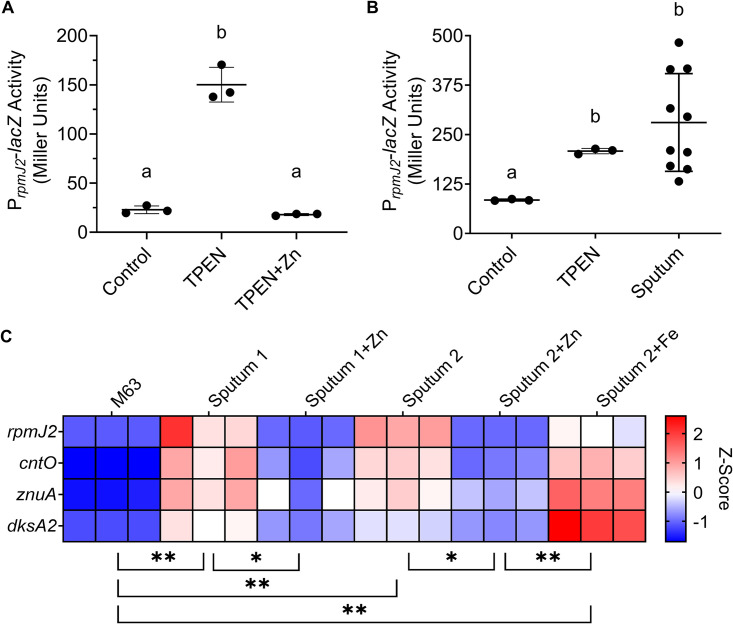
We constructed a lacZ fusion to the promoter of rpmJ2 on the chromosome of P. aeruginosa strain PAO1 (PAO1 att::PrpmJ2-lacZ). Activation of the rpmJ2 promoter was first confirmed by measuring activity by P. aeruginosa grown in culture medium (LB), medium containing TPEN (N,N,N′,N′-tetrakis-2-pyridylmethyl-ethylenediamine), or medium containing both TPEN and zinc (Fig. 1A). TPEN is a membrane-permeable metal ion chelator with a high affinity for zinc (49) and was therefore used to induce a zinc starvation response in P. aeruginosa. P. aeruginosa grown for 3 h in LB had little promoter activity (∼23 Miller units [MU]), while growth in medium containing TPEN resulted in a 7-fold increase in promoter activity (∼150 MU) (Fig. 1A). The addition of TPEN and an excess (1 mM) of zinc did not stimulate promoter activity (Fig. 1A). The ability of sputum to activate the rpmJ2 promoter was then determined by growing P. aeruginosa in M63 minimal medium containing 0.2% glucose (M63), M63 plus TPEN (positive control), or expectorated CF sputum from 10 different donors (Fig. 1B). While P. aeruginosa grown for 3 h in M63 exhibited greater promoter activity (∼85 MU) than that grown in LB (∼23 MU), growth in CF sputum resulted in a 3-fold increase in promoter activity (∼281 MU). Average promoter activation in CF sputum was statistically the same as promoter activity induced by TPEN (Fig. 1B).
FIG 1.
P. aeruginosa inoculated into expectorated CF sputum from different donors exhibits a zinc starvation response. (A) P. aeruginosa strain PAO1 PrpmJ2-lacZ was grown in LB (Control), LB with 50 μM TPEN (TPEN), or LB with 50 μM TPEN and 1 mM ZnSO4 · 7 H2O (TPEN+Zn) for 3 h. The data shown represent the mean ± standard deviation (SD) from three independent experiments. (B) P. aeruginosa strain PAO1 PrpmJ2-lacZ was grown in M63 (Control), M63 with 50 μM TPEN (TPEN), or expectorated CF sputum (Sputum) for 3 h. Each point in the sputum set indicates a separate sample from a different donor. The data were analyzed by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple-comparison test. (C) P. aeruginosa strain PAO1 was inoculated into M63 (M63) or into sputum from two different donors (Sputum 1 and Sputum 2). The sputum was divided and left untreated (Sputum), treated with 1 mM ZnSO4 · 7 H2O (Sputum+Zn), or treated with 1 mM (NH4)2Fe(SO4)2 · 6 H2O (Sputum+Fe). Each condition was analyzed in triplicate. Expression of select Zur-regulated genes was measured by NanoString (codeset PAV4). Expression values are normalized to the geometric mean of loading controls and Z-score was scaled by gene. The data in panel A and panel C were analyzed by one-way ANOVA with Tukey’s multiple-comparison test. Samples marked with the same letter are not significantly different; samples marked with different letters are significantly different (P < 0.05). *, P < 0.05, **, P < 0.01.
To further assess the activity of Zur in CF sputum, we used a multiplex method to assess expression of rpmJ2 and three additional Zur-regulated genes. To do so, we used NanoString technology, which is a hybridization-based method that is quantitative, is not hindered by contaminating DNA in sputum, and requires only a small amount of RNA. Consequently, NanoString works well for the analysis of small clinical sample aliquots (e.g., sputum) as previously demonstrated (50, 51). In this study, NanoString technology allowed for the analysis of a subset of Zur-regulated genes: rpmJ2, cntO, znuA, and dksA2. Analysis showed an induction of these Zur-regulated genes in P. aeruginosa grown in sputum compared to those grown in M63 (Fig. 1C). Amending samples with excess (1 mM) zinc was sufficient to reduce the expression of Zur-regulated genes (Fig. 1C). Studies have shown regulatory cross talk between iron and zinc, as iron starvation was previously shown to increase expression of Zur-regulated genes cntO, cntM, and amiA but not znuA (52). However, amending sputum samples with excess (1 mM) ferrous iron did not reduce expression of Zur-regulated genes (Fig. 1C). Together, these data support the model that P. aeruginosa has limited access to zinc in sputum and that zinc and iron limitation are separate signals.
Activation of the Zur-regulated rpmJ2 promoter in CF sputum is inversely correlated with concentration of zinc in sputum samples.
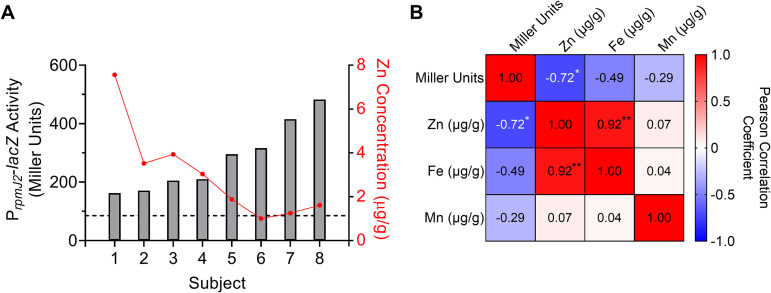
While promoter activity of P. aeruginosa grown in CF sputum samples was overall higher than that of P. aeruginosa grown in medium controls, there was a range of promoter activity across sputum samples from different subjects (Fig. 1B). We hypothesized that differences in promoter activities between sputum samples from different CF patients were due to differences in sputum zinc concentrations. To test this, inductively coupled plasma-mass spectrometry (ICP-MS) was performed on homogenized CF sputum samples to measure total metal (i.e., zinc, iron, and manganese) concentrations. The ability of these same sputum samples to activate the rpmJ2 promoter in reporter strain PAO1 att::PrpmJ2-lacZ was tested in parallel. The data showed a significant inverse correlation between sputum zinc concentration and induction of the rpmJ2 promoter across tested sputum samples (Fig. 2). There was no significant correlation between sputum iron or manganese concentrations and induction of the rpmJ2 promoter (Fig. 2B; Fig. S2A and B). Induction of the rpmJ2 promoter was also compared to clinical information, primarily lung function (percent of forced expiratory volume in 1 s, FEV1%) at the time of sputum collection, but there was no correlation found between FEV1% and rpmJ2 promoter activity (Fig. S2C). Therefore, the derepression of Zur-regulated genes in P. aeruginosa grown in CF sputum inversely correlates with the total zinc concentration in sputum samples.
FIG 2.
Activation of the rpmJ2 promoter in CF sputum by P. aeruginosa is inversely correlated with total sputum zinc concentration. (A) P. aeruginosa strain PAO1 PrpmJ2-lacZ was inoculated into 8 different CF sputum samples. Zinc concentration of the same 8 CF sputum samples was determined by ICP-MS. β-Gal activity on the left y axis (bars) was then compared to sputum zinc concentration on the right y axis (dots). (B) Pearson correlation matrix comparing β-Gal activity (Miller units), sputum zinc concentration, sputum iron concentration, and sputum manganese concentration. *, P < 0.05, **, P < 0.01.
Recombinant CP induces a P. aeruginosa zinc starvation response in vitro and in expectorated CF sputum.
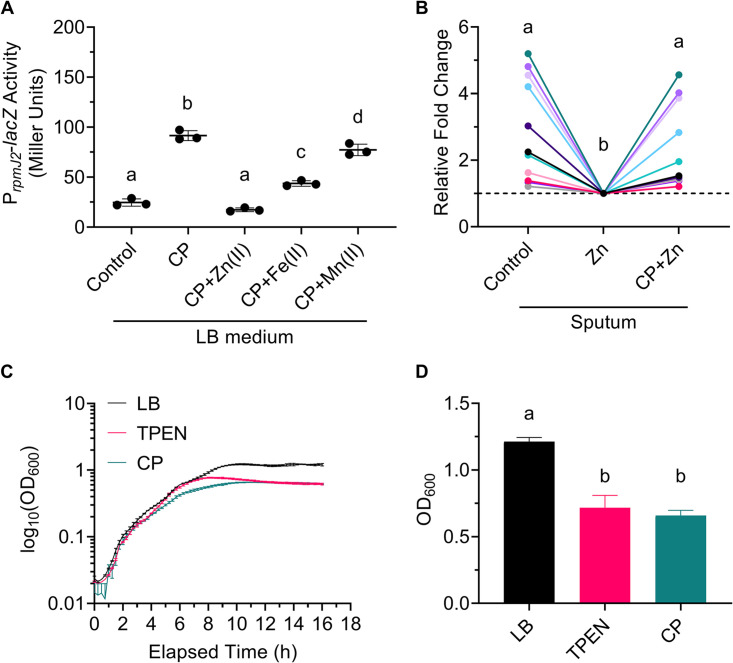
Studies report elevated levels of zinc in CF sputum (1, 2). Our ICP-MS data show that the sputum sample in our study that elicited the strongest zinc starvation response had a zinc concentration of ∼2 μg/g (∼2,000 μg/liter, ∼31 μM) (Fig. 2A). Given the concomitant high zinc concentration in our CF sputum samples and the elevated zinc starvation response in P. aeruginosa grown in these CF sputum samples, it is likely that the zinc in our CF sputum samples is bound by zinc-sequestering proteins. CP is one such host zinc-sequestering protein that is found in high concentrations in the sputum of CF patients (2, 22, 23). CP has also been shown to induce expression of Zur-regulated genes in P. aeruginosa strain PA14 (53). Therefore, we hypothesized that CP binds zinc to induce a zinc starvation response in P. aeruginosa grown in CF sputum. To test this, we first expressed and purified recombinant human CP as previously described (54) and as illustrated in Fig. S3. The ability of our recombinant CP to induce a zinc starvation response was tested by growing P. aeruginosa strain PAO1 att::PrpmJ2-lacZ in culture medium (LB), medium containing CP, or medium containing CP and divalent zinc, iron, or manganese (Fig. 3A). CP concentrations in the sputum of CF patients have been reported to reach up to 3 mg/ml with an interquartile range of 0.5 mg/ml to 1 mg/ml (2, 23); we used 1 mg/ml (∼40 μM) CP, which is at the highest end of the interquartile range, for all CP-based experiments. Growing P. aeruginosa in medium containing 1 mg/ml CP resulted in a 4-fold increase in promoter activation (∼92 MU) compared to that of the control (∼25 MU) (Fig. 3A). The addition of excess (1 mM) zinc in the presence of CP completely prevented promoter activation (∼17 MU) (Fig. 3A). The addition of 1 mM iron had an intermediate effect (∼44 MU), while the addition of 1 mM manganese had a small though statistically significant effect (∼77 MU) (Fig. 3A). These results suggest that CP primarily binds zinc and iron, but not manganese, under the conditions tested. Furthermore, given the intermediate effect of iron, these results suggest that excess iron displaces zinc from the His6 binding site but not from the His3Asp site, consistent with reported findings (55), effectively causing CP to bind half the amount of zinc. These results confirm that our purified recombinant human CP can induce a zinc starvation response in P. aeruginosa which is quenched with the addition of exogenous zinc.
FIG 3.
Recombinant human CP added to CF sputum and culture medium induces a zinc starvation response by P. aeruginosa. (A) P. aeruginosa strain PAO1 PrpmJ2-lacZ was grown in culture medium (Control), medium with 40 μM CP (CP), or medium with 40 μM CP and 1 mM ZnSO4 · 7 H2O [CP+Zn(II)], (NH4)2Fe(SO4)2 · 6 H2O [CP+Fe(II)], or MnCl2 · 4 H2O [CP+Mn(II)] for 3 h. The data shown represent the mean ± SD from three independent experiments. (B) P. aeruginosa strain PAO1 PrpmJ2-lacZ was inoculated into CF sputum from 11 different donors. The sputum was divided and left untreated (Control), treated with 100 μM ZnSO4 · 7 H2O (Zn), or treated with 40 μM CP and 100 μM ZnSO4 · 7 H2O (CP+Zn) for 3 h. Different-color dots represent samples from different donors. The same-color dots connected by a line are from the same CF sputum donor. Data were analyzed by RM one-way ANOVA with Tukey’s multiple-comparison test. (C) Representative growth curves of P. aeruginosa strain PAO1 PrpmJ2-lacZ grown in LB, LB containing 50 μM TPEN, or LB containing 40 μM CP. Data shown represent the mean ± SD from three technical replicates and are representative of three independent experiments. (D) OD600 at 16 h of P. aeruginosa strain PAO1 PrpmJ2-lacZ grown in LB, LB containing 50 μM TPEN, or LB containing 40 μM CP. Data shown represent the mean ± SD from three independent experiments. The data in panel A and panel D were analyzed by one-way ANOVA with Tukey’s multiple-comparison test. Samples marked with the same letter are not significantly different; samples marked with different letters are significantly different (P < 0.05).
Despite the reportedly high concentrations of CP in the serum, sputum, and BALF of CF patients (2, 21–24), P. aeruginosa appears to be able to access enough zinc to persist. Various environmental factors may influence CP zinc binding, such as calcium concentrations (56), pH (57), or the presence of oxidants (58, 59). Additionally, while CP in its tetrameric state is resistant to proteolytic degradation, CP is susceptible to oxidation, which in turn makes it susceptible to proteolytic degradation by both host and bacterial proteases (58, 59). Because it was unclear if CP in sputum would remain intact and/or active to bind zinc, we tested the ability of recombinant human CP to bind zinc and thereby induce a zinc starvation response in P. aeruginosa grown in CF sputum. P. aeruginosa strain PAO1 att::PrpmJ2-lacZ was grown in unamended CF sputum, sputum supplemented with 1 mM zinc, and sputum supplemented with both 1 mM zinc and ∼40 μM CP (Fig. 3B). The addition of zinc lowered rpmJ2 promoter activity in sputum (Fig. 3B), supporting our NanoString data (Fig. 1C), while addition of CP to zinc-amended sputum significantly prevented reduction of promoter activity (Fig. 3B). These data confirm that recombinant CP added to CF sputum remains intact to bind zinc, which induces a zinc starvation response in colonizing P. aeruginosa.
While recombinant CP added to zinc-amended sputum increased P. aeruginosa rpmJ2 promoter activity on average compared to that of zinc-amended sputum controls, the CF sputum samples tested varied in their responses (Fig. 3B; Fig. 1B). The inverse correlation between sputum zinc concentrations and induction of the rpmJ2 promoter suggests that sputum samples that result in high promoter activity have lower concentrations of zinc than those of samples that induce low promoter activity, comparatively (Fig. 2). The high promoter activity by P. aeruginosa was readily quenched by the addition of zinc but remained high when CP was also added (Fig. 3B; green, lavender, and lilac). Conversely, the low promoter activity by P. aeruginosa grown in sputum samples with presumably high zinc was not affected greatly by the addition of zinc or CP (Fig. 3B; pink, light pink, and gray). Overall, these data show that addition of recombinant CP to zinc-amended sputum can induce a zinc starvation response dependent on sputum zinc concentration.
Previous studies have shown that CP (60) or a biologically equivalent CP derivative (55, 61, 62) affects P. aeruginosa growth rate and yield in culture to different extents depending on culture conditions. The concentrations of TPEN and CP that we found to induce a zinc starvation response in P. aeruginosa in LB medium (Fig. 1A; Fig. 3A) had minor effects on growth. In this study, we found that both TPEN and CP did not affect growth rate (Fig. 3C) but caused entry into stationary phase at a lower optical density (OD) (Fig. 3D).
Zinc metalloproteases are enriched among P. aeruginosa-secreted zinc-binding proteins.
CP, which is not membrane permeable, may have effects on pathogens beyond limiting zinc availability for growth by binding or competing for metals in the extracellular environment. We performed a UniProt Knowledgebase (UniProtKB) analysis of the P. aeruginosa strain PAO1 proteome, which identified at least 72 zinc-binding proteins (Table 2). Of those 72, 64 were described by gene ontology (GO) molecular function as having catalytic activity (Table 2), which is consistent with the role of zinc as a cofactor. Of those 64 zinc-binding enzymes, 12 were further described as proteases and 5 of those were secreted zinc metalloproteases LasB, LasA, AprA, ImpA, and PepB (Table 2). We performed a second UniProtKB analysis of the P. aeruginosa strain PAO1 proteome that identified at least 34 secreted proteins, of which 6 were proteases and included the 5 aforementioned zinc metalloproteases in addition to protease IV (PA4175). While protease IV is not a zinc metalloprotease, its enzymatic activity is dependent on zinc. Protease IV enzymatic activity is reduced under zinc-limited conditions and in a P. aeruginosa mutant lacking the zinc importer-encoding gene znuA (14), which may be due to lower LasB activity, which is required to activate protease IV (36, 37). These analyses suggest that while 83% of secreted proteases, important virulence factors, are zinc metalloproteases, 100% of secreted proteases are either directly or indirectly zinc dependent. Overall, previously published studies and curated databases suggest that CP (2, 22, 23) and P. aeruginosa-secreted zinc metalloproteases (63) are abundant in the extracellular milieu of the CF mucus environment.
TABLE 2.
Characteristics of zinc-binding proteins in P. aeruginosa as annotated by UniProtKBa
| GO molecular functionb | No. of proteins total | No. of proteins with indicated subcellular localization |
|||
|---|---|---|---|---|---|
| Secreted | Inner membrane | Cytoplasm | Not listed | ||
| Zinc binding | 72 | 8 | 5 | 21 | 38 |
| Catalytic activity: nonpeptidase | 52 | 3 | 16 | 33 | |
| Catalytic activity: peptidase | 12 | 5 | 3 | 1 | 3 |
| Structural binding activity | 2 | 2 | |||
| Molecular function regulator | 2 | 1 | 1 | ||
| ATPase-coupled protein transmembrane transporter activity | 1 | 1 | |||
From protein knowledgebase (UniProtKB) website, https://www.uniprot.org/uniprot/.
Gene ontology (GO).
Zinc chelation inhibits LasB-mediated proteolysis.
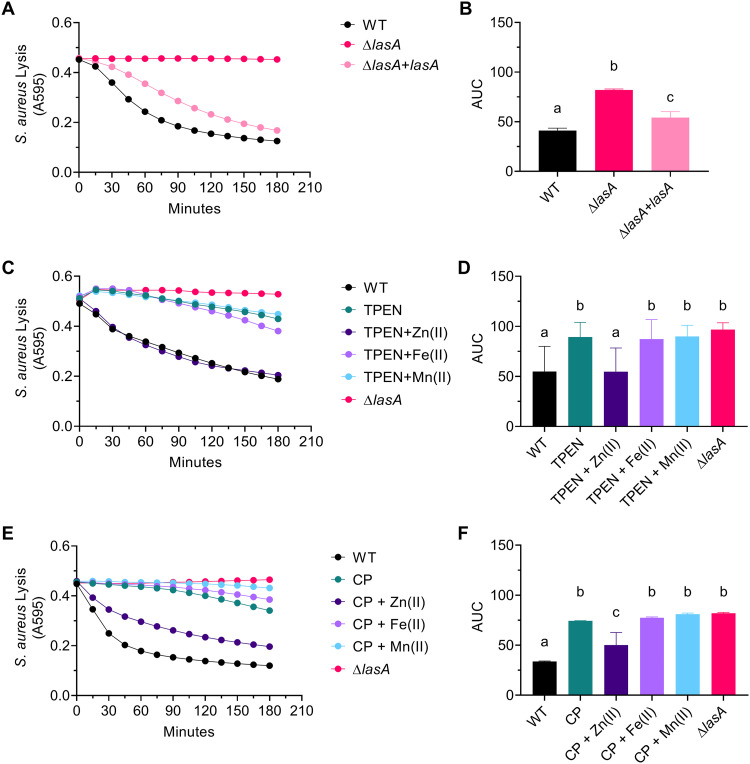
Given the importance of zinc to the activity of extracellular zinc metalloenzymes, we hypothesized that zinc chelation by TPEN and CP would inhibit the activity of secreted zinc metalloproteases. Our initial studies suggested that LasB and LasA accounted for the majority of proteolytic activity by P. aeruginosa strain PAO1 (wild type [WT]) because filtered supernatants from ΔlasAB cultures spotted onto milk plates cleared the milk plates substantially less than did filtered WT supernatants (Fig. 4A, insets i and ii). As a result, this study focuses on the effect of zinc chelation on LasB and LasA activity.
FIG 4.
Zinc chelation inhibits LasB enzymatic activity. (A) Filtered supernatants from 16-h WT, ΔlasA, and ΔlasAB cultures were incubated with 2% azocasein for 15 min. Inset are images showing the ability of WT (i) and ΔlasAB (ii) cell-free supernatants to clear milk plates after 16 h. (B) Filtered supernatants from WT 16 h cultures were left untreated (Control), treated with 50 μM TPEN (TPEN), or treated with 50 μM TPEN and 1 mM ZnSO4 · 7 H2O [TPEN+Zn(II)], (NH4)2Fe(SO4)2 · 6 H2O [TPEN+Fe(II)], or MnCl2 · 4 H2O [TPEN+Mn(II)] for an additional 16 h. Supernatants were then incubated with 2% azocasein for 15 min. The data shown represent the mean ± SD from three independent experiments. (C) Filtered supernatants from WT 16 h cultures were left untreated (Control), treated with 40 μM CP (CP), or treated with 40 μM CP and 1 mM ZnSO4 · 7 H2O [CP+Zn(II)], (NH4)2Fe(SO4)2 · 6 H2O [CP+Fe(II)], or MnCl2 · 4 H2O [CP+Mn(II)] for an additional 16 h. Supernatants were then incubated with 2% azocasein for 15 min. Samples marked with the same letter are not significantly different; samples marked with different letters are significantly different (P < 0.05). An enzyme unit (U) is defined as 1 μmol min−1.
To test the above hypothesis, LasB activity was determined quantitatively using azocasein as a substrate. The azocasein degradation assay was previously described to measure total proteolytic activity (14). However, by comparing the ability of P. aeruginosa WT, ΔlasA, and ΔlasAB supernatants to degrade azocasein, we found that azocasein degradation was LasB-dependent under the conditions tested (Fig. 4A). As a result, we tested the effect of TPEN and CP on LasB activity using the azocasein degradation assay. P. aeruginosa supernatants were filtered and then left untreated, treated with TPEN or CP, or treated with both TPEN or CP and divalent zinc, iron, or manganese. Treatment with TPEN or CP inhibited LasB enzymatic activity, while addition of 1 mM zinc, but not of iron or manganese, in the presence of TPEN or CP restored LasB activity (Fig. 4B and C). Furthermore, treatment of ΔlasAB supernatants with TPEN (Fig. S4A) or CP (Fig. S4B) without or with the addition of excess zinc did not alter azocasein degradation. Therefore, treatment of P. aeruginosa cell-free supernatants with zinc chelators TPEN and CP inhibits LasB-mediated caseinolytic activity.
Zinc chelation inhibits LasA-mediated lysis of S. aureus.
LasA activity was determined by monitoring the decrease in absorbance at 595 nm of a heat-killed S. aureus suspension as previously described (14). Use of P. aeruginosa strain PAO1 (WT), ΔlasA, and ΔlasA+lasA (complemented mutant) supernatants confirmed that LasA is necessary for the lysis of S. aureus and that this assay measures LasA-mediated lysis of S. aureus under the conditions tested (Fig. 5A and B). This assay was then used to measure LasA activity in P. aeruginosa cell-free supernatants left untreated, treated with TPEN or CP, or treated with both TPEN or CP and divalent zinc, iron, or manganese. Treatment of supernatants with TPEN or CP inhibited LasA activity, while treatment with TPEN or CP in the presence of excess zinc (500 μM and 160 μM, respectively), but not iron or manganese, restored LasA activity (Fig. 5C to F). Furthermore, treatment of ΔlasA supernatants with zinc, TPEN, or CP had no effect on lysis of S. aureus, confirming that treatment of supernatants did not have LasA-independent cytotoxic effects on S. aureus (Fig. S6). Therefore, treatment of P. aeruginosa cell-free supernatants with zinc chelators TPEN and CP inhibits LasA-mediated lysis of S. aureus.
FIG 5.
Zinc chelation inhibits LasA enzymatic activity. (A and B) Lysis of heat-killed S. aureus strain SH1000 by cell-free supernatants from WT, ΔlasA, and ΔlasA+lasA (ΔlasA complemented in trans under the control of arabinose-inducible PBAD) 16 h cultures. (C and D) Lysis of heat-killed S. aureus strain SH1000 by WT and ΔlasA cell-free supernatants. WT supernatant was divided and left untreated (WT), treated with 50 μM TPEN (TPEN), or treated with 50 μM TPEN and 500 μM ZnSO4 · 7 H2O [TPEN+Zn(II)], (NH4)2Fe(SO4)2 · 6 H2O [TPEN+Fe(II)], or MnCl2 · 4 H2O [TPEN+Mn(II)]. (E and F) Lysis of heat-killed S. aureus strain SH1000 by WT and ΔlasA cell-free supernatants. WT supernatant was divided and left untreated (WT), treated with 40 μM CP (CP), or treated with 40 μM CP and 160 μM ZnSO4 · 7 H2O [CP+Zn(II)], (NH4)2Fe(SO4)2 · 6 H2O [CP+Fe(II)], or MnCl2 · 4 H2O [CP+Mn(II)]. (A, C, and E) The data represent the mean from three independent experiments. Error bars have been omitted for clarity. (B, D, and F) Quantification of data in panels A, C, and E, respectively, using area under the curve (AUC). Data are the mean ± SD from three independent experiments. Samples marked with the same letter are not significantly different; samples marked with different letters are significantly different (P < 0.05).
DISCUSSION
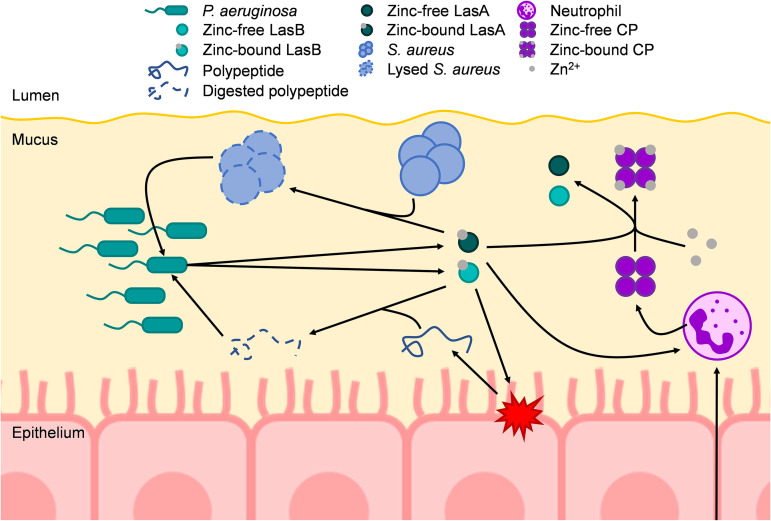
Here, we show that P. aeruginosa strain PAO1 grown in aliquots of expectorated CF sputum exhibits a zinc starvation response despite relatively high concentrations of zinc in the sputum samples. Treatment with recombinant host CP was sufficient to induce a zinc starvation response in P. aeruginosa grown in zinc-amended CF sputum samples from different subjects, demonstrating that CP retains its function in sputum. Furthermore, treatment of P. aeruginosa supernatants with CP inhibited the activity of secreted, extracellular zinc metalloproteases LasB and LasA. The data presented in this study support a model in which CP released from recruited neutrophils sequesters zinc from the environment to induce a zinc starvation response in P. aeruginosa and sequesters zinc from secreted virulence factors, including zinc-dependent metalloproteases LasA and LasB, inhibiting S. aureus lysis, degradation of peptides, and/or nutrient acquisition (Fig. 6).
FIG 6.
Model of the effects of CP-mediated zinc chelation in the CF lung on P. aeruginosa. P. aeruginosa colonizes the mucus in the airways of CF patients to high densities, which in part requires the uptake and utilization of zinc. At high densities, P. aeruginosa secretes a variety of quorum sensing-dependent virulence factors, including zinc metalloproteases such as LasB and LasA. LasB is a protease that can degrade host proteins, such as elastin, as well as peptides. These degraded proteins/peptides can then be taken up and utilized as nutrients by P. aeruginosa. LasA is a protease that lyses S. aureus by cleaving pentaglycine bridges of peptidoglycan. LasA-mediated lysis of S. aureus allows P. aeruginosa to take up nutrients released from lysed S. aureus as well as to outcompete S. aureus in the CF lung. During infection, neutrophils are recruited to sites of infection/inflammation. Neutrophils may then release cellular contents such as CP. CP can then bind bioavailable zinc away from P. aeruginosa, thus reducing the overall abundance of P. aeruginosa, while also inducing a zinc starvation response by P. aeruginosa. Additionally, CP can bind zinc away from both LasB and LasA, thereby inhibiting their proteolytic activity. Furthermore, LasB and LasA activity have been shown to induce neutrophil extracellular traps (NETs). Therefore, CP-mediated inhibition of LasB and LasA activity may lead to less NET formation and, subsequently, less CP release. Arrows indicate a positive interaction.
A variety of strategies have been used to learn about the environment that P. aeruginosa encounters in the CF lung, including analysis of bacteria grown in buffered media supplemented with CF sputum compared to bacteria grown in laboratory media (8, 9) and direct analysis of gene expression by bacteria in expectorated CF sputum (4, 5, 64). While studies have varied in their techniques, transcriptomic analyses have found that genes induced by low intracellular zinc are elevated in sputum samples relative to those in controls (4–9). Our model differs from previous models, as it measures the transcriptional response of P. aeruginosa grown directly in expectorated sputum from a variety of CF patients. Our study also found that P. aeruginosa activates its zinc starvation response in CF sputum on average but revealed differences across samples from different CF donors (Fig. 1B and C; Fig. 3B). These findings taken together underscore the fact that P. aeruginosa growth in laboratory media would not recapitulate the effect of low-zinc conditions in the context of CF. To this end, our CF sputum model is one way to provide a low-zinc environment and allows for investigation of the response of P. aeruginosa across sputum samples from different donors which vary in levels of host factors like CP. This same approach would also enable the investigation of different P. aeruginosa strains in sputum aliquots from a single donor.
CP concentrations in the sputum of CF patients vary but have been reported to reach up to 3 mg/ml (∼120 μM), with the majority of sputum samples measuring between 0.5 mg/ml (∼20 μM) and 1 mg/ml (∼40 μM) (2, 23). Zinc concentrations in CF sputum similarly vary but are generally high relative to those in sputum from non-CF individuals and other biological compartments. Smith et al. (1) found that the zinc concentration of 45 CF sputum samples ranged from 678 μg/liter (∼10 μM) to 1,181 μg/liter (∼18 μM) compared to 103 μg/liter (∼2 μM) to 597 μg/liter (∼9 μM) in 8 non-CF sputum samples. Li et al. (3) reported that the zinc concentration of 118 CF sputum samples ranged from ∼5 μM to ∼145 μM. Gray et al. reported that among 23 CF sputum samples, the median concentration was 135 μg/liter (∼2 μM) with an interquartile range of 54 to 210 μg/liter (∼0.8 to 3 μM) (84). In this study, the zinc concentration of 8 CF sputum samples ranged from 1 μg/g (∼15 μM) to 8 μg/g (∼116 μM) (Fig. 2A). The persistence of P. aeruginosa infections in the CF lung is thus likely influenced by levels of zinc, CP, other host and microbial zinc-binding factors, and its own cellular zinc requirements in this specific environment.
There is mounting evidence that divalent-metal sequestration by CP affects P. aeruginosa. Wakeman et al. (53) demonstrated that CP-mediated genetic responses in P. aeruginosa were reversed upon treatment with zinc in vitro and that P. aeruginosa and CP colocalized at sites of inflammation within a CF lung explant. D’Orazio et al. showed that CP-mediated growth inhibition was enhanced in P. aeruginosa strain ΔznuA, which is a mutant lacking the gene encoding the small zinc-binding protein of the ZnuABC zinc importer resulting in reduced intracellular zinc accumulation (13, 14). Zygiel et al. (61) showed that treatment with CP significantly reduced intracellular iron and manganese in P. aeruginosa but did not significantly affect intracellular zinc, though intracellular zinc trended downward (61). Our data show that CP induces a Zur-regulated zinc starvation response in vitro and in expectorated CF sputum which is repressed upon the addition of excess zinc (Fig. 3A and B). We also observed CP-mediated growth defects in vitro (Fig. 3C) similar to those reported by Zygiel et al. (61) which were previously attributed to ferrous iron chelation by CP. Taken together, the data show that P. aeruginosa and CP colocalize at sites of inflammation in the CF lung and that CP is capable of inducing zinc and/or iron starvation responses depending on test conditions.
Additionally, while Filkins et al. (65) showed that in vitro coculture of P. aeruginosa and S. aureus on CF bronchial epithelial cells reduced the viability of S. aureus, Wakeman et al. (53) showed that zinc chelation by CP promotes P. aeruginosa and S. aureus coculture in in vitro, in vivo, and ex vivo models, in part by downregulating genes encoding anti-staphylococcal factors such as pyocyanin, hydrogen cyanide, and Pseudomonas quinolone signal/2-heptyl-4-hydroxyquinoline N-oxide (PQS/HQNO). Interestingly, treatment of P. aeruginosa with CP did not reduce the expression of lasA, though the functionality of LasA was not tested (53). In this study, we show that CP-mediated zinc chelation inhibits LasA-mediated lysis of S. aureus by P. aeruginosa in vitro (Fig. 5E and F). Therefore, while LasA may be expressed and secreted by P. aeruginosa in the presence of CP, CP may posttranslationally inhibit LasA activity via zinc sequestration. Furthermore, colonization of the CF airways is usually described as a pattern of succession where S. aureus is the predominant colonizer early on in younger patients before being outcompeted by P. aeruginosa in older patients (65). However, Fischer et al. (66) recently showed that P. aeruginosa and S. aureus chronically cocolonize the CF lung. Wakeman et al. also showed that P. aeruginosa, S. aureus, and CP colocalize in CF lung explants (53). Further studies are required to determine if CP modulates protease-dependent and/or protease-independent cocolonization of P. aeruginosa and S. aureus in the CF lung.
Notably, P. aeruginosa strains chronically adapted to the CF lung, including lasR loss-of-function (LasR−) mutants, have a reduced capacity to outcompete S. aureus (67). LasR is a QS regulator that positively regulates the expression and secretion of several virulence factors, including zinc metalloproteases LasB, LasA, AprA, ImpA, PepB, and protease IV (34, 35). However, LasR− strains commonly arise during chronic CF infection and are associated with worse lung function (68–73). While LasR− strains are common in CF infections, virulence factors regulated by LasR such as zinc metalloproteases are still reported to be abundant in CF sputum (63). Recent work by Mould et al. showed that when LasR+ and LasR− strains were cocultured, the LasR+ strain increased production of RhlR-controlled virulence factors by the LasR− strain (74). Interestingly, LasB and LasA are reportedly regulated by both the LasR and RhlR QS regulators (35). Therefore, further investigation is needed to understand how intra- and interspecies interactions within populations colonizing the CF airway affect the secretion and function of virulence factors such as zinc metalloproteases LasB and LasA.
LasB is an abundant protease with broad substrate specificity that is implicated in amino acid liberation and consumption (75). In addition to nutrient acquisition, LasB also plays a role in the ability of P. aeruginosa to invade host epithelial cells (39) and to evade host immune responses via processes such as degrading cytokines (40). Interestingly, degradation of proinflammatory cytokines interleukin 8 (IL-8) and IL-6 by LasB reduces neutrophil recruitment and the overall IL-8 and IL-6 response (40). While LasB-mediated cytokine degradation has been reported to reduce neutrophil recruitment, LasB can also induce neutrophil extracellular traps (NETs) (76, 77). Neutrophils recruited to sites of inflammation can release CP through processes such as NET formation (78), and in this study we show that CP-mediated zinc chelation inhibits the activity of secreted LasB (Fig. 4C). Taken together, there appears to be a complex interplay between LasB, neutrophils, and CP during the course of infection which may contribute to exacerbations in CF. Furthermore, recent work suggests that secreted LasB activates protease IV which then predominantly processes and activates LasA (36, 37). Therefore, CP-mediated inhibition of secreted LasB activity may have downstream effects on the processing and activity of other secreted zinc metalloproteases.
In conclusion, the results of our study show that CP can induce a zinc starvation response in P. aeruginosa in CF sputum as well as chelate zinc to inhibit the activity of virulence-associated zinc metalloproteases. Other zinc-binding host proteins (79) or zincophores from other microbes (31) may also modulate the activity of P. aeruginosa zinc metalloproteases. Future studies will focus on how competition for zinc in a zinc-limited or zinc-chelating environment such as CF mucus shapes polymicrobial infections and patient outcomes, particularly considering the observed variability in zinc concentration and availability across CF patients.
MATERIALS AND METHODS
Strains and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. P. aeruginosa and Escherichia coli strains were maintained on lysogeny broth (LB) (1% tryptone, 0.5% yeast extract, 0.5% NaCl) with 1.5% agar and routinely grown in LB on a roller drum at 37°C LB. P. aeruginosa plasmid strains were maintained by supplementing media with 300 μg/ml carbenicillin or 60 μg/ml gentamicin. E. coli plasmid strains were maintained by supplementing media with 100 μg/ml carbenicillin. S. aureus SH1000 was maintained on Trypticase soy with 1.5% agar (TSA) or grown in Trypticase soy broth (TSB) on a roller drum at 37°C. Saccharomyces cerevisiae strains for cloning were maintained on yeast-peptone-dextrose (YPD) medium with 2% agar.
Construction of plasmids.
Primers used for plasmid construction are listed in Table S2. All plasmids were sequenced at the Molecular Biology Core at the Geisel School of Medicine at Dartmouth. Plasmid GH121_PrpmJ2-lacZ (DH3229) was constructed using a S. cerevisiae recombination technique as previously described (80). Plasmid GH121_PpqsA-lacZ served as the vector backbone for this construct. GH121_PrpmJ2-lacZ was purified from yeast using Zymoprep Yeast Plasmid Miniprep II according to the manufacturer’s protocol and transformed into electrocompetent E. coli strain S17 by electroporation. The plasmid was introduced into P. aeruginosa by conjugation, and recombinants were obtained using sucrose counterselection and genotype screening by PCR.
Complementation plasmid pMQ70_lasA was generated using the NEBuilder HiFi DNA assembly cloning kit (New England BioLabs). P. aeruginosa strain PAO1V ΔlasA was complemented in trans by inserting a functional copy of lasA amplified from PAO1V genomic DNA into plasmid pMQ70 under the control of the arabinose-inducible BAD promoter generating plasmid pMQ70_lasA. Plasmid pMQ70_lasA was transformed into ΔlasA by electroporation.
Cystic fibrosis (CF) sputum collection.
Sputum samples were collected in accordance with protocols approved by the Committee for the Protection of Human Subjects at Dartmouth. Expectorated sputum samples used in this study were collected from adult subjects with CF during a routine office visit or upon admission for treatment of a disease exacerbation. Sputum samples were frozen upon collection and stored at −80°C until use.
Beta-galactosidase assay.
P. aeruginosa cells with a promoter fusion to lacZ integrated at the att locus were grown in 5 ml cultures of LB at 37°C for 16 h. Overnight cultures were diluted 1:50 in 50 ml of culture medium (LB or M63) and then grown to an optical density at 600 nm (OD600) of 0.5. The cells were then centrifuged at 4,500 × g for 10 min, resuspended in culture medium, centrifuged at 10,000 × g for 2 min, and then resuspended in 500 μl of culture medium. Ten μl of cell suspension were added per 100 μl of culture medium or sputum sample in a 2-ml microcentrifuge tube. Samples were incubated at 37°C with shaking for 3 h. Beta-galactosidase (β-Gal) activity was measured as described by Miller (81) using 50 μl of sample.
RNA isolation and NanoString analysis.
Unamended sputum or sputum amended with 1 mM ZnSO4 · 7 H2O or (NH4)2Fe(SO4)2 · 6 H2O (100 μl) was added to 2-ml microcentrifuge tubes. P. aeruginosa strain PAO1 was grown in 5 ml cultures of LB at 37°C for 16 h. Overnight cultures were diluted 1:50 in 50 ml M63 minimal medium with 0.2% glucose and then grown to an OD600 of 0.5. The cells were then centrifuged at 4,500 × g for 10 min, washed with water, centrifuged, and then resuspended in 500 μl of water. Ten μl of cell suspension were added per 100 μl M63 minimal medium with 0.2% glucose (control) or sputum sample in a 2-ml microcentrifuge tube. Samples were then incubated at 37°C with shaking for 3 h. TRIzol (900 μl) was added to 100 μl of sputum containing 10 μl of PAO1 cell suspension. Samples were stored overnight. RNA was prepared following DirectZol kit instructions and eluted in 50 μl of water.
For NanoString, 5 μl of a 1:10 dilution of RNA was used. Diluted RNA was applied to the codeset PaV4 and processed as previously reported (51). Counts were normalized to the geometric mean of spiked-in technical controls. Normalized counts were used for Z-score calculations and heatmap construction.
Measurement of zinc in sputum samples.
Sputum samples for zinc analysis were stored at −80°C until processed. Sputum zinc was quantified by inductively coupled plasma-mass spectrometry (ICP-MS) following nitric acid digestion of organic material according to the method of Heck et al. and is expressed as micrograms of zinc per gram of sputum (82). ICP-MS was performed by the Dartmouth Trace Element Analysis (TEA) Core.
Expression and purification of recombinant calprotectin.
Plasmid S100A8/A9 was obtained from Futami et al. (54), and recombinant calprotectin (CP) was expressed and purified as previously described with minor modification. Plasmid S100A8/A9 was first confirmed by Sanger sequencing and then transformed into E. coli T7 Express cells. Transformed T7 Express cells were then grown in LB containing 100 μg/ml carbenicillin at 37°C with shaking and induced at about an OD600 of 0.5 with 0.5 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) for 3 h. Cultures were centrifuged at 13,260 × g for 10 min at 4°C. Supernatant was discarded. Cell pellets were resuspended in 30 ml of wash solution (150 mM NaCl), transferred to a 50-ml conical tube, and then centrifuged at 3,210 × g for 10 min at 4°C. Supernatant was discarded. Pellets were weighed and then stored at −20°C.
Cell pellets were resuspended in 85 ml of lysis buffer (50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 5 mM MgCl2) supplemented with Benzonase-HC to control viscosity of the sample. Cells were then lysed using the microfluidizer with 3 passages at 18,000 lb/in2. Final volume was about 100 ml. Fifteen percent polyethylenimine (PEI) was added dropwise to a final concentration of 0.7% to precipitate nucleic acids (about 5 ml). Samples were then centrifuged at 23,280 × g for 10 min at 4°C. Pellet containing intact cells and precipitated nucleic acids was discarded. NH4SO4 (61.27 g) was added slowly to clarified supernatant (about 115 ml) while stirring at 4°C until a saturation of 80%. The sample became gradually turbid. Sample was stirred for an additional 30 min after complete saturation. Sample was then centrifuged at 23,280 × g for 10 min at 4°C. Supernatant was discarded and the pellet was dissolved in about 30 ml of solubilization buffer (50 mM Tris-HCl [pH 7.5], 30 mM dithiothreitol [DTT]) and incubated for 1 h at 37°C. Dissolved pellet was transferred to dialysis cassettes and dialyzed overnight in 50 mM sodium phosphate (pH 6.0) at 4°C using 3.5 kDa cutoff dialysis cassettes to change buffer. Sample was then centrifuged at 23,280 × g for 10 min at 4°C to remove any pellet.
CP was then purified using a HiTrap SP column (stored in 20% ethanol). The column was washed with 5 column volumes (CV) of H2O at about 5 ml/min. The column was then washed with 5 CV of 100% SP Sepharose HP buffer B (50 mM sodium phosphate [pH 6.0], 1 mM DTT, 1 M NaCl; filtered/degassed) at about 5 ml/min. The column was equilibrated with 10 CV of SP Sepharose HP buffer A (50 mM sodium phosphate [pH 6.0], 1 mM DTT; filtered/degassed) at about 5 ml/min. A superloop was assembled with the appropriate volume for sample application. Sample was then loaded in the column using the superloop at 2.5 ml/min. The column was then washed with 10 CV of SP Sepharose HP buffer A at about 5 ml/min. The column was then washed with a step gradient of SP Sepharose HP buffer B: 5 CV of 5% SP Sepharose HP buffer B, 10 CV at 30% SP Sepharose HP buffer B, and 5 CV at 100% SP Sepharose HP buffer B at about 5 ml/min.
Fractions were analyzed using SDS-PAGE (15% gel) and the appropriate fractions were then pooled.
CP was then purified using a HiLoad 26/600 Sephadex S75 and CP buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM DTT; filtered/degassed). Sample (about 13 ml) was loaded in a 50 ml superloop. Sample was then run on the HiLoad 26/600 Superdex 75p, a program composed of 2 CV equilibration, injection of 12 ml of sample, and elution with 1.2 CV at 2.6 ml/min. Flow rate is 2.6 ml/min and collection of 7 ml/tube. Tubes corresponding to three different fractions were pooled to make fractions F1_I, F2_I, and F3_I. All other tubes containing calprotectin from both HiTrap runs were concentrated using YM-10 Amicon centrifugal filters and reloaded in the HiLoad 26/600 superdex 75 as before. Tubes corresponding to three different fractions were pooled to make fractions F1_II, F2_II, and F3_II. Samples from all six fractions were analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (4 to 12% gel). Fractions F1_I and F1_II, F2_I and F2_II, and F3_I and F3_II were combined to make fractions F1, F2, and F3, respectively. Fractions were concentrated with YM-10 Amicon centrifugal filters. The final concentrations of the fractions were determined using a Bradford protein assay.
Growth assays.
Growth of P. aeruginosa in the presence of TPEN or CP was measured as previously described (61) with modification. P. aeruginosa was grown for 16 h in 5 ml LB with rolling at 37°C. Samples were then centrifuged and washed once in LB before resuspending the pellet in LB. Washed samples were adjusted to an OD600 of 1.0 in LB. Samples were then diluted 1:10 in LB, LB plus 50 μM TPEN, or LB plus 40 μM CP to an OD600 of 0.1. In triplicate, 100-μl aliquots of samples or media alone (negative controls) were added to wells of a 96-well flat-bottom plate containing 100 μl of LB, LB plus 50 μM TPEN, or LB plus 40 μM CP. Plates were incubated statically at 37°C in a plate reader for 16 h, during which time the OD600 was measured every 15 min following a brief shake.
Protease assays.
P. aeruginosa culture supernatants were used for protease assays. Five milliliters of overnight cultures in LB was centrifuged at 4,500 × g for 10 min. Supernatants were then filter sterilized using a 0.22-μm syringe filter. For TPEN experiments, undiluted supernatants were used. For CP experiments, stored aliquots of CP were first diluted to 3 mg/ml in CP buffer without DTT (50 mM Tris-HCl [pH 7.5], 150 mM NaCl). Then, 1 part 3 mg/ml CP was added to 2 parts supernatant for a final concentration of 1 mg/ml.
Caseinolytic activity was determined qualitatively by spotting P. aeruginosa supernatants onto 1% milk plates or quantitatively using azocasein as a substrate as previously described (14) with modification. In brief, P. aeruginosa culture supernatants were treated overnight (16 h) with 50 μM TPEN or an equivalent volume of 100% ethanol (EtOH), 1 mg/ml (∼40 μM) CP or an equivalent volume of CP buffer without DTT, and/or 1 mM ZnSO4 · 7 H2O or an equivalent volume of diH2O. Treatment of WT supernatants with 50 μM to 2 mM ZnSO4 · 7 H2O was found not to affect LasB activity (Fig. S4C). The supernatants were then incubated at 37°C overnight (16 h). Supernatants (25 μl) were mixed with 150 μl 2% azocasein in 10 mM Tris-HCl, 8 mM CaCl2 (pH 7.4). Samples were incubated at 37°C for 15 min. To each sample, 228 μl of 10% TCA was added and the sample was vortexed and then incubated at room temperature for 15 min. Samples were then centrifuged for 10 min at 10,000 × g. Cleared supernatants (100 μl) were added to wells of a 96-well flat-bottom polystyrene plate containing 200 μl of 1 M NaOH. Absorbance was read at 440 nm.
Staphylolytic activity was determined by monitoring the decrease in absorbance at 595 nm of a heat-killed S. aureus suspension as previously described (14) with modification. S. aureus strain SH1000 (83) was cultured in TSB overnight (16 h) at 37°C with rolling. Cultures were centrifuged at 4,500 × g for 10 min, resuspended in 20 mM Tris-HCl (pH 8.8) to a final OD600 of 1.0, and then killed by heating at 100°C for 30 min. Heat-killed S. aureus suspensions were cooled to room temperature before use. P. aeruginosa culture supernatants were treated overnight (16 h) with 50 μM TPEN or an equivalent volume of 100% EtOH, 1 mg/ml (∼40 μM) CP or an equivalent volume of CP buffer without DTT, and/or 160 to 500 μM ZnSO4 · 7 H2O or an equivalent volume of diH2O. Because increasing concentrations of zinc were previously reported to inhibit LasA activity (46), an appropriate concentration of zinc to use in add-back experiments was determined experimentally. For undiluted WT supernatants, the addition of 500 μM zinc had no effect on LasA activity, while increasing concentrations of zinc inhibited LasA-mediated lysis of S. aureus (Fig. S5A and B). Therefore, we used 500 μM zinc for TPEN-based experiments. For CP buffer-diluted WT supernatants, the addition of 50 μM zinc had no effect on LasA activity, while increasing concentrations of zinc inhibited LasA-mediated lysis of S. aureus (Fig. S5C and D). To ensure that zinc would be in excess in CP-based experiments, we used 160 μM zinc, which was four times the concentration of CP but still less than 250 μM zinc, which was the concentration tested that started to inhibit LasA activity independent of CP. P. aeruginosa supernatants (20 μl) were added to 180 μl of heat-killed S. aureus in wells of a 96-well flat-bottom polystyrene plate. Staphylolytic activity was determined by monitoring the change in absorbance at 595 nm every 15 min for 3 h using a plate reader. The plate was shaken before each read.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 8 and results were expressed as the mean values plus or minus standard deviations. Unless otherwise noted, one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test was performed to determine statistical significance of the data. See the figure legends for other specific statistical tests used.
ACKNOWLEDGMENTS
We thank Pat Occhipinti for generating the promoter-lacZ fusion reporter strain used in this study, Andreia Verissimo of the Institute for Biomolecular Targeting (bioMT) Molecular Tools Core (MTC) for her assistance in expressing and purifying recombinant calprotectin used in this study, and Nick Jacobs and Georgia Doing for their insightful comments and feedback during manuscript preparation.
Research reported in this publication was supported by the Cystic Fibrosis Foundation (CFF) grants GIFFOR1610 and GIFFOR17Y5 awarded to A.H.G. and CFF grant HOGAN19G0 awarded to D.A.H. Support for the project was also provided by bioMT through NIH NIGMS grant P20 GM113132, the Dartmouth Cystic Fibrosis Research Center (DartCF) through NIH NIDDK grant P30 DK117469, the CFF Research Development Program (CF RDP) through CFF grant STANTO19R0, and the Dartmouth Trace Element Analysis (TEA) Core through NIH NIEHS P42 ES007373.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources.
Footnotes
Supplemental material is available online only.
Contributor Information
Deborah A. Hogan, Email: dhogan@dartmouth.edu.
Laurie E. Comstock, Brigham and Women’s Hospital/Harvard Medical School
REFERENCES
- 1.Smith DJ, Anderson GJ, Bell SC, Reid DW. 2014. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J Cyst Fibros 13:289–295. 10.1016/j.jcf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Gray RD, Duncan A, Noble D, Imrie M, O'Reilly DS, Innes JA, Porteous DJ, Greening AP, Boyd AC. 2010. Sputum trace metals are biomarkers of inflammatory and suppurative lung disease. Chest 137:635–641. 10.1378/chest.09-1047. [DOI] [PubMed] [Google Scholar]
- 3.Li K, Gifford AH, Hampton TH, O’Toole GA. 2019. Availability of zinc impacts interactions between Streptococcus sanguinis and Pseudomonas aeruginosa in coculture. J Bacteriol 202 10.1128/JB.00618-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornforth DM, Dees JL, Ibberson CB, Huse HK, Mathiesen IH, Kirketerp-Moller K, Wolcott RD, Rumbaugh KP, Bjarnsholt T, Whiteley M. 2018. Pseudomonas aeruginosa transcriptome during human infection. Proc Natl Acad Sci U S A 115:E5125–E5134. 10.1073/pnas.1717525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornforth DM, Diggle FL, Melvin JA, Bomberger JM, Whiteley M. 2020. Quantitative framework for model evaluation in microbiology research using Pseudomonas aeruginosa and cystic fibrosis infection as a test case. mBio 11 10.1128/mBio.03042-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mastropasqua MC, Lamont I, Martin LW, Reid DW, D'Orazio M, Battistoni A. 2018. Efficient zinc uptake is critical for the ability of Pseudomonas aeruginosa to express virulence traits and colonize the human lung. J Trace Elem Med Biol 48:74–80. 10.1016/j.jtemb.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Tan J, Doing G, Lewis KA, Price CE, Chen KM, Cady KC, Perchuk B, Laub MT, Hogan DA, Greene CS. 2017. Unsupervised extraction of stable expression signatures from public compendia with an ensemble of neural networks. Cell Syst 5:63–71.e6. 10.1016/j.cels.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer KL, Mashburn LM, Singh PK, Whiteley M. 2005. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol 187:5267–5277. 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner KH, Wessel AK, Palmer GC, Murray JL, Whiteley M. 2015. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc Natl Acad Sci U S A 112:4110–4115. 10.1073/pnas.1419677112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison ML, Farrow JM, Farrow JM, Parrish W, Danell AS, Pesci EC. 2013. The transcriptional regulator Np20 is the zinc uptake regulator in Pseudomonas aeruginosa. PLoS One 8:e75389. 10.1371/journal.pone.0075389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikhaylina A, Ksibe AZ, Scanlan DJ, Blindauer CA. 2018. Bacterial zinc uptake regulator proteins and their regulons. Biochem Soc Trans 46:983–1001. 10.1042/BST20170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novichkov PS, Brettin TS, Novichkova ES, Dehal PS, Arkin AP, Dubchak I, Rodionov DA. 2012. RegPrecise web services interface: programmatic access to the transcriptional regulatory interactions in bacteria reconstructed by comparative genomics. Nucleic Acids Res 40:W604–W608. 10.1093/nar/gks562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pederick VG, Eijkelkamp BA, Begg SL, Ween MP, McAllister LJ, Paton JC, McDevitt CA. 2015. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci Rep 5:13139. 10.1038/srep13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Orazio M, Mastropasqua MC, Cerasi M, Pacello F, Consalvo A, Chirullo B, Mortensen B, Skaar EP, Ciavardelli D, Pasquali P, Battistoni A. 2015. The capability of Pseudomonas aeruginosa to recruit zinc under conditions of limited metal availability is affected by inactivation of the ZnuABC transporter. Metallomics 7:1023–1035. 10.1039/C5MT00017C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastropasqua MC, D'Orazio M, Cerasi M, Pacello F, Gismondi A, Canini A, Canuti L, Consalvo A, Ciavardelli D, Chirullo B, Pasquali P, Battistoni A. 2017. Growth of Pseudomonas aeruginosa in zinc poor environments is promoted by a nicotianamine-related metallophore. Mol Microbiol 106:543–561. 10.1111/mmi.13834. [DOI] [PubMed] [Google Scholar]
- 16.Lhospice S, Gomez NO, Ouerdane L, Brutesco C, Ghssein G, Hajjar C, Liratni A, Wang S, Richaud P, Bleves S, Ball G, Borezée-Durant E, Lobinski R, Pignol D, Arnoux P, Voulhoux R. 2017. Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline. Sci Rep 7:17132. 10.1038/s41598-017-16765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makarova KS, Ponomarev VA, Koonin EV. 2001. Two C or not two C: recurrent disruption of Zn-ribbons, gene duplication, lineage-specific gene loss, and horizontal gene transfer in evolution of bacterial ribosomal proteins. Genome Biol 2:RESEARCH 0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaby-Haas CE, Furman R, Rodionov DA, Artsimovitch I, de Crecy-Lagard V. 2011. Role of a Zn-independent DksA in Zn homeostasis and stringent response. Mol Microbiol 79:700–715. 10.1111/j.1365-2958.2010.07475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabriel SE, Helmann JD. 2009. Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J Bacteriol 191:6116–6122. 10.1128/JB.00802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barthe C, Figarella C, Carrere J, Guy-Crotte O. 1991. Identification of 'cystic fibrosis protein' as a complex of two calcium-binding proteins present in human cells of myeloid origin. Biochim Biophys Acta 1096:175–177. 10.1016/0925-4439(91)90057-g. [DOI] [PubMed] [Google Scholar]
- 22.Gray RD, MacGregor G, Noble D, Imrie M, Dewar M, Boyd AC, Innes JA, Porteous DJ, Greening AP. 2008. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med 178:444–452. 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. 2010. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros 9:193–198. 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 24.MacGregor G, Gray RD, Hilliard TN, Imrie M, Boyd AC, Alton EW, Bush A, Davies JC, Innes JA, Porteous DJ, Greening AP. 2008. Biomarkers for cystic fibrosis lung disease: application of SELDI-TOF mass spectrometry to BAL fluid. J Cyst Fibros 7:352–358. 10.1016/j.jcf.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. 1991. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem 266:7706–7713. 10.1016/S0021-9258(20)89506-4. [DOI] [PubMed] [Google Scholar]
- 26.Gebhardt C, Németh J, Angel P, Hess J. 2006. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 72:1622–1631. 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Strupat K, Rogniaux H, Van Dorsselaer A, Roth J, Vogl T. 2000. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J Am Soc Mass Spectrom 11:780–788. 10.1016/S1044-0305(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 28.Korndörfer IP, Brueckner F, Skerra A. 2007. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol 370:887–898. 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 29.Zygiel EM, Nolan EM. 2018. Transition metal sequestration by the host-defense protein calprotectin. Annu Rev Biochem 87:621–643. 10.1146/annurev-biochem-062917-012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, Pasquali P, Lane TE, Chazin WJ, Vogl T, Roth J, Skaar EP, Raffatellu M. 2012. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11:227–239. 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grim KP, San Francisco B, Radin JN, Brazel EB, Kelliher JL, Parraga SP, Kim PC, McDevitt CA, Kehl-Fie TE. 2017. The metallophore staphylopine enables Staphylococcus aureus to compete with the host for zinc and overcome nutritional immunity. mBio 8 10.1128/mBio.01281-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaksen B, Fagerhol MK. 2001. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol 54:289–292. 10.1136/mp.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. 10.1128/jb.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nouwens AS, Beatson SA, Whitchurch CB, Walsh BJ, Schweizer HP, Mattick JS, Cordwell SJ. 2003. Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology (Reading) 149:1311–1322. 10.1099/mic.0.25967-0. [DOI] [PubMed] [Google Scholar]
- 36.Li XH, Lee JH. 2019. Quorum sensing-dependent post-secretional activation of extracellular proteases in Pseudomonas aeruginosa. J Biol Chem 294:19635–19644. 10.1074/jbc.RA119.011047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh J, Li XH, Kim SK, Lee JH. 2017. Post-secretional activation of protease IV by quorum sensing in Pseudomonas aeruginosa. Sci Rep 7:4416. 10.1038/s41598-017-03733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruce MC, Poncz L, Klinger JD, Stern RC, Tomashefski JF, Dearborn DG. 1985. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis 132:529–535. 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- 39.Cowell BA, Twining SS, Hobden JA, Kwong MSF, Fleiszig SMJ. 2003. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology (Reading) 149:2291–2299. 10.1099/mic.0.26280-0. [DOI] [PubMed] [Google Scholar]
- 40.LaFayette SL, Houle D, Beaudoin T, Wojewodka G, Radzioch D, Hoffman LR, Burns JL, Dandekar AA, Smalley NE, Chandler JR, Zlosnik JE, Speert DP, Bernier J, Matouk E, Brochiero E, Rousseau S, Nguyen D. 2015. Cystic fibrosis–adapted Pseudomonas aeruginosa quorum sensing lasR mutants cause hyperinflammatory responses. Sci Adv 1:e1500199. 10.1126/sciadv.1500199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bardoel BW, Hartsink D, Vughs MM, de Haas CJ, van Strijp JA, van Kessel KP. 2012. Identification of an immunomodulating metalloprotease of Pseudomonas aeruginosa (IMPa). Cell Microbiol 14:902–913. 10.1111/j.1462-5822.2012.01765.x. [DOI] [PubMed] [Google Scholar]
- 42.Laarman AJ, Bardoel BW, Ruyken M, Fernie J, Milder FJ, van Strijp JA, Rooijakkers SH. 2012. Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J Immunol 188:386–393. 10.4049/jimmunol.1102162. [DOI] [PubMed] [Google Scholar]
- 43.Kessler E, Safrin M, Olson JC, Ohman DE. 1993. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508. 10.1016/S0021-9258(18)53203-8. [DOI] [PubMed] [Google Scholar]
- 44.Spencer J, Murphy LM, Conners R, Sessions RB, Gamblin SJ. 2010. Crystal structure of the LasA virulence factor from Pseudomonas aeruginosa: substrate specificity and mechanism of M23 metallopeptidases. J Mol Biol 396:908–923. 10.1016/j.jmb.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Cahan R, Axelrad I, Safrin M, Ohman DE, Kessler E. 2001. A secreted aminopeptidase of Pseudomonas aeruginosa: identification, primary structure, and relationship to other aminopeptidases. J Biol Chem 276:43645–43652. 10.1074/jbc.M106950200. [DOI] [PubMed] [Google Scholar]
- 46.Kessler E, Safrin M, Abrams WR, Rosenbloom J, Ohman DE. 1997. Inhibitors and specificity of Pseudomonas aeruginosa LasA. J Biol Chem 272:9884–9889. 10.1074/jbc.272.15.9884. [DOI] [PubMed] [Google Scholar]
- 47.Bremer H, Dennis PP. 2008. Modulation of chemical composition and other parameters of the cell at different exponential growth rates. EcoSal Plus 3 10.1128/ecosal.5.2.3. [DOI] [PubMed] [Google Scholar]
- 48.Gilston BA, Wang S, Marcus MD, Canalizo-Hernández MA, Swindell EP, Xue Y, Mondragón A, O'Halloran TV. 2014. Structural and mechanistic basis of zinc regulation across the E. coli Zur regulon. PLoS Biol 12:e1001987. 10.1371/journal.pbio.1001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertuchi FR, Papai R, Ujevic M, Gaubeur I, Cerchiaro G. 2014. General chelating action of copper, zinc and iron in mammalian cells. Analytical Methods 6:8488–8493. 10.1039/C4AY01912A. [DOI] [Google Scholar]
- 50.Gifford AH, Willger SD, Dolben EL, Moulton LA, Dorman DB, Bean H, Hill JE, Hampton TH, Ashare A, Hogan DA. 2016. Use of a multiplex transcript method for analysis of Pseudomonas aeruginosa gene expression profiles in the cystic fibrosis lung. Infect Immun 84:2995–3006. 10.1128/IAI.00437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grahl N, Dolben EL, Filkins LM, Crocker AW, Willger SD, Morrison HG, Sogin ML, Ashare A, Gifford AH, Jacobs NJ, Schwartzman JD, Hogan DA. 2018. Profiling of bacterial and fungal microbial communities in cystic fibrosis sputum using RNA. mSphere 3:e00292-18. 10.1128/mSphere.00292-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson CE, Huang W, Brewer LK, Nguyen AT, Kane MA, Wilks A, Oglesby-Sherrouse AG. 2019. Proteomic analysis of the Pseudomonas aeruginosa iron starvation response reveals PrrF small regulatory RNA-dependent iron regulation of twitching motility, amino acid metabolism, and zinc homeostasis proteins. J Bacteriol 201 10.1128/JB.00754-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakeman CA, Moore JL, Noto MJ, Zhang Y, Singleton MD, Prentice BM, Gilston BA, Doster RS, Gaddy JA, Chazin WJ, Caprioli RM, Skaar EP. 2016. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat Commun 7:11951. 10.1038/ncomms11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Futami J, Atago Y, Azuma A, Putranto EW, Kinoshita R, Murata H, Sakaguchi M. 2016. An efficient method for the preparation of preferentially heterodimerized recombinant S100A8/A9 coexpressed in Escherichia coli. Biochem Biophys Rep 6:94–100. 10.1016/j.bbrep.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakashige TG, Zhang B, Krebs C, Nolan EM. 2015. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol 11:765–771. 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephan JR, Nolan EM. 2016. Calcium-induced tetramerization and zinc chelation shield human calprotectin from degradation by host and bacterial extracellular proteases. Chem Sci 7:1962–1975. 10.1039/C5SC03287C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen T, Nolan EM. 2020. Metal sequestration and antimicrobial activity of human calprotectin are pH-dependent. Biochemistry 59:2468–2478. 10.1021/acs.biochem.0c00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoskin TS, Crowther JM, Cheung J, Epton MJ, Sly PD, Elder PA, Dobson RCJ, Kettle AJ, Dickerhof N. 2019. Oxidative cross-linking of calprotectin occurs in vivo, altering its structure and susceptibility to proteolysis. Redox Biol 24:101202. 10.1016/j.redox.2019.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephan JR, Yu F, Costello RM, Bleier BS, Nolan EM. 2018. Oxidative post-translational modifications accelerate proteolytic degradation of calprotectin. J Am Chem Soc 140:17444–17455. 10.1021/jacs.8b06354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. 2013. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A 110:3841–3846. 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zygiel EM, Nelson CE, Brewer LK, Oglesby-Sherrouse AG, Nolan EM. 2019. The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J Biol Chem 294:3549–3562. 10.1074/jbc.RA118.006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brophy MB, Hayden JA, Nolan EM. 2012. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc 134:18089–18100. 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaffar-Bandjee MC, Lazdunski A, Bally M, Carrère J, Chazalette JP, Galabert C. 1995. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J Clin Microbiol 33:924–929. 10.1128/JCM.33.4.924-929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossi E, Falcone M, Molin S, Johansen HK. 2018. High-resolution in situ transcriptomics of Pseudomonas aeruginosa unveils genotype independent patho-phenotypes in cystic fibrosis lungs. Nat Commun 9:3459. 10.1038/s41467-018-05944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filkins LM, Graber JA, Olson DG, Dolben EL, Lynd LR, Bhuju S, O'Toole GA. 2015. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a cystic fibrosis model. J Bacteriol 197:2252–2264. 10.1128/JB.00059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer AJ, Singh SB, LaMarche MM, Maakestad LJ, Kienenberger ZE, Peña TA, Stoltz DA, Limoli DH. 2021. Sustained coinfections with Staphylococcus aureus and Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med 203:328–338. 10.1164/rccm.202004-1322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baldan R, Cigana C, Testa F, Bianconi I, De Simone M, Pellin D, Di Serio C, Bragonzi A, Cirillo DM. 2014. Adaptation of Pseudomonas aeruginosa in cystic fibrosis airways influences virulence of Staphylococcus aureus in vitro and murine models of co-infection. PLoS One 9:e89614. 10.1371/journal.pone.0089614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marvig RL, Sommer LM, Molin S, Johansen HK. 2015. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet 47:57–64. 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 69.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hansen SK, Rau MH, Johansen HK, Ciofu O, Jelsbak L, Yang L, Folkesson A, Jarmer H, Aanæs K, von Buchwald C, Høiby N, Molin S. 2012. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J 6:31–45. 10.1038/ismej.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kohler T, Buckling A, van Delden C. 2009. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc Natl Acad Sci U S A 106:6339–6344. 10.1073/pnas.0811741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffman LR, Kulasekara HD, Emerson J, Houston LS, Burns JL, Ramsey BW, Miller SI. 2009. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros 8:66–70. 10.1016/j.jcf.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiricny N, Molin S, Foster K, Diggle SP, Scanlan PD, Ghoul M, Johansen HK, Santorelli LA, Popat R, West SA, Griffin AS. 2014. Loss of social behaviours in populations of Pseudomonas aeruginosa infecting lungs of patients with cystic fibrosis. PLoS One 9:e83124. 10.1371/journal.pone.0083124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mould DL, Botelho NJ, Hogan DA. 2020. Intraspecies signaling between common variants of Pseudomonas aeruginosa increases production of quorum-sensing-controlled virulence factors. mBio 11 10.1128/mBio.01865-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flynn JM, Phan C, Hunter RC. 2017. Genome-wide survey of Pseudomonas aeruginosa PA14 reveals a role for the glyoxylate pathway and extracellular proteases in the utilization of mucin. Infect Immun 85 10.1128/IAI.00182-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skopelja S, Hamilton BJ, Jones JD, Yang ML, Mamula M, Ashare A, Gifford AH, Rigby WF. 2016. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight 1:e88912. 10.1172/jci.insight.88912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skopelja-Gardner S, Theprungsirikul J, Lewis KA, Hammond JH, Carlson KM, Hazlett HF, Nymon A, Nguyen D, Berwin BL, Hogan DA, Rigby WFC. 2019. Regulation of Pseudomonas aeruginosa-mediated neutrophil extracellular traps. Front Immunol 10:1670. 10.3389/fimmu.2019.01670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5:e1000639. 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cunden LS, Gaillard A, Nolan EM. 2016. Calcium ions tune the zinc-sequestering properties and antimicrobial activity of human S100A12. Chem Sci 7:1338–1348. 10.1039/C5SC03655K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O’Toole GA. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol 72:5027–5036. 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller JH. 1992. A short course in bacterial genetics. Cold Spring Harbor Press. [Google Scholar]
- 82.Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, Duell EJ. 2009. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect 117:1718–1723. 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184:5457–5467. 10.1128/jb.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gray RD, Duncan A, Noble D, Imrie M, O'Reilly DS, Innes JA, Porteous DJ, Greening AP, Boyd AC. 2010. Sputum trace metals are biomarkers of inflammatory and suppurative lung disease. Chest 137:635–641. 10.1378/chest.09-1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S6 and Tables S1 and S2. Download JB.00100-21-s0001.pdf, PDF file, 690 KB (689.8KB, pdf)