ABSTRACT
Sepsis causes half of acute kidney injuries in the intensive care unit (ICU). ICU patients may need continuous renal replacement therapy (CRRT), which will affect their antimicrobial exposure. We aimed to build a cefepime population pharmacokinetic (PK) model in CRRT ICU patients and perform simulations to assess target attainment. Patients who were ≥18 years old, were admitted to the ICU, and received cefepime 2 g every 8 h as a 4-h infusion while on CRRT were enrolled prospectively. Samples were collected from the predialyzer ports, postdialyzer ports, and effluent fluid at 1, 2, 3, 4, and 8 h after the first dose and at steady state. Age, sex, weight, urine output, and CRRT parameters were recorded. Pmetrics was used for population PK and simulations. The target exposure was 100% of the dosing interval during which the free beta-lactam concentration is above the MIC (fT>MIC). Ten patients were included; their mean age was 53 years, and mean weight was 119 kg. Seventy percent were males. Cefepime was described by a five-compartment model. The downtime was applied to the CRRT flow rates, which were used to describe the rates of transfer between the compartments. At MICs of ≤8 mg/liter, intermittent infusion of 2 g cefepime every 8 h achieved good target attainment both early in therapy and at steady state. Only extended- and continuous-infusion regimens achieved good target attainment at MICs of 16 mg/liter. In conclusion, 2 g cefepime infused over 30 min followed by extended infusion of 2 g every 8 h achieved good target attainment at MICs of ≤16 mg/liter with different CRRT flow rates and may be considered in resistant bacterial infections.
KEYWORDS: CRRT, Monte Carlo simulation, cefepime, population pharmacokinetics
INTRODUCTION
Sepsis is a major problem in the intensive care unit (ICU). The number of sepsis cases is increasing, and the associated mortality is 25% globally (1–3). Sepsis causes half of the acute kidney injuries in the ICU (4). As such, patients receive antimicrobial therapy while on renal replacement therapy, which adds to the variability in drug exposure in these patients (5–7). Continuous renal replacement therapy (CRRT) provides better tolerability while efficiently maintaining fluid, electrolytes, and acid-base balance (8). In patients who receive CRRT, the antimicrobial exposure can be affected by CRRT parameters, drug characteristics, and patient’s pharmacokinetics (PK) (9–11). If insufficient doses of antimicrobials are administered to ICU patients, resistance and treatment failure can develop, given the suboptimal antimicrobial exposure that is not adequate to eradicate the pathogen, resulting in clinical worsening and even death (12, 13).
Beta-lactams are commonly prescribed in the ICU for suspected or confirmed Gram-negative bacterial infections. Beta-lactam therapy is optimized by achieving a high percentage of the dosing interval during which the free beta-lactam concentration is above the MIC (fT>MIC), which is the pharmacokinetic/pharmacodynamic (PK/PD) target (14). Given the changes in CRRT factors and the variability between the ICU patients, beta-lactam regimens recommended by the earlier studies may be insufficient to achieve the optimal PK/PD targets (9, 15, 16). In addition, there is a conflict in the literature concerning the best cefepime dosing regimen to achieve the appropriate PK/PD target and the impact of CRRT intensity on cefepime exposure in ICU patients (15, 17–19). In this study, we aimed to build a cefepime population PK model and assess the optimal PK/PD target attainment using Monte Carlo simulation (MCS) in ICU patients receiving CRRT.
RESULTS
Patient characteristics.
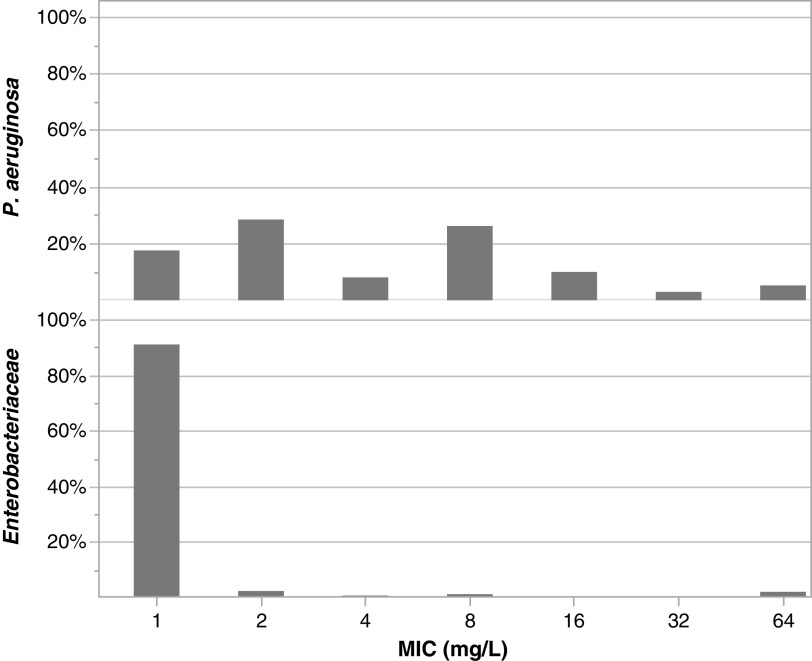
Ten patients contributed 162 plasma and 79 effluent samples, with an average of 24 samples drawn from each patient (16 plasma and 8 effluent). Table 1 shows the baseline characteristics. The mean (standard deviation [SD]) age was 53.2 (11.3) years, and mean weight was 118.8 (26.6) kg. Seventy percent (n = 7) were males; two patients received continuous venovenous hemodialysis (CVVHD), and eight received continuous venovenous hemofiltration (CVVH). Figure 1 shows the institutional Pseudomonas aeruginosa and Enterobacteriaceae MIC data.
TABLE 1.
Baseline characteristics and CRRT settingsa
| Characteristic | Value for patients (n = 10) |
|---|---|
| Age, yrs | 53.2 (11.3) |
| No. (%) of males | 7 (70) |
| Wt, kg | 118.8 (26.8) |
| No. (%) with CRRT modality | |
| CVVH | 8 (80) |
| CVVHD | 2 (20) |
| Urine output,b ml | 14.4 (43.2) |
| Blood flow rate, ml/min | 296 (49) |
| Ultrafiltrate rate,c ml/kg/h | 0.6 (0.9) |
| Therapy fluid rate,c ml/kg/h | 29.6 (5.2) |
| CRRT downtime,b h | 0.1 (0.5) |
| No. of samples | |
| Predialyzer serum | 82 |
| Postdialyzer serum | 80 |
| Effluent filtrate | 79 |
Data are means (SD) unless otherwise specified. CRRT, continuous renal replacement therapy; CVVH, continuous venovenous hemofiltration; CVVHD, continuous venovenous hemodialysis.
Calculated as total volume or time during the dosing interval.
Calculated as average rate during the dosing interval.
FIG 1.
Local Pseudomonas aeruginosa and Enterobacteriaceae MIC distribution for cefepime.
Population pharmacokinetic model.
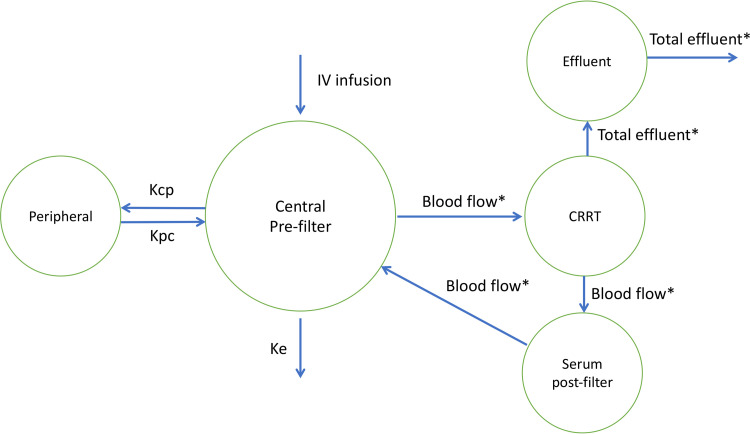
In this population, cefepime was best described by a five-compartment model: two compartments for the patient and three for the CRRT machine (Fig. 2). The CRRT blood and total effluent flow rates were used as flow rates in the model. The CRRT downtime covariate was applied to the rates of transfer as run time and as a fraction of the dosing interval: k = (flow rate/V) × [(dosing interval − CRRT downtime)/dosing interval], where k is the rate of transfer and V is the volume of distribution of the compartment.
FIG 2.
Cefepime five-compartment model in patients on CRRT. *, CRRT downtime was applied as run time and as a fraction of the dosing interval on these flow rates. CRRT, continuous renal replacement therapy; IV, intravenous; Kcp, transfer rate from the central to the peripheral compartment; Ke, rate of elimination; Kpc, transfer rate from the peripheral to the central compartment.
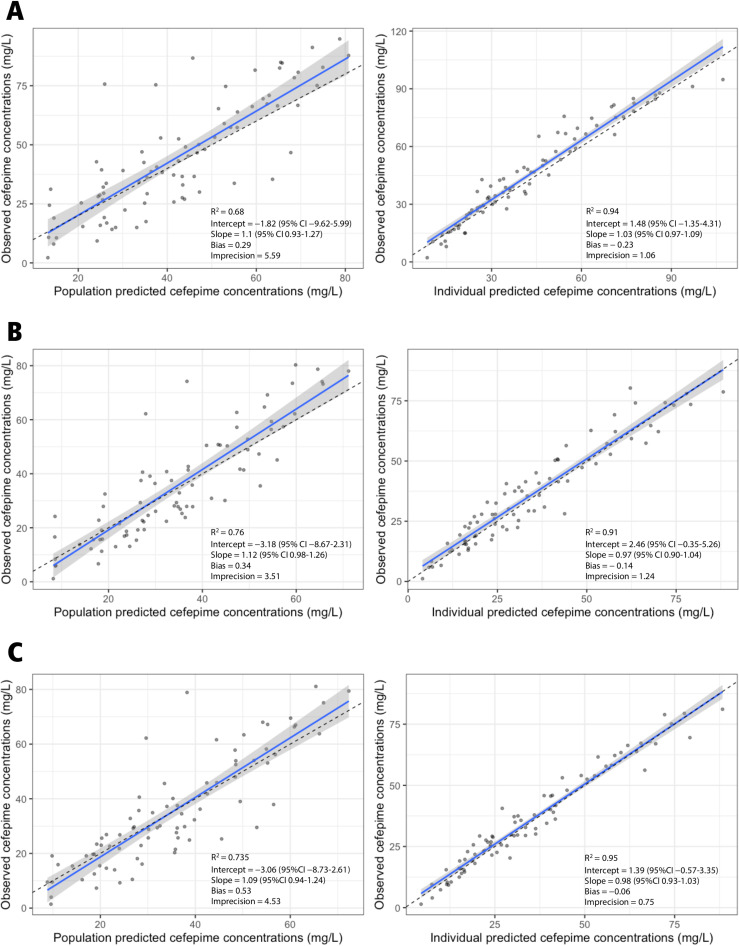
Table 2 shows the parameter estimates for the final model. The mean (SD) for the rate of elimination is 0.07 (0.05) h−1, the rate of transfer from the central to the peripheral compartment is 0.83 (0.64) h−1, the rate of transfer from the peripheral to the central compartment is 2.47 (2.25) h−1, and the volume of distribution in the central compartment is 26.76 (15.09) liters. Figure 3 shows the observed versus population and individual predicted predialyzer, postdialyzer, and effluent concentrations.
TABLE 2.
Population parameter estimates for cefepime final modela
| Parameter (unit) | Median | 95% credibility interval | Mean | SD | CV (%) | Shrinkage (%) |
|---|---|---|---|---|---|---|
| kel (h−1) | 0.07 | 0.04–0.09 | 0.07 | 0.05 | 71.73 | 0.31 |
| kcp (h−1) | 0.75 | 0.13–1.64 | 0.83 | 0.64 | 76.80 | 4.80 |
| kpc (h−1) | 3.23 | 0.25–5.00 | 2.47 | 2.25 | 90.94 | 1.50 |
| Vcentral (liters) | 18.67 | 9.18–45.02 | 26.76 | 15.09 | 56.37 | 1.44 |
| VCRRT (liters) | 2.09 | 0.01–3.47 | 1.98 | 1.69 | 85.41 | 3.27 |
| Veffluent (liters) | 0.18 | 8 × 10−4–0.85 | 0.57 | 0.66 | 116.25 | 3.72 |
| Vpostdialyzer (liters) | 1.28 | 0.81–3.38 | 1.79 | 1.34 | 74.90 | 10.75 |
CRRT, continuous renal replacement therapy compartment; CV, coefficient of variation; kcp, rate of transfer from the central to the peripheral compartment; kel, elimination rate constant; kpc, rate of transfer from the peripheral to the central compartment; SD, standard deviation; V, volume of distribution.
FIG 3.
Observed versus population and individual predicted cefepime concentrations in predialyzer (A), effluent (B), and postdialyzer (C) compartments.
Monte Carlo simulations.
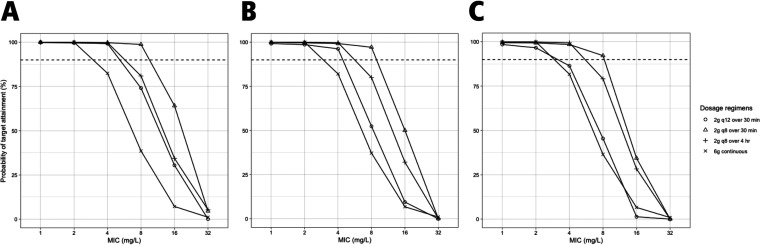
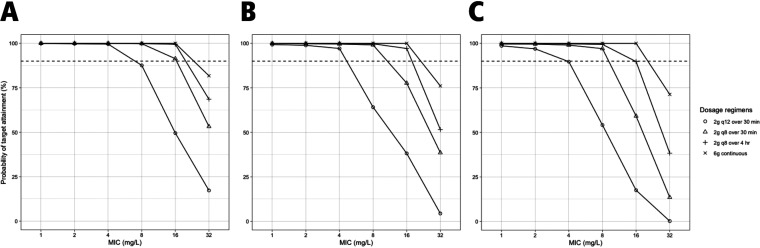
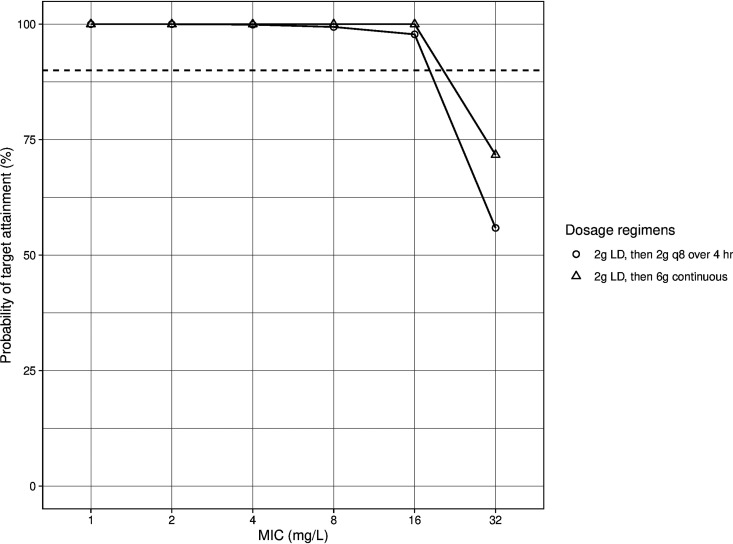
Figure S1a and b show the results of simulating cefepime 2 g intravenously (i.v.) infused over 4 h at 8-h intervals along with different values of CRRT blood flow rate, total effluent flow rate, and downtime to assess the impact of these parameters on 100% fT>MIC target attainment. Total effluent flow rate impacted target attainment the most in this population. Figures 4 and 5 show the probability of target attainment (PTA) at target 100% fT>MIC within the first 24 h and after 72 h, respectively, at different total effluent flow rates. For the first 24 h of therapy, all regimens achieved ≥90% target attainment at MICs of 1 and 2 mg/liter at all total effluent flow rates. At MICs of 4 mg/liter, PTA dropped below 90% with continuous infusion (CI) at all flow rates and with intermittent infusion (II) every 12 h at a 40-ml/kg/h flow rate. At a MIC of 8 mg/liter, cefepime given via CI, extended infusion (EI) every 8 h, and II every 12 h had a PTA of <90% at all flow rates. PTA within the first 24 h dropped significantly for all regimens at MIC of ≥16 mg/liter. After 72 h of therapy, all regimens achieved good target attainment with all flow rates at MICs of 1, 2, and 4 mg/liter. At MICs of 8 mg/liter, II of 2 g every 12 h achieved a PTA close to 90% at a total effluent flow rate of 20 ml/kg/h but dropped significantly at higher flow rates. At MICs of 16 mg/liter, the PTA for II of 2 g every 12 h was ≤50% at all flow rates, while for the every-8-h II regimen, it was <90% at flow rates of ≥30 ml/kg/h. All regimens had PTA of <90% at MICs of 32 mg/liter, with the highest PTA achieved with the CI regimen. To improve the PTA early in therapy, we simulated a 2-g loading dose (LD) administered over 30 min before the EI and CI regimens (i.e., an 8-g total dose for the first day) (Fig. 6). Both regimens achieved PTA of >90% at target 100% fT>MIC up to a MIC of 16 mg/liter. At 60% fT>4×MIC, all regimens achieved good target attainment at MICs of ≤4 mg/liter except for cefepime 2 g every 12 h, both within the first 24 h and after 72 h of cefepime therapy. All regimens had a PTA of <90% at a MIC of 8 mg/liter (Fig. S2).
FIG 4.
Probability of target attainment (100% fT>MIC) for cefepime within the first 24 h with total effluent flow rates of 20 (A), 30 (B), and 40 (C) ml/kg/h. The dashed lines indicate 90% probability of target attainment. The mean (SD) weight used for the simulations was 119 kg (27 kg). Blood flow rate and CRRT downtime were fixed at 300 ml/min and 0 h, respectively.
FIG 5.
Probability of target attainment (100% fT>MIC) for cefepime after 72 h with total effluent flow rate of 20 (A), 30 (B), and 40 (C) ml/kg/h. Dashed lines indicate 90% probability of target attainment. The mean (SD) weight used for the simulations was 119 kg (27 kg). Blood flow rate and CRRT downtime were fixed at 300 ml/min and 0 h, respectively.
FIG 6.
Probability of target attainment (100% fT>MIC) for cefepime loading dose followed by extended and continuous infusion within the first 24 h. Dashed lines indicate 90% probability of target attainment. The mean (SD) weight used for the simulations was 119 kg (27 kg). Total effluent flow rate, blood flow rate, and CRRT downtime were fixed at 30 ml/kg/h, 300 ml/min, and 0 h, respectively. LD, loading dose.
Figure S3 shows the probability of achieving free trough concentrations of ≥20 and ≥70 mg/liter. Within the first 24 h, the probability is low for both targets with all regimens. After 72 h, the EI and CI regimens had the highest probability of achieving a trough concentration of 20 mg/liter but not 70 mg/liter. The probability of achieving these targets might change with changes in CRRT flow rates and downtime.
DISCUSSION
We describe a cefepime population PK model in ICU patients receiving CRRT using the machine flow rates to describe the drug elimination and transfer between compartments. This may provide flexibility to clinicians if this model is to be used to optimize cefepime therapy in similar patients while providing the blood flow rate, total effluent flow rate, and CRRT downtime. We assessed the impact of different CRRT flow rates and downtimes on the clearance of cefepime and found that total effluent rate had the highest impact on the drug clearance, especially after 72 h of therapy. CRRT downtime had the second-highest impact on cefepime clearance; however, it is not expected to happen with every dose of cefepime. Based on our simulations, II of 2 g cefepime every 8 h achieved good target attainment at MICs of ≤8 mg/liter. Both EI and CI did not initially attain the target if the MIC was 16 mg/liter (intermediate susceptibility for P. aeruginosa and resistant for Enterobacterales). Thus, a 2-g LD will be needed before EI and CI so that better target attainment is achieved early in therapy. This dosing strategy should allow clinicians to maximize the potential efficacy of cefepime.
Carlier et al. (17) built a cefepime one-compartment model using blood (arterial) samples drawn at 0, 1, 2, 5, and 6 or 12 h after the start of cefepime infusion, from 13 patients receiving CRRT. Ultrafiltration rate was used as a covariate on the volume of distribution and clearance. Based on the simulations, cefepime at 1 g every 6 h or 2 g every 8 h as an II achieved good target attainment (100% fT>MIC) at a MIC of 8 mg/liter and ultrafiltration rates of 1,000 to 2,000 ml/h. The PTA dropped below 90% at a MIC of 16 mg/liter (17). This is consistent with our findings that 2 g cefepime given as an II every 8 h had good target attainment at all total effluent rates at MICs of ≤8 mg/liter but not 16 mg/liter. In a prospective PK study conducted on 12 ICU patients who received II of 1 to 2 g cefepime every 12 to 24 h during CRRT, premembrane blood, postmembrane blood, and ultrafiltrate or dialysate samples were collected at 1, 2, 4, 8, and 12 or 24 h after the end of cefepime infusion. The mean blood flow was 150 ml/min, and the ultrafiltration rate was ∼1,000 ml/h. The authors measured the total concentration and used standard PK methods to calculate the T>MIC. The authors suggested that 2 g/day might be sufficient for treating susceptible pathogens and 4 g/day for those with higher MICs (18). However, given the method of calculating the PK/PD parameters, not accounting for the free drug concentration and use of different ultrafiltration rates, these regimens might be suboptimal for dynamic patients with changes in the CRRT flow rates. Seyler and colleagues (16) evaluated the PK of beta-lactams in patients receiving the recommended CRRT doses. Blood samples were drawn at times 0, 1, 2, 5, and 6 or 12 h after the antibiotic administration. The mean blood flow was 150 ml/min, and the ultrafiltration rate was 22 ml/kg/h. Of 53 patients, eight received II of 2 g cefepime every 12 h. The median (range) total trough concentration was 11 mg/liter (3 to 22 mg/liter), and none of the patients achieved the 70% T>4×MIC target as specified by the authors (16).
A few previous studies assessed the impact of CRRT intensity on cefepime clearance. A retrospective study included 50 ICU patients who received unadjusted doses of beta-lactams and had therapeutic drug monitoring. Nine patients received cefepime. The authors found a weak, but significant, correlation between beta-lactam clearance and CRRT intensity (r = 0.32, P = 0.03) (19). On the other hand, simulations performed using published PK values suggested that CRRT intensity may not have a significant impact on cefepime PK/PD target attainment (15, 20). In our simulations, we found that the total effluent rate might have an impact on target attainment at MICs of ≥8 mg/liter after 72 h of therapy. There are differences in the simulations published previously and the one in this study. Assuming a normal distribution of cefepime PK parameters, previous work used the mean and standard deviation values of PK and CRRT parameters and used certain fixed CRRT parameters (i.e., less intensive versus intensive flow rates). On the other hand, our simulations were semiparametric where support points, each with a value for each PK parameter (e.g., elimination rate constant and volume of distribution) in the model and an associated probability which corresponds to the number of patients having these PK parameter values, serve as the mean of one multivariate normal distribution in a multimodal, multivariate joint distribution. The weight of each multivariate distribution is equal to the probability of the support point (21).
Different cefepime PK/PD targets were specified in the literature with a common range of 50% to 74% fT>MIC, and very few studies evaluated 100% fT>MIC. Based on some preclinical studies, cephalosporins may have static effect at fT>MIC values of >30% to 40% while maximal killing is achieved at 60% to 70% (14, 22). To prevent resistance, a trough-to-MIC ratio of >3.8 was needed (23). Higher targets (i.e., 100% fT>MIC and fT>4×MIC) might be desirable in the clinical setting (24–26). The differences in favorable targets between the preclinical and clinical studies might be due to the fact that suggested preclinical targets are assumed to be at the site of infection, whereas clinically, plasma concentrations are usually measured, which may not correlate with the concentration at the site of infection. In case of pneumonia, a common infection in the ICU, beta-lactams and cefepime may have variable and poor penetration to the lung tissue and secretions in critically ill patients (6, 27), which may indicate that higher drug plasma concentrations are desirable to achieve the optimal exposure at the site of infection. This has been shown previously with meropenem CI (28).
As the cefepime concentration goes up, optimizing efficacy, neurotoxicity may be a concern, which researchers have tried to correlate to plasma exposure. Current evidence on this topic is still weak due to retrospective study design, trough-only sampling, measurement of total concentration in plasma, difficulty in defining the event, and presence of confounders affecting the neurotoxic event, which is common in the ICU (29–32). In this study, the probability of achieving a free trough plasma concentration of ≥20 mg/liter was the highest with CI and EI after 72 h of therapy, while the probability of achieving a trough concentration of ≥70 mg/liter was much lower. The CRRT flow rates and downtime will affect these probabilities, given the impact on cefepime clearance. Eventually, more investigation is still needed in the area of cefepime neurotoxicity to better define thresholds. Therapeutic drug monitoring will have a major role in this.
In this study, we showed that CRRT ICU patients might benefit from extended-infusion cefepime regimens rather than intermittent therapy. The strengths of this study are that five samples were drawn per site and occasion, samples were drawn from the three different sites, and unbound cefepime concentration was measured. On the other hand, some of the limitations were that sample size was limited, clinical outcomes were not evaluated, and the results may not be generalizable because of the use of different CRRT machines or filters. Future studies should have a larger sample size and use different CRRT filters to help identify more support points in the PK model and assess the impact of different filters on cefepime exposure.
Conclusions.
In patients receiving CRRT, cefepime was described by a five-compartment model, and CRRT flow rates were used to describe the drug transfer between compartments. A 2-g LD of cefepime followed by EI of 2 g every 8 h achieved a high PTA at MICs of ≤16 mg/liter (at target 100% fT>MIC) and at MICs of ≤4 mg/liter (at target 60% fT>4×MIC) with a very low probability of reaching a trough concentration of 70 mg/liter.
MATERIALS AND METHODS
This was a prospective, PK study at the University of Cincinnati Medical Center (NCT02458261) which included patients who were ≥18 years old, were admitted to the ICU, and received cefepime 2 g i.v. every 8 h as a 4-h infusion while on CVVH or CVVHD. Exclusion criteria were pregnancy, cystic fibrosis, incarceration, admission for burns, and unmeasured or >400 ml of urine output in the last 24 h. Data collected include age, sex, weight, urine output, and CRRT parameters, including blood, dialysate, therapy fluid, and ultrafiltrate flow rates. The total effluent rate is the sum of therapy fluid and ultrafiltrate rates. This study was reviewed and approved by the Institutional Review Board at the University of Cincinnati (IRB no. 00000180) and the University of Florida (IRB202003071), and informed consent was obtained from all participants (33).
CRRT machines.
NxStage CRRT machines with Purema high-permeability polysulfone membrane filters (NxStage Medical Inc., Lawrence, MA) were used to provide the CVVH and CVVHD therapy. NxStage PureFlow fluids 400, 401, 402, 453, and 454 were used as appropriate. Patients who received CVVH received precircuit replacement fluid.
Cefepime samples and assay.
Two sets of predialyzer serum, postdialyzer serum, and effluent samples were collected after both the first and the fourth to sixth cefepime doses. If the CRRT was not running, a single arterial sample was collected. The samples were collected at 1, 2, 3, 4, and 8 h after the start of cefepime extended infusion in nonheparinized tubes and stored at −80°C. The concentration of unbound cefepime in the samples was measured at the Antimicrobial Research Laboratory at the University of Cincinnati James L. Winkle College of Pharmacy using high-performance liquid chromatography with UV detection (298 nm). Microcon 30-kDa filters (Millipore, Cork, Ireland) were used, and samples were centrifuged at 12,000 × g for 10 min at room temperature to obtain the protein-free ultrafiltrate for drug quantification. The cefepime range of detection was 1 to 200 mg/liter for all matrices (r > 0.999, n = 11). The intra- and interday coefficients of variation were ≤8.2% for low (2-mg/liter), medium (25-mg/liter), and high (100-mg/liter) controls (33).
Population PK analysis and simulations.
The nonparametric adaptive grid in Pmetrics v1.9.7 was used to build cefepime population PK model and perform the Monte Carlo simulations (34). Cefepime values from the three sites of sampling (predialyzer, postdialyzer, and effluent) were used to build the model. Starting with the simplest model, we tested a two-compartment model assuming one compartment for the patient and another for the CRRT machine and kept testing additional compartments up to a five-compartment model. The following covariates were tested and added on PK parameters in a forward stepwise fashion: weight, urine output, CRRT downtime, blood, ultrafiltrate, therapy fluid, and total effluent flow rates. We examined the model on each step, and the final model was chosen based on the lowest Akaike information criterion, highest coefficient of determination (R2) of observed versus predicted plots for both population and Bayesian, and lowest imprecision and bias. The assay error (standard deviation) and environmental noise were accounted for using error polynomials as a function of observed concentration (SD = C0 + [C1 × observed concentration]) using C0 (intercept) and C1 (slope) values of 1 and 0.1, respectively. The gamma multiplicative error model was used to estimate residual error (error = SD × gamma) (35).
To assess the impact of CRRT parameters included in the model, a fixed cefepime dose of 2 g i.v. infused over 4 h at 8-h intervals along with different values for the CRRT parameters were simulated. A total of 2,500 subjects were simulated for each of the following regimens: 2 g every 8 and 12 h over 30 min, 2 g every 8 h over 4 h, and 6 g as a CI. Also, a 2-g LD over 30 min followed by EI and CI regimens was simulated. The covariates included in the final model were simulated either as mean and SD or fixed at certain values. The MICs chosen for simulation were 1, 2, 4, 8, 16, and 32 mg/liter. The PK/PD targets chosen were 100% fT>MIC and 60% fT>4×MIC. In addition, we assessed the probability of achieving free trough concentrations of ≥20 mg/liter and ≥70 mg/liter as potential thresholds for neurotoxicity (15). Given that unbound cefepime concentration was measured, there was no assumption of free fraction. We calculated the PTA for the first 24 h and after 72 h of therapy (from 72 h to the end of the dosing interval).
Footnotes
Supplemental material is available online only.
aac.00144-21-s000s1.pdf (309.4KB, pdf)
REFERENCES
- 1.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K, International Forum of Acute Care Trialists. 2016. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 193:259–272. 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellomo R. 2014. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 311:1308–1316. 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 3.McPherson D, Griffiths C, Williams M, Baker A, Klodawski E, Jacobson B, Donaldson L. 2013. Sepsis-associated mortality in England: an analysis of multiple cause of death data from 2001 to 2010. BMJ Open 3:e002586. 10.1136/bmjopen-2013-002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A. 2017. Acute kidney injury in sepsis. Intensive Care Med 43:816–828. 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 5.Chapuis TM, Giannoni E, Majcherczyk PA, Chioléro R, Schaller MD, Berger MM, Bolay S, Décosterd LA, Bugnon D, Moreillon P. 2010. Prospective monitoring of cefepime in intensive care unit adult patients. Crit Care 14:R51. 10.1186/cc8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonçalves-Pereira J, Póvoa P. 2011. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care 15:R206. 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases. 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepa C, Muralidhar K. 2012. Renal replacement therapy in ICU. J Anaesthesiol Clin Pharmacol 28:386–396. 10.4103/0970-9185.98357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Li X, Xia Y, Chu Y, Zhong H, Li J, Liang P, Bu Y, Zhao R, Liao Y, Yang P, Lu X, Jiang S. 2020. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol 29:786. 10.3389/fphar.2020.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JA, Joynt G, Lee A, Choi G, Bellomo R, Kanji S, Mudaliar MY, Peake SL, Stephens D, Taccone FS, Ulldemolins M, Valkonen MM, Agbeve J, Baptista JP, Bekos V, Boidin C, Brinkmann A, Buizen L, Castro P, Cole CL, Creteur J, De Waele JJ, Deans R, Eastwood GM, Escobar L, Gomersall C, Gresham R, Ain Jamal J, Kluge S, König C, Koulouras VP, Lassig-Smith M, Laterre P, Lei K, Leung P, Lefrant J, Llauradó-Serra M, Martin-Loeches I, Mat Nor MB, Ostermann M, Parker SL, Rello J, Roberts DM, Roberts MS, Richards B, Rodríguez A, Roehr AC, Roger C, Seoane L, Sinnollareddy M, Sousa E, Soy D, Spring A, et al. 2020. The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: data from the multinational SMARRT Study. Clin Infect Dis 9:ciaa224. 10.1093/cid/ciaa224. [DOI] [PubMed] [Google Scholar]
- 11.Pea F, Viale P, Pavan F, Furlanut M. 2007. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet 46:997–1038. 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 12.Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. 2005. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:4920–4927. 10.1128/AAC.49.12.4920-4927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts JA, Kruger P, Paterson DL, Lipman J. 2008. Antibiotic resistance—what’s dosing got to do with it? Crit Care Med 36:2433–2440. 10.1097/CCM.0b013e318180fe62. [DOI] [PubMed] [Google Scholar]
- 14.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 15.Chaijamorn W, Charoensareerat T, Srisawat N, Pattharachayakul S, Boonpeng A. 2018. Cefepime dosing regimens in critically ill patients receiving continuous renal replacement therapy: a Monte Carlo simulation study. J Intensive Care 6:61. 10.1186/s40560-018-0330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seyler L, Cotton F, Taccone FS, De Backer D, Macours P, Vincent J, Jacobs F. 2011. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit Care 15:R137. 10.1186/cc10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlier M, Taccone FS, Beumier M, Seyler L, Cotton F, Jacobs F, Roberts JA. 2015. Population pharmacokinetics and dosing simulations of cefepime in septic shock patients receiving continuous renal replacement therapy. Int J Antimicrob Agents 46:413–419. 10.1016/j.ijantimicag.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Malone RS, Fish DN, Abraham E, Teitelbaum I. 2001. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 45:3148–3155. 10.1128/AAC.45.11.3148-3155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beumier M, Casu GS, Hites M, Seyler L, Cotton F, Vincent J, Jacobs F, Taccone FS. 2014. β-Lactam antibiotic concentrations during continuous renal replacement therapy. Crit Care 18:R105. 10.1186/cc13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang SM, Pai MP, Shaw AR, Mueller BA. 2019. Antibiotic exposure profiles in trials comparing intensity of continuous renal replacement therapy. Crit Care Med 47:e863–e871. 10.1097/CCM.0000000000003955. [DOI] [PubMed] [Google Scholar]
- 21.Goutelle S, Woillard J, Neely M, Yamada W, Bourguignon L. 26October2020, posting date. Nonparametric methods in population pharmacokinetics. J Clin Pharmacol 10.1002/jcph.1650. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. 10.1016/s0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 23.Tam VH, Chang K-T, Zhou J, Ledesma KR, Phe K, Gao S, Van Bambeke F, Sánchez-Díaz AM, Zamorano L, Oliver A, Cantón R. 2017. Determining β-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J Antimicrob Chemother 72:1421–1428. 10.1093/jac/dkx001. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, Rai V, Wong KK, Hasan MS, Abd Rahman AN, Jamal JA, Wallis SC, Lipman J, Staatz CE, Roberts JA. 2016. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 42:1535–1545. 10.1007/s00134-015-4188-0. [DOI] [PubMed] [Google Scholar]
- 25.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. 2002. Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother 50:425–428. 10.1093/jac/dkf130. [DOI] [PubMed] [Google Scholar]
- 27.Heffernan AJ, Sime FB, Lipman J, Dhanani J, Andrews K, Ellwood D, Grimwood K, Roberts JA. 2019. Intrapulmonary pharmacokinetics of antibiotics used to treat nosocomial pneumonia caused by Gram-negative bacilli: a systematic review. Int J Antimicrob Agents 53:234–245. 10.1016/j.ijantimicag.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Benítez-Cano A, Luque S, Sorlí L, Carazo J, Ramos I, Campillo N, Curull V, Sánchez-Font A, Vilaplana C, Horcajada JP, Adalia R, Bermejo S, Samsó E, Hope W, Grau S. 2020. Intrapulmonary concentrations of meropenem administered by continuous infusion in critically ill patients with nosocomial pneumonia: a randomized pharmacokinetic trial. Crit Care 24:55. 10.1186/s13054-020-2763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boschung-Pasquier L, Atkinson A, Kastner LK, Banholzer S, Haschke M, Buetti N, Furrer DI, Hauser C, Jent P, Que YA, Furrer H, Babouee Flury B. 2020. Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect 26:333–339. 10.1016/j.cmi.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Beumier M, Casu GS, Hites M, Wolff F, Cotton F, Vincent JL, Jacobs F, Taccone FS. 2015. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol 81:497–506. [PubMed] [Google Scholar]
- 31.Al-Shaer MH, Peloquin CA. 2021. Using precision dosing to minimize cefepime-induced neurotoxicity: the challenge of targets. J Infect Chemother 10.1016/j.jiac.2021.02.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Lamoth F, Buclin T, Pascual A, Vora S, Bolay S, Decosterd LA, Calandra T, Marchetti O. 2010. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob Agents Chemother 54:4360–4367. 10.1128/AAC.01595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philpott CD, Droege CA, Droege ME, Healy DP, Courter JD, Ernst NE, Harger NJ, Foertsch MJ, Winter JB, Carter KE, Van Fleet SL, Athota K, Mueller EW. 2019. Pharmacokinetics and pharmacodynamics of extended-infusion cefepime in critically ill patients receiving continuous renal replacement therapy: a prospective, open-label study. Pharmacotherapy 39:1066–1076. 10.1002/phar.2332. [DOI] [PubMed] [Google Scholar]
- 34.Neely MN, Van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jelliffe RW. 2012. Some comments and suggestions concerning population pharmacokinetic modeling, especially of digoxin, and its relation to clinical therapy. Ther Drug Monit 34:368–377. 10.1097/FTD.0b013e31825c88bb. [DOI] [PMC free article] [PubMed] [Google Scholar]