Abstract
A subset of diabetic COVID-19 patients treated with steroids, oxygen, and/or prolonged intensive care admission develop rhino-orbito-cerebral mucormycosis. Radiologists must have a high index of suspicion for early diagnosis, which prompts immediate institution of antifungal therapy that limits morbidity and mortality. Assessment of disease extent by imaging is crucial for planning surgical debridement. Complete debridement of necrotic tissue improves survival. Imaging features reflect the angioinvasive behaviour of fungal hyphae from the Mucoraceae family, which cause necrotising vasculitis and thrombosis resulting in extensive tissue infarction. Contrast-enhanced magnetic resonance imaging (MRI) is the imaging technique of choice. The classic “black turbinate” on contrast-enhanced imaging represents localised invasive fungal rhinosinusitis (IFRS). A striking radiological feature of disseminated craniofacial disease is non-enhancing devitalised and necrotic soft tissue at the orbits and central skull base. Sinonasal and extrasinonasal non-enhancing lesions in IFRS are secondary to coagulative necrosis induced by fungal elements. Multicompartmental and extrasinonasal tissue infarction is possible without overt bone involvement and caused by the propensity of fungal elements to disseminate from the nasal cavity via perineural and perivascular routes. Fungal vasculitis can result in internal carotid artery occlusion and cerebral infarction. Remnant non-enhancing lesions after surgical debridement portend a poor prognosis. Assessment for the non-enhancing MRI lesion is crucial, as it is a sole independent prognostic factor for IFRS-specific mortality. In this review, we describe common and uncommon imaging presentations of biopsy-proven rhino-orbito-cerebral mucormycosis in a cohort of nearly 40 COVID-19 patients.
Introduction
India is witnessing a devastating outbreak of opportunistic rhino-orbito-cerebral mucormycosis in the second wave of the coronavirus disease 2019 (COVID-19) pandemic. Prior to 2021, most cases of mid-face and skull-base invasive mucormycosis were seen in immunocompromised individuals with poorly controlled diabetes mellitus, haematological diseases, and following transplantation or chemotherapy. In the second wave of the pandemic in India, a new and unexpected epidemiological pattern has emerged. A subset of diabetic COVID-19 patients treated with steroids, oxygen, and/or prolonged intensive care admission develop rhino-orbito-cerebral mucormycosis.1 Rampant steroid use to suppress severe COVID-19 inflammatory syndromes in ventilated diabetic patients has been incriminated as a cause. Fulminant progression is common and sometimes leads to death in less than a week after presentation. The government in India has asked the states to notify all cases of the so-called “black fungus” under the “Epidemic Diseases Act”2 (Box 1 ).
Box 1. The Epidemic Diseases Act (1897) in India and its relevance to public health epidemiology.
-
•
The Epidemic Diseases Act (1897) is first enacted to control bubonic plague in former British India. The law provides special powers required for implementing containment measures.
-
•
In May 2021, the central government in India directs all states to notify “black fungus” or mucormycosis cases under the Epidemic Diseases Act (1897).2
-
•
The new notification mandates that all confirmed or suspected cases of mucormycosis be reported to the Health Ministry. A letter from the Health Ministry directs “all government and private health facilities to follow guidelines for screening, diagnosis and management of mucormycosis”. These guidelines are issued by the Indian Council of Medical Research (ICMR).
-
•
Declaring a disease “notifiable” allows authorities to monitor and track the disease, collate information, and issue early warnings to improve management. Other notifiable diseases in the India are tuberculosis, diphtheria and cholera.
Alt-text: Box 1
Imaging is requisitioned for a variety of reasons that includes early diagnosis, pre-surgical mapping of extent, post-treatment follow-up, and for prognosis. Central to early diagnosis is a high index of suspicion by the clinician and the radiologist. Consequent early institution of antifungal therapy can limit morbidity and mortality. In diagnosed cases, imaging is imperative to map extent, detect early orbital involvement, and demonstrate possible extension to the skull base, anterior, and middle cranial fossa, which may be clinically silent. Exact imaging demonstration of disease extent determines the extent of surgical debridement. Finally, imaging is important to assess response to medical treatment and adequacy of surgical debridement. The modality of choice is contrast-enhanced magnetic resonance imaging (MRI) in view of its superior contrast resolution for soft-tissue and marrow abnormalities. Computed tomography (CT) is complementary to MRI to better demonstrate bone erosion and demineralisation.3 In this review, we describe common and uncommon radiological presentations of biopsy/fungal culture-proven rhino-orbito-cerebral mucormycosis in a cohort of 40 COVID-19 patients imaged in the months of April and May 2021 at Kokilaben Dhirubhai Ambani Hospital, Mumbai, India. COVID-19 was confirmed by a positive RT-PCR (reverse transcriptase polymerase chain reaction) test.
Clinical presentation
Since the primary site of inoculation of fungus is the nose and paranasal sinuses, clinical presentation in early disease is with facial numbness, pain, or swelling, dark coloured nasal discharge and/or nasal stuffiness. Some patients present with predominant dental symptoms such as jaw pain or loosening of teeth. Clinical features of disseminated disease include blurred vision, proptosis, ptosis, ophthalmoplegia, facial paresis, altered sensorium, and stroke. Unfortunately, many patients from remote areas present late and require debilitating craniofacial resections and orbital exenteration.
Radiological features
Imaging features of mucormycosis closely reflect the angioinvasive behaviour of fungal hyphae from the Mucoraceae family, which invade blood vessels, cause necrotising vasculitis and thrombosis resulting in extensive tissue infarction. The radiological appearances of COVID-19-associated rhino-orbito-cerebral mucormycosis are similar to invasive fungal rhinosinusitis (IFRS) historically identified in immunocompromised individuals with poorly controlled diabetes mellitus, haematological malignancies, and following transplantation or chemotherapy.
The classic imaging sign of the “black turbinate” (BT; Fig 1 ) refers to lack of contrast enhancement of invaded mucosa secondary to occlusion of small vessels.4 Caution is warranted while reporting BT as diagnostic of invasive fungal rhinosinusitis (IFRS), as up to 30% of patients without IFRS may have heterogeneous enhancement or even lack of enhancement (particularly in the posterior part of the turbinate).5 The lack of enhancement in the posterior aspect of the turbinates in patients without IFRS improves temporally if delayed imaging after contrast medium administration is obtained and has been referred to as “benign BT”.5 In contradistinction, in IFRS, lack of enhancement of the BT persists until the delayed phase of contrast-enhanced imaging. The most common site of involvement of IFRS is the middle turbinate, which filters the major volume of the nasal airflow explaining the penchant for fungal seeding in this region.6 A similar lack of enhancement is seen in the involved mucosa of the paranasal sinuses (Fig 2 ) and sometimes referred to as sinonasal lack of contrast enhancement (LoCE) lesion. 7 A diagnostic pitfall is obscuration of normal enhancement in the sinus by susceptibility artefacts at the air–mucosa interface and adjacent to metallic dental implants. On unenhanced MRI, the fungus has variable signal; however, low T2 signal is characteristic with occasional cases showing restricted diffusion in the invaded mucosa4 and in the soft-tissue abnormality. The low T2 signal (Fig 2) may be related to the presence of paramagnetic iron and magnesium in the fungal elements.8 Mucosa of non-aggressive inflammatory sinusitis has high T2 signal, enhances avidly, and usually coexists with IFRS. Very early fungal sinusitis with superficial involvement mimics non-specific or bacterial sinusitis and can be easily missed. The presence of an air–fluid level is suggestive of acute bacterial sinusitis. Absence of fluid levels in the involved sinus is a historically described feature of fungal sinusitis.9 Diagnosis of fungal sinusitis based on signal alteration can be confounded by altered signal intensity of secretions or haemorrhage (especially in the postoperative setting). Obliteration of the periantral fat is an early diagnostic sign of extrasinus invasion10 and known to be the best individual predictor of IFRS on CT.11 Predominant unilateral involvement (Fig 2) is specific for IFRS.12
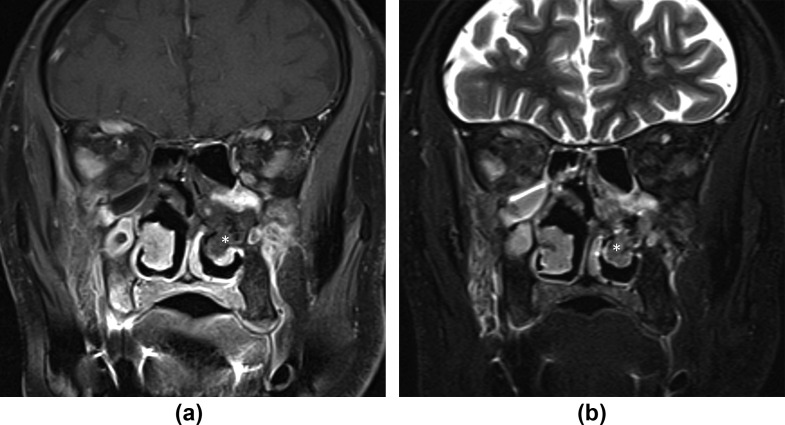
Figure 1.
Black turbinate sign. (a) Coronal contrast-enhanced, fat-saturated T1 and (b) T2-STIR image show a left inferior turbinate (asterisks) with lack of enhancement and relatively low T2 signal. A 72-year-old, diabetic man with left facial pain and nasal stuffiness. Treated for COVID-19 with injectable corticosteroids and invasive ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 16 days. Patient died 12 days after first imaging from mucormycosis-related complications.
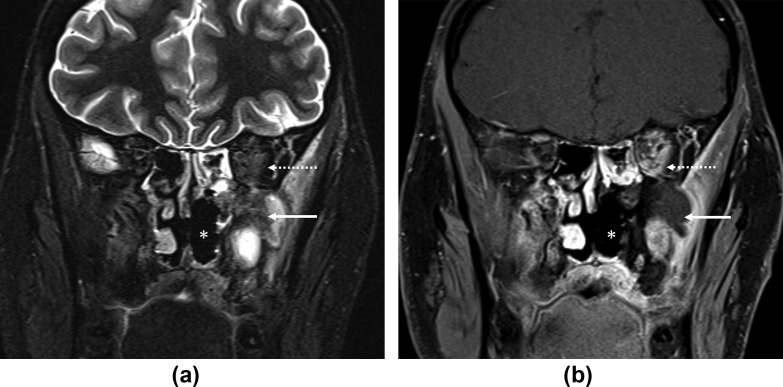
Figure 2.
Low signal residual lesion after surgery. (a) Coronal fat-saturated T2-STIR and (b) contrast-enhanced, fat-saturated T1 images through the posterior aspect of the maxillary sinuses show a remnant T2 hypointense, non-enhancing mass (arrows) in the left maxillary sinus extending into the left orbit (dotted arrows). Prior left turbinectomies are seen (asterisks). A 27-year-old, non-diabetic man with left facial numbness, hearing loss, and headache. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 33 days.
T1 without fat saturation, T2-STIR (short tau inversion recovery) and T1 contrast-enhanced fat-saturated sequences in the axial and coronal planes are particularly useful in evaluation for mucormycosis. These sequences assist in the differentiation of mucormycosis from other non-specific inflammatory sinonasal processes, given the significant overlap in imaging findings. At Kokilaben Dhirubhai Ambani Hospital, dedicated thin sections with these sequences are obtained through the face/paranasal sinuses with the following parameters: coronal T1 non-fat-saturated, T2-STIR and T1 contrast-enhanced, fat-saturated fast spin echo (FSE) images (3 mm section thickness, 1 mm gap, 17 cm field of view [FOV]) from nose tip to brainstem; axial T2-STIR and T1 contrast-enhanced, fat-saturated FSE images (3 mm section thickness, 1 mm gap, 17 cm FOV) from top of hyoid to frontal sinuses. Diffusion images (b = 800) are obtained in select cases in the axial or coronal planes. Imaging of the brain with angiograms is added when required. Multidetector CT study of the paranasal sinuses (1 mm section thickness, 210 mm FOV) with both soft-tissue and sharp (bone) kernels, is performed without contrast medium from the top of hyoid to the top of the frontal sinuses. Reconstructions are obtained in the sagittal and coronal plane as needed.
Prior studies have shown contrast-enhanced MRI to demonstrate mucormycosis at considerable distance from the primary focus of infection by perineural spread in addition to perivascular spread. The fungi invade the perineural connective tissue of the cranial nerves and their branches.13 Perineural spread is known along the trigeminal nerve14 , 15 and rarely along the labyrinthine segment of the facial nerve and geniculate ganglion with retrograde extension into the internal auditory canal (IAC) (Fig 3 a). Involvement of the pterygopalatine fossa (Fig 4 ) ipsilateral to the diseased nasal cavity in the absence of bone destruction is explained by perineural spread along the superior nasal nerves and sphenopalatine artery.11 Similarly, involvement of the periantral fat is common without intervening bone destruction. Thus, although bone dehiscence is a specific marker for IFRS, due to low sensitivity it is not a useful exclusion criterion.11
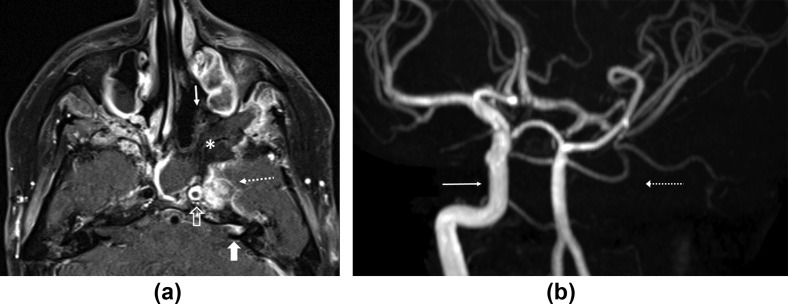
Figure 3.
Perineural spread, vasculitis, and ICA occlusion. (a) Axial contrast-enhanced fat-saturated T1 image through the sphenoid sinus shows a large area of non-enhancing necrotic soft tissue in the left sphenoid and ethmoid sinuses (asterisk) with lack of mucosal enhancement (arrow). Abnormal asymmetric enhancing soft tissue is seen in the left cavernous sinus (dotted arrow) with thrombosis of the left ICA (open arrow). Note perineural extension of disease from the left cavernous sinus (along the greater superficial petrosal nerve) into the distal left internal auditory canal (thick arrow). (b) Time-of-flight MR angiogram brain image with maximum intensity projection algorithm shows loss of flow enhancement in the left intracranial ICA (dotted arrow). Normal flow enhancement is seen in the right intracranial ICA (arrow). A 27-year-old, non-diabetic man with left facial numbness, hearing loss and headache. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 33 days.
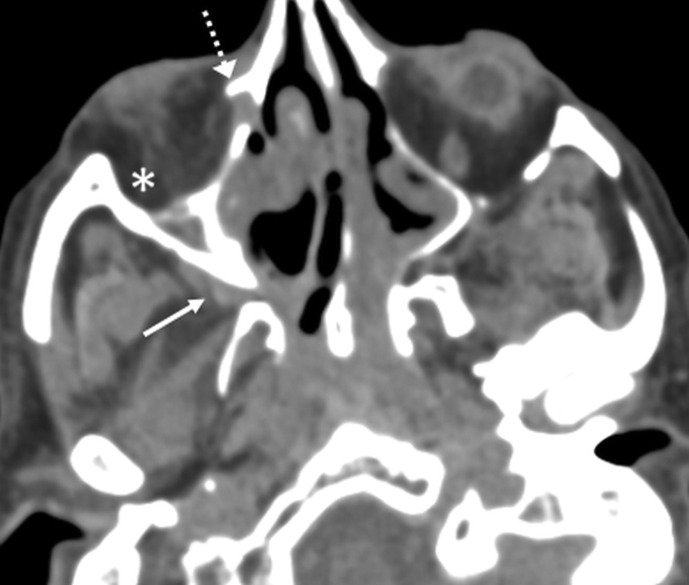
Figure 4.
Pterygopalatine fossa involvement. Axial CT image with soft-tissue algorithm/window through the inferior aspect of the orbits shows soft-tissue prominence with fat effacement in the right pterygopalatine fossa (arrow). Also seen is involvement of the ipsilateral nasolacrimal duct (dotted arrow) and infiltration of the intraorbital fat (asterisk) compatible with an orbital cellulitis. A 70-year-old, diabetic man with right facial pain, and watering and pain in the right eye. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 16 days.
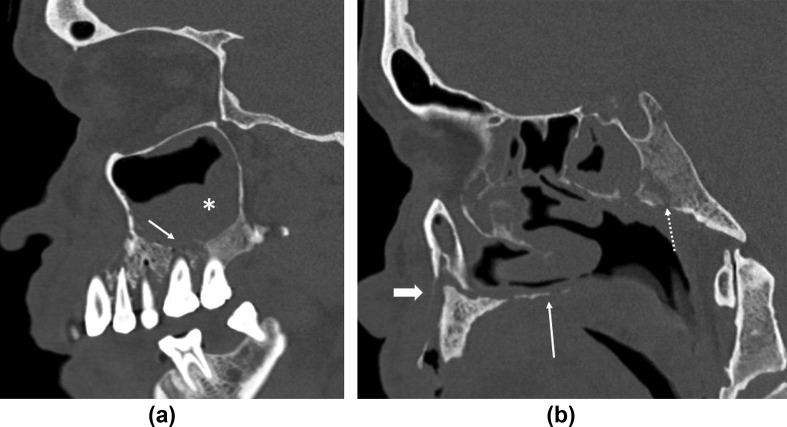
In the present epidemic, bone necrosis of the facial skeleton indicating an advanced stage is often seen at presentation16 (nearly 25% of the present cases), highlighting the fulminant nature of the disease in immunocompromised individuals. Bony nasal septal involvement may cause perforation. Bony destruction of the ipsilateral maxillary alveolus and hard palate (Fig 5 ) can lead to an oro-antral fistula and lucencies around the teeth indicating loosening. Extensive bone destruction of the skull base (Fig 5b) including the cranial nerve foramina and canals (Fig 6 ) is not uncommon.
Figure 5.
Bony destruction of the facial skeleton and skull base. (a) Sagittal CT reconstructed image in bone algorithm/window through the maxillary sinus shows bony destruction of the alveolus with tooth loosening (arrow). Extensive maxillary sinus opacification (asterisk) is seen. (b) Sagittal CT reconstructed image in bone algorithm/window through the palate shows bony destruction of the palate (thin arrow). Extensive bony destructive changes of the clivus with osteomyelitis (dotted arrow) and associated inflammatory soft tissue are seen. Also note osseous disruption of the alveolar ridge (thick arrow). A 65-year-old, diabetic man with loosening of the teeth, facial pain, and severe headache. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 21 days.
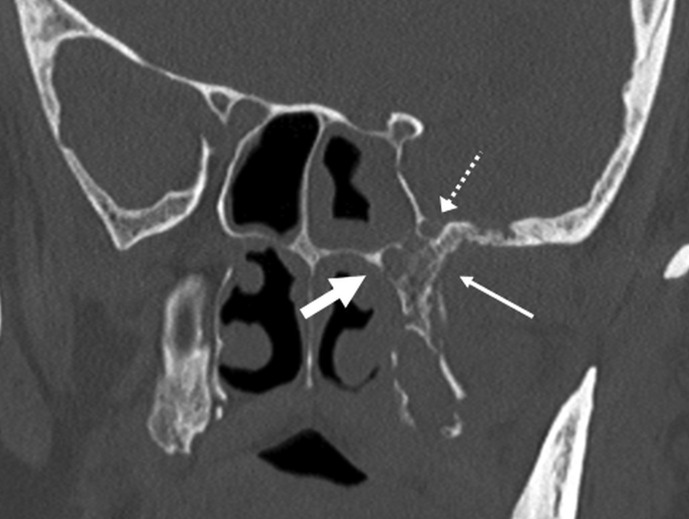
Figure 6.
Skull base foraminal involvement. Coronal reconstructed CT image through the central skull base shows bony destruction of the left pterygoid plates and sphenoid body/greater wing (arrow) with involvement of the vidian canal (thick arrow) and foramen rotundum (dotted arrow). A 65-year-old, diabetic man with loosening of the teeth, facial pain, and severe headache. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 21 days.
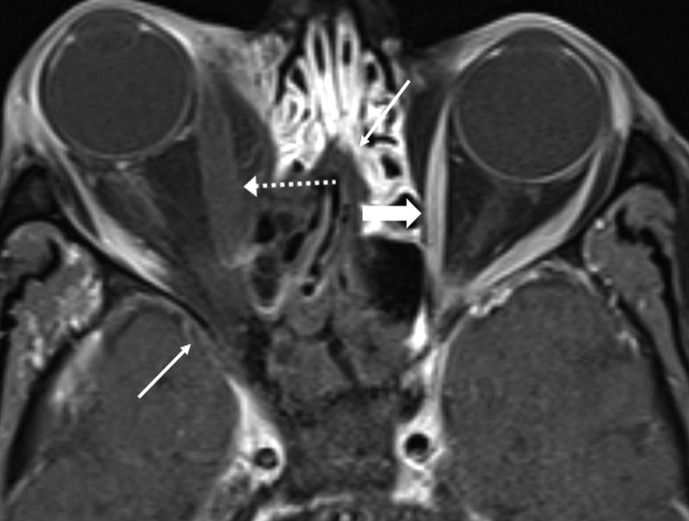
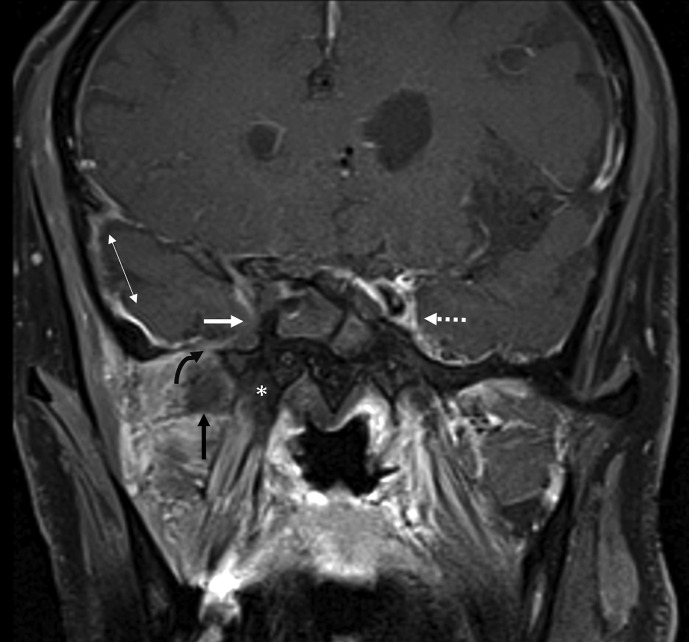
Intraorbital invasion is from ethmoid and/or frontal disease (Figure 7, Figure 8 ) or via the nasolacrimal duct17 (Fig 4) and presents with a spectrum of findings including thickened, non-enhancing extraocular muscles, pre-septal and retrobulbar oedema, optic nerve sheath enhancement, and orbital apex soft-tissue abnormality. Lack of enhancement of the extra ocular muscles (Fig 8) and low T2 signal non-enhancing orbital apex soft tissue are not seen in bacterial orbital cellulitis. Involvement of the ipsilateral cavernous sinus may present with asymmetric bulging contour (thrombophlebitis)18 (Fig 3a) or non-enhancement (thrombosis; Fig 9 ) with prominence of the superior ophthalmic vein.
Figure 7.
Orbital extension. Coronal contrast-enhanced, fat-supressed T1 image through the frontal sinuses shows extension of fungal disease from the left frontal sinus (asterisk) into the superior extraconal space of the left orbit (arrow). Compare with normal extraconal space of the right orbit (dotted arrow). A 78-year-old, diabetic woman with left proptosis, orbital pain, and headache. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first paranasal sinus imaging was 25 days.
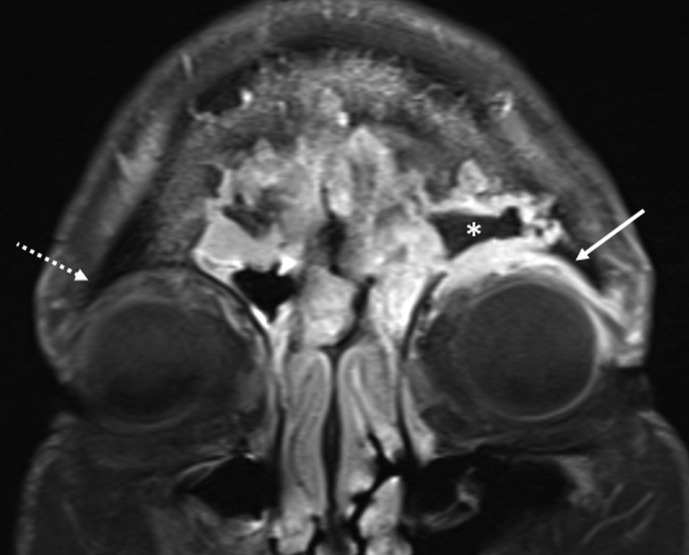
Figure 8.
Orbital involvement with non-enhancing medial rectus. Axial contrast-enhanced, fat-saturated T1 image through the orbits shows large non-enhancing areas (arrows) of devitalised soft tissue, centred in the right posterior ethmoidal air cells and sphenoid sinus extending into the right orbit. Thickening of the right medial rectus muscle without enhancement (dotted arrow) is notable. Compare with left medial rectus muscle, which shows normal enhancement (thick arrow). A 65-year-old, diabetic man with right orbital pain and headache. Treated for COVID-19 with injectable corticosteroids and invasive ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 17 days.
Figure 9.
Cavernous sinus thrombosis. Coronal contrast-enhanced, fat-saturated image at the level of the pterygoid plates shows non-enhancing right cavernous sinus (white arrow). Compare with normal enhancement of left cavernous sinus (dotted arrow). Non-enhancing fungal disease involves the right pterygoid plates (asterisk). Non-enhancing soft tissue is seen in the right infratemporal fossa (black arrow). Note extension of fungal disease into the right foramen ovale (curved arrow). Intracranial dural thickening is prominent in the right middle cranial fossa (double arrow). A 65-year-old diabetic man with right orbital pain and headache. Treated for COVID-19 with injectable corticosteroids and invasive ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 17 days.
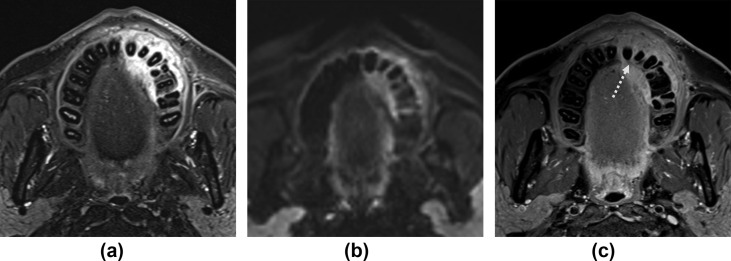
A striking feature of skull-base osteomyelitis (SBO) in mucormycosis is extensive soft-tissue infarction and necrosis, which appears as large areas of non-enhancing profoundly low signal devitalised soft tissue in and around the bony central skull base (Fig 3a) and along the nasopharynx.19 This is sometimes referred to as extrasinonasal LoCE lesion7 and not encountered in bacterial SBO.20 Abscess formation seen in bacterial SBO is not a feature of fungal SBO. Thus, necrosis or tissue infarction are features of fungal SBO contrasting with suppuration, which is the hallmark of bacterial/pyogenic SBO.21 While fungal SBO originates from fungal inoculation in the nasal cavities, bacterial SBO is usually otogenic in origin. Enhancement patterns of IFRS are known to be diverse.22 Occasionally, infected soft tissue in maxillofacial or skull base fungal disease may show an alternative contrast enhancement pattern; presenting as variable signal mass, such as soft-tissue thickening, that enhances avidly and also displays restricted diffusion (Fig 10 ).
Figure 10.
Variable patterns of signal and enhancement in a left maxillary alveolus lesion. Axial (a) T2-STIR, (b) diffusion (b = 800), and (c) contrast-enhanced, fat-saturated T1 images show bony erosion in the left maxillary alveolus (dotted arrow) with an adjacent soft-tissue mass that displays high T2 signal, restricted diffusion and avid contrast enhancement. A 50-year-old, diabetic man presented with loosening of teeth, gum swelling, and midface pain. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 19 days.
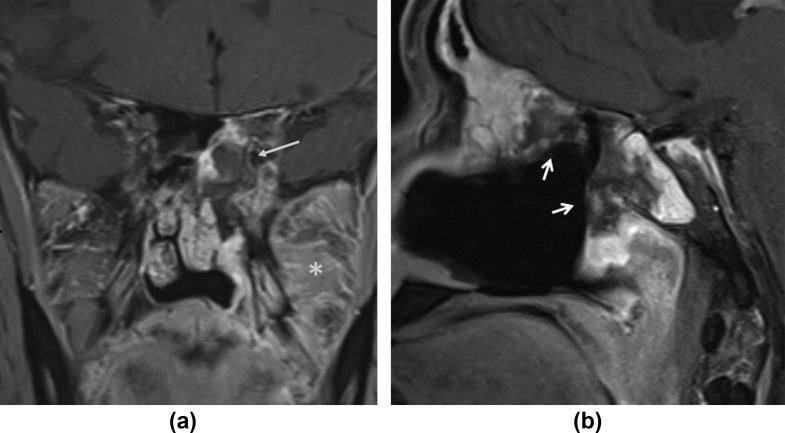
On postoperative MRI obtained after surgical exenteration, any residual LoCE lesions are associated with a higher mortality and merit further debridement (Fig 11 ). When infarcted soft tissue cannot be completely eradicated by surgery due to poor access (pterygopalatine fossa) or possibility of complications (cavernous sinus), the prognosis is grim; however, residual contrast-enhancing lesions (including in the orbits and brain) may be conserved with antifungal medication.7
Figure 11.
Remnant LoCE lesion. (a) Coronal and (b) sagittal contrast-enhanced, fat-saturated T1 images show remnant non-enhancing lesions (arrows) in the left sphenoid and ethmoid sinuses following debridement of the ipsilateral nasal cavity and maxilla. Note enhancing soft-tissue inflammation in the left infratemporal fossa (asterisk). Repeat exploration and debridement confirmed presence of invasive fungal hyphae. A 56-year-old diabetic male with left facial pain. Treated for COVID-19 with injectable corticosteroids and invasive ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 15 days.
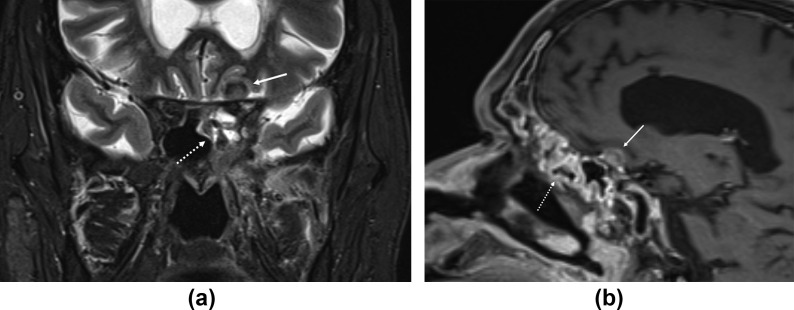
Large-vessel vasculitis is another distinct feature of rhino-cerebral mucormycosis, seen as mural thickening and enhancement of the intracranial internal carotid artery (ICA) (Fig 3a), sometimes leading to ICA occlusion (Fig 3b) and cerebral infarction. The vasculitis is secondary to direct invasion of the vessel wall by fungal elements. The infarcts from fungal vasculitis may be either bland or laden with fungal elements. Intracranial extension with pachymeningitis, dural-based mass-like lesions (Fig 12 ) and cerebritis in the frontal or temporal lobes is also encountered. Vascular invasion and neurotropism are established pathological attributes of invasive mucormycosis.23
Figure 12.
Intracranial extension. (a) Coronal T2-STIR and (b) sagittal contrast-enhanced T1 images show extension from the paranasal sinuses (dotted arrows) into the anterior cranial fossa (solid arrows). Intracranial extension appears as a dural based, T2 hypointense, enhancing lesion along the left planum sphenoidale. A 64-year-old, diabetic man presented with left facial pain and headache. Treated for COVID-19 with injectable corticosteroids and mechanical ventilation. Duration between RT-PCR positivity and first maxillofacial imaging was 33 days.
Validation of imaging diagnosis and treatment
In clinically or radiologically suspected cases, diagnosis is validated by potassium hydroxide (KOH) wet-mount preparation and histopathology of specimens obtained from the nasal cavity and/or paranasal sinuses that demonstrate broad aseptate filamentous fungi branching at right angles with tissue invasion. Culture on Sabouraud's dextrose agar shows growth of Mucor species.
Systemic amphotericin B with surgical debridement of sinuses, orbital exenterations, and diabetes control remain the mainstay of treatment. Notably, recent studies show higher survival rates in patients diagnosed promptly with use of early imaging.18 , 24
Conclusions
A subset of diabetic COVID-19 patients treated with steroids, oxygen, and/or prolonged intensive care admission develop rhino-orbito-cerebral mucormycosis.25 Radiologists must have a high index of suspicion for early diagnosis. IFRS is a fulminant condition that can lead to devastating blindness, stroke, and death as early as 48 h after presentation. Early diagnosis prompts immediate institution of antifungal therapy that limits morbidity and mortality. Assessment of disease extent by imaging is crucial for planning extent of surgical debridement. Complete debridement of necrotic tissue improves survival.26
Contrast-enhanced MRI with complementary plain CT are the imaging techniques of choice. The classic “black turbinate” on contrast-enhanced imaging represents localised IFRS. A striking radiological feature of disseminated/advanced craniofacial disease is non-enhancing devitalised and necrotic soft tissue at the orbits and central skull base. Sinonasal and extrasinonasal non-enhancing lesions in IFRS are secondary to coagulative necrosis induced by fungal elements. Multicompartmental and extrasinonasal tissue infarction is possible without overt bone involvement and caused by propensity of fungal elements to disseminate from the nasal cavity via perivascular and perineural routes. Assessment for the non-enhancing MRI lesion is crucial as it is a sole independent prognostic factor for IFRS-specific mortality.22
Conflict of interest
The authors declare no conflict of interest
References
- 1.Moorthy A., Gaikwad R., Krishna S., et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021; Mar;6:1–8. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notify mucormycosis under Epidemic Act: centre to states as cases rise . May, 2021. The times of India 21 may 2021.http://timesofindia.indiatimes.com/articleshow/82818309.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst/ [Google Scholar]
- 3.Groppo E.R., El-Sayed I.H., Aiken A.H., et al. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2011;137:1005–1010. doi: 10.1001/archoto.2011.170. [DOI] [PubMed] [Google Scholar]
- 4.Safder S., Carpenter J.S., Roberts T.D., et al. The “black turbinate” sign: an early MR imaging finding of nasal mucormycosis. AJNR Am J Neuroradiol. 2010;3:771–774. doi: 10.3174/ajnr.A1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Q., Escott E.J. The black turbinate sign, a potential diagnostic pitfall: evaluation of the normal enhancement patterns of the nasal turbinates. AJNR Am J Neuroradiol. 2019;40:855–861. doi: 10.3174/ajnr.A6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie M.B., O’Malley B.W., Jr., Francis H.W. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg. 1998;124:520–526. doi: 10.1001/archotol.124.5.520. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.H., Kang B.C., Lee J.H., et al. The prognostic value of gadolinium-enhanced magnetic resonance imaging in acute invasive fungal rhinosinusitis. J Infect. 2015;70:88–95. doi: 10.1016/j.jinf.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Terk M.R., Underwood D.J., Zee C.S., et al. MR imaging in rhinocerebral and intracranial mucormycosis with CT and pathologic correlation. Magn Reson Imaging. 1992;10:81–87. doi: 10.1016/0730-725x(92)90376-b. [DOI] [PubMed] [Google Scholar]
- 9.Green W.H., Goldberg H.I., Wohl G.T. Mucormycosis infection of the craniofacial structures. Am J Roentgenol Radium Ther Nucl Med. 1967;101:802–806. doi: 10.2214/ajr.101.4.802. [DOI] [PubMed] [Google Scholar]
- 10.Silverman C.S., Mancuso A.A. Periantral soft-tissue infiltration and its relevance to the early detection of invasive fungal sinusitis: CT and MR findings. AJNR Am J Neuroradiol. 1998;19:321e5. [PMC free article] [PubMed] [Google Scholar]
- 11.Middlebrooks E.H., Frost C.J., De Jesus R.O., et al. Acute invasive fungal rhinosinusitis: a comprehensive update of CT findings and design of an effective diagnostic imaging model. AJNR Am J Neuroradiol. 2015;36:1529–1535. doi: 10.3174/ajnr.A4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni Mhurchu E., Ospina J., Janjua A.S., et al. Fungal rhinosinusitis: a radiological review with intraoperative correlation. Can Assoc Radiol J. 2017;68:178–186. doi: 10.1016/j.carj.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Sravani T., Uppin S.G., Uppin M.S., et al. Rhinocerebral mucormycosis: pathology revisited with emphasis on perineural spread. Neurol India. 2014;62:383–386. doi: 10.4103/0028-3886.141252. [DOI] [PubMed] [Google Scholar]
- 14.McLean F.M., Ginsberg L.E., Stanton C.A. Perineural spread of rhinocerebral mucormycosis. AJNR Am J Neuroradiol. 1996;17:114–116. [PMC free article] [PubMed] [Google Scholar]
- 15.Orgue S., Yuceturk A.V., Demir M.A., et al. Rhinocerebral mucormycosis: perineural spread via the trigeminal nerve. J Clin Neurosci. 2005;12:484–486. doi: 10.1016/j.jocn.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Joshi A.R., Muthe M.M., Patankar S.H., et al. CT and MRI findings of invasive mucormycosis in the setting of COVID-19: experience from a single center in India. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.26205. June 23. [DOI] [PubMed] [Google Scholar]
- 17.Herrera D.A., Dublin A.B., Ormsby E.L., et al. Imaging findings of rhinocerebral mucormycosis. Skull Base. 2009;19:117–125. doi: 10.1055/s-0028-1096209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae M.S., Kim E.J., Lee K.M., et al. Rapidly progressive rhino-orbito-cerebral mucormycosis complicated with unilateral internal carotid artery occlusion: a case report. Neurointervention. 2012;7:45–49. doi: 10.5469/neuroint.2012.7.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo J., Kim H.J., Chung S.K., et al. Cervicofacial tissue infarction in patients with acute invasive fungal sinusitis: prevalence and characteristic MR imaging findings. Neuroradiology. 2013;55:467–473. doi: 10.1007/s00234-013-1147-8. [DOI] [PubMed] [Google Scholar]
- 20.Álvarez Jáñez F., Barriga L.Q., Iñigo T.R., et al. Diagnosis of skull base osteomyelitis. RadioGraphics. 2021;41:156–174. doi: 10.1148/rg.2021200046. [DOI] [PubMed] [Google Scholar]
- 21.Gaviani P., Schwartz R.B., Hedley-Whyte E.T., et al. Diffusion-weighted imaging of fungal cerebral infection. AJNR Am J Neuroradiol. 2005;26:1115–1121. [PMC free article] [PubMed] [Google Scholar]
- 22.Choi Y.R., Kim J.H., Min H.S., et al. Acute invasive fungal rhinosinusitis: MR imaging features and their impact on prognosis. Neuroradiology. 2018;60:715–723. doi: 10.1007/s00234-018-2034-0. [DOI] [PubMed] [Google Scholar]
- 23.Morace G., Borghi E. Invasive mold infections: virulence and pathogenesis of mucorales. Int J Microbiol. 2012;2012:349278. doi: 10.1155/2012/349278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S., Goyal R., Kaore N.M. Rhino-orbital-cerebral mucormycosis: battle with the deadly enemy. Indian J Otolaryngol Head Neck Surg. 2020;72:104–111. doi: 10.1007/s12070-019-01774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel A., Agarwal R., Rudramurthy S.M., et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021; Jun;4:27. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong H.L., Lee Y.M., Kim T., et al. Risk factors for mortality in patients with invasive mucormycosis. Infect Chemother. 2013;45:292e8. doi: 10.3947/ic.2013.45.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]