Abstract
Since the beginning of vaccination programs against COVID-19 in different countries, several populations such as patients with specific immunological conditions have been considered as the priorities for immunization. In this regard, patients with autoimmune diseases or those receiving immunosuppressive agents and anti-cancer therapies, need special attention. However, no confirmed data is presently available regarding COVID-19 vaccines in these populations due to exclusion from the conducted clinical trials. Given the probable suppression or over-activation of the immune system in such patients, reaching a consensus for their vaccination is critical, besides gathering data and conducting trials, which could probably clarify this matter in the future. In this review, besides a brief on the available COVID-19 vaccines, considerations and available knowledge about administering similar vaccines in patients with cancer, hematopoietic stem cell transplantation, solid organ transplantation, multiple sclerosis (MS), inflammatory bowel disease (IBD), and rheumatologic and dermatologic autoimmune disorders are summarized to help in decision making. As discussed, live-attenuated viruses, which should be avoided in these groups, are not employed in the present COVID-19 vaccines. Thus, the main concern regarding efficacy could be met using a potent COVID-19 vaccine. Moreover, the vaccination timing for maximum efficacy could be decided according to the patient’s condition, indicated medications, and the guides provided here. Post-vaccination monitoring is also advised to ensure an adequate immune response. Further studies in this area are urgently warranted.
Keywords: Transplantation, Cancer, Multiple sclerosis, Inflammatory bowel disease, Vaccination, Hematologic malignancies
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has become a disastrous pandemic since its first outbreak in Dec. 2019 [1]. Until July 7, 2021, more than 184 million people were infected by COVID-19 leading to over four million deaths [2]. While vaccines and drugs are two arms in controlling the pandemic, from the beginning of the pandemic, many efforts have been devoted to the development of both solutions [3], [182], especially safe and effective vaccines as one of the most reliable interventions to suppress viral transmission [5].
Presently, several vaccines are available worldwide with different platforms, administration schedules, and efficacies. However, the race is being continued with many other vaccines under evaluation in different clinical trial phases, which can boost the vaccination process if approved, especially those that are already in phase 3 (All vaccines in phase 3 and 4 clinical trials are summarized in Table 1 ). In the United States, three COVID-19 vaccines are authorized for emergency use, namely Pfizer-BioNTech, Moderna, and Janssen vaccines, which are all non-living vaccines. Pfizer-BioNTech and Moderna vaccines are nucleoside-modified mRNA vaccines; and the Janssen vaccine is a recombinant replication-incompetent adenovirus type 26 (Ad26) [6]. In Britain, the United Kingdom Medicines and Healthcare Products Regulatory Agency approved the Oxford-AstraZeneca vaccine, which is a recombinant replication-incompetent chimpanzee adenovirus vector encoding SARS-CoV-2 spike (S) protein [7]. Two other adenovirus vector vaccines are produced in Russia and China named Sputnik V® and CoronaVac®, respectively, which are authorized by their origin countries [8], [9] and are being used in some other parts of the world as well.
Table 1.
COVID-19 vaccines that are in phase 3 or 4 of clinical trials, based on WHO report on July 6, 2021 [56].
| Developer | Platform | Clinical trial phase | Number of doses | Dose schedule in healthy cases (day) | Study report | |
|---|---|---|---|---|---|---|
| 1 | Sinovac Research and Development Co., Ltd | Inactivated virus | 4 | 2 | 0, 14 | [57], [59] |
| 2 | Sinopharm + China National Biotec Group Co + Wuhan Institute of Biological Products | Inactivated virus | 3 | 2 | 0, 21 | – |
| 3 | Sinopharm + China National Biotec Group Co + Beijing Institute of Biological Products | Inactivated virus | 4 | 2 | 0, 21 | – |
| 4 | Institute of Medical Biology + Chinese Academy of Medical Sciences | Inactivated virus | 3 | 2 | 0, 28 | [61] |
| 5 | Research Institute for Biological Safety Problems, Rep of Kazakhstan | Inactivated virus | 3 | 2 | 0, 21 | – |
| 6 | Bharat Biotech International Limited | Inactivated virus | 3 | 2 | 0, 14 | [62] |
| 7 | Shifa Pharmed Industrial Co | Inactivated virus | 2/3 | 2 | 0, 14 | – |
| 8 | Shenzhen Kangtai Biological Products Co., Ltd. | Inactivated virus | 3 | 2 | 0, 28 | – |
| 9 | Valneva. National Institute for Health Research. | Inactivated virus | 3 | 2 | 0, 21 | – |
| 10 | Erciyes University, Turkey | Inactivated virus | 3 | 2 | 0, 21 | – |
| 11 | AstraZeneca + University of Oxford | Viral vector (Non-replicating) | 4 | 1–2 | 0, 28 | [7], [45] |
| 12 | CanSino Biological Inc./ Beijing Institute of Biotechnology | Viral vector (Non-replicating) | 4 | 1 | 0 | [64] |
| 13 | Gamaleya Research Institute; Health Ministry of the Russian Federation | Viral vector (Non-replicating) | 3 | 2 | 0, 21 | [8] |
| 14 | Janssen Pharmaceuticals | Viral vector (Non-replicating) | 4 | 1–2 | 0 or 0, 56 | [65] |
| 15 | ReiThera + Leukocare + Univercells | Viral vector (Non-replicating) | 2/3 | 1 | 0 | – |
| 16 | Medicago Inc. | Virus like particle (VLP) | 2/3 | 2 | 0, 21 | [66] |
| 17 | Novavax | Protein subunit | 3 | 2 | 0, 21 | [67] |
| 18 | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Protein subunit | 3 | 2–3 | 0, 28 or 0, 28, 56 | [68] |
| 19 | Clover Biopharmaceuticals Inc./GSK/Dynavax | Protein subunit | 2/3 | 2 | 0, 21 | – |
| 20 | Sanofi Pasteur + GSK | Protein subunit | 3 | 2 | 0, 21 | – |
| 21 | EpiVacCorona (EpiVacCorona vaccine based on peptide antigens for the prevention of COVID-19) | Protein subunit | 3 | 2 | 0, 21 | – |
| 22 | Instituto Finlay de Vacunas | Protein subunit | 3 | 2 | 0, 28 | – |
| 21 | Center for Genetic Engineering and Biotechnology (CIGB) | Protein subunit | 3 | 3 | 0, 14, 28 or 0, 28, 56 | – |
| 23 | Vaxxinity | Protein subunit | 2/3 | 2 | 0, 28 | – |
| 24 | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” | Protein subunit | 3 | 2 | 0, 21 | – |
| 25 | West China Hospital + Sichuan University | Protein subunit | 3 | 2 | 0, 28 | – |
| 26 | Inovio Pharmaceuticals + International Vaccine Institute + Advaccine (Suzhou) Biopharmaceutical Co., Ltd | DNA based vaccine | 2/3 | 2 | 0, 28 | [69] |
| 27 | AnGes + Takara Bio + Osaka University | DNA based vaccine | 2/3 | 2 | 0, 14 | – |
| 28 | Zydus Cadila | DNA based vaccine | 3 | 3 | 0, 28, 56 | – |
| 29 | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | RNA based vaccine | 4 | 2 | 0, 28 | [70] |
| 30 | Pfizer/BioNTech + Fosun Pharma | RNA based vaccine | 4 | 2 | 0, 21 | [53], [71] |
| 31 | CureVac AG | RNA based vaccine | 3 | 2 | 0, 28 | – |
| 32 | Academy of Military Sciences (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | RNA based vaccine | 3 | 2 | 0, 14 or 0, 28 | – |
| 33 | Moderna TX. Inc. | RNA based vaccine | 2/3 | 3 | 0, 21, 35 | – |
Considering the importance of vaccination to control the pandemic, immunization of special subgroups such as elderlies and patients with chronic diseases, is of high importance. It is proven that the incidence of severe COVID-19 is much more in individuals with underlying comorbidities [10], [11]. Given this, patients with specific immunological deficits, such as patients with autoimmune diseases or those receiving immunosuppressive or anti-cancer agents, need special attention. Besides, vaccination in these patients is somehow problematic due to the probable suppression or over-activation of the immune system. Despite a vast number of conducted studies in regards to vaccination and the approval of several SARS-CoV-2 vaccines, such special patient groups were excluded in the performed trials. Hence, there remain questions about the efficacy and safety of vaccination in immunocompromised patients, and the lack of data to evaluate the risk/benefit balance of vaccination in these groups makes decision-making difficult. In light of these problems, formulating an effective and safe vaccination program for patients with immunological diseases requires attention and further investigations in this field. In this study, we tried to collect the presently published data on COVID-19 vaccination in immunocompromised patients and the recommendations from different societies so far to suggest some advice for safe and efficient vaccination in this population.
2. COVID-19 and immune system
As a pathogen enters the body, both innate and adaptive immunities are activated in order to eliminate the pathogen. If the immune system cannot act efficiently against pathogens, an illness appears. The innate immunity provides a fast and non-specific response, while the adaptive immunity responses are slow, pathogen-specific, and long-lasting. With this aim, helper T cells make B cells produce antibodies. Besides, cytotoxic T cells invade the cells infected by viruses and force them to die [12]. Although the above-mentioned general immunity procedure is usually induced against SARS-CoV-2, some defects might be caused by the virus in the immune system mechanism, such as interrupting the interferon signaling pathway [13], [183].
3. COVID-19 vaccines
3.1. Inactivated vaccines
Inactivated vaccines are a kind of whole-cell vaccines in which the pathogenic materials of the causing pathogen or a very similar one are destroyed by chemicals (such as formaldehyde or beta-propiolactone), radiation, or heat. This demolishes the pathogen’s ability to replicate while keeping its immunization potential [14]. Therefore, the organism cannot revert to a more pathogenic form, and the chance of interference is quite low [15]. Both neutralizing antibody (nAb) response and seroconversion have been observed in inactivated vaccines. Besides, it is shown that there would be a more potent response when the interval between two doses expands from 14 days to 21 days (increased seroconversion from 85.7% to 100%) [16], [17]. In general, these vaccines are quite safe and very stable and have shown good results in immunocompromised patients [18]. A downside of inactivated vaccines is the high quantity of the immunogen required to reach an adequate antibody response. Additionally, they can create a substantial humoral response but only a slight cellular immunity [19]. Other drawbacks include major laboratory facilities needed to grow the pathogen [20]. Moreover, producing the vaccine on a large scale and the inactivation process can be time-consuming thereby limiting vaccine production and making difficulties in developing vaccines with sufficient titers. Hence, due to the large amounts of vaccine required to evoke sufficient antibody response, the cost might increase drastically. Besides, the physical or chemical methods used to inactivate the pathogen could weaken its immunogenicity due to altering the DNA sequence, capsid, and protein structure [21]. In general, these changes might diminish the expected cell-mediated and mucosal immune responses, which could decrease the spectrum and effectiveness of the vaccine [15]. However, utilizing two or three booster doses can help to maintain adequate immunity in long term [18], and adding adjuvants such as aluminum or MF59 can help improve cellular or humoral responses depending on the adjuvant type [21], [22].
One of the inactivated vaccines manufactured for the COVID-19 infection is CoronaVac® or Sinovac® containing the inactivated virus and aluminum as an adjuvant. After the second injection, high neutral antibodies can be seen in patients, but the T cell response is quite low. However, the added aluminum adjuvant can help to initiate a T-helper 2 response [17], [23]. Studies indicated that the level of post-injection neutral antibody decreased with aging. Thus, it is recommended to elevate the dosage in [25].
3.2. Protein subunit vaccines
Subunit vaccines merely consist of the immunogenic proteins derived from a pathogen, which can stimulate the host’s immune system [4]. The proteins can be easily produced by recombinant DNA techniques [184]. They have some other advantages due to their structure, such as lacking active pathogens and triggering a specific immune response against the main antigenic proteins or epitopes of the pathogen. These characteristics give rise to their high safety [4]. However, they usually generate a weak immune response [26] due to mounting a poor innate immune response, inadequate activation of antigen-presenting cells (APCs), and limited stimulation of B cells and T cells [27]. Therefore, their immunogenicity needs to be enhanced by other strategies such as using adjuvants [4], [184], carriers, or nanotechnology approaches [26]. Moreover, multiple doses of this kind of vaccine are advised for long-lasting immunization. To overcome these limitations, an empty virus shell with coronavirus’ structure was suggested. However, manufacturing of this structure is complicated [28]. In the case of SARS-CoV-2, the S protein and its fragments including S1, as well as the RBD (receptor-binding domain) and N (nucleocapsid) proteins are candidate for subunit vaccine development. Most of the in-development vaccines have employed RBD as the antigen, since immunization against it may protect the host cells from viral binding to ACE2 and invasion into host cells [29]. As reported, an anti-SARS-CoV vaccine based on RBD antigen and alum had a high protective immune response, and interestingly, RBD vaccines could minimize the risk of host immunopotentiation [30], [31]. It was shown that protein subunit vaccines together with adjuvants generated a potent response against SARS-CoV-2. In the case of Novavax, nAbs were 100-times higher after the second dose and 4-times higher than symptomatic outpatients after 35 days [32].
3.3. DNA vaccines
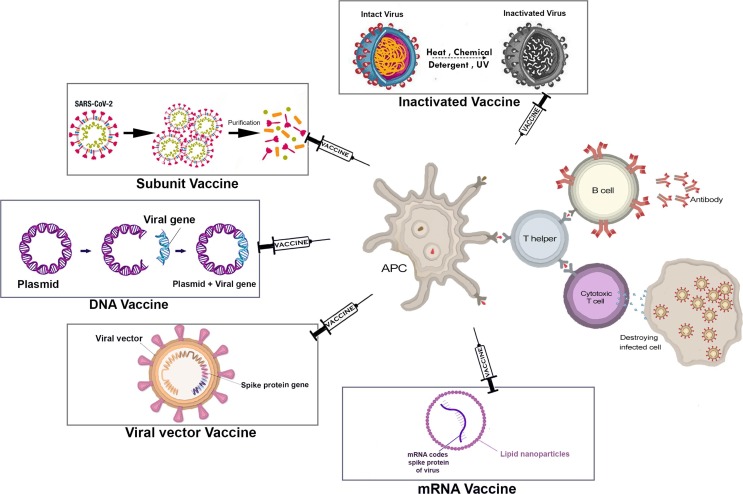
DNA vaccines are produced by inserting the gene of an antigenic protein into a bacteria-derived plasmid and delivering it to the host cells. The protein-producing machinery of the host cell will translate DNA to mRNA and finally, a protein that can stimulate the immune system (Fig. 1 ). Formulation of these vaccines is designed to translocate the gene to host cells’ nuclei. Though, some other methods such as electroporation, gene gun, and jet injection might be used to enhance DNA entry into cells [33]. APCs are among the most important recipients of DNA plasmids, which translate them to mRNA and protein. Myocytes and dendritic cells (DCs) are other recipients of these DNA plasmids. It is shown that DNA vaccines can activate both cellular and humoral immunity. This might be due to the cross-presentation of antigens on MHC-I and MHC-II and consequently, stimulation of both CD4+ and CD8+ T cells [34]. Moreover, some intrinsic factors such as non-methylated CpG sequences can activate the innate immunity and enhance the response of adaptive immunity to that specific antigen [35], [36].
Fig. 1.
Schematic representation of COVID-19 vaccine platforms and their mechanism of immunogenicity.
Some advantages and disadvantages are already addressed for DNA vaccines. Broad immunity response in both humoral and cellular arms, no risk of pathogen replication, supporting multiple antigens in a single vaccine, low-cost and large-scale production, and high storage stability are some of the benefits of this vaccine platform [35]. On the other hand, some concerns are mentioned about DNA vaccines. There is a small risk of integration of the plasmid into the host chromosome, which should be considered specifically in the enhanced DNA uptake systems. Furthermore, the long-term persistence of these vaccines increases the risk of integration and the following threat of mutagenesis and oncogenesis. Besides, antibiotic resistance markers in vector plasmids had raised some concerns about antibiotic resistance, which has been replaced in the newer generation of DNA vaccines. Furthermore, some adjuvants employed in the formulation of these vaccines to enhance vaccine immunogenicity, for example, pattern recognition protein (PRP) ligands and IL-12, may cause unintended adverse effects [33]. Among COVID-19 in-development vaccines, Inovio is a DNA vaccine whose intradermal injection would be followed by CELLECTRA® electroporation [37]. However, this should be considered that electroporation device, as an essential step of administration of this vaccine, might not be available everywhere.
3.4. Viral vector vaccines
A viral vector is a modified virus used as a means to deliver a target gene to the host cell. Modified virus is obtained by inserting an immunogenic part of the desired virus, which is the S protein gene for SARS-CoV2 into a safe and non-pathogenic virus as a vector [38]. The virus used as a vector does not contain pathogenic genes and cannot cause severe adverse outcomes. Thus, they are not a threat to the patients' safety [38], [39]. Different viruses are used as vectors, such as cytomegalovirus, measles, vesicular stomatitis virus, modified adenovirus, and many others [40].
As viral vector vaccines enter the cells, they use the cells’ protein-making machinery to produce their antigens, which are S proteins displayed on the cells' surface. When these antigens are expressed on the cell surface, they trigger an immune response in the patient including both cellular and humoral responses [26], [39].
There are two types of viral vector vaccines: non-replicating and replicating. The non-replicating vectors do not have genes responsible for replication, so they cannot replicate and only produce virus antigens. On the other hand, replicating vectors, multiply in the body and infect other cells, causing all cells to produce antigens [39].
There are four non-replicating adenoviral vector vaccines for COVID-19 at phases 3 and 4 of clinical trials. The AstraZeneca-Oxford vaccine is composed of a chimpanzee adenovirus that is in phase 4. Moreover, the Cansino Biologics vaccine, Janssen vaccine, and Sputnik V are at phase 3. Additionally, Beijing Wantai Biological Pharmacy, the University of Xiamen, and the University of Hong Kong are working together on the development of an intranasal vaccine containing a replicating viral vector based on influenza, which is in clinical trial phase 2 [42], [43].
Advantages of viral vectors include efficient gene transduction and delivery of the gene to the target cells. Furthermore, they trigger a strong immune response including T cells and antibodies produced by B cells [44]. Data obtained from the AstraZeneca-Oxford clinical trials showed that vaccination led to both T cell responses and nAbs formation. The first dose caused the immunological memory and the second dose fixed it. T cells peaked in 28 days after the first dose and stayed elevated in the body for 56 days. The second dose could be administered 4–12 weeks after the first dose. Moreover, the second administration did not affect T cell elevation but could end in more nAb production. Trials have represented a relationship between dose intervals and nAb concentration. As the time gap between the first and second doses gets longer, more nAb responses would be observed [17], [45].
Due to strong immune responses, this type of vaccine usually does not need any additive adjuvants. However, in some vectors, adjuvants may be useful [33]. Moreover, designing, modifying, and large-scale manufacturing of adenovirus vaccines are easy [17], [38]. Generally, the virus genome does not integrate into the host’s DNA [38]; but some viral vectors have an integration mechanism to express genes that may cause cancers [44].
There are also some challenges including the complex technology needed to manufacture the viral vectors. Moreover, due to previous exposure to the virus acting as a vector, such as an adenovirus that causes the common cold [38], patients may have pre-existing immunity to those viruses, leading to reduced vaccine efficacy [44]. To overcome this obstacle, scientists use uncommon viruses or viruses from other species (such as chimpanzees) for designing viral vector vaccines [47]. The same issue could also occur in the second-dose vaccination due to re-exposure to the same vector [39]. In this regard, using different types of adenoviruses for the first and second doses can help to reach the desired efficacy. For instance, Sputnik V vaccine utilizes the Ad26 serotype for the first dose followed by a boost dose with the Ad5 vector, 21 days later [48].
3.5. mRNA vaccines
mRNA vaccines area type of nucleic acid vaccines. They should reach the ribosomes on the endoplasmic reticulum to be translated into specific proteins that could trigger the immune response in a patient. mRNA vaccines are usually made of mRNA molecules encapsulated in lipid nanoparticles (LNP), as shown in Fig. 1. LNP facilitate mRNA penetration into the host cell so that the translation process can be initiated [14]. The cellular immune response is seen when the S proteins are presented to immune cells either by the MHC class I or II complex, activating both CD4+ and CD8+ cells. However, their main mechanism is activating the humoral immune response. When naïve B cells are activated due to their exposure to the S proteins, they start to proliferate and differentiate into either memory B cells or plasma cells with the ability to secret antibodies. Hence, when the virus carrying the antigen enters the body, the antibody will bind to the antigen, block it, and prevent the infection [50], [51].
Two important mRNA vaccines for COVID-19 are mRNA-1273 by Moderna TX, Inc and BNT162b1 by BioNTech-FosunPharma-Pfizer.
-
–
The mRNA-1273 vaccine encodes the stabilized pre-fusion form of the S proteins ofSARS-CoV-2 and can trigger extremely high specific antiviral responses against the S protein as well as nAbs, especially after the second injection [52].
-
–
BNT162b1 encodes the SARS-CoV-2 RBD. Moreover, adding a T4 fibritin (fold on)-derived trimerization domain to the RBD antigen led to an increase in vaccine efficacy [52]. BNT162b1 induced high titers of nAbs against SARS-CoV-2. Virus-specific T-helper1 and CD8+ T cells were also seen after administering the second dose [53], [54].
Some benefits of mRNA vaccines include their inability to cause infection or insertional mutagenesis, fast uptake, and expression by the host cells, which can be attained with the right formulation and carrier molecules attached to the mRNA. Furthermore, their rapid and inexpensive production due to the high efficacy of in vitro transcription is of great value in manufacturing mRNA vaccines [55].
A significant disadvantage of these vaccines is their unstable nature there by requiring a storage temperature of −70 to −20 °C, which can cause difficulties in their access and distribution. Although, utilizing specific mutations of the gene and chemical stabilizers have allowed keeping the mRNA vaccines at a temperature between 2 and 8 °C for a short period [14].
4. Vaccination in immunocompromised patients
Generally, live-attenuated vaccines are not usually recommended in primary or acquired immunodeficient patients because of the risk of infection development by the live pathogens present in the vaccine [73]. Live-attenuated vaccines are not currently under development or approved for COVID-19. However, almost all other types of vaccines are found in the list of approved or understudy vaccines (Table 1).
4.1. Cancer and hematopoietic stem cell transplantation
Numerous studies imply that cancer patients are at a greater risk of developing severe COVID-19. The more susceptibility of cancer patients to COVID-19 is due to the immunosuppression caused by different employed therapeutic approaches such as chemotherapy, radiotherapy, and stem cell transplantation. For instance, a retrospective cohort study in which 39% of patients were on anticancer therapies and 43% had active cancer, demonstrated that patients with cancer had a higher 30-day all-cause mortality rate, which was related to the risk factors exclusive to cancer [74]. In another study on patients with cancer and COVID-19 infection, patients with tumors showed an increased risk for developing severe COVID-19 infection, poor clinical outcomes, and death [38]. These shreds of evidence make cancer patients a priority for COVID-19 vaccination.
Since cancer patients have a different immunological situation due to receiving anti-cancer medications and the nature of the tumor itself, their possible response to COVID-19 vaccines might be affected. Immune suppression in cancer patients who are on chemotherapy is not complete, and they can show a response against vaccination. This response varied between 10% in patients with acute lymphoblastic leukemia, who received HBV (hepatitis B virus) vaccine, to 100% in patients immunized by tetanus and diphtheria vaccines [75], [76]. A similar response was observed in patients who received the inactivated influenza vaccine. Overall, it seems that vaccination can generate a proper immune response except for periods of intensive chemotherapy [26].
Patients who are on targeted therapies, such as tyrosine kinase inhibitors, could produce seroprotection against the influenza vaccine, which was comparable to healthy individuals [77]. Controversially, 7–26% of patients on ibrutinib had impaired response and experienced seroconversion following an influenza vaccine, while about 75% of patients could respond to the varicella-zoster virus (VZV) subunit vaccine [78], [79]. However, it seems that patients with cancer that are receiving targeted therapies can still produce enough immune response against vaccines [26].
Plasma-cell-depleting and lymph depleting therapies, such as anti-CD20 and anti-CD38 monoclonal antibodies, reduced the peripheral B cells for at least four months [80]. During this period, immunization with influenza, Streptococcus pneumonia, and Haemophilus influenza vaccines was impaired [81]. This suggests that patients on these medications have to postpone the vaccination for at least six months after anti-B cell therapies [82]. It should be noted that these patients are at an even higher risk for disease severity, long-time viral shedding, and death [83], [84]. On the other hand, it is also proposed that since vaccines produce stronger immune responses than SARS-CoV-2 infection itself, the possible importance of strong T cell protective immunity has to be considered as well [85].
For patients who are on immune checkpoint inhibitors (ICIs), one expects that a proper immune response would be produced, but there is a concern for immune-related adverse effects (IRAEs). IRAEs are ICI-related toxicities that are due to unintended activation of the immune system. IRAEs may occur in every organ system, but the most affected organs are the skin, gastrointestinal system/liver, endocrine, and pulmonary systems. The symptoms of IRAEs are different depending on the affected organ. Dermatologic toxicity symptoms vary from dry mouth, rash, pruritis, and mucositis to Steven-Johnson syndrome. Diarrhea, colitis, pancreatitis, and hepatitis are examples of the GI symptoms. Pulmonary symptoms include pneumonitis, sarcoidosis, and pleural effusion [86]. One study showed a great risk of IRAE in patients receiving ICIs after influenza vaccination [87]. Although, three other similar studies did not show such effects [88]. Moreover, it seems that radiotherapy could not completely impair the immune system and consequent ineffectiveness of vaccine immunization. As a result, vaccination in patients on radiotherapy could be safe [26].
COVID-19 prognosis is poor in patients receiving hematopoietic stem cell transplantation (HSCT) [89]. The 30-days overall survival for HSCT recipients was about 67% [90]. HSCT recipients should be considered as newborns, since they are immunologically naive. It is suggested that recognizing new antigens and producing an immune response would take 6–12 months in these patients. The immune response to vaccines in these patients depends on the age of vaccination, the type of vaccine, and confrontation with the pathogen before or after HSCT [91], [92]. There are different recommendations for post-HSCT vaccination based on the type of vaccine.
Inactivated vaccines should be administrated six months after HSCT. For example, inactivated trivalent influenza vaccine should be used six months after transplantation [93]. Guidelines recommend that the influenza vaccines could be administered four months after HSCT in epidemiological conditions, and a second dose should be given four weeks later. Live-attenuated vaccines are not recommended and if necessary should be administered at least 24 months after HSCT in patients who did not have graft versus host disease (GVHD) and took no immunosuppressive drugs in the last three months [93]. As an instance, live-attenuated influenza vaccines are contraindicated in HSCT recipients [94].
The trial studies regarding the effectiveness or safety of COVID-19 vaccines in cancer patients are rare, but there is one published clinical trial on the effect of COVID-19 vaccines in cancer patients. The authors showed that administration of one dose of Pfizer-BioNTech vaccine in patients with cancer was well tolerated even in those who were under immunotherapy; though, the immune response was improper at least for five weeks. Data declared that the IgG response against SARS-CoV-2 was 13% and 39% in hematologic and solid cancer patients, respectively, which is very low compared to healthy controls (97%). However, all immunological indices improved following the 21-day boost in these patients [95]. This indicates the important role of on-time second dose administration of COVID-19 vaccines in cancer patients, both for pandemic control and the individuals.
Overall, most cancer treatments could not impair proper immune response against vaccines. Moreover, most patients with cancer who participated in a trial had a functional adaptive immunity during COVID-19 infection [96]. Although, the immune response might be weak in some cancer patients’ subgroups including those who are on intensive immunosuppression or HSCT [97]. Furthermore, it should be considered that live vaccines should not be administered in patients on cytotoxic, lymphodepleting, or targeted therapies [98]. Giesen et al. suggested mRNA vaccines would be safe in cancer patients [97]. Besides, many oncology professional scientific societies including the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), Society for Immunotherapy of Cancer (SITC), and the American Association for Cancer Research (AACR) strongly recommended vaccinating patients with cancer [99].
It is suggested that patients with advanced disease, particularly lung cancer, who can postpone their systematic treatment for one month and patients undergoing radical surgery who are candidate for adjuvant systematic treatments should be vaccinated first [100]. It is proposed that the first dose of vaccine for radical surgery-undergoing patients is administered within 7–10 days after surgery. In breast cancer screening trials, due to the risk of transient lymphadenopathy after vaccination, screening examinations should be either before the first vaccine injection or 2–3 weeks after the second dose. Presently, revaccination after immunosuppressive therapies in those who received a vaccine previously is not recommended, but administration of booster doses might be helpful [101]. In another study, patients with active disease, hematologic malignancies, and recently diagnosed solid tumors were recommended as a priority for COVID-19 vaccination [97]. However, a clinical trial on using COVID-19 vaccines in patients with cancer is presently recruiting (NCT04715438), which can shed light on more details about vaccination in these patients [99].
4.1.1. Summary
It seems that vaccination in all cancer patients is safe, effective, and recommended, except for those who are on anti-B cell therapies, for whom a 4–6 months interval is needed after medication. It is important to complete the vaccination schedule. All types of vaccines are safe and effective in these patients, except live-attenuated vaccines. Additional data on the efficacy of the Pfizer-BioNTech vaccine has been presented.
4.2. Solid organ transplantation
Solid organ transplant (SOT) recipients are at a higher risk of severeCOVID-19 due to the immunosuppression needed for avoiding graft rejection [103]. Furthermore, it seems these patients experience prolonged SARS-CoV-2 shedding due to corticosteroid usage, which has been linked to the emergence of viral mutants [104], [105]. Therefore, vaccination is a crucial intervention to prevent infections and their resultant morbidity and mortality. However, the safety, efficacy, magnitude, and duration of SARS-CoV-2 vaccine response in SOT recipients are unknown because these groups have been excluded from the COVID-19 vaccine trials till now.
Previous studies reported lower antibody titers in immunosuppressed SOT recipients than the general populations following influenza, hepatitis A and B virus, and pneumococcal vaccinations [106], [107]. Taking immunosuppressive medications as well as an underlying chronic disease may weaken the immune reaction of these patients to immunization. A meta-analysis showed a substantially decreased response rate following seasonal influenza vaccination in transplant recipients receiving antimetabolites such as mycophenolate mofetil (MMF) [108]. Another concern about the effectiveness of COVID-19 vaccines is the anti-CD20-based immunosuppressive regimen in transplant patients. Rituximab depletes naive B cells and impairs humoral immunity [109]. In a study on early influenza vaccination (4–8 weeks) versus late vaccination (6–10 months) following rituximab, the early vaccination group did not exhibit IgM or IgG responses, while a significant IgG response (2.82-fold) was observed in patients receiving the vaccine 6–10 months after rituximab usage in rheumatoid arthritis [110]. Therefore, some studies recommend a few months’ interval between rituximab administration and vaccination [111], [112], [113], but more clinical studies are required on this subject. Boyarsky et al. reported that only 17% of 436 SOT recipients undergoing SARS-CoV-2 mRNA vaccination developed humoral response after the first dose of these vaccines [56]. Furthermore, older transplant recipients (adjusted incidence rate ratio [IRR], 0.83 [95% CI, 0.73–0.93] per 10 years, P = .002) and those receiving anti-metabolite maintenance immunosuppression therapy (adjusted [IRR], 0.22 [95% CI, 0.15–0.34], P < .001) were less likely to develop an antibody response according to the results of this study. Thus, some studies suggested that vaccination could be done prior to transplantation or be delayed until three to six months later than transplantation, when the intensity of immunosuppression reaches its lowest level [114], [115].
There are also some important issues in regards to the safety of the COVID-19 vaccines among SOT recipients. First, historically, vaccines seem to be able to induce donor-specific and non-donor-specific antibodies but have not been associated with graft rejection in SOT recipients [116]. In this regard, the results of the Boyarsky et al. study also showed no cases of acute rejection or allograft dysfunction following SARS-CoV-2 mRNA vaccination of SOT patients [117]. Considering that some SARS-CoV-2 vaccine platforms employing protein-based structures and adjuvants have not been widely studied in transplant recipients, theoretical concerns for possible graft rejection were also raised, which need further studies [118]. Secondly, live-attenuated virus vaccines are usually contraindicated in SOT recipients due to the risk of disseminated infection [119]. Presently, no approved or in-Phase-3-trial SARS-CoV-2 vaccine is available that employs an attenuated live virus platform; though, if such a vaccine is approved, it could be suitable just for some non-immunocompromised pre-transplant patients who are stable on the waitlist. Thirdly, with increasing vaccination rates around the world, some misleading information and concerns about SARS-CoV-2 vaccines are spreading, especially on social media. Some people suspect that the recently approved mRNA SARS-CoV-2 vaccines can integrate into the human genome and induce genetic manipulation [120]; while the reality is that mRNA cannot replicate or integrate into the human genome, as mRNA would be degraded following translation. Moreover, some individuals are worried about the viral vector vaccines to induce viral infections, especially in immunocompromised patients, but the COVID-19 (chimpanzee) adenoviral vector vaccine (ChAdOx1-nCoV-19) is replication-incompetent, which is encouraging when considering vaccination of the immunocompromised transplant recipient [121].
Although concluding there commendations regarding vaccination among SOT patients is still early, and we should wait for the results of ongoing studies in this field, according to the published guidelines so far [111], [114], [115], the following points can be mentioned:
-
1.
All healthcare workers and household members who care for these patients should be prioritized for COVID-19 vaccination.
-
2.
SOT candidates should get vaccinated against COVID-19 before transplantation whenever possible to support a proper immune response. The best time for COVID-19 vaccine indication in the post-transplantation period is probably a minimum of three months after transplantation when immunosuppression is diminished and other prophylactic medications are reduced.
-
3.
COVID-19 vaccination should be avoided in acute cellular rejection (ACR) status until ACR episodes are resolved, and corticosteroid high dose usage is not required anymore.
-
4.
To induce a better immune response, waiting for six weeks after the transplantation surgery is recommended before injection of the second dose of the vaccine.
-
5.
Double dosing of COVID-19 vaccines is not recommended in SOT recipients.
-
6.
For SOT patients who received anti-CD20 monoclonal antibody, six months interval between the last rituximab dose and COVID-19 vaccination is recommended.
4.2.1. Summary
SOT candidates should get vaccinated against COVID-19 before transplantation. The best time for vaccination is probably minimum three months after transplantation when ACR is not presented.
4.3. Multiple sclerosis
Multiple Sclerosis (MS) is defined as a chronic immune-mediated inflammatory disease, which causes demyelination and neurodegeneration in the central nervous system (CNS) and has become a concerning issue in the COVID-19 pandemic [122]. There are contradictive reports regarding the risk of COVID-19 infection in MS patients. Some studies stated that MS patients are at no higher risk than the general population except for patients under long-term treatment with anti-CD20 medications, probably [123]; whereas others have shown that patients with MS are at a greater risk of COVID-19 infection. However, most of them do not need hospitalization and would recover despite their comorbidity and treatment with disease-modifying therapies [124]. Bsteh et al. demonstrated that only less than 1% of MS patients are at an increased mortality risk due to COVID-19 infection [125].
MS is usually associated with other health issues. The treatments used in MS patients can affect their immune response even in mild infections due to the immunomodulatory and immunosuppressive properties of the drugs. Therefore, temporary exacerbation or even relapse and progression of MS in COVID-19 infection should be considered. Furthermore, studies showed that age, obesity [126], and increased disability were related to severe clinical indications and even death [127], [128]. Shreds of evidence indicated that infectious diseases, especially infections in the upper respiratory tracts, were correlated with MS progression and relapse. It was revealed that about 30–40% of MS patients experienced post-upper respiratory infection relapse; though, the mechanism is still unknown [129]. Even, cases displaying exacerbation, progression, and demyelination observed by MRI were reported during COVID-19 infection. This highlights the importance of vaccinating MS patients and paying special attention to them with following valid guidelines [130].
Vaccination can be an effective way to prevent infections, but MS patients may not display the anticipated immunization response because of administering immunomodulatory and immunosuppressive therapies. Moreover, discontinuing the routine therapeutic regimen for vaccination could cause progression and relapse, which needs to be taken into consideration [131], [132].
Three main questions need to be asked regarding the vaccination of MS patients:
-
(1)
Can the vaccine cause relapse or progression in people with MS?
-
(2)
Will the vaccine have appropriate efficacy in immunizing the patients under MS treatment?
-
(3)
What are the guidelines for vaccinating patients under treatment?
-
1.
Many studies have been conducted on the vaccines, questioning their ability to induce MS or cause relapse and progression. For instance, several reports suggest that live-inactivated H1N1 influenza vaccination could worsen the MS status. But finally, this hypothesis was disapproved. Thus, today there is no reliable proof of a connection between vaccination and relapse or progression in MS patients [133].
-
2.
Regarding vaccine efficacy, especially in patients using therapies such as immunomodulatory and immunosuppressive drugs, a post-vaccination checkup is necessary for MS patients to make sure that the immunization has occurred properly [131]. If an adequate response is not seen, it is recommended to add another booster vaccine [134].
-
3.
Because COVID-19 vaccination is still at its early stages, there are not many reports on vaccinating patients with MS. Moreover, there is not much information on the interaction between COVID-19 vaccines and therapies used in MS patients or the measures that need to be taken to maximize vaccine efficacy and safety. But, in a recent study discussing the relationship between different medications used in MS and COVID-19, mRNA vaccines including Moderna and Pfizer-BioNTech vaccines, the following points were concluded [131]:
-
–
Patients using ß-interferons, teriflunomide, natalizumab, glatiramer acetate, or dimethyl fumarate probably have no issues regarding vaccine safety and efficacy. Thus, the vaccine could be administered at any given time [135].
-
–
In patients using fingolimod, alemtuzumab, ocrelizumab, rituximab, or oral cladribine, there is a possibility of insufficient response to the vaccine [136]. In particular, an inadequate response to the Pfizer-BioNTech COVID-19 mRNA vaccine was reported in a 52-year-old patient with relapsing-remitting MS using ocrelizumab (a B-cell-depleting therapy) probably due to vaccination less than two weeks after the last ocrelizumab infusion [137]. It proves the importance of post-vaccination monitoring and choosing the right time for vaccination to maximize the efficacy, considering the patient’s therapeutic regimen. For instance, it was suggested to vaccinate patients starting B-cell-depleting therapies, such as ocrelizumab or rituximab, 4–6 weeks before their first dose. If the patient has started therapy, the best vaccination time is 4–6 months after the last infusion [138], [139]. In the case of alemtuzumab, if the medicine has been used in the last 12–24 months, a reduction in the vaccine efficacy may happen. Thus, it is recommended to postpone the treatment until vaccination is completed [140].
-
–
In patients on immunosuppressive treatments, such as mitoxantrone, cyclophosphamide, azathioprine, and methotrexate, vaccination is probably safe, but proper immunization might not occur.
Regarding high dose corticosteroid therapy, the golden time of vaccine administration is 4–6 weeks following the last corticosteroid therapy to achieve optimal immunization [131], [141].
4.3.1. Summary
Vaccination is recommended in all MS patients without discontinuing their disease-modifying therapies, which could increase the risk of relapse and progression. Right timing is of great importance to maximize efficacy and immunization, especially in patients using high-dose corticosteroids and B cell-depleting therapies such as rituximab and ocrelizumab.
4.4. Inflammatory bowel diseases
Ulcerative colitis (UC) and Crohn’s disease (CD) are two types of inflammatory bowel diseases (IBD) in which the immune response of the gastrointestinal system is dysregulated [142]. As COVID-19 has become a global issue, a concern about the susceptibility and increased risk of COVID-19infectionin IBD patients due to immunosuppressive, immunomodulatory, or biological treatments has been raised.
Studies have shown that potential risk factors for SARS-CoV-2 infection include age, nutritional status, high comorbidities, and IBD activity [143]. Some studies demonstrated no association between IBD treatments and COVID-19 severe infection or mortality [144], [145]. On the other hand, in the SECURE-IBD clinical trial, the use of systemic corticosteroids and sulfasalazine was shown as serious risk factors for poor clinical outcomes in COVID-19 patients [146], [147].
Moreover, in the cohort study of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD), UC diagnosis was shown as a risk factor for COVID-19 infection [145]. Additionally in a recent report, vitamin D deficiency was mentioned to be probably related to the severity or increased risk of COVID-19 in patients with IBD [148]. Based on the recent data, IBD patients presented high GI symptoms such as diarrhea because ofCOVID-19 infection [149].
As a result, the impact of IBD on the risk of COVID-19 infection is controversial. Despite the above-mentioned information, there is no solid evidence indicating that IBD patients are at a higher risk of COVID-19 infection. Moreover, many studies declared no significant difference between the incidence and mortality of COVID-19 in IBD patients and the general population [144], [149].
Considering the importance of vaccination to protect against COVID-19, the safety, adverse effects, and immunogenicity of vaccines in immunosuppressed patients such as individuals with IBD have been the subject of debate.
Due to the exclusion of IBD patients from the third phase of clinical trials for approved vaccines, there is a lack of data regarding vaccination benefits, side effects, and the risk of disease activation. Nevertheless, these vaccines are not contraindicated for people with IBD [150].
Based on previous experiences in vaccinating IBD patients for infectious diseases such as pneumococcus, hepatitis A virus (HAV), HBV, and influenza, it is possible to conclude that immunosuppressive treatments can impair the immune reaction to vaccines and diminish the immunization process efficacy. However, administration of the mentioned vaccines in IBD patients has a low risk [150]. Moreover, the vaccination of patients with active IBD might be impaired due to concurrent anemia [151].
Although less immune response might be observed in IBD patients, non-live vaccines are generally safe, and most of them are recommended for these patients [152]. However, there is a concern about the safety of live-attenuated and inactivated vaccines as well as replicating vaccines that may make trouble in immunosuppressed individuals [152], [153]. In this context, vaccines using adenoviruses as vectors such as the AstraZeneca-Oxford vaccine seem appropriate in IBD patients, because they provoke the immune system without integrating the viral genome into the hosts’ DNA. Thus, they are highly capable of producing a proper immune response [153].
Regarding the interaction between IBD treatment and vaccination, studies indicated that patients using anti-TNFα drugs, such as infliximab and adalimumab, displayed impaired immune response and reduced antibody titers to influenza [154], HBV [155], and pneumococcal vaccines [156]. Treatment with vedolizumab was associated with reduced efficacy of oral cholera vaccine; but, it did not impede influenza vaccination [157]. Hence, it may pertain to the mucosal vaccine delivery, which should be taken into account for some in-development SARS-CoV-2 vaccines [152].
The British Society of Gastroenterology (BSG) published a set of recommendations on COVID-19 vaccination of IBD patients. According to BSG, patients with IBD should receive vaccination as soon as possible irrespective of the medication used, even in patients with active IBD. However, in patients with severe IBD flares or those who need hospitalization, a delay in vaccination is preferred to help them recover and be prepared for the vaccine injection. Additionally, IBD patients should receive both doses of the COVID-19 vaccines [150].
Moreover, based on the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) international consensus meeting, the following statements should be considered:
-
–
There is no association between vaccination and the onset, flares, and exacerbation of IBD.
-
–
IBD patients can receive non-live SARS-CoV-2 vaccines regardless of their immune-modifying treatments.
-
–
mRNA, replication-incompetent vector, inactivated, and recombinant SARS-CoV-2 vaccines are safe for IBD patients.
-
–
Live-attenuated COVID-19 vaccines are not safe in patients receiving immune-modifying medications or those going to receive such therapies in the next eight weeks.
-
–
Immune-modifying medications taken by people with IBD should not force them to postpone getting the vaccine [152].
Due to the reduction of vaccine efficacy in patients receiving systemic corticosteroids, it is recommended to vaccinate IBD patients when the dose of corticosteroids is at their lowest level [150].
The CLARITY IBD study indicated that antibody responses after the first dose of vaccine in patients treated with infliximab were lower than vedolizumab. This data was obtained following administration of Pfizer-BioNTech and AstraZeneca vaccines. Furthermore after two doses, there was inadequate antibody response in 18% of patients who used infliximab and 8% of those administered vedolizumab [158]. Even though, patients taking infliximab should not postpone receiving their second dose of vaccine [159].
4.4.1. Summary
In patients with IBD, vaccination is suggested as soon as possible. Non-live vaccines are safe in such patients. However, in patients with severe IBD flares or those who need hospitalization, a delay in vaccination is preferred. It is recommended to get a vaccine when they take the lowest dose of corticosteroid medications.
4.5. Rheumatologic and dermatologic diseases
Patients with rheumatic diseases have been found to be at an increased risk of infection due to the underlying inflammation and use of immunomodulatory drugs [160]. Moreover, a higher risk of COVID-19 morbidity and mortality might be possible due to the comorbidities present in rheumatic disease patients [161]. Although data regarding clinical outcomes in COVID-19 patients with an underlying rheumatic disease is very limited, the use of moderate to high doses of corticosteroids in such patients, possibly increases the chance of hospitalization. On the other hand, administration of TNFα inhibitors reduced hospitalization odds, while using NSAIDs (non-steroidal anti-inflammatory drugs), DMARDs (disease-modifying anti-rheumatic drugs), and JAK (Janus kinase) inhibitors resulted in no significant change in the probability of hospitalization [162].
The innate immune system does not recognize the nucleoside-modified mRNAs employed in Pfizer-BioNTech and Moderna vaccine platforms, which indicates that these vaccines seem to be as safe as any other protein conjugated vaccines. Moreover, 62 subjects (0.3%) out of all subjects in Pfizer-BioNTech clinical trials had rheumatic diseases and received BNT162b2. These subjects showed no relapse or flare-up of the underlying rheumatic condition, although data is still limited [71]. Though, there is a slight probability for disease flares after vaccination [163]. Subjects with rheumatic diseases were excluded from Moderna vaccine clinical trials [70], and probably none has been included in the clinical trials of the Janssen vaccine [164]. Vaccination against COVID-19 in subjects with an underlying rheumatic disease as a part of vulnerable subject groups has been highly recommended by the United States Center for Disease Control and Prevention (US CDC) [6], the British Society of Rheumatology (BSR) [165], and the American College of Rheumatology (ACR) [166] in spite of probable adverse effects following vaccination. In this context, vaccination in rheumatic disease subjects during the quiescent state of the disease is highly recommended as is the case for other vaccines in such patients [167], [168]. While in subjects with active rheumatic diseases, data is still limited regarding vaccine immunogenicity despite studies showing no evident correlation between the immunogenicity of vaccines and the state of the underlying rheumatic disease such as juvenile systemic lupus erythematosus (SLE) [169].
Another burning question in this matter is whether to continue or discontinue pharmacologic therapies in rheumatic disease patients respective to SARS-CoV-2 vaccine administration. A meta-analysis in 2018 indicated that the use of methotrexate had no significant effect on influenza vaccine immunogenicity while diminishing immunogenicity was conveyed by the pneumococcal vaccine [170]. Moreover, it was shown that discontinuation of methotrexate for two weeks before seasonal influenza vaccine administration and two weeks after that, improved the achieved immunogenicity [113], [171], [172]. Rituximab use was shown to suppress humoral immunity as opposed to cellular immunity, following the reduction of nAbs [173]. Thus, the timing of rituximab use is critical in achieving better vaccine immunogenicity [170]. In light of these findings, in vaccinated subjects, rituximab usage is recommended to be started a few weeks after vaccine administration [113], [174], [175]. Moreover, ACR and European League against Rheumatism (EULAR) have recommended the administration of pneumococcal and seasonal influenza vaccines at least six months after the last rituximab dose administration [168], [176]. In a recent update on the COVID-19 vaccination guide, BSR has advised all rheumatic disease patients to receive Medicines and Healthcare products Regulatory Agency (MHRA) approved vaccines (Pfizer-BioNTech and Oxford-AstraZeneca) but suggests that administration of rituximab in these subjects begin with at least two weeks’ lay off from vaccination. It also advises in favor of completing the two-dose vaccination before induction of immunosuppression with rituximab to achieve maximum vaccine immunogenicity. Although, administration of rituximab should not be delayed in cases with severe organ-threatening states, and vaccination should be carried out along with the use of rituximab. Additionally, in patients with rheumatic diseases, an alternative therapy should be considered instead of rituximab, if the use of DMARDs or other biological treatments is needed, noting that treatment with other agents is appropriate and available [165]. Similarly, the continuation of other immunosuppressive drugs has been recommended along with vaccine administration [177], [178], [179]. The use of TNFα inhibitors and IL-17 inhibitors also was shown to have minimal effect on vaccine efficacy, while data regarding the effect of abatacept on vaccine efficacy is still limited [180]. Overall, further studies should be carried out regarding the efficacy and safety of COVID-19 vaccination along with the use of DMARDs in rheumatic disease patients [181]. Given that some autoimmune dermatologic diseases, such as psoriasis and lupus, are the result of disorders in the immune system activity and inflammatory conditions, they can be regarded as similar to rheumatologic diseases in respect to vaccination considerations (as in Table 2 ).
Table 2.
A brief on present considerations for COVID-19 vaccination in immunological diseases.*
| Immunological conditions | Considerations regarding COVID-19 vaccination |
|---|---|
| Cancer and HSCT |
|
| SOT |
|
| MS |
|
| IBD |
|
| Rheumatology and Dermatology |
|
HSCT: hematopoietic stem cell transplantation; ICI: immune checkpoint inhibitors; IRAE: immune-related adverse effects; SOT: solid organ transplantation; MS: multiple sclerosis; IBD: inflammatory bowel diseases; BSG: British Society of Gastroenterology; BSR: British Society of Rheumatology; COVID-19: coronavirus disease-2019.
These data are based on the present knowledge on this subject (date: July 2021).
4.5.1. Summary
Rheumatologic patients should be vaccinated as soon as possible, and mRNA vaccines seem to be safe in this group. A two-week gap of methotrexate administration before and after vaccination is recommended. It is recommended to consider at least two weeks’ lay off from vaccination for rituximab use or to change the medication to other DMARDs.
5. Conclusion
Based on the available data, patients with immunosuppressive diseases or patients on immunosuppressive medications are prioritized to receive current COVID-19 vaccines, besides following general precautions such as social distancing and using masks, to prevent COVID-19 infection. There is a paradox between this subject and the fact that immunocompromised patients were excluded in the main conducted trials of these vaccines, which are already published. Therefore, coming to a consensus for the administration of vaccines to this population is very critical, while gathering data and conducting trials that can clarify the outcomes and conditions is also an urgent need. Though the suitability of new COVID-19 vaccines such as mRNA or viral-vectored vaccines for these patients is unknown, choosing an effective prophylactic COVID-19 vaccine and avoiding live-attenuated vaccines are recommended. Since there is no live-attenuated vaccine available or understudy for COVID-19, all available vaccines could be regarded as almost safe. On the other hand, the efficacy would be the main concern, because the immunosuppressive medications may impair the response to vaccines. Hence, decision on delaying immunosuppressive therapy because of COVID-19 vaccination will need to be evaluated and discussed taking into account the prescribed medications and condition of each patient.
With time, dedicated COVID-19 vaccine studies on patients with immunosuppressive diseases or those who take immunosuppressant will reveal the pros and cons of vaccination in this heterogeneous population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Negahdaripour M. The battle against COVID-19: where do we stand now? Iran. J. Med. Sci. 2020;45(2):81. doi: 10.30476/ijms.2020.46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, WHO Coronavirus (COVID-19) Dashboard, https://covid19.who.int/.
- 3.Owji H., Negahdaripour M., Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020 doi: 10.1016/j.intimp.2020.106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negahdaripour M., et al. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol. Adv. 2017;35(5):575–596. doi: 10.1016/j.biotechadv.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negahdaripour M. COVID-19 Vaccine Global Access Is an Urgency. Iran. J. Med. Sci. 2021;46(2):79–80. doi: 10.30476/ijms.2021.47336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(CDC). Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. March 5, 2021; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html.
- 7.Voysey M., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logunov D.Y., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. The Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet. Infect. Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson S., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers L.C., et al. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury M.A., et al. Immune response in COVID-19: A review. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagheri A., et al. Interferon-inducer antivirals: Potential candidates to combat COVID-19. Int. Immunopharmacol. 2021;91 doi: 10.1016/j.intimp.2020.107245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakidis N.C., et al. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines. 2021;6(1):1–17. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D.W. Vaughn, S.S. Whitehead, and A.P. Durbin, Dengue, in Vaccines for Biodefense and Emerging and Neglected Diseases. 2009, Elsevier Inc. pp. 285–324.
- 16.Xia S., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma O., et al. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020;11:2413. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.C.J. Burrell, C.R. Howard, F.A. Murphy, Fenner and white's medical virology, 2016, Academic Press.
- 19.Cohen N.D., Bordin A.I. Principles of Vaccination. Equine Clin. Immunol. 2015:263. [Google Scholar]
- 20.World Health Organization, The different types of COVID-19 vaccines, https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained. 2021.
- 21.Pasquale A., et al. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines. 2015;3(2):320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grun J.L., Maurer P.H. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell. Immunol. 1989;121(1):134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 23.Hwang J.K., et al. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J. Hematol. Oncol. 2021;14(1) doi: 10.1186/s13045-021-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Y.Z. Zhang, et al., Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medrxiv, 2020. 10.1101/2020.07.31.20161216. [DOI]
- 26.Hwang J.K., et al. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J. Hematol. Oncol. 2021;14(1):38. doi: 10.1186/s13045-021-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell R.L., Pelka P., Mark B.L. Frontrunners in the race to develop a SARS-CoV-2 vaccine. Can. J. Microbiol. 2021;67(3):189–212. doi: 10.1139/cjm-2020-0465. [DOI] [PubMed] [Google Scholar]
- 28.Velikova T., Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021;41(3):509–518. doi: 10.1007/s00296-021-04792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belete T.M. A review on Promising vaccine development progress for COVID-19 disease. Vacunas. 2020;21(2):121–128. doi: 10.1016/j.vacun.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang S., et al. Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expert Rev. Vacc. 2012;11(12):1405–1413. doi: 10.1586/erv.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W.H., et al. The SARS-CoV-2 Vaccine Pipeline: an Overview. Curr. Trop. Med. Rep. 2020:1–4. doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keech C., et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauch S., et al. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hobernik D., Bros M. DNA vaccines—how far from clinical use? Int. J. Mol. Sci. 2018;19(11):3605. doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silveira M.M., Moreira G., Mendonca M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021;267 doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee L.Y.Y., Izzard L., Hurt A.C. A review of DNA vaccines against influenza. Front. Immunol. 2018;9:1568. doi: 10.3389/fimmu.2018.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tebas P., et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.What are viral vector-based vaccines and how could they be used against COVID-19? https://www.gavi.org/vaccineswork/what-are-viral-vector-based-vaccines-and-how-could-they-be-used-against-covid-19. 2021.
- 40.Humphreys I.R., Sebastian S. Novel viral vectors in infectious diseases. Immunology. 2018;153(1):1–9. doi: 10.1111/imm.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Draft landscape and tracker of COVID-19 candidate vaccines https://cdn.who.int/media/docs/default-source/blue-print/06.04.2021-novel-coronavirus_landscape_covid-19.xlsx033cfc8f-b99b-4edf-aedb-765a272e9eb5.zip?sfvrsn=d6471b19_3&download=true. 2021.
- 43.Tregoning J.S., et al. Vaccines for COVID-19. Clin. Exp. Immunol. 2020;202(2):162–192. doi: 10.1111/cei.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines. 2014;2(3):624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voysey M., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. The Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Hara G.A., et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J. Infect. Dis. 2012;205(5):772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundstrom K. Viral vectors for COVID-19 vaccine development. Viruses. 2021;13(2):317. doi: 10.3390/v13020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anand P., Stahel V.P. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf. Surg. 2021;15(1):1–9. doi: 10.1186/s13037-021-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park J.W., et al. mRNA vaccines for COVID-19: what, why and how. Int. J. Biol. Sci. 2021;17(6):1446. doi: 10.7150/ijbs.59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaur S.P., Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh E.E., et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahin U., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 55.Jackson L.A., et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization, COVID-19 - Landscape of Novel Coronavirus Candidate Vaccine Development Worldwide, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. 2021.
- 57.Palacios R., et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac–PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):1–3. doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bueno S.M., et al. Interim report: Safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy chilean adults in a phase 3 clinical trial. medRxiv. 2021 doi: 10.1101/2021.03.31.21254494. [DOI] [Google Scholar]
- 61.Pu J., et al. The safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in Chinese adults aged 18–59 years: A phase I randomized, double-blinded, controlled trial. Vaccine. 2021;39(20):2746–2754. doi: 10.1016/j.vaccine.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.R. Ella et al., Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomised controlled trial) and the persistence of immune responses from a phase 1 follow-up report. medRxiv, 2020. 10.1101/2020.12.21.20248643. [DOI]
- 64.Zhu F.C., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadoff J., et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.P. Gobeil, et al., Interim report of a phase 2 randomized trial of a plant-produced virus-like particle vaccine for covid-19 in healthy adults aged 18-64 and older adults aged 65 and older. medRxiv, 2021. doi: 10.1101/2021.05.14.21257248. [DOI]
- 67.Heath P.T., et al. Efficacy of the NVX-CoV2373 Covid-19 vaccine against the B. 1.1.7 variant. medRxiv. 2021 doi: 10.1101/2021.05.13.21256639. [DOI] [Google Scholar]
- 68.Yang S., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet. Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mammen M.P., et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv. 2021 doi: 10.1101/2021.05.07.21256652. [DOI] [Google Scholar]
- 70.Baden L.R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shinjoh M., et al. Effective and safe immunizations with live-attenuated vaccines for children after living donor liver transplantation. Vaccine. 2008;26(52):6859–6863. doi: 10.1016/j.vaccine.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 74.Kuderer N.M., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. The Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goyal S., et al. Hepatitis B vaccination in acute lymphoblastic leukemia. Leuk. Res. 1998;22(2):193–195. doi: 10.1016/s0145-2126(97)00155-0. [DOI] [PubMed] [Google Scholar]
- 76.Ercan T.E., et al. Antibody titers and immune response to diphtheria-tetanus-pertussis and measles-mumps-rubella vaccination in children treated for acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2005;27(5):273–277. doi: 10.1097/01.mph.0000163214.37147.5a. [DOI] [PubMed] [Google Scholar]
- 77.Mulder S.F., et al. Cancer patients treated with sunitinib or sorafenib have sufficient antibody and cellular immune responses to warrant influenza vaccination. Clin. Cancer Res. 2011;17(13):4541–4549. doi: 10.1158/1078-0432.CCR-11-0253. [DOI] [PubMed] [Google Scholar]
- 78.Zent C.S., et al. Short term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial BTK inhibitor therapy for CLL or lymphoplasmacytic lymphoma. Leukemia. 2020:1–4. doi: 10.1038/s41375-020-01074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun C., et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. 2016;2(12):1656–1657. doi: 10.1001/jamaoncol.2016.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cho A., et al. Robust memory responses against influenza vaccination in pemphigus patients previously treated with rituximab. JCI Insight. 2017;2(12) doi: 10.1172/jci.insight.93222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nazi I., et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946–1953. doi: 10.1182/blood-2013-04-494096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rieger C., et al. Anti-infective vaccination strategies in patients with hematologic malignancies or solid tumors—Guideline of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO) Ann. Oncol. 2018;29(6):1354–1365. doi: 10.1093/annonc/mdy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loarce-Martos J., et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol. Int. 2020:1–7. doi: 10.1007/s00296-020-04699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Damiani G., et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol. Ther. 2020;33(5) doi: 10.1111/dth.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribas A., et al. AACR COVID-19 and cancer task force. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11(2):233–236. doi: 10.1158/2159-8290.CD-20-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Darnell E.P., et al. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020;22(4):39. doi: 10.1007/s11912-020-0897-9. [DOI] [PubMed] [Google Scholar]
- 87.Läubli H., et al. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J. ImmunoTher. Cancer. 2018;6(1):1–10. doi: 10.1186/s40425-018-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gambichler T., et al. On the use of immune checkpoint inhibitors in patients with viral infections including COVID-19. J. ImmunoTherapy Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leclerc M., Maury S. A rationale to prioritise vaccination of HSCT patients against COVID-19. The Lancet Haematology. 2021 doi: 10.1016/S2352-3026(21)00008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma A., et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. The Lancet Haematology. 2021;8(3):e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ljungman P., et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44(8):521–526. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 92.Parkman R., Weinberg K.I. Immune reconstitution following hematopoietic cell transplantation. Thomas' Hematopoietic Cell Transpl.: Stem Cell Transpl. 2009:222–231. [Google Scholar]
- 93.Issa N.C., Baden L.R. Current issues in vaccines for adult patients with hematologic malignancies. J. Natl. Compr. Canc. Netw. 2012;10(11):1447–1454. doi: 10.6004/jnccn.2012.0147. [DOI] [PubMed] [Google Scholar]
- 94.Kamboj M., Shah M.K. Vaccination of the stem cell transplant recipient and the hematologic malignancy patient. Infect. Dis. Clinics. 2019;33(2):593–609. doi: 10.1016/j.idc.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.L. Monin-Aldama, et al., Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. medRxiv, 2021. doi: 10.1101/2021.03.17.21253131. [DOI]
- 96.Fendler A., et al. Adaptive immunity to SARS-CoV-2 in cancer patients. The CAPTURE study. medRxiv. 2020 doi: 10.1101/2020.12.21.20248608. [DOI] [Google Scholar]
- 97.Giesen N., et al. 2021 Update of the AGIHO guideline on evidence-based management of COVID-19 in cancer patients regarding diagnostics, viral shedding, vaccination and therapy. Eur. J. Cancer. 2021:154–160. doi: 10.1016/j.ejca.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mikulska M., et al. Vaccination of patients with haematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7) Lancet Infect. Dis. 2019;19(6):e188–e199. doi: 10.1016/S1473-3099(18)30601-7. [DOI] [PubMed] [Google Scholar]
- 99.van der Veldt A.A., et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat. Med. 2021:1–2. doi: 10.1038/s41591-021-01240-w. [DOI] [PubMed] [Google Scholar]
- 100.Silvestris N., et al. COVID vaccination in cancer patients: what vaccination priority strategies should there be? Front. Oncol. 2021;11:168. doi: 10.3389/fonc.2021.641388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desai A., et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat. Rev. Clin. Oncol. 2021;18(5):313–319. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mirjalili M., Shafiekhani M., Vazin A. Coronavirus disease 2019 (COVID-19) and transplantation: pharmacotherapeutic management of immunosuppression regimen. Therapeut. Clin. Risk Manage. 2020;16:617. doi: 10.2147/TCRM.S256246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson K.M., et al. Managing COVID-19 in renal transplant recipients: a review of recent literature and case supporting corticosteroid-sparing immunosuppression. Pharmacother.: J. Hum. Pharmacol Drug Therapy. 2020;40(6):517–524. doi: 10.1002/phar.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cano E.J., et al. Impact of corticosteroids in COVID-19 outcomes: systematic review and meta-analysis. Chest. 2020 doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chong P.P., Avery R.K. A comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin. Ther. 2017;39(8):1581–1598. doi: 10.1016/j.clinthera.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Härmälä S., et al. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis. BMJ Open. 2019;9(9) doi: 10.1136/bmjopen-2019-031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chong P.P., Handler L., Weber D.J. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin. Infect. Dis. 2018;66(11):1802–1811. doi: 10.1093/cid/cix1081. [DOI] [PubMed] [Google Scholar]
- 109.Baker D., et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020;202(2):149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Westra J., et al. Rituximab impairs immunoglobulin (Ig) M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients. Clin. Exp. Immunol. 2014;178(1):40–47. doi: 10.1111/cei.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kronbichler A., et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol. Dial. Transplant. 2021;36(7):1160–1168. doi: 10.1093/ndt/gfab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parrino J., et al. Safety and immunogenicity of inactivated varicella-zoster virus vaccine in adults with hematologic malignancies receiving treatment with anti-CD20 monoclonal antibodies. Vaccine. 2017;35(14):1764–1769. doi: 10.1016/j.vaccine.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 113.Sonani B., et al. COVID-19 vaccination in immunocompromised patients. Clin. Rheumatol. 2021;40(2):797–798. doi: 10.1007/s10067-020-05547-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cornberg M., et al. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J. Hepatol. 2021;74(4):944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fix O.K., et al. AASLD Expert Panel Consensus Statement: Vaccines to Prevent COVID-19 Infection in Patients with Liver Disease. Hepatology. 2021 doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mulley W.R., et al. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. J. Heart Lung Transplant. 2018;37(7):844–852. doi: 10.1016/j.healun.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 117.Boyarsky B.J., et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56–e57. doi: 10.1097/TP.0000000000003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haddadin Z., et al. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am. J. Transplant. 2020;21(3):938–949. doi: 10.1111/ajt.16295. [DOI] [PubMed] [Google Scholar]
- 119.Danziger-Isakov L., Kumar D., Practice A.I.C.O. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019;33(9) doi: 10.1111/ctr.13563. [DOI] [PubMed] [Google Scholar]
- 120.Aslam S., et al. COVID-19 vaccination in our transplant recipients: The time is now. J. Heart Lung Transplant. 2021;40(3):169–171. doi: 10.1016/j.healun.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lefebvre M., et al. Covid-19 vaccines: frequently asked questions and updated answers. Infect. Dis. Now. 2021 doi: 10.1016/j.idnow.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lassmann H. Multiple Sclerosis Pathology. Cold Spring Harbor Perspect. Med. 2018;8(3) doi: 10.1101/cshperspect.a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zabalza A., et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021 doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 124.Sepúlveda M., et al. Incidence and Impact of COVID-19 in MS. Neurol. – Neuroimmunol. Neuroinflamm. 2021;8(2) doi: 10.1212/NXI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bsteh G., et al. Multiple sclerosis and COVID-19: how many are at risk? Eur. J. Neurol. 2020 doi: 10.1111/ene.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sellner J., Rommer P.S. Multiple Sclerosis and SARS-CoV-2 Vaccination: Considerations for Immune-Depleting Therapies. Vaccines. 2021;9(2):99. doi: 10.3390/vaccines9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salter A., et al. Outcomes and Risk Factors Associated With SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mares J., Hartung H.-P. Multiple sclerosis and COVID-19. Biomed. Pap. 2020;164(3):217–225. doi: 10.5507/bp.2020.033. [DOI] [PubMed] [Google Scholar]
- 129.Steelman A.J. Infection as an Environmental Trigger of Multiple Sclerosis Disease Exacerbation. Front. Immunol. 2015;6 doi: 10.3389/fimmu.2015.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sadeghmousavi S., Rezaei N. COVID-19 and Multiple Sclerosis: Predisposition and Precautions in Treatment. SN Comprehens. Clin. Med. 2020;2(10):1802–1807. doi: 10.1007/s42399-020-00504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nojszewska M., et al. COVID-19 mRNA vaccines (Pfizer-BioNTech and Moderna) in patients with multiple sclerosis: a statement by a working group convened by the Section of Multiple Sclerosis and Neuroimmunology of the Polish Neurological Society. Neurol. Neurochir. Pol. 2021;55(1):8–11. doi: 10.5603/PJNNS.a2021.0016. [DOI] [PubMed] [Google Scholar]
- 132.Mailand M.T., Frederiksen J.L. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017;264(6):1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- 133.Zrzavy T., et al. Vaccination in Multiple Sclerosis: Friend or Foe? Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bhise V., Dhib-Jalbut S. Potential Risks and Benefits of Multiple Sclerosis Immune Therapies in the COVID-19 Era: Clinical and Immunological Perspectives. Neurotherapeutics. 2021 doi: 10.1007/s13311-021-01008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morales F.S., et al. Risk factors for lymphopenia in patients with relapsing–remitting multiple sclerosis treated with dimethyl fumarate. J. Neurol. 2020;267(1):125–131. doi: 10.1007/s00415-019-09557-w. [DOI] [PubMed] [Google Scholar]
- 136.Zheng C., et al. Multiple Sclerosis Disease-Modifying Therapy and the COVID-19 Pandemic: Implications on the Risk of Infection and Future Vaccination. CNS Drugs. 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chilimuri S., et al. COVID-19 Vaccine Failure in a Patient with Multiple Sclerosis on Ocrelizumab. Vaccines. 2021;9(3):219. doi: 10.3390/vaccines9030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Safavi F., Nourbakhsh B., Azimi A.R. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Multiple Sclerosis Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seachrist E.J. Multiple sclerosis, B cell therapy, and the COVID-19 vaccine. Eneurologicalsci. 2021;22 doi: 10.1016/j.ensci.2021.100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCarthy C.L., et al. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology. 2013;81(10):872–876. doi: 10.1212/WNL.0b013e3182a35215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ciotti J.R., Valtcheva M.V., Cross A.H. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Multiple Sclerosis Relat. Disord. 2020;45 doi: 10.1016/j.msard.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kennedy N.A., et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69(6):984–990. doi: 10.1136/gutjnl-2020-321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rizzello F., et al. COVID-19 in IBD: The experience of a single tertiary IBD center. Digest. Liver Dis. 2021;53(3):271–276. doi: 10.1016/j.dld.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Al-Ani A.H.R., et al. Review article: prevention, diagnosis and management of COVID-19 in the IBD patient. Aliment Pharmacol. Ther. 2020;52(1):54–72. doi: 10.1111/apt.15779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bezzio C., et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69(7):1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 146.Brenner E.J., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mazza S., et al. A fatal case of COVID-19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut. 2020;69(6):1148–1149. doi: 10.1136/gutjnl-2020-321183. [DOI] [PubMed] [Google Scholar]
- 148.Rhodes J.M., et al. 12th edition. Vol. 51. Wiley Online Library; 2020. Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity; p. 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taxonera C., et al. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2020;52(2):276–283. doi: 10.1111/apt.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alexander J.L., et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol. Hepatol. 2021 doi: 10.1016/S2468-1253(21)00024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stoffel N.U., et al. Iron deficiency anemia at time of vaccination predicts decreased vaccine response and iron supplementation at time of vaccination increases humoral vaccine response: a birth cohort study and a randomized trial follow-up study in Kenyan infants. Front. Immunol. 2020;11:1313. doi: 10.3389/fimmu.2020.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siegel C.A., et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70(4):635–640. doi: 10.1136/gutjnl-2020-324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kumar A., et al. COVID-19 vaccinations in patients with inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2020;5(11):965–966. doi: 10.1016/S2468-1253(20)30295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.deBruyn J., et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm. Bowel Dis. 2016;22(3):638–647. doi: 10.1097/MIB.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 155.Pratt P.K., Jr, et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm. Bowel Dis. 2018;24(2):380–386. doi: 10.1093/ibd/izx001. [DOI] [PubMed] [Google Scholar]
- 156.Fiorino G., et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm. Bowel Dis. 2012;18(6):1042–1047. doi: 10.1002/ibd.21800. [DOI] [PubMed] [Google Scholar]
- 157.Wyant T., et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64(1):77–83. doi: 10.1136/gutjnl-2014-307127. [DOI] [PubMed] [Google Scholar]
- 158.Prentice R.E., et al. SARS‐CoV‐2 vaccination in patients with inflammatory bowel disease. GastroHep. 2021;6(3):218–224. doi: 10.1002/ygh2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kennedy N.A., et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021 doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 160.Doran M.F., et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 161.Kuo C.F., et al. Temporal relationships between systemic lupus erythematosus and comorbidities. Rheumatology. 2019;58(5):840–848. doi: 10.1093/rheumatology/key335. [DOI] [PubMed] [Google Scholar]
- 162.Gianfrancesco M., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Watad A., et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.FDA Briefing Document Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. 2021 March 24th, 2021]; Available from: https://www.fda.gov/media/146217/download.
- 165.Principles for COVID-19 Vaccination in Musculoskeletal and Rheumatology for Clinicians. March 10, 2021 [accessed at March 25th, 2021]; Available from: http://arma.uk.net/covid-19-vaccination-and-msk/.
- 166.Curtis J.R., et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients with Rheumatic and Musculoskeletal Diseases-Version 1. Arthritis Rheumatol. 2021;73(7):1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guerrini G., et al. Italian recommendations for influenza and pneumococcal vaccination in adult patients with autoimmune rheumatic diseases. Clin. Exp. Rheumatol. 2019;38(2):245–256. [PubMed] [Google Scholar]
- 168.Furer V., et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020;79(1):39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 169.Campos L.M., et al. High disease activity: an independent factor for reduced immunogenicity of the pandemic influenza a vaccine in patients with juvenile systemic lupus erythematosus. Arthritis Care Res. 2013;65(7):1121–1127. doi: 10.1002/acr.21948. [DOI] [PubMed] [Google Scholar]
- 170.Subesinghe S., et al. A systematic review and metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J. Rheumatol. 2018;45(6):733–744. doi: 10.3899/jrheum.170710. [DOI] [PubMed] [Google Scholar]
- 171.Park J.K., et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann. Rheum. Dis. 2018;77(6):898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Park J.K., et al. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann. Rheum. Dis. 2019;78(9):1283–1284. doi: 10.1136/annrheumdis-2019-215187. [DOI] [PubMed] [Google Scholar]
- 173.Arad U., et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29(8):1643–1648. doi: 10.1016/j.vaccine.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 174.Bingham C.O., III, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheumat.: Off. J. Am. College Rheumatol. 2010;62(1):64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 175.Papp K.A., et al. Vaccination guidelines for patients with immune-mediated disorders taking immunosuppressive therapies: executive summary. J. Rheumatol. 2019;46(7):751–754. doi: 10.3899/jrheum.180784. [DOI] [PubMed] [Google Scholar]
- 176.Singh J.A., et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 177.Bühler S., et al. Vaccination recommendations for adult patients with autoimmune inflammatory rheumatic diseases. Swiss Med. Week. 2015;145 doi: 10.4414/smw.2015.14159. [DOI] [PubMed] [Google Scholar]
- 178.Seo Y.B., et al. The Practice Guideline for Vaccinating Korean Patients with Autoimmune Inflammatory Rheumatic Disease. Infect. Chemother. 2020;52(2):252. doi: 10.3947/ic.2020.52.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Santosa A., et al. Recommendations for COVID-19 vaccination in people with rheumatic disease: developed by the Singapore Chapter of Rheumatologists. Int. J. Rheum. Dis. 2021;24:746–757. doi: 10.1111/1756-185X.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Day A.L., et al. The effect of disease-modifying antirheumatic drugs on vaccine immunogenicity in adults. Cleveland Clin. J. Med. 2020;87(11):695. doi: 10.3949/ccjm.87a.20056. [DOI] [PubMed] [Google Scholar]
- 181.Calabrese L., Winthrop K.L. Rheumatology and COVID-19 at 1 year: facing the unknowns. Ann. Rheum. Dis. 2021;80:679–681. doi: 10.1136/annrheumdis-2021-219957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mosaddeghi P., et al. Harnessing the non-specific immunogenic effects of available vaccines to combat COVID-19. Hum. Vaccin. Immunother. 2021;17(6):1650–1661. doi: 10.1080/21645515.2020.1833577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mosaddeghi P., et al. Therapeutic approaches for COVID-19 based on the interferon-mediated immune responses. Curr. Signal Transduct. Ther. 2021;16 doi: 10.2174/1574362416666210120104636. [DOI] [Google Scholar]
- 184.Negahdaripour M., et al. Structural vaccinology considerations for in silico designing of a multi-epitope vaccine. Infect. Genet. Evol. 2018;58:96–109. doi: 10.1016/j.meegid.2017.12.008. [DOI] [PubMed] [Google Scholar]