ABSTRACT
Two herpes zoster (HZ) vaccines licensed in the United States are recommended by the Advisory Committee on Immunization Practices (ACIP): (i) live-attenuated vaccine (ZVL) using vOka strain varicella-zoster virus (VZV) and (ii) recombinant adjuvanted vaccine (RZV) containing recombinant varicella-zoster virus (VZV) glycoprotein E (gE). Two phase 3 clinical trials of RZV led the Advisory Committee on Immunization Practices (ACIP) to recommend it with preferred status. VZV T cell-mediated immunity (CMI), but not humoral immunity, is considered essential for protection against HZ. Published studies of humoral immunity focused on VZV-specific IgG concentration. To complement reports comparing the CMI responses to these vaccines, we compared humoral responses in ZVL and RZV recipients, emphasizing functional qualities (avidity and neutralization). Baseline avidities to a VZV glycoprotein mixture (gp) were near the upper limit of detection, but avidity to gE was much lower. Small increases in gp avidity were observed for both RZV and ZVL vaccination (19 and 12 avidity index units [AIU], respectively). RZV boosted both gE avidity and VZV neutralizing antibody significantly more than ZVL (mean gE avidity boost, 47 AIU versus 22 AIU; mean neutralizing antibody boost, 22-fold versus 8-fold). Increases in neutralizing antibodies strongly correlated with gE avidity increases (r = 0.5) and moderately with gp avidity increases (r = 0.23). After 1 year, 81% of RZV recipients and only 18% of ZVL recipients retained >50% of their peak avidity boosts. These results are consistent with the CMI responses to these vaccines: RZV responses are skewed to long-term memory, whereas ZVL preferentially induces transient effector responses.
IMPORTANCE These observations further distinguish the immunogenicity and duration of the immune response of the two vaccines. In addition, measurements of functional humoral immunity (IgG avidity and neutralizing antibody) in response to zoster immunization, alone or combined with other immune markers, might contribute to practical in vitro correlates of protection. Combined with previous observations of the cell-mediated response to these vaccines, this study suggests that vaccine development will benefit from more expansive and granular assessments of acquired immunity during early phase 1 immunogenicity trials.
KEYWORDS: herpes zoster, vaccine, avidity, humoral immunity, neutralizing antibodies
INTRODUCTION
Herpes zoster (HZ) results from reactivation of varicella-zoster virus (VZV) latent in neurons in dorsal root sensory and cranial nerve ganglia, causing an often painful and sometimes debilitating rash illness (1, 2). HZ risk increases dramatically in persons with reduced VZV-specific T cell-mediated immunity (CMI), such as those with immune deficiency or immune-compromising conditions or therapies or because of the immune senescence that accompanies aging (2–7). VZV-specific CMI responses are considered to play the dominant role in reducing the risk of HZ. For example, HZ risk increases with age and immune-compromising illnesses, in correlation with a decline in VZV-specific CMI but not with a decline in VZV-specific antibody (8–12).
The worldwide burden of HZ is very large (13) and expected to increase, because the population in most countries is aging, and increasing age correlates with the incidence and severity of HZ. Two vaccines, a live vaccine based on an attenuated VZV (Zostavax; ZVL) and an adjuvanted vaccine based on recombinant VZV glycoprotein E (gE) (Shingrix; RZV), are licensed to prevent HZ (14–16). RZV has a preferred recommendation because it has greater efficacy, especially in older vaccinees, and appears to have a longer persistence of protection (17). The unique immunologic responses that explain the clinical differences between these two vaccines are unknown. However, the immunological profile of VZV-specific CMI and humoral immunity after administration of each vaccine has been described in substudies of their separate phase III trials (18, 19). In addition, determination of a large array of T cell responses in recipients of either vaccine delineated differences that distinguish the two vaccines (20).
The current report focuses on antibody responses to each vaccine, including functional antibody responses not previously measured in recipients of HZ vaccines. In general, studies of antibody responses to HZ and HZ vaccines have used a lentil lectin purified mixture of glycoproteins extracted from VZV-infected cells as the antigen source for an enzyme-linked immunosorbent assay (gpELISA), whereas limited attention has been given to evaluating functional qualities of the immunoglobulin G (IgG) response to VZV antigens, such as avidity. Affinity maturation is characteristic of the IgG response after exposure to an antigen (21). For several months following antigen activation of B cells, the mean affinity (avidity) of antibodies for their specific antigen increases exponentially (21). Affinity maturation is driven by two independent processes, somatic hypermutation (SHM) and the culling of low-affinity clones through apoptosis (22, 23). The process coincides with a class switch from IgM to IgG after activated B cells are recruited to germinal centers, during which memory B cells and long-lived plasma cells are established (23–25).
In this study, we compared VZV gp- and gE-specific IgG immune responses in recipients of either ZVL or RZV measured by ELISA and by assays of functional responses, such as neutralization and avidity. These were evaluated in middle-aged and older individuals and in separate cohorts with or without prior HZ vaccination. The primary objective was to compare VZV gp- and gE-specific antibody responses to ZVL and RZV, as measured by three distinct assays, and to determine the correlation of each type of antibody response with the age of the vaccinee and prior HZ immunization.
RESULTS
IgG serology determined by ELISA.
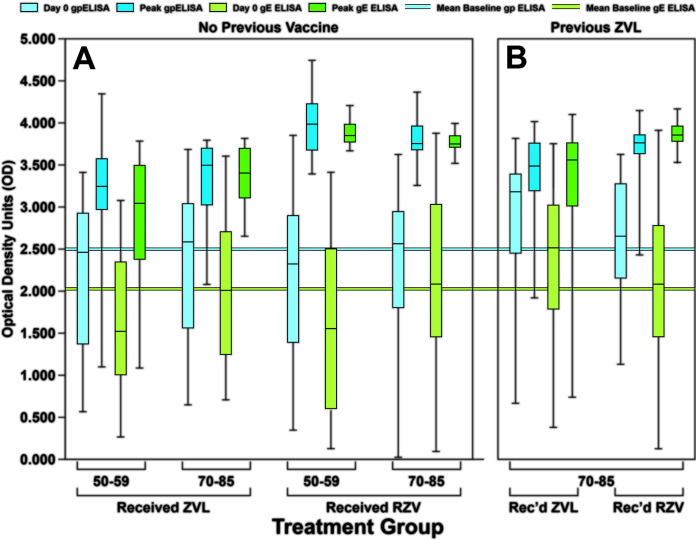
We compared VZV-specific IgG responses by participants in all six study groups, before and after they received HZ vaccines, using gpELISA and gE ELISA. The day 0 (baseline) optical density (OD) was compared with the peak OD during the first year of the study (Fig. 1). For all participants, the mean day 0 OD (before any vaccination) by gpELISA was 2.499, and for gE ELISA it was 2.027 (Fig. 1). Among participants in the four groups that had no previous HZ immunization (Fig. 1A), almost all day 0 VZV-specific IgG levels to gp were very high (average of 2.3 OD units), and postimmunization increases were small with both vaccines (average boost of 1.3 OD units). Although there was no difference in baseline gp OD values (2.3 OD units in RZV vaccinees versus 2.4 OD units in ZVL vaccinees, P = 0.5953), there was a statistically significantly greater gp OD boost in RZV vaccinees (1.6 OD units) versus ZVL vaccinees (0.9 OD units) (P < 0.0001). In contrast, day 0 levels of gE-specific IgG were substantially lower (average of 1.9 OD units) and, on average, underwent much larger gE-specific IgG boosts (average of 1.6 OD units) to both vaccines than was observed with gp-specific IgG (P = 0.0019 when comparing day 0 gp to day 0 gE levels; P = 0.0153 when comparing boosts in gp and gE). Mean boosts in gE antibody ranged from 1.3 to 2.3 OD units across all four treatment categories, whereas the mean boost in gp-specific antibody ranged from 0.8 to 1.8 OD units. There was no difference in day 0 gE OD values (1.9 OD units in either RZV and ZVL vaccinees; P = 0.9384), but there was a statistically significant difference in the gE OD boosts (2.0 OD units in RZV vaccinees versus 1.3 OD units in ZVL vaccinees; P = 0.0013). Predictably, mean boosts to gE were almost always higher in response to RZV than to ZVL (Fig. 1). These patterns were not different as a function of the age of the participant or prior ZVL immunization (Fig. 1). Boosts in gE OD were higher in RZV vaccinees than ZVL vaccinees (P = 0.0025 for 50 to 59 year olds; P = 0.0001 for 70 to 85 year olds) when stratified by age group and when stratified by prior ZVL immunization (P < 0.0001 for previously unimmunized; P = 0.0001 for previously immunized).
FIG 1.
Baseline and peak postimmunization VZV IgG levels measured by gpELISA and gE ELISA. (A) Participants with no previous shingles vaccination history, subdivided into two age groups. (B) Participants who received one dose of ZVL at least 5 years prior to study entry. Blue figures represent gpELISA, and green figures represent gE ELISA. Box-and-whisker plots subdivide the respondents into quartiles, with the colored boxes representing the middle 50% of respondents and the whiskers representing the extreme 50% of respondents. Baseline horizontal lines represent titers across all 6 groups and are indicated for gpELISA (blue) and gE ELISA (green).
Antibody avidity studies.
To evaluate functional properties of the VZV-specific IgG antibodies before and after administration of ZVL or RZV, we measured IgG avidity using either gp or gE as the antigen source. The comparison of day 0 prevaccination gp and gE avidity levels are shown in Fig. 2 for participants with no prior HZ vaccine grouped by participant age. There was no difference in the day 0 gp and gE avidity levels by age groups (average of 61 to 62 avidity index units [AIU] for both age groups; P = 0.9365), but there was a large difference between IgG avidity levels to gp versus gE (average of 74 AIUs for gp and 49 AIUs for gE; P < 0.0001). For gp, 91% (80/88) of participants displayed high-avidity IgG (≥60 AIU) compared to 24% (21/86) of the same participants having high avidity to gE (P < 0.0001). This indicated that measurements of avidity to a mixture of all VZV glycoproteins could mask low levels of avidity to individual glycoproteins.
FIG 2.
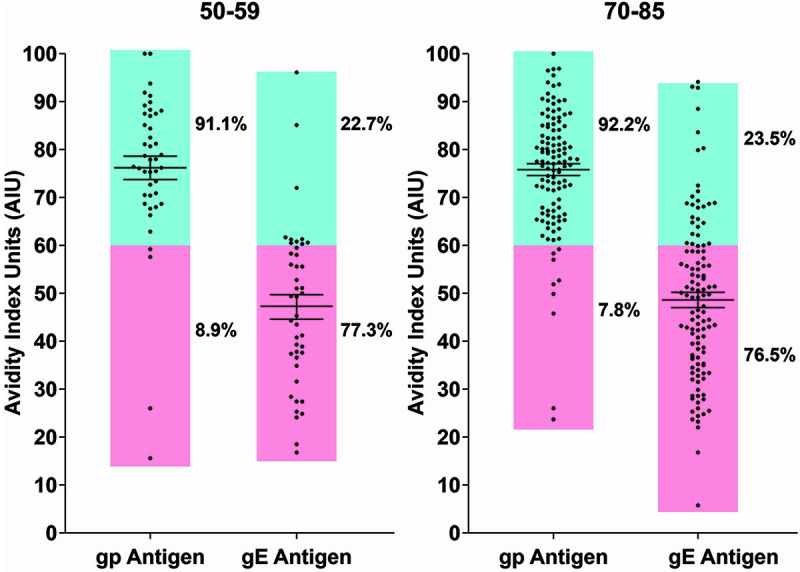
Comparison of preimmunization (day 0) avidity to gp antigens and purified gE antigen. Blue sector, >60 AIU; pink sector, ≤60 AIU.
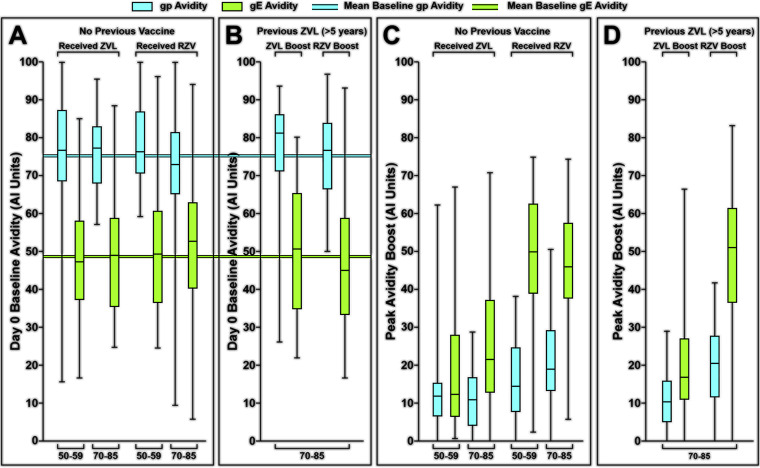
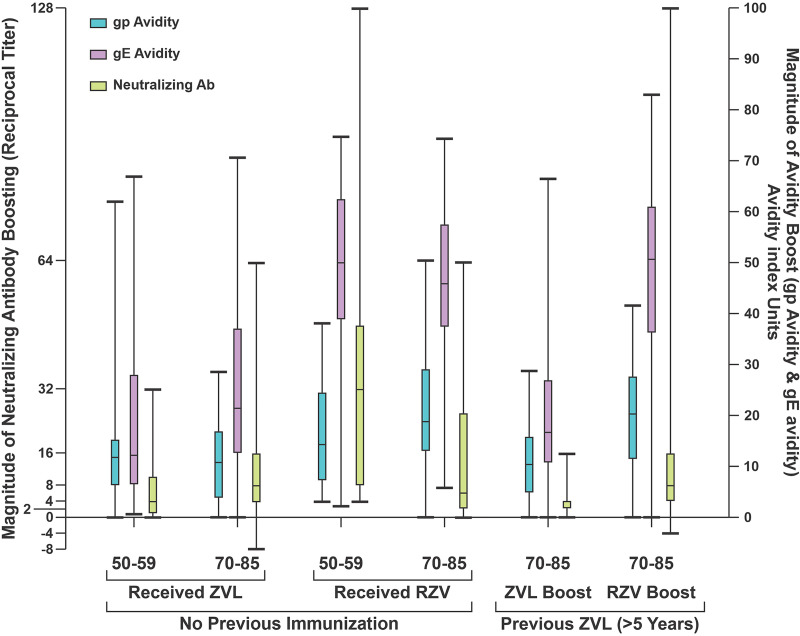
We then measured the capacity of ZVL and RZV to increase gp-specific IgG avidity among all study participants, including those previously immunized with HZ vaccine (Fig. 3). The mean day 0 avidities for gp and gE (Fig. 3A and B) recapitulate the striking differences in day 0 avidities to gp versus gE shown in Fig. 2. Mean avidity for gp was 75.2 AIU across all treatment groups, compared with 48.6 AIU for gE, a difference of 26.6 AIU. Only small increments in boosts of gp ELISA-based avidity were observed in either ZVL or RZV (average of 12 for ZVL vaccinees and 19 for RZV vaccinees) (Fig. 3). This was predictable given that most of the day 0 avidities for the gp mixture were already in the high-avidity range. ZVL recipients generally mounted low to moderate boosts in avidity over baseline to gE (average of 22 AIUs), whereas participants immunized with RZV produced pronounced boosts in avidity to gE (48% [38/80] displayed boosts of ≥50 AIU and 23% [18/80] displayed boosts of ≥60 AIU). A more granular display of the individual avidity boosts shows that the level of boosting of gE-specific avidity was approximately 2-fold higher in RZV recipients than ZVL recipients (∼20 AIU) (47 versus 22; P < 0.0001). These differences in gE avidity boosting remained statistically significant even after adjusting for both age group and prior vaccination with ZVL (P < 0.0001). In a univariate analysis, there were no statistically significant differences in either gE avidity boosting by age group (P = 0.4633) or prior vaccination with ZVL (P = 0.8000). However, among participants 70 to 85 years old who were previously immunized with ZVL (Fig. 3C and D), no recipient of a second dose of ZVL recipients boosted gE avidity to the limit of detection (0/34; 3%), whereas 16/34 (44%) recipients of boosting with RZV developed avidity levels at the limit of detection.
FIG 3.
Comparison of day 0 gp and gE avidities with peak postimmunization avidities. Results are displayed in quartiles as per Fig. 1. (A) Day 0 avidities among participants with no previous shingles immunization. (B) Peak boosts in avidity among previously unimmunized persons. (C) Day 0 avidities among participants previously immunized with ZVL. (D) Peak boosts in avidity among participants previously immunized with RZV.
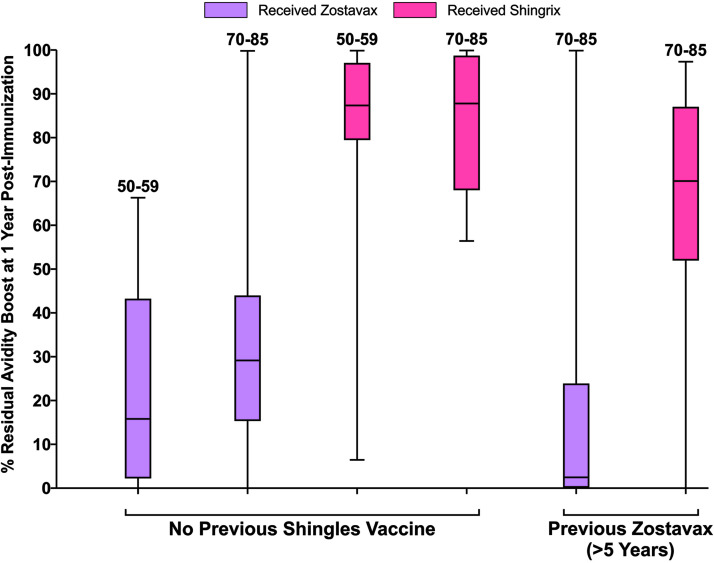
The generally superior increase in gE-specific avidity after RZV was also observed to be more durable (Fig. 4). Among those previously unimmunized who received ZVL, gE avidity declined by more than 60% by the end of 1 year in 67% (30/45) and by more than 70% in 60% (27/45) (Fig. 4). Among previously immunized ZVL recipients, 85% (29/34) had declines of ≥60%, and more than half (53%, 18/34) had 1-year declines in the vaccine-induced boost to gE avidity by more than 95%. In contrast, among RZV recipients, with or without prior ZVL, the boosts in gE avidity were maintained to a great extent at 1 year across all ages and groups. A higher proportion of RZV vaccinees retained ≥50% of the gE avidity boost at 1 year compared to ZVL vaccinees (81% versus 18%, P < 0.0001 by χ2; relative risk [RR] of 4.5 [2.8 to 7.3], P < 0.0001, when adjusted by age group and prior vaccination), and a higher proportion also retained ≥70% of the gE avidity boost at 1 year (65% versus 10%, P < 0.0001 by χ2; RR of 6.4 [3.3 to 12.6], P < 0.0001, when adjusted by age group and prior vaccination).
FIG 4.
Residual gE avidity boost, 1 year postimmunization. Purple plots represent the percentage of peak avidity retained for ZVL recipients; pink plots represent the percentage of peak avidity retained for RZL recipients.
Neutralizing antibody studies.
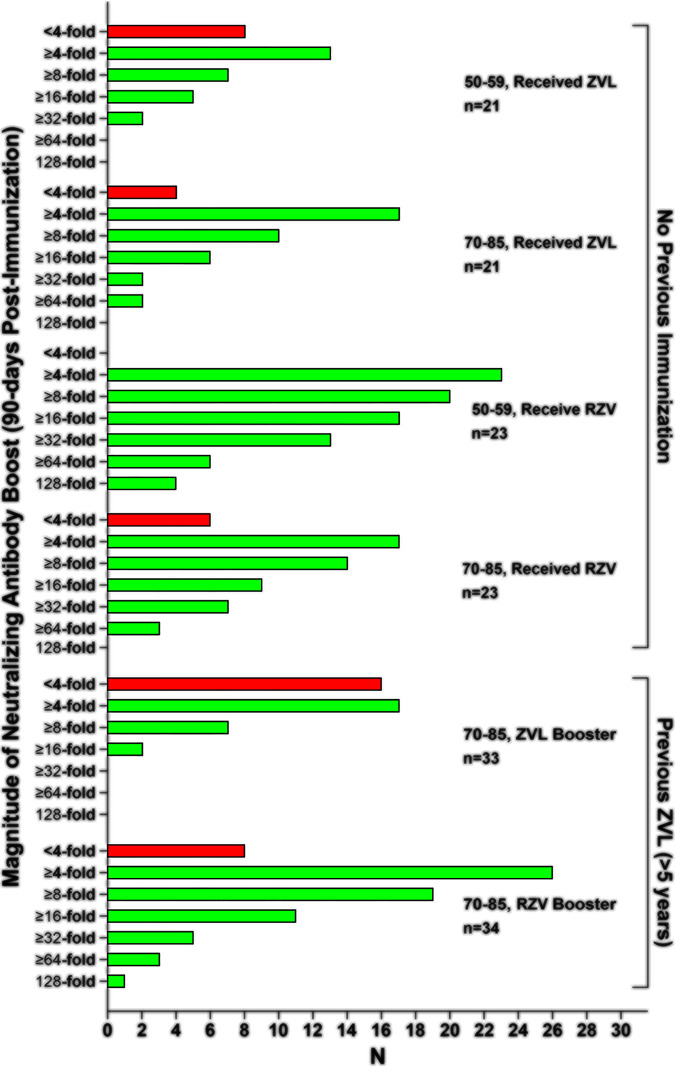
Complement-dependent immunocolorimetric neutralizing antibody (NA) assays were performed on day 0 and day 90 samples from all 6 treatment groups. In all categories, more than half of the participants displayed at least a 4-fold boost in NA titer (ranging from 52% to 100% by group); however, both the percentage (P = 0.0059) and magnitude of boosting (P = 0.0002) differed substantially between ZVL and RZV recipients (average NA boost of 8-fold in ZVL vaccinees versus 22-fold in RZV vaccinees) (Fig. 5). Comparing all ZVL recipients with all RZV recipients, 37% of ZVL recipients versus 6% of RZV recipients failed to achieve an NA boost of at least 4-fold. RZV induced superior neutralizing antibody boosts at every measured 2-fold endpoint (4-fold to 128-fold). For example, no ZVL recipients achieved NA boosts of ≥128-fold compared with 8% of RZV recipients. Results for each of the 6 treatment groups are displayed in Fig. 5.
FIG 5.
Relative level of boosting of VZV neutralizing antibody. Red bars indicate the number of participants who failed to develop at least a 4-fold increase in NA. Green bars indicate the total number of participants who developed boosts in NA greater than or equal to the indicated fold boost. As such, the magnitude of boosting declines with each successive boost level.
Comparative boosts in gp avidity, gE avidity, and NA titer.
We compared the boosts in gp avidity, gE avidity, and NA antibody in each treatment group (Fig. 6). Although different scales were used for each of the two measurements, the ordinates for each were scaled to reflect the maximum observed boosts for NA and avidity, respectively. Boosting of NA was more strongly associated with boosting in gE avidity (Spearman’s rank correlation coefficient [rs] = 0.50, P < 0.0001) than gp avidity (rs = 0.23, P = 0.0038) and remained significantly associated for boosting in NA and gE avidity (rs = 0.40, P < 0.0001, by Spearman’s partial correlation coefficient) when adjusted by HZ vaccine type, age, and prior vaccination with ZVL. In all cases, the level of boosting for both gE avidity and VZV-specific NA antibody was statistically higher for recipients of RZV than for ZVL recipients (P ≤ 0.0001 for gE avidity boost; P = 0.0002 for NA boost). Overall, the level of boosting of NA was lower for participants previously vaccinated with ZVL compared with previously unvaccinated participants (average NA boost of 10-fold in previously vaccinated versus 20-fold in previously unvaccinated persons, P = 0.0098), but RZV was more effective at boosting NA than ZVL in previously ZVL-vaccinated persons (average NA boost of 15-fold in RZV vaccinees versus 4-fold in ZVL vaccinees, P = 0.0178). The lower overall NA boosting among previously immunized 70- to 85-year-old participants compared to those not previously immunized was not explained by the possibility that the previously immunized group had higher NA titers at baseline. Among previously unimmunized 70- to 85-year-old participants, 42% (19/45) had baseline titers of ≤1:40; 60% (27/45) had baseline titers of ≤1:80; and 87% (39/45) had ≤1:160 baseline titers. The corresponding baseline titer percentages for previously vaccinated participants were 34% (23/68), ≤1:40; 66% (45/68), ≤1:80; and 87% (59/68), ≤1:160. There was no statistically significant difference in the proportions of 70 to 85 year olds in these 4 baseline NA titer groups by prior vaccination status (P > 0.05). Whatever the explanation for this discrepancy between previously vaccination and unvaccinated participants, the level of NA boosting among previously immunized participants was clearly much higher for the group that was boosted with RZV. NA boosting remained significantly associated with type of HZ vaccine when adjusted for age group, prior vaccination with ZVL, and baseline NA titers (P < 0.0001).
FIG 6.
Comparison of boosting in gp avidity, gE avidity, and neutralizing antibody titer among study participants. Ordinates for avidity boosting and NA antibody boosting were scaled to roughly reflect the maximum observed responses in the two categories of measurements.
DISCUSSION
The IgG avidity to a lectin-purified VZV glycoprotein mixture was uniformly high, irrespective of age group, before receiving an HZ vaccine and increased very little after vaccination. In contrast, avidity to purified gE was mostly in the moderate-to-low avidity range prior to vaccination, which indicates that the affinity maturation to gE differs from that of other VZV glycoproteins across individuals. It is conceivable that the immune dominance of VZV glycoproteins varies with the host. Ultimately all individuals achieve very high overall avidities to VZV gp. RZV, which contains only gE, induced substantial increases in gE avidity in the vast majority of vaccinees that persisted through the first year postimmunization, whereas gE avidity boosts by ZVL were less pronounced and declined by the end of 1 year.
A large majority of RZV recipients boosted to maximum gE avidity. When affinity maturation of antibody was first described, the authors measured the association constant, K0, and observed up to 10,000-fold increases in mean antibody binding strength over a 2-month period (21). Avidity is now measured by determining the ratio of residual bound antibody after chemical displacement to antibody bound prior to displacement. This effectively imposes a linear scale onto an exponential phenomenon. We may be underestimating the true scale of affinity maturation. Evaluation of a subset of the sera from this study for changes in the gE association constant should shed light on this possibility.
gE has multiple roles in the biology of VZV, including in cell-to-cell spread of the virus (26–28), replication (29–32), possibly cell entry (33), neurovirulence (34), and viral maturation (27, 35). In general, evidence for overt differences in the pathogenicity of individual VZV strains is lacking; however, a single-amino-acid change in gE in a clinical isolate of VZV (strain MSP) exhibited enhanced cell-to-cell spread in cell culture (36).
Functional aspects of the humoral immune response, such as antibody avidity, are augmented or specifically driven by components of the CMI response. CMI responses to VZV antigens are readily detected in older people without prior HZ or HZ vaccination, although the magnitude of these responses declines as age increases beyond ∼40 years (5). In contrast, CMI responses to gE are low or absent in older people, even though these responses are induced by varicella (20). Glycoprotein E represents a significant proportion of glycoproteins produced by VZV, and anti-gE antibodies are also a major component of the antibody response to varicella (10, 36–39) or after reexposure to VZV (40, 41). Our observations here suggest that engagement of the immune system by gE differs from the response to at least some of the other glycoproteins, leading to a decline in antibody to gE. Similarly, immunization with Oka strain VZV stimulates primarily VZV-specific CMI and only minimal gE-specific CMI (20). In contrast, immunization with RZV stimulates strong VZV- and gE-specific CMI responses, further indicating that gE is a significant component of VZV immunity. The overall peak VZV-specific CMI response after ZVL administration is generally 2-fold and is progressively lower as the age of the vaccinee increases (18). In contrast, gE-specific CMI peak responses after RZV administration are increased >20-fold with little effect of age on this response (18, 20).
The high titers of anti-gp antibodies detected in older people probably represent immunity that developed after varicella in childhood, possibly enhanced by exogenous or endogenous exposure throughout life (38, 40, 41). Levels of these antibodies were similar in the two age groups compared in this report, confirming previous evidence that VZV-specific antibodies do not decline with aging (10, 18). In contrast to total VZV-specific antibodies measured by gpELISA, levels of antibodies measured by gE ELISA were much lower. The low level of anti-gE antibodies, which was masked when total anti-gp antibodies were measured, presumably due to high concentrations of antibody directed against other gp antigens, was similar in the two age groups studied. While we are observing this effect in isolation for gE, it is possible that avidity to other VZV glycoproteins (i.e., gC) also diminish with age. This is something that should be studied experimentally, e.g., with other purified VZV glycoproteins or through selective antibody depletion.
Mean antibody avidity boost to gE was nearly 3.5 times higher after RZV administration than after ZVL. Since ample gE is present in ZVL, it appears that affinity maturation after exposure to gE during natural infection is limited and/or transient in individual hosts. Baseline avidity levels for gE were equivalent among previously ZVL-immunized and unimmunized participants, indicating that any boosting in gE avidity by ZVL had declined completely by 5 years postimmunization. By extension, these limitations in the development of matured gE IgG probably apply to reexposures to VZV and endogenous reactivation of the virus. In contrast, RZV appears to have overcome the naturally limited affinity maturation of anti-gE IgG, likely through a mechanism orchestrated by the adjuvant. This effect of RZV on the avidity profile was observed among both younger and older study participants. These results closely parallel measurements of CMI responses; memory and regulatory T cell responses are strongly engaged by RZV, in contrast to the transient induction of effector T cell responses by ZVL (20).
Among ZVL recipients, gE avidity boosts declined by >50% by the end of 1 year. In contrast, 75% of RZV recipients retained at least 50% of their peak avidity boost at 1 year, and half retained at least 75%. This suggests that the adjuvanted vaccine more efficiently stimulates the production of gE-specific memory B cells and long-lived plasma cells than ZVL. Thus, avidity produced in response to RZV is expected to be more durable than that produced in response to ZVL. Both vaccines appear to drive class switch recombination to gE IgG and, at least transiently, affinity maturation, which is consistent with observations of different effects of the vaccines on CMI responses.
Both class switch recombination (CSR) and somatic hypermutation (SHM) in antibody-producing cells are mediated by the same enzyme, activation-induced cytidine deaminase (AID). Follicular T helper cells (Tfh) within germinal centers influence the multiple roles that are driven by AID and that result in the production of memory B cells and long-lived plasma cells (42–44). CSR and SHM can be uncoupled experimentally, reflecting the presence of distinct peptide moieties responsible for targeting the enzyme for each activity (45). Our observations suggest that vaccine strain VZV Oka does not optimally increase the T cell interaction with B cells and are consistent with independent engagement of SHM and CSR in response to varicella vaccination (46). Supporting evidence is found in of 2-dose study of varicella vaccine in adult health care professionals (HCP); among 61 VZV IgG-seropositive HCP, 11.5% had antibodies with low avidity. In contrast, 132/142 (93%) of sera from persons with a history of natural VZV infection were in the high-avidity range, and the remainder were in the moderate range (46). These measurements used a VZV gp mixture as the target antigen.
Importantly, the boost in gE avidity was highly correlated with the boost in neutralizing antibodies, whereas the correlation between gp avidity and neutralization was significant but less robust, confirming that gE is an important target for neutralization.
In summary, RZV was markedly more effective at amplifying two important functional qualities of the antibody response to VZV. Notably, these findings are analogous to the findings for the CMI response by these same study participants. The CMI response to RZV was much larger than that to ZVL (20). Moreover, RZV preferentially engaged memory T cell and regulatory T cell responses, whereas ZVL preferentially stimulated an effector T cell response (20). The CMI, humoral, and demographic data from this study are now being evaluated to determine how these two acquired immune responses correlate.
Conclusions.
For roughly half a century, evaluations of vaccine immunogenicity have been limited to simple quantitative antibody measurements and somewhat limited studies of cell-mediated immunity. The superior clinical outcome of RZV administration is the result of the immune response to gE as facilitated by the ASO1B adjuvant, which must play an essential role in directing the acquired immune responses in favor of long-term memory. This also suggests the potential for enhancing the avidity and neutralizing antibodies to other important VZV glycoproteins, although this is evidently not required for an effective HZ vaccine.
The current evaluations of the acquired immune responses to two licensed vaccines provide additional insight into the underlying mechanisms of vaccine efficacy. The findings from this comparison study and others (20, 47) make a cogent argument that more comprehensive assessments of the immunogenicity of an investigational vaccine will better inform the decision to proceed to large clinical trials. It also demonstrates that in-depth immunological analysis of a relatively small number of participants can reveal unique information regarding vaccine performance. The potential role(s) of high-avidity gE IgG and neutralizing antibodies was not established in this study and will be evaluated in future efforts.
MATERIALS AND METHODS
Clinical study design.
This comparison study (ClinicalTrials.gov registration no. NCT02114333) was previously described in detail (20). The CDC’s participation was reviewed by the CDC IRB and determined as “Human Subjects Research, CDC not engaged,” as no personal identifying information was provided. RZV is an adjuvanted recombinant subunit vaccine; each dose contains 50 μg each of VZV gE, monophosphoryl lipid, and QS-21 saponin, the latter two components comprising the adjuvant system. ZVL is a live vaccine containing approximately 19,400 PFU of the same attenuated Oka strain of VZV. The vaccine also contains a substantial quantity of antigen in the form of nonviable viral particles and unpackaged proteins. Evaluation of a commercial vial of ZVL found that gE content was 5.25 μg/19,400 PFU of VZV (Hannah Nam, personal communication). There were 4 arms (total of 160 participants) that received an HZ vaccine. Arms A and B had not previously received an HZ vaccine (total of 90 participants, 45 in each arm). Arm A received one dose of ZVL followed 2 months later by placebo, and arm B received 2 doses of RZV separated by 2 months. Both arms were further stratified by age (50 to 59 years and 70 to 85 years; 22 to 23 in each age group in each arm). Arms C and D consisted of 35 participants per arm aged 70 to 85 years who had been immunized with ZVL at least 5 years previously. Arm C received a booster dose of ZVL and placebo, and arm D received 2 doses of RZV at the intervals specified for arms A and B.
Blood was obtained for immunologic assessment on days 0 (prevaccine), 30 (1 month after ZVL or RZV), 90 (2 months after placebo or RZV), and 365 from all participants. Additional blood was obtained for arm A on day 7 and for arm B on days 7 and 67. Peripheral blood mononuclear cells (PBMCs), plasma, and serum were cryopreserved within 4 h of acquisition (48, 49). Serum samples were provided to the CDC without personal identifiable information (PII), in part to preserve the study blind. The study was determined exempt (not human subject research) by the CDC IRB based on the assurance that under no circumstances would PII be provided.
VZV IgG assays.
The glycoprotein (gp) methods and glycoprotein E (gE) assays were performed based on methods developed by Merck & Co. (Kenilworth, NJ) (50) and GlaxoSmithKline (London, England) (51) and with antigen preparations they provided under material transfer agreements. In both cases, the antigen preparations were the same as those used in the respective vaccines. Normal tissue control antigen was procured from the same source.
(i) gp ELISA. Both lectin-purified gp antigens and uninfected tissue control antigens were diluted to a concentration of 1.0 μg/ml in phosphate-buffered saline (PBS) and added to wells of a 96-well plate at 100 μl/well. Plates were held at 4°C for 18 to 24 h, washed, and blocked with 5% skim milk for 30 min at ambient temperature. Test serum samples were diluted at a preoptimized 1:20 dilution and added to antigen-coated wells at 100 μl/well (duplicate test and normal tissue control wells). Plates were incubated at ambient temperature for 30 min, fluid was aspirated, and plates were washed 4 times with PBS-Tween 20. Goat anti-human IgG-alkaline phosphatase conjugate, diluted 1:1,000 in PBS, was added at 100 μl/well and incubated for 30 min. Disodium nitrophenyl phosphate substrate was added per the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO), and the enzymatic reaction proceeded for 10 min before being stopped with 100 μl of 2.5N NaOH solution. Plates were read on a spectrophotometer at 405 nm. Adjusted OD was calculated as mean test OD minus mean control OD.
(ii) gE ELISA. Purified recombinant gE was diluted to a concentration of 1.0 μg/ml and added to wells at 100 μl/well in 96-well plates. No normal tissue control antigen was required, as no other proteins are present in the preparation. All other specifications for this assay were the same as those for the gpELISA method.
(iii) gp and gE avidity. Plates were prepared and run as for the gp- and gE ELISA methods, except that duplicate plates were prepared. For avidity assessment, one plate was used for the conventional ELISAs described previously; the second plate was prepared in the same fashion except that it was washed 4 times with PBS-Tween 20 containing 35 mM diethylamine (DEA) before the color was determined. Avidity was calculated as AIU = (mean OD DEA plate/mean OD PBS wash plate) × 100.
sICNA.
Cell-free VZV preparations were produced as follows. Primary human lung fibroblasts (HLF) (ATCC, Manassas, VA) were grown in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS). Confluent monolayers were infected with VZV-infected cells (Webster strain) at a ratio of 1 infected cell to 5 uninfected cells. After 2 days of incubation at 37°C, when cytopathic effect (CPE) reached 80%, infected monolayers were scraped into overlay medium and sonicated twice using a Branson SFX450 digital sonifier (Atkinson, NH) set to 20% amplitude. Sonications were performed on ice for 15 s with a 30-s rest between two runs. Cellular debris was removed by centrifugation (2,000 rpm at 4°C for 10 min) and the supernatant aliquoted and stored at −70°C. For the soluble immunocolorimetric neutralization assay (sICNA) protocol, HLF were grown in MEM supplemented with 10% fetal calf serum. Confluent cells were infected with cell-free strain Webster VZV at an inoculum that produced a colorimetric optical density (wavelength, 450 nm) of roughly 1.000 at day 3, establishing the optimal inoculum for the working virus stock. This inoculum was roughly equivalent to 25 PFU of viable virus. Prior to infection, the VZV inoculum was incubated at 37°C for 30 min with duplicate serial dilutions of test or control serum previously heat inactivated at 56°C for 30 min (52). Since the neutralizing antibody to gE is complement dependent (47), 2.5 U of guinea pig complement was added to serum-virus mixtures and incubated a further 30 min at 37°C. The serum-virus mixtures were added to confluent HLF monolayers in 96-well plates, supplied with MEM, and incubated at 37°C for 1 h. Medium was aspirated from wells to remove unattached virus and test sera, replenished with 200 μl of fresh complete MEM, and incubated at 37°C for 3 days in a humidified CO2 incubator. Medium was removed on day 3, and the cells were fixed with methanol. Mouse anti-VZV antibody (Chemicon International, Inc., Temecula, CA) was added to wells for 30 min at ambient temperature, followed by anti-mouse IgG-tetramethylbenzidine conjugate. Colorimetric development was halted with dilute sulfuric acid. Plates were read in a spectrophotometer at 450 nm. The neutralization endpoint was the dilution at which the input virus (no serum) colorimetric signal was reduced by at least 50%. This method has been run in parallel with a conventional plaque reduction neutralization assay and demonstrated to correlate closely (47, 53, 54).
Statistical analysis.
The peak gp- and gE ELISA OD and avidity values were defined as the highest ELISA and avidity values taken at any visit during the study period. The magnitude of boosting of gp- and gE ELISA and avidity were calculated by taking the peak gp- and gE ELISA and avidity values minus their respective values at baseline (day 0). The magnitude of boosting in sICNA titer was calculated by taking the reciprocal sICNA titer at day 90 divided by the reciprocal titer at baseline. The percentage of gp and gE avidity boosting remaining at 1 year was calculated by taking the difference in the peak gp and gE values taken at any time point minus the respective gp and gE avidity values at 1 year, divided by the respective peak gp and gE avidity values.
We used t tests for comparing means of continuous variables and chi-square (χ2) or Fisher’s exact tests for comparing categorical variables. Additionally, to assess the association in boosts in gE avidity and sICNA by type of vaccine (ZVL and RZV), a multivariable log-gamma logistic regression model was used to control for age group (50 to 59 and 70 to 85 years) and prior vaccination with ZVL; baseline sICNA values were also included in the model to assess boosts in sICNA by vaccine type. To assess whether the proportion of participants who maintained ≥50% or ≥70% of gE avidity boost at 1 year differed by vaccine type, we used a generalized linear mixed-effect logistic regression model to estimate the relative risk (RR) and 95% confidence interval (CI), adjusting for age group and prior vaccination with ZVL. Spearman’s rank correlation coefficient (rs) was used to investigate the correlation between boosts in gp and gE avidity and sICNA titers. Partial correlation coefficients were computed to control for the type of HZ vaccine, age, and prior vaccination with ZVL. Two-sided P values of <0.05 were considered statistically significant. Data were analyzed using SAS 9.4 (SAS Institute, Inc., Cary, NC).
ACKNOWLEDGMENT
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Contributor Information
D. Scott Schmid, Email: dss1@cdc.gov.
Joanna L. Shisler, University of Illinois at Urbana Champaign
REFERENCES
- 1.Levin MJ. 2017. Zoster vaccine, p 1268–1281. In Plotkin S, Orenstein W, Offitt P, Edwards KM (ed), Vaccines, 7th ed. Elsevier, Philadelphia, PA. [Google Scholar]
- 2.Levin MJ, Schmader KS, Oxman MN. 2019. Chapter 165. Varicella and herpes zoster, p 3035–3064. In Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, Orringer JS (ed), Fitzpatrick’s dermatology, 9th ed. McGraw-Hill Education, New York, NY. [Google Scholar]
- 3.Chen SY, Suaya JA, Li Q, Galindo CM, Misurski D, Burstin S, Levin MJ. 2014. Incidence of herpes zoster in patients with altered immune function. Infection 42:325–334. 10.1007/s15010-013-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvin AM, Pollard RB, Rasmussen LE, Merigan TC. 1980. Cellular and humoral immunity in the pathogenesis of recurrent herpes viral infections in patients with lymphoma. J Clin Invest 65:869–878. 10.1172/JCI109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, Levin MJ. 2010. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis 201:1024–1030. 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolin R, Reichman RC, Mazur MH, Whitley RJ. 1978. Herpes zoster varicella infections in immunosuppressed patients. Ann Intern Med 89:375–388. 10.7326/0003-4819-89-3-375. [DOI] [PubMed] [Google Scholar]
- 7.Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, Saag KG, Singh J, Curtis JR. 2016. Risk of herpes zoster in autoimmune and inflammatory diseases. Implications for vaccination. Arthritis Rheumatol 68:2328–2337. 10.1002/art.39670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger R, Florent G, Just M. 1981. Decrease of the lymphoproliferative response to varicella-zoster virus in the aged. Infect Immun 32:24–27. 10.1128/IAI.32.1.24-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. 1982. Immune responses to varicella-zoster virus in the aged. Arch Intern Med 142:291–293. 10.1001/archinte.1982.00340150091017. [DOI] [PubMed] [Google Scholar]
- 10.Tang H, Moriishi E, Okamoto S, Okuno Y, Iso H, Asada H, Yamanishi K, Mori Y. Shozu Herpes Zoster Study Group. 2012. A community-based survey of varicella-zoster virus-specific immune responses in the elderly. J Clin Virol 55:46–50. 10.1016/j.jcv.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg A, Levin MJ. 2010. VZV T cell-mediated immunity. Curr Top Microbiol Immunol 342:341–357. 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- 12.Hata A, Asanuma H, Rinki M, Sharp M, Wong RM, Blume K, Arvin AM. 2002. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 347:26–34. 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 13.Kawai K, Gebremeskel BG, Acosta CJ. 2014. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 4:e004833. 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh S-S, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan ISF, Wang WWB, Annunziato PW, Silber JL. Shingles Prevention Study Group. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352:2271–2284. 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 15.Lal H, Cunningham AL, Godeaux O, Chlibek R, Díez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC. ZOE-50 Study Group. 2015. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 372:2087–2096. 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham AL, Lal H, Kovac R, Chlibek R, Hwang S-J, Díez-Comingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, Abeele CV, Vesikari T, Watanabe D, Zahaf T, Ahonen A, Athan E, Barba-Gomez JF, Campora L, de Looze F, Downey HJ, Ghesquiere W, Gorfinkel I, Korhonen R, Leung E, McNeil SA, Oostvogels L, Rombo L, Smetana J, Weckx L, Yeo W, Heineman TC. Study Group Z-7. 2016. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 375:1019–1032. 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 17.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. 2018. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep 67:103–108. 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan ISF, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A. VA Cooperative Studies Program Shingles Prevention Study Investigators. 2008. VZV-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 197:825–835. 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham A, Heineman TC, Lal H, Godeaux O, Chlibek R, Hwang S-J, McElhaney JE, Vesikari T, Andrews C, Choi WS, Esen M, Ikematsu H, Choma MK, Pauksens K, Ravault S, Salaun B, Schwarz TF, Smetana J, Abeele CV, Van de Steen P, Vastiau I, Weckx LY, Levin MJ. ZOE-50/70 Study Group. 2018. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis 217:1750–1760. 10.1093/infdis/jiy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin MJ, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N, Weinberg A. 2018. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest 128:4429–4440. 10.1172/JCI121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisen HN, Siskind GW. 1964. Variations in affinities of antibodies during the immune response. Biochemistry 3:996–1008. 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 22.Victora GD, Nussenzweig MC. 2012. Germinal centers. Annu Rev Immunol 30:429–457. 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 23.Tarlinton DM, Smith GC. 1997. Apoptosis and the B cell response to antigen. Int Rev Immunol 15:53–71. 10.3109/08830189709068171. [DOI] [PubMed] [Google Scholar]
- 24.Amanna IJ, Slifka MK. 2010. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev 236:125–138. 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelsoe G. 1996. The germinal center: a crucible for lymphocyte selection. Semin Immunol 8:179–184. 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- 26.Grose C, Tyler S, Peters G, Hiebert J, Stephens GM, Ruyechan WT, Jackson W, Storlie J, Tipples GA. 2004. Complete DNA sequence analyses of the first two varicella-zoster virus glycoprotein E (D150N) mutant viruses found in North America: evolution of genotypes with an accelerated cell spread phenotype. J Virol 78:6799–6807. 10.1128/JVI.78.13.6799-6807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berarducci B, Rajamani J, Reichelt M, Sommer M, Zerboni L, Arvin AM. 2009. Deletion of the first cysteine-rich region of the varicella-zoster virus glycoprotein E ectodomain abolishes the gE and gI interaction and differentially affects cell-cell spread and viral entry. J Virol 83:228–240. 10.1128/JVI.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo C, Schneeberger EE, Arvin AM. 2000. Glycoprotein E of varicella-zoster virus enhances cell-cell contact in polarized epithelial cells. J Virol 74:11377–11387. 10.1128/jvi.74.23.11377-11387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berarducci B, Ikoma M, Stamatis S, Sommer M, Grose C, Arvin AM. 2006. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J Virol 80:9481–9496. 10.1128/JVI.00533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moffat J, Ito H, Sommer M, Taylor S, Arvin AM. 2002. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J Virol 76:8468–8471. 10.1128/jvi.76.16.8468-8471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffat J, Mo C, Cheng JJ, Sommer M, Zerboni L, Stamatis S, Arvin AM. 2004. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J Virol 78:12406–12415. 10.1128/JVI.78.22.12406-12415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mo C, Lee J, Sommer M, Grose C, Arvin AM. 2002. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology 304:176–186. 10.1006/viro.2002.1556. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Mir A, Cohen JI. 2006. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 127:305–316. 10.1016/j.cell.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zerboni L, Berarducci B, Rajamani J, Jones CD, Zehnder JL, Arvin AM. 2011. Varicella-zoster virus glycoprotein E is a critical determinant of virulence in the SCID mouse-human model of neuropathogenesis. J Virol 85:98–111. 10.1128/JVI.01902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alconada A, Bauer U, Baudoux L, Piette J, Hoflack B. 1998. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J Biol Chem 273:13430–13436. 10.1074/jbc.273.22.13430. [DOI] [PubMed] [Google Scholar]
- 36.Santos RA, Hatfield CC, Cole NL, Padilla JA, Moffat JF, Arvin AM, Ruyechan WT, Hay J, Grose C. 2000. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology 275:306–317. 10.1006/viro.2000.0507. [DOI] [PubMed] [Google Scholar]
- 37.Haumont M, Jurdan M, Kangro H, Jacquet A, Massaer M, Deleersnyder V, Garcia L, Bosseloir A, Bruck C, Bollen A, Jacobs P. 1997. Neutralizing antibody responses induced by varicella-zoster virus gE and gB glycoproteins following infection, reactivation or immunization. J Med Virol 53:63–68. . [DOI] [PubMed] [Google Scholar]
- 38.Chlibek R, Pauksens K, Rombo L, van Rijckevorsel G, Richardus JH, Plassmann G, Schwarz TF, Catteau G, Lal H, Heineman TC. 2016. Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine 34:863–868. 10.1016/j.vaccine.2015.09.073. [DOI] [PubMed] [Google Scholar]
- 39.Arvin AM. 2008. Humoral and cellular immunity to varicella-zoster virus: an overview. J Infect Dis 197:S58–S60. 10.1086/522123. [DOI] [PubMed] [Google Scholar]
- 40.Arvin AM, Koropchak CM, Wittek AE. 1983. Immunological evidence of reinfection with varicella-zoster virus. J Infect Dis 148:200–205. 10.1093/infdis/148.2.200. [DOI] [PubMed] [Google Scholar]
- 41.Ogunjimi B, van den Bergh J, Meysman P, Heynderick S, Bergs K, Jansens H, Leuridan E, Vorsters A, Goossens H, Laukens K, Cools N, Van Tendeloo V, Hens N, Van Damme P, Smits E, Beutels PH. 2017. Multidisciplinary study of the secondary immune response in grandparents re-exposed to chickenpox. Sci Rep 7:1077. 10.1038/s41598-017-01024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ettinger R, Sims GP, Fairhurst A-M, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. 2005. IL-21 induces differentiation of human naïve and memory B cells into antibody-secreting plasma cells. J Immunol 175:7867–7879. 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Shi J, Yan J, Xiao Z, Hou X, Lu P, Hou S, Mao T, Liu W, Ma Y, Zhang L, Yang X, Qi H. 2017. Germinal center development of memory B cells driven by IL-9 from follicular helper T cells. Nat Immunol 18:921–930. 10.1038/ni.3788. [DOI] [PubMed] [Google Scholar]
- 44.Good-Jacobson KI, Szumila CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. 2010. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol 11:535–542. 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barreto V, Reina-San-Martin B, Ramiro AR, McBride KM, Nussenzweig MC. 2003. C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol Cell 12:501–508. 10.1016/S1097-2765(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 46.Behrman A, Lopez AS, Chaves SS, Watson BM, Schmid DS. 2013. Varicella immunity in vaccinated healthcare workers. J Clin Virol 57:109–114. 10.1016/j.jcv.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan NL, Reuter-Monslow MA, Sei J, Durr E, Davis CW, Chang C, McCausland M, Wieland A, Krah D, Rouphael N, Mehta AK, Mulligan MJ, Pulendran B, Ahmed R, Vora KA. 2018. Breadth and functionality of varicella-zoster virus glycoprotein-specific antibodies identified after zostavax vaccination in humans. J Virol 92:e00269-18. 10.1128/JVI.00269-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberg A, Song LY, Wilkening CL, Fenton T, Hural J, Louzao R, Ferrari G, Etter PE, Berrong M, Canniff JD, Carter D, Defawe OD, Garcia A, Garrelts TL, Gelman R, Lambrecht LK, Pahwa S, Pilakka-Kanthikeel S, Shugarts DL, Tustin NB. 2010. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J Immunol Methods 363:42–50. 10.1016/j.jim.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song L-Y, Wilkening C, Sevin A, Blais B, Louzao R, Stein D, Defechereux P, Durand D, Riedel E, Raftery N, Jesser R, Brown B, Keller MF, Dickover R, McFarland E, Fenton T. Pediatric ACTG Cryopreservation Working Group. 2009. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol 16:1176–1186. 10.1128/CVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasmuth EH, Miller WJ. 1990. Sensitive enzyme-linked immunosorbent assay for antibody to varicella-zoster virus using purified VZV glycoprotein antigen. J Med Virol 32:189–193. 10.1002/jmv.1890320310. [DOI] [PubMed] [Google Scholar]
- 51.Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. 2013. Safety and immunogenicity of an adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults >=50 years of age. J Infect Dis 208:1953–1961. 10.1093/infdis/jit365. [DOI] [PubMed] [Google Scholar]
- 52.Miao C, Place R, Hao L, Chen MH, Icenogle J, Schmid S. 2019. Development of a soluble immunocolorimetric neutralization assay (sICNA) for measuring antibody to varicella-zoster virus. 44th Int Herpesvirus Workshop, Knoxville, TN, 20 to 24 July 2019.
- 53.Chen MH, Zhu Z, Zhang Y, Favors S, Xu W-B, Featherstone DA, Icenogle JP. 2007. An indirect immunocolorimetric assay to detect rubella virus infected cells. J Virol Methods 146:414–418. 10.1016/j.jviromet.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Lambert ND, Pankratz VS, Larrabee BR, Ogee-Nwankwo A, Chen M-H, Icenogle JP, Poland GA. 2014. High-throughput assay optimization and statistical interpolation of rubella-specific neutralizing antibody titers. Clin Vaccine Immunol 21:340–346. 10.1128/CVI.00681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]