ABSTRACT
Limited pharmacokinetic (PK) data suggest that currently recommended pediatric dosages of colistimethate sodium (CMS) by the Food and Drug Administration and European Medicines Agency may lead to suboptimal exposure, resulting in plasma colistin concentrations that are frequently <2 mg/liter. We conducted a population PK study in 17 critically ill patients 3 months to 13.75 years (median, 3.3 years) old who received CMS for infections caused by carbapenem-resistant Gram-negative bacteria. CMS was dosed at 200,000 IU/kg/day (6.6 mg colistin base activity [CBA]/kg/day; 6 patients), 300,000 IU/kg/day (9.9 mg CBA/kg/day; 10 patients), and 350,000 IU/kg/day (11.6 mg CBA/kg/day; 1 patient). Plasma colistin concentrations were determined using ultraperformance liquid chromatography combined with electrospray ionization-tandem mass spectrometry. Colistin PK was described by a one-compartment disposition model, including creatinine clearance, body weight, and the presence or absence of systemic inflammatory response syndrome (SIRS) as covariates (P < 0.05 for each). The average colistin plasma steady-state concentration (Css,avg) ranged from 1.11 to 8.47 mg/liter (median, 2.92 mg/liter). Ten patients had Css,avg of ≥2 mg/liter. The presence of SIRS was associated with decreased apparent clearance of colistin (47.8% of that without SIRS). The relationship between the number of milligrams of CBA per day needed to achieve each 1 mg/liter of plasma colistin Css,avg and creatinine clearance (in milliliters per minute) was described by linear regression with different slopes for patients with and without SIRS. Nephrotoxicity, probably unrelated to colistin, was observed in one patient. In conclusion, administration of CMS at the above doses improved exposure and was well tolerated. Apparent clearance of colistin was influenced by creatinine clearance and the presence or absence of SIRS.
KEYWORDS: colistin, population pharmacokinetics, children, creatinine clearance, systemic inflammatory response syndrome
TEXT
The emergence of nosocomial infections caused by multidrug-resistant Gram-negative bacteria, observed worldwide for both adult and pediatric patients, has led to the resurgence of use of colistin. Also called polymyxin E, colistin was introduced in clinical practice in the 1950s but was soon replaced by newer antibiotics due to nephro- and neurotoxicity. It is currently active against many carbapenem-resistant isolates of Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Susceptibility MIC breakpoints for A. baumannii, P. aeruginosa, and the Enterobacterales are ≤2 mg/liter (CLSI, EUCAST). Colistin is administered parenterally in its inactive prodrug form, colistimethate sodium (CMS), which is subsequently converted to its active metabolite, colistin. Early pharmacokinetic (PK) studies of CMS in humans were based on microbiologic assays for determination of colistin concentrations in biological fluids and therefore overestimated colistin concentrations because of continuous, ex vivo conversion of CMS to colistin during the incubation period (1). Over the last decade, PK studies in adults using reliable high-performance liquid chromatography (HPLC) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods for quantification of colistin concentrations have led to proposed dosage recommendations for these patients (2–9). These studies have revealed significant interpatient variability in plasma concentrations among adult critically ill patients; population PK analysis also identified creatinine clearance (CrCL) as an important covariate for the clearance of CMS and the apparent clearance of colistin (3, 7, 8).
The U.S. Food and Drug Administration (FDA)- and the European Medicines Agency (EMA)-recommended doses of CMS for children with normal renal function are currently 75,000 to 150,000 IU/kg/day (equivalent to ∼2.5 to 5 mg of colistin base activity [CBA]/kg/day) in 2 to 4 divided doses (10, 11). There have been only a few PK studies of CMS in pediatric patients using modern, valid assays for determination of colistin concentrations, with each study consisting of single or small series of patients (12–15). Results reported from another study involving 7 pediatric patients suggested dose-normalized plasma colistin concentrations substantially higher than those previously reported (16). That report prompted correspondence questioning the results (17); subsequently, the authors of the original report indicated that the conditions used in the LC-MS/MS assay to quantify colistin in the plasma of the 7 pediatric patients resulted in artificially high plasma colistin concentrations in samples containing CMS (18). A recently published PK study of 20 patients included children aged ≥2 years; traditional noncompartmental PK analysis was conducted, and covariates of the apparent clearance of colistin were not identified (19). Results of several of these studies suggest that administering CMS at doses currently recommended by the FDA or EMA for pediatric patients frequently results in plasma colistin concentrations of <2 mg/liter (12–15, 19), which is the breakpoint for susceptibility of nosocomial Gram-negative pathogens to colistin. Population PK covariate analysis in 5 patients with a median age of 1.75 (range, 0.1 to 6.25) years also demonstrated that the clearances of CMS and colistin were related to CrCL (15).
In the present study, we aimed to investigate colistin PK in critically ill pediatric patients, aged 1 month to 14 years, who were treated with doses higher than those recommended by the FDA and EMA. We performed population PK analysis in order to investigate relationships between various demographic or clinical factors, such as CrCL or the presence of systemic inflammatory response syndrome (SIRS), with the apparent clearance of colistin. Based upon the population PK covariates identified, we investigated approaches to possibly develop a dosing algorithm for determination of CMS dose needed to achieve each 1 mg/liter of average steady-state plasma colistin concentration and evaluated generated equations using patient data from two previous studies (12, 15).
RESULTS
Patient characteristics.
A total of 17 patients (6 female) were studied, aged from 3 months to 13.75 years (median age, 3.3 years). Fourteen of these patients were hospitalized in pediatric intensive care units (PICU) and 3 in neurosurgical and pediatric oncology units. Six patients (median age, 9.2 years) received CMS at 200,000 IU/kg/day (6.6 mg CBA/kg/day), 10 patients (median age, 2.8 years) were dosed at 300,000 IU/kg/day (9.9 mg CBA/kg/day), and one infant (5 months old) was dosed at 350,000 IU/kg/day (11.6 mg CBA/kg/day) (Table 1). SIRS data, pediatric risk of mortality (PRISM) score, and pediatric logistic organ dysfunction (PELOD) score were provided only for PICU patients. Most patients received CMS for sepsis or ventilator-associated pneumonia (VAP) caused by Gram-negative organisms, such as A. baumannii, K. pneumoniae, and P. aeruginosa. Seven of these patients had documented bacteremia. In all cases, CMS was coadministered with other antimicrobials possibly active against Gram-negative bacteria. Duration of CMS treatment ranged between 10 and 28 days (Table 2).
TABLE 1.
Patients’ demographic characteristics, CMS doses, and presence of SIRSa
| Estimated CrCL (ml/min/1.73 m2) for dose |
CMS dose [IU/kg/day | SIRS presence at dose |
||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age range | Wt (kg) | 1 | 5 | (mg CBA/kg/day)] | 1 | 5 |
| 1 | F | 2 yr to <4 yr | 9.5 | 126.3 | 133.6 | 200,000 (6.6) | Yes | Yes |
| 2 | M | 1 mo to <2 yr | 13 | 111.3 | 106.1 | 300,000 (9.9) | Yes | Yes |
| 3 | M | 2 yr to <4 yr | 9 | 127.4 | 146.7 | 300,000 (9.9) | No | No |
| 4 | M | 1 mo to <2 yr | 5 | 72.5 | 74.6 | 300,000 (9.9) | No | No |
| 5 | M | 1 mo to <2 yr | 6.2 | 65.1 | 65.1 | 300,000 (9.9) | Yes | Yes |
| 6 | M | 6 yr to <8 yr | 22 | 113.9 | 115.9 | 300,000 (9.9) | NR | NR |
| 7 | M | 1 mo to <2 yr | 3.8 | 61.9 | 61.9 | 350,000 (11.6) | No | No |
| 8 | F | 8 yr to <10 yr | 34 | 176.8 | NR | 200,000 (6.6) | NR | NR |
| 9 | M | 8 yr to <10 yr | 27 | 138.0 | 146.3 | 300,000 (9.9) | NR | NR |
| 10 | F | 1 mo to <2 yr | 6.5 | 168.7 | 168.7 | 300,000 (9.9) | Yes | Yes |
| 11 | M | 10 yr to <12 yr | 40 | 131.2 | 175.0 | 200,000 (6.6) | Yes | Yes |
| 12 | F | 8 yr to <10 yr | 50 | 130.7 | 135.2 | 200,000 (6.6) | Yes | Yes |
| 13 | M | 4 yr to <6 yr | 18 | 113.2 | 127.9 | 300,000 (9.9) | Yes | No |
| 14 | F | 12 yr to 14 yr | 47 | 149.8 | 127.8 | 200,000 (6.6) | Yes | No |
| 15 | M | 1 mo to <2 yr | 5.8 | 85.9 | NA | 200,000 (6.6) | Yes | NA |
| 16 | M | 12 yr to 14 yr | 44 | 244.2 | 305.3 | 300,000 (9.9) | Yes | Yes |
| 17 | F | 2 yr to <4 yr | 15 | 172.3 | 172.3 | 300,000 (9.9) | Yes | Yes |
CrCL, creatinine clearance; CMS, colistimethate sodium; CBA, colistin base activity; SIRS, systemic inflammatory response syndrome; F, female; M, male; NR, not recorded; NA, not applicable (patient did not receive the fifth dose).
TABLE 2.
Type of infection, Gram-negative organism implicated, coadministered antimicrobial agents, outcome, and renal toxicitya
| Patient | Type of infection | Treatment duration (days) | Organism implicated | Site of isolation | Coadministered antimicrobial agents | Outcome | Renal toxicity |
|---|---|---|---|---|---|---|---|
| 1 | VAP | 28 | P. aeruginosa | BA | TZP, CLR | Death | No |
| 2 | Fever unresponsive to broad-spectrum ABx, colonization with CR, P. aeruginosa | 10 | P. aeruginosa | Throat swab | MEM, AMK, TEC | Cure | No |
| 3 | Possible VAP, colonization with P. aeruginosa and A. baumannii | 20 | P. aeruginosa, A. baumannii | BA | CIP, TGC | Cure | No |
| 4 | VAP | 20 | P. aeruginosa | BAL fluid | FEP, TEC | Cure | No |
| 5 | Sepsis | 19 | A. baumannii | Blood | MEM, AMK, LZD | Cure | No |
| 6 | Bacteremia | 10 | K. pneumoniae | Blood | MEM, GEN, SXT | Cure | No |
| 7 | VAP | 20 | A. baumannii | BA | MEM, AMK | Clinical improvement | No |
| 8 | VAP | 10 | A. baumannii | BA | MEM, TEC | Cure | No |
| 9 | Bacteremia | 10 | A. baumannii | Blood | MEM, GEN, TZP | Cure | No |
| 10 | Septic shock, VAP | 15 | Stenotrophomonas maltophilia | BAL fluid | MEM | Death | No |
| 11 | Septic shock | 17 | P. aeruginosa | Blood | MEM, VAN, RIF, CIP, AZM | Clinical improvement | No |
| 12 | VAP, bacteremia | 10 | A. baumannii | Blood, BA | TGC, TEC, AMK, TZP | Cure | No |
| 13 | Sepsis | 23 | K. pneumoniae | Blood | MEM, GEN, TEC, TGC, FOF, SXT | Death | Yes |
| 14 | Surgical trauma infection | 20 | A. baumannii | Trauma site, BAL fluid | MEM, LZD, TGC | Cure | No |
| 15 | VAP | 28 | A. baumannii, K. pneumoniae, P. aeruginosa | BAL fluid | GEN, TGC | Cure | No |
| 16 | Septic shock | 20 | P. aeruginosa | Blood | MEM, GEN, TZP, SXT | Clinical improvement | No |
| 17 | Sepsis | 17 | S. maltophilia | Blood | CIP, TEC | Cure | No |
ABx, antibiotics; AMK, amikacin; BA, bronchial aspirate; BAL, bronchoalveolar lavage; CIP, ciprofloxacin; CLR, clarithromycin; CR, carbapenem-resistant; FEP, cefepime; FOF, fosfomycin; GEN, gentamicin; LZD, linezolid; MEM, meropenem; RIF, rifampin; SXT, trimethoprim-sulfamethoxazole; TEC, teicoplanin; TGC, tigecycline; TZP, piperacillin-tazobactam; VAN, vancomycin; VAP, ventilator-associated pneumonia.
Pharmacokinetic analysis.
The population PK of formed colistin was described by a one-compartment disposition model (see Fig. S1A in the supplemental material). The final model included a first-order process that described the increase in formed colistin concentrations due to conversion of CMS to colistin and estimated a first-order formation rate constant. The apparent clearance and volume of distribution of formed colistin, including their interindividual variabilities, were also estimated. Interindividual variability of the formation rate constant was not estimable. Interoccasion variability in the apparent volume of distribution was incorporated in the model. Covariate effects that were included in the final model were total body weight and the presence or absence of SIRS on the apparent clearance of formed colistin, CrCL on the estimated relative fraction of CMS that was converted to formed colistin (20), and total body weight on the apparent volume of distribution of colistin. All covariates were included as time-varying covariates. Following inclusion of these covariate effects, no further biologically plausible effects of other potential covariates, such as height, age, sex, PRISM, or PELOD, were identified. The final model described the data well, as indicated by the diagnostic plots and model fits (Fig. S1B and C).
The population mean parameters were estimated with good precision by the final model (all standard errors [SE] were <30%) (Table 3). The apparent population mean clearance was 4.10 liters/h for a typical subject with 15 kg body weight and without SIRS. The presence of SIRS was correlated with a substantially decreased apparent clearance of colistin, which was 47.8% of that without SIRS. The relative fraction of CMS converted to colistin was inversely correlated with CrCL. A potential effect of the outlying concentrations that were omitted from the final modeling data set was evaluated. The final model was rerun with the two outlying concentrations from patient 7 included in the data set, which resulted in very similar population PK parameter estimates but increased the unexplained interindividual variability.
TABLE 3.
Population PK parameter estimates, interindividual and interoccasion variability, and precision of estimates
| Parameter (unit)a | % CV (% shrinkage) |
|||
|---|---|---|---|---|
| Population estimate | Interindividual | Interoccasion | % SE | |
| Apparent clearance (liters/h/15 kg0.75) in absence of SIRS | 4.10 | 53 (4.1) | 20 | |
| Apparent vol of distribution (liters/15 kg) | 20.7 | 40 (37) | 62 (dose 1, 41; dose 5, 8.1) | 21 |
| Formation rate constant (h−1) | 1.50 | 17 | ||
| RFMslope [(48 ml/min)−1] | 0.146 | 12 | ||
| Fraction of clearance in the presence of SIRS | 0.478 | 26 | ||
| Terminal half-life of colistin (h) | 4.96b | |||
| SDslope (%) | 20 | |||
| SDintercept (mg/liter) | 0.300 | |||
Apparent clearance and volume of distribution for a creatinine clearance of 0 ml/min; RFM, relative fraction of CMS converted to colistin {1 − [RFMslope × creatinine clearance/(48 ml/min)]} (14); RFMslope, slope of RFM change by creatinine clearance; SDslope, proportional residual error; SDintercept, additive residual error (ɛ shrinkage was 12.6%).
Median of the individual estimates calculated based on the apparent clearance and apparent volume of distribution (10th to 90th percentile 2.57 to 10.80 h).
In the current study, the clinician-selected daily dose of CMS ranged from 200,000 to 350,000 IU/kg/day (6.6 to 11.6 mg CBA/kg/day), and the resulting average steady-state plasma colistin concentration (Css,avg) derived from the population PK analysis ranged from 1.11 to 8.47 mg/liter, with a median of 2.92 mg/liter. Ten of the 17 patients achieved a plasma colistin Css,avg of ≥2 mg/liter, five of whom had a concentration of ≥4 mg/liter. The median (range) plasma colistin Css,avg expected to be achieved if the 17 patients had been administered the lower (2.5 mg/kg/day) or upper (5 mg/kg/day) limit of the FDA- and EMA-approved doses were 0.75 (0.29 to 3.21) mg/liter and 1.50 (0.58 to 6.42) mg/liter, respectively; i.e., at each dose, there was an expected 11-fold range of concentrations.
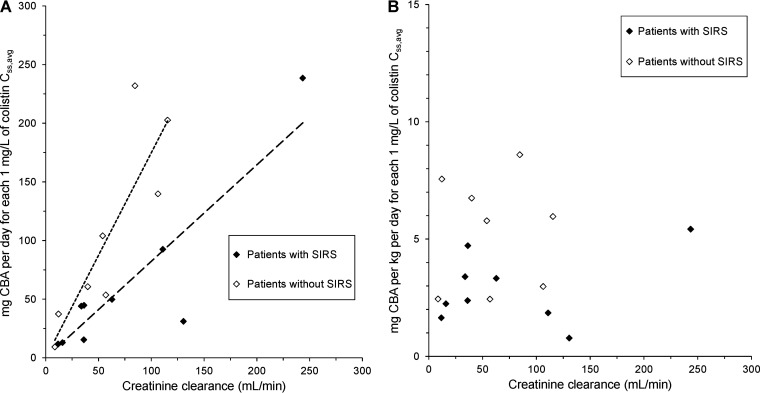
A number of graphical approaches were explored in an attempt to identify a possible path to formulation of dosing guidelines to target a desired plasma colistin concentration. From knowledge of the plasma colistin Css,avg achieved from the administered daily dose of CBA in each patient, it was possible to calculate the daily dose of CBA needed to achieve each 1 mg/liter of plasma colistin Css,avg. These daily doses of CBA are presented in Fig. 1 as a function of the covariates of the apparent clearance of colistin identified in the population PK analysis, namely, CrCL, the presence or absence of SIRS, and total body weight. In Fig. 1A, the relationship between the number of milligrams per day of CBA needed to achieve each 1 mg/liter of plasma colistin Css,avg and CrCL (in milliliters per minute) was described by linear regression both for patients with SIRS and for those without SIRS; the slope of the relationship for patients with SIRS (slope = 0.823; R2 = 0.81) was 47% of that for patients without SIRS (slope = 1.748; R2 = 0.74). In contrast, there was no relationship between the body weight-normalized daily dose needed to achieve each 1 mg/liter of plasma colistin Css,avg and CrCL for patients either with SIRS (R2 = 0.12) or without SIRS (R2 = 0.001) (Fig. 1B); there was a 6.95-fold range of daily doses for patients with SIRS (0.78 to 5.42 mg/kg/day) and a 3.52-fold range for patients without SIRS (2.44 to 8.59 mg/kg/day).
FIG 1.
Relationships between the daily dose of colistin base activity (CBA), expressed as milligrams per day (A) and as milligrams per kilogram per day (B), needed for each 1 mg/liter of the average steady-state plasma concentration of colistin (Css,avg) and creatinine clearance (in milliliters per minute). In panel A, data for patients with SIRS (filled symbols; R2 = 0.81) and without SIRS (empty symbols; R2 = 0.74) were regressed separately.
The corresponding plots of the daily dose of CBA to achieve each 1 mg/liter of plasma colistin Css,avg versus CrCL normalized to body surface area (i.e., milliliters per minute per 1.73 m2) are presented in Fig. S2. The relationships in Fig. S2A had lower coefficients of determination (R2) and very large intercepts on the x axis relative to the corresponding relationships for CrCL expressed in milliliters per minute (Fig. 1). Although patient age was not identified as a covariate in the population PK analysis, that variable was investigated for possible relationships with the daily dose of CBA for each 1 mg/liter plasma colistin Css,avg (Fig. S3). The R2 values for the relationships presented in Fig. S3A were low relative to the relationships for patients with and without SIRS presented in Fig. 1A; the relationships in Fig. S3A almost certainly arose because there were strong relationships (R2 = 0.87 and 0.85, respectively) (Fig. S4) between the physiological variable, CrCL, and the demographic variable, age.
The equations for the regression lines in Fig. 1A for patients with and without SIRS were used to estimate for each patient the dose of CMS, expressed as milligrams of CBA per day, needed to achieve each 1 mg/liter of plasma colistin Css,avg. Those doses were then applied back to the patients to estimate the plasma colistin Css,avg predicted to be achieved had those doses been administered. For the 8 patients without SIRS, the median (range) plasma colistin Css,avg predicted was 1.07 (0.56 to 1.86) mg/liter, a 3.3-fold range; for the 9 patients with SIRS, the corresponding values were 0.99 (0.63 to 3.44) mg/liter, a 5.5-fold range. Across all 17 patients, the median predicted Css,avg was 1.00 mg/liter with a 6.1-fold range when the respective regression equations for patients with and without SIRS were applied. When the same equations were applied to the 5 cases reported by Antachopoulos et al. (12) and the 5 patients reported by Ooi et al. (15), the predicted plasma colistin Css,avg were 0.67 (0.21 to 1.02) mg/liter and 0.69 (0.25 to 1.07) mg/liter, respectively. When the respective regression equations for patients with and without SIRS were applied to all patients across the present and those two previous studies (i.e., 27 cases) the median (range) predicted Css,avg was 0.91 (0.21 to 3.44) mg/liter, a >16-fold range. In Fig. 1B, the median body weight-normalized daily doses of CMS needed to achieve each 1 mg/liter of plasma colistin Css,avg for patients with and without SIRS were 2.38 and 5.87 mg/kg/day, respectively. When those doses were applied back to each of the 17 patients in the current study to estimate the plasma colistin Css,avg predicted to be achieved had those doses been administered, the median (range) plasma colistin Css,avg predicted for the 8 patients without SIRS was 1.00 (0.68 to 2.41) mg/liter, a 3.5-fold range; for the 9 patients with SIRS the corresponding values were 1.00 (0.44 to 3.05) mg/liter, a 7.0-fold range. When the median doses (in milligrams per kilogram per day) for patients with and without SIRS (Fig. 1B) were applied to the 5 cases reported by Antachopoulos et al. (12) and the 5 patients reported by Ooi et al. (15), the predicted plasma colistin Css,avg were 0.88 (0.28 to 1.19) mg/liter and 0.30 (0.15 to 2.04) mg/liter, respectively. When the respective median body weight-normalized daily doses of CMS needed to achieve each 1 mg/liter of plasma colistin Css,avg for patients with and without SIRS were applied to all patients across the present and those two previous studies (i.e., 27 cases), the median (range) predicted Css,avg was 0.98 (0.15 to 3.05) mg/liter, a >20-fold range.
Patient outcome and adverse events.
In 14 of 17 patients a favorable outcome was observed, with cure or clinical improvement. Death occurred in 3 patients. Renal toxicity was observed in only one patient treated with 300,000 IU/kg/day (9.9 mg CBA/kg/day), who had rapidly progressing Burkitt lymphoma and was also receiving gentamicin and teicoplanin (patient 13 [Pt13]) (Tables 1 and 2). Three patients (Pt7, Pt11, and Pt14) (Tables 1 and 2) had moderate and transient increase of transaminases during the second week of treatment with CMS (maximum alanine aminotransferase values, 105, 132, and 113 U/liter, respectively, which for Pt7 and Pt14 normalized while they were still on CMS treatment). One patient (Pt12) developed urticarial rash on the last (10th) day of treatment. Finally, Pt11 developed seizures on the last (17th) day of treatment, which recurred 1 and 2 weeks after the discontinuation of CMS administration.
DISCUSSION
In the present study, we investigated colistin PK in critically ill hospitalized pediatric patients across a wide range of ages. The patients received CMS at doses higher than currently recommended by the FDA and EMA for treatment of severe infections caused by multidrug-resistant Gram-negative bacteria. These doses (200,000, 300,000, and 350,000 IU/kg/day, or 6.6, 9.9, 11.6 mg CBA/kg/day, respectively) were employed by the treating physicians based on published data suggesting suboptimal exposure for pediatric patients treated with lower doses (12–15). We performed population PK analysis in order to identify factors affecting colistin apparent clearance and possibly develop a dosing algorithm. As there are no studies on the use of such high CMS doses in children, we closely monitored our patients for clinical and laboratory signs of toxicity.
The population PK analysis was based on concentrations of colistin, as unfortunately plasma concentrations of the inactive prodrug, CMS, were not available. The modeling approach was as described by Lee et al. (20), which involved a parameter (the relative fraction of CMS converted to colistin) to reflect the influence of renal function on the fraction converted, and hence on the apparent clearance of formed colistin. A one-compartment structural model satisfactorily described the PK of colistin formed in vivo after the administration of CMS, in keeping with previous studies in pediatric patients (14, 15) and healthy and sick adults (2, 3, 5, 7, 20, 21).
A number of potential covariates were examined in the population PK modeling. While the relatively small number of 17 subjects in the study may have resulted in a risk of selection bias, it should be noted that eta shrinkage was small and that this is to date the largest population PK study of colistin in pediatric patients. Total body weight was found to be a covariate of the apparent volume of distribution of formed colistin, in agreement with a small-population PK study in pediatric patients reported previously (15). Since the apparent clearance of colistin is the PK parameter that influences the daily dose of CMS required to achieve a desired target plasma colistin Css,avg, an important finding was that the apparent clearance was influenced by CrCL and the presence or absence of SIRS.
Creatinine clearance has been reported as a covariate of the apparent clearance of formed colistin in both pediatric and adult patients (3, 7, 8, 15, 20). The apparent clearance of formed colistin is strongly influenced by renal function because the latter modulates the proportion of each dose of the extensively renally cleared prodrug (CMS) that is available for conversion to colistin; as renal function declines, the fraction of a CMS dose converted to colistin increases, thereby decreasing the apparent clearance of formed colistin (3, 5, 7, 22). SIRS (or sepsis) has been reported to influence the PK of some antibiotics (23) but not, thus far, of colistin. The dysregulated inflammatory and other responses associated with SIRS (or sepsis) can lead to a wide range of pathophysiological changes in the functioning of numerous organs, including the heart, lungs, liver, and kidneys (24). In the case of the kidneys, outcomes can range from augmented renal clearance (ARC) relatively early in the pathogenesis to renal impairment and acute kidney injury (AKI) if sepsis is not controlled (23, 25–29). ARC (creatinine clearance > 130 ml/min/1.73 m2) was observed in 9 of the 17 patients in this study and has been reported to be common in pediatric patients (25, 30, 31). The impact of ARC and/or renal impairment in pediatric patients on the PK of antibiotics in general (32, 33), and colistin in particular (14, 15), has been reported.
The duration of SIRS has been observed to modulate the PK of vancomycin, a renally cleared drug; vancomycin clearance in patients with a SIRS duration of <2 days was higher than that for patients with a SIRS duration of ≥6 days (34). The impact of SIRS on PK is not restricted to drugs that are primarily excreted in urine. A population PK study of pantoprazole, a proton pump inhibitor that is extensively metabolized, in pediatric patients reported that the presence of SIRS was associated with a significantly lower clearance of the drug, a finding considered to be the result of SIRS-related inflammatory processes impairing cytochrome P450 enzymes (35). In the current study, the apparent clearance of colistin at a given CrCL in patients with SIRS was only ∼47% (based on both the population PK modeling and the relative slopes in Fig. 1A) of that in patients without SIRS. This may have been partly due to an effect on the nonrenal excretory clearance of formed colistin, although metabolic and other elimination pathways for colistin have not been elucidated.
We sought to investigate possible ways to select a daily dose of CMS for an individual pediatric patient. First, we predicted the plasma colistin Css,avg expected if each patient in the study had received the lower- and upper-limit doses approved by the FDA and EMA for pediatric patients, 2.5 and 5 mg CBA/kg/day, respectively. That analysis revealed that only 1 and 7, respectively, of the 17 patients were expected to achieve a plasma colistin Css,avg of ≥2 mg/liter, a proposed initial target concentration intended to cover isolates with an MIC at the clinical breakpoint (2 mg/liter) (6, 7, 9). At the higher of the two doses, 6 patients would be likely to achieve a Css,avg of <1 mg/liter. The observation in the current study of an inability to reliably achieve a plasma colistin Css,avg of ≥2 mg/liter with FDA- and EMA-approved doses adds to the same finding from two studies involving a total of 6 pediatric patients (14, 15). This observation should be considered in the context of a recently reported study based on prescribing information from 2 international data collection networks spanning 6 World Health Organization regions. That study found that 63.8% of children and 58.8% of neonates were prescribed intravenous doses of colistin below the lowest FDA and EMA recommendation (2.5 mg CBA/kg/day) (36). It is likely that many, perhaps most, of those pediatric patients would have had plasma colistin Css,avg substantially lower than the suggested target of 2 mg/liter (6, 7, 9).
Of the various graphical approaches explored as possible means to develop a dosing algorithm for pediatric patients, the most promising were the relationships, for patients with and without SIRS, between the number of milligrams of CBA per day needed to achieve each 1 mg/liter of plasma colistin Css,avg and CrCL in milliliters per minute (Fig. 1A), the same as the relationship employed to develop dosing suggestions for individual adult critically ill patients (7, 9). When the algorithm-derived doses were applied back to each of the 17 patients in the current study, the median predicted Css,avg was ∼1 mg/liter, but wide interpatient variability was observed, similar to that reported previously in adult patients (7). When the derived doses were applied to patients reported in previous studies (12, 15), the median predicted Css,avg values were only ∼0.7 mg/liter and the maxima were only slightly greater than 1 mg/liter. Variability within and across the three studies in the predicted plasma colistin Css,avg is very likely the result of batch-to-batch variability in the multicomponent composition of CMS products, which influences the fraction of each dose of the prodrug that is converted to colistin, this fraction being a key determinant of the apparent clearance of colistin (3, 7, 8, 37, 38).
While it would be beneficial to have data from a larger number of pediatric patients, the current analyses suggest that it is not likely to be a satisfactory way to select individualized doses for pediatric patients. The findings highlight the importance of (i) closely monitoring each patient for clinical and microbiological signs of improvement or deterioration in infection status, (ii) careful observation to detect any deterioration in renal function or evidence of other adverse effects, and (iii) undertaking therapeutic drug monitoring whenever possible to fine-tune CMS dosing based on measurement of plasma colistin concentrations (9, 37).
The clinical outcomes across the 17 patients were very good. Clinical cure or improvement of infection occurred in 14 patients. A formal PK/pharmacodynamic (PD) analysis was not possible for three reasons. First, the total number of patients in the study was relatively small, and there were only three cases where death occurred during treatment (in those cases the plasma colistin Css,avg were 1.40, 4.16, and 1.71 mg/liter). Second, all of the patients received one or more other antibiotics for treatment of their infection. Third, information on colistin susceptibility (MIC) for implicated organisms was mostly based on the Vitek2 automated system (bioMérieux, Marcy l’Étoile, France), which may yield high rates of erroneous susceptible results (39). Similarly, a formal PK/toxicodynamic (TD) analysis was not possible because there was only one patient who manifested renal toxicity, and that was considered to be due to Burkitt lymphoma, and the patient succumbed; the plasma colistin Css,avg was 1.71 mg/liter. The renal findings were remarkable given that all patients received doses higher than the upper limit dose approved by the FDA and EMA; indeed, ∼65% of patients received a dose approximately double that of the maximum label dose. Five of the patients achieved plasma colistin Css,avg of ≥4 mg/liter, higher than the value (≥2 mg/liter) associated with increased incidence and severity of AKI in adult patients (9, 40–42), and most of the patients received one or more other potentially nephrotoxic antibiotics. Recent reports and reviews have concluded that coadministration of colistin with other antibiotics was associated with a favorable outcome in the majority of pediatric patients and a relatively low rate of nephrotoxicity (43, 44). The results of the current and previous studies may serve to prompt the FDA and EMA to review the appropriateness of pediatric dosing guidelines in the product label.
In conclusion, intravenous administration of CMS at doses higher than those recommended by the FDA and EMA in critically ill pediatric patients was well tolerated and resulted in improved exposure, although wide interpatient variability was observed, similar to that previously reported for adult patients. Colistin PK was satisfactorily described by a one-compartment disposition model and its apparent clearance was influenced by creatinine clearance and the presence or absence of SIRS. The relationship between the dose of CBA (expressed in milligrams per day) required to achieve each 1 mg/liter of plasma colistin Css,avg and creatinine clearance (expressed in milliliters per minute) was described by linear regression with different slopes for patients with and without SIRS. However, when the equation-derived doses were applied back to patients of the current and previous studies, considerable variability in the predicted plasma colistin Css,avg was observed, precluding the development of a generalizable dosing algorithm for pediatric patients. When available, therapeutic drug monitoring is recommended to assist in dose titration to achieve a desired level of exposure.
MATERIALS AND METHODS
Patients and study design.
This was a prospective, open-label PK study, enrolling patients from two pediatric intensive care units (PICU), a neurosurgical and a pediatric oncology unit, located at Hippokration Hospital of Thessaloniki and University Hospital of Heraklion, Greece. The study was approved by the Ethics Committees of the two hospitals. Written informed consent was obtained from patients’ parents or alternative legal representatives.
Patients eligible for the study were children 1 month to 14 years old, who were hospitalized from June 2014 to December 2017 and received CMS because of possible or documented infection by carbapenem-resistant Gram-negative bacteria. The decision to initiate or discontinue CMS was taken by the treating physicians, with no involvement of the study team. Criteria for exclusion were (i) administration of CMS through other routes (intraventricular, intrathecal, or via inhalation); (ii) cystic fibrosis; (iii) renal impairment, manifested as serum creatinine above the upper limit for age (>0.4 mg/dl, 0.7 mg/dl, and 1.0 mg/dl for infants, children, and adolescents, respectively); (iv) great difficulty in vascular access; and (v) parental refusal of consent.
CMS administration.
CMS (Norma Hellas S.A., Athens, Greece) was administered at doses of 200,000 IU/kg/day (6.6 mg CBA/kg/day), 300,000 IU/kg/day (9.9 mg CBA/kg/day), or 350,000 IU/kg/day (11.6 mg CBA/kg/day), at 8-h intervals, infused intravenously over 20 min in 50 ml of normal saline. The decision on the dose of CMS to be administered was taken by the patients’ treating physicians in consultation with the attending infectious diseases specialist. The above doses were chosen based on our previous study (12) as well as preliminary PK data. In general, a dose of 200,000 IU/kg/day (6.6 mg CBA/kg/day) was administered to younger patients (1 month to 8 years old) with possible infection and to older children (9 to 14 years old) with possible or documented infection of moderate severity. The dose of 300,000 IU/kg/day (9.9 mg CBA/kg/day) was administered to children 9 to 14 years old with severe infection and to those 1 month to 8 years old with probable or documented infection of any severity. The dose of 350,000 IU/kg/day (11.6 mg CBA/kg/day) was chosen as an exception for patients 1 month to 2 years old with very severe sepsis.
PK sampling.
Blood samples (0.5 to 1 ml) were collected immediately before and at 30, 60, 90, 120, 240, and 360 min after the end of infusion of the first and fifth doses of CMS. The precise time of collection was documented for each sample. After immediate centrifugation of samples at 4°C, collected plasma was stored at −80°C until assayed to minimize potential ex vivo conversion of CMS to colistin (1, 45).
Determination of colistin concentrations in plasma.
Colistin concentrations in plasma were determined using ultraperformance liquid chromatography (UPLC) combined with electrospray ionization (ESI) tandem mass spectrometry (MS/MS) on a hybrid quadrupole time of flight (QTOF) instrument, as previously described (46). The method presented good fit (regression coefficient, ≥0.998) over the quantitation ranges of 0.02 to 3 and 0.03 to 4.5 mg/liter, with the lower limit of quantitation (LLOQ) being 0.02 and 0.03 mg/liter for colistin A and colistin B in human plasma, respectively. Samples for which the measured concentrations of colistin A or B exceeded the upper limit of the calibration curve (3 or 4.5 mg/liter, respectively) were diluted with blank drug-free plasma in order to bring the concentrations into the above-mentioned ranges. The intraday and interday precision of the method was 14.2% and 13.2%, respectively, while the relative error did not exceed 14.9%. The colistin plasma concentrations reported here are the sum of colistin A and colistin B concentrations for each patient and time point.
Patient data.
Various parameters were recorded that could possibly be implicated in colistin PK, such as sex, age, body weight, height, body surface area, serum creatinine, calculated CrCL, the presence of SIRS, pediatric risk of mortality (PRISM) score, and pediatric logistic organ dysfunction (PELOD) score at the time of sampling. Serum creatinine levels were determined using the Jaffe method. CrCL was calculated using the Schwartz formula (estimated CrCL = k × height/serum creatinine concentration, where k = 0.45 for infants, 0.55 for children and adolescent [>10 years old] girls, and 0.70 for adolescent boys, CrCL is measured in milliliters per minute per 1.73 m2, height is measured in centimeters, and serum creatinine concentration is measured in milligrams per deciliter) (47). The presence of SIRS was determined after assessment of patients’ core temperature, heart rate, respiratory rate, and blood leukocyte count, using previously published age-specific criteria for pediatric patients (48). PRISM and PELOD scores were calculated as previously described (49, 50).
In addition, infection- or treatment-related data were recorded, such as type of infection for which CMS was administered, causative organism (if isolated), duration of CMS treatment, coadministered antibiotics and potentially nephrotoxic agents, and outcome. Patients had daily clinical assessment and laboratory workup (including full blood count, liver function tests, electrolytes, and renal function). Any clinical or laboratory adverse events that could be attributed to CMS treatment were recorded. Renal toxicity was identified as increase of serum creatinine above the upper limit for age.
Pharmacokinetic analysis.
Population PK modeling of colistin in plasma was conducted using the first-order conditional estimation algorithm with interaction (FOCE+I) in NONMEM version 7.2 (ICON Development Solutions, Ellicott City, MD). After careful examination of the observed data, the only concentration-time point data not included were as follows: (i) 26 samples (22 of which were collected after the first dose) for which the concentration of colistin A was below the lower limit of quantification, as the PK analysis was based on the sum of concentrations of colistin A and colistin B as described previously (2–4, 7, 8); (ii) for patient 7, data for the 32- and 63-min samples after the fifth dose, as the concentrations (15.8 and 20.9 mg/liter, respectively) were implausibly high compared to the subsequent concentrations for this dose in the same subject and also relative to the concentrations at the corresponding time points after the first dose. All remaining colistin concentrations from all 17 subjects were modeled simultaneously.
Models with one and two disposition compartments for formed colistin were evaluated. The formation of colistin from CMS was described by a first-order process. The interindividual variability in the PK parameters was described by a log-normal distribution. Models with and without covariance were tested. Inclusion of interoccasion variability was evaluated to describe differences in the formed colistin concentrations between the first and fifth dose within a subject, which were not explained by the increase of formed colistin concentrations to steady state. The residual unexplained variability was incorporated through a combined proportional plus additive error model.
The possible effects of patient characteristics, including renal function (creatinine clearance) (47), total body weight, presence or absence of SIRS (48), height, age, sex, PRISM, and PELOD on the disposition of formed colistin were explored. The effect of body weight on the PK parameters was based on allometric scaling, using standard power coefficients of 0.75 for clearance and 1.0 for volume of distribution. Inclusion of covariates was based on graphical analysis, change in the objective function, the reduction in unexplained interindividual variability, and biological plausibility. Candidate models were evaluated based on plots of observed versus individual-fitted and observed versus population-fitted concentrations, conditional weighted residuals, the normalized prediction distribution error, and the log-likelihood ratio test for nested models.
The population PK analysis enabled determination for each patient of the average plasma steady-state concentration (Css,avg) of colistin achieved with the respective administered daily dose of CMS, expressed as CBA. This enabled the following analyses. First, the performance of the current FDA and EMA pediatric dosing guidelines (10, 11) was investigated as follows. Using direct proportionality, based on linear PK of colistin (3, 5, 7, 8, 21), the plasma colistin Css,avg expected to be achieved had each patient received the upper- and lower-limit FDA- and EMA-recommended daily doses were calculated (6, 15). Second, to explore possible approaches to formulating new dosage guidelines, the daily dose of CBA required to achieve each 1 mg/liter of plasma colistin Css,avg, a surrogate of the apparent clearance of formed colistin, was also determined for each patient (3, 7). This enabled graphical approaches to investigate possible clinically useful relationships with patient characteristics (e.g., CrCL and other clinical characteristics) to develop a dosing algorithm. Promising approaches were further evaluated using patient data from previous studies by Antachopoulos et al. (12) and Ooi et al. (15). In the first of those studies peak and trough plasma colistin concentrations were reported (12); for the current analyses, the plasma colistin Css,avg for each patient was determined as the logarithmic average of the peak and trough concentrations.
ACKNOWLEDGMENTS
This work was supported by a grant from the Greek Ministry of Education, Department of Research and Technology (ARISTEIA II no. 5393).
We thank all the nurses and physicians of the hospital departments who helped to conduct the study as well as the parents of the patients who consented that their children participate in the study.
Footnotes
Supplemental material is available online only.
aac.00002-21-s0001.pdf (395.1KB, pdf)
Contributor Information
Roger L. Nation, Email: roger.nation@monash.edu.
Emmanuel Roilides, Email: roilides@med.auth.gr.
REFERENCES
- 1.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregoire N, Mimoz O, Megarbane B, Comets E, Chatelier D, Lasocki S, Gauzit R, Balayn D, Gobin P, Marchand S, Couet W. 2014. New colistin population pharmacokinetic data in critically ill patients suggesting analternativeloading dose rational. Antimicrob Agents Chemother 58:7324–7330. doi: 10.1128/AAC.03508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karaiskos I, Friberg LE, Pontikis K, Ioannidis K, Tsagkari V, Galani L, Kostakou E, Baziaka F, Paskalis C, Koutsoukou A, Giamarellou H. 2015. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 59:7240–7248. doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Turnidge JD, Forrest A, Silveira FP. 2016. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, Li J, Silveira FP. 2017. Dosing guidance for intravenous colistin in critically ill patients. Clin Infect Dis 64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristoffersson AN, Rognas V, Brill MJE, Dishon-Benattar Y, Durante-Mangoni E, Daitch V, Skiada A, Lellouche J, Nutman A, Kotsaki A, Andini R, Eliakim-Raz N, Bitterman R, Antoniadou A, Karlsson MO, Theuretzbacher U, Leibovici L, Daikos GL, Mouton JW, Carmeli Y, Paul M, Friberg LE. 2020. Population pharmacokinetics of colistin and the relation to survival in critically ill patients infected with colistin susceptible and carbapenem-resistant bacteria. Clin Microbiol Infect 26:1644–1650. doi: 10.1016/j.cmi.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Infectious Diseases Society of America (IDSA), International Society for anti-Infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. 2013. Approved drug products. Label andapproval history for Coly-Mycin M, NDA 050108. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050108s030lbl.pdf. Accessed 6 December 2020.
- 11.European Medicines Agency. 2014. Polymyxin-containing medicines. Polymyxin Article 31 referral—Annex III amendments to relevant sections of the summary of product characteristics and the package leaflets. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Polymyxin_31/WC500176332.pdf. Accessed 6 December 2020.
- 12.Antachopoulos C, Karvanen M, Iosifidis E, Jansson B, Plachouras D, Cars O, Roilides E. 2010. Serum and cerebrospinal fluid levels of colistin in pediatric patients. Antimicrobial Agents Chemother 54:3985–3987. doi: 10.1128/AAC.01799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakwan N, Usaha S, Chokephaibulkit K, Villani P, Regazzi M, Imberti R. 2016. Pharmacokinetics of colistin following a single dose of intravenous colistimethate sodium in critically ill neonates. Pediatr Infect Dis J 35:1211–1214. doi: 10.1097/INF.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 14.Magreault S, Petyt C, Senneville E, Fron D, Nectoux E, Loiez C, Marchand S, Gregoire N, Couet W. 2018. Pharmacokinetics of colistin in a 8-year-old child with acute bone infection. Clin Microbiol Infect 24:1025–1026. doi: 10.1016/j.cmi.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Ooi MH, Ngu SJ, Chor YK, Li J, Landersdorfer CB, Nation RL. 2019. Population pharmacokinetics of intravenous colistin in pediatric patients: implications for the selection of dosage regimens. Clin Infect Dis 69:1962–1968. doi: 10.1093/cid/ciz067. [DOI] [PubMed] [Google Scholar]
- 16.Mesini A, Loy A, Gattorno M, Moscatelli A, Bandettini R, Faraci M, Cangemi G, Castagnola E. 2018. Colistin area under the time-concentration in children treated with intravenous loading dose and maintenance therapy. Clin Infect Dis 66:808–809. doi: 10.1093/cid/cix757. [DOI] [PubMed] [Google Scholar]
- 17.Nation RL. 2018. Dose suggestions for intravenous colistin in pediatric patients: caution required. Clin Infect Dis 66:810–811. doi: 10.1093/cid/cix1048. [DOI] [PubMed] [Google Scholar]
- 18.Barco S, Castagnola E, Mesini A, Tripodi G, Cangemi G. 2019. Potential pitfalls in LC-MS/MS quantification of colistin for therapeutic drug monitoring of patients treated with colistimethate. J Pharm Biomed Anal 170:193–195. doi: 10.1016/j.jpba.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Wacharachaisurapol N, Phasomsap C, Sukkummee W, Phaisal W, Chanakul A, Wittayalertpanya S, Chariyavilaskul P, Puthanakit T. 2020. Greater optimisation of pharmacokinetic/pharmacodynamic parameters through a loading dose of intravenous colistin in paediatric patients. Int J Antimicrob Agents 55:105940. doi: 10.1016/j.ijantimicag.2020.105940. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Han S, Jeon S, Hong T, Song W, Woo H, Yim DS. 2013. Population pharmacokinetic analysis of colistin in burn patients. Antimicrob Agents Chemother 57:2141–2146. doi: 10.1128/AAC.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. 2011. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther 89:875–879. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 23.Phe K, Heil EL, Tam VH. 2020. Optimizing pharmacokinetics-pharmacodynamics of antimicrobial management in patients with sepsis: a review. J Infect Dis 222:S132–S141. doi: 10.1093/infdis/jiaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caraballo C, Jaimes F. 2019. Organ dysfunction in sepsis: an ominous trajectory from infection to death. Yale J Biol Med 92:629–640. [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai K, Ihara S, Kinae A, Ikegaya K, Suzuki M, Hirano K, Itoh K. 2016. Augmented renal clearance in pediatric patients with febrile neutropenia associated with vancomycin clearance. Ther Drug Monit 38:393–397. doi: 10.1097/FTD.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 26.Baptista JP, Roberts JA, Udy AA. 2019. Augmented renal clearance: a real phenomenon with an uncertain cause. Anaesth Crit Care Pain Med 38:335–336. doi: 10.1016/j.accpm.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 27.AtkinsonAJ, Jr.2018. Augmented renal clearance. Transl Clin Pharmacol 26:111–114. doi: 10.12793/tcp.2018.26.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blatt NB, Srinivasan S, Mottes T, Shanley MM, Shanley TP. 2014. Biology of sepsis: its relevance to pediatric nephrology. Pediatr Nephrol 29:2273–2287. doi: 10.1007/s00467-013-2677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C,. Acute Dialysis Quality Initiative Consensus XIII Work Group. 2016. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27:371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA. 2020. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35:25–39. doi: 10.1007/s00467-018-4120-2. [DOI] [PubMed] [Google Scholar]
- 31.Van Der Heggen T, Dhont E, Peperstraete H, Delanghe JR, Vande Walle J, De Paepe P, De Cock PA. 2019. Augmented renal clearance: a common condition in critically ill children. Pediatr Nephrol 34:1099–1106. doi: 10.1007/s00467-019-04205-x. [DOI] [PubMed] [Google Scholar]
- 32.Downes KJ, Hayes M, Fitzgerald JC, Pais GM, Liu J, Zane NR, Goldstein SL, Scheetz MH, Zuppa AF. 2020. Mechanisms of antimicrobial-induced nephrotoxicity in children. J Antimicrob Chemother 75:1–13. doi: 10.1093/jac/dkz325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartman SJF, Brüggemann RJ, Orriëns L, Dia N, Schreuder MF, de Wildt SN. 2020. Pharmacokinetics and target attainment of antibiotics in critically ill children: a systematic review of current literature. Clin Pharmacokinet 59:173–205. doi: 10.1007/s40262-019-00813-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuma M, Makishima M, Imai T, Tochikura N, Sakaue T, Kikuchi N, Kinoshita K, Kaburaki M, Yoshida Y. 2016. Duration of systemic inflammatory response syndrome influences serum vancomycin concentration in patients with sepsis. Clin Ther 38:2598–2609. doi: 10.1016/j.clinthera.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Pettersen G, Mouksassi MS, Théorêt Y, Labbé L, Faure C, Nguyen B, Litalien C. 2009. Population pharmacokinetics of intravenous pantoprazole in paediatric intensive care patients. Br J Clin Pharmacol 67:216–227. doi: 10.1111/j.1365-2125.2008.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin MKY, Hsia Y, Goossens H, Versporten A, Bielicki J, Sharland M, Donà D. 2020. Evidence of dose variability and dosing below the FDA and EMA recommendations for intravenous colistin (polymyxin E) use in children and neonates. Pediatr Infect Dis J 39:1032–1034. doi: 10.1097/INF.0000000000002847. [DOI] [PubMed] [Google Scholar]
- 37.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2015. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 38.He H, Li JC, Nation RL, Jacob J, Chen G, Lee HJ, Tsuji BT, Thompson PE, Roberts K, Velkov T, Li J. 2013. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 68:2311–2317. doi: 10.1093/jac/dkt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dafopoulou K, Vourli S, Tsakris A, Pournaras S. 2019. An update on polymyxin susceptibility testing methods for Acinetobacter baumannii. Expert Rev Anti Infect Ther 17:699–713. doi: 10.1080/14787210.2019.1667230. [DOI] [PubMed] [Google Scholar]
- 40.Sorli L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, Alvarez-Lerma F, Knobel H, Benito N, Horcajada JP. 2013. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forrest A, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Li J, Silveira FP, Nation RL. 2017. Pharmacokinetic/toxicodynamic analysis of colistin-associated acute kidney injury in critically ill patients. Antimicrob Agents Chemother 61:e01367-17. doi: 10.1128/AAC.01367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horcajada JP, Sorli L, Luque S, Benito N, Segura C, Campillo N, Montero M, Esteve E, Mirelis B, Pomar V, Cuquet J, Marti C, Garro P, Grau S. 2016. Validation of a colistin plasma concentration breakpoint as a predictor ofnephrotoxicity in patients treated with colistin methanesulfonate. Int JAntimicrob Agents 48:725–727. doi: 10.1016/j.ijantimicag.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Antachopoulos C, Iosifidis E. 2017. Colistin use in neonates and children with infections due to carbapenem-resistant bacteria. Pediatr Infect Dis J 36:905–907. doi: 10.1097/INF.0000000000001655. [DOI] [PubMed] [Google Scholar]
- 44.Sahbudak Bal Z, Kamit Can F, Yazici P, Berna Anil A, Duyu M, Yilmaz Ciftdogan D, Nisel Yilmaz O, Cilli F, Karapinar B. 2018. The evaluation of safety and efficacy of colistin use in pediatric intensive care unit: results from two reference hospitals and review of literature. J Infect Chemother 24:370–375. doi: 10.1016/j.jiac.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Dudhani RV, Nation RL, Li J. 2010. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J Antimicrob Chemother 65:1412–1415. doi: 10.1093/jac/dkq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gikas E, Bazoti FN, Katsimardou M, Anagnostopoulos D, Papanikolaou K, Inglezos I, Skoutelis A, Daikos GL, Tsarbopoulos A. 2013. Determination of colistin A and colistin B in human plasma by UPLC-ESI high resolution tandem MS: application to a pharmacokinetic study. J Pharm Biomed Anal 83:228–236. doi: 10.1016/j.jpba.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz GJ, Brion LP, Spitzer A. 1987. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein B, Giroir B, Randolph A. 2005. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 49.Pollack MM, Ruttimann UE, Getson PR. 1988. Pediatric risk of mortality (PRISM) score. Crit Care Med 16:1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. 2003. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]