Abstract
Background
Refractory status epilepticus (RSE) represents a serious medical condition requiring early and targeted therapy. Given the increasing number of elderly or multimorbid patients with a limitation of life-sustaining therapy (LOT) or within a palliative care setting (PCS), guidelines-oriented therapy escalation options for RSE have to be omitted frequently.
Objectives
This systematic review sought to summarize the evidence for fourth-line antiseizure drugs (ASDs) and other minimally or non-invasive therapeutic options beyond guideline recommendations in patients with RSE to elaborate on possible treatment options for patients undergoing LOT or in a PCS.
Methods
A systematic review of the literature in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, focusing on fourth-line ASDs or other minimally or non-invasive therapeutic options was performed in February and June 2020 using the MEDLINE, EMBASE and Cochrane databases. The search terminology was constructed using the name of the specific ASD or therapy option and the term ‘status epilepticus’ with the use of Boolean operators, e.g. “(brivaracetam) AND (status epilepticus)”. The respective Medical Subject Headings (MeSH) and Emtree terms were used, if available.
Results
There is currently no level 1, grade A evidence for the use of ASDs in RSE. The best evidence was found for the use of lacosamide and topiramate (level 3, grade C), followed by brivaracetam, perampanel (each level 4, grade D) and stiripentol, oxcarbazepine and zonisamide (each level 5, grade D). Regarding non-medicinal options, there is little evidence for the use of the ketogenic diet (level 4, grade D) and magnesium sulfate (level 5, grade D) in RSE. The broad use of immunomodulatory or immunosuppressive treatment options in the absence of a presumed autoimmune etiology cannot be recommended; however, if an autoimmune etiology is assumed, steroid pulse, intravenous immunoglobulins and plasma exchange/plasmapheresis should be considered (level 4, grade D). Even if several studies suggested that the use of neurosteroids (level 5, grade D) is beneficial in RSE, the current data situation indicates that there is formal evidence against it.
Conclusions
RSE in patients undergoing LOT or in a PCS represents a challenge for modern clinicians and epileptologists. The evidence for the use of ASDs in RSE beyond that in current guidelines is low, but several effective and well-tolerated options are available that should be considered in this patient population. More so than in any other population, advance care planning, advance directives, and medical ethical aspects have to be considered carefully before and during therapy.
Key Points
| There is sustainable evidence for the efficacy of fourth-line antiseizure drugs (ASDs) in status epilepticus (SE) and refractory SE (RSE). |
| Lacosamide, topiramate, perampanel, brivaracetam, and stiripentol should be considered in patients with RSE in limited therapy settings. |
| Besides ASDs, the ketogenic diet can be a therapy option that must be individually evaluated for every case. |
| There is minor evidence for the use of magnesium sulfate in non-eclamptic SE as an additional therapeutic approach. |
| A central part of any medical therapeutic decision in RSE is the reflection of medical ethical aspects in the context of the patient’s will. |
Introduction
Status epilepticus (SE) is a frequent and potentially life-threatening medical condition that must be treated immediately to prevent further harm from befalling the affected patient. SE can not only manifest in patients with known epilepsy but can also have many other triggers, such as infection, brain damage, electrolyte imbalance, and autoimmune or paraneoplastic antibodies [1, 2]. According to German and other national and international clinical practice guidelines, benzodiazepines (BZDs) represent the first-line therapy in the treatment of SE. The International League Against Epilepsy defines all tonic–clonic seizures lasting for more than 5 min, as well as focal seizures with impaired consciousness or absence seizures lasting for more than 10 min, as SE [3]. An SE that lasts for more than 10 min despite adequately administered therapy with BZDs should be treated with intravenous antiseizure drugs (ASDs) such as levetiracetam (LEV), valproic acid (VPA), or (fos-)phenytoin (PHT) [4]. In the recently published ESETT trial, all three drugs showed similar efficacy profiles, at 47% (LEV), 45% (fosPHT), and 46% (VPA), in terminating an established SE, while having similar incidence rates of adverse events (AEs) [4]. If the combination of one BZD and one ASD fails, then criteria for a refractory SE (RSE) are met and further treatment in the intensive care unit (ICU), as well as the consequent use of anesthetic drugs together with the need for mechanical ventilation, are recommended for generalized tonic–clonic SE [5–8]. Failure to control SE with anesthetic drugs defines a super-refractory SE (SRSE) that is associated with increased mortality, decreased quality of life, and poor chance of response to further therapeutics [2, 9–14]. Due to demographic changes such as an increasing number of elderly and multimorbid patients [15, 16], as well as the growing importance of self-determination for, in particular, severely ill patients within a palliative care setting (PCS) or with another limitation of life-sustaining therapy (LOT) [17], most of the recommended therapeutic options for RSE and SRSE fail due to invasiveness or the requirement of an ICU/IMC stay, invasive monitoring or mechanical ventilation [18, 19]. Moreover, based on the increasing implementation of advanced care planning (ACP), many patients have decided already in times of good health conditions or at the onset of a chronic or potentially life-threatening disease to waive specific medical treatment options [20]. However, many patients and their relatives or caregivers insist on a therapeutic attempt in spite of the given LOT or PCS, as SE per se does not always result in an additional permanent disability [21]. Moreover, many patients with acute or pre-existing relevant cardiopulmonary or hepatorenal conditions cannot be treated with several second- or third-line ASDs due to their high interaction potential (i.e. with phenobarbital [PB], PHT, or VPA), proarrhythmic effects (e.g. PHT), or contraindications to their use in combination with other drugs (e.g. enhanced VPA metabolism due to carbapenem antibiotics) [5, 19, 22].
This systematic review sought to summarize the evidence for fourth-line ASDs and other minimally or non-invasive therapeutic options beyond guideline recommendations in patients with RSE to elaborate on possible treatment options for patients undergoing LOT or in a PCS.
Methods
Characteristics of the Review
To evaluate the possible therapeutic options for patients undergoing ACP or LOT, or in a PCS, beyond the guideline recommendations, a systematic review of the literature was performed in February 2020 using the MEDLINE, EMBASE, and Cochrane databases, using the PubMed gateway, focusing on minimally or non-invasive treatment options that do not require invasive monitoring or admission to the ICU. Based on reviewers’ recommendations, a second literature study was performed in June 2020 focusing on immunomodulatory and immunosuppressive treatment options. Studies with patients of all age ranges were included. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were closely followed; the PRISMA checklist for this review is available on request [23, 24]. The search terminology was constructed using the name of the specific ASD or therapy option and the term ‘status epilepticus’ with the use of Boolean operators, e.g. “(brivaracetam) AND (status epilepticus)”. The respective Medical Subject Headings (MeSH) and Emtree terms were used, if available. The protocol for this systematic review has not been previously registered on PROSPERO [25]. As other minimally or non-invasive therapeutic options, the intravenous application of magnesium sulfate (MgSO4), neurosteroids (e.g. allopregnanolone), and the ketogenic diet (KD), as well as steroid pulse (SP), intravenous immunoglobulins (IVIGs) and plasma exchange/plasmapheresis (PLEX), were considered based on recent publications [1, 2]. No further filter was applied to avoid the falsification of the results, and two reviewers (LMW, AS) screened the identified articles for eligibility. Publications were defined as ‘relevant’ if the following criteria were met: (1) original publication; (2) human study; (3) at least one enrolled patient; and (4) sufficient data to enable a comparison with other publications. Reasons to exclude publications from this review included the unavailability of a version in English, French, German, Italian, or Spanish. Available reviews with a total of over 300 enrolled subjects for the use of a single ASD or other therapeutic options were used to cross-check the selected publications [26–41]. Processed data (e.g. number of subjects, age range, study design, characterization of SE, severity of SE, loading dose, maintenance dose, responder rate, rate of AEs in studies on ASDs) of the individual studies were manually transferred into a previously conceived assessment form. In addition, other aspects such as nutrition regimen, number of patients achieving ketosis (of those on KD), treatment days, relapse of seizures after the discontinuation of therapy (MgSO4), treatment days, and titration and maintenance (if taking neurosteroids) doses were accessed. Regarding immunomodulatory and immunosuppressive therapies, treatment options and the specific response was recorded. Moreover, individual aspects of particular interest, and the strengths and weaknesses of each study, were assessed. Due to the use of processed data, cross-checking for double entries of single individuals was impossible and no additional post hoc subgroup analysis was performed. The risk of a specific bias was assessed for each publication; however, due to the low number of available publications with predominantly small numbers of enrolled patients, no studies were excluded, and major biases were discussed, except one study with previously proven fundamental statistical errors. The responder rate was defined as the primary outcome, while safety and tolerability (AEs) were defined as secondary outcomes for this study.
Level of Evidence
The well-established and internationally accepted Oxford classification scheme from the Oxford Centre for Evidence-Based Medicine (CEBM; March 2009) [42] was used to graduate the existing evidence based on the available quality and design of the published studies and reports in the following order.
Randomized controlled trials (RCT) or meta-analyses of RCTs with homogenous results.
Poorly designed RCTs, prospective cohort studies, or meta-analyses of level 2 studies.
Retrospective cohort studies, case–control studies, or meta-analyses of level 3 studies.
Case series.
Case reports, expert opinions, or personal observations.
Based on the available amount, quality, and consistency of the available publications, the following different grades of recommendation can be concluded.
-
(A)
Consistent level 1 studies.
-
(B)
Consistent level 2 or 3 studies or extrapolations from level 1 studies.
-
(C)
Level 4 studies or extrapolations from level 2 or 3 studies.
-
(D)
Level 5 evidence or troublingly inconsistent or inconclusive studies of any level.
In addition to the Oxford classification scheme, which focuses on the existing evidence, the Grading Recommendations Assessment, Development, and Evaluation (GRADE) scale was adopted to access the strength of recommendations [43]. The GRADE significance is subdivided into the following four quality levels.
-
(I)
‘High’, wherein we are very confident that the true effect lies close to that of the estimate of the effect.
-
(II)
‘Moderate’, wherein we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect but there is a possibility that it is substantially different.
-
(III)
‘Low’, wherein our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
-
(IV)
‘Very low’, wherein we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect.
The displayed level of evidence and grade of recommendation for each ASD or therapeutic option represents the consensus of all authors of this publication.
Definitions and Terminology
Definitions and terminology for seizures, epilepsy, and SE used for this review follow the international recommendations of the International League against Epilepsy [3, 44, 45]. Due to the method-inherent character of this systematic review, no reappraisal of the reported responder or AE rates was performed, which means that all patients with a reported response or AE were counted without any further subcategorization. If AEs were not explicitly named or their absence was reported, AEs were classified as not available. Definitions and terms in relation to therapeutic restrictions, palliation, and end-of life care follow the current doctrines [20, 46] (please refer to the Infobox for more information).
Data Analysis and Reporting
All numbers with decimals were rounded to the nearest full number except those of dose recommendations in pediatric patients. No further data analysis or processing was performed for this systematic review.
Results
General Aspects
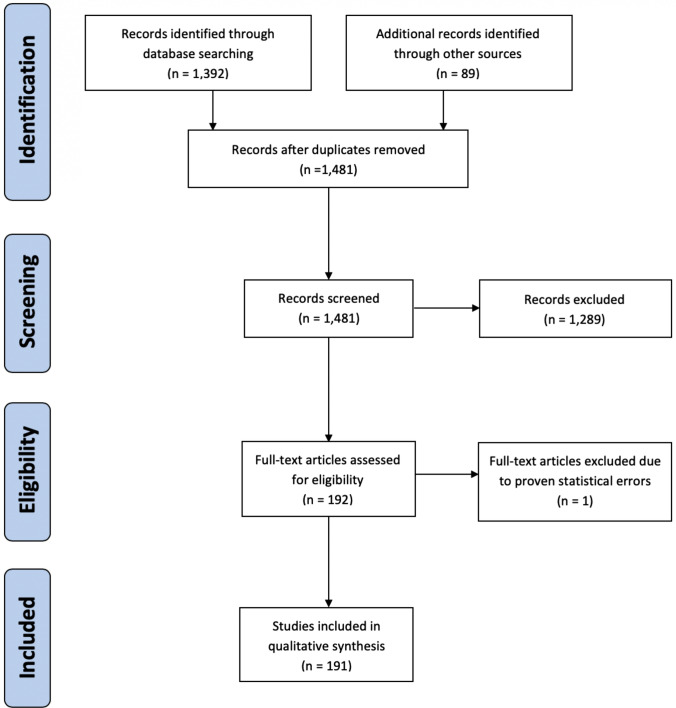
A total of 1481 publications were screened, resulting in the identification of 191 (13%) publications that were relevant to the topic of this systematic review. For more details on the PRISMA diagram, please refer to Fig. 1. Of these 191 publications, 62 (32%) focused on ASDs and 129 (68%) focused on other therapeutic options. Among fourth-line ASDs, the highest number of relevant publications was available for lacosamide (LCM; n = 27, 44%), followed by topiramate (TPM; n = 15, 24%), perampanel (PER; n = 9, 15%), brivaracetam (BRV; n = 5, 8%), stiripentol (STP; n = 3, 5%) and lamotrigine (LTG), oxcarbazepine (OXC) or zonisamide (ZNS; n = 1 each, 2%). Regarding other therapeutic options, most relevant publications reported on immunomodulatory and immunosuppressive therapies (n = 72, 65%), followed by the KD (n = 30, 23%), MgSO4 (n = 23, 18%) and neurosteroids (n = 4, 3%).
Fig. 1.
PRISMA flow diagram. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Antiseizure Drugs (ASDs)
BRV is a novel third-generation ASD available in tablet, oral, and intravenous solution forms and is approved for monotherapy (US FDA) or add-on therapy (European Medicines Agency) in focal epilepsy (FE). BRV is a derivate of LEV, acting mainly as a ligand at the synaptic vesicle glycoprotein 2A (SV2A). In comparison with LEV, BRV has a tenfold increased affinity to its molecular target and boasts a lower potential for inducing psychobehavioral adverse effects [37, 47, 48]. BRV is not approved for use in SE; the evidence for its use is limited to five retrospective case series and reports offering a total of 77 adult cases with responder rates for convulsive SE (CSE) and non-convulsive SE (NCSE) of between 27 and 54%, as well as an acceptable tolerability profile [49–53]. The largest available retrospective multicenter cohort included 43 adult patients and reported a responder rate of 54% as well as good tolerability, with AEs observed in 14% of the enrolled subjects [50]. Two studies provided a subgroup analysis of a connection between responders and dosage showing a significant correlation of a response with a loading dose of 1.82–2.0 mg/kg body weight or greater [50, 53]. According to the current state of knowledge, a loading dose of 50–400 mg, considering the given minimum dose of approximately 2 mg/kg body weight and a maintenance dose of 100–400 mg/day, has been shown to be well tolerated and effective (Table 1b).
Table 1.
Current evidence for the use of second- and third-line ASDs in refractory and super-refractory status epilepticus
| Study design | Status epilepticus | Therapy regimen | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Layout | Study population | Type | Severity | Loading dose (mg) | Maintenance dose (mg/day) | Response (%) | AEs (%) | ||
| n | Age (years) | ||||||||
| (a) Lacosamide (LCM) | |||||||||
| Kellinghaus et al. (2011) [55] | rs, mc | 39 | 18–90 | CSE | SE, RSE | 200–400 | – | 44 | 77 |
| Albers et al. (2011) [56] | rs, sc | 7 | 33–83 | CSE | SE, RSE | 400 | 400 | 100 | – |
| Goodwin et al. (2011) [57] | rs, sc | 9 | 47–89 | CSE, NCSE | RSE | 200 | 400 | 0 | 22 |
| Höfler et al. (2011) [58] | rs, mc | 31 | 22–95 | CSE, NCSE | SE, RSE, SRSE | 200–400 | 0–400 | 81 | – |
| Koubeissi et al. (2011) [59] | rs, sc | 4 | 53–79 | NCSE | RSE | 50–200 | 100–200 | 100 | 0 |
| Rantsch et al. (2011) [60] | rs, sc | 9 | 52–84 | NCSE | RSE | 50–100 | – | 20 | – |
| Jain and Harvey (2011) [61] | rs, mc | 31 | 12–17 | CSE, NCSE | SE, RSE | 50–200 | 0–400 | 75–100 | 4 |
| Cherry et al. (2012) [62] | rs, sc | 24 | 24–80 | NCSE, CSE | RSE | 100–400 | 300–400 | 38 | 31 |
| Mnatsakanyan et al. (2012) [63] | rs, sc | 10 | 16–90 | NCSE | RSE | 200–300 | 200–400 | 70 | – |
| Belcastro et al. (2013) [64] | ps, sc | 16 | 77 ± 7 | NCSE | SE | 400 | 400 | 50 | - |
| Miró et al. (2013) [65] | ps, mc | 34 | 22–86 | CSE, NCSE | RSE | 100–400 | 100–600 | 65 | 6 |
| Santamarina et al. (2013) [66] | rs, sc | 31 | 21- 85 | CSE, NCSE | SE, RSE | 200–600 | 400 | 85 | 13 |
| Sutter et al. (2013) [67] | rs, sc | 86 | 65 ± 15 | CSE, NCSE | SE, RSE, SRSE | 200 | 200 | 93 | 0 |
| Legros et al. (2014) [68] | ps, cs | 25 | 36–74 | CSE, NCSE | SE, RSE | 200–400 | 400 | 36 | 20 |
| Kellinghaus et al. (2014) [69] | rs, sc | 21 | 18–90 | CSE, NCSE | RSE | 200–800 | 200–800 | 33 | – |
| Garcés et al. (2014) [70] | rs, mc | 55 | 18–90 | CSE, NCSE | SE, RSE | 50–400 | 50–400 | 71 | 15 |
| Grosso et al. (2014) [71] | rs, mc | 11 | 3–16 | CSE, NCSE | RSE | 6.7–9.9a | 8.8–13.9a | 45 | 0 |
| Poddar et al. (2016) [72] | rs, sc | 9 | 0–16 | CSE | SE, RSE | 3.3–10a | – | 78 | 11 |
| Moreno Morales et al. (2015) [73] | ps, ob | 53 | 19–73 | CSE, NCSE | SE, RSE, SRSE | 400 | 400 | 57 | – |
| d´Orsi et al. (2015) [74] | ps, sc | 23 | 33–85 | CSE, NCSE | SE, RSE, SRSE | 200 | 200–400 | 80 | 20 |
| Lang et al. (2016) [75] | ps, mc | 51 | 1–92 | CSE, NCSE | SE, RSE, SRSE | 300 | 93–1200 | 70 | 13 |
| Santamarina et al. (2018) [76] | rs, mc | 165 | av. 64 | NCSE | SE, RSE | 100–600 | 200–400 | 63 | 10 |
| Reif et al. (2018) [77] | rs, sc | 1 | 28 | ASE | RSE | 400 | 400 | 100 | 0 |
| Ngampoopun et al. (2018) [78] | ps, sc | 11 | 7–16 | NCSE | SE, RSE | 227 | 225 | 73 | 9 |
| Perrenoud et al. (2017) [79] | rs, mc | 40 | 34–88 | – | SE, RSE | 100–800 | – | 40 | – |
| Newey et al. (2017) [80] | rs, sc | 51 | av. 60 | CSE, NCSE | RSE | 188b | 362b | 82 | – |
| Toledo et al. (2018) [81] | rs, mc | 15 | 19–81 | CSE, NCSE | SE, RSE | 100–600 | 100–800 | 73 | 53 |
| (b) Brivaracetam (BRV) | |||||||||
| Strzelczyk et al. (2017) [49] | rs, mc | 11 | 34–85 | NCSE, CSE | RSE, SRSE | 50–400 | 100–400 | 27 | 0 |
| Kalss et al. (2018) [51] | rs, sc | 7 | 29–79 | NCSE, CSE | RSE | 50–200 | 100–300 | 43 | 0 |
| Strzelczyk et al. (2018) [52] | rs, mc | 2 | 23–38 | ASE | SE | 200–300 | – | 0 | – |
| Aicua-Rapun et al. (2019) [53] | rs, sc | 14 | 33–80 | NCSE, CSE | RSE, SRSE | 100–200 | 200–300 | 50 | – |
| Santamarina et al. (2019) [50] | rs, mc | 43 | 56 ± 32 | CSE, NCSE | SE, RSE, SRSE | 50–400 | – | 54 | 14 |
| (c) Perampanel (PER) | |||||||||
| Redecker et al. (2015) [88] | rs, cs | 9 | 57–82 | NCSE | RSE | 2–6 | – | 22 | – |
| Rohracher et al. (2015) [89] | rs, cs | 12 | 60–91 | CSE, NCSE | RSE | 2–12 | 4–12 | 16 | 0 |
| Beretta et al. (2018) [90] | ps, cs | 8 | 26–71 | NCSE | SRSE | 6–12 | 6–12 | 100 | 0 |
| Rohracher et al. (2018) [91] | rs, cs | 30 | 18–91 | NCSE, CSE | RSE | 2–32 | 12–20 | 17 | 0 |
| Newey et al. (2019) [92] | ps, cr | 4 | 28–69 | NCSE, GSE | RSE | 32 | 6–12 | 100 | 0 |
| Ho et al. (2019) [93] | rs, cs | 22 | 26–89 | NCSE, CSE | RSE, SRSE | 2–8 | 2–12 | 36 | – |
| Rhabani et al. (2019) [94] | rs, cr | 1 | 72 | CSE | SRSE | 8 | – | 100 | – |
| Santamarina et al. (2019) [95] | ps, cr | 3 | 19–39 | CSE, NCSE | RSE | 8–16 | – | 100 | 0 |
| Strzelczyk et al. (2019) [87] | rs, mc | 52 | 19–91 | NCSE, CSE | RSE, SRSE | 2–24 | 4–24 | 37 | 4 |
| (d) Stiripentol (STP) | |||||||||
| Strzelczyk et al. (2015) [96] | rs, sc | 5 | 65–88 | CSE, NCSE | SRSE | 2000–5000 | 4000–6000 | 60 | 0 |
| Uchida et al. (2017) [98] | rs, sc | 1 | 20 | NCSE | RSE | 500 | 2500 | – | 0 |
| Uchida et al. (2018) [99] | rs, sc | 5 | 18–65 | – | SRSE | 500 | 2500 | 100 | 0 |
| (e) Lamotrigine (LTG) | |||||||||
| Pisani et al. (1991) [83] | rs, sc | 1 | 17 | CSE | RSE | 600 | 400 | 100 | 0 |
| (f) Topiramate (TPM) | |||||||||
| Kahriman et al. (2003) [100] | rs, sc | 3 | 0–11 | CSE, NCSE | RSE | 2–3a | 5–6a | 100 | 0 |
| Bensalem and Fakhoury (2003) [101] | rs, sc | 3 | 31–75 | CSE | RSE, SRSE | 500 | 200–500 | 67 | – |
| Blumkin et al. (2005) [102] | rs, sc | 2 | 0 | CSE | RSE | 2–5a | 22–25a | 100 | 0 |
| Perry et al. (2006) [103] | ps, sc | 3 | 0–6 | CSE | RSE | 10a | 5a | 100 | – |
| Soler et al. (2009) [104] | rs, sc | 3 | 24–59 | – | RSE | – | – | 100 | – |
| Akyildiz and Kumandas (2011) [105] | ps, sc | 14 | 1–12 | CSE | RSE, SRSE | 5a | 5a | 86 | 21 |
| Kim et al. (2011) [106] | rs, sc | 16 | 31–79 | CSE, NCSE | SE, RSE | 300–1000 | – | 81 | 0 |
| Synowiec et al. (2011) [107] | rs, sc | 35 | 19–84 | – | RSE, SRSE | 100–1000 | 100–800 | 40 | 0 |
| Bragatti et al. (2011) [108] | rs, sc | 1 | 16 | CSE | RSE | 2,5a | 2,5a | 100 | – |
| Hottinger et al. (2012) [109] | rs, sc | 35 | 19–83 | CSE, NCSE | RSE, SRSE | 800c | 800c | 71 | – |
| Stojanova and Rossetti (2012) [110] | rs, sc | 11 | 16–80 | CSE | RSE, SRSE | – | 50–800 | 18 | – |
| Shelton et al. (2014) [111] | rs, sc | 1 | 12 | CSE | RSE | – | 1000 | 100 | 0 |
| Asadi-Pooya et al. (2015) [112] | ps, sc | 20 | 18–92 | CSE | RSE, SRSE | – | – | 25 | – |
| Madzar et al. (2016) [113] | rs, sc | 17 | 37–70 | CSE, NCSE | RSE, SRSE | 50–400 | 150–1000 | 100 | – |
| Fechner et al. (2019) [114] | rs, mc | 106 | 18–95 | CSE, NCSE | RSE, SRSE | 25–500 | 25–900 | 27 | 36 |
| (g) Oxcarbazepine (OXC) | |||||||||
| Kellinghaus et al. (2014) [85] | rs, sc | 13 | 56–86 | CSE | RSE | 600–1800 | 1200–2400 | 62 | 23 |
| (h) Zonisamide (ZNS) | |||||||||
| Hubert et al. (2020) [116] | rs, mc | 34 | 19–91 | CSE, NCSE | SE, RSE, SRSE | 25–300 | 25–600 | 16 | 10 |
AEs adverse events, ASDs antiseizure drugs, ASE absence SE, av average, cs case series, CSE convulsive SE, cr case report, mc multicenter, NCSE nonconvulsive SE, ob observational, ps prospective, rs retrospective, RSE refractory SE, sc single-center, SE status epilepticus, SRSE super-refractory SE
aMilligrams/kilogram body weight
bAverage dose
cUp to
Carbamazepine (CBZ) is a second-generation ASD available in tablet and oral solution forms only, unfolding its anticonvulsive effect via voltage-gated sodium (Na+) channels and being approved for both monotherapy and add-on therapy in FE [54]. There is currently no evidence supporting the efficacy, safety, or tolerability of CBZ in SE.
Eslicarbazepine (ESL) is a third-generation ASD approved for monotherapy and add-on therapy for FE, and is available in an oral formulation with the same mechanism of action as that of OXC, i.e. the modulation of voltage-gated Na+ channels [54]. There is currently no evidence supporting the efficacy, safety, or tolerability of ESL in SE.
LCM is a third-generation ASD available in tablet, oral, and intravenous forms and is approved for both monotherapy and add-on therapy in FE, which unfolds its effect by antagonism at voltage-gated Na+ channels. LCM is currently not approved for use in SE. There are 20 retrospective case reports or series, as well as seven prospective studies, for a total of 862 pediatric and adult patients with CSE and NCSE, showing a responder rate ranging from 0 to 100%. AEs have been reported in 0–77% of the enrolled patients, with bradycardia, hypotension, and relevant atrioventricular blockage as relevant substance-specific symptoms [55–81]. The largest retrospective multicenter cohort enrolled 165 patients with SE/RSE and reported a responder rate of 63% as well as good tolerability, with 10% of subjects reporting AEs [76]. Given this review focuses on patients in a PCS or with a LOT, one publication with a cohort of 15 adult patients with active cancer of various etiologies and SE/RSE has to be highlighted. Here, a responder rate of 54% and AEs in 14% of the enrolled subjects were reported [81]. According to the current state of knowledge, a loading dose of 5 mg/kg (200–400 mg) or greater and a maintenance dose of 200–400 mg/day seem feasible (Table 1a).
LTG is a third-generation ASD approved for use in FE and genetic generalized epilepsy (GGE) as well as in Lennox–Gastaut syndrome, albeit available in oral tablet form only. Via the modulation of Na+ channels, LTG suppresses the release of glutamate and aspartate as the dominant excitatory neurotransmitters. Based on its strong potential for interactions as well as the high risk of life-threatening skin reactions, including Stevens–Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, and toxic epidermal necrolysis, LTG must be titrated slowly, usually requiring weeks to reach a therapeutic dosage [82]. Moreover, LTG has a high interaction potential with other drugs due to its exceptional hepatic metabolization. There is only one reported case of the successful use of LTG in RSE, from 1991 [83]. However, due to the mentioned aspects, there is currently no evidence supporting the efficacy, safety, or tolerability of LTG (Table 1e).
OXC is a third-generation ASD derivate of CBZ and a member of the dibenzazepine family approved for FE. OXC is a prodrug that is metabolized to its pharmacologically active derivate licarbazepine (MHD), which acts mainly by inactivating voltage-gated Na+ channels. OXC is available as both a tablet and oral solution. Moreover, an anticonvulsive effect via enhanced potassium conductance and the modulation of voltage-gated calcium channels has been reported for this medication [84]. OXC is currently not approved for SE and there is only one retrospective case series with 13 enrolled adult patients supporting its efficacy, safety, and tolerability in RSE, with a responder rate of 62%. AEs, especially hyponatremia, were reported in 23% of patients [85]. According to the current state of knowledge, a loading dose of 600 mg and a maintenance dose of between 600 and 1800 mg/day seem feasible (Table 1g).
PER is one of the newest established third-generation ASDs. It is approved as add-on therapy in FE and GGE, and is available as both a tablet and oral formulation (with an intravenous solution under development). PER acts as a non-competitive antagonist at AMPA receptors [37]. Regarding the considerable glutamatergic proportion in the pathomechanism of SE [86], PER was granted special interest in the context of RSE and SRSE [37, 86, 87]. PER is not approved for use in SE but nine published case series and reports involving a total of 141 patients with CSE and NCSE are available that suggest a responder rate of 16–100% [87–95]. The largest available retrospective, multicenter study with 53 adult patients reports a responder rate of 37% and good tolerability, with only sporadic AEs in 8% (4/53) of the enrolled subjects [87]. According to the current state of knowledge, a loading dose of between 8 and 12 mg (up to 32 mg in few reported cases) and a maintenance dose of 10–12 mg/day seem feasible (Table 1c).
STP is currently only approved for administration in patients with Dravet syndrome and refractory generalized tonic–clonic seizures as add-on therapy with VPA and clobazam, and is available only for oral application as a tablet or dissoluble granulate. STP acts mainly via activating the subunits α3 and δ of GABAA receptors. In addition, STP has been shown to increase extracellular GABA concentration via an unknown mechanism [96]. Being metabolized via the hepatic cytochrome P450 isoenzymes, STP secondarily increases the serum concentrations of many other ASDs, such as CBZ, VPA, PHT, and PB and many BZDs, supporting additional anticonvulsive properties [97]. There are three case reports or series in RSE/SRSE involving a total of 11 adult patients, revealing a responder rate of 0–100% as well as good tolerability of the treatment [96, 98, 99]. The largest case series includes five elderly patients between 65 and 88 years of age, and reports a responder rate of 60% without any AEs [96]. According to the current state of knowledge, a loading dose of 2000–4000 mg and a maintenance dose of 2000–3000 mg/day seem feasible. However, the comparably expensive costs of STP of approximately €100 ($110 or £85) per day have to be considered (Table 1d).
TPM is an orally available third-generation ASD approved for monotherapy and add-on therapy in FE and GGE that acts mainly via the modulation of voltage-gated Na+ channels, and also via calcium channels, by increasing GABA levels and antagonism at the excitatory AMPA receptors. In both FE and GGE, TPM has been proven to be an effective and well-tolerated therapeutic option. TPM is not approved for use in SE. To date, there are 15 case reports and series with a total of 268 pediatric and adult subjects with RSE/SRSE and a responder rate of 17–100% [100–114]. AEs (predominantly hyperammonemia) in seven reports (47%), with a frequency of between 0 and 36%, were reported, mostly during the simultaneous intake of VPA [100, 102, 105–107, 111, 114]. The largest retrospective multicenter study enrolled 106 adult patients and reported a responder rate of 27%, as well as AEs in 36% of patients, additionally confirming the risk for relevant hyperammonemia in patients being simultaneously treated with VPA or PB [114]. Moreover, metabolic acidosis has been reported as a frequent AE [115]. According to the current state of knowledge, a loading dose of 100–200 mg and a maintenance dose of 200–600 mg/day, or 2–5 mg/kg and 5 mg/kg in pediatric patients, seem feasible (Table 1f).
ZNS is a third-generation ASD approved as add-on therapy in FE with seizures with or without secondary generalization. ZNS is only available as an oral formulation and unfolds its anticonvulsive effect most likely via the modulation of voltage-gated Na+ and T-type calcium channels. To date, only one study is available comprising 34 patients with SE using a median ZNS maintenance dose of 400 mg/day after loading with a median dose of 100 mg. A clinical effect attributed to ZNS was observed in 16.2% of the SE patients, and possible AEs occurred in 10.4% of the cohort [116].
Other Minimally or Non-Invasive Therapeutic Options
MgSO4 is a well-established prophylactic and therapeutic option in pregnant patients with pre-eclampsia and eclampsia-associated seizures; however, its use in non-eclamptic RSE is not approved [26]. Although its exact anticonvulsive mechanism of action is not well-understood at this time, MgSO4 is supposed to modulate and thereby inhibit N-methyl D-aspartate (NMDA) receptors. Regarding its use in non-eclamptic RSE/SRSE, 23 case reports and case series involving a total of 32 adult and pediatric patients are available. A response to MgSO4 was reported in 38% (12/32) of patients, while 47% (7/15) showed a recurrence of seizures after discontinuation. AEs were addressed in four reports, including in detail in three of four subjects (75%) [117–139]. In general, the use of MgSO4 has been controversially discussed by modern epileptologists; a bolus of 4 g followed by a continuous infusion of 2–6 g/h is thought to increase the serum Mg level by approximately 3.5 mmol/L and has been suggested as appropriate (Table 2) [1, 2, 26].
Table 2.
Overview of the current evidence for the use of magnesium sulfate (MgSO4) in refractory and super-refractory status epilepticus
| Study design | Status epilepticus | Therapy regimen | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Layout | Study population | Type | Severity | Duration of treatment (days) | Recurrence after withdrawal | Response (n/n) | AEs (n/n) | ||
| n | Age (years) | ||||||||
| Baxter et al. (2003) [117] | rs, sc | 1 | 6 | CSE | RSE | – | – | 0/1 | – |
| Berkley et al. (2015) [118] | rs, sc | 1 | 23 | CSE | RSE | – | – | 0/1 | – |
| Broomall et al. (2014) [119] | rs, sc | 1 | 11 | CSE | RSE | 4 | – | 0/1 | – |
| Dionisio et al. (2013) [120] | rs, sc | 1 | 17 | CSE | RSE | – | – | 0/1 | – |
| Fisher et al. (1988) [121] | rs, sc | 1 | 22 | CSE | RSE | 2 | 1/1 | 0/1 | 1/1 |
| Gedik et al. (2014) [122] | rs, sc | 1 | 5 | CSE | RSE | 4 | – | 0/1 | – |
| Nandakumar et al. (2008) [123] | rs, sc | 1 | 24 | CSE | RSE | – | – | 0/1 | – |
| Neligan et al. (2011) [124] | rs, sc | 1 | 33 | – | SE, RSE | – | 0/1 | 1/1 | – |
| Pandey et al. (2010) [125] | rs, sc | 1 | 18 | CSE | RSE | 5 | – | 0/1 | – |
| Robakis and Hirsch (2006) [126] | rs, sc | 1 | 30 | CSE | RSE | 7 | – | 0/1 | – |
| Sadeh et al. (1991) [127] | rs, sc | 1 | 16 | CSE | RSE | 7 | 1/1 | 1/1 | 1/1 |
| Sahin et al. (2001) [128] | rs, sc | 2 | 13, 15 | CSE | RSE | – | – | 1/2 | – |
| Savard et al. (2012) [129] | rs, sc | 1 | 27 | CSE | RSE | – | – | 0/1 | – |
| Shin et al. (2011) [130] | rs, sc | 1 | 57 | NCSE | RSE | 1 | 0/1 | 1/1 | – |
| Storchheim (1933) [131] | rs, sc | 8 | – | – | SE, RSE | – | 4/8 | 4/8 | – |
| Strzelczyk et al. (2013) [132] | rs, sc | 1 | 21 | CSE | RSE | 1 | – | 0/1 | 1/1 |
| Tan et al. (2013) [133] | rs, sc | 1 | 2 | CSE | RSE | – | 0/1 | 1/1 | 0/1 |
| Valle-Morales et al. (2009) [134] | rs, sc | 1 | 29 | CSE | RSE | – | – | 0/1 | – |
| Visser et al. (2011) [135] | rs, sc | 2 | 19,17 | CSE | RSE | 6 | 1/2 | 2/2 | – |
| Madisia and Bergkeley (2014) [136] | rs, sc | 1 | 23 | CSE | RSE | – | – | 0/1 | – |
| Zaatreh (2005) [137] | rs, sc | 1 | 48 | CSE | RSE | – | – | 1/1 | – |
| Hatch et al. (2016) [138] | rs, sc | 1 | 31 | CSE | RSE | – | – | 1/1 | – |
| Sahin and Riviello (2001) [139] | rs, sc | 1 | 15 | CSE | RSE | – | – | 0/1 | – |
AEs adverse events, CSE convulsive SE, NCSE nonconvulsive SE, rs retrospective, RSE refractory SE, sc single-center study, SE status epilepticus
The KD is a subtype of low-carb, high-fat and high-protein nutrition used as an established therapeutic approach in GLUT-1 deficiency and pyruvate dehydrogenase deficit, as well as refractory epilepsy. KD unfolds its anticonvulsive action by way of offering a limited neuronal energy supply, thereby prompting a general decrease in neuronal excitability. The presence of ketones in the blood and urine can be used as an indicator of a sufficient nutrition change. In the area of RSE and SRSE, 30 publications including a total of 157 pediatric and adult patients are available, using mainly KD in a 3:1 to 5:1 ratio (27/30; 90%) [27, 31, 98, 132, 140–165]. KD is usually initiated as an enteral solution but may also be induced by intravenous means [132]. Moreover, the sparse use of low glycemic index treatment, the adoption of medium-chain triglycerides, and additional use of an SGLT-2 inhibitor in a case of insufficient ketosis under KD have been reported (each 1/30; 3%) [145, 160, 164]. Overall, 80% (125/157) of patients were responders and relevant AEs were observed in 47% (55/117), including mostly gastroesophageal reflux, acidosis, or lipid/electrolyte unbalance. However, AEs were not addressed in all included publications (Table 3).
Table 3.
Overview of the current evidence for the use of a ketogenic diet in refractory and super-refractory status epilepticus
| Study design | Status epilepticus | Therapy regimen | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Layout | Study population | Type | Severity | Diet type | Achieved level of ketosis (n/n) | Response (n/n) | AEs (n/n) | ||
| n | Age (years) | ||||||||
| Bodenant et al. (2008) [140] | rs, sc | 1 | 54 | CSE | RSE | KD 4:1 | 1/1 | – | |
| Wusthoff et al. (2010) [141] | rs, sc | 2 | 29–34 | CSE, NCSE | SRSE | KD 4:1 | 2/2 | 2/2 | – |
| Nabbout et al. (2010) [142] | rs, sc | 9 | 0–6 | CSE | RSE | KD 4:1 | 8/9 | 7/9 | – |
| Cervenka (2011) [31] | rs, sc | 1 | 49 | FSE | SRSE | KD 4:1 | 1/1 | 1/1 | – |
| Kramer et al. (2011) [143] | rs, mc | 4 | 7–27 | – | SRSE | – | – | 1/4 | – |
| Nam et al. (2011) [144] | ps, sc | 5 | 4–40 | – | RSE | KD 4:1 | 5/5 | 5/5 | 5/5 |
| Martikainen et al. (2012) [145] | rs, sc | 1 | 22 | CSE | SRSE | LGIT | – | 1/1 | 0/1 |
| Vaccarezza et al. (2012) [146] | rs, sc | 5 | 1–14 | CSE | SRSE | – | – | 4/5 | – |
| Sort et al. (2013) [147] | rs, sc | 3 | 3–11 | CSE | SRSE | – | 3/3 | 2/3 | 1/3 |
| Strzelczyk et al. (2013) [132] | rs, sc | 1 | 26 | CSE | SRSE | KD 4:1 | 1/1 | 1/1 | – |
| Caraballo et al. (2014) [148] | rs, sc | 10 | 0–16 | CSE | SRSE | KD 4:1 | 10/10 | 7/10 | 3/10 |
| Cobo et al. (2014) [149] | rs, sc | 4 | 0–13 | CSE | SRSE | KD 4:1 | 4/4 | 4/4 | 1/4 |
| Thakur et al. (2014) [150] | rs, mc | 10 | 23–51 | – | SRSE | KD 3–4:1 | 9/10 | 9/10 | 3/10 |
| Amer et al. (2015) [151] | rs, sc | 1 | 21 | – | SRSE | KD 4:1 | 1/1 | 1/1 | – |
| Caraballo et al. (2015) [152] | rs, sc | 2 | 0–1 | CSE | SRSE | KD 4:1 | 2/2 | 2/2 | 0/2 |
| Cash (2015) [153] | rs, sc | 1 | CSE | SRSE | KD 4:1 | 1/1 | 1/1 | – | |
| Fung et al. (2015) [154] | rs, sc | 4 | 8–16 | CSE | SRSE | KD 4:1 | 3/4 | 1/4 | 2/4 |
| Lin et al. (2015) [155] | rs, sc | 1 | 6 | CSE | SRSE | KD 4:1 | 1/1 | 1/1 | |
| Appavu et al. (2016) [27] | rs, sc | 10 | 2–16 | CSE | SRSE | KD 4–5:1 | 9/10 | 9/10 | 1/10 |
| Caraballo et al. (2017) [156] | rs, sc | 6 | 2–9 | CSE | RSE | KD 4:1 | – | 5/6 | 0/6 |
| Cervenka et al. (2017) [157] | ps, mc | 15 | 18–82 | – | SRSE | KD 4:1 | 15/15 | 11/15 | 10/15 |
| Smith and Press (2017) [158] | rs, sc | 9 | SRSE | – | 9/9 | 8/9 | 2/9 | ||
| Uchida et al. (2017) [98] | rs, sc | 1 | 20 | NCSE | RSE | – | – | 1/1 | – |
| Arya et al. (2018) [159] | ps, mc | 14 | 0–19 | – | RSE | KD 4:1 | 14/14 | 10/14 | 3/14 |
| Blunk et al. (2018) [160] | rs, sc | 1 | 42 | CSE | RSE | KD/SGLT2 | 1/1 | 0/1 | 0/0 |
| Francis et al. (2019) [161] | rs, sc | 11 | 21–73 | – | RSE | – | 10/11 | 8/11 | 10/11 |
| Park et al. (2019) [162] | rs, sc | 16 | 0–21 | CSE | RSE | 3–4:1 | 14/16 | 15/16 | 10/16 |
| Peng et al. (2019) [163] | rs, sc | 7 | 1–13 | – | RSE | 3–4:1 | 6/7 | 7/7 | 3/7 |
| Prasoppokakorn et al. (2019) [164] | rs, sc | 1 | 19 | CSE | SRSE | MCT KD | 1/1 | 1/1 | 0/1 |
| Noviawaty et al. (2020) [165] | rs, sc | 1 | 38 | CSE | SRSE | KD 4:1 | 1/1 | 1/1 | |
AEs adverse events, CSE convulsive SE, FSE febrile SE, KD ketogenic diet, LGIT low glycemic index treatment diet, mc multicenter, MCT KD medium-chain triglyceride ketogenic diet, NCSE nonconvulsive SE, ps prospective, rs retrospective, RSE refractory SE, sc single-center, SE status epilepticus, SGLT2 sodium-glucose co-transporter-2, SRSE super-refractory SE
Neurosteroids with allopregnanolone (brexanolone) as the main representative of this class are endogenous derivates of progesterone acting mainly as positive allosteric modulators at the GABAA receptors. Allopregnanolone has faced high expectations as a new potential option to wean patients from anesthetic drugs during the treatment of RSE and SRSE. Three studies with a total of 29 patients have been published to date, showing promising results [119, 166, 167]; however, a placebo-controlled RCT with 132 enrolled subjects (67 receiving verum vs. 65 receiving placebo) analyzing the safety and efficacy of allopregnanolone to wean from third-line ASDs in SRSE was aborted by the sponsor after preliminary results suggested no significant difference was present between the groups (p = 0.878). Within the RCT population, allopregnanolone was well-tolerated [168]. Moreover, the use of neurosteroids outside weaning of intravenous or inhalable anesthetic drugs has not been analyzed thus far.
Other Immunomodulatory or Immunosuppressive Treatment Options (IT)
Over the past decades, several ITs have been used in SE, such as SP, IVIGs or plasmapheresis/plasma exchange (PLEX). Based on their direct effects, these options have been classified as first-line ITs to allow a better differentiation from rituximab, cyclophosphamide, or other long-term immunosuppressants (second-line ITs). There were 72 studies with 175 patients who used first-line ITs, of which 14 (19%) used a single therapy regimen, 20 (28%) used a double therapy regimen, and 38 (53%) used a triple or quadruple therapy regimen. Overall, 46% (n = 81) of the patients treated with IT showed an attributable response. The data situation was insufficient to allow for analyzing the AEs. For more information on the included studies, please refer to Table 4.
Table 4.
Overview of the current evidence for the use of neurosteroids and immunomodulatory/immunosuppressive treatment in refractory and super-refractory status epilepticus
| Study design | Status epilepticus | Therapy characteristics | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Layout (phase) | Study population | Type | Severity | Duration (days) | Loading dose (µg/kg/h) | Rate (µg/kg/h) | Response (n/n) | ||
| n | Age (years) | ||||||||
| (a) Neurosteroids (allopregnanolone) | |||||||||
| Broomall et al. (2014) [119] | rs, sc | 2 | 2, 11 | CSE, NCSE | SRSE | 5 | – | 28–86 | Successful weaning |
| Vaitkevicius et al. (2017) [167] | rs, sc | 2 | 23, 28 | CSE, NCSE | SRSE | 5 | – | 86 | Successful weaning |
| Rosenthal et al. (2017) [166] | ps, mc (I/II) | 25 | 10–76 | – | SRSE | 5 | 286 | 86–156 | 17/25 |
| NCT02477618 (2019) [168] | ps, mc (III) | 67 | 41.3 | CSE, NCSE | SRSE | 6 | 300 | 90–150 | Not superior to placebo (p = 0.878) |
| (b) Immunomodulatory/immunosuppressive treatment with SP, IVIG or PLEX | |||||||||
| Agan et al. (2015) [199] | rs, sc | 1 | 19 | AI CSE | RSE | PLEX | 1/1 | ||
| Agirre-Arrizubieta and Moran (2012) [200] | rs, sc | 1 | 28 | – | RSE | PLEX, IVMP | 0/1 | ||
| Al-Ajlan et al. (2014) [201] | rs, sc | 1 | 18 | AI CSE | RSE | SP, IVIG | 0/1 | ||
| Alam et al. (2013) [202] | rs, sc | 1 | 60 | AI CSE | RSE | SP, IVIG, PLEX, RTX | 0/1 | ||
| Alam et al. (2014) [203] | rs, sc | 1 | 20 | CSE | RSE | SP, IVIG | 0/1 | ||
| Amer et al. (2015) [151] | rs, sc | 1 | 21 | AI CSE | RSE | SP, IVIG, PLEX | 0/1 | ||
| Armas et al. (2013) [204] | rs, sc | 1 | 69 | RSE | SP, IVIG, AZA | 1/1 | |||
| Barnes et al. (2013) [205] | rs, sc | 1 | 33 | CSE | RSE | SP, PLEX | 0/1 | ||
| Bobb et al. (2014) [206] | rs, sc | 1 | 28 | – | RSE | IVIG | 1/1 | ||
| Brigo et al. (2018) [207] | rs, sc | 1 | 22 | AI CSE | RSE | SP | 1/1 | ||
| Buenache et al. (2012) [208] | rs, sc | 1 | 4 | FIRES | RSE | SP, IVIG, PLEX | 1/1 | ||
| Buerger et al. (2015) [209] | rs, sc | 1 | 74 | CSE | RSE | SP, PLEX | 0/1 | ||
| Calabrace and Witherspoon (2014) [210] | rs, sc | 1 | 19 | AI NCSE | RSE | SP, PLEX, RTX | 0/1 | ||
| Caputo et al. (2017) [211] | rs, sc | 1 | 13 | FIRES | RSE | SP, IVIG | 1/1 | ||
| Caraballo et al. (2013) [212] | rs, sc | 10 | - | FIRES | RSE | IVIG, PLEX, RTX | 3/10 | ||
| Charles et al. (2014) [213] | rs, sc | 1 | 57 | NCSE | RSE | IVIG | 1/1 | ||
| Chevret et al. (2008) [214] | rs, sc | 4 | 9a | FIRES | RSE | PLEX | 1/4 | ||
| Chevret et al. (2011) [215] | rs, sc | 8 | 6a | FIRES | RSE | PLEX | 1/8 | ||
| Costas et al. (2015) [216] | rs, sc | 1 | 5 | FIRES | RSE | IVIG, PLEX | 0/1 | ||
| Dishong et al. (2013) [217] | rs, sc | 1 | 14 | FIRES | RSE | SP, IVIG, PLEX, Anakinra | 0/1 | ||
| Gall et al. (2013) [218] | rs, sc | 2 | 30a | NORSE | RSE | PLEX, IVIG | 2/2 | ||
| Gedik et al. (2014) [122] | rs, sc | 1 | 5 | AI CSE | RSE | SP, PLEX | 1/1 | ||
| Ghamande et al. (2013) [219] | rs, sc | 1 | 41 | – | RSE | SP, IVIG, RTX | 0/1 | ||
| Gonzales et al. (2011) [220] | rs, sc | 1 | 23 | NORSE | RSE | SP, IVIG | 0/1 | ||
| Hainsworth et al. (2014) [221] | rs, sc | 1 | 23 | AI CSE | RSE | IVIG, SP, PLEX, RTX | 1/1 | ||
| Hakimi et al. (2014) [222] | rs, sc | 1 | 44 | AI CSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Hoang et al. (2014) [223] | rs, sc | 1 | 40 | NCSE | RSE | IVIG, SP, PLEX | 0/1 | ||
| Howell et al. (2012) [224] | rs, sc | 1 | 14 | FIRES | RSE | PLEX, RTX | 0/1 | ||
| Hribljan et al. (2013) [225] | rs, sc | 4 | – | FIRES | RSE | SP, IVIG, PLEX | 0/4 | ||
| Incecik et al. (2015) [226] | rs, sc | 1 | 16 | – | RSE | SP, IVIG, PLEX | 0/1 | ||
| Kaneko et al. (2012) [227] | rs, sc | 1 | 17 | AI CSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Katsuse et al. (2019) [228] | rs, sc | 1 | 48 | AI NCSE | RSE | SP | 1/1 | ||
| Khawaja et al. (2014) [229] | rs, sc | 1 | 22 | AI CSE | RSE | IVIG, SP, PLEX, RTX | 1/1 | ||
| Khawaja et al. (2015) [230] | rs, sc | 7 | 35a | AI/viral CSE | RSE | IVIG, SP, PLEX, RTX | 0/7 | ||
| Kirkpatrick et al. (2011) [231] | rs, sc | 1 | 19 | AI CSE | RSE | SP, PLEX, RTX | 1/1 | ||
| Kong et al. (2018) [232] | rs, sc | 24 | 17a | AI CSE | RSE | SP, IVIG, PLEX, RTX, CP | 20/24 | ||
| Korff et al. (2009) [233] | rs, sc | 1 | 9 | FIRES | RSE | SP, PLEX | 0/1 | ||
| Kramer et al. (2011) [234] | rs, sc | 1 | – | FIRES | RSE | SP, IVIG, PLEX | 0/1 | ||
| Labate et al. (2013) [235] | rs, sc | 1 | 17 | AI CSE | RSE | SP, IVIG | 1/1 | ||
| Le Moigno et al. (2014) [236] | rs, sc | 1 | 6 | AI CSE | RSE | SP, PLEX, RTX | 1/1 | ||
| Lenoir et al. (2012) [237] | rs, sc | 1 | 17 | AI CSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Li et al. (2013) [238] | rs, sc | 3 | 39, 43, 51 | CSE, NCSE | RSE | SP, IVIG | 2/3 | ||
| Lin et al. (2018) [239] | rs, sc | 45 | 1–18 | CSE | RSE | SP, IVIG vs. IVIG | 10/45 | ||
| Lousa et al. (2000) [240] | rs, sc | 1 | 14 | – | RSE | PLEX | 1/1 | ||
| Madisi and Berkley (2014) [241] | rs, sc | 1 | 23 | NORSE, CSE | RSE | IVIG, SP, PLEX, RTX | 1/1 | ||
| Malaga et al. (2015) [242] | rs, sc | 1 | 1 | AI CSE | RSE | SP, IVIG, PLEX, RTX | 1/1 | ||
| Mann et al. (2013) [243] | rs, sc | 1 | 20 | AI CSE | RSE | IVIG, SP, PLEX | 1/1 | ||
| Marques et al. (2014) [244] | rs, sc | 1 | 30 | AI CSE | RSE | SP, IVIG | 1/1 | ||
| Milh et al. (2012) [245] | rs, sc | 1 | 5 | AI CSE | RSE | IVIG | 1/1 | ||
| Mirás Veiga et al. (2016) [246] | rs, sc | 1 | 4 | FIRES | RSE | SP, PLEX, IVIG | 0/1 | ||
| Moeller et al. (2012) [247] | rs, sc | 1 | 27 | AI CSE | RSE | IVIG, PLEX | 0/1 | ||
| Nakamura (2015) [248] | rs, sc | 1 | 1 | FIRES | RSE | SP, IVIG, PLEX | 0/1 | ||
| Neligan et al. (2011) [124] | rs, sc | 1 | 33 | AI CSE | RSE | IVIG, RTX | 1/1 | ||
| Noviawaty et al. (2015) [249] | rs, sc | 1 | 13 | AI CSE | RSE | PLEX | 0/1 | ||
| Ogawa et al. (2013) [250] | rs, sc | 1 | 11 | AERRPS | RSE | PLEX | 0/1 | ||
| Pari et al. (2014) [251] | rs, sc | 1 | 19 | AI NCSE | RSE | SP, PLEX | 1/1 | ||
| Ramos et al. (2019) [169] | rs, sc | 4 | 51–75 | CSE, NCSE | RSE | SP | 4/4 | ||
| Rypulak et al. (2016) [252] | rs, sc | 1 | 23 | AI CSE | RSE | IVIG, SP, | 1/1 | ||
| Sawicka et al. (2016) [253] | rs, sc | 1 | 18 | AI CSE | RSE | IVIG, PLEX, RTX | 1/1 | ||
| Shatzmiller et al. (2011) [254] | rs, sc | 1 | 19 | AI CSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Shrivastava et al. (2017) [255] | rs, sc | 1 | 24 | NORSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Soldatos and Gorman (2012) [256] | rs, sc | 1 | 6 | FIRES | RSE | SP, IVIG, PLEX | 1/1 | ||
| Thomas et al. (2012) [257] | rs, sc | 1 | 19 | AI NCSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Ting et al. (2017) [258] | rs, sc | 1 | 16 | AI CSE | RSE | SP, PLEX, CP | 1/1 | ||
| Triplet et al. (2018) [259] | rs, sc | 1 | 21 | AI CSE | RSE | SP, IVIG, PLEX, RTX, CP | 1/1 | ||
| Van Baalen et al. (2012) [260] | rs, sc | 6 | 2–12 | FIRES | RSE | SP, IVIG, PLEX | 1/6 | ||
| Villani et al. (2001) [261] | rs, sc | 1 | 45 | CSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Wilder-Smith et al. (2005) [262] | rs, sc | 3 | – | NORSE, CSE | RSE | IVIG | 0/1 | ||
| Yamamoto et al. (2014) [263] | rs, sc | 1 | 35 | AI CSE | RSE | SP, IVIG, PLEX | 1/1 | ||
| Yeo et al. (2009) [264] | rs, sc | 1 | 32 | AI CSE | RSE | IVIG | 1/1 | ||
| Yoshida et al. (2019) [265] | rs, sc | 1 | 68 | PNS NCSI | RSE | SP, PLEX | 1/1 | ||
AERRPS acute encephalitis with refractory repetitive partial seizures, AI autoimmune, AZA azathioprine, CP cyclophosphamide, CSE convulsive SE, FIRES febrile infection-related epilepsy syndrome, IVIG intravenous immunoglobulins, mc multicenter study, NCSE non-convulsive SE, NORSE new-onset refractory status epilepticus, PLEX plasma exchange or plasmapheresis, PNS paraneoplastic neurological syndrome, ps prospective, rs retrospective, RSE refractory SE, RTX rituximab, sc single-center, SE status epilepticus, SP steroid pulse, SRSE super-refractory SE
aMedian
Discussion
Based on a survey of existing publications, no level 1, grade A evidence is available for the use of any of the reviewed fourth-line ASDs in RSE. The best data were found for LCM and TPM, with a level 3 evidence and grade C recommendation. Moreover, there was level 4, grade D evidence for the use of BRV and PER, or level 5, grade D evidence for STP, OXC, and ZNS in RSE. Regarding the other assessed minimally or non-invasive, non-medicinal therapeutic options, level 4, grade D evidence supporting the benefit of the KD, and level 5, grade D evidence supporting the use of MgSO4, in the context of non-eclamptic RSE, were available. The evidence for ITs, such as SP, IVIG, or PLEX, was level 4, grade D, with the additional limitation that most studies showing an effect of ITs included patients with autoimmune SE. Even if single studies supported the hypothesis, the SP use could be a feasible option as an abortive treatment for refractory seizures or status epilepticus [169], but is no reliable evidence for their broad use in RSE in general [170]. There were contradictory results and no reliable evidence regarding the use of neurosteroids in RSE, with the evidence formally rated as level 5, grade D (Table 4). Based on these results, the use of neurosteroids in RSE treatment cannot be recommended (Table 4), although studies of ganaxolone in humans are in progress [171]. All mentioned medicinal or non-medicinal therapeutic options do not require a stay in the ICU or other invasive measures beyond demand-oriented continuous monitoring that is usually affected by ACP or LOT, or within a moderate PCS. Regarding PLEX, establishing a central venous catheter, as well as the often-exhausting experience of therapeutic cycles, has to be reflected upon and discussed in regard to the individual advance directives. Nearly all reviewed therapy options can be applied orally or enterally via a nasogastric tube, which underlines their applicability in patients with impairment of consciousness as well as dysphagia or aphagia. Due to rarely reported cardiac arrhythmia or de novo atrioventricular blockage [26], MgSO4 should be applied intravenously for improved controllability and to prepare for the possibility of rapid discontinuation in the case of the above-mentioned symptoms. Due to the obligatory need for advanced cardiopulmonary monitoring of established anesthetic drugs, such as ketamine, propofol, thiopental or midazolam, in low doses and not requiring mechanical ventilation, they were not included in this systematic review but may also be individually considered [2, 172, 173]. For the same reasons, sporadically used neuromodulation techniques in RSE, such as vagal nerve stimulation (VNS), electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), and deep brain stimulation (DBS) were also not included in this review [174, 175].
As with any systemic review, this publication may suffer from several methodical limitations, such as possible missing relevant publications not identified by the prescribed search algorithm or the cross-check via pre-existing reviews on this topic. Moreover, the exclusion of articles not available in the English, French, German, Italian, or Spanish languages, as well as the limitation of the research on the MEDLINE, EMBASE and Cochrane databases, represents possible biases of this review. In particular, all studies on RSE and SRSE are subject to bias due to consecutive add-on therapy with several ASDs or other therapeutic options, which makes it difficult to link a specific effect to one of the used interventions. Most included publications try to solve this bias by temporal correlation of therapy onset and the observed effect; however, this established way of analysis does not exclude the occurrence of a prolonged effect of previously used interventions or a cumulative effect of different therapeutic approaches [176, 177]. To minimize the mentioned limitations and to offer a structured analysis, PRISMA guidelines were closely followed [178].
The management of RSE in patients with a LOT or a PCS represents a challenge for modern clinicians, intensive care medics, and epileptologists. Due to patients’ advanced directives or specific health conditions, recommendations of existing guidelines have to be frequently omitted as invasive treatment in the ICU may be refused or individual contraindications for specific second- or third-line treatment options exist. In the case of a sustained wish for a conservative therapeutic attempt, clinicians have to fall back on fourth-line ASDs as the last therapeutic resort. The evidence for the use of fourth-line ASDs in RSE and SRSE is limited; however, there are several therapeutic options that have been shown to be effective, safe, and well-tolerated whose use can be translated in the context of ACP, LOT, and PCS. In accordance with frequent ACP, LOT or PCS, all ASDs as well as KD can be applied enterally without the need to adopt invasive measures such as a central venous catheter or port catheter. In all patients, basal therapy with BZDs should be initiated if possible [19, 179]. The highest level of evidence in RSE/SRSE exists to support the use of LCM and TPM (level 3, grade C), which have been proven to be effective and well-tolerated in patients with RSE [36, 114]. Moreover, there is sufficient evidence for the use of PER (level 4, grade D) [87], BRV (level 4, grade D), OXC (level 5, grade D) [85], ZNS (level 5, grade D) and STP (level 5, grade D) [96] in patients with RSE or SRSE (Table 1). Here, the reduced potential for psychobehavioral adverse effects, as well as the availability of an intravenous formulation for BRV and the pleiotropic effects of STP, have to be mentioned [37, 96]. The use of LTG, ESL, and CBZ cannot be recommended based on the current state of knowledge. In line with conventional clinical experience, data from rodent studies suggested that early and aggressive combination therapy in RSE treatment is beneficial, which was attributed to an early maladaptive internalization of synaptic GABAA receptors and the externalization of NMDA receptors [180]. Here, the unique mode of action of LEV, BRV, PER or STM could be of particular advantage, which has to be further elucidated by future trials and research work [37, 96]. However, even if these aspects should not be the focus of the therapy decision, economic aspects of different ASD may also have to be considered [181, 182].
Considering non-medicinal therapeutic options, there is low evidence for the use of KD (level 4, grade D) in RSE based on more than 150 reported cases. This therapy should be started and monitored by a physician experienced in KD, or a nutritional expert, to avoid electrolyte or vitamin imbalance or hypoglycemia, and standardized protocols should be followed [157]. A recent study on the nutritional state in SE showed that the induction of ketogenesis might improve treatment outcomes and will surely stimulate the conduct of further research into the adoption of KD in SE [183]. The use and benefits of MgSO4 in non-eclamptic RSE are controversially discussed within the field [26, 184] and are only supported by weak evidence (level 5, grade D). There is currently no evidence available for the use of neurosteroids in RSE, and, furthermore, there is even evidence against their use [168]. The use of immunotherapies such as steroids, plasmapheresis, or IVIGs also cannot be recommended unless an autoimmune or paraneoplastic etiology is proven or seems obvious [185, 186].
Following the GRADE classification, the authors of this manuscript came to a consensus for a ‘high’ recommendation for the use of LCM, TPM, and BRV; a ‘moderate’ recommendation for the use of PER and STM; a ‘low’ recommendation for the use of OXC, ZNS, and KD; and a ‘very low’ recommendation for the use of MgSO4 and ITs in patients without presumptive autoimmune etiology of SE. The level of evidence, grade of recommendation, feasible loading and maintenance doses, route of application, and relevant AEs for every mentioned therapeutic option are given in Table 5.
Table 5.
Feasible non- or minimally invasive therapeutic options for RSE in patients undergoing LOT or in a PCS, beyond current recommendations of existing guidelines
| Substance | Level of evidence | Dosing | ApplicationApplication | Important treatment issues | ||||
|---|---|---|---|---|---|---|---|---|
| Name | INN | Oxford [42] | GRADE [43] | Loading (mg) | Maintenance (mg/day) | Regimen | Route | |
| Antiseizure drugs | ||||||||
| Topiramatea | TPM | 3C | I | 400 | 400–600 | bid | po/os | Hyperammonemia, metabolic acidosis |
| Brivaracetama | BRV | 4D | I | 2b | 100–200 | bid | po/os/iv | Psychobehavioral changes |
| Perampanela | PER | 4D | II | 8–12 | 8–12 | qd | po/os | Dizziness, vertigo |
| Lacosamidea | LCM | 3C | I | 5b | 200–400 | bid | po/os/iv | AV block ≥ °III, relevant bradycardia |
| Stiripentola | STP | 5D | II | 2000–3000 | 3000–4000 | bid | po/os | High daily treatment costs |
| Oxcarbazepinea | OXC | 5D | III | 600–1200 | 1200–1800 | bid | po/os | Hyponatremia |
| Zonisamidea | ZNS | 5D | III | 200–300 | 200–600 | bid | po/os | Ataxia, skin rash |
| Other minimally or non-invasive therapeutic options | ||||||||
| Magnesium sulfate | MgSo4 | 5D | IV | 4000 | 2000–6000c | cont | iv | Cardiac arrhythmia |
| Ketogenic dieta | KD | 4D | III | KD 4:1 ratio | KD 4:1 | cont | po | Acidosis, lipid or electrolyte disbalance, GER |
| Steroid pulsea | IVMP | 4D | III | 1000–2000 | 1000–2000 | qd | iv | Only if autoimmune etiology is assumed or confirmed |
| Immunoglobulinsa | IVIG | 4D | III | 2d | tid | iv | Only if autoimmune etiology is assumed or confirmed | |
| Plasma exchangea | PLEX | 4D | III | NA | NA | daily | iv | Only if autoimmune etiology is assumed or confirmed |
AV atrioventricular, bid twice daily, Cont. continuous application, GER gastroesophageal reflux, GRADE Grading Recommendations Assessment, Development, and Evaluation, iv intravenous, IVMP intravenous methylprednisolone, KD ketogenic diet, LOT limitation of life-sustaining therapy, NA not applicable, os oral solution available, po orally, PCS palliative care setting, qd once daily, RSE refractory status epilepticus, tid three times daily
aUse and dose recommendations not in line with the official approval
bMilligram/kilogram body weight
cGrams/hour as a continuous infusion
dGrams/kilogram body weight, distributed over 2–5 days
In otherwise healthy patients with RSE/SRSE, rational diagnostics tests should be performed to identify the possible causes or factors leading to disease persistence. According to the German clinical practice guidelines on SE, routine point-of-care and laboratory testing (including blood glucose, hemography, inflammation parameters, electrolytes, liver, pancreas, renal and thyroid function, and creatine kinase) and cerebral imaging (e.g. computed tomography, magnetic resonance imaging) should be performed, supplemented by lumbar puncture where appropriate. Possible causal, maintenance or trigger factors for RSE/SRSE should be addressed and treated adequately as long as the therapy administered is consistent with the patient’s ACP, LOT, or PCS [5, 187].
More so than in any other population, ACP, advance directives, and medical ethical aspects have to be considered carefully in these patients before the initiation of therapy, and should be re-evaluated during the course of treatment. According to the literature, only a small portion (4.6–39.0%) of patients with SE or other critical neurological diseases had written advance directives or directives disclosed by designated healthcare agents (also known as healthcare proxy, i.e. a person who can act and decide on medical matters if you are not able to do so yourself), which were mostly effecting a do-not-resuscitate (DNR) order [188–190]. Here, the availability of an informed healthcare agent was associated with a slightly increased proportion of patients with DNR orders. Advance directives are largely accepted by physicians regarding both the adaption of the therapy setting and the withholding of resuscitation [190].
Realistic expectations of the outcome of treatment and the timely anticipation of a further disease course are warranted. In this context, the potential harm of prolonged iatrogenic coma and its secondary risks due to supportive aids and medications (e.g. vasopressors, paralysis of the bowels) that have been previously shown in elderly and multimorbid patients has to be addressed [190–193]. Referring to the written advance directive directly or indirectly (through conversation) will guide the therapeutic approach, with treatment steps checked for their compatibility and discussed with the patient, caregivers, or healthcare agents. For example, before introducing artificial enteral feeding via a nasogastric tube or intravenously as an indispensable step for the KD, it should be considered whether the patient would have agreed to this procedure and artificial nutrition itself, and whether the resulting decrease in quality of life due to both measures would have been accepted. In the case of refusal, the probability for reaching ketosis over the stay as a consequence of catabolic metabolism is also high [194], but less controllable and of unclear therapeutic relevance. Moreover, fundamental therapeutic aspects, such as the renunciation of resuscitation or the immediate switching to a supportive care setting in the case of acute deterioration, should be addressed early if they were not already mentioned in the ACP documentation. In this context, the withdrawal and withholding of life-supporting treatment, including ASDs, as a possible measure of LOT should also be addressed [188–190]. A re-evaluation of the health condition, therapeutic approaches, and further diagnostic or therapeutic steps should be performed at regular intervals and discussed with the patient, their caregivers or their healthcare agents where appropriate. If any doubts exist as to the medical ethical tenability of the chosen therapy regimen and the patient’s advance directive, a third neutral party, such as an ethics committee should be consulted. In addition, the well-being and possible illness-specific consequences of caregivers should be addressed during and after therapy [195].
The approximate mortality rate for RSE and SRSE in unrestricted therapy settings is 35–40% [12, 196–198], supporting such an outcome in a far higher proportion of patients with ACP, undergoing LOT or in a PCS. In the case of a continuing refractory course or an acute deterioration of the health state, palliative sedation and analgesia using BZDs, propofol, or morphine should be considered within the setting of a dying patient, with the aim of anxiolysis, freedom from pain and convulsive seizures, and mitigation of respiratory distress [18, 19].
Conclusion
Refractory SE in patients undergoing LOT or in a PCS represents a difficult challenge for modern clinicians, intensivists and epileptologists. The evidence for the use of ASDs and other minimally or non-invasive therapy options in RSE beyond that in current guidelines is low, but several effective and well-tolerated therapies are available that should be considered in this patient population. In particular, newer third- and fourth-generation ASDs such as LCM, TPM, BRV and PER seem to be a feasible escalation option for the treatment of RSE in patients undergoing LOT or in a PCS. These drugs can be rapidly uptitrated and applied orally, via nasogastric tube, and two of them are also available as an intravenous solution. In addition, the KD represents a practicable non-invasive therapy option that may be considered individually. More so than in any other population, advance care planning, advance directives, and medical ethical aspects have to be considered carefully before the initiation of therapy. Furthermore, the determination of no further escalation of therapy with ASDs, or their discontinuation in the context of terminal care, has to be considered and re-evaluated on a regular basis depending on the individual’s clinical course and the ACP or directives.
Compliance with Ethical Standards
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors were supported via the Center for Personalized and Translational Epilepsy Research with a LOEWE grant from the State of Hessen.
Conflict of interest
Felix Rosenow reports grants and personal fees from UCB Pharma, Arvelle Therapeutics, and Desitin Arzneimittel; personal fees from Eisai, GW Pharmaceuticals, Novartis, Medtronic, Cerbomed, Sandoz, BayerVital, and Shire; and grants from the European Union, Deutsche Forschungsgemeinschaft, the LOEWE-Programm of the state of Hesse, and the Detlev-Wrobel-Fonds for Epilepsy Research. Adam Strzelczyk reports personal fees and grants from Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceuticals, LivaNova, Marinus Pharmaceuticals, Medtronic, Sage Therapeutics, UCB Pharma, and Zogenix. Laurent M. Willems, Sebastian Bauer, Martin Voss, and Kolja Jahnke report no conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Consent for publication
All authors approved the final manuscript for submission.
Author contributions
LMW developed the idea for this review. AS and LMW drafted the concept and performed the literature search and data analysis. LMW and AS wrote the manuscript, and SB, KJ, MV and FR critically revised the work. All authors contributed to the final manuscript.
Footnotes
The original online version of this article was revised due to a retrospective Open Access order.
Change history
7/27/2021
A Correction to this paper has been published: 10.1007/s40263-021-00845-6
References
- 1.Trinka E, Kalviainen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73. doi: 10.1016/j.seizure.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134(Pt 10):2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 3.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 4.Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. 2019;381(22):2103–2113. doi: 10.1056/NEJMoa1905795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenow F. AWMF-Leitlinie: Status epilepticus im Erwachsenenalter; Deutsche Gesellschaft für Neurologie (DGN). In: Diener H, Weimar C, editors. Leitlinien für Diagnostik und Therapie in der Neurologie, Kapitel Anfälle und Bewusstseinsstörungen. Stuttgart: Thieme Verlag; 2017.
- 6.Meierkord H, Boon P, Engelsen B, Gocke K, Shorvon S, Tinuper P, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17(3):348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 7.Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 9.Gomes D, Pimentel J, Bentes C, Aguiar de Sousa D, Antunes AP, Alvarez A, et al. Consensus protocol for the treatment of super-refractory status epilepticus. Acta Med Port. 2018;31(10):598–605. doi: 10.20344/amp.9679. [DOI] [PubMed] [Google Scholar]
- 10.Holtkamp M. Pharmacotherapy for refractory and super-refractory status epilepticus in adults. Drugs. 2018;78(3):307–326. doi: 10.1007/s40265-017-0859-1. [DOI] [PubMed] [Google Scholar]
- 11.Schubert-Bast S, Zöllner JP, Ansorge S, Hapfelmeier J, Bonthapally V, Eldar-Lissai A, et al. Burden and epidemiology of status epilepticus in infants, children, and adolescents: a population-based study on German health insurance data. Epilepsia. 2019;60(5):911–920. doi: 10.1111/epi.14729. [DOI] [PubMed] [Google Scholar]
- 12.Strzelczyk A, Ansorge S, Hapfelmeier J, Bonthapally V, Erder MH, Rosenow F. Costs, length of stay, and mortality of super-refractory status epilepticus: a population-based study from Germany. Epilepsia. 2017;58(9):1533–1541. doi: 10.1111/epi.13837. [DOI] [PubMed] [Google Scholar]
- 13.Kortland LM, Alfter A, Bähr O, Carl B, Dodel R, Freiman TM, et al. Costs and cost-driving factors for acute treatment of adults with status epilepticus: a multicenter cohort study from Germany. Epilepsia. 2016;57(12):2056–2066. doi: 10.1111/epi.13584. [DOI] [PubMed] [Google Scholar]
- 14.Kortland LM, Knake S, von Podewils F, Rosenow F, Strzelczyk A. Socioeconomic outcome and quality of life in adults after status epilepticus: a multicenter, longitudinal, matched case-control analysis from Germany. Front Neurol. 2017;8:507. doi: 10.3389/fneur.2017.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etkind SN, Bone AE, Gomes B, Lovell N, Evans CJ, Higginson IJ, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med. 2017;15(1):102. doi: 10.1186/s12916-017-0860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupu D, Quigley L, Mehfoud N, Salsberg ES. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manag. 2018;55(4):1216–1223. doi: 10.1016/j.jpainsymman.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 17.McCormick AJ. Self-determination, the right to die, and culture: a literature review. Soc Work. 2011;56(2):119–128. doi: 10.1093/sw/56.2.119. [DOI] [PubMed] [Google Scholar]
- 18.Gronheit W, Popkirov S, Wehner T, Schlegel U, Wellmer J. Practical management of epileptic seizures and status epilepticus in adult palliative care patients. Front Neurol. 2018;9:595. doi: 10.3389/fneur.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalviainen R, Reinikainen M. Management of prolonged epileptic seizures and status epilepticus in palliative care patients. Epilepsy Behav. 2019;101(Pt B):106288. doi: 10.1016/j.yebeh.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Landefeld J, Incze MA. Advanced care planning—what should i know? JAMA Intern Med. 2019 doi: 10.1001/jamainternmed.2019.0005. [DOI] [PubMed] [Google Scholar]
- 21.Zubek L. Options for the improvement of communication and self-determination in end-of-life decisions in intensive care units [in Hungarian] Orv Hetil. 2016;157(17):669–674. doi: 10.1556/650.2016.30459. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, Takahashi K, Mizutani T. Interaction between valproic acid and carbapenem antibiotics. Drug Metab Rev. 2007;39(4):647–657. doi: 10.1080/03602530701690341. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011;22(1):128. doi: 10.1097/EDE.0b013e3181fe7825. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38(2):171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 26.Zeiler FA, Matuszczak M, Teitelbaum J, Gillman LM, Kazina CJ. Magnesium sulfate for non-eclamptic status epilepticus. Seizure. 2015;32:100–108. doi: 10.1016/j.seizure.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Appavu B, Vanatta L, Condie J, Kerrigan JF, Jarrar R. Ketogenic diet treatment for pediatric super-refractory status epilepticus. Seizure. 2016;41:62–65. doi: 10.1016/j.seizure.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Bauer S, Willems LM, Paule E, Petschow C, Zollner JP, Rosenow F, et al. The efficacy of lacosamide as monotherapy and adjunctive therapy in focal epilepsy and its use in status epilepticus: clinical trial evidence and experience. Ther Adv Neurol Disord. 2017;10(2):103–126. doi: 10.1177/1756285616675777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brigo F, Bragazzi NL, Igwe SC, Nardone R, Trinka E. Topiramate in the treatment of generalized convulsive status epilepticus in adults: a systematic review with individual patient data analysis. Drugs. 2017;77(1):67–74. doi: 10.1007/s40265-016-0672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brigo F, Lattanzi S, Rohracher A, Russo E, Meletti S, Grillo E, et al. Perampanel in the treatment of status epilepticus: a systematic review of the literature. Epilepsy Behav. 2018;86:179–186. doi: 10.1016/j.yebeh.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Cervenka MC, Hartman AL, Venkatesan A, Geocadin RG, Kossoff EH. The ketogenic diet for medically and surgically refractory status epilepticus in the neurocritical care unit. Neurocrit Care. 2011;15(3):519–524. doi: 10.1007/s12028-011-9546-3. [DOI] [PubMed] [Google Scholar]
- 32.Farrokh S, Bon J, Erdman M, Tesoro E. Use of newer anticonvulsants for the treatment of status epilepticus. Pharmacotherapy. 2019;39(3):297–316. doi: 10.1002/phar.2229. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez EM, Franck AJ. Lacosamide for the treatment of refractory status epilepticus. Ann Pharmacother. 2011;45(11):1445–1449. doi: 10.1345/aph.1Q461. [DOI] [PubMed] [Google Scholar]
- 34.Strzelczyk A, Klein KM, Willems LM, Rosenow F, Bauer S. Brivaracetam in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus. Expert Rev Clin Pharmacol. 2016;9(5):637–645. doi: 10.1586/17512433.2016.1156529. [DOI] [PubMed] [Google Scholar]
- 35.Strzelczyk A, Willems LM, Willig S, Rosenow F, Bauer S. Perampanel in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus. Expert Rev Clin Pharmacol. 2015;8(6):733–740. doi: 10.1586/17512433.2015.1091303. [DOI] [PubMed] [Google Scholar]
- 36.Strzelczyk A, Zöllner JP, Willems LM, Jost J, Paule E, Schubert-Bast S, et al. Lacosamide in status epilepticus: systematic review of current evidence. Epilepsia. 2017;58(6):933–950. doi: 10.1111/epi.13716. [DOI] [PubMed] [Google Scholar]
- 37.Willems LM, Bauer S, Rosenow F, Strzelczyk A. Recent advances in the pharmacotherapy of epilepsy: brivaracetam and perampanel as broad-spectrum antiseizure drugs for the treatment of epilepsies and status epilepticus. Expert Opin Pharmacother. 2019;20(14):1755–1765. doi: 10.1080/14656566.2019.1637420. [DOI] [PubMed] [Google Scholar]
- 38.Williams TJ, Cervenka MC. The role for ketogenic diets in epilepsy and status epilepticus in adults. Clin Neurophysiol Pract. 2017;2:154–160. doi: 10.1016/j.cnp.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeiler FA, Matuszczak M, Teitelbaum J, Kazina CJ, Gillman LM. Intravenous immunoglobulins for refractory status epilepticus, part I: a scoping systematic review of the adult literature. Seizure. 2017;45:172–180. doi: 10.1016/j.seizure.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Zeiler FA, Matuszczak M, Teitelbaum J, Kazina CJ, Gillman LM. Plasmapheresis for refractory status epilepticus. Part II: a scoping systematic review of the pediatric literature. Seizure. 2016;43:61–68. doi: 10.1016/j.seizure.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Zeiler FA, Matuszczak M, Teitelbaum J, Kazina CJ, Gillman LM. Plasmapheresis for refractory status epilepticus, part I: a scoping systematic review of the adult literature. Seizure. 2016;43:14–22. doi: 10.1016/j.seizure.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Oxford Centre for Evidence-based Medicine . Levels of Evidence (March 2009) Oxford: Centre for Evidence-Based Medicine; 2009. [Google Scholar]
- 43.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 45.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salins N, Gursahani R, Mathur R, Iyer S, Macaden S, Simha N, et al. Definition of terms used in limitation of treatment and providing palliative care at the end of life: the Indian Council of Medical Research Commission Report. Indian J Crit Care Med. 2018;22(4):249–262. doi: 10.4103/ijccm.IJCCM_165_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willems LM, Bertsche A, Bosebeck F, Hornemann F, Immisch I, Klein KM, et al. Efficacy, retention, and tolerability of brivaracetam in patients with epileptic encephalopathies: a multicenter cohort study from Germany. Front Neurol. 2018;9:569. doi: 10.3389/fneur.2018.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schubert-Bast S, Willems LM, Kurlemann G, Knake S, Müller-Schlüter K, Rosenow F, Strzelczyk A. Postmarketing experience with brivaracetam in the treatment of focal epilepsy in children and adolescents. Epilepsy Behav. 2018;89:89–93. doi: 10.1016/j.yebeh.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Strzelczyk A, Steinig I, Willems LM, Reif PS, Senft C, Voss M, et al. Treatment of refractory and super-refractory status epilepticus with brivaracetam: a cohort study from two German university hospitals. Epilepsy Behav. 2017;70:177–181. doi: 10.1016/j.yebeh.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Santamarina E, Parejo Carbonell B, Sala J, Gutierrez-Viedma A, Miro J, Asensio M, et al. Use of intravenous brivaracetam in status epilepticus: a multicenter registry. Epilepsia. 2019;60(8):1593–1601. doi: 10.1111/epi.16094. [DOI] [PubMed] [Google Scholar]
- 51.Kalss G, Rohracher A, Leitinger M, Pilz G, Novak HF, Neuray C, et al. Intravenous brivaracetam in status epilepticus: a retrospective single-center study. Epilepsia. 2018;59(Suppl 2):228–233. doi: 10.1111/epi.14486. [DOI] [PubMed] [Google Scholar]
- 52.Strzelczyk A, Kay L, Bauer S, Immisch I, Klein KM, Knake S, et al. Use of brivaracetam in genetic generalized epilepsies and for acute, intravenous treatment of absence status epilepticus. Epilepsia. 2018;59(8):1549–1556. doi: 10.1111/epi.14476. [DOI] [PubMed] [Google Scholar]
- 53.Aicua-Rapun I, Andre P, Rossetti AO, Decosterd LA, Buclin T, Novy J. Intravenous brivaracetam in status epilepticus: correlation between loading dose, plasma levels and clinical response. Epilepsy Res. 2019;149:88–91. doi: 10.1016/j.eplepsyres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Willems LM, Zollner JP, Paule E, Schubert-Bast S, Rosenow F, Strzelczyk A. Eslicarbazepine acetate in epilepsies with focal and secondary generalised seizures: systematic review of current evidence. Expert Rev Clin Pharmacol. 2018;11(3):309–324. doi: 10.1080/17512433.2018.1421066. [DOI] [PubMed] [Google Scholar]
- 55.Kellinghaus C, Berning S, Immisch I, Larch J, Rosenow F, Rossetti AO, et al. Intravenous lacosamide for treatment of status epilepticus. Acta Neurol Scand. 2011;123(2):137–141. doi: 10.1111/j.1600-0404.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 56.Albers JM, Moddel G, Dittrich R, Steidl C, Suntrup S, Ringelstein EB, et al. Intravenous lacosamide—an effective add-on treatment of refractory status epilepticus. Seizure. 2011;20(5):428–430. doi: 10.1016/j.seizure.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Goodwin H, Hinson HE, Shermock KM, Karanjia N, Lewin JJ., 3rd The use of lacosamide in refractory status epilepticus. Neurocrit Care. 2011;14(3):348–353. doi: 10.1007/s12028-010-9501-8. [DOI] [PubMed] [Google Scholar]
- 58.Höfler J, Unterberger I, Dobesberger J, Kuchukhidze G, Walser G, Trinka E. Intravenous lacosamide in status epilepticus and seizure clusters. Epilepsia. 2011;52(10):e148–e152. doi: 10.1111/j.1528-1167.2011.03204.x. [DOI] [PubMed] [Google Scholar]
- 59.Koubeissi MZ, Mayor CL, Estephan B, Rashid S, Azar NJ. Efficacy and safety of intravenous lacosamide in refractory nonconvulsive status epilepticus. Acta Neurol Scand. 2011;123(2):142–146. doi: 10.1111/j.1600-0404.2010.01430.x. [DOI] [PubMed] [Google Scholar]
- 60.Rantsch K, Walter U, Wittstock M, Benecke R, Rosche J. Efficacy of intravenous lacosamide in refractory nonconvulsive status epilepticus and simple partial status epilepticus. Seizure. 2011;20(7):529–532. doi: 10.1016/j.seizure.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Jain V, Harvey AS. Treatment of refractory tonic status epilepticus with intravenous lacosamide. Epilepsia. 2012;53(4):761–762. doi: 10.1111/j.1528-1167.2012.03419.x. [DOI] [PubMed] [Google Scholar]
- 62.Cherry S, Judd L, Muniz JC, Elzawahry H, LaRoche S. Safety and efficacy of lacosamide in the intensive care unit. Neurocrit Care. 2012;16(2):294–298. doi: 10.1007/s12028-011-9662-0. [DOI] [PubMed] [Google Scholar]
- 63.Mnatsakanyan L, Chung JM, Tsimerinov EI, Eliashiv DS. Intravenous lacosamide in refractory nonconvulsive status epilepticus. Seizure. 2012;21(3):198–201. doi: 10.1016/j.seizure.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Belcastro V, Vidale S, Pierguidi L, Sironi L, Tancredi L, Striano P, et al. Intravenous lacosamide as treatment option in post-stroke non convulsive status epilepticus in the elderly: a proof-of-concept, observational study. Seizure. 2013;22(10):905–907. doi: 10.1016/j.seizure.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 65.Miró J, Toledo M, Santamarina E, Ricciardi AC, Villanueva V, Pato A, et al. Efficacy of intravenous lacosamide as an add-on treatment in refractory status epilepticus: a multicentric prospective study. Seizure. 2013;22(1):77–79. doi: 10.1016/j.seizure.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Santamarina E, Toledo M, Sueiras M, Raspall M, Ailouti N, Lainez E, et al. Usefulness of intravenous lacosamide in status epilepticus. J Neurol. 2013;260(12):3122–3128. doi: 10.1007/s00415-013-7133-6. [DOI] [PubMed] [Google Scholar]
- 67.Sutter R, Marsch S, Rüegg S. Safety and efficacy of intravenous lacosamide for adjunctive treatment of refractory status epilepticus: a comparative cohort study. CNS Drugs. 2013;27(4):321–329. doi: 10.1007/s40263-013-0049-y. [DOI] [PubMed] [Google Scholar]
- 68.Legros B, Depondt C, Levy-Nogueira M, Ligot N, Mavroudakis N, Naeije G, et al. Intravenous lacosamide in refractory seizure clusters and status epilepticus: comparison of 200 and 400 mg loading doses. Neurocrit Care. 2014;20(3):484–488. doi: 10.1007/s12028-013-9882-6. [DOI] [PubMed] [Google Scholar]
- 69.Kellinghaus C, Berning S, Stogbauer F. Intravenous lacosamide or phenytoin for treatment of refractory status epilepticus. Acta Neurol Scand. 2014;129(5):294–299. doi: 10.1111/ane.12174. [DOI] [PubMed] [Google Scholar]
- 70.Garcés M, Villanueva V, Mauri JA, Suller A, Garcia C, Lopez Gonzalez FJ, et al. Factors influencing response to intravenous lacosamide in emergency situations: LACO-IV study. Epilepsy Behav. 2014;36:144–152. doi: 10.1016/j.yebeh.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 71.Grosso S, Zamponi N, Bartocci A, Cesaroni E, Cappanera S, Di Bartolo R, et al. Lacosamide in children with refractory status epilepticus. A multicenter Italian experience. Eur J Paediatr Neurol. 2014;18(5):604–608. doi: 10.1016/j.ejpn.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Poddar K, Sharma R, Ng YT. Intravenous lacosamide in pediatric status epilepticus: an open-label efficacy and safety study. Pediatr Neurol. 2016;61:83–86. doi: 10.1016/j.pediatrneurol.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 73.Moreno Morales EY, Fernandez Peleteiro M, Bondy Pena EC, Dominguez Lorenzo JM, Pardellas Santiago E, Fernandez A. Observational study of intravenous lacosamide in patients with convulsive versus non-convulsive status epilepticus. Clin Drug Investig. 2015;35(7):463–469. doi: 10.1007/s40261-015-0295-5. [DOI] [PubMed] [Google Scholar]
- 74.d'Orsi G, Pascarella MG, Martino T, Specchio LM. Lacosamide in absence status epilepticus: effective or ineffective? Seizure. 2015;25:32. doi: 10.1016/j.seizure.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Lang N, Lange M, Schmitt FC, Bos M, Weber Y, Evers S, et al. Intravenous lacosamide in clinical practice—results from an independent registry. Seizure. 2016;39:5–9. doi: 10.1016/j.seizure.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Santamarina E, Gonzalez-Cuevas M, Toledo M, Jimenez M, Becerra JL, Quilez A, et al. Intravenous lacosamide (LCM) in status epilepticus (SE): weight-adjusted dose and efficacy. Epilepsy Behav. 2018;84:93–98. doi: 10.1016/j.yebeh.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 77.Reif PS, Manner A, Willems LM, Kay L, Zöllner JP, Klein KM, et al. Intravenous lacosamide for treatment of absence status epilepticus in genetic generalized epilepsy: a case report and review of literature. Acta Neurol Scand. 2018;138(3):259–262. doi: 10.1111/ane.12935. [DOI] [PubMed] [Google Scholar]
- 78.Ngampoopun M, Suwanpakdee P, Jaisupa N, Nabangchang C. Effectiveness and adverse effect of intravenous lacosamide in nonconvulsive status epilepticus and acute repetitive seizures in children. Neurol Res Int. 2018;2018:8432859. doi: 10.1155/2018/8432859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perrenoud M, Andre P, Alvarez V, Stahli C, Decosterd LA, Rossetti AO, et al. Intravenous lacosamide in status epilepticus: correlation between loading dose, serum levels, and clinical response. Epilepsy Res. 2017;135:38–42. doi: 10.1016/j.eplepsyres.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Newey CR, Le NM, Ahrens C, Sahota P, Hantus S. The safety and effectiveness of intravenous lacosamide for refractory status epilepticus in the critically ill. Neurocrit Care. 2017;26(2):273–279. doi: 10.1007/s12028-016-0322-2. [DOI] [PubMed] [Google Scholar]
- 81.Toledo M, Molins A, Quintana M, Santamarina E, Martinez-Ricarte F, Martinez-Saez E, et al. Outcome of cancer-related seizures in patients treated with lacosamide. Acta Neurol Scand. 2018;137(1):67–75. doi: 10.1111/ane.12809. [DOI] [PubMed] [Google Scholar]
- 82.Yasam VR, Jakki SL, Senthil V, Eswaramoorthy M, Shanmuganathan S, Arjunan K, et al. A pharmacological overview of lamotrigine for the treatment of epilepsy. Expert Rev Clin Pharmacol. 2016;9(12):1533–1546. doi: 10.1080/17512433.2016.1254041. [DOI] [PubMed] [Google Scholar]
- 83.Pisani F, Gallitto G, Di Perri R. Could lamotrigine be useful in status epilepticus? A case report. J Neurol Neurosurg Psychiatry. 1991;54(9):845–846. doi: 10.1136/jnnp.54.9.845-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bialer M, Soares-Da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia. 2012;53(6):935–946. doi: 10.1111/j.1528-1167.2012.03519.x. [DOI] [PubMed] [Google Scholar]
- 85.Kellinghaus C, Berning S, Stogbauer F. Use of oxcarbazepine for treatment of refractory status epilepticus. Seizure. 2014;23(2):151–154. doi: 10.1016/j.seizure.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5(3):246–256. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 87.Strzelczyk A, Knake S, Kälviäinen R, Santamarina E, Toledo M, Willig S, et al. Perampanel for treatment of status epilepticus in Austria, Finland, Germany, and Spain. Acta Neurol Scand. 2019;139(4):369–376. doi: 10.1111/ane.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Redecker J, Wittstock M, Benecke R, Rösche J. Efficacy of perampanel in refractory nonconvulsive status epilepticus and simple partial status epilepticus. Epilepsy Behav. 2015;45:176–179. doi: 10.1016/j.yebeh.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 89.Rohracher A, Höfler J, Kalss G, Leitinger M, Kuchukhidze G, Deak I, et al. Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav. 2015;49:354–358. doi: 10.1016/j.yebeh.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 90.Beretta S, Padovano G, Stabile A, Coppo A, Bogliun G, Avalli L, et al. Efficacy and safety of perampanel oral loading in postanoxic super-refractory status epilepticus: a pilot study. Epilepsia. 2018;59(Suppl 2):243–248. doi: 10.1111/epi.14492. [DOI] [PubMed] [Google Scholar]
- 91.Rohracher A, Kalss G, Neuray C, Hofler J, Dobesberger J, Kuchukhidze G, et al. Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit: a single-center audit of 30 patients. Epilepsia. 2018;59(Suppl 2):234–242. doi: 10.1111/epi.14494. [DOI] [PubMed] [Google Scholar]
- 92.Newey CR, Mullaguri N, Hantus S, Punia V, George P. Super-refractory status epilepticus treated with high dose perampanel: case series and review of the literature. Case Rep Crit Care. 2019;2019:3218231. doi: 10.1155/2019/3218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho CJ, Lin CH, Lu YT, Shih FY, Hsu CW, Tsai WC, et al. Perampanel treatment for refractory status epilepticus in a neurological intensive care unit. Neurocrit Care. 2019;31(1):24–29. doi: 10.1007/s12028-019-00704-9. [DOI] [PubMed] [Google Scholar]
- 94.Rahbani A, Adwane G, Jomaa N. Oral perampanel for the treatment of super-refractory status epilepticus. Case Rep Neurol Med. 2019;2019:8537815. doi: 10.1155/2019/8537815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santamarina E, Alpuente A, Maisterra O, Sueiras M, Sarria S, Guzman L, et al. Perampanel: a therapeutic alternative in refractory status epilepticus associated with MELAS syndrome. Epilepsy Behav Case Rep. 2019;11:92–95. doi: 10.1016/j.ebcr.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strzelczyk A, Kortland LM, Knake S, Rosenow F. Stiripentol for the treatment of super-refractory status epilepticus. Acta Neurol Scand. 2015;132(6):435–439. doi: 10.1111/ane.12403. [DOI] [PubMed] [Google Scholar]
- 97.Fisher JL. The anti-convulsant stiripentol acts directly on the GABA(A) receptor as a positive allosteric modulator. Neuropharmacology. 2009;56(1):190–197. doi: 10.1016/j.neuropharm.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uchida Y, Kato D, Toyoda T, Oomura M, Ueki Y, Ohkita K, et al. Combination of ketogenic diet and stiripentol for super-refractory status epilepticus: a case report. J Neurol Sci. 2017;15(373):35–37. doi: 10.1016/j.jns.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 99.Uchida Y, Terada K, Madokoro Y, Fujioka T, Mizuno M, Toyoda T, et al. Stiripentol for the treatment of super-refractory status epilepticus with cross-sensitivity. Acta Neurol Scand. 2018;137(4):432–437. doi: 10.1111/ane.12888. [DOI] [PubMed] [Google Scholar]
- 100.Kahriman M, Minecan D, Kutluay E, Selwa L, Beydoun A. Efficacy of topiramate in children with refractory status epilepticus. Epilepsia. 2003;44(10):1353–1356. doi: 10.1046/j.1528-1157.2003.11803.x. [DOI] [PubMed] [Google Scholar]
- 101.Bensalem MK, Fakhoury TA. Topiramate and status epilepticus: report of three cases. Epilepsy Behav. 2003;4(6):757–760. doi: 10.1016/j.yebeh.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 102.Blumkin L, Lerman-Sagie T, Houri T, Gilad E, Nissenkorn A, Ginsberg M, et al. Pediatric refractory partial status epilepticus responsive to topiramate. J Child Neurol. 2005;20(3):239–241. doi: 10.1177/08830738050200031701. [DOI] [PubMed] [Google Scholar]
- 103.Perry MS, Holt PJ, Sladky JT. Topiramate loading for refractory status epilepticus in children. Epilepsia. 2006;47(6):1070–1071. doi: 10.1111/j.1528-1167.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- 104.Soler B, Godoy J, Mellado TP. Treatment of refractory status epilepticus with topiramate. Report of three cases. Rev Med Chil. 2009;137(7):936–939. [PubMed] [Google Scholar]
- 105.Akyildiz BN, Kumandas S. Treatment of pediatric refractory status epilepticus with topiramate. Childs Nerv Syst. 2011;27(9):1425–1430. doi: 10.1007/s00381-011-1432-y. [DOI] [PubMed] [Google Scholar]
- 106.Kim W, Kwon SY, Cho AH, Lim SC, Kim YI, Shon YM. Effectiveness of topiramate in medically complicated patients with status epilepticus or acute refractory seizures. J Epilepsy Res. 2011;1(2):52–56. doi: 10.14581/jer.11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Synowiec AS, Yandora KA, Yenugadhati V, Valeriano JP, Schramke CJ, Kelly KM. The efficacy of topiramate in adult refractory status epilepticus: experience of a tertiary care center. Epilepsy Res. 2012;98(2–3):232–237. doi: 10.1016/j.eplepsyres.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 108.Bragatti JA, Torres CM, Netto CB, Vedolin L, Garzon E, Rieder CR, et al. Topiramate is effective for status epilepticus and seizure control in neuraminidase deficiency. Arq Neuropsiquiatr. 2011;69(3):565–566. doi: 10.1590/s0004-282x2011000400031. [DOI] [PubMed] [Google Scholar]
- 109.Hottinger A, Sutter R, Marsch S, Ruegg S. Topiramate as an adjunctive treatment in patients with refractory status epilepticus: an observational cohort study. CNS Drugs. 2012;26(9):761–772. doi: 10.2165/11633090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 110.Stojanova V, Rossetti AO. Oral topiramate as an add-on treatment for refractory status epilepticus. Acta Neurol Scand. 2012;125(2):e7–e11. doi: 10.1111/j.1600-0404.2011.01562.x. [DOI] [PubMed] [Google Scholar]
- 111.Shelton CM, Alford EL, Storgion S, Wheless J, Phelps SJ. Enteral topiramate in a pediatric patient with refractory status epilepticus: a case report and review of the literature. J Pediatr Pharmacol Ther. 2014;19(4):317–324. doi: 10.5863/1551-6776-19.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Asadi-Pooya AA, Jahromi MJ, Izadi S, Emami Y. Treatment of refractory generalized convulsive status epilepticus with enteral topiramate in resource limited settings. Seizure. 2015;24:114–117. doi: 10.1016/j.seizure.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 113.Madzar D, Kuramatsu JB, Gerner ST, Huttner HB. Assessing the value of topiramate in refractory status epilepticus. Seizure. 2016;38:7–10. doi: 10.1016/j.seizure.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Fechner A, Hubert K, Jahnke K, Knake S, Konczalla J, Menzler K, et al. Treatment of refractory and superrefractory status epilepticus with topiramate: a cohort study of 106 patients and a review of the literature. Epilepsia. 2019;60(12):2448–2458. doi: 10.1111/epi.16382. [DOI] [PubMed] [Google Scholar]
- 115.Mirza N, Marson AG, Pirmohamed M. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009;68(5):655–661. doi: 10.1111/j.1365-2125.2009.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hubert K, Knake S, Bauer S, Voss M, Rosenow F, Strzelczyk A. Treatment of status epilepticus with zonisamide: a multicenter cohort study of 34 patients and review of literature. Epilepsy Behav. 2020;14(109):107139. doi: 10.1016/j.yebeh.2020.107139. [DOI] [PubMed] [Google Scholar]
- 117.Baxter P, Clarke A, Cross H, Harding B, Hicks E, Livingston J, et al. Idiopathic catastrophic epileptic encephalopathy presenting with acute onset intractable status. Seizure. 2003;12(6):379–387. doi: 10.1016/s1059-1311(02)00340-0. [DOI] [PubMed] [Google Scholar]
- 118.Berkeley J, Foreman P, Foroughi A, Tirol F. Successful treatment of febrile illness related epilepsy syndrom (FIRES) and new onset refractory status epilepticus (NORSE) with plasma exchange. Epilepsy Curr. 2015;15:275. [Google Scholar]
- 119.Broomall E, Natale JE, Grimason M, Goldstein J, Smith CM, Chang C, et al. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann Neurol. 2014;76(6):911–915. doi: 10.1002/ana.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dionisio S, Brown H, Lander C, Airey C, Lehn A, Nooruddin H, et al. Immunoglobulin-responsive refractory epilepsy—3 cases with a similar EEG pattern. Seizure. 2013;22(5):403–408. doi: 10.1016/j.seizure.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 121.Fisher RS, Kaplan PW, Krumholz A, Lesser RP, Rosen SA, Wolff MR. Failure of high-dose intravenous magnesium sulfate to control myoclonic status epilepticus. Clin Neuropharmacol. 1988;11(6):537–544. doi: 10.1097/00002826-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 122.Gedik AH, Demirkol D, Tatli B, Bayraktar S, Alkan A, Karabocuoglu M, et al. Therapeutic plasma exchange for malignant refractory status epilepticus: a case report. Pediatr Neurol. 2014;50(4):407–410. doi: 10.1016/j.pediatrneurol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Nandakumar A, Andrezejowski J, Turnbull D. Case report: new onset drug resistant status epilepticus (NODRSE) J Neurosurg Anesthesiol. 2008;20:218. [Google Scholar]
- 124.Neligan A, Oomeer S, Ziso B, Chrisofi G, Turner B. A case of prolonged status epilepticus with a good outcome: the importance of etiology in determining prognosis. Epilepsia. 2011;52:83. [Google Scholar]
- 125.Pandey M, Gupta A, Baduni N, Vijfdar H, Sinha S, Jain A. Refractory status epilepticus–magnesium as rescue therapy. Anaesth Intensive Care. 2010;38(5):962. [PubMed] [Google Scholar]
- 126.Robakis TK, Hirsch LJ. Literature review, case report, and expert discussion of prolonged refractory status epilepticus. Neurocrit Care. 2006;4(1):35–46. doi: 10.1385/NCC:4:1:035. [DOI] [PubMed] [Google Scholar]
- 127.Sadeh M, Blatt I, Martonovits G, Karni A, Goldhammer Y. Treatment of porphyric convulsions with magnesium sulfate. Epilepsia. 1991;32(5):712–715. doi: 10.1111/j.1528-1157.1991.tb04714.x. [DOI] [PubMed] [Google Scholar]
- 128.Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42(11):1461–1467. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- 129.Savard M, Dupre N, Turgeon AF, Desbiens R, Langevin S, Brunet D. POLG mitochondrial disorder heralded by propofol infusion syndrome: a case report. Neurol Sci. 2012;39:24. doi: 10.1212/WNL.0b013e3182a1aa78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shin RK, Rosenbaum AV, Frost N. A case of magnesium-responsive paraneoplastic non-convulsive status epilepticus. Ann Neurol. 2011;70:35–36. [Google Scholar]
- 131.Storcheim F. Status epilepticus treated by magnesium sulfate injected intravenously. JAMA. 1933;101:1313–1314. [Google Scholar]
- 132.Strzelczyk A, Reif PS, Bauer S, Belke M, Oertel WH, Knake S, et al. Intravenous initiation and maintenance of ketogenic diet: proof of concept in super-refractory status epilepticus. Seizure. 2013;22(7):581–583. doi: 10.1016/j.seizure.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 133.Tan WW, Chan DWS, Lee JH, Thomas T, Menon AP, Chan YH. Use of magnesium sulfate infusion for the management of febrile illness-related epilepsy syndrome: a case series. Child Neurol Open. 2015;2(1):2329048X14550067. doi: 10.1177/2329048X14550067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Valle-Morales L, Cortes-Cros E, Santana A, Barber M, Figueras T, Garcia-Hernandez JA. Epileptic status refractory to conventional treatment caused by vitamin B6 deficiency. J Perinatol. 2009;29(3):252–253. doi: 10.1038/jp.2008.180. [DOI] [PubMed] [Google Scholar]
- 135.Visser NA, Braun KP, Leijten FS, van Nieuwenhuizen O, Wokke JH, van den Bergh WM. Magnesium treatment for patients with refractory status epilepticus due to POLG1-mutations. J Neurol. 2011;258(2):218–222. doi: 10.1007/s00415-010-5721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Madisia N, Bergkeley JL. Successful treatment of prolonged refravtory status epilepticus with plasma exchange and rituximab. Neurocrit Care. 2014;21:280. [Google Scholar]
- 137.Zaatreh MM. Levetiracetam in porphyric status epilepticus: a case report. Clin Neuropharmacol. 2005;28(5):243–244. doi: 10.1097/01.wnf.0000185828.80561.ad. [DOI] [PubMed] [Google Scholar]
- 138.Hatch DM, Atito-Narh E, Herschmiller EJ, Olufolabi AJ, Owen MD. Refractory status epilepticus after inadvertent intrathecal injection of tranexamic acid treated by magnesium sulfate. Int J Obstet Anesth. 2016;26:71–75. doi: 10.1016/j.ijoa.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 139.Sahin M, Riviello JJ., Jr Prolonged treatment of refractory status epilepticus in a child. J Child Neurol. 2001;16(2):147–150. doi: 10.1177/088307380101600218. [DOI] [PubMed] [Google Scholar]
- 140.Bodenant M, Moreau C, Sejourne C, Auvin S, Delval A, Cuisset JM, et al. Interest of the ketogenic diet in a refractory status epilepticus in adults [in French] Rev Neurol (Paris) 2008;164(2):194–199. doi: 10.1016/j.neurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 141.Wusthoff CJ, Kranick SM, Morley JF, Christina Bergqvist AG. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. 2010;51(6):1083–1085. doi: 10.1111/j.1528-1167.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 142.Nabbout R, Mazzuca M, Hubert P, Peudennier S, Allaire C, Flurin V, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51(10):2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 143.Kramer U, Chi CS, Lin KL, Specchio N, Sahin M, Olson H, et al. Febrile infection-related epilepsy syndrome (FIRES): does duration of anesthesia affect outcome? Epilepsia. 2011;52(Suppl 8):28–30. doi: 10.1111/j.1528-1167.2011.03230.x. [DOI] [PubMed] [Google Scholar]
- 144.Nam SH, Lee BL, Lee CG, Yu HJ, Joo EY, Lee J, et al. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. 2011;52(11):e181–e184. doi: 10.1111/j.1528-1167.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 145.Martikainen MH, Paivarinta M, Jaaskelainen S, Majamaa K. Successful treatment of POLG-related mitochondrial epilepsy with antiepileptic drugs and low glycaemic index diet. Epileptic Disord. 2012;14(4):438–441. doi: 10.1684/epd.2012.0543. [DOI] [PubMed] [Google Scholar]
- 146.Vaccarezza M, Silva W, Maxit C, Agosta G. Super-refractory status epilepticus: treatment with ketogenic diet in pediatrics. Rev Neurol. 2012;55(1):20–25. [PubMed] [Google Scholar]
- 147.Sort R, Born AP, Pedersen KN, Fonsmark L, Uldall P. Ketogenic diet in 3 cases of childhood refractory status epilepticus. Eur J Paediatr Neurol. 2013;17(6):531–536. doi: 10.1016/j.ejpn.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 148.Caraballo RH, Flesler S, Armeno M, Fortini S, Agustinho A, Mestre G, et al. Ketogenic diet in pediatric patients with refractory focal status epilepticus. Epilepsy Res. 2014;108(10):1912–1916. doi: 10.1016/j.eplepsyres.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 149.Cobo NH, Sankar R, Murata KK, Sewak SL, Kezele MA, Matsumoto JH. The ketogenic diet as broad-spectrum treatment for super-refractory pediatric status epilepticus: challenges in implementation in the pediatric and neonatal intensive care units. J Child Neurol. 2015;30(2):259–266. doi: 10.1177/0883073813516192. [DOI] [PubMed] [Google Scholar]
- 150.Thakur KT, Probasco JC, Hocker SE, Roehl K, Henry B, Kossoff EH, et al. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. 2014;82(8):665–670. doi: 10.1212/WNL.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Amer S, Shah P, Kommineni V. Refractory status epilepticus from NMDA receptor encephalitis successfully treated with an adjunctive ketogenic diet. Ann Indian Acad Neurol. 2015;18(2):256–257. doi: 10.4103/0972-2327.150620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Caraballo RH, Valenzuela GR, Armeno M, Fortini S, Mestre G, Cresta A. The ketogenic diet in two paediatric patients with refractory myoclonic status epilepticus. Epileptic Disord. 2015;17(4):491–495. doi: 10.1684/epd.2015.0781. [DOI] [PubMed] [Google Scholar]
- 153.Cash C. Use of the ketogenic diet in an adult with myoclonic status epilepticus. Clin Nutr ESPEN. 2015;10(5):e199–e200. doi: 10.1016/j.clnesp.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 154.Fung EL, Chang SK, Yam KK, Yau PY. Ketogenic diet as a therapeutic option in super-refractory status epilepticus. Pediatr Neonatol. 2015;56(6):429–431. doi: 10.1016/j.pedneo.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 155.Lin JJ, Lin KL, Chan OW, Hsia SH, Wang HS, Group CS Intravenous ketogenic diet therapy for treatment of the acute stage of super-refractory status epilepticus in a pediatric patient. Pediatr Neurol. 2015;52(4):442–445. doi: 10.1016/j.pediatrneurol.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 156.Caraballo R, Darra F, Reyes G, Armeno M, Cresta A, Mestre G, et al. The ketogenic diet in patients with myoclonic status in non-progressive encephalopathy. Seizure. 2017;51:1–5. doi: 10.1016/j.seizure.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 157.Cervenka MC, Hocker S, Koenig M, Bar B, Henry-Barron B, Kossoff EH, et al. Phase I/II multicenter ketogenic diet study for adult superrefractory status epilepticus. Neurology. 2017;88(10):938–943. doi: 10.1212/WNL.0000000000003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smith G, Press CA. Ketogenic diet in super-refractory status epilepticus. Pediatr Neurol Briefs. 2017;31(3):8. doi: 10.15844/pedneurbriefs-31-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Arya R, Peariso K, Gainza-Lein M, Harvey J, Bergin A, Brenton JN, et al. Efficacy and safety of ketogenic diet for treatment of pediatric convulsive refractory status epilepticus. Epilepsy Res. 2018;144:1–6. doi: 10.1016/j.eplepsyres.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 160.Blunck JR, Newman JW, Fields RK, Croom JE. Therapeutic augmentation of ketogenic diet with a sodium-glucose cotransporter 2 inhibitor in a super-refractory status epilepticus patient. Epilepsy Behav Case Rep. 2018;10:61–64. doi: 10.1016/j.ebcr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Francis BA, Fillenworth J, Gorelick P, Karanec K, Tanner A. The feasibility, safety and effectiveness of a ketogenic diet for refractory status epilepticus in adults in the intensive care unit. Neurocrit Care. 2019;30(3):652–657. doi: 10.1007/s12028-018-0653-2. [DOI] [PubMed] [Google Scholar]
- 162.Park EG, Lee J, Lee J. The ketogenic diet for super-refractory status epilepticus patients in intensive care units. Brain Dev. 2019;41(5):420–427. doi: 10.1016/j.braindev.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 163.Peng P, Peng J, Yin F, Deng X, Chen C, He F, et al. Ketogenic diet as a treatment for super-refractory status epilepticus in febrile infection-related epilepsy syndrome. Front Neurol. 2019;10:423. doi: 10.3389/fneur.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Prasoppokakorn T, Jirasakuldej S, Lakananurak N. Medium-chain triglyceride ketogenic diet is effective for treatment of an adult with super-refractory status epilepticus: a case report and literature review. Eur J Clin Nutr. 2019;73(12):1594–1597. doi: 10.1038/s41430-019-0471-4. [DOI] [PubMed] [Google Scholar]
- 165.Noviawaty I, Olaru E, Rondello C, Fitzsimmons B, Raghavan M. Clinical reasoning: ketogenic diet in adult super-refractory status epilepticus. Neurology. 2020;94(12):541–546. doi: 10.1212/WNL.0000000000009137. [DOI] [PubMed] [Google Scholar]
- 166.Rosenthal ES, Claassen J, Wainwright MS, Husain AM, Vaitkevicius H, Raines S, et al. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann Neurol. 2017;82(3):342–352. doi: 10.1002/ana.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vaitkevicius H, Husain AM, Rosenthal ES, Rosand J, Bobb W, Reddy K, et al. First-in-man allopregnanolone use in super-refractory status epilepticus. Ann Clin Transl Neurol. 2017;4(6):411–414. doi: 10.1002/acn3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.SageTherapeutics. A study with SAGE-547 for super-refractory status epilepticus. ClinicalTrails.gov; 2019. https://clinicaltrials.gov/ct2/show/NCT02477618.
- 169.Ramos AB, Cruz RA, Villemarette-Pittman NR, Olejniczak PW, Mader EC., Jr Dexamethasone as abortive treatment for refractory seizures or status epilepticus in the inpatient setting. J Investig Med High Impact Case Rep. 2019;7:2324709619848816. doi: 10.1177/2324709619848816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Trinka E, Hofler J, Leitinger M, Brigo F. Pharmacotherapy for status epilepticus. Drugs. 2015;75(13):1499–1521. doi: 10.1007/s40265-015-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zolkowska D, Wu CY, Rogawski MA. Intramuscular allopregnanolone and ganaxolone in a mouse model of treatment-resistant status epilepticus. Epilepsia. 2018;59(Suppl 2):220–227. doi: 10.1111/epi.13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rosati A, Ilvento L, Lucenteforte E, Pugi A, Crescioli G, McGreevy KS, et al. Comparative efficacy of antiepileptic drugs in children and adolescents: a network meta-analysis. Epilepsia. 2018;59(2):297–314. doi: 10.1111/epi.13981. [DOI] [PubMed] [Google Scholar]
- 173.Rosati A, De Masi S, Guerrini R. Ketamine for refractory status epilepticus: a systematic review. CNS Drugs. 2018;32(11):997–1009. doi: 10.1007/s40263-018-0569-6. [DOI] [PubMed] [Google Scholar]
- 174.Dibue-Adjei M, Brigo F, Yamamoto T, Vonck K, Trinka E. Vagus nerve stimulation in refractory and super-refractory status epilepticus: a systematic review. Brain Stimul. 2019;12(5):1101–1110. doi: 10.1016/j.brs.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 175.San-Juan D, Davila-Rodriguez DO, Jimenez CR, Gonzalez MS, Carranza SM, Hernandez Mendoza JR, et al. Neuromodulation techniques for status epilepticus: a review. Brain Stimul. 2019;12(4):835–844. doi: 10.1016/j.brs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 176.Ferlazzo E, Sueri C, Gasparini S, Russo E, Cianci V, Ascoli M, et al. Methodological issues associated with clinical trials in epilepsy. Expert Rev Clin Pharmacol. 2017;10(10):1103–1108. doi: 10.1080/17512433.2017.1356720. [DOI] [PubMed] [Google Scholar]
- 177.Perucca E, Wiebe S. Not all that glitters is gold: a guide to the critical interpretation of drug trials in epilepsy. Epilepsia Open. 2016;1(1–2):9–21. doi: 10.1002/epi4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hopp JL, Sanchez A, Krumholz A, Hart G, Barry E. Nonconvulsive status epilepticus: value of a benzodiazepine trial for predicting outcomes. Neurologist. 2011;17(6):325–329. doi: 10.1097/NRL.0b013e31822f688c. [DOI] [PubMed] [Google Scholar]
- 180.Niquet J, Baldwin R, Norman K, Suchomelova L, Lumley L, Wasterlain CG. Simultaneous triple therapy for the treatment of status epilepticus. Neurobiol Dis. 2017;104:41–49. doi: 10.1016/j.nbd.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Willems LM, Richter S, Watermann N, Bauer S, Klein KM, Reese JP, et al. Trends in resource utilization and prescription of anticonvulsants for patients with active epilepsy in Germany from 2003 to 2013 — A ten-year overview. Epilepsy Behav. 2018;83:28–35. doi: 10.1016/j.yebeh.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 182.Willems LM, Hamer HM, Knake S, Rosenow F, Reese JP, Strzelczyk A. General Trends in Prices and Prescription Patterns of Anticonvulsants in Germany between 2000 and 2017: Analysis of National and Cohort-Based Data. Appl Health Econ Health Policy. 2019;17(5):707–722. doi: 10.1007/s40258-019-00487-2. [DOI] [PubMed] [Google Scholar]
- 183.Rybitschka A, Semmlack S, Kaplan PW, De Marchis GM, Ruegg S, Marsch S, et al. Calorie intake during status epilepticus and outcome: a 5-year cohort study. Crit Care Med. 2019;47(8):1106–1115. doi: 10.1097/CCM.0000000000003828. [DOI] [PubMed] [Google Scholar]
- 184.Shorvon S. Super-refractory status epilepticus: an approach to therapy in this difficult clinical situation. Epilepsia. 2011;52(Suppl 8):53–56. doi: 10.1111/j.1528-1167.2011.03238.x. [DOI] [PubMed] [Google Scholar]
- 185.Arya R, Rotenberg A. Dietary, immunological, surgical, and other emerging treatments for pediatric refractory status epilepticus. Seizure. 2019;68:89–96. doi: 10.1016/j.seizure.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 186.Bayrlee A, Ganeshalingam N, Kurczewski L, Brophy GM. Treatment of super-refractory status epilepticus. Curr Neurol Neurosci Rep. 2015;15(10):66. doi: 10.1007/s11910-015-0589-2. [DOI] [PubMed] [Google Scholar]
- 187.Mattozzi S, Sabater L, Escudero D, Arino H, Armangue T, Simabukuro M, et al. Hashimoto encephalopathy in the 21st century. Neurology. 2020;94(2):e217–e224. doi: 10.1212/WNL.0000000000008785. [DOI] [PubMed] [Google Scholar]
- 188.Kassab M, Oatel A, Shah H, Schulte A, Jani V, Burghardt T. Demographic, regional and social predictors of do not resuscitate order utilization in generalized convulsive status epilepticus (GCSE): a national perspective (P1.066). Neurology. 2016;86(16 Suppl).
- 189.Lunagariya A, Pateel A, Grayson J, Heilmann K. Ethical dilemma: a national perspective on utilization of do-not-resuscitate orders in status epilepticus (P1.355). Neurology. 2016;86(16 Suppl).
- 190.Sutter R, Meyer-Zehnder B, Baumann SM, Marsch S, Pargger H. Advance directives in the neurocritically ill: a systematic review. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004388. [DOI] [PubMed] [Google Scholar]
- 191.Sutter R, De Marchis GM, Semmlack S, Fuhr P, Rüegg S, Marsch S, et al. Anesthetics and outcome in status epilepticus: a matched two-center cohort study. CNS Drugs. 2017;31(1):65–74. doi: 10.1007/s40263-016-0389-5. [DOI] [PubMed] [Google Scholar]
- 192.Marchi NA, Novy J, Faouzi M, Stahli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med. 2015;43(5):1003–1009. doi: 10.1097/CCM.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 193.Kowalski RG, Ziai WC, Rees RN, Werner JK, Jr, Kim G, Goodwin H, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med. 2012;40(9):2677–2684. doi: 10.1097/CCM.0b013e3182591ff1. [DOI] [PubMed] [Google Scholar]
- 194.Naisbitt C, Davies S. Starvation, exercise and the stress response. Anaesth Intensive Care Med. 2017;18(10):508–512. [Google Scholar]
- 195.Riechmann J, Willems LM, Boor R, Kieslich M, Knake S, Langner C, et al. Quality of life and correlating factors in children, adolescents with epilepsy, and their caregivers: A cross-sectional multicenter study from Germany. Seizure. 2019;69:92–98. doi: 10.1016/j.seizure.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 196.Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(Pt 8):2314–2328. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 197.Kantanen AM, Sairanen J, Kalviainen R. Incidence of the different stages of status epilepticus in Eastern Finland: a population-based study. Epilepsy Behav. 2019;101(Pt B):106413. doi: 10.1016/j.yebeh.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 198.Delaj L, Novy J, Ryvlin P, Marchi NA, Rossetti AO. Refractory and super-refractory status epilepticus in adults: a 9-year cohort study. Acta Neurol Scand. 2017;135(1):92–99. doi: 10.1111/ane.12605. [DOI] [PubMed] [Google Scholar]
- 199.Agan K, Midi I, Alibas H, Gonul O. Refractory status epilepticus with possible autoimmune etiology treated with plasma exchange. J Neurol Sci. 2015;357:e142–e160. [Google Scholar]
- 200.Agirre-Arrizubieta Z, Moran N. Super-refractory status-epilepticus: there is always hope. Epilepsia. 2012;53.
- 201.Al-Ajlan FS, Althobiti A, Baz S, Al-Attas A. Autoimmune encephalopathy and drug refractory seizures with the presence of two autoantibodies specific for the neuronal cell surface. Epilepsy Behav Case Rep. 2014;2:199–202. doi: 10.1016/j.ebcr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Alam H, Kassar D, Chand P, Iyadurai S. Treatment of recurrent status epilepticus secondary to Hashimoto’s encephalitis by thyroidectomy. Neurocrit Care. 2013;19:S306. [Google Scholar]
- 203.Alam M, Deb R, Reddy K, Ratnam B, Lath R, Ranjan A. A rare case of status epilepticus—Rasmussen’s encephalitis. Ind J Crit Care Med. 2014;18(Suppl.1).
- 204.Armas S, Miro J, Veciana M, Pedro J, Crorral L, Castaner S. Long-term immunosuppressive treatment in a patient with recurrent refractory status epilepticus. Epilepsia. 2013;54:111. [Google Scholar]
- 205.Barnes B, Kaplan P, Venkatesan A, Geocadin R. Recovery of consciousness in comatose patients with acute encephalitis and super refractory status epilepticus: impact of EEG sleep intrusions. Neurocrit Care. 2013;19:S316. [Google Scholar]
- 206.Bobb W, Kolls B, Ummat M, Husain A. Allopregnanolone to treat refractory status epilepticus. J clin Neurophysiol. 2014;31:297. [Google Scholar]
- 207.Brigo F, Vogrig A, Bratti A, Tavernelli V, Nardone R, Trinka E. Probable dysimmune epilepsia partialis continua manifesting as epileptic moving toes syndrome: electroclinical features of a challenging case. Epileptic Disord. 2018;20(4):301–312. doi: 10.1684/epd.2018.0983. [DOI] [PubMed] [Google Scholar]
- 208.Buenache R, Morillo P, Mesequer M, Perez Caballaro C, Lorenzo G. Refractory epileptic encephalopathy related to mycoplasma pneumoniae infection. Epilepsia. 2012;53:544. [Google Scholar]
- 209.Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015: bcr2015210522. [DOI] [PMC free article] [PubMed]
- 210.Calabrace J, Witherspoon B. Acute psychosis as the initial presentation of a 19yo with mediastinal teratoma. Neurocrit Care. 2013;19:321. [Google Scholar]
- 211.Caputo D, Iorio R, Vigevano F, Fusco L. Febrile infection-related epilepsy syndrome (FIRES) with super-refractory status epilepticus revealing autoimmune encephalitis due to GABAAR antibodies. Eur J Paediatr Neurol. 2018;22(1):182–185. doi: 10.1016/j.ejpn.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 212.Caraballo RH, Reyes G, Avaria MF, Buompadre MC, Gonzalez M, Fortini S, et al. Febrile infection-related epilepsy syndrome: a study of 12 patients. Seizure. 2013;22(7):553–559. doi: 10.1016/j.seizure.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 213.Charles S, Hainaut E, Cante V, Valette C, Levillain P, Guillet G. Dermato-neuro syndrome during scleromyxedema: efficacy of plasmapheresis and intravenous immunoglobulin [in French]. Ann Dermatol Venereol. 2014;141(8–9):523–7. [DOI] [PubMed]
- 214.Chevret L, Husson B, Nguefack S, Nehlig A, Bouilleret V. Prolonged refractory status epilepticus with early and persistent restricted hippocampal signal MRI abnormality. J Neurol. 2008;255(1):112–116. doi: 10.1007/s00415-008-0713-1. [DOI] [PubMed] [Google Scholar]
- 215.Chevret L, Falcon E, Durand O, Essouri S, Balu L, Bouilleret V. Plasmapheresis in malignant status epilepticus. Pediatr Crit Care Med. 2011;12:648. [Google Scholar]
- 216.Costas K, Tasker RC, Soul J, Lamb N, Li L, Bergin A. Pentobarbital, propylene glycol, and ketosis in refractory status epilepticus. Epilepsia. 2015;15:546. [Google Scholar]
- 217.Dishong M, Rutledge C, Winkler M, Prabahakara P. Refractory status epilepticus in a previously healthy child: a case report. J Invest Med. 2013;61:434–435. [Google Scholar]
- 218.Gall CR, Jumma O, Mohanraj R. Five cases of new onset refractory status epilepticus (NORSE) syndrome: outcomes with early immunotherapy. Seizure. 2013;22(3):217–220. doi: 10.1016/j.seizure.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 219.Ghamande S, Akiode I, HD W, Prince W, Fiocco G. Rituximab for refractory lupus cerebritis with status epilepticus. Am J Respir Crit Care Med. 2013;187:A2988.
- 220.Gonzalez R, Chari G, Peguero N, Ramirez M. New onset refractory status epilepticus. Epilepsy Curr. 2011;11(3):1114. [Google Scholar]
- 221.Hainsworth J, Shishido A, Theeler B, Carroll C, Fasano R. Treatment responsive GABA(B)-receptor limbic encephalitis presenting as a new-onset super-refractory status epilepticus (NORSE) in a Deployed U.S. Soldier. Epileptic Disord. 2014;16(4):486–93. [DOI] [PubMed]
- 222.Hakimi R, Butchee M, Duvall J, Fields E, Hakimi A. Should a ketogenic died be considered earlier in the treatment course of adults with super-refractory status epilepticus? Neurocrit Care. 2014;21:227. [Google Scholar]
- 223.Hoang Q, Wohlt P, Rosenburg N. Treatment of super-refractory status epilepticus with perampanel in an intensive care unit. Crit Care Med. 2014;42:1250. [Google Scholar]
- 224.Howell KB, Katanyuwong K, Mackay MT, Bailey CA, Scheffer IE, Freeman JL, et al. Long-term follow-up of febrile infection-related epilepsy syndrome. Epilepsia. 2012;53(1):101–110. doi: 10.1111/j.1528-1167.2011.03350.x. [DOI] [PubMed] [Google Scholar]
- 225.Hribljan M, Tesovic G, Mise B, Roglic S. Febrile infection-related epilepsy syndrome in four Croatian boys. Epilepsia. 2013;54:58. [Google Scholar]
- 226.Incecik F, Horoz O, Herguner O, Yildizdas D, Altunbasak S. Electroconvulsive therapy for refractory status epilepticus in a child: a case report. Indian Acad Neurol. 2015;18:364–365. doi: 10.4103/0972-2327.157250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kaneko J, Iizuka T, Uchino A, Hamada J. Immunotherapy-responsive acute encephalopathy presenting as generalize convulsive status epilepticus with fever: autoimmune epileptic syndrome? Clin Exp Neuroimmun. 2012;3:54. [Google Scholar]
- 228.Katsuse K, Kurihara M, Sugiyama Y, Kodama S, Takahashi M, Momose T, et al. Aphasic status epilepticus preceding tumefactive left hemisphere lesion in anti-MOG antibody associated disease. Mult Scler Relat Disord. 2019;27:91–94. doi: 10.1016/j.msard.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 229.Khawaja A, Amara AW. Refractory status epilepticus: a report of two cases to illustrate the significance of GAD antibody. Neurology. 2014;82(10 Suppl).
- 230.Khawaja A, DeWolfe J, Miller D, Szaflarski J. New-onset refractory status epilepticus (NORSE)—the potneital role for immunotherapy. Epilepsy Behav. 2015;47:17–23. doi: 10.1016/j.yebeh.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 231.Kirkpatrick MP, Clarke CD, Sonmezturk HH, Abou-Khalil B. Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti-NMDA receptor antibody encephalitis. Epilepsy Behav. 2011;20(2):392–394. doi: 10.1016/j.yebeh.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 232.Kong SS, Chen YJ, Su IC, Lin JJ, Chou IJ, Chou ML, et al. Immunotherapy for anti-NMDA receptor encephalitis: experience from a single center in Taiwan. Pediatr Neonatol. 2019;60(4):417–422. doi: 10.1016/j.pedneo.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 233.Korff C, Fluss J, Valenza N, Deglise S, Heritier-Barras A-C, Picard F. Febrile infection responsive encephalopathy of school-age (FIRES) with unexpected favorable evolution. Epilepsia. 2009;50:84. [Google Scholar]
- 234.Kramer U, Chi CS, Lin KL, Specchio N, Sahin M, Olson H, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. 2011;52(11):1956–1965. doi: 10.1111/j.1528-1167.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- 235.Labate A, Quattrone A, Dalmau J, Gambardella A. Anti-N-methyl-d-aspartate-glutamic-receptor encephalitis presenting as paroxysmal exercise-induced foot weakness. Mov Disord. 2013;28(6):820–822. doi: 10.1002/mds.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Le Moigno L, Ternant D, Paintaud G, Thibault G, Cloarec S, Tardieu M, et al. N-methyl-d-aspartate receptor antibody encephalitis: Value of immunmodulatory therapy [in French] Arch Pediatr. 2014;21:620–623. doi: 10.1016/j.arcped.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 237.Lenoir X, Sindic C, van Pesch V, El Sankari S, de Toutchaninoff M, Denays R, et al. Anti-N-Methyl-d-aspartat receptor encepthalitis with favourable outcome despite prolongued status epilepticus. Neurocrit Care. 2013;18:89–92. doi: 10.1007/s12028-012-9788-8. [DOI] [PubMed] [Google Scholar]
- 238.Li J, Saldivar C, Maganti R. Plasma exchange in cryptogenic new onset refractory status epilepticus. Seizure. 2013;22(1):70–73. doi: 10.1016/j.seizure.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 239.Lin JJ, Wang Y, Lan SY, Chan OW, Hsia SH, Chou ML, et al. Combination of intravenous immunoglobulin and steroid pulse therapy improves outcomes of febrile refractory status epilepticus. Epilepsy Res. 2018;142:100–105. doi: 10.1016/j.eplepsyres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 240.Lousa M, Sanchez-Alonso S, Rodriguez-Diaz R, Dalmau J. Status epilepticus with neuron-reactive serum antibodies: response to plasma exchange. Neurology. 2000;54:2163–2165. doi: 10.1212/wnl.54.11.2163. [DOI] [PubMed] [Google Scholar]
- 241.Madisi N, Berkley J. Successful treatment of prolonged status epilepticus with plasma exchange and rituximab. Neurocrit Care. 2014;21:S280. [Google Scholar]
- 242.Malaga A, Roncero I, Santovena-Gonzalez L, Blanco-Lago R, Rekarte S, Armangue T. PP13.2-3042: Infantile spasm in an infant with anti-NMDA receptor encephalitis secondary to HSV-1 encephalitis. Euro J Ped Neurol. 2015;19(Suppl 1):S82.
- 243.Mann M, Sekhon M, Javidan M. Voltage-gated potassium channel antibody associated with limbic encephalitis presenting as a rapidly progressive refractory status epilepticus: a case report and review of the literature. Neurology. 2013;80(7 Suppl).
- 244.Marques I, Teotónia R, Cunha C, Bento C, Sales F. Anti-NMDA receptor encephalitis presenting with total insomnia—a case report. J Neurol Sci. 2014;336(1–2):276–280. doi: 10.1016/j.jns.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 245.Milh M, Villeneuve N, Chapon F, Gavaret M, Girard N, Mancini J, et al. New onset refractory convulsive status epilepticus associated with serum neuropil auto-antibodies in a school aged child. Brain Dev. 2011;33(8):687–691. doi: 10.1016/j.braindev.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 246.Miras Veiga A, Moreno DC, Menendez AI, Siscart IM, Fernandez MD, Sanchez EG, et al. Effectiveness of electroconvulsive therapy for refractory status epilepticus in febrile infection-related epilepsy syndrome. Neuropediatrics. 2017;48(1):45–48. doi: 10.1055/s-0036-1584939. [DOI] [PubMed] [Google Scholar]
- 247.Moeller JJ, Friedman D, Dugan P, Akman CI. Refractory status epilepticus associated with anti-SSA (anti-Ro) antibodies. Can J Neurol Sci. 2012;39(5):660–663. doi: 10.1017/s0317167100015444. [DOI] [PubMed] [Google Scholar]
- 248.Nakamura L. Febrile infection-related epilepsy syndrome (FIRES): a rare but very severe epileptic encephalopathy. Pediatr Radiol. 2015;45:166. [Google Scholar]
- 249.Noviawaty I, Valappil A, Zeft A, Lachhwani D. Super refractory status epilepticus: a case report. Epilepsy Curr. 2015;15:87. [Google Scholar]
- 250.Ogawa C, Natsume J, Takeuchi T, Yamamoto H, Azuma Y, Kidokoro H. First autopsy report of a patient with acute encephalitis with refractory, repetitive partial seizures. Epilepsia. 2013;54:315. [Google Scholar]
- 251.Pari E, Rinaldi F, Premi E, Codella M, Rao R, Paghera B, et al. A follow-up (1)(8)F-FDG brain PET study in a case of Hashimoto's encephalopathy causing drug-resistant status epilepticus treated with plasmapheresis. J Neurol. 2014;261(4):663–667. doi: 10.1007/s00415-013-7228-0. [DOI] [PubMed] [Google Scholar]
- 252.Rypulak E, Borys M, Piwowarczyk P, Fijalkowska M, Potrec B, Sysiak J, et al. Successful treatment of anti-NMDA receptor encephalitis with a prompt ovarian tumour removal and prolonged course of plasmapheresis: a case report. Mol Clin Oncol. 2016;5(6):845–849. doi: 10.3892/mco.2016.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sawicka K, Cooley R, Hunter G. New onset refractory status epilepticus (NORSE) lasting 110 days resulting in a positive outcome. Neurology. 2016;86(16 Suppl).
- 254.Schatzmiller R, Apelian R, Cho J, Ko D, Millet D. Asian women presenting with new onset refractory status epilepticus: cyclophosphamide-responsive NMDA receptor encephalitis without tumor. Epilepsy Curr. 2011;11.
- 255.Shrivastava M, Chouhan S, Navaid S. Plasma exchange as a therapeutic modality in a rare case of cryptogenic new onset refractory status epilepticus (NORSE). J Clin Diagn Res. 2017;11(7):ED33-ED4. [DOI] [PMC free article] [PubMed]
- 256.Soldatos A, Gorman M. Rituximab treatment in a girl with voltage-gated potassium channel associated FIRES (fever-induced refractory epileptic encephalopathy in school-aged children) J Neuroimmunol. 2012;253:13–14. [Google Scholar]
- 257.Thomas D, Livingston M, Currey K, Krumholz A. Anti-NMDA receptor encephalitis associated with an ovarian teratoma presenting as non convulsive status epilepticus with atypical ictal paroxysmal fast activity. Epilepsy Curr. 2012;1(Suppl. 1).
- 258.Ting IP, Abdul Halim S, Adnan A, Jaafar H. Status epilepticus as the initial presentation of antibody-negative Goodpasture's syndrome. BMJ Case Rep. 2017;2017: bcr2017219628. [DOI] [PMC free article] [PubMed]
- 259.Tripplet J, Vijayan S, MacDonald A, Lawn N, McLean-Tooke A, Bynevelt M, et al. Fulminant Anti-GAR antibody encephalitis, presenting with status epilepticus requiering aggressive immunosuppression. J Neuroimmunol. 2018;323:119–124. doi: 10.1016/j.jneuroim.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 260.van Baalen A, Häusler M, Plecko-Startinig B, Strautmanis J, Vlaho S, Gebhardt B, et al. Febrile infection-related epilepsy syndrome without detectable autoantibodies and response to immunotherapy: a case series and discussion of epileptogenesis in FIRES. Neuropediatrics. 2012;43:209–216. doi: 10.1055/s-0032-1323848. [DOI] [PubMed] [Google Scholar]
- 261.Villani F, Spreafico R, Farina L, Giovagnoli A, Bernasconi P, Granata T. Positive response to immunomodulatory therapy in an adult patient with Rassmussen’s encephalitis. Neurology. 2001;56(2):248–250. doi: 10.1212/wnl.56.2.248. [DOI] [PubMed] [Google Scholar]
- 262.Wilder-Smith E, Lim E, Tehoh H, Sharma V, Tan J, Chan B. The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singap. 2005;34(7):417–420. [PubMed] [Google Scholar]
- 263.Yamamoto D, Uchiyama T, Bunai T, Sato K, Shimizu T, Tanaka K, et al. Acute encephalitis with refractory partial status epilepticus treated with early immunotherapies including plasma exchange: a case report. Rinsho Shinkeigaku. 2014;54(9):715–720. doi: 10.5692/clinicalneurol.54.715. [DOI] [PubMed] [Google Scholar]
- 264.Yeo L, Loh P, Tan J, Chan Y. Different presentations and outcomes in NMDAR antibody encephalitis. Eur J Neurol. 2009;16:574. [Google Scholar]
- 265.Yoshida H, Furugori M, Kanatsuka Y, Yamaguchi S, Shigeta H. Case of anti-Hu antibody-mediated encephalopathy associated with ovarian cancer. J Obstet Gynaecol Res. 2019;45(9):1948–1951. doi: 10.1111/jog.14031. [DOI] [PubMed] [Google Scholar]