Abstract
Pro- and anti-inflammatory B cell subsets that localize to unperturbed and inflamed skin are newly emerging components of the skin immune system. To test the relevance of regulatory B cells (Bregs) in the suppression of cutaneous inflammation, we asked whether impaired migration of these cells into the skin exacerbates skin inflammation. Using a mouse model with a B cell-specific tamoxifen-inducible deletion of α4β1-integrin we demonstrate that selective disruption of α4β1-integrin expression in B cells significantly decreases IL-10+ Bregs in inflamed skin while not affecting their counterparts in lymphoid tissues. Impaired skin-homing and reduced cutaneous accumulation of IL-10+ Bregs leads to a significant increase in clinical and histopathological parameters of inflammation in both psoriasiform skin inflammation and cutaneous delayed contact hypersensitivity. Thus, our data show a crucial function of skin-homing IL-10+ Bregs in the suppression of skin inflammation, supporting the notion that Bregs are critical players in the cutaneous environment during inflammatory skin diseases.
INTRODUCTION
The skin is a key barrier organ that provides protection from physical and infectious insults and is also the target of inflammatory diseases such as psoriasis. Cells of the skin immune system orchestrate a local balance of host defense and suppression of overt inflammation in response to insults. B cells were only relatively recently demonstrated in unperturbed mouse and human skin (Geherin et al., 2016, Saul et al., 2016) and also found to continuously traffic through the healthy dermis in sheep (Geherin et al., 2012). The principal function of B cells is the secretion of antibodies following differentiation into antibody secreting cells. Additionally, B cells are effective antigen presenting cells and through secretion of pro- and anti-inflammatory cytokines, they can promote and suppress inflammation, respectively (Matsushita, 2019).
A growing body of work shows both pro- and anti-inflammatory roles of skin B cells depending on the predominant B cell subset as well as the type of inflammation (reviewed in (Debes and McGettigan, 2019)). Specifically, B cells can accumulate in the skin to initiate development of cutaneous tertiary lymphoid structures and B-cell-T-cell aggregates that accelerate chronic skin inflammation (Debes and McGettigan, 2019, Yuan et al., 2017). Cutaneous production of IL-6 (Matsushita et al., 2018) or pathogenic antibodies (Yuan et al., 2017) present additional mechanisms by which skin B cells contribute to skin inflammation. Moreover, skin inflammation upregulates localized expression of the B cell survival cytokines BAFF and APRIL, which in turn promote accumulation of antibody secreting cells in skin (Wilson et al., 2019). On the other hand, B cells can act as regulatory B cells (Bregs) that suppress skin inflammation by virtue of IL-10 production in various types of skin inflammation such as models of psoriasis, contact hypersensitivity (CHS), and scleroderma (Alrefai et al., 2016, Matsushita et al., 2018, Nakashima et al., 2010, Yanaba et al., 2013) with evidence for such a role also in human disease, e.g. psoriasis (Gran et al., 2020, Mavropoulos et al., 2017). Indeed, unperturbed human and mouse skin harbors IL-10+ B cells, suggesting a regulatory role at steady state (Geherin et al., 2016). Opposing roles of different B cell subsets also explains the apparent conflicting results obtained with pan-B cell-depleting therapies, such as rituximab (anti-CD20). Rituximab can induce or exacerbate autoimmune conditions, including psoriasis (reviewed in (Kersh and Feldman, 2018)), while being an effective treatment to curb skin inflammation in pemphigus vulgaris and other inflammatory skin entities (Joly et al., 2017, Nagel et al., 2009). Thus, systemic B cell depletion can eliminate IL-10+ Bregs and provoke a pro-inflammatory environment that enhances autoimmunity while in other cases depletes disease-driving pathogenic B cells.
Lymphocyte migration from blood into tissues is mediated by a multi-step adhesion cascade involving adhesion and chemoattractant receptors on leukocytes and cognate interactions with their respective endothelial ligands. While the receptor-ligand pairs controlling skin homing of T cells are well established (Schön et al., 2003), the molecules that mediate B cell migration into skin are less well defined (Egbuniwe et al., 2015) and do not include canonical skin-homing chemokine receptors, such as CCR4 (Geherin et al., 2016). However, IL-10+ innate-like B cell migration into granulomatous skin inflammation is dependent on α4β1-integrin (Geherin et al., 2016). α4β1-integrin, also known as very late antigen-4 (VLA-4), is a heterodimer consisting of α4 and β1 subunits and is expressed by several leukocytes. α4β1-integrin mediates leukocyte migration through interactions with ligands such as vascular cell adhesion molecule-1 (VCAM-1), which is constitutively expressed by skin vasculature (Quinlan et al., 1999) and inducible by inflammatory signals in many tissues (Mitroulis et al., 2015). α4β1-integrin is clinically recognized for its roles in inflammatory diseases, such as multiple sclerosis and colitis as well as in cancer development and stem cell mobilization (Mitroulis et al., 2015). The α4-subunit can alternatively pair with β7 to form α4β7-integrin and mediate lymphocyte trafficking to mucosal sites by binding to MadCAM-1, which is absent from skin except for rare instances of extraintestinal inflammatory bowel disease (Adams and Eksteen, 2006).
In this study, we found that IL-10+ Breg skin homing and accumulation critically depend on B-cell expression of α4β1-integrin. Impaired migration of Bregs into skin exacerbates inflammation and leads to delayed resolution of inflammation during both psoriasiform skin inflammation and cutaneous contact hypersensitivity. Thus, IL-10+ Bregs fulfill a critical role in limiting inflammation within the skin microenvironment and disruption of their recruitment into skin can enhance clinical parameters of skin inflammation.
RESULTS
Short-term deletion of α4β1-integrin in B cells does not affect B cell numbers in unperturbed skin or secondary lymphoid tissues.
To study the role of α4β1-integrin in skin-associated B cells without affecting α4β1-integrin-dependent B cell development (Arroyo et al., 1996), we crossed hCD20TAMCre mice (Khalil et al., 2012), which express a B-cell specific tamoxifen (TAM)-inducible IRES-Cre/ERT2, with mice that carry a floxed integrin α4 gene (Itga4flox/flox; (Scott et al., 2003)). Treatment with TAM almost completely abrogated α4β1-integrin expression on B cells from spleen, skin draining lymph nodes (dLN) and skin in hCD20TAMCreItga4flox/flox mice seven days after the last treatment, without affecting B cells in hCD20TAMCreItga4flox/WT control mice (p <0.001; Fig. 1a and b, Sup Fig. 1a). As expected, inducing B cell-specific α4 deletion did not affect α4β1-integrin expression on CD4+ T cells or their numbers in skin and secondary lymphoid tissues (p >0.05; Fig. 1a, b, and d; Fig. S1a). Interestingly, induced deletion of α4 in B cells did not change the numbers of B cells in spleen, lymph nodes or skin of hCD20TAMCreItga4flox/flox mice relative to hCD20TAMCreItga4flox/WT control mice (p >0.05; Fig. 1c). Comparing hCD20TAMCreItga4flox/flox mice to hCD20TAMCreItga4flox/WT control mice seven days after the last TAM treatment, we did not detect statistically significant differences in the numbers of IL-10+ total B cells (CD19+ cells), IL-10+ conventional B2 cells (CD19+B220highCD43–), or IL-10+ innate-like B1 cells (CD19+B220–/lowCD43+) in unperturbed skin (Fig. 1e, Fig. S1b). Similarly, no differences were observed in the numbers of IL-10+ CD4+ T cells (Fig. 1e, Fig. S1b). Together, these data indicate that IL-10+ Breg turnover in unperturbed skin is independent of α4β1-integrin at least for the timeframe analyzed.
Figure 1: α4β1-integrin deletion in B cells of adult mice does not affect skin B cell numbers.
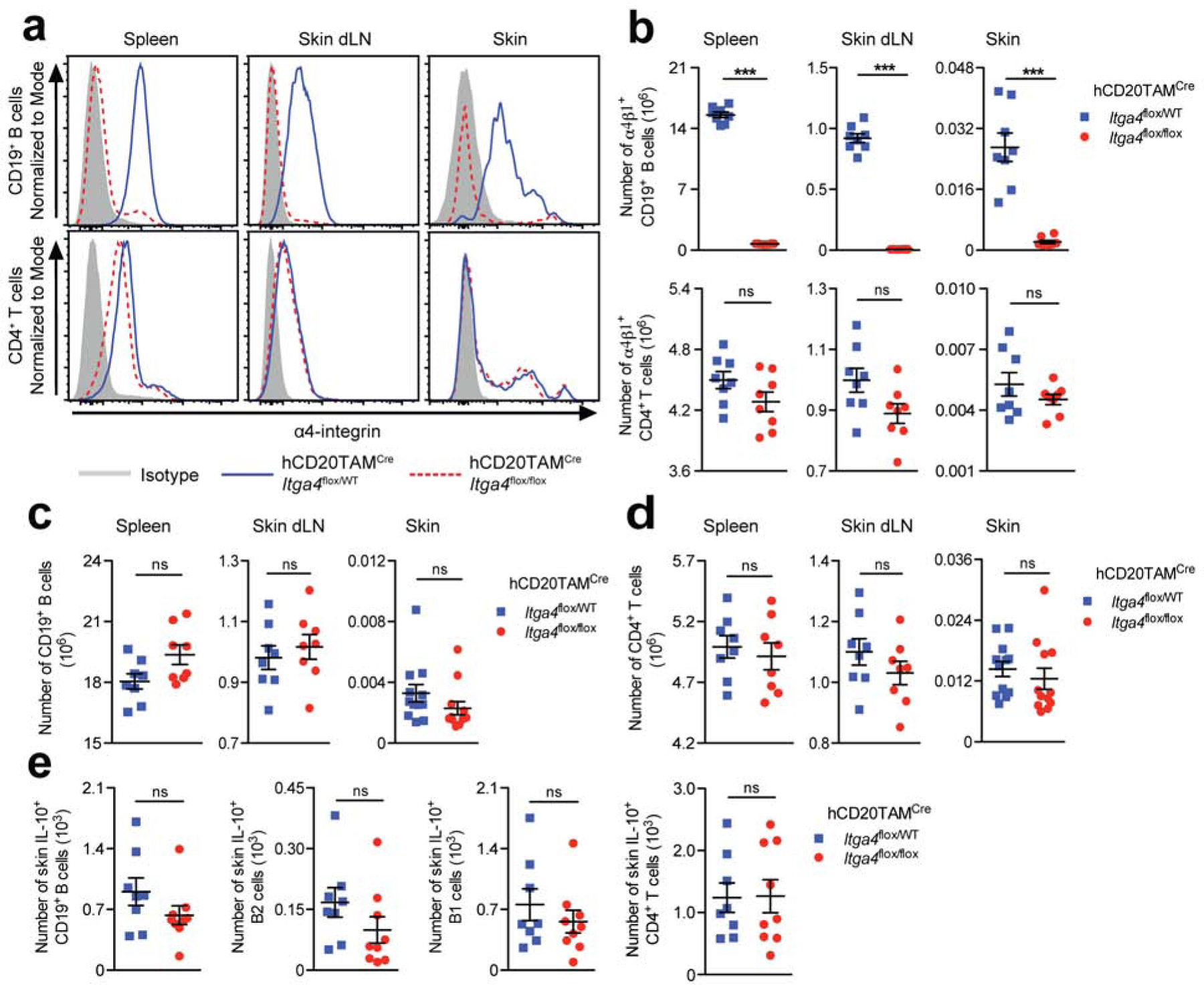
hCD20TAMCreItga4flox/flox and hCD20TAMCreItga4flox/WT mice were treated thrice with tamoxifen (TAM). Seven days after the last TAM administration, tissue lymphocytes were analyzed by flow cytometry for (a) α4 expression levels, and (b-d) subset quantification. (e) Skin lymphocytes were stained for intracellular IL-10 after 4-hour stimulation. B cells were identified as L/D Aqua–CD45+CD19+ single cells with lymphocyte scatter, distinguishing B220–/lowCD43+ B1 and B220highCD43– B2 cells. Data points represent one representative staining (a) or individually analyzed mice and the mean ± SEM of each group (b-e) from a minimum of 2 independent experiments analyzing a minimum of 8 mice per group. ***p <0.001 using the Mann-Whitney test. dLN, draining lymph nodes; ns, not significant.
α4β1-integrin deletion in B cells delays the resolution of psoriasis-like skin inflammation.
We induced a psoriasiform skin inflammation by daily application of imiquimod (IMQ; (van der Fits et al., 2009)) to the shaved back skin of hCD20TAMCreItga4flox/flox mice and hCD20TAMCreItga4flox/WT control mice three days after the last treatment with TAM. Consistent with the unaltered presence of IL-10+ Bregs and IL-10+ CD4+ T cells (Fig. 1), we did not observe differences between the IMQ-treated groups in clinical parameters of skin inflammation, such as redness (erythema) and transepidermal water loss (TEWL), in the early phase (d3) and at the peak of inflammation (d6) (p >0.05; Fig 2a). In contrast, a significant increase in skin erythema (p <0.01) and TEWL (p <0.05) was measured in hCD20TAMCreItga4flox/flox mice compared with hCD20TAMCreItga4flox/WT control mice during the resolution phase of skin inflammation (d8; Fig. 2a). The delayed resolution of inflammation was associated with a significant increase in epidermal hyperplasia as assessed by histology (p <0.05; Fig. 2b and c) and increased infiltration of inflammatory monocytes and neutrophils (p <0.05; Fig. S2a). In contrast, the numbers of cutaneous IL-17 or IFN-γ producing T cells or cutaneous expression of several inflammatory cytokines such as IL-6, IL-1β, TNF-α, IL-23, and IL-17 were not affected (p >0.05; Fig. S2b and c). The deletion of α4 was confirmed by flow cytometry analyzing total B cells, B1 cells, and B2 cells on day 8 after induction of inflammation with IMQ (p <0.001; Fig. 2d, Fig. S2d). Unlike at the steady state (Fig. 1), during the resolution phase of inflammation there was a significant decrease in the number of total B cells and B1 cells in the inflamed skin of hCD20TAMCreItga4flox/flox mice compared with hCD20TAMCreItga4flox/WT control mice (p <0.05; Fig. 2e). Strikingly, a similar reduction in IL-10+ total B cells and IL-10+ B1 cells was observed in the inflamed skin of mice with α4β1-integrin deletion in B cells (p <0.01; Fig. 2f), while, as expected, numbers of IL-10+ CD4+ T cells were unchanged in skin or other sites (Fig. 2f, Fig. S2e and f). Furthermore, no differences were detected in the number of IL-10+ B cells in skin dLN comparing both genotypes (Fig. S2f). However, there was a significant increase of IL-10+ total B and IL-10+ B1 cells in the spleens of hCD20TAMCreItga4flox/flox mice compared with hCD20TAMCreItga4flox/WT control mice (Fig. S2e). Thus, the delayed resolution of psoriasis-like inflammation observed in hCD20TAMCreItga4flox/flox mice was linked to a significant decrease in skin-associated B cells and, particularly, in IL-10+ Bregs.
Figure 2: α4β1-integrin deletion in B cells delays resolution of psoriasis-like inflammation.
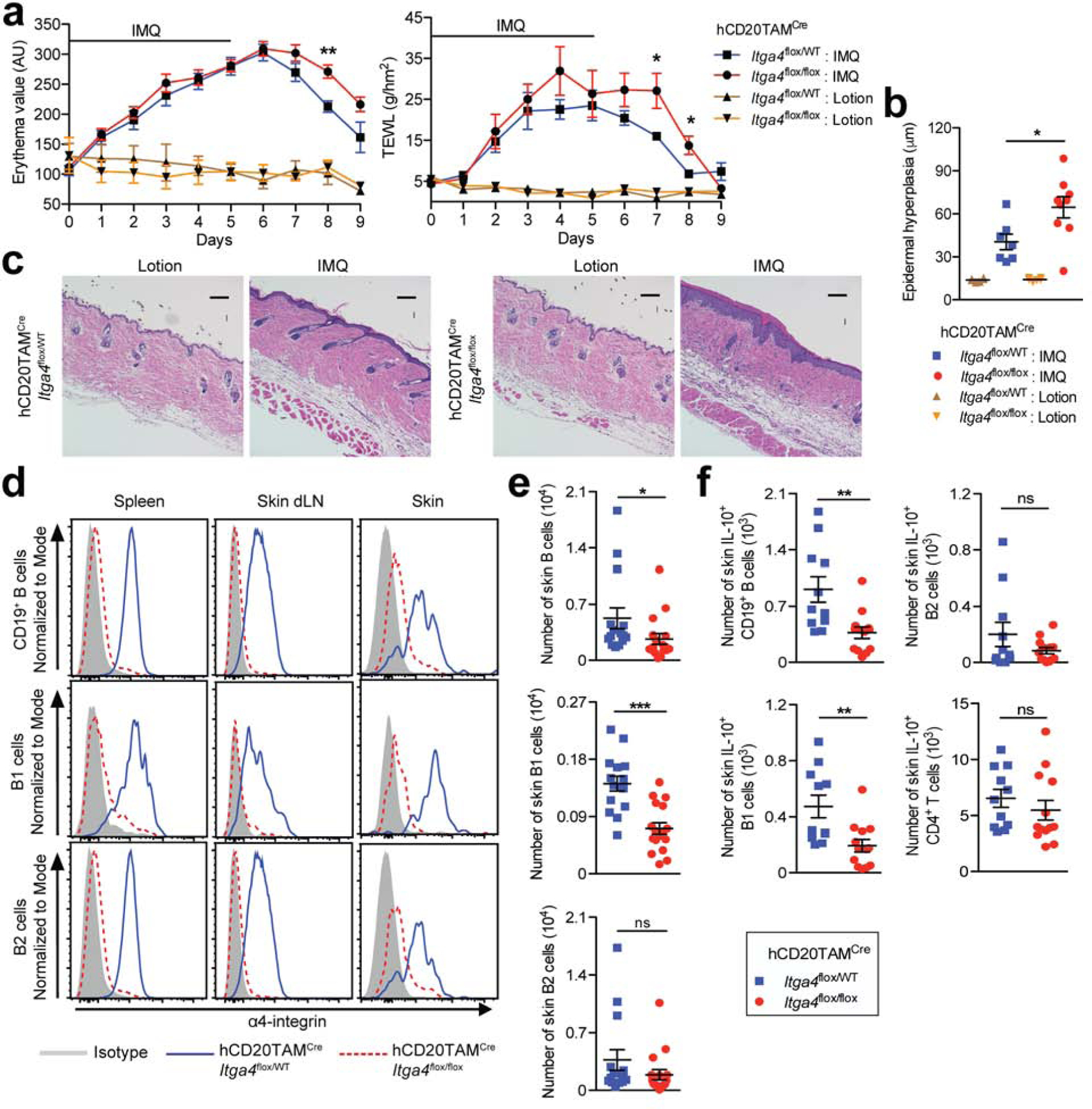
hCD20TAMCreItga4flox/flox and hCD20TAMCreItga4flox/WT mice were treated with TAM prior to daily epicutaneous application of IMQ or control lotion for six days. (a) Erythema and transepidermal water loss (TEWL) were measured. (b and c) Paraffin skin sections were stained with hematoxylin and eosin and epidermal hyperplasia quantified; scale bars = 100 μm. (d-f) On d8 after initiation of IMQ treatment, lymphocytes were isolated from indicated tissues of IMQ-treated mice, enumerated, and analyzed by flow cytometry. (f) Skin lymphocytes were stained for intracellular IL-10. Data points represent mean ± SEM of each group (a, b, e, f) and/or individually analyzed mice (b, e and f), or one representative staining (c and d) from a minimum of 3 independent experiments, analyzing a minimum of 3 lotion-treated mice (a-c) and/or 9–17 IMQ-treated mice per group (a-f). *p < 0.05, **p < 0.01, ***p < 0.001 using the Mann-Whitney test. AU, arbitrary units; IMQ, imiquimod; dLN, draining lymph nodes; ns, not significant; WT, wildtype.
α4β1-integrin deletion in B cells exacerbates hapten-induced CHS
To address whether deletion of α4β1-integrin in B cells would affect the course of non-psoriasiform skin inflammation, we sensitized hCD20TAMCreItga4flox/flox mice and hCD20TAMCreItga4flox/WT control mice through epicutaneous application of the hapten 2,4-dinitrofluorobenzene (DNFB). Prior to challenge (elicitation) with DNFB, which induces a CHS phenotype (Honda et al., 2013) the mice were treated with TAM. The DNFB challenge caused a significantly stronger erythema and TEWL in the affected back skin of hCD20TAMCreItga4flox/flox mice compared with hCD20TAMCreItga4flox/WT controls (p <0.05; Fig. 3a). Moreover, hCD20TAMCreItga4flox/flox mice exhibited a significantly enhanced swelling response in ear skin relative to hCD20TAMCreItga4flox/WT control mice (p <0.05; Fig. 3a). The exacerbated inflammation in hCD20TAMCreItga4flox/flox mice was also reflected in an increased weight of 8-mm punch biopsies of ear skin (p <0.05; Fig. 3b) as well as aggravated epidermal hyperplasia assessed by histology (p < 0.05; Fig. 3c and d). As in the psoriasis model, α4 expression was largely abrogated on total B cells, B1 cells and B2 cells (p <0.001; Fig. 3e, Fig. S3a). The inflamed skin of hCD20TAMCreItga4flox/flox mice harbored significantly fewer B1 cells relative to hCD20TAMCreItga4flox/WT control mice, while there were no differences in the total numbers of cutaneous total B cells or B2 cells (Fig. 3f). Importantly, the increased skin inflammation in hCD20TAMCreItga4flox/flox mice was associated with significantly reduced numbers of cutaneous IL-10+ B cells, including IL-10+ B1 cells and IL-10+ B2 cells (p <0.05) while not affecting IL-10+ skin CD4+ T cells (Fig. 3g) or IL-10+ CD4+ T cells in other sites (Fig. S3b and c). Notably, IL-10+ B cells were significantly increased in spleen and skin dLN of mice whose B cells lack α4β1-integrin (p <0.05; Fig. S3b and c). These data suggest that enhanced skin inflammation observed in hCD20TAMCreItga4flox/flox mice is governed by impaired α4β1-integrin-mediated migration of IL-10+ Bregs into skin but is not influenced by Bregs in lymphoid tissues.
Figure 3: α4β1-integrin deletion in B cells prevents cutaneous Breg accumulation and exacerbates CHS.
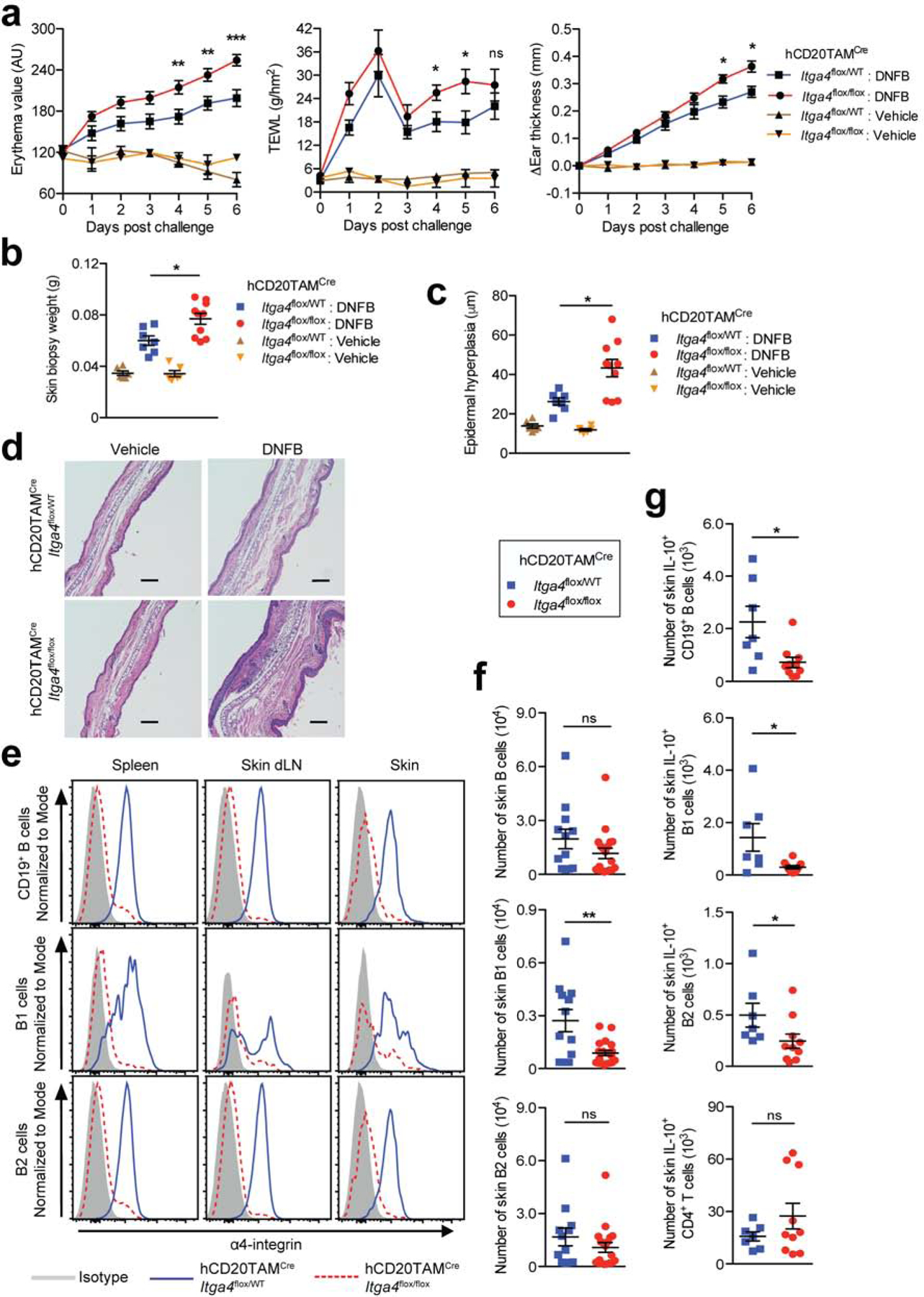
DNFB-sensitized hCD20TAMCreItga4flox/flox and hCD20TAMCreItga4flox/WT mice were treated with TAM before challenge with DNFB or vehicle on back and ear skin. A contact hypersensitivity (CHS) phenotype was evaluated by measuring (a) skin erythema, TEWL, and change in ear thickness. (b) On day 6 post challenge, 8-mm punch biopsies of ears were weighed and used for (c and d) histological epidermal hyperplasia quantification. Scale bars = 100 μm. (e-g) On day 6 post challenge, lymphocytes were isolated from indicated tissues of DNFB-treated mice, enumerated, and analyzed by flow cytometry. (g) Skin lymphocytes were stained for intracellular IL-10. Data points represent mean ± SEM of each group (a, b, c, f, g) and/or individually analyzed mice (b, c, f, g), or one representative staining (d, e) from a minimum of two independent experiments analyzing a minimum of 4 vehicle-treated mice (a-c) and/or 7–19 DNFB-treated mice per group (a-f). *p <0.05, **p <0.01, ***p <0.001 using the Mann-Whitney test. AU, arbitrary units; DNFB, 2,4-dinitrofluorobenzene; dLN, draining lymph nodes; ns, not significant; TEWL, transepidermal water loss; WT, wildtype.
α4β1-integrin required for Breg homing into psoriasis-like skin inflammation
Cd19Cre/Cre mice (Rickert et al., 1997) lack CD19 and in line with the described phenotype of traditional Cd19-null mice exhibit alterations in B cell subsets, including an almost complete lack of IL-10+ Bregs (Fig. S4a and b; (Engel et al., 1995, Yanaba et al., 2008). As expected (Yanaba et al., 2013), the severe reduction of IL-10+ Bregs was associated with an enhanced psoriasis-like phenotype after application of IMQ treatment with a significant increase in skin erythema in Cd19Cre/Cre mice compared with wildtype (WT) mice (Fig. 4a). To test whether α4β1-integrin mediates B cell homing into psoriasiform skin inflammation, we performed a competitive homing assay, comparing the migration of differentially labeled B cells from hCD20TAMCreItga4flox/flox mice and hCD20TAMCreItga4flox/WT control mice in IMQ-treated Cd19Cre/Cre mice. Sixteen hours after IV transfer, there was a greater than 80% reduction in the number of recovered α4β1-integrin-deficient (hCD20TAMCreItga4flox/flox) B cells in peritoneal cavity and skin relative to α4β1-integrin+ (hCD20TAMCreItga4flox/WT) control B cells (p <0.05 and p <0.001, respectively; Fig. 4b). The severely impaired migration of α4β1-integrin-deficient B cells into inflamed skin was also reflected in a 5-fold reduced homing index for skin and a 9-fold enhanced accumulation in blood relative to control B cells (p <0.01; Fig. 4c). Importantly, almost all IL-10+ skin B cells expressed α4β1-integrin (Fig 4d), suggesting that they use the molecule to localize to skin. Indeed, in short-term homing assays, IL-10+ B cell migration into peritoneal cavity and inflamed skin was significantly reduced when donor cells lacked α4β1-integrin (p <0.01 and p <0.05, respectively; Fig. 4e), demonstrating that α4β1-integrin is critical for IL-10+ Breg migration from blood into psoriasiform skin inflammation.
Figure 4. α4β1-integrin mediates B-cell homing into IMQ-induced psoriasiform skin inflammation.
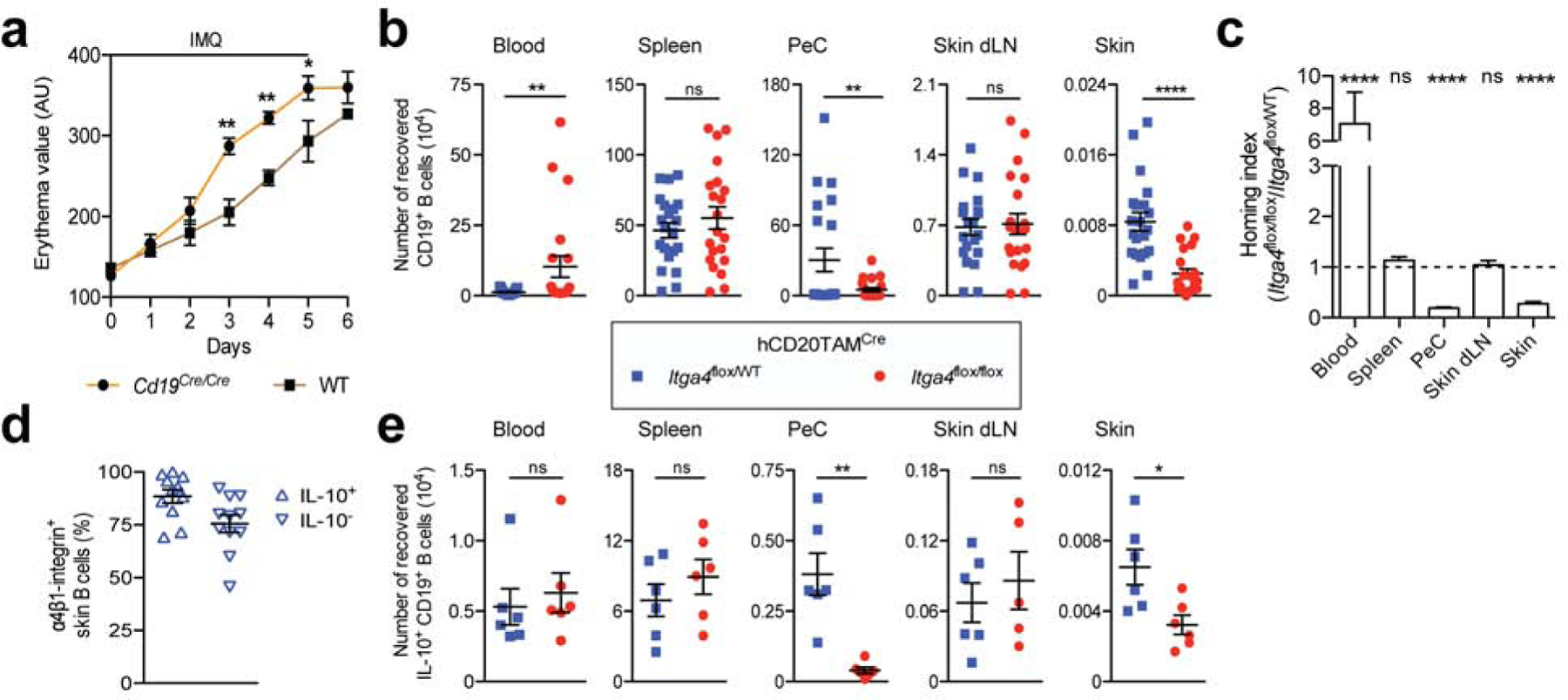
(a) Cd19Cre/Cre and WT mice were treated daily with IMQ to induce psoriasiform skin inflammation and skin erythema was measured. (b) α4β1-deleted (hCD20TAMCreItga4flox/flox) and α4β1-integrin+ (hCD20TAMCreItga4flox/WT) B cells were differentially labeled with fluorescent dyes and i.v. co-transferred into Cd19Cre/Cre recipient mice on day 5 of IMQ treatment. Sixteen hours later, indicated tissues were analyzed for transferred cells using flow cytometry, enumerating labeled donor B cells. (c) The homing index is the ratio of recovered α4β1-integrin-deleted B cells to α4β1-integrin+ B cells, corrected for their injected ratio. The dotted line indicates a homing index of 1 with no migratory difference between groups. (d) α4β1-integrin expression on IL-10+ and IL-10– B cells from IMQ-treated skin of hCD20TAMCreItga4flox/WT mice. (e) Recovered lymphocytes were stained for intracellular IL-10, and migrated IL-10+ B cells were enumerated. Data points represent means ± SEM of each group (a-e) and individual recipient mice (b, d and e) from one experiment analyzing a minimum of 6 mice per group (a, e) or 3 combined experiments analyzing a total of 11 mice (d) or 21 mice per group (b and c). *p <0.05 **p <0.01, ****p <0.0001 using the Mann-Whitney test (a, b and e) or the Wilcoxon signed rank test comparing migration to a theoretical homing index of 1 (c). AU, arbitrary units; IMQ, imiquimod; dLN, draining lymph nodes; ns, not significant; PeC, peritoneal cavity; WT, wildtype.
Breg homing into the skin ameliorates psoriasis-like inflammation
Adoptive transfer of B cells ameliorates IMQ-induced psoriasiform skin inflammation in Cd19-null mice in an IL-10-dependent manner (Yanaba et al., 2013), and we found that B-cell homing into and IL-10+ Breg accumulation in psoriasis-like skin inflammation are dependent on α4β1-integrin (Figs. 2 and 4). Thus, we wondered whether the ability of B cells to limit psoriasiform inflammation was dependent on their capacity to home into skin. To address this, we transferred α4β1-integrin-deficient (hCD20TAMCreItga4flox/flox) or α4β1-integrin+ (hCD20TAMCreItga4flox/WT) B cells into Cd19Cre/Cre mice the day before starting IMQ administration. WT mice and Cd19Cre/Cre mice without IMQ treatment or cell transfer served as controls. Throughout the course of inflammation and at its peak on day 6, transfer of α4+ B cells from hCD20TAMCreItga4flox/WT mice significantly improved skin erythema (p <0.05) and other parameters of skin inflammation, such as weight of skin biopsies (p <0.001) and epidermal hyperplasia (p <0.05) of Cd19Cre/Cre recipient mice (Fig. 5a to d). In contrast, transfer of α4β1-integrin-deficient B cells from hCD20TAMCreItga4flox/flox mice did not improve skin inflammation in Cd19Cre/Cre mice (p >0.05; Fig. 5a to d). To address the relevance of IL-10 production by B cells in our experimental set-up, we generated inducible B cell-specific IL-10-deficient mice (Fig. S5) and transferred α4β1-integrinWT IL-10 competent (hCD20TAMCreIl10flox/WT) B cells or α4β1-integrinWT IL-10-deficient (hCD20TAMCreIl10flox/flox) B cells into Cd19Cre/Cre mice the day before starting IMQ administration. Similar to α4β1-integrin+ (hCD20TAMCreItga4flox/WT) B cells, the transfer of α4β1WT IL-10-competent B cells into Cd19Cre/Cre recipient mice significantly improved parameters of skin inflammation, such as skin erythema (p <0.01), TEWL (p <0.01), weight of skin biopsies (p <0.05), and epidermal hyperplasia (p <0.05) (Fig. 5e to h). In contrast, transfer of α4β1WT IL-10-deficient (hCD20TAMCreIl10flox/flox) B cells did not improve skin inflammation of Cd19Cre/Cre mice (p >0.05; Fig. 5e to h). We conclude that α4β1-integrin-dependent migration of Bregs cells into the inflamed skin and B cell-derived IL-10 are critical to limiting psoriasis-like skin inflammation.
Figure 5. Skin-homing B cells ameliorate psoriasiform skin inflammation.
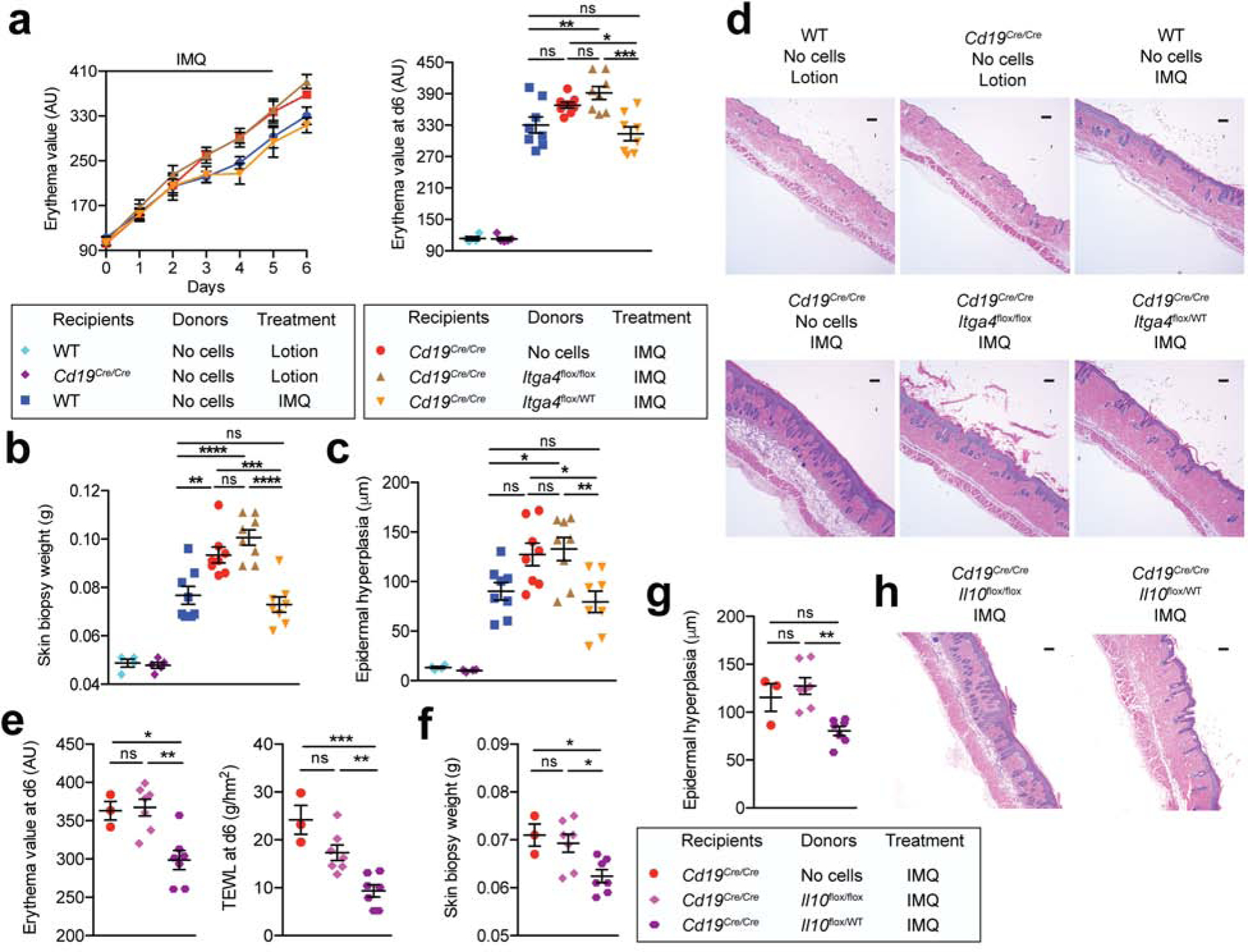
One day prior to induction of psoriasiform skin inflammation with IMQ, Cd19Cre/Cre recipient mice were reconstituted i.v. with B cells from tamoxifen-treated donor mice. (a-d) α4β1-integrin-deleted (hCD20TAMCreItga4flox/flox) compared to α4β1-integrin+ (hCD20TAMCreItga4flox/WT) B cells served as donors. IMQ- or control lotion-treated WT and Cd19Cre/Cre mice without transferred cells (PBS) served as controls. (a) Skin erythema was measured. (b) Weights of 8-mm punch biopsies from treated back skin. (c and d) Histological epidermal hyperplasia quantification with one representative section shown in (d) Scale bars, 50 μm. (e-h) IL-10-deficient (hCD20TAMCreIl10flox/flox) compared to IL-10-competent B cells (hCD20TAMCreIl10flox/WT) served as donors. (e) Skin erythema and transepidermal water loss were measured. (f) Weights of 8-mm punch biopsies from treated back skin. (g and h) Histological epidermal hyperplasia quantification with one representative section shown in (h) Scale bars, 50 μm. Data points represent means ± SEM (a) and/or individual mice (a-c; e-g) or one representative staining (d and h) in two combined independent experiments using 4 mice per group in lotion controls and 7–8 mice in all IMQ-treated groups. *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001 using One-way ANOVA with Bonferroni’s multiple comparisons post-test. AU, arbitrary units; IMQ, imiquimod; ns, not significant; TEWL, transepidermal water loss; WT, wildtype.
DISCUSSION
Recent studies demonstrate B cells as cells of the skin immune system with roles in the regulation of cutaneous immunity at steady state, acute and chronic inflammation, as well as cancer (reviewed in (Debes and McGettigan, 2019, Egbuniwe et al., 2015)). Here, we illustrated that impaired B cell recruitment into inflamed skin prevents accumulation of IL-10+ Bregs in the cutaneous environment and promotes the development of skin inflammatory states, indicating that B cells, and particularly IL-10+ Bregs, are vital to the resolution and dampening of skin inflammation.
Our previous work had shown that innate-like B cells, many of which are IL-10+ Bregs, require α4β1-integrin to home into granulomatous skin inflammation (Geherin et al., 2016). Here we found that skin B cell numbers, including those of IL-10+ Bregs were not significantly affected by induced deletion of α4β1-integrin in B cells over a 7-day period. Thus, steady-state B cell migration into skin and/or cutaneous retention appear to be independent of the VCAM-1-α4β1-integrin axis, or B cell turnover is slower in skin, preventing detection of significant decreases in α4β1-dependent skin-homing B cells. The latter explanation would also raise the possibility that some skin B cells are resident in skin for extended periods of time akin to skin-resident T cells (Gebhardt et al., 2018). In support of potential skin-resident B cells are recent studies demonstrating non-recirculatory spleen-resident T-bethi B cells (Johnson et al., 2020).
Similar to innate-like B cell homing into skin granulomas (Geherin et al., 2016), we reveal that B cell migration into psoriasiform skin inflammation as well as total B cell and IL-10+ Breg accumulation in both CHS and psoriasiform skin inflammation are critically dependent on B-cell expressed α4β1-integrin. Thus, we establish a universal role for α4β1-integrin in mediating B cell and Breg trafficking into and accumulation in inflamed skin. Blocking B-cell accumulation in the inflamed skin affected IL-10+ Bregs as well as B1 cells, consistent with elevated expression of activated α4β1-integrin on B1-like Bregs relative to conventional B2 cells (Geherin et al., 2016). However, we detected an additional reduction of IL-10+ B2 cells in the CHS model when α4 was deleted in B cells, indicating that also IL-10+ B cells from the conventional B2 lineage use α4β1-integrin to localize to inflamed skin. α4β1-integrin is not only important for the trafficking of B cells and T cells into skin, it also mediates effector T cell and B cell migration across the blood-brain barrier and its blockade with natalizumab represents an effective therapy for multiple sclerosis (Baiula et al., 2019, Lehmann-Horn et al., 2015, von Andrian and Engelhardt, 2003). Induced deletion of α4β1-integrin in B cells decreases accumulation of both effector B cells and Bregs in the central nervous system (CNS) and can positively or negatively influence the severity of experimental autoimmune encephalitis depending on the dominant role of recruited effector vs. regulatory B cells in the particular experimental autoimmune encephalitis model (Glatigny et al., 2016, Lehmann-Horn et al., 2015, Lehmann-Horn et al., 2016). Therefore, it is possible that in some instances α4β1-integrin is also used by proinflammatory B cells to enter the skin. Future studies that address this question are critical and would also aid assessing the utility of α4β1-integrin-targeting drugs to prevent undesired Breg accumulation in skin during chronic infection or malignancies. For example, a recent study reported intratumoral accumulation of IL-10+ Bregs in a mouse model of cutaneous melanoma with evidence for Breg suppression of localized anti-tumor T cell responses (Aira and Debes, 2019, Kobayashi et al., 2019).
Several studies reported that IL-10+ Bregs exert their function by acting in secondary lymphoid organs (Hussain et al., 2019, Matsushita et al., 2008), and constitutive lack of α4β1-integrin in B cells has been linked to a reduction of IL-10+ Bregs in spleen and exacerbated CNS inflammation in experimental autoimmune encephalitis (Glatigny et al., 2016). However, in our study, increased inflammation in both CHS-like and psoriasiform skin inflammation was paralleled by a decrease in cutaneous IL-10+ Breg numbers and a concomitant increase of their counterparts in spleen. Therefore, we conclude that a main mechanism by which Bregs limit cutaneous inflammation is by acting directly at the site of inflammation. Differences to the study by Glatigny et al. are likely due to differences in experimental systems: they employed constitutive α4β1-integrin deletion in B cells (Glatigny et al., 2016), which is known to affect B cell development and B cell reconstitution of peripheral tissues (Arroyo et al., 1996). In contrast, our system involved inducible deletion of α4-integrin in B cells of adult mice with physiological WT B cell repertoire and distribution (Khalil et al., 2012). Alternatively, it is possible that CNS vs. skin inflammation differentially affect Bregs in spleen. However, several studies of extracutaneous inflammation (i.e. CNS inflammation, colitis, and osteoarthritis) also support a localized role for Bregs and link the suppressive function of IL-10+ Bregs to the site of inflammation (Lehmann-Horn et al., 2016, Mishima et al., 2019, Sun et al., 2019).
In both the CHS and psoriasis skin inflammation models used in our study, inhibition of cutaneous IL-10+ Breg accumulation after TAM treatment led to an enhanced CHS response and prolonged psoriasiform inflammation, revealing an important role for Bregs in situ both at the peak and during the resolution phase of inflammation. These data are in line with the reported key anti-inflammatory function of Bregs throughout the course of psoriasiform inflammation (Alrefai et al., 2016, Yanaba et al., 2013) and the elicitation phase of CHS (Watanabe et al., 2007, Yanaba et al., 2008), as well as a potent role for innate-like IL-10+ Bregs in accelerating remission of CHS responses (Nakashima et al., 2010). Of note, we only detected very small to negligible effects of α4β1-integrin deletion in B cells on skin inflammation severity at the early and peak stages of psoriasiform inflammation (days 1–6 of IMQ challenge), reflecting the unaltered presence of cutaneous B cell populations immediately after induction of α4β1 deletion. However, our adoptive transfer studies support an additional role for Bregs in the earlier stages of psoriasiform skin inflammation because transfer of α4β1-integrin+ skin-homing B cells (hCD20TAMCreItga4flox/WT) was able to ameliorate the exacerbated early and peak skin inflammation of IMQ-treated Breg-deficient Cd19Cre/Cre recipients. In contrast, α4β1-integrin– (hCD20TAMCreItga4flox/flox) B cells with reduced ability to home into skin, were unable to rescue the phenotype. Similarly, B cells deficient in IL-10 were also unable to ameliorate the enhanced skin inflammation. Based on these data, we conclude that early inflammatory events in psoriasiform skin inflammation are also limited by Bregs in situ, consistent with data of others (Alrefai et al., 2016, Yanaba et al., 2013).
Mouse models clearly demonstrate a role for IL-10+ Bregs in the suppression of psoriasiform skin inflammation (Alrefai et al., 2016, Yanaba et al., 2013) and our data extend those findings by emphasizing a key role for skin-homing Bregs and IL-10 production by B cells. In support of a protective role of B cells in human psoriasis are studies that B cell depletion with rituximab treatment can induce or exacerbate autoimmune conditions, including psoriasis (Kersh and Feldman, 2018). Moreover, IL-10+ Breg numbers are reduced in the blood of individuals with psoriasis (Hayashi et al., 2016, Mavropoulos et al., 2017). While there are case reports of psoriasis development upon therapeutic targeting of α4β1-integrin with natalizumab (Lambrianides et al., 2018, Millan-Pascual et al., 2012), and our data in mice reveal that IL-10+ Bregs use α4β1-integrin to migrate into skin, it remains unknown how much the lack of Breg recruitment into skin contributes to the human disease. As IL-10+ Bregs are present in lesional skin in psoriasis (Toussi et al., 2019), it is conceivable that different frequencies of Bregs reside in psoriasis-affected skin, which in turn may modulate the level of disease activity and severity. In support of this idea are findings in osteoarthritis, in which disease severity is negatively correlated with the number of lesion-infiltrating IL-10+ Bregs (Sun et al., 2019). Thus, future studies are important to establish the potential relationship between the number of Bregs in lesional psoriasis skin and the level of disease activity (PASI).
The role of B cell-derived IL-10 in the suppression of CHS and psoriasis are well established and further supported by our data. Various stimuli, including bacterial products and aryl hydrocarbon receptor ligands can potently stimulate IL-10 production in B cells (Mishima et al., 2019, Piper et al., 2019). Our targeting of B cell migration into skin largely affected IL-10+ Breg homing and accumulation, however, it is conceivable that additional or alternative B cell-derived factors besides IL-10 were responsible for our observed B-cell homing-dependent suppression of skin inflammation. Bregs can suppress inflammation via alternative mechanisms, including adenosine and IL-35 production, expansion of regulatory T cells, and expression of programmed death 1 (PD-1) ligand PD-L1 (Mauri and Menon, 2017, Ray and Dittel, 2017). While most studies assume that IL-10 Bregs exert a direct suppressive effect on T cells, after deletion of α4β1-integrin in B cells and impaired Breg accumulation in skin, we did not detect changes in the number of cutaneous IL-17+ or IFN-γ+ T cells that correlated with the enhanced inflammation. Instead, when Bregs were unable to traffic into skin and skin inflammation was prolonged, we found increased numbers of inflammatory monocytes and neutrophils in the affected skin. These data suggest that Bregs suppress further recruitment of innate effectors or aid their clearance from the inflamed site thereby accelerating the resolution of inflammation. Future studies are required to determine the detailed mechanisms by which skin Bregs are able to limit cutaneous inflammation and the stimuli that drive their local anti-inflammatory activities.
In conclusion, our results reveal a critical role for Bregs within the cutaneous environment with their homing into skin as a key factor in downmodulation of inflammation in both a CHS and a psoriasis model, two very different entities of skin inflammation. Thus, Breg homing into skin and suppression of inflammation in situ is likely a universal mechanism to curb skin immune responses, and manipulation of Breg migration into skin may offer novel therapeutic avenues for inflammatory skin diseases or in the treatment of cutaneous malignancies.
MATERIALS AND METHODS
Mice, skin inflammation models
All mice were on C57BL/6 background, bred and housed under specific pathogen-free conditions and fed with standard laboratory feed in our animal facility. Sex and age-matched groups of male and female mice, 8–12 weeks of age were used in experiments. No differences between male and female mice were detected in the skin inflammation models. C57BL/6 WT and Cd19Cre/Cre mice (Rickert et al., 1997) were obtained from The Jackson Laboratory, hCD20TAMCre mice (Khalil et al., 2012) from Mark Shlomchik (University of Pittsburgh), Itga4flox/flox mice (Scott et al., 2003) from Thalia Papayannopoulou (University of Washington), and Il10flox/flox mice (Roers et al., 2004) from Joseph Craft (Yale University).
A psoriasis-like phenotype was induced by daily administration of 50 mg of 5% IMQ cream (Perrigo) on the shaved backs of mice for six days. Control mice were treated with lotion (Lubriderm). CHS was induced by sensitization of naïve mice on shaved abdominal skin with 30 μl of 0.5% DNFB (Sigma-Aldrich) in acetone (Sigma-Aldrich) and olive oil (3:1) for two consecutive days. Two weeks later, CHS was elicited by 30 μl of 0.5% DNFB on shaved backs and 12 μl on ear skin. Control mice were treated with acetone and olive oil alone. Skin erythema and TEWL were measured using electronic skin probes (Courage + Khazaka Electronic, Cologne, Germany) S004WL Mexameter® MX 18 WL and S008WL Tewameter® TM 300 WL, respectively. Ear thickness was evaluated using a digital engineer’s caliper (ThermoFisher). ∆ear thickness was determined as the current thickness measured minus baseline thickness. All clinical assessments of inflammation were performed in a blinded fashion. To induce B-cell specific deletion of Itga4, hCD20TAMCreItga4flox/flox mice were gavaged thrice with 3 mg of TAM (Sigma-Aldrich) diluted in 200 μl of olive oil, administered every other day with the last TAM treatment 3 days prior to starting IMQ treatments or performing the DNFB challenge of mice. Control hCD20TAMCreItga4flox/WT mice were treated in the same manner. All animal experiments were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
B cell homing and adoptive transfer
For B cell homing assays, recipient Cd19Cre/Cre mice were daily treated with IMQ for six days. Combined splenocytes and peritoneal cells from TAM-treated hCD20TAMCreItga4flox/flox and hCD20TAMCreItga4flox/WT served as donor cells. Donor B cells of each genotype were labeled with CFSE or eF670, mixed at equal ratios, and 1.8x107 total cells injected i.v. into recipients. Sixteen hours later recipient mice were sacrificed, lymphocytes isolated from tissues, enumerated, stained for B cells, and analyzed by flow cytometry to determine exact ratios of labeled B cells prior to injection and after recovery. For adoptive transfers, spleen and peritoneal cavity B cells from TAM-treated hCD20TAMCre+ mouse strains were isolated using magnetic cell sorting with CD19 microbeads (Miltenyi Biotec), and 0.7–1.1x107 B cells were IV injected into recipient Cd19Cre/Cre mice the day prior to starting the IMQ treatment.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Shannon McGettigan and Van Duc Dang for stimulating discussions, Tim Manser for critical comments on the manuscript, Anna Lynch for excellent technical assistance, Lei Yu and Amir Yarmahmoodi for flow cytometry support and histology support by Steve Prouty at the Penn Skin Biology and Diseases Resource-based Center (P30-AR069589). We are indebted to Thalia Papayannopoulou, Mark Shlomchik, and Joseph Craft for kindly providing mice. This work was supported in parts by NIH grants R01AR067751 and R01AI127389 to GFD.
Abbreviations:
- Bregs
B regulatory cells
- CHS
contact hypersensitivity
- CNS
central nervous system
- DNFB
2,4-dinitrofluorebenzene
- IMQ
imiquimod
- TAM
tamoxifen
- TEWL
transepidermal water loss
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL MATERIAL AND METHODS
Details concerning cell isolation and labeling, flow cytometry, histology, tissue dissociation, RNA extraction, qPCR, and statistical analysis are provided in the Supplementary Materials and Methods.
DATA AVAILABILITY STATEMENT
No datasets were generated or analyzed during the current study. All the data that support the findings are available upon request from Dr. Gudrun Debes at Thomas Jefferson University.
CONFLICT OF INTEREST STATEMENT
The authors state no conflict of interest.
REFERENCES
- Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol 2006;6(3):244–51. [DOI] [PubMed] [Google Scholar]
- Aira LE, Debes GF. B Cells and Melanoma Pathogenesis. J Invest Dermatol 2019;139(7):1422–4. [DOI] [PubMed] [Google Scholar]
- Alrefai H, Muhammad K, Rudolf R, Pham DA, Klein-Hessling S, Patra AK, et al. NFATc1 supports imiquimod-induced skin inflammation by suppressing IL-10 synthesis in B cells. Nat Commun 2016;7:11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell 1996;85(7):997–1008. [DOI] [PubMed] [Google Scholar]
- Baiula M, Spampinato S, Gentilucci L, Tolomelli A. Novel Ligands Targeting alpha4beta1 Integrin: Therapeutic Applications and Perspectives. Front Chem 2019;7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes GF, McGettigan SE. Skin-Associated B Cells in Health and Inflammation. J Immunol 2019;202(6):1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbuniwe IU, Karagiannis SN, Nestle FO, Lacy KE. Revisiting the role of B cells in skin immune surveillance. Trends Immunol 2015;36(2):102–11. [DOI] [PubMed] [Google Scholar]
- Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 1995;3(1):39–50. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Palendira U, Tscharke DC, Bedoui S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev 2018;283(1):54–76. [DOI] [PubMed] [Google Scholar]
- Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, et al. The skin, a novel niche for recirculating B cells. J Immunol 2012;188(12):6027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geherin SA, Gomez D, Glabman RA, Ruthel G, Hamann A, Debes GF. IL-10+ Innate-like B Cells Are Part of the Skin Immune System and Require alpha4beta1 Integrin To Migrate between the Peritoneum and Inflamed Skin. J Immunol 2016;196(6):2514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatigny S, Wagner CA, Bettelli E. Cutting Edge: Integrin alpha4 Is Required for Regulatory B Cell Control of Experimental Autoimmune Encephalomyelitis. J Immunol 2016;196(9):3542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran F, Kerstan A, Serfling E, Goebeler M, Muhammad K. Current Developments in the Immunology of Psoriasis. Yale J Biol Med 2020;93(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Yanaba K, Umezawa Y, Yoshihara Y, Kikuchi S, Ishiuji Y, et al. IL-10-producing regulatory B cells are decreased in patients with psoriasis. J Dermatol Sci 2016;81(2):93–100. [DOI] [PubMed] [Google Scholar]
- Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol 2013;133(2):303–15. [DOI] [PubMed] [Google Scholar]
- Hussain RZ, Cravens PD, Miller-Little WA, Doelger R, Granados V, Herndon E, et al. alpha4-integrin deficiency in B cells does not affect disease in a T-cell-mediated EAE disease model. Neurol Neuroimmunol Neuroinflamm 2019;6(4):e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Rosenthal RL, Knox JJ, Myles A, Naradikian MS, Madej J, et al. The Transcription Factor T-bet Resolves Memory B Cell Subsets with Distinct Tissue Distributions and Antibody Specificities in Mice and Humans. Immunity 2020;52(5):842–55 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet 2017;389(10083):2031–40. [DOI] [PubMed] [Google Scholar]
- Kersh AE, Feldman RJ. Autoimmune Sequelae Following Rituximab Therapy: A Review of the Literature and Potential Immunologic Mechanisms. J Clin Rheumatol 2018;24(8):427–35. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science 2012;336(6085):1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Oishi K, Okamura A, Shintaro M, Komuro A, Hamaguchi Y, et al. Regulatory B1a cells suppress melanoma tumor immunity via IL-10 production and inhibiting Th1 cytokine production in tumor-infiltrating CD8(+) T cells. J Invest Dermatol 2019. [DOI] [PubMed] [Google Scholar]
- Lambrianides S, Kinnis E, Leonidou E, Pantzaris M. Does Natalizumab Induce or Aggravate Psoriasis? A Case Study and Review of the Literature. Case Rep Neurol 2018;10(3):286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Horn K, Sagan SA, Bernard CC, Sobel RA, Zamvil SS. B-cell very late antigen-4 deficiency reduces leukocyte recruitment and susceptibility to central nervous system autoimmunity. Ann Neurol 2015;77(5):902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Horn K, Sagan SA, Winger RC, Spencer CM, Bernard CC, Sobel RA, et al. CNS accumulation of regulatory B cells is VLA-4-dependent. Neurol Neuroimmunol Neuroinflamm 2016;3(2):e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T. Regulatory and effector B cells: Friends or foes? J Dermatol Sci 2019;93(1):2–7. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Kobayashi T, Mizumaki K, Kano M, Sawada T, Tennichi M, et al. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci Adv 2018;4(7):eaas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 2008;118(10):3420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest 2017;127(3):772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavropoulos A, Varna A, Zafiriou E, Liaskos C, Alexiou I, Roussaki-Schulze A, et al. IL-10 producing Bregs are impaired in psoriatic arthritis and psoriasis and inversely correlate with IL-17- and IFNgamma-producing T cells. Clin Immunol 2017;184:33–41. [DOI] [PubMed] [Google Scholar]
- Millan-Pascual J, Turpin-Fenoll L, Del Saz-Saucedo P, Rueda-Medina I, Navarro-Munoz S. Psoriasis during natalizumab treatment for multiple sclerosis. J Neurol 2012;259(12):2758–60. [DOI] [PubMed] [Google Scholar]
- Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest 2019;129(9):3702–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther 2015;147:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel A, Hertl M, Eming R. B-cell-directed therapy for inflammatory skin diseases. J Invest Dermatol 2009;129(2):289–301. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, et al. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J Immunol 2010;184(9):4637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper CJM, Rosser EC, Oleinika K, Nistala K, Krausgruber T, Rendeiro AF, et al. Aryl Hydrocarbon Receptor Contributes to the Transcriptional Program of IL-10-Producing Regulatory B Cells. Cell Rep 2019;29(7):1878–92 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan KL, Song IS, Naik SM, Letran EL, Olerud JE, Bunnett NW, et al. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol 1999;162(3):1656–61. [PubMed] [Google Scholar]
- Ray A, Dittel BN. Mechanisms of Regulatory B cell Function in Autoimmune and Inflammatory Diseases beyond IL-10. J Clin Med 2017;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 1997;25(6):1317–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med 2004;200(10):1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul L, Ilieva KM, Bax HJ, Karagiannis P, Correa I, Rodriguez-Hernandez I, et al. IgG subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci Rep 2016;6:29736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön MP, Zollner TM, Boehncke WH. The molecular basis of lymphocyte recruitment to the skin: clues for pathogenesis and selective therapies of inflammatory disorders. J Invest Dermatol 2003;121(5):951–62. [DOI] [PubMed] [Google Scholar]
- Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol 2003;23(24):9349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhang Y, Song W, Yin L, Wang G, Yu D, et al. IgM(+)CD27(+) B cells possessed regulatory function and represented the main source of B cell-derived IL-10 in the synovial fluid of osteoarthritis patients. Hum Immunol 2019;80(4):263–9. [DOI] [PubMed] [Google Scholar]
- Toussi A, Merleev A, Barton VR, Le ST, Marusina A, Luxardi G, et al. Transcriptome mining and B cell depletion support a role for B cells in psoriasis pathophysiology. J Dermatol Sci 2019;96(3):181–4. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009;182(9):5836–45. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med 2003;348(1):68–72. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, Yazawa N, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol 2007;171(2):560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RP, McGettigan SE, Dang VD, Kumar A, Cancro MP, Nikbakht N, et al. IgM Plasma Cells Reside in Healthy Skin and Accumulate with Chronic Inflammation. J Invest Dermatol 2019;139(12):2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008;28(5):639–50. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Kamata M, Ishiura N, Shibata S, Asano Y, Tada Y, et al. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J Leukoc Biol 2013;94(4):563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Zhou S, Liu Z, Cong W, Fei X, Zeng W, et al. Pivotal Role of Lesional and Perilesional T/B Lymphocytes in Pemphigus Pathogenesis. J Invest Dermatol 2017;137(11):2362–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


