Abstract
Objective:
To measure adherence rates to swallowed topical steroids in children with eosinophilic esophagitis (EoE), describe factors related to adherence, and determine the association between adherence, symptoms, perceived disease severity, and quality of life in children with EoE.
Study design:
Subjects in this cross-sectional study of 117 children between 5–18 years old with EoE completed the Pediatric Eosinophilic Esophagitis Symptoms Score V2.0 (PEESS), Pediatric Quality of Life Inventory Eosinophilic Esophagitis Module (PedsQL EoE), a Medication-Taking Checklist (MTC), and a demographics questionnaire. Adherence rate was calculated based on reported number of missed doses/prescribed doses in the last week. Parent-reported measures were used for children aged 5–12 years and self-report was used for children aged 13–18 years.
Results:
Adolescents had lower adherence rates than younger children (76.2 ± 24.5% versus 88.6 ± 16.7%, P =.002). Adherence rates were not associated with disease history, PEESS or PedsQL™ EoE scores, but instead correlated with MTC scores (Pearson r o f0.65, P<.001 for child-report and Pearsonr of 0.74, P<.001 for parent-report). Symptomatology was associated with worse quality of life (PEESS Frequency: r=-0.7, P<0.001; PEESS Severity: r=-0.71, P<.001 for children 5–12 years old; PEESS Frequency: r-0.61, P<0.001; PEESS Severity: r=-0.5, P<.001 for adolescents).
Conclusions:
Unrelated to their clinical history, demographic factors, symptoms, and quality of life, adolescents with EoE have lower medication adherence rates. The MTC may serve as a clinical tool to discuss adherence and provide targeted educational counseling regarding adherence interventions.
Given high nonadherence rates in the adolescent population, adolescents requiring chronic medication use – such as those with solid-organ transplantation, chronic respiratory conditions, or inflammatory bowel disease – are at increased risk for disease complications.1, 2 Eosinophilic esophagitis (EoE) is a chronic allergen-mediated inflammatory disease of the esophagus with an increasing prevalence.3When compared with other chronic gastrointestinal diseases, EoE across all ages is now almost as common as pediatric inflammatory bowel disease4 and has similar healthcare associated costs with estimated United States annual health care cost between $0.5 and $1.4 billion.5 Therefore, understanding adherence rates, describing factors related to adherence, and developing interventions to improve adherence may not only improve patient care but also decrease healthcare-related spending.
Untreated EoE can lead to oral aversion and feeding disorders,6 esophageal strictures,7 esophageal food bolus impactions,8and decreased quality of life across all ages.9 Swallowed topical steroids (administered through a metered-dose inhaler or as a homemade oral viscous solution) are a common first line treatment for EoE and not only improve mucosal inflammation10, 11 but also lower risks for complications such as esophageal food bolus impactions.12Conversely, delayed treatment appears to increase the risks of stricturing disease.7 Because discontinuation of therapy results in diseasere occurrence,13chronic and continuous treatment is recommended to both control inflammation and prevent complications. Despite this, the adherence rates and the factors influencing or impeding adherence are largely unknown in the EoE population. Our prior work has suggested that adolescents with EoE have low medication adherence rates and that planning is a major barrier to adherence,14but no large scale studies have measured this to date. Thus, the aims of this study are to quantify self-reported treatment adherence rates to swallowed steroids, describe factors related to adherence, and determine the association between adherence, symptoms, perceived disease severity, and quality of life in pediatric patients with EoE. We hypothesized that adolescents would have lower adherence rates when compared with younger children and that adherence rates would be unrelated to symptomatology.
Methods
Participants included children between the ages of 5 to 18 years who met the diagnostic criteria for EoE15 and were treated with once or twice daily swallowed steroids. Participants were excluded if they were unable to read and write in English. Children and their parents were recruited from February 2018 to March 2020 in a large pediatric tertiary care center in both the general gastroenterology clinic and a multidisciplinary clinic specializing in EoE. Children were categorized as adolescents if they were between the ages of 13–18 years old inclusive. This study was approved by the institutional review board.
Measures
All participants completed the following questionnaires: Pediatric Eosinophilic Esophagitis Symptoms Score V2.0 (PEESS), Pediatric Quality of Life Inventory Eosinophilic Esophagitis Module (PedsQL EoE), a Medication-Taking Checklist (MTC), and a disease history and demographics questionnaire. As part of the disease history and demographics questionnaire, parents were asked to categorize their child’s disease as mild, moderate, or severe. PEESS is an age-specific validated index measuring EoE symptomatology16 and includes a frequency score and severity score. Scores range from 0 to 100, with a higher score indicative of more frequent and/or severe symptoms. The PedsQL EoE Module is an age-specific validated instrument to measure quality of life in patients with EoE.17It consists of 33 items and encompasses 7 scales: symptoms related to pain, symptoms related to dysphagia, treatment, worry, communication, food and eating, and food feelings. Scoring ranges from 0 to 100 with higher scores indicative of better quality of life. The MTC is a study-specific clinical tool designed with the input of gastroenterologists, nurses, patients, and a health behavior psychologist to gauge behaviors associated with medication-taking (Appendix; available at www.jpeds.com). Because planning and overcoming barriers are known factors that influence adherence,18–22 the MTC includes 9 items that assess overall self-reported adherence including 7 situations in which adherence may be challenged, and 1 on planning behavior. Scores on the MTC range from 0 – 18 with higher scores indicative of having positive medication-taking behaviors (such as routines and plans). Because neighborhood characteristics and social environment can impact medication adherence,23home addresses were inputted into the Area Deprivation Index (ADI) – a validated measure of neighborhood disadvantage based on United States Census Data and American Community Survey Data.24, 25National ADI rankings range from 1–100 with higher scores indicative of the most disadvantaged neighborhoods. Adherence rate was determined based on reported number of doses taken in the last week over the total number of prescribed doses per week. Child-reported and parent-reported adherence rates were calculated for all participants.
Statistical Analyses
Kappa statistics and the McNemar-Bowker Ptestas well as Bland-Altman analysis were used to assess the agreement of categorical variables between child and parent report. Parent-report measures for adherence rates, PEESS, Peds QL™ EoE, and MTC were used for children ages 5–12 and child-report measures were used for adolescents. Categorization of disease severity is based on parental report only for all age groups. Descriptive statistics, multiple linear regression, and ANOVA were used to analyze the data. Pearson coefficients were calculated to determine associations between adherence rates and responses to the PEESS, PedsQL™ EoE, and the MTC. A sample size of 117 allowed detection of a Pearson correlation of 0.26 with 80% power at 5% significance. A P-value of < .05 was considered statistically significant.
Results
Adherence rates
Patient and parent pairs (n- 117) agreed to participate including 60 children between the ages 5–12 years and 57 children between the ages 13–18 years. Patient age was an average of 12.0 (±3.4) years. Table I depicts participant characteristics and whether these characteristics were associated with child-reported adherence. Children reported an average adherence rate of 82.3 ± 22.4% and parents reported an average of 83.9 ± 21.7%. There was good agreement between parent and child-reported adherence; by Bland-Altman analysis, child-report was 1.4% lower (P=NS) with a limit of agreement of 22.6% (-25.4%, 22.6%). Adherence rates were not associated with age of diagnosis, disease duration, sex, race, ethnicity, ADI ranking, household size, method of steroid administration, dosing frequency, concurrent or prior treatments, history of food impaction, history of dilation, perceived severity of disease, or person in charge of taking medications. Patient age at enrollment negatively correlated with adherence (P=.001). Adolescents had lower self-reported adherence rates than younger children (76.2 ± 24.5% versus 88.6 ± 16.7%, P=.002).
Table 1:
Subject characteristics and type III p-values based on linear regression for association with adherence. Parent-reported adherence is used for children aged 5–12 years and self-reported adherence is used for children 13 years and older.
| Subject Characteristic | P-value | |
|---|---|---|
| Gender (N, %) | .78 | |
| Female | 39 (33.3) | |
| Male | 78 (66.7) | |
| Race (N, %) | .41 | |
| American Indian or Alaskan | 1 (0.9) | |
| Asian | 2 (1.7) | |
| Black/African American | 1 (0.9) | |
| Other | 8 (6.8) | |
| Prefer not to answer | 4(3.4) | |
| White/Caucasian | 101 (86.3) | |
| Ethnicity (N, %) | .37 | |
| Hispanic or Latino | 15 (12.8) | |
| Not Hispanic or Latino | 99 (84.6) | |
| Prefer not to answer | 3 (2.6) | |
| Area Deprivation Index national ranking (mean ± SD) | 25.5 (19.2) | .97 |
| Household size (mean ± SD?) | 4.7(±1.5) | .23 |
| Age group, categorical (N, %) | .002 | |
| 5–12 years old | 60 (51.3) | |
| Adolescent (13–18 years old) | 57 (48.7) | |
| Age in years at enrollment (mean ± SD) | 12.0 (±3.4) | .001 |
| Age in years at diagnosis (mean ± SD) | 7.2 (±4.7) | .21 |
| Disease duration in years (mean ± SD) | 4.9 (±3.8) | .20 |
| History of food impaction (N, %) | 8 (6.8) | .68 |
| History of dilation (N, %) | 20 (17.2) | .49 |
| Modality of swallowed steroid (N, %) | .15 | |
| Metered-dose inhaler | 94 (80.3) | |
| Oral viscous solution | 23 (19.7) | |
| Dosing Frequency (N, %) | .61 | |
| Once daily | 20 (17.1) | |
| Twice daily | 97 (82.9) | |
| Concurrently avoiding food for EoE (N, %) | 29 (24.8) | .17 |
| Proton-pump inhibitor use (N, %) | 45 (38.5) | .85 |
| Person “in charge” of taking medicines (N, %) | .55 | |
| Both child and parent/guardian | 54 (46.6) | |
| Child | 31 (26.7) | |
| Parent/Guardian | 31 (26.7) | |
| Perceived severity of disease (N, %) | .80 | |
| Mild | 73 (62.4) | |
| Moderate | 37 (31.6) | |
| Severe | 7 (6.0) | |
Medication-taking Checklist (MTC)
Child-reported MTC scores ranged from 0–18 with a mean of 13.3±4.0. Parent-reported MTC scores ranged from 1–18 with a mean of 13.7±3.9. Figure 2 (available at www.jpeds.com) shows the distribution of MTC scores, stratified by age. For all statistical analysis involving MTC scores, parent-report was used for children ages 5–12 and self-report for ages 13–18. Table 2 describes associations between adherence rate, total MTC score, and individual items on the MTC. Higher adherence rates were associated with both the total score on the MTC as well as each individual MTC item (aside from taking medications when there is difficulty swallowing) for both parent and child-report. Total MTC score was strongly correlated with child-reported adherence rate (Pearson r of 0.65, P<.001) and parent-reported adherence rate (Pearson r of 0.74, P<.001). There was agreement between parent-report and child-report on all MTC items except for taking medicines when something unexpected happened and making plans for when to take medicines (P=.02 and P<.001 respectively).
Figure 2.
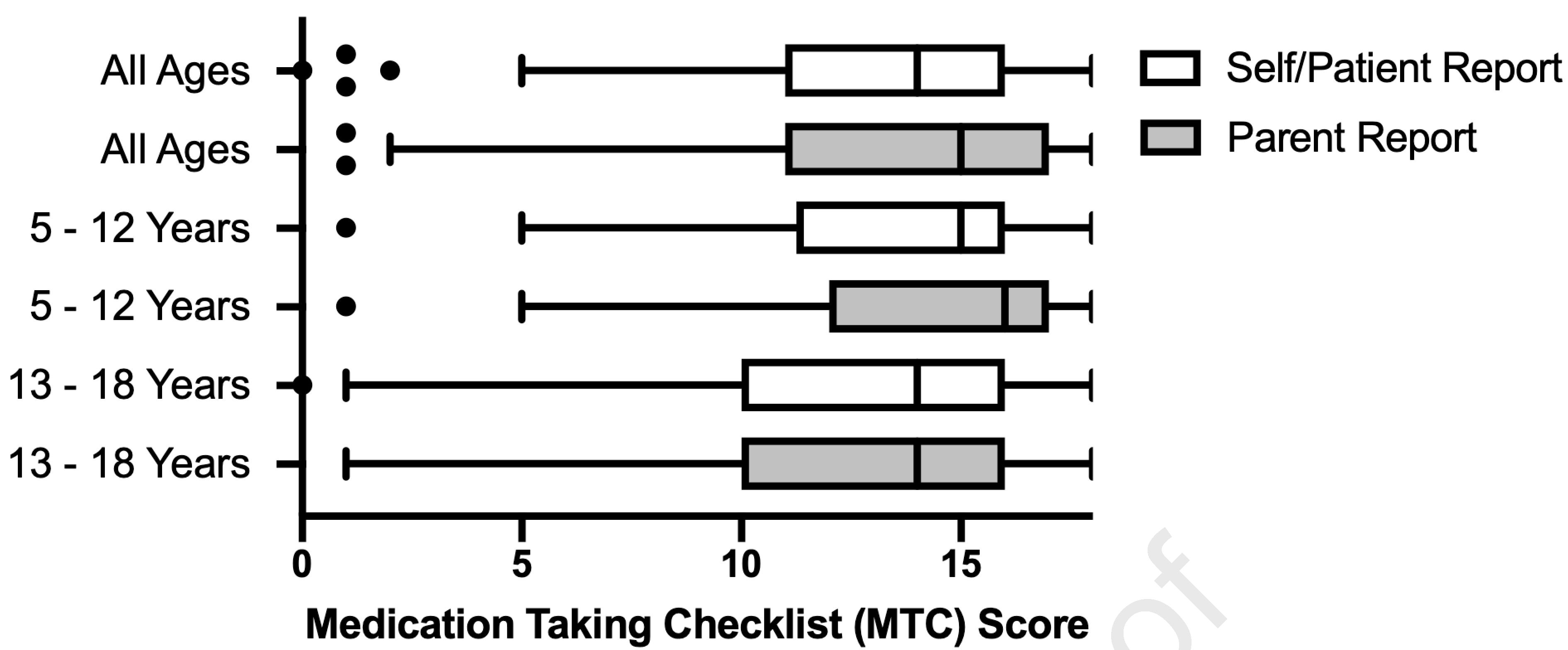
online only: Distribution of scores on the Medication-Taking Checklist (MTC), stratified by age. Possible scores range from 0–18 with higher scores indicative of having positive medication-taking behaviors. Self-report and parent-report was captured for all participants; however, for all statistical analysis involving MTC scores, parent-report was used for children ages 5–12 and self-report for ages 13–18.
Table 2:
Association between the Medication Taking Checklist and adherence rates. Parent-reported adherence is used for children aged 5–12 years and self-reported adherence is used for children 13 years and older.
| Children (ages 5– 12 years) | Adolescents (ages 13 through 18 years) | ||||
|---|---|---|---|---|---|
| Medication-Taking Checklist | Association with adherence (Pearson r) | P-value | Medication-Taking Checklist | Association with adherence (Pearson r) | P-value |
| Total Score | 0.65 | <.001 | Total Score | 0.74 | <.001 |
| Individual items | Individual items | ||||
| Takes swallowed steroids “as instructed” | 0.56 | <.001 | Takes swallowed steroids “as instructed” | 0.69 | <.001 |
| Takes swallowed steroids when difficulty swallowing | 0.11 | .21 | Takes swallowed steroids when difficulty swallowing | 0.18 | 0.058 |
| Takes swallowed steroids when feeling well | 0.54 | <.001 | Takes swallowed steroids when feeling well | 0.65 | <.001 |
| Takes swallowed steroids even when very busy | 0.45 | <.001 | Takes swallowed steroids even when very busy | 0.63 | <.001 |
| Takes swallowed steroids on school days | 0.59 | <.001 | Takes swallowed steroids on school days | 0.62 | <.001 |
| Takes swallowed steroids on weekends/holidays | 0.51 | <.001 | Takes swallowed steroids on weekends/holidays | 0.68 | <.001 |
| Takes swallowed steroids when something unexpected happens | 0.52 | <.001 | Takes swallowed steroids when something unexpected happens | 0.52 | <.001 |
| Takes swallowed steroids when traveling | 0.40 | <.001 | Takes swallowed steroids when traveling | 0.57 | <.001 |
| Makes plans for when he/she will take swallowed steroids | 0.35 | <.001 | Makes plans for when he/she will take swallowed steroids | 0.38 | <.001 |
Symptomatology and quality of life
PEESS and PedsQL™ EoE scores stratified by age are depicted in Figure 1. Adherence rates were not strongly associated with the PEESS Frequency Score, PEESS Severity Score, or the total PedsQL™ EoE score in both the 5–12-year-old group (Pearson r of -0.03, -0.10, and 0.24 respectively) and adolescents (Pearson r of 0.13, 0.17, and 0.01 respectively). In children aged 5–12, the PedsQL™ EoE communication subscore and PedsQLTM EoE food and eating subscore were positively associated with better adherence (r=0.26, P=.05 and r=0.38, P=.008). There were no significant associations between PedsQL™ EoE subscores and adherence rates in adolescents. PEESS Frequency and PEESS severity scores were associated with lower quality of life as measured by the PedsQL™ EoE in both the 5–12-year-old group (Frequency: r=-0.7, P<0.001; Severity: r=-0.71, P<.001) and adolescents (Frequency: r -0.61, P<0.001; Severity: r=-0.5, P<.001).
Figure 1.
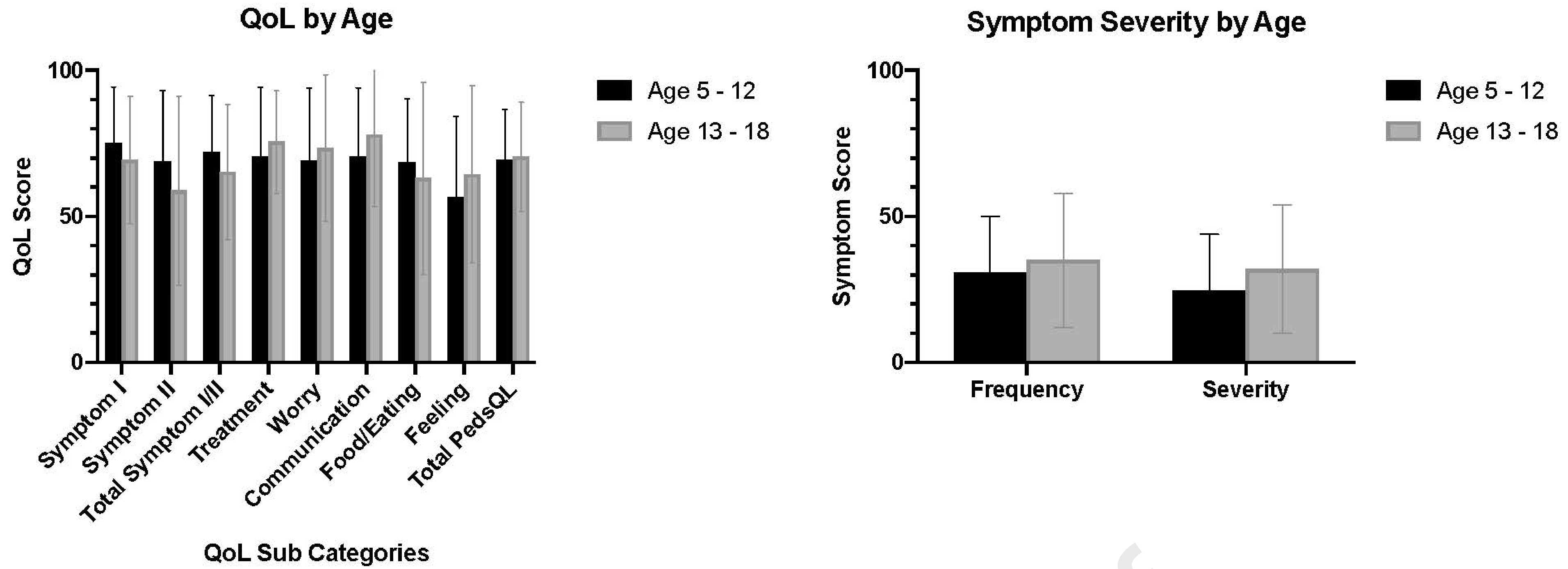
Scores on the Pediatric Eosinophilic Esophagitis Symptoms Score V2.0 (PEESS) and Pediatric Quality of Life Inventory™ Eosinophilic Esophagitis Module (PedsQL EoE) stratified by age. Scores on both the PEESS and PedsQL EoE range from 0 to 100, with a higher score indicative of more frequent and/or severe symptoms and better quality of life, respectively.
Effects of perceived disease severity
Perceived severity was not associated with adherence but was independently associated with PEESS and quality of life. Table 3 depicts perceived severity, categorized by age, and its association with PEESS and quality of life. Perceived disease severity was associated with worse quality of life scores, even after adjusting for PEESS scores (P=.03 in the 5–12-year age group and P=.04 in adolescents).
Table 3:
Association of perceived eosinophilic esophagitis (EoE) severity with the Pediatric Eosinophilic Esophagitis Symptoms Score V2.0 (PEESS) and the Pediatric Quality of Life Inventory™ Eosinophilic Esophagitis Module (PedsQL™ EoE). Scores on both the PEESS and PedsQL EoE range from 0 to 100, with a higher score indicative of more frequent and/or severe symptoms and better quality of life, respectively.
| Age group: 5–12 years old | |||||||
| Perceived Severity | N | PEESS Frequency Score (mean ± SD) | P-value | PEESS Severity Score (mean ± SD) | P-value | PedsQL™ EoE Score (mean ± SD) | P-value |
| Mild | 40 | 25.5 ± 18.8 | .002* | 20.3 ± 19.1 | .02* | 77.4 ± 18.4 | .003* |
| Moderate | 19 | 41.6 ± 15.5 | 33.0 ± 18.0 | 61.4 ± 17.5 | |||
| Severe | 1 | 40.9 | 33.3 | 48.5 | |||
| Age group: 13 – 18 years old | |||||||
| Perceived Severity | N | PEESS Frequency Score | P-value | PEESS Severity Score | P-value | PedsQL™ EoE Score | P-value |
| Mild | 33 | 24.6 ± 13.3 | <.001 | 18.7 ± 17.9 | <.001 | 74.3 ± 18.0 | <.001 |
| Moderate | 18 | 43.2.6 ± 23.7 | 40.6 ± 23.5 | 56.8 ± 21.6 | |||
| Severe | 6 | 59.8 ± 24.5 | 50.5 ± 20.1 | 40.0 ± 28.8 | |||
Due to the small sample size, the subject with perceived severe disease was not included in the statistical analysis.
Discussion
In chronic conditions with the potential for progressive disease such as EoE, adequately characterizing adherence is critical to disease management. Clinical assessment of adherence may decrease the frequency of invasive and expensive endoscopies for disease surveillance. Moreover, interventions targeting adherence may prevent complications like food bolus impactions.12In this study, we found adherence rates to be lower in adolescents as compared with younger children and that adherence rates did not correlate with symptoms, perceived disease severity, or quality of life. However, adherence strongly correlated with a newly developed medication-taking behavior checklist that assessed common medication-taking behaviors.
Adherence rates
Reported swallowed steroid adherence rates were relatively high among pediatric patients with EoE. There were no identifiable demographic or disease features associated with adherence rates other than participant age. Specifically, adolescents had significantly lower adherence rates than younger children. These findings are consistent with other chronic pediatric conditions.1, 2This may be related to an adolescent’s struggle with independence and self-esteem or the fact that adolescents frequently have lower levels of characteristics consistent with executive function – the organization, planning, self-monitoring, and problem solving required to manage complex tasks, such as following a medical regimen.26
Medication taking is a complex behavioral process without a one-size-fits-all solution. Numerous studies have shown that healthcare providers are unable to correctly predict medication adherence patterns in clinical practice based on disease history or demographics alone.27–31 In our study, both the total score on the MTC as well as most questions on the MTC correlated with adherence. Future studies of the MTC are needed to determine if there are score cut-offs that are associated with objectively measured adherence or disease outcomes.
Given the brevity of the MTC and its strong association with self-reported adherence in this study, the MTC may serve as a clinical tool in practice. In fact, the feeling of not getting support from health professionals is independently associated with poor medication adherence. 32The family environment plays a large role in medication adherence33 which may suggest a group discussion with the entire family is warranted. When children are young, parents are actively involved in their medication-taking. However, as they get older, responsibility is often transferred to the child themselves and children may be less able to self-manage medication taking.34Additionally, a recent systematic review showed that adolescents with chronic diseases tend to have difficulty planning and increased forgetfulness and this impacts medication adherence.35Because a stable family involvement, increased parental involvement (but not rigidity), and a home environment that promotes emotional expressiveness and support all lead to better medication adherence in adolescents,36–38 perhaps health providers can employ the MTC as a conversation-starter surrounding specific medication-behaviors that need problem solving.
Interventions that focus solely on reminders do not improve long-term adherence patterns.39Additionally, a recent meta-analysis showed that interventions that focus on habit formation and behavior change rather than education and attitudes are most successful at improving adherence.40In adults, self-management plans or “action plans” - a type of behavioral change technique, have proven to be successful in improving health outcomes; however, pediatric-specific action plans are limited in availability.41Thus, our findings, in conjunction with the literature, suggest a need to develop and test interventions aimed at improving adolescent adherence by targeting behaviors that support planning, which may improve disease outcomes in EoE.
Adherence, symptomatology, and quality of life
Although numerous studies have shown swallowed steroids can improve health-related quality of life in EoE,42–44 and that increasing symptomatology results in lower quality of life,45, 46 we found no association between adherence and PEESS. This is not all together surprising as PEESS does not always strongly correlate with histology.16, 47, 48Prior studies that have shown improvement in symptoms with initiation of steroids have focused on newly diagnosed EoE.10, 49, 50This suggests that assessment of symptom severity alone is not a reliable marker of mucosal inflammation. The lack of association between adherence and symptomatology is important, as it can be more difficult to continue long-term medication adherence when patients cannot directly observe effects of medication taking on how they feel.51Our findings may also lend further credence to newer evidence suggestive of phenotypic variations in EoE including variable response to treatments.52Future studies comparing adherence to disease activity (histological improvement, tissue remodeling) are needed to better understand the complicated relationship between symptoms, disease activity, and medication effect.
Quality of life was also not associated with adherence rates. These findings are fitting with adult EoE studies which have shown that quality of life is associated with symptom severity but not endoscopic or histologic features.53Consistent with other published literature, participants in our study had low quality of life scores44, 54 and quality of life was strongly associated with symptomatology. Our study had similar PedsQL™ EoE scores to a large multicenter study of children with EoE, which also found that children with more symptoms had worse quality of life.47In adults with EoE, the combined effects of symptoms, endoscopic, and histologic findings only account for 60% of variation in quality of life55 and patients report unmet needs in multiple domains.56In food protein–induced enterocolitis syndrome – another non IgE-mediated food allergy disease, perception of disease management has been associated with quality of life.57Similarly, in our study, perceived disease severity was associated with lower quality of life even after accounting for PEESS scores. Although this may be due to unmeasured variables such as endoscopic and histological findings, or mental health disorders which are common among EoE patients,58, 59further research should explore what drives patient and caregiver perception of disease severity and whether interventions in this arena can improve quality of life.
This study had several limitations. First, in this cross-sectional study, we directly compared PedsQL™ EoE scores and PEESS at one point in time, however we did not assess for other known factors associated with quality of life such as depression and anxiety59 and could not assess for changes in quality of life longitudinally. Second, the cohort in this study had relatively high neighborhood advantage with a mean ADI ranking of 25. Future prospective research on quality of life and adherence are needed in the EoE population and should include a more diverse patient population and include both PEESS and validated mental health assessments. Additionally, this study used self-reported adherence, which may overestimate true medication adherence.14, 60Although we have used electronic sensors attached to metered-dose inhalers in prior work to objectively measure adherence in EoE,14there is no objective method of assessing adherence to swallowed topical steroids administered as an oral viscous solution. Finally, although we did not measure endoscopic activity or histological activity, this is one of few studies to quantify adherence in patients with EoE and compare it with symptomatology.
Unrelated to their clinical history, demographic factors, and quality of life, adolescents with EoE have lower self-reported adherence to swallowed steroid therapy relative to younger children. Conversely, adherence was strongly related to a newly developed clinical tool – the Medication-Taking Questionnaire (MTC). Perceived disease severity (from both parents and children) was associated with both quality of life and more comprehensive measures of symptom severity, such that a brief severity assessment may serve as a pragmatic indicator of symptom severity and quality of life. Therefore, there may be two separate but important outcomes that physicians caring for EoE patients should measure and treat longitudinally symptom severity and quality of life, and adherence and mucosal healing. Future studies aimed at determining the effect of adherence on EoE disease severity as well as testing effects of adherence interventions on disease outcomes for adolescents with EoE are needed. Such research may further enhance our understanding of treatment response and disease phenotypes in children and adolescents with EoE.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (K23 DK109263 [to C.K.]) and the NASPGHAN Foundation/APGNN Susan Moyer Nursing Research Grant (to S.S.). The authors declare no conflicts of interest.
Abbreviations:
- ADI
Area Deprivation Index
- EoE
eosinophilic esophagitis
- PEESS
Pediatric Eosinophilic Esophagitis Symptoms Score V2.0
- PedsQL™ EoE
Pediatric Quality of Life Inventory™ Eosinophilic Esophagitis Module
- MTC
Medication-Taking Checklist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pooja Mehta, Children’s Hospital Colorado, Department of Pediatrics, University of Colorado School of Medicine.
Zhaoxing Pan, Department of Biostatistics, University of Colorado School of Medicine.
Stephanie Skirka, Children’s Hospital Colorado.
Bethany M. Kwan, Department of Family Medicine, University of Colorado School of Medicine.
Calies Menard-Katcher, Children’s Hospital Colorado, Department of Pediatrics, University of Colorado School of Medicine.
References
- 1.McQuaid EL, Kopel SJ, Klein RB, et al. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol 2003;28:323–33. [DOI] [PubMed] [Google Scholar]
- 2.Foster BJ, Dahhou M, Zhang X, et al. Association between age and graft failure rates in young kidney transplant recipients. Transplantation 2011;92:1237–43. [DOI] [PubMed] [Google Scholar]
- 3.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018;154:319–332 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellon ES, Jensen ET, Martin CF, et al. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014;12:589–96 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen ET, Kappelman MD, Martin CF, et al. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol 2015;110:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P, Furuta GT, Brennan T, et al. Nutritional State and Feeding Behaviors of Children With Eosinophilic Esophagitis and Gastroesophageal Reflux Disease. J Pediatr Gastroenterol Nutr 2018;66:603–608. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Kim HP, Sperry SL, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79:577–85 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperry SL, Crockett SD, Miller CB, et al. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc 2011;74:985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukkada V, Falk GW, Eichinger CS, et al. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin Gastroenterol Hepatol 2018;16:495–503 e8. [DOI] [PubMed] [Google Scholar]
- 10.Remedios M, Campbell C, Jones DM, et al. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc 2006;63:3–12. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology 2002;122:1216–25. [DOI] [PubMed] [Google Scholar]
- 12.Kuchen T, Straumann A, Safroneeva E, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy 2014;69:1248–54. [DOI] [PubMed] [Google Scholar]
- 13.Greuter T, Bussmann C, Safroneeva E, et al. Long-Term Treatment of Eosinophilic Esophagitis With Swallowed Topical Corticosteroids: Development and Evaluation of a Therapeutic Concept. Am J Gastroenterol 2017;112:1527–1535. [DOI] [PubMed] [Google Scholar]
- 14.Mehta P, Pan Z, Furuta GT, et al. Mobile health tool detects adherence rates in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol Pract 2019;7:2437–2439. [DOI] [PubMed] [Google Scholar]
- 15.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018;155:1022–1033 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin LJ, Franciosi JP, Collins MH, et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol 2015;135:1519–28 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franciosi JP, Hommel KA, Bendo CB, et al. PedsQL eosinophilic esophagitis module: feasibility, reliability, and validity. J Pediatr Gastroenterol Nutr 2013;57:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50:179–211. [Google Scholar]
- 19.Godin G, Kok G. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot 1996;11:87–98. [DOI] [PubMed] [Google Scholar]
- 20.Plaza V, Fernandez-Rodriguez C, Melero C, et al. Validation of the ‘Test of the Adherence to Inhalers’ (TAI) for Asthma and COPD Patients. J Aerosol Med Pulm Drug Deliv 2016;29:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwasnicka D, Dombrowski SU, White M, et al. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev 2016;10:277–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarzer R. Self-efficacy in the adoption and maintenance of health behaviours: Theoretical approaches and a new model. In: Schwarzer R, ed. Self-Efficacy: Thought Control of Action. Washington Taylor and Francis, 1992:217–243. [Google Scholar]
- 23.Hu J, Kind AJH, Nerenz D. Area Deprivation Index Predicts Readmission Risk at an Urban Teaching Hospital. Am J Med Qual 2018;33:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible The Neighborhood Atlas. N Engl J Med 2018;378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.University of Wiscons in School of Medicine and Public Health. 2018. Area Deprivation Index v3.0. Downloaded from https://www.neighborhoodatlas.medicine.wisc.edu/ February 1.
- 26.Gutierrez-Colina AM, Eaton CK, Lee JL, et al. Executive Functioning, Barriers to Adherence, and Nonadherence in Adolescent and Young Adult Transplant Recipients. J Pediatr Psychol 2016;41:759–67. [DOI] [PubMed] [Google Scholar]
- 27.Zeller A, Taegtmeyer A, Martina B, et al. Physicians’ ability to predict patients’ adherence to antihypertensive medication in primary care. Hypertens Res 2008;31:1765–71. [DOI] [PubMed] [Google Scholar]
- 28.Norell SE. Accuracy of patient interviews and estimates by clinical staff in determining medication compliance. Soc Sci Med E 1981;15:57–61. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert JR, Evans CE, Haynes RB, et al. Predicting compliance with a regimen of digoxin therapy in family practice. Can Med Assoc J 1980;123:119–22. [PMC free article] [PubMed] [Google Scholar]
- 30.Bangsberg DR, Hecht FM, Clague H, et al. Provider assessment of adherence to HIV antiretroviral therapy. J Acquir Immune Defic Syndr 2001;26:435–42. [DOI] [PubMed] [Google Scholar]
- 31.Gross R, Bilker WB, Friedman HM, et al. Provider inaccuracy in assessing adherence and outcomes with newly initiated antiretroviral therapy. AIDS 2002;16:1835–7. [DOI] [PubMed] [Google Scholar]
- 32.Thorell LB, Dahlstrom K. Children’s self-reports on perceived effects on taking stimulant medication for ADHD. J Atten Disord 2009;12:460–8. [DOI] [PubMed] [Google Scholar]
- 33.Kraenbring MM, Zelikovsky N, Meyers KEC. Medication adherence in pediatric renal transplant patients: The role of family functioning and parent health locus of control. Pediatr Transplant 2019;23:e13346. [DOI] [PubMed] [Google Scholar]
- 34.Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med 2012;74:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanghoj S, Boisen KA. Self-reported barriers to medication adherence among chronically ill adolescents: a systematic review. J Adolesc Health 2014;54:121–38. [DOI] [PubMed] [Google Scholar]
- 36.Guilfoyle SM, Goebel JW, Pai AL. Efficacy and flexibility impact perceived adherence barriers in pediatric kidney post-transplantation. Fam Syst Health 2011;29:44–54. [DOI] [PubMed] [Google Scholar]
- 37.Zelikovsky N, Schast AP, Palmer J, et al. Perceived barriers to adherence among adolescent renal transplant candidates. Pediatr Transplant 2008;12:300–8. [DOI] [PubMed] [Google Scholar]
- 38.Killian MO, Schuman DL, Mayersohn GS, et al. Psychosocial predictors of medication non-adherence in pediatric organ transplantation: A systematic review. Pediatr Transplant 2018;22:e13188. [DOI] [PubMed] [Google Scholar]
- 39.Choudhry NK, Krumme AA, Ercole PM, et al. Effect of Reminder Devices on Medication Adherence: The REMIND Randomized Clinical Trial. JAMA Intern Med 2017;177:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: Systematic review and meta-analysis. Prev Med 2017;99:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lozano P, Houtrow A. Supporting Self-Management in Children and Adolescents With Complex Chronic Conditions. Pediatrics 2018;141:S233–S241. [DOI] [PubMed] [Google Scholar]
- 42.Bergquist H, Larsson H, Johansson L, et al. Dysphagia and quality of life may improve with mometasone treatment in patients with eosinophilic esophagitis: a pilot study. Otolaryngol Head Neck Surg 2011;145:551–6. [DOI] [PubMed] [Google Scholar]
- 43.Larsson H, Bergman K, Finizia C, et al. Erratum to: Dysphagia and health-related quality of life in patients with eosinophilic esophagitis: a long-term follow-up. Eur Arch Otorhinolaryngol 2015;272:3841. [DOI] [PubMed] [Google Scholar]
- 44.Kruszewski PG, Russo JM, Franciosi JP, et al. Prospective, comparative effectiveness trial of cow’s milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus 2016;29:377–84. [DOI] [PubMed] [Google Scholar]
- 45.Klinnert MD, Atkins D, Pan Z, et al. Symptom Burden and Quality of Life Over Time in Pediatric Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2019;69:682–689. [DOI] [PubMed] [Google Scholar]
- 46.Lynch MK, Barnes MJ, Dimmitt RA, et al. Disease-Related Predictors of Health-Related Quality of Life in Youth With Eosinophilic Esophagitis. J Pediatr Psychol 2018;43:464–471.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aceves SS, King E, Collins MH, et al. Alignment of parent- and child-reported outcomes and histology in eosinophilic esophagitis across multiple CEGIR sites. J Allergy Clin Immunol 2018;142:130–138 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pentiuk S, Putnam PE, Collins MH, et al. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2009;48:152–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dellon ES, Woosley JT, Arrington A, et al. Efficacy of Budesonide vs Fluticasone for Initial Treatment of Eosinophilic Esophagitis in a Randomized Controlled Trial. Gastroenterology 2019;157:65–73 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clin Proc 2003;78:830–5. [DOI] [PubMed] [Google Scholar]
- 51.Parthan A, Vincze G, Morisky DE, et al. Strategies to improve adherence with medications in chronic, ‘silen’ diseases representing high cardiovascular risk. Expert Rev Pharmacoecon Outcomes Res 2006;6:325–36. [DOI] [PubMed] [Google Scholar]
- 52.Ruffner MA, Cianferoni A. Phenotypes and endotypes in eosinophilic esophagitis. Ann Allergy Asthma Immunol 2020;124:233–239. [DOI] [PubMed] [Google Scholar]
- 53.Stern E, Taft T, Zalewski A, et al. Prospective assessment of disease-specific quality of life in adults with eosinophilic esophagitis. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 54.Varni JW, Burwinkle TM, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329–41. [DOI] [PubMed] [Google Scholar]
- 55.Safroneeva E, Coslovsky M, Kuehni CE, et al. Eosinophilic oesophagitis: relationship of quality of life with clinical, endoscopic and histological activity. Aliment Pharmacol Ther 2015;42:1000–10. [DOI] [PubMed] [Google Scholar]
- 56.Hiremath G, Kodroff E, Strobel MJ, et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol 2018;42:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenhawt M, Schultz F, DunnGalvin A. A validated index to measure health-related quality of life in patients with food protein-induced enterocolitis syndrome. J Allergy Clin Immunol 2016;137:1251–1253 e5. [DOI] [PubMed] [Google Scholar]
- 58.Chehade M, Jones SM, Pesek RD, et al. Phenotypic Characterization of Eosinophilic Esophagitis in a Large Multicenter Patient Population from the Consortium for Food Allergy Research. J Allergy Clin Immunol Pract 2018;6:1534–1544 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hommel KA, Franciosi JP, Gray WN, et al. Behavioral functioning and treatment adherence in pediatric eosinophilic gastrointestinal disorders. Pediatr Allergy Immunol 2012;23:494–9. [DOI] [PubMed] [Google Scholar]
- 60.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015;5:470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


