Abstract
Bone stress injuries (BSIs) occur at inopportune times to invariably interrupt training. All BSIs in runners occur due to an ‘error’ in workload wherein the interaction between the number and magnitude of bone tissue loading cycles exceeds the ability of the tissue to resist the repetitive loads. There is not a single optimal bone workload rather a range which is influenced by the prevailing scenario. In prepubertal athletes, optimal bone workload consists of low-repetitions of fast, high-magnitude, multidirectional loads introduced a few times per day to induce bone adaptation. Premature sports specialization should be avoided so as to develop a robust skeleton that is structurally optimized to withstand multidirectional loading. In the mature skeleton, optimal workload enables gains in running performance, but minimizes bone damage accumulation by sensibly progressing training, particularly training intensity. When indicated (e.g. following repeated BSIs) attempts to reduce bone loading magnitude should be considered, such as increasing running cadence. Determining the optimal bone workload for an individual athlete to prevent and manage BSIs requires consistent monitoring. In the future, it may be possible to clinically determine bone loads at the tissue level to facilitate workload progressions and prescriptions.
Keywords: exercise, relative energy deficiency in sport, running, stress fracture, stress reaction
Bone stress injuries (BSIs), including those presenting as a radiographically-confirmed cortical defect (i.e. stress fracture), are frustrating. Frequently occurring in the lead up to a major running event, these injuries invariably require interruption to training as the prodromal pain and risk of progression to complete fracture are real. It is well established cumulative loading, inherent to running, contributes to bone fatigue, which presents as microscopic damage (i.e. microdamage) in bone tissue [1, 2]. Microdamage is a normal and necessary phenomenon occurring in all skeletons independent from athletic ability. It triggers targeted remodeling where bone-resorbing osteoclasts remove damaged regions of bone before bone-forming osteoblasts fill the void with new, undamaged bone [3].
It can be argued all BSIs occur due to an ‘error’ in workload, whereby microdamage accumulation in response to cumulative loading outweighs the ability to repair or resist the damage. Assuming a suitable workload and healthy athlete, microdamage is removed at the same rate new microdamage forms; however, the process takes time. Osteoclast activation and resorption in cortical bone takes approximately 4 weeks, and replacement with new bone can take three months and up to a year for full mineralization [4]. The process is longer in trabecular bone, which may explain why BSIs at trabecular rich sites have more prolonged healing times [5].
Within the remodeling timeline, the transition period between resorption and formation is consequential to BSIs. Bone resorption creates porosity, which influences mechanical properties and reduces fatigue resistance (Fig. 1) [6]. This creates the potential for a feedforward loop whereby suboptimal workload (e.g. too rapid progression of training) can heighten microdamage formation, and its accumulation, coalescence, and progression to a BSI. BSIs begin appearing approximately 3-4 weeks following a major workload ‘error’, as observed in military recruits experiencing a large change in workload as they transitioned from a sedentary lifestyle to the rigors of basic training [7].
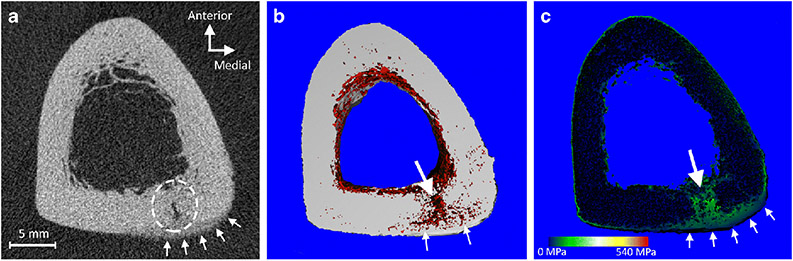
Figure 1.
Bone stress injuries (BSI) cause porosity and reduced localized mechanical properties. A) Tomographic image of a posteromedial tibial cortex BSI (broken circle) in a 22-year-old female distance runner, acquired using high-resolution peripheral quantitative computed tomography (voxel resolution = 61 μm). Note the presence of undermineralized callus bridging the periosteal surface at the injury site (arrows). B) 3D map showing regions of porosity in red. The majority of the tibial cortex has limited porosity, including the newly formed undermineralized callus. However, there is prevalent porosity at the BSI site (large arrow) and branching medially and laterally along the original periosteal layer of bone (small arrows). C) Finite element model of the stress distribution in response to axial compressive loading. Stresses are concentrated on the regions of the BSI (large arrow) and the immature undermineralized callus (small arrows).
What is bone workload?
There is no uniform definition of workload. From the perspective of athletic performance, there is consensus workload comprises a combination of internal and external factors which combine to determine training and/or competition stress [8]. Because microdamage occurs at the tissue level, bone workload leading to microdamage formation and its progression to a BSI is best described at this level.
Applied loads produce stresses and strains within bone tissue which in turn can produce microdamage. Here, stress is a localized measure of load intensity defined as the force per unit area of tissue and strain is a normalized measure of tissue deformation created by stress. Microdamage formation is threshold-driven and depends on the interaction between the number of times a bone is loaded, and the magnitude and rate that stresses and strains are generated. Of these factors, load magnitude appears most important in terms of bone fatigue and BSI risk [9].
There is a strong correlation between the magnitude of bone tissue stress/strain and the number of cycles before bone fatigue failure. The relationship is often described by an inverse power law which indicates small increases in stress/strain dramatically reduce the number of cycles until fatigue failure, and vice versa. In vitro, the rate of microdamage accumulation increases with strain magnitude by an exponent of 17 [10]. For the loads relevant to running, it has been estimated a 10% increase in tissue stress/strain results in halving the number of loading cycles before failure [11].
The stresses and strains experienced within a bone depend on the magnitude of the applied load and the ability of the tissue to resist this load. Greater applied loads generate greater stresses and strains whereas weaker bones experience greater stresses and strains for a given applied load. Thus, workload approaches to minimize microdamage and reduce BSI risk aim to: 1) improve the ability of the skeleton to resist load by inducing mechanoadaptation and 2) manage the loads being introduced to the skeleton to reduce damage accumulation. These two approaches are somewhat paradoxical, suggesting loading both protects against and causes BSI development. Ultimately, optimal bone workload can be defined as: the interaction between the number and magnitude of bone loading cycles that induces adaptation to best enhance function and reduce the risk of re/injury.
Optimal workload to induce skeletal mechanoadaptation
The ability of the skeleton to resist load is determined by its mass, structure and material quality. There is great potential for the skeleton to adapt to mechanical loads to improve its strength. For instance, baseball players have nearly double the strength of the humeral diaphysis in their throwing arm compared to their contralateral non-throwing arm [12]. The adaptation reduces tissue stresses and strains such that they remain safely below fracture thresholds during throwing. If the same throwing-related forces were introduced to the contralateral non-adapted humerus it would catastrophically fail in a single pitch [12]. In terms of BSI risk, an animal study showed a moderate (<10%) gain in bone mass induced by a loading program can generate a large and exponential (>100-fold) gain in bone fatigue resistance as a result of less tissue level strain being experienced during each fatigue loading cycle [13].
Not all athletes have good skeletons
There is a presumption all athletes have good bone health as they are regularly exposed to elevated loads; however, this is not always true. The mechanosensitive machinery in bone responds best to high magnitude loads introduced at high rates. Weight bearing activities incorporating impulsive loading, particularly those involving some degree of intermittent, explosive jumping and/or sprinting with rapid changes in direction, have the greatest osteogenic potential (Fig. 2a).
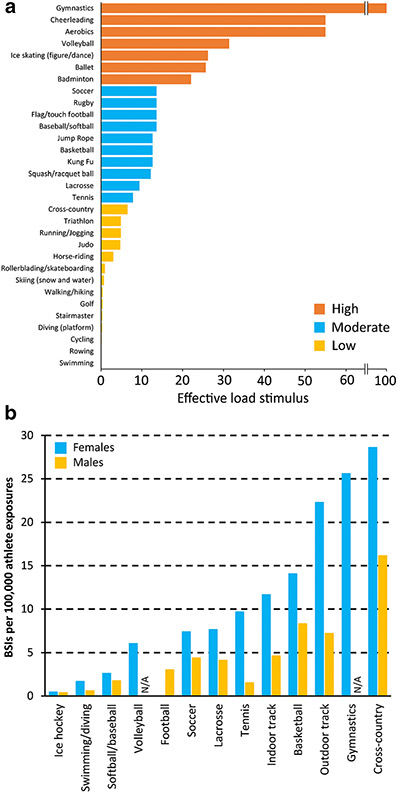
Figure 2.
A) Lower extremity effective load ratings for common physical activities, with higher load ratings being representative of a greater bone osteogenic stimulus. Effective load ratings were estimated from the magnitude and rate of ground reaction force generation during representative actions (or similar actions when reaction forces could not be directly measured). Data from Weeks and Beck.[86] B) Incidence of bone stress injuries (BSIs) in females and males across collegiate sports in the United States over a 10-year period. N/A = data not available. Data from Rizzone et al.[15]
Gymnastic activities generate some of the greatest osteogenic stimuli and accordingly gymnasts have high bone mass [14]. Interestingly, gymnasts also have a high incidence of BSIs [15] suggesting a finite ability by their well-adapted skeletons to tolerate applied loads (Fig. 2b). Similarly, basketball players experience a relatively high number of BSIs despite typically good skeletal health. Conversely, swimmers and cyclists experience limited osteogenic stimuli resulting in low bone mass compared to other athletes [16], yet experience few BSIs due to low bone workloads (Fig. 2b) [15]. These findings underscore the importance of understanding the balance between applied bone loads and underlying bone health across a spectrum of athletes.
Distance runners have some of the highest incidences of BSIs (Fig. 2b). A contributing factor in many is poor bone health. For instance, up to 40% of female adolescent cross-country runners have a dual-energy x-ray absorptiometry (DXA) z-score of below −1 for spine areal bone mineral density (aBMD) [17]. There are numerous possible reasons for the inferior bone health in cross-country athletes. The most obvious is the high occurrence of Relative Energy Deficiency in Sport (RED-S) and Female Athlete Triad in this population, with up to 50% meeting criteria for disordered eating and/or reporting menstrual dysfunction [17-19]. However, another important contributing factor is that distance running simply is not a good bone building activity. The latter is supported by the observation that gymnasts exhibit higher bone mass than runners despite both populations having a similar prevalence of menstrual dysfunction [20].
Distance running does not build good bones
Bone cells desensitize or become ‘deaf’ to repetitive loading. They lose 95% of their mechanosensitivity after only 20 back-to-back loading cycles and introducing additional cycles does not yield proportional adaptation [21]. The implication is that after a few minutes of running, bone cells find the monotonous, unidirectional loading to be boring and they stop responding.
A period of relative rest enables the system to regain mechanosensitivity to support further adaptation. Over 90% of mechanosensitivity is restored with 4-8 hours of rest between repeat loading bouts [22]. Thus, a few minutes of a bone-centric exercise (e.g. plyometrics) later in the day after a running session may generate further bone adaptation over and above that generated solely by running. The addition of short bouts of bone-centric exercises requires consideration of cumulative bone workload, but the approach has been used in other sports to improve bone health while limiting exposure to excessive loading cycles [23].
Use periodization to build more bone
Bone cells also lose sensitivity over a block of training (e.g. across a sport season). The adaptive response of bone cells to loading is proportional to the difference between the applied and routine loads [24, 25]. When routine bone loads are high, a greater stimulus is required to create an adaptive response as the threshold to respond is raised [26]. Progressive overload does not address this issue as each load increment leads to accommodation creating a situation where the difference between the new and routine load is small [27].
Periodization can be used to improve bone cell mechanosensitivity, particularly in individuals participating in year-round sports such as distance running. In an animal model, bone adaptation was compared between groups that received either continuous loading for 15 weeks versus a periodized approach of two 5-week blocks separated by a 5-week ‘rest’ period [28]. Despite receiving one-third less cumulative load, bone adaptation in the periodized group was greater since the rest period restored the mechanosensitivity prior to the recommencement of loading. Clinically, “rest” would involve other conditioning activities (such as cycling, swimming, water running) that load alternate skeletal sites, rather than absolute rest.
Early sport specialization likely contributes to BSIs
Early sport specialization (i.e. intensive prepubertal participation in a single sport for more than 8-months of year at the expense of other sports) has been associated with an increased risk of overuse injury [29], which may include BSIs. The years from birth to the pubertal growth period provide a window of opportunity to accrue bone mass. This was eloquently shown in racquet sport players. Girls who began playing before puberty had more than twice as much adaptation between their racquet and non-racquet arms compared to girls who began playing after puberty [30]. Athletes who specialize early in sports with low osteogenic potential (e.g. distance running, swimming, cycling) may enter adolescence and young adulthood with low bone mass and elevated BSI risk [31, 32].
Structural optimization is critical and only develops before puberty
More importantly than facilitating bone mass accrual, loading during growth provides a once-in-a-lifetime opportunity to optimize bone size. Bone size develops principally due to modeling (not remodeling) which involves new bone forming on existing surfaces without prior resorption. Growth is associated with rapid modeling on the periosteal/outer surface which increases bone girth. Loading when young encourages additional bone to be added periosteally to further increase bone size [33] and disproportionately increase bone strength and fatigue resistance for the amount of material added (Fig. 3) [13, 34]. Also, the bone size, but not mass, benefits of loading when young persist long term [12, 35]. In contrast, there is no consistent evidence mechanical loading impacts bone size once skeletal maturity is reached, with the less consequential gains in bone mass occurring at the inner endocortical surface post-puberty [33].
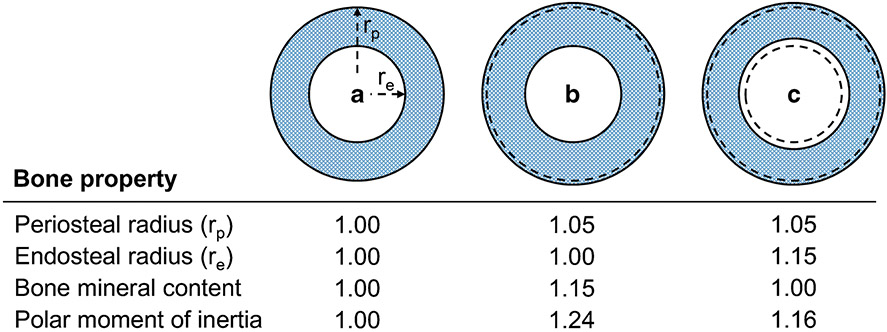
Figure 3.
Loading-induced addition of bone on the outer periosteal surface is functionally important, helping the skeleton meet its dual needs of being strong to resist injury, but lightweight for energy efficient motion. A) The polar moment of inertia (i.e. strength) of a bone is proportional to the radii of its outer periosteal (rp) and inner endocortical (re) surfaces according to the relationship π(rp4-re4)/2. This relationship illustrates that periosteal surface changes have a greater influence on strength than changes on the endocortical surface. B) For example, a 5% increase in rp (equating to a 15% increase in bone mineral content [i.e. mass]) results in a disproportionate 24% increase in strength, assuming constant bone material properties (i.e. volumetric bone mineral density) and an initial rp-to-re ratio of 1.8. C) If the same mass of bone added to the periosteal surface was simultaneously removed from the endocortical surface, re would increase by 15%, but the bone would still be 16% stronger than the bone with same mass in A) because of its greater size (i.e. 5% greater rp). Broken lines in B) and C) indicate the original bone surfaces in A).
Load in multiple directions to build a robust skeleton
Bone adaptation to loading has directionality, with bone mass being added and size developing in accordance with the direction of loading [36]. The implication is that athletes who specialize in unidirectional sports from a young age (e.g. long-distance road running) may not have the ability to resist loading in alternate directions (e.g. such as during trail running, with running on natural terrain changing the principal loading axes [37]).
Activities requiring jumping and landing in different directions or running with rapid changes in direction should be encouraged during growth, such as occurs during basketball, volleyball, soccer, and gymnastics, to name a few sports. Participants in these sports exhibit more structurally robust lower extremity bones which may be more resistant to BSI [38, 39]. Indeed, military recruits with a prior history of playing ball sports had nearly 1/3rd the odds of developing a BSI in basic training than those without a history of playing these sports [40]. Multidirectional activities should be encouraged from a very young age when the skeleton is most permissive, and single sport specialization should be delayed at least until high school so to develop a robust skeleton that can withstand multidirectional loading.
Managing the load being introduced to the skeleton
If skeletal mechanoadaptation requires proactive attention when very young and continuing through puberty, what do we do for skeletally mature athletes? In this case, attempts at improving skeletal health are still important, particularly in athletes with poor bone health (e.g. distance runners suffering from RED-S with associated low bone mass and a history of repeat BSIs). Nevertheless, bone gains are more difficult to achieve in mature athletes, particularly in terms of optimizing bone size. For those who have reached skeletal maturity, attention shifts more towards managing applied loads.
The load applied to bone represents the summation of external and internal forces, which are influenced by a range of variables including biomechanical factors, muscle performance, and environmental characteristics (e.g. training surface/s, shoes and inserts, etc.). However, training factors are by far the leading contributor with which all other factors interact.
All BSIs occur due to training errors
All running injuries are training load injuries [41], and BSIs are no exception. Evidence suggests rapid increases in training loads increase the risk of running injuries [42, 43]. Historically, the ‘10% rule’ has been used to guide increases in running volume on a weekly basis [44]. More recently, an athlete’s acute:chronic workload ratio (ACWR) [45] has been proposed to guide training load prescriptions. The ACWR is most commonly defined by the ratio of workload for the previous week to the average workload for the previous three to six weeks, with the training goal being to avoid large increases or ‘spikes’ in this measure. However, there is much individual variability with respect to tolerance to changes in training loads and it is unlikely a single “rule” can be uniformly applied to eliminate running injuries [46]. Also, different skeletal sites may respond differently to changes in workload [47]. Training workloads should be individualized since two runners may have identical training loads but have different injury patterns. Individualized risk of BSI likely relates to the complex interaction between rapid changes in workload and a runner’s biomechanics, psychology, physiology, musculoskeletal qualities, and energy availability [48, 49].
Changes in workload occur at specific times depending on sport
Identifying scenarios when large changes in workload are likely enables implementation of preventative strategies. The most hazardous times for BSIs in seasonal sports (e.g. basketball, soccer, outdoor track) are during preseason and with the intensity increase from preseason to competition [15, 50]. These times present particular risk for those returning from off-season surgery and those transitioning between competitive levels (e.g. moving from high-school to collegiate to professional level).
Athletes competing in seasonal sports should be afforded a workload reduction at the end of the competitive season to take advantage of periodization. However, a progressive bone loading and general conditioning program should be considered leading up to preseason to dampen the spike in workload with the return from the off-season and to help reduce seasonal variations in bone health. A lead-in program is particularly important in those with history of BSis as they are at high risk of another BSI [51].
In more year-round sports where training is more constant and progressive (e.g. gymnastics and cross-country), BSI risk progressively increases across the competitive season [15]. However, the risk patterns vary by sex. Risk in male cross-country runners is fairly consistent across the season, whereas it progressively increases in females [15]—possibly due to increasing effects of RED-S as training progresses, with RED-S being more common in females [52]. In athletes competing in more year-round sports, BSI mitigating strategies include substituting training sessions with activities requiring reduced load (discussed later) and incorporating rest periods (e.g. at least 1 rest day per week and 1-to-2 weeks rest every 3 months [53]).
Monitoring bone workload
The often weeks delay between workload change and BSI development necessitates clinicians, coaches, and athletes closely track workload. While the majority of runners tend to overestimate their training volume [54], individuals who develop a BSI tend to under-report their training volume and intensities when compared with wearable monitors [49]. Thus, using objective means to monitor training load should be the cornerstone of managing risk of BSI.
Unfortunately, there is no prospectively established workload metric that accurately predicts BSIs. The proposed microdamage origin of BSIs suggests monitoring of tissue level loading is the ultimate goal, but determining bone tissue loading is neither trivial or currently available clinically. Surrogate metrics, such as GRFs and segmental accelerations, and the development of wearable sensors accompanied with software algorithms and user interfaces (collectively known as ‘wearables’) are an attempt to fill this void [55].
GRFs and segmental accelerations are used to quantify and monitor the intensity of foot-ground impacts, with the presumption that their magnitudes and rates are related to BSI risk. However, limited evidence supports a causal link between foot-ground impact characteristics and BSIs, with currently available data derived mostly from retrospective cohort studies [56]. The lack of data supporting causality has raised conjecture as to the relative contributions of the initial foot-ground impact versus muscle contraction in BSI genesis [57].
Internal tissue loads are much greater than those predicted from external measures because of the pull of muscle [58]; this was illustrated decades ago for running [59]. Differentiating impact-related versus muscle-generated bone loading is challenging given most impulsive (i.e. high impact) activities are also those with greatest muscular loading. However, muscle (and, consequently, bone) loads peak well after initial foot-ground impact and nearer midstance of the running gait cycle—near the second or ‘active’ peak of the ground reaction force (Fig. 4) [57, 60, 61]. Foot-ground impact may generate greater loading rates, but the majority of cadaveric research suggests bone is able to better withstand repetitive loads when applied over shorter durations (i.e. higher loading rate) [11], tilting the balance towards greater importance of high-magnitude muscle-derived bone loading. Unfortunately, at the moment, clinicians cannot capture muscle-derived loading.
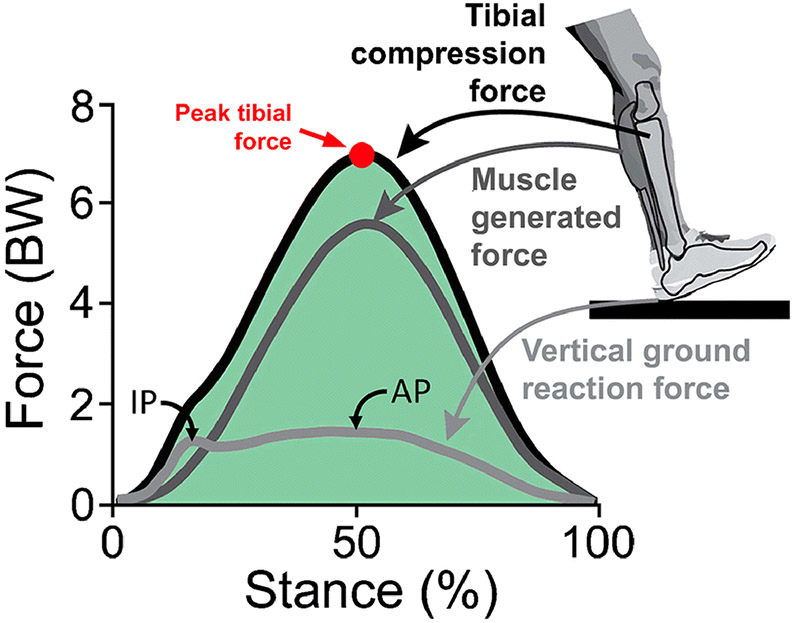
Figure 4.
Vertical ground reaction force, and computed muscle generated and tibial compression forces during running with a typical rear-foot strike pattern. The external ground reaction force has two peaks—an initial rapidly reached impact peak (IP) and a second slower, but higher magnitude active peak (AP). Internal muscle generated and tibial compressive forces, computed via subject-specific musculoskeletal modeling, far exceed ground reaction forces and peak near the active peak of the ground reaction force. The later peak of tibial forces has raised the question of the relative contribution of initial foot-ground impact versus later muscle generated forces in BSI genesis. Image adapted from: Matijevich E, Scott L, Volgyesi P, Derry K, Zelik K. Combining wearable sensor signals, machine learning and biomechanics to estimate tibial bone force and damage during running. Human Movement Science 2020;74:102690, with permission from Elsevier.
Progress training duration before intensity
The non-linear relationship between loading cycles and their magnitude before bone fatigue failure can be used to guide training progression. Assuming all other risk factors remain constant (e.g. energy availability) there is a linear one-to-one increase in BSI risk for an increase in running volume (i.e. number of loading cycles). In contrast, the disproportionate reduction in bone fatigue life with increasing loading magnitude means BSI risk increases more rapidly with increases in running velocity. These observations suggest it is safer to initially increase training volume than intensity, and have led to the concept of high volume, low velocity training (e.g. ‘train slow to race fast’). In a probabilistic model, running the same distance but with decreased speed from 3.5 to 2.5 m/sec reduced tibial BSI likelihood by half [62]. Ultimately, bouts of high-speed running should be performed judiciously and progressions in high speed running should be coupled with temporary reductions in running volume.
Training with reduced bone workload
Beyond careful management of high-speed running volume, there are other practical methods of reducing bone loads without compromising training benefits. For example, treadmill running may engender lower tibial bone strain than overground training [63], despite minimal differences in running mechanics and physiological metrics between the two running modes [64]. Thus, treadmill running may be substituted for one or more overground sessions per week to reduce cumulative bone workload.
Treadmill running can also be coupled with body weight support (e.g. with lower body positive pressure or a mounted upper body support system) to further reduce bone workload, and be performed on steady incline. The role of incline running (and, similarly stairclimbing) on BSI risk remains unknown. On one hand, incline running may reduce BSI risk as it reduces impact loads and accelerations [65]. On the other hand, it may increase risk by increasing muscle-induced bone loading or by shifting risk to an alternate site (e.g. metatarsals).
Can we alter running mechanics to alter bone workload?
It may be possible to alter bone workload through gait retraining. Gait retraining is usually reserved for runners with repeat BSIs and currently involves implementing techniques to reduce GRFs and/or bone accelerations, despite evidence lacking that these metrics are valid surrogates for tissue-level loading. Techniques currently being applied include increasing stride rate (i.e. cadence) [66], cueing a softer landing by providing feedback on peak positive tibial acceleration [67], or transitioning an athlete to a forefoot (FFS) strike pattern [68]. Ultimately, retraining interventions should aim to reduce bone tissue loads, not surrogates for bone loads such as vertical GRFs [57]. For instance, cueing a softer landing may reduce GRFs but may require higher muscle forces ultimately resulting in greater bone loads [69].
Of the techniques listed, cueing an increase in cadence appears to have considerable clinical promise. Increasing running cadence above a preferred rate results in a proportional decrease in stride length at a given speed. The net result is an increase in the number of loading cycles for a given running distance, but a concomitant reduction in the vertical excursion and velocity of the center of mass, reduced peak hip adduction angle and moment, reduced GRFs and tibial accelerations, and reduced demands on lower extremity joints [70, 71]. The combination of these changes may improve running economy [72] and has been modeled to reduce internal loading, which was predicted to more than offset any increase in tibial BSI risk associated with the increased loading cycles [73]. In high school cross-country runners, those in the lowest quartile for step rate were 6.7 times more likely to experience a shin injury compared to runners in the highest step rate quartile [74], and prescriptive decreases in step length (e.g. via increased cadence) contributed to a reduction in the incidence of BSIs in female military recruits [75, 76]. Runners can be retrained to increase their running cadence easily in the clinic, and most commercially available running watches enable runners to retrain and monitor cadence during routine runs [66].
Does muscle strengthening increase or decrease BSI risk?
There is no doubt muscle loads bone, but debate remains as to whether muscle-induced loading is causative or protective of BSIs. Biomechanical data suggests most bone loading is muscle-induced [59, 57]; however, clinical data points to a potential protective role of muscle on BSI risk. In particular, prospective clinical studies demonstrated BSI susceptibility was directly related to muscle size (girth and cross-sectional area) [7, 77, 78] and strength [79].
Enhanced muscle properties may aid in diffusing forces across the bone cortex during running or reduce bending moments induced by external loads. Alternatively, they may protect against the skeletal consequences of fatigue. Runners exhibit greater tibial stress and strain during running after an exertion protocol [80, 81], suggesting poor endurance may elevate the risk of BSI. In addition, intense running can lead to altered kinematics, which may modify the direction of bone loading resulting in increased strain at less accustomed sites [82].
Overall, these data suggest improving muscular endurance and strength may benefit runners at risk for BSI. Unfortunately, much of the evidence supporting resistance training to reduce BSI risk is retrospective. For example, female military cadets with <7 months of resistance training prior to basic combat training had a 4-fold greater risk of sustaining a BSI than cadets who habitually strength trained [83], and adolescent runners who did not strength train were more likely to sustain a BSI during a cross country season [84]. Lastly, greater bone density is observed in runners and athletes who regularly participate in heavy resistance training compared with those who solely did their sport [85].
Summary
Optimal bone workload promotes beneficial adaptation to best enhance function and reduce the risk of re/injury. There is not a single optimal workload, rather a range which are influenced by the current scenario. In athletes before their adolescent pubertal growth period, optimal bone workload consists of low-repetitions of fast, high-magnitude, multidirectional, novel loads introduced a few times per day. Care needs to be taken to avoid premature sports specialization to develop a robust skeleton that is structurally optimized and can withstand loading in multiple directions. In the mature skeleton, tracking of workload is indicated to avoid acute spikes. Rest periods should be incorporated into each program, at least 1 d/wk and 1 wk every 3 months. When indicated (e.g. following repeated BSIs), attempts to reduce bone loading magnitude such as increasing cadence should be considered.
Acknowledgments
Funding: This contribution was partly made possible by support from the NBA & GE Healthcare Orthopedics and Sports Medicine Collaboration (BSI-030, D776) and National Institutes of Health (P30 AR072581).
Footnotes
Conflicts of interest/competing interests: The authors have no financial disclosures or conflicts of interest.
Ethics approval: not applicable.
Consent to participate: not applicable.
Consent for publication: not applicable.
Availability of data and material: not applicable.
Code availability: not applicable.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. [DOI] [PubMed] [Google Scholar]
- 2.Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44(10):749–65. doi: 10.2519/jospt.2014.5334. [DOI] [PubMed] [Google Scholar]
- 3.Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30(1):2–4. [DOI] [PubMed] [Google Scholar]
- 4.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11(4):219–27. doi: 10.1007/s11154-010-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nattiv A, Kennedy G, Barrack MT, Abdelkerim A, Goolsby MA, Arends JC et al. Correlation of MRI grading of bone stress injuries with clinical risk factors and return to play: a 5-year prospective study in collegiate track and field athletes. Am J Sports Med. 2013;41(8):1930–41. doi: 10.1177/0363546513490645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loundagin LL, Haider IT, Cooper DML, Edwards WB. Association between intracortical microarchitecture and the compressive fatigue life of human bone: A pilot study. Bone Reports. 2020;12:100254. doi: 10.1016/j.bonr.2020.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong DW III., Rue J-PH, Wilckens JH, Frassica FJ. Stress fracture injury in young military men and women. Bone. 2004;35:806–16. [DOI] [PubMed] [Google Scholar]
- 8.Bourdon PC, Cardinale M, Murray A, Gastin P, Kellmann M, Varley MC et al. Monitoring athlete training loads: consensus statement. Int J Sports Physiol Perform. 2017;12(Suppl 2):S2161–70. doi: 10.1123/ijspp.2017-0208. [DOI] [PubMed] [Google Scholar]
- 9.Loundagin LL, Schmidt TA, Edwards WB. Mechanical fatigue of bovine cortical bone using ground reaction force waveforms in running. J Biomech Eng. 2018;140(3):0310031–5. doi: 10.1115/1.4038288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotton JR, Winwood K, Zioupos P, Taylor M. Damage rate is a predictor of fatigue life and creep strain rate in tensile fatigue of human cortical bone samples. J Biomech Eng. 2005;127(2):213–9. doi: 10.1115/1.1865188. [DOI] [PubMed] [Google Scholar]
- 11.Edwards WB. Modeling overuse injuries in sport as a mechanical fatigue phenomenon. Exerc Sport Sci Rev. 2018;46(4):224–31. doi: 10.1249/jes.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 12.Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A. 2014;111:5337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20:809–16. [DOI] [PubMed] [Google Scholar]
- 14.Jurimae J, Gruodyte-Raciene R, Baxter-Jones ADG. Effects of gymnastics activities on bone accrual during growth: a systematic review. Journal of sports science & medicine. 2018;17(2):245–58. [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzone KH, Ackerman KE, Roos KG, Dompier TP, Kerr ZY. The epidemiology of stress fractures in collegiate student-athletes, 2004-2005 through 2013-2014 academic years. J Athl Train. 2017;52(10):966–75. doi: 10.4085/1062-6050-52.8.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scofield KL, Hecht S. Bone health in endurance athletes: runners, cyclists, and swimmers. Curr Sports Med Rep. 2012;11(6):328–34. doi: 10.1249/JSR.0b013e3182779193. [DOI] [PubMed] [Google Scholar]
- 17.Barrack MT, Rauh MJ, Nichols JF. Prevalence of and traits associated with low BMD among female adolescent runners. Med Sci Sports Exerc. 2008;40(12):2015–21. doi: 10.1249/MSS.0b013e3181822ea0. [DOI] [PubMed] [Google Scholar]
- 18.Rauh MJ, Barrack M, Nichols JF. Associations between the female athlete triad and injury among high school runners. International journal of sports physical therapy. 2014;9(7):948–58. [PMC free article] [PubMed] [Google Scholar]
- 19.Skorseth P, Segovia N, Hastings K, Kraus E. Prevalence of female athlete triad risk factors and iron supplementation among high school distance runners: results from a triad risk screening tool. Orthopaedic journal of sports medicine. 2020;8(10):2325967120959725. doi: 10.1177/2325967120959725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J Bone Miner Res. 1995; 10(1):26–35. [DOI] [PubMed] [Google Scholar]
- 21.Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30(5):781–6. [DOI] [PubMed] [Google Scholar]
- 22.Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204(Pt 19):3389–99. [DOI] [PubMed] [Google Scholar]
- 23.Vlachopoulos D, Barker AR, Ubago-Guisado E, Williams CA, Gracia-Marco L. A 9-month jumping intervention to improve bone geometry in adolescent male athletes. Med Sci Sports Exerc. 2018;50(12):2544–54. doi: 10.1249/mss.0000000000001719. [DOI] [PubMed] [Google Scholar]
- 24.Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23:399–407. [DOI] [PubMed] [Google Scholar]
- 25.Turner CH. Toward a mathematical description of bone biology: the principal of cellular accommodation. Calcif Tissue Int. 1999;65:466–71. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo: the strain threshold for an osteogenic response varies with location. J Bone Miner Res. 2001;16(12):2291–7. [DOI] [PubMed] [Google Scholar]
- 27.Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accomodation and the response of bone to mechanical loading. J Biomech. 2005;38:1838–45. [DOI] [PubMed] [Google Scholar]
- 28.Saxon LK, Robling AG, Alam I, Turner CH. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone. 2005;36(3):454–64. doi:S8756-3282(04)00469-7 [pii] 10.1016/j.bone.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Bell DR, Post EG, Biese K, Bay C, Valovich McLeod T. Sport specialization and risk of overuse injuries: a systematic review with meta-analysis. Pediatrics. 2018;142(3):e20180657. doi: 10.1542/peds.2018-0657. [DOI] [PubMed] [Google Scholar]
- 30.Kannus P, Haapasalo H, Sankelo M, Sievänen H, Pasanen M, Heinonen A et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123(1):27–31. [DOI] [PubMed] [Google Scholar]
- 31.Rauh MJ, Tenforde AS, Barrack MT, Rosenthal MD, Nichols JF. Sport specialization and low bone mineral density in female high school distance runners. J Athl Train. 2020;55(12):1239–46. doi: 10.4085/1062-6050-0547.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenforde AS, Fredericson M. Influence of sports participation on bone health in the young athlete: a review of the literature. PM R. 2011;3(9):861–7. doi: 10.1016/j.pmrj.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17(12):2274–80. [DOI] [PubMed] [Google Scholar]
- 34.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17(8):1545–54. [DOI] [PubMed] [Google Scholar]
- 35.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22(2):251–9. [DOI] [PubMed] [Google Scholar]
- 36.Warden SJ, Carballido-Gamio J, Weatherholt AM, Keyak JH, Yan C, Kersh ME et al. Heterogeneous spatial and strength adaptation of the proximal femur to physical activity: a within-subject controlled cross-sectional study. J Bone Miner Res. 2020;35(4):681–90. doi: 10.1002/jbmr.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milgrom C, Finestone AS, Voloshin A. Differences in the principal strain angles during activities performed on natural hilly terrain versus engineered surfaces. Clin Biomech. 2020;80:105146. doi: 10.1016/j.clinbiomech.2020.105146. [DOI] [PubMed] [Google Scholar]
- 38.Nikander R, Sievanen H, Uusi-Rasi K, Heinonen A, Kannus P. Loading modalities and bone structures at nonweight-bearing upper extremity and weight-bearing lower extremity: a pQCT study of adult female athletes. Bone. 2006;39(4):886–94. [DOI] [PubMed] [Google Scholar]
- 39.Schipilow JD, Macdonald HM, Liphardt AM, Kan M, Boyd SK. Bone micro-architecture, estimated bone strength, and the muscle-bone interaction in elite athletes: an HR-pQCT study. Bone. 2013;56(2):281–9. doi: 10.1016/j.bone.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Milgrom C, Simkin A, Eldad A, Nyska M, Finestone A. Using bone's adaptation ability to lower the incidence of stress fractures. Am J Sports Med. 2000;28:245–51. [DOI] [PubMed] [Google Scholar]
- 41.Hreljac A. Impact and Overuse Injuries in Runners. Medicine & Science in Sports & Exercise. 2004:845–9. doi: 10.1249/01.mss.0000126803.66636.dd. [DOI] [PubMed] [Google Scholar]
- 42.Damsted C, Glad S, Nielsen RO, Sørensen H, Malisoux L. Is there evidence for an association between changes in training load and running-related injuries? A systematic review. International journal of sports physical therapy. 2018;13(6):931. [PMC free article] [PubMed] [Google Scholar]
- 43.Rauh MJ. Summer training factors and risk of musculoskeletal injury among high school cross-country runners. J Orthop Sports Phys Ther. 2014;44(10):793–804. doi: 10.2519/jospt.2014.5378. [DOI] [PubMed] [Google Scholar]
- 44.Johnston CA, Taunton JE, Lloyd-Smith DR, McKenzie DC. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Gabbett TJ. The training-injury prevention paradox: should athletes be training smarter and harder? Br J Sports Med. 2016;50(5):273–80. doi: 10.1136/bjsports-2015-095788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen RO, Buist I, Sorensen H, Lind M, Rasmussen S. Training errors and running related injuries: a systematic review. Int J Sports Phys Ther. 2012;7(1):58–75. [PMC free article] [PubMed] [Google Scholar]
- 47.Finestone A, Milgrom C, Wolf O, Petrov K, Evans R, Moran D. Epidemiology of metatarsal stress fractures versus tibial and femoral stress fractures during elite training. Foot Ankle Int. 2011;32(1):16–20. doi: 10.3113/fai.2011.0016. [DOI] [PubMed] [Google Scholar]
- 48.Bertelsen ML, Hulme A, Petersen J, Brund RK, Sørensen H, Finch CF et al. A framework for the etiology of running-related injuries. Scand J Med Sci Sports. 2017;27(11):1170–80. doi: 10.1111/sms.12883. [DOI] [PubMed] [Google Scholar]
- 49.Moran DS, Evans R, Arbel Y, Luria O, Hadid A, Yanovich R et al. Physical and psychological stressors linked with stress fractures in recruit training. Scand J Med Sci Sports. 2013;23(4):443–50. doi: 10.1111/j.1600-0838.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 50.Khan M, Madden K, Burrus MT, Rogowski JP, Stotts J, Samani MJ et al. Epidemiology and impact on performance of lower extremity stress injuries in professional basketball players. Sports health. 2018;10(2):169–74. doi: 10.1177/1941738117738988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright AA, Taylor JB, Ford KR, Siska L, Smoliga JM. Risk factors associated with lower extremity stress fractures in runners: a systematic review with meta-analysis. Br J Sports Med. 2015;49(23):1517–23. doi: 10.1136/bjsports-2015-094828. [DOI] [PubMed] [Google Scholar]
- 52.Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C et al. The IOC consensus statement: beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014;48(7):491–7. doi: 10.1136/bjsports-2014-093502. [DOI] [PubMed] [Google Scholar]
- 53.Krabak BJ, Tenforde AS, Davis IS, Fredericson M, Harrast MA, d'Hemecourt P et al. Youth distance running: strategies for training and injury reduction. Curr Sports Med Rep. 2019;18(2):53–9. doi: 10.1249/jsr.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 54.Dideriksen M, Soegaard C, Nielsen RO. Validity of Self-Reported Running Distance. J Strength Cond Res. 2016;30(6):1592–6. doi: 10.1519/JSC.0000000000001244. [DOI] [PubMed] [Google Scholar]
- 55.Moore IS, Willy RW. Use of wearables: tracking and retraining in endurance runners. Curr Sports Med Rep. 2019;18(12):437–44. doi: 10.1249/jsr.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 56.Zadpoor AA, Nikooyan AA. The relationship between lower-extremity stress fractures and the ground reaction force: a systematic review. Clin Biomech. 2011;26(1):23–8. doi: 10.1016/j.clinbiomech.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Matijevich ES, Branscombe LM, Scott LR, Zelik KE. Ground reaction force metrics are not strongly correlated with tibial bone load when running across speeds and slopes: implications for science, sport and wearable tech. PLoS ONE. 2019;14(1):e0210000. doi: 10.1371/journal.pone.0210000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avin KG, Bloomfield SA, Gross TS, Warden SJ. Biomechanical aspects of the muscle-bone interaction. Curr Osteoporos Rep. 2015;13(1):1–8. doi: 10.1007/s11914-014-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott SH, Winter DA. Internal forces at chronic running injury sites. Med Sci Sports Exerc. 1990;22:357–69. [PubMed] [Google Scholar]
- 60.Matijevich E, Scott L, Volgyesi P, Derry K, Zelik K. Combining wearable sensor signals, machine learning and biomechanics to estimate tibial bone force and damage during running. Human Movement Science. 2020;74:102690-undefined. doi: 10.1016/j.humov.2020.102690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang P-F, Sanno M, Ganse B, Koy T, Bruggemann G-P, Müller LP et al. Torsion and Antero-Posterior Bending in the In Vivo Human Tibia Loading Regimes during Walking and Running. PLoS ONE. 2014;9(4):e94525. doi: 10.1371/journal.pone.0094525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edwards WB, Taylor D, Rudolphi TJ, Gillette JC, Derrick TR. Effects of running speed on a probabilistic stress fracture model. Clin Biomech 2010;25(4):372–7. doi: 10.1016/j.clinbiomech.2010.01.001 S0268-0033(10)00002-1[pii]. [DOI] [PubMed] [Google Scholar]
- 63.Milgrom C, Finestone A, Segev S, Olin C, Arndt T, Ekenman I. Are overground or treadmill runners more likely to sustain tibial stress fracture? Br J Sports Med. 2003;37(2):160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Hooren B, Fuller JT, Buckley JD, Miller JR, Sewell K, Rao G et al. Is motorized treadmill running biomechanically comparable to overground running? A systematic review and meta-analysis of cross-over studies. Sports Med. 2020;50(4):785–813. doi: 10.1007/s40279-019-01237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vernillo G, Giandolini M, Edwards WB, Morin J-B, Samozino P, Horvais N et al. Biomechanics and physiology of uphill and downhill running. Sports Med. 2017;47(4):615–29. doi: 10.1007/s40279-016-0605-y. [DOI] [PubMed] [Google Scholar]
- 66.Willy RW, Buchenic L, Rogacki K, Ackerman J, Schmidt A, Willson JD. In-field gait retraining and mobile monitoring to address running biomechanics associated with tibial stress fracture. Scand J Med Sci Sports. 2016;26(2): 197–205. doi: 10.1111/sms.12413. [DOI] [PubMed] [Google Scholar]
- 67.Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78–83. doi: 10.1016/j.clinbiomech.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baggaley M, Willy RW, Meardon SA. Primary and secondary effects of real-time feedback to reduce vertical loading rate during running. Scand J Med Sci Sports. 2016. doi: 10.1111/sms.12670. [DOI] [PubMed] [Google Scholar]
- 69.Mills C, Pain MT, Yeadon MR. Reducing ground reaction forces in gymnastics’ landings may increase internal loading. Journal of Biomechanics. 2009;42(6):671–8. [DOI] [PubMed] [Google Scholar]
- 70.Baggaley M, Vernillo G, Martinez A, Horvais N, Giandolini M, Millet GY et al. Step length and grade effects on energy absorption and impact attenuation in running. Eur J Sport Sci. 2019:1–11. doi: 10.1080/17461391.2019.1664639. [DOI] [PubMed] [Google Scholar]
- 71.Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports health. 2014;6(3):210–7.doi: 10.1177/1941738113508544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quinn TJ, Dempsey SL, LaRoche DP, Mackenzie AM, Cook SB. Step frequency training improves running economy in well-trained female runners. J Strength Cond Res. doi: 10.1519/jsc.0000000000003206. [DOI] [PubMed] [Google Scholar]
- 73.Edwards WB, Taylor D, Rudolphi TJ, Gillette JC, Derrick TR. Effects of stride length and running mileage on a probabilistic stress fracture model. Med Sci Sports Exerc. 2009;41(12):2177–84. doi: 10.1249/MSS.0b013e3181a984c4. [DOI] [PubMed] [Google Scholar]
- 74.Luedke LE, Heiderscheit BC, Williams DS, Rauh MJ. Influence of step rate on shin injury and anterior knee pain in high school runners. Med Sci Sports Exerc. 2016;48(7):1244–50. doi: 10.1249/mss.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 75.Hill PF, Chatterji S, Chambers D, Keeling JD. Stress fracture of the pubic ramus in female recruits. J Bone Joint Surg Br. 1996;78(3):383–6. [PubMed] [Google Scholar]
- 76.Pope RP. Prevention of pelvic stress fractures in female army recruits. Mil Med. 1999;164(5):370–3. [PubMed] [Google Scholar]
- 77.Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437–44. [DOI] [PubMed] [Google Scholar]
- 78.Bennell KL, Malcolm SA, Thomas SA, Reid SJ, Brukner PD, Ebeling PR et al. Risk factors for stress fractures in track and field athletes: a twelve-month prospective study. Am J Sports Med. 1996;24:810–8. [DOI] [PubMed] [Google Scholar]
- 79.Hoffman JR, Chapnik L, Shamis A, Givon U, Davidson B. The effect of leg strength on the incidence of lower extremity overuse injuries during military training. Mil Med. 1999;164(2):153–6. [PubMed] [Google Scholar]
- 80.Milgrom C, Radeva-Petrova DR, Finestone A, Nyska M, Mendelson S, Benjuya N et al. The effect of muscle fatigue on in vivo tibial strains. J Biomech. 2007;40(4):845–50. [DOI] [PubMed] [Google Scholar]
- 81.Rice H, Weir G, Trudeau MB, Meardon S, Derrick T, Hamill J. Estimating tibial stress throughout the duration of a treadmill run. Medicine & Science in Sports & Exercise. 2019. [DOI] [PubMed] [Google Scholar]
- 82.Yoshikawa T, Mori S, Santiesteban AJ, Sun TC, Hafstad E, Chen J et al. The effects of muscle fatigue on bone strain. J Exp Biol. 1994;188:217–33. [DOI] [PubMed] [Google Scholar]
- 83.Rauh MJ, Macera CA, Trone DW, Shaffer RA, Brodine SK. Epidemiology of stress fracture and lower-extremity overuse injury in female recruits. Med Sci Sports Exerc. 2006;38(9):1571–7. doi: 10.1249/01.mss.0000227543.51293.9d. [DOI] [PubMed] [Google Scholar]
- 84.Nussbaum ED, Bjornaraa J, Gatt CJ Jr. Identifying Factors That Contribute to Adolescent Bony Stress Injury in Secondary School Athletes: A Comparative Analysis With a Healthy Athletic Control Group. Sports health. 2019;11(4):375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duplanty AA, Levitt DE, Hill DW, McFarlin BK, DiMarco NM, Vingren JL. Resistance training is associated with higher bone mineral density among young adult male distance runners independent of physiological factors. The Journal of Strength & Conditioning Research. 2018;32(6):1594–600. [DOI] [PubMed] [Google Scholar]
- 86.Weeks BK, Beck BR. The BPAQ: a bone-specific physical activity assessment instrument. Osteoporos Int. 2008; 19(11):1567–77. doi: 10.1007/s00198-008-0606-2. [DOI] [PubMed] [Google Scholar]