Abstract
Torsade de Pointes (TdP), a rare but lethal ventricular arrhythmia, is a toxic side effect of many drugs. To assess TdP risk, safety regulatory guidelines require quantification of hERG channel block in vitro and QT interval prolongation in vivo for all new therapeutic compounds. Unfortunately, these have proven to be poor predictors of torsadogenic risk, and are likely to have prevented safe compounds from reaching clinical phases. While this has stimulated numerous efforts to define new paradigms for cardiac safety, none of the recently developed strategies accounts for patient conditions. In particular, despite being a well-established independent risk factor for TdP, female sex is vastly underrepresented in both basic research and clinical studies, and thus current TdP metrics are likely biased toward the male sex. Here, we apply statistical learning to synthetic data, generated by simulating drug effects on cardiac myocyte models capturing male and female electrophysiology, to develop new sex-specific classification frameworks for TdP risk. We show that (1) TdP classifiers require different features in females vs. males; (2) male-based classifiers perform more poorly when applied to female data; (3) female-based classifier performance is largely unaffected by acute effects of hormones (i.e., during various phases of the menstrual cycle). Notably, when predicting TdP risk of intermediate drugs on female simulated data, male-biased predictive models consistently underestimate TdP risk in women. Therefore, we conclude that pipelines for preclinical cardiotoxicity risk assessment should consider sex as a key variable to avoid potentially life-threatening consequences for the female population.
Keywords: Quantitative pharmacology, computational biology, mathematical modeling, cardiology, adverse drug reactions
1. Introduction
During drug development, promising therapeutic compounds are tested to evaluate their potential risk of inducing Torsade de Pointes (TdP), a specific form of polymorphic ventricular tachycardia that can precipitate ventricular fibrillation and cause sudden cardiac death.1 While TdP is a very rare adverse event, amounting to less than one case out of 100,000 exposures for some non-antiarrhythmic drugs, cardiac safety concerns have caused withdrawal from the market of several drugs, including antihistamines, antidepressants, chemotherapeutics, pain medications, that had been associated with TdP proclivity in patients.2 The most simple mechanistic explanation of torsadogenicity involves a reduction of the rapid delayed rectifier K+ current (IKr), carried by the human Ether-à-go-go-Related Gene (hERG) channel, which importantly contributes to cardiac action potential (AP) repolarization.3 Pharmacological block of the hERG channel, which is a very promiscuous target interacting with cardiac and non-cardiac drugs, produces AP duration (APD) and QT interval prolongation, and leads to an increased susceptibility to pro-arrhythmic events. Based on this evidence, current safety regulatory guidelines require the measurement of hERG channel block in vitro and QT interval prolongation in vivo to estimate TdP risk.4,5 Since their adoption, these guidelines have successfully avoided that cardiotoxic drugs could endanger the welfare of people. However, it has also become apparent that these biomarkers are poor predictors of torsadogenic risk, and have in all probability prevented safe treatments from reaching the market.6,7
In response to this problem, recent efforts have led to several proposed new paradigms for the prediction of TdP. One notable example is the Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative, an international multi-group initiative by regulatory, industry, and academic partners including the US Food and Drug Administration.8 This paradigm relies upon the idea of combining in vitro studies to measure the drugs effects on each of the different types of ion channels and in silico models of cardiac myocyte electrophysiology to understand how these effects combine to influence cardiac function, thus creating a novel tool for TdP risk assessment of new drugs.9 Mathematical models of cardiac electrophysiology, in fact, make it possible to simulate extreme conditions with precision (e.g., dangerously supratherapeutic drug concentrations), and to obtain insights generally only accessible via animal experiments. Thus, computational approaches have become essential components of numerous strategies to predict torsadogenic risk.10–12 In addition, simulated measurements extracted from the biophysical model simulations can also be applied to machine learning (ML) pipelines, as demonstrated by the Sobie group,13 with the potential to bring out mechanistic insights buried in the data that could be otherwise ignored.
To our knowledge, however, no simulation-based approach has considered any risk factor for TdP in their predictive pipelines. An emblematic example is provided by known sex-differences: it is well established that women are more susceptible to TdP than men when treated with QT-prolonging drugs,14,15 suggesting that TdP risk classifiers could benefit from inclusion of biological sex as a predictive variable. However, female sex is highly underrepresented in both basic research16 and clinical studies17 involved in the drug development process, with important consequences for the identification of accurate TdP predictors. In vitro studies tend to use mostly male animals,16 raising concerns on the generalizability of findings to the whole population. This sex bias propagates onto the mathematical models of cardiac cells,18 which are parameterized based on male-dominated datasets. The issue of underestimating potential health risks for women is then aggravated by the fact that female sex is also underrepresented in clinical cohorts,17 making training of classifiers harder due to the lack of reliable ground truth data.
Here, we combined simulations of these mathematical models with machine learning to generate sex-specific TdP risk classifiers. We simulated the effects of 59 training drugs under different conditions using in silico models of human ventricular myocytes with sex-specific parameterizations.19,20 We fed the resulting datasets of simulated biomarkers to machine learning algorithms to generate male and female classifiers of torsadogenic risk. Finally, we evaluated the effects of using sex-specific models for risk prediction on a separated set of 36 drugs, which are deemed at intermediate risk of TdP. Our results show that TdP classifiers trained on sex-specific datasets identify distinct and not interchangeable sets of optimal features, suggesting potential different drivers of drug-induced arrhythmias, and that the use of sex-biased predictive models underestimates the torsadogenic risk of drugs with intermediate risk of TdP in females, which could potentially lead to life-threatening consequences for women.
2. Methods
A schematic diagram describing the complete workflow used in the generation of the ML classifiers for TdP risk is shown in Fig 1, and a detailed Methods section is provided in the Supplementary Materials. Source code and documentation are freely available at http://elegrandi.wixsite.com/grandilab/downloads and https://github.com/drgrandilab.
Figure 1:
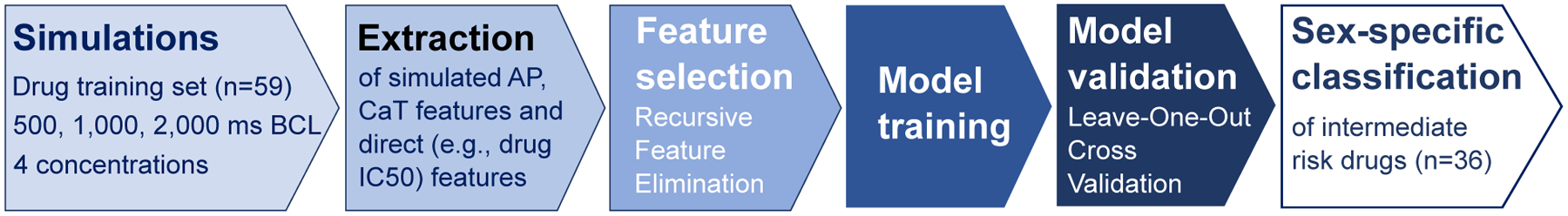
Workflow for the creation and testing of the sex-specific classifiers for torsadogenic risk.
2.1. Drug simulations
We updated the male and female human epicardial ventricular cardiomyocyte models developed by the Clancy lab,20 based on the O’Hara-Rudy model21 to recapitulate observed functional sex differences in Ca2+ handling.22–24 Drug effects were simulated with male and female models to build the complete set of biomarkers used to train the ML classifiers. The drug dataset used in this study (Table S1) was obtained by combining 83 compounds analyzed in the study by the Lancaster and Sobie with 12 CiPA compounds.13,25 Each drug was simulated using a pore block model based on the available IC50 values and Hill coefficients for various ion channels (a full list is available in Table S1), at various concentrations ranging from 1 to 4 times their effective free therapeutic plasma concentration (EFTPC), and various basic cycle lengths (BCL), ranging from 500 to 2,000 ms. We extracted 27 “simulated” biomarkers (Table S2) for each simulation, and added “direct” measures, such as IC50 values for IKr, INa (fast Na+ current) and ICaL (L-type Ca2+ current), for a total of 340 features per drug. To account for differences in the sex-specific baseline models, biomarkers are calculated as deviations from drug-free conditions. For ion current/transporter integrals, the difference is calculated between the absolute values of the integrals (e.g., positive values for both inward and outward currents).
2.2. Drug labels
Unfortunately, we are not aware of any clinical source of torsadogenic risk categorization that takes in account sex as an independent variable. In order to assign a unique binary label to each drug, we took advantage of the TdP risk classification available at www.crediblemeds.org,27 which reviews and analyzes adverse event reports for placing drugs in three broad categories: known, possible, and conditional risk of inducing TdP. Compounds characterized by known risk of inducing TdP in CredibleMeds were considered TdP+ in our analysis, while safe compounds (i.e., not included in any of the CredibleMeds categories) were considered TdP−. Given that sex is one of the potential risk factors for TdP, and thus determines the categorization of drugs in the possible or conditional risk categories, it would have been circular to assign these compounds to a specific class in our binary classification problem. Therefore, we did not use these drugs in training our classifiers. A similar selection was performed on the training set of drugs proposed by CiPA, which separates the compounds in high, intermediate, and low torsadogenic risk. Drugs classified differently by CredibleMeds and CiPA (Astemizole, Chlorpromazine, Cisapride, Droperidol, Ondansetron, Pimozide, Ranolazine, and Terfenadine) were excluded from the training dataset. Out of the 95 drugs in our initial dataset, 59 drugs met the requirements above and were used for creating our classifiers. The remaining 36 drugs, which are more likely to be associated with sex-specific effects, were later used to test our TdP classifiers and investigate the consequences of performing male-sex-biased vs. sex-specific predictions.
2.3. Machine Learning
In order to select the biomarkers contributing to the most accurate prediction of torsadogenic outcome, we applied a recursive feature elimination (RFE) algorithm, as detailed in the Supplementary Materials. For the sake of feature interpretability, we limited the ML modeling algorithms to logistic regression and support vector machine (SVM) with linear kernel. All the results shown here were obtained using SVM models, which outperformed logistic regression models on our datasets.
3. Results
3.1. Sex-specific biophysical models for action potential and Ca2+ transient
The Yang and Clancy model20 nicely recapitulates the prolonged AP observed in females vs. males.28 Conversely, Ca2+ loading and transient (CaT) amplitude are predicted much larger in the female vs. male model in disagreement with data in rodents.24 Our updated female model, with modifications in Ca2+-handling processes described in the Methods, better reflects the sex-specific differences reported in experiments (Fig 2Aii). Namely, the female CaT amplitude is modestly reduced (Fig 2Biv) and decays slightly more slowly (CaT duration at 50% of decay or CaD50, Fig 2Bv) than in male. At the same time, the diastolic [Ca2+] and sarcoplasmic reticulum (SR) content do not appreciably differ in male and female (Fig 2Biii,Bvi). These results are similar to Ca2+ measurements in myocytes from patients with cardiac hypertrophy (Fig 2 insets), whereby no significant sex differences were detected in systolic or diastolic Ca2+ levels, decay rate, and SR Ca2+content.29–32 Notably, AP properties are very modestly affected by the modifications we introduced in the model parameters, thus preserving the typical differences in repolarization between the two sexes in the original Yang and Clancy model (Fig 2Ai,Bi,Bii).20
Figure 2: Sex-specific biophysical models of cardiac electrophysiology and Ca2+ handling.
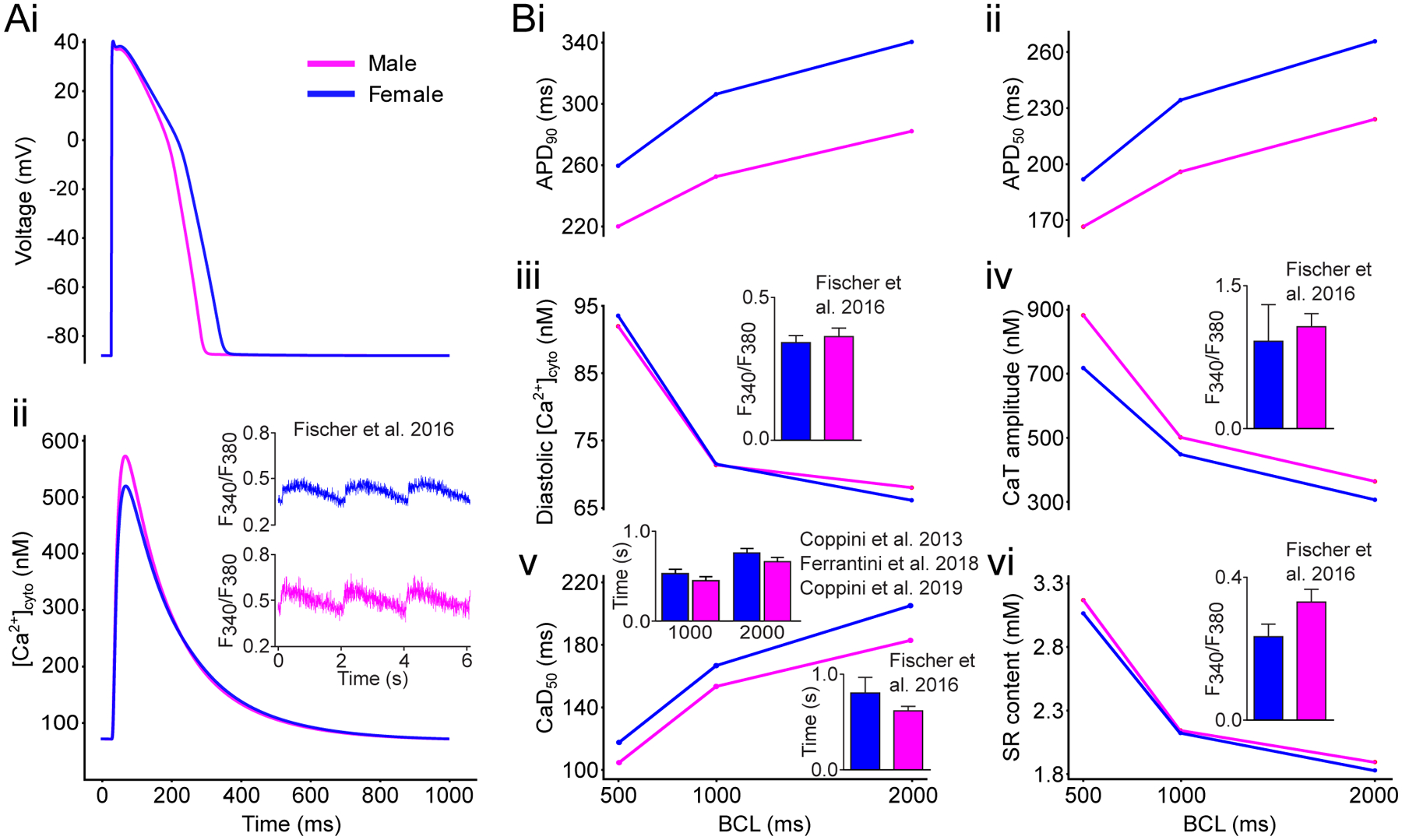
A: simulated AP (i) and CaT (ii) traces pacing at a BCL of 1,000 ms the baseline models of male (magenta) and female (blue) ventricular epicardial cardiomyocyte. B: Rate dependency of simulated biomarkers: measurements of voltage- (i,ii) and Ca2+-related (iii-vi) biomarkers as a function of the BCL tested. Insets show representative and summary data for CaT characteristics measured in myocytes from patients with cardiac hypertrophy paced at a BCL of 2,000 ms unless noted otherwise (reproduced with permission from Fischer et al. 2016,29 Coppini et al. 2013,30 Ferrantini et al. 2018,31 and Coppini et al. 201932).
3.2. Sex-specific TdP risk classifiers
With the original male and updated female models, we simulated the effects of the 59 training drugs under different pacing and drug regimen conditions (see Methods). Representative simulated traces reflecting the ranges of variations induced by the drugs on the AP and CaT are shown in Fig 3A (male) and B (female). For each simulated condition, several biomarkers (Table S2) were extracted and, together with the IC50 values available for each drug, generated the final feature datasets used by the RFE algorithm. This process led to optimized male- and female-specific TdP classifiers.
Figure 3: Sex-specific TdP risk classifiers.
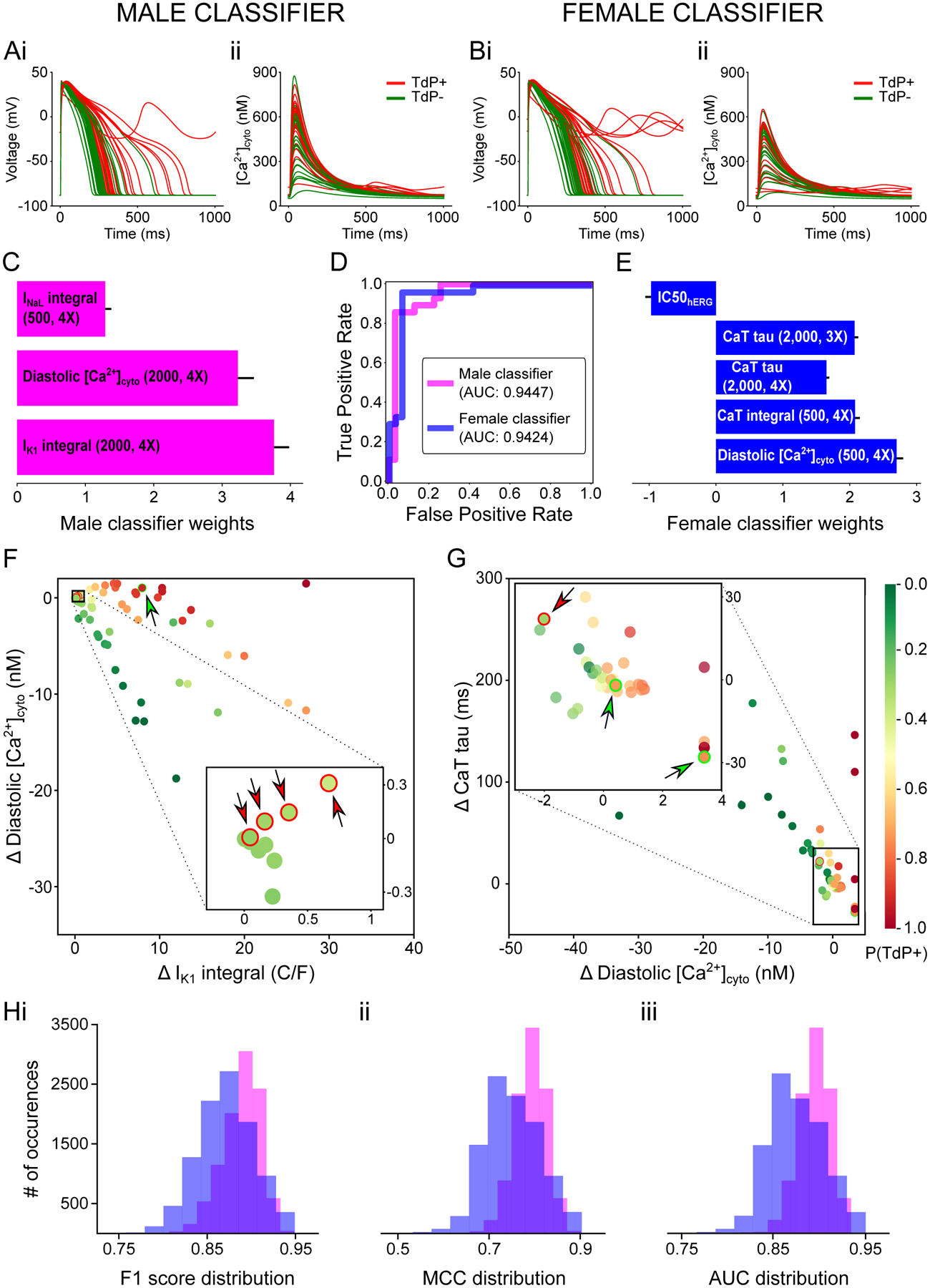
A,B: simulated effects of the 59 training drugs on the AP (i) and CaT (ii) with male (A) and female (B) biophysical myocyte models paced at a BCL of 1,000 ms. Traces belonging to drugs TdP+ and TdP− are colored in red and green, respectively. C,E: best performing set of features selected through RFE and used to build the male (C) and female (E) TdP classifiers. Uncertainty of the feature weights is measured using LOO-CV (mean + SD). D: Receiver operating characteristic curve for male (magenta) and female (blue) TdP classifiers. Area under the curve (AUC) is 0.9447 and 0.9424 for male and female, respectively. F,G: scatterplot of the training drugs created using the two features with the largest weight for the male (F) and female (G) TdP classifiers. The estimated TdP risk probability for each drug is indicated by the color of the filling. The misclassified drugs are indicated by arrows, and the color of the arrow and the stroke specifies the right class (green for TdP−, red for TdP+). H: distributions of performance metrics for the male (magenta) and female (blue) TdP classifiers after injecting random normally-distributed (μ=0, σ=0.1) noise to the original data (10,000 repetitions).
The final sets of features and relative weights selected and used by the best performing male and female TdP risk classifiers are illustrated in Fig 3C and 3E, respectively. Except for IC50 values, all biomarkers are deviations from baseline values. A positive (negative) weight indicates a positive (negative) correlation between a feature and the risk of torsade. TdP risk predictions for the male dataset are based on the current integrals of the inward rectifier IK1 and late Na+ current INaL, and the diastolic [Ca2+]. With LOO-CV, this ML model can correctly classify 54 out of 59 drugs, whereby 4 proarrhythmic drugs are predicted safe (4 false negatives, FNs – Amiodarone 1, Amiodarone 2, Cilostazol, Donepezil, where Amiodarone is simulated using different available IC50 values as shown in Table S1) and 1 safe drug is predicted harmful (1 false positive, FP – Prenylamine). The best performing female classifier is built on a set of 5 features. Four of the features are measurements extracted from the CaT (decay times, integral, and diastolic [Ca2+]), while the fifth one is the value of IC50 for the hERG channel. The predictive model optimized on the female dataset misclassifies 3 drugs: 1 FN (Procainamide) and 2 FPs (Ajmaline, Prenylamine). Despite the different set of features involved, both classifiers clearly demonstrate good ability in distinguishing torsadogenic from safe drugs. To evaluate model performances with commonly used metrics, we calculated F1 scores of 0.9148 and 0.9492, and MCCs of 0.8336 and 0.8987 for male and female TdP risk classifier, respectively (Table 1). Robust performances were confirmed by AUC values of 0.9447 and 0.9424 (Fig 3D) values for male and female classifier, respectively.
Table 1:
Summary of prediction performances for various combinations of TdP classifiers and feature datasets.
| F1 score | MCC | AUC | FN | FP | Misclassified drugs | |
|---|---|---|---|---|---|---|
| M on M | 0.9148 | 0.8336 | 0.9447 | 4 | 1 | Amiodarone 1, Amiodarone 2, Cilostazol, Donepezil, Prenylamine |
| F on F | 0.9492 | 0.8987 | 0.9424 | 1 | 2 | Ajmaline, Prenylamine, Procainamide |
| M on F | 0.7693 | 0.5945 | 0.9159 | 12 | 1 | Amiodarone 1, Amiodarone 2, Bepridil 2, Bepridil CiPA, Cilostazol, Donepezil, Flecainide, Halofantrine, Prenylamine, Procainamide, Quinidine 1, Sotalol, Thioridazine 1 |
| M retrained on F | 0.8980 | 0.7972 | 0.9217 | 4 | 2 | Ajmaline, Amiodarone 1, Amiodarone 2, Cilostazol, Donepezil, Prenylamine |
| M+F on M+F | 0.9066 | 0.8137 | 0.9507 | 7 | 4 | Ajmaline M, Amiodarone 1 M, Cilostazol M, Donepezil M, Mexiletine CiPA M, Prenylamine M, Amiodarone 1 F, Amiodarone 2 F, Cilostazol F, Donepezil F, Prenylamine F |
| F on EF | 0.9322 | 0.8665 | 0.9516 | 1 | 3 | Ajmaline, Prenylamine, Procainamide, Telbivudine |
| F on LF | 0.9492 | 0.8987 | 0.9516 | 1 | 2 | Ajmaline, Prenylamine, Procainamide |
| F on LU | 0.9492 | 0.8987 | 0.9505 | 1 | 2 | Ajmaline, Prenylamine, Procainamide |
To visualize the TdP risk predictions of the classifiers, we created 2D scatterplots of the training compounds using the two most influential features used by each model (Fig 3F for male, Fig 3G for female). In this representation, the farther the features from the origin, i.e., the larger the drug effect on the two most influential features, the more extreme are the predicted probabilities (darker colors). Interestingly, most of the misclassified drugs are located in the features space around the origin (insets of Fig 3F and 3G), and are associated with probabilities close to the classification threshold of 0.5. To evaluate the robustness of our classifiers, we added random normally distributed noise (μ=0, σ=0.1) to the features and evaluated their performances by repeating this procedure 10,000 times (Fig 3H). The noise injection tends to increase the misclassification rate of the classifiers, whereby the drugs that are more frequently misclassified are those located in close proximity to the decision boundary of the SVM (r = −0.7653 and −0.8808 for male and female, respectively). However, despite being negatively affected, the performances of the sex-specific classifiers remain satisfactory even in presence of confounding noise in the data.
3.3. Increased performance of TdP classifiers accounting for sex-specific features
To verify our contention that the creation of sex-specific classifiers is indeed critical to obtain accurate predictions, we evaluated the performance of the male classifier in predicting TdP risk of the training drugs applied to the female simulated features. When applied to female data, the predictive accuracy of the male classifier dropped, producing 1 FP and 12 FNs (Fig 4A). One possible explanation for this poor performance could be that the male classifier was trained on a different dataset. To test this idea, we retrained the male classifier using the data generated from the simulations with the female biophysical model (weights are compared in the insert of Fig 4B) and re-evaluated its performance. The retrained male ML model still produced more misclassified drugs compared to our female classifier (Fig 4A). This result demonstrates that the set of features used by the female classifier has superior predictive ability for drug-induced arrhythmias in females, and confirms the need for considering sex when estimating drug-induced torsadogenic risk in preclinical compounds (and in patients). Interestingly, a single classifier trained on both female and male simulated datasets yielded a set of features remarkably similar to those of the female classifier (Figure S1A). While this new sex-agnostic classifier performs well, the number if misclassified drugs is larger than with sex-specific predictions (6 vs. 5 and 5 vs. 3 misclassified drugs in male and female, respectively – Table 1). Finally, comparing the predictivity of existing TdP biomarkers on our male and female datasets (Table S3) revealed that the performances of classifiers based on previously published features are generally worse for females, and worse than those achieved by our sex-specific classifiers.
Figure 4: Predictions of TdP risk on female data with male-specific features.
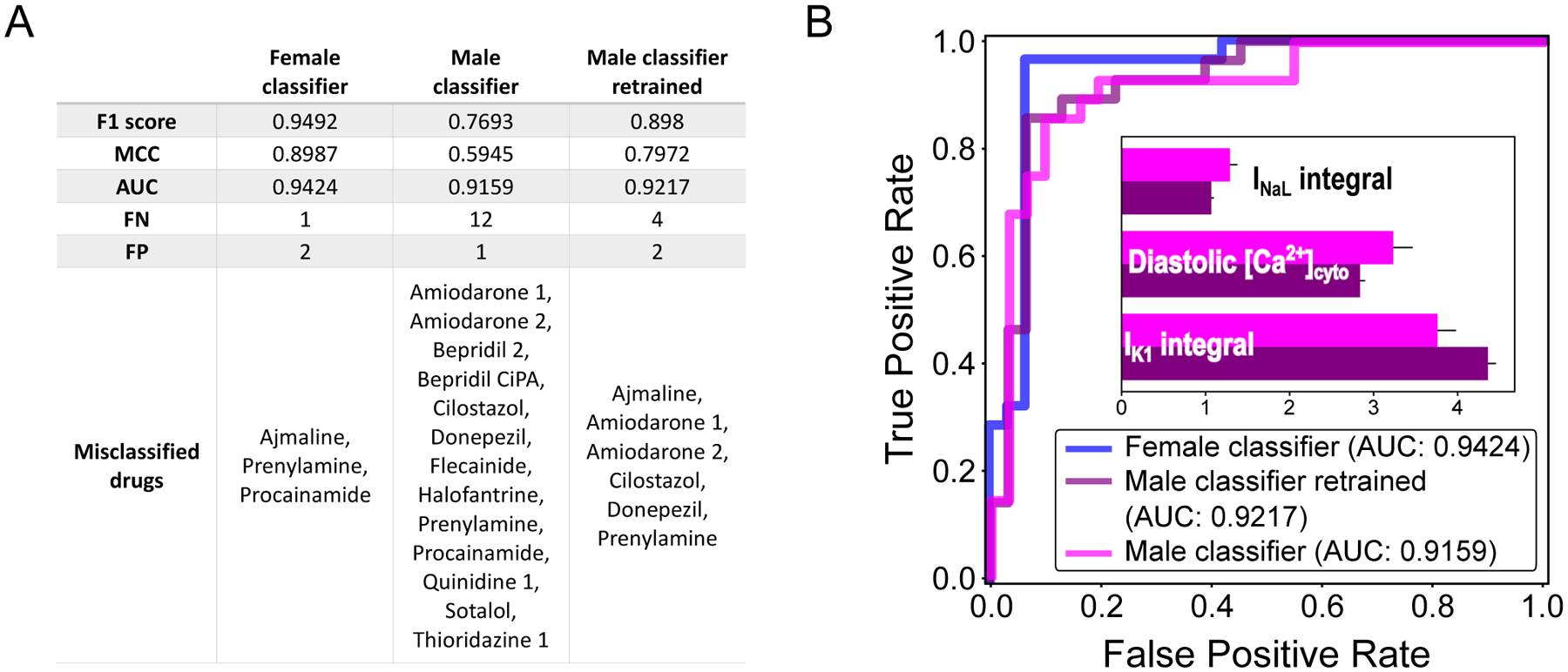
A: TdP classifier performances are evaluated on the dataset created with the female biophysical model. The results obtained with the female classifier (left column) are compared with the ones of the original male classifier (center column) and of a male classifier retrained on the female dataset (right column). B: Receiver operating characteristic curve for female (blue), original male (blue), and retrained male (purple) TdP classifiers applied on female data; area under the curve (AUC) is 0.9424, 0.9159, and 0.9217, respectively. In the inset, bar plot comparing the weights of the original (magenta) and retrained (purple) male classifiers. Uncertainty of the feature weights is measured using LOO-CV (mean + SD).
3.4. Sex-specific prediction of drugs with intermediate risk of TdP
From the initial list of drugs in our possession, 36 drugs had not been included in the training phase for belonging to the so-called intermediate torsadogenic risk category. In these drugs, TdP risk is associated with the presence of one or more risk factors. Indeed, when simulating these compounds with the sex-specific cardiomyocyte models, the observed changes on the AP (Fig 5Ai,Bi) and CaT (Fig 5Aii,Bii) are milder compared to the drugs with higher risk (Fig 3A,B). In order to explore how sex could affect the estimated torsadogenic risk of the drugs in the intermediate category, we applied the sex-specific TdP classifiers to the simulated male and female features and compared the predicted TdP probabilities (Fig 5Ci). In general, intermediate TdP risk drugs tend to have more dangerous outcomes in women, whereby a larger number of compounds is predicted to have higher probability of TdP in women (Fig 5Cii). Interestingly, a similar conclusion is reached with our sex-agnostic classifier trained on both male and female data (Fig S1B). Moreover, if the male-specific classifier is used on female data (right columns, Fig 5Ci), the predicted risk of the compounds is consistently underestimated, confirming the results obtained with the training dataset.
Figure 5: Sex-specific TdP predictions for intermediate-risk drugs.
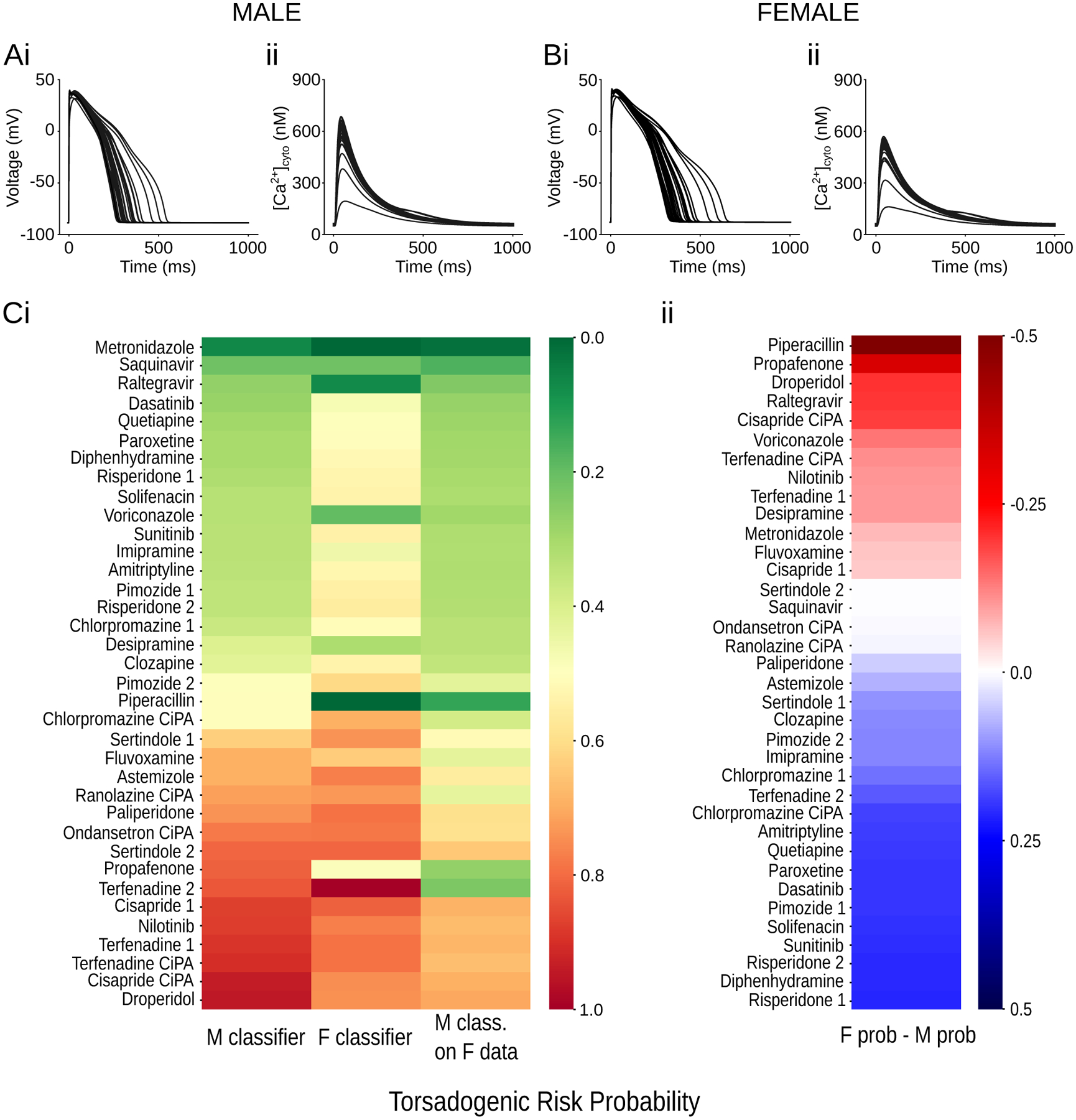
A,B: simulated effects of the 36 intermediate drugs on the AP (i) and CaT (ii) on the male (A) and female (B) biophysical models paced at a BCL of 1,000 ms. C: heatmaps of the predicted torsadogenic risk probability estimated by the sex specific TdP classifiers. (i) Left and center columns show the predictions obtained in male and female, respectively. The torsadogenic risk probabilities predicted by the male TdP classifier on the female dataset are visualized in the right column. Drugs are sorted by the risk probability predicted by the male TdP classifier. (ii) Difference between torsadogenic risk probabilities predicted by female and male TdP classifiers.
3.5. Effect of hormones on TdP risk prediction
It is well-established in the literature that circulating levels of hormones affect cardiac electrophysiology.33 To test how the performance of the female TdP classifier is influenced by hormones, we simulated the drug effects during the different phases of the menstrual cycle as described previously (see Supplementary Materials).20 Notably, the performance of the female classifier is almost unaltered by the hormonal effects (Fig 6A), with some modest changes in prediction in the late follicular phase. This is explained by hormones having minimal consequences on the CaT, which strongly influences the female predictions, while prolonging the AP duration considerably (Fig 6B). The consistency of the female classifier predictions in response to hormonal perturbations is also reflected in the probabilities forecasted for the drugs at intermediate TdP risk (Fig 6C). Similar results were obtained for the male classifier simulating the effects of testosterone (not shown). Taken together, these results show that this torsadogenic classifier is robust to acute changes in the levels of the sex hormones.
Figure 6: Effects of sex hormone fluctuations on female TdP classifier predictions.
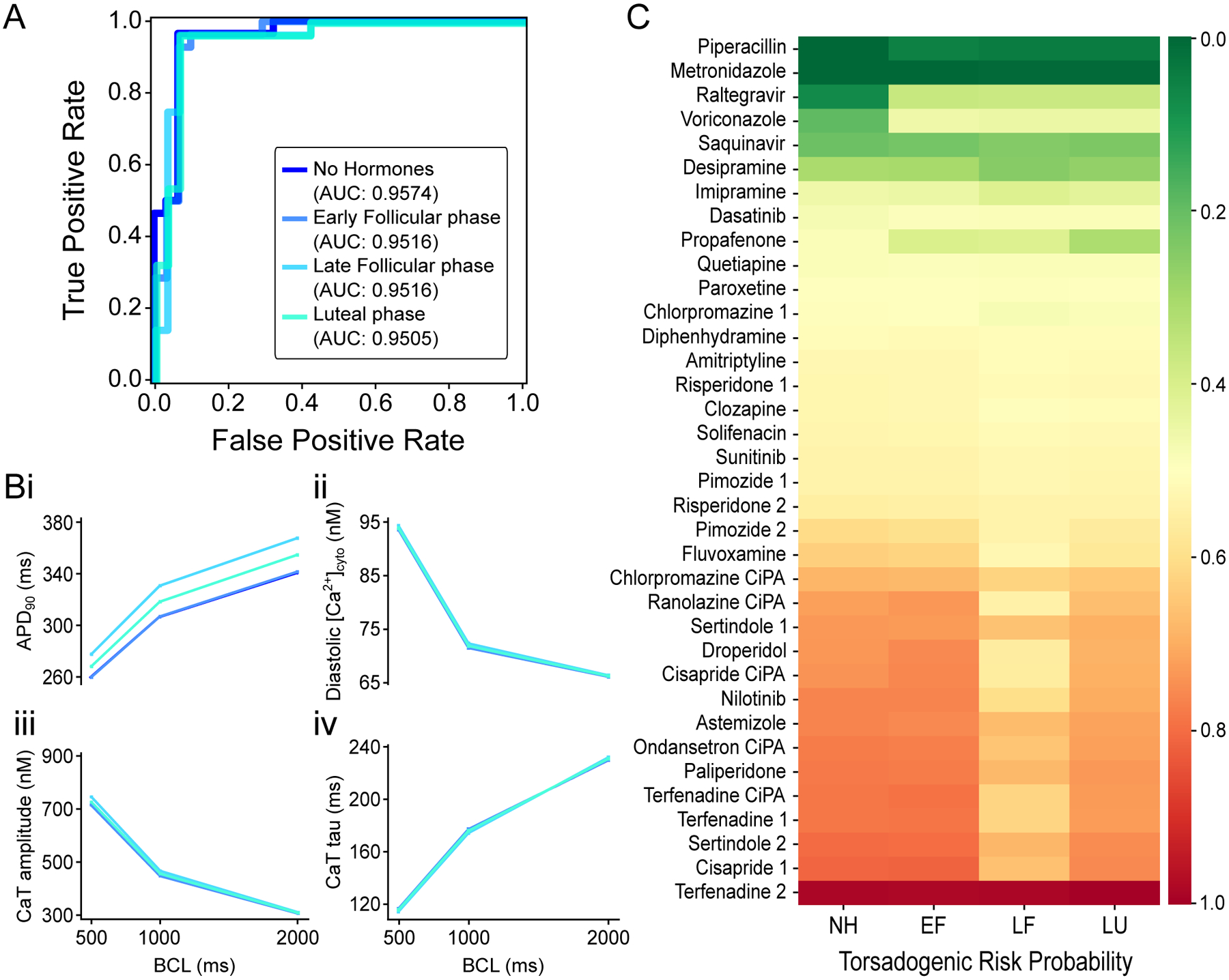
A: Receiver operating characteristic curve for female TdP classifier applied on training drugs dataset in absence of hormonal effects (navy blue) or simulating early follicular (EF, azure blue), late follicular (LF, sky blue), and luteal (LU, turquoise) phases. Area under the curve (AUC) is 0.9574, 0.9516, 0.9516, and 0.9505, respectively. B: Rate dependency of biomarkers simulated during different menstrual phases: measurements of voltage- (i) and Ca2+-related (ii-iv) biomarkers as a function of the BCL tested. C: heatmap of the predicted torsadogenic risk probabilities of intermediate TdP risk drugs simulated during different menstrual phases.
4. Discussion
Despite its important role in susceptibility to Torsade de Pointes,14,15 sex is rarely considered when developing predictive frameworks for torsadogenic risk. In the current study, we updated a published model of the human ventricular epicardial myocyte with sex-specific parameterizations20 to include experimentally observed differences in Ca2+ handling,22–24 and used it to simulate the effects of drugs belonging to different TdP risk categories. We then fed the simulated data to machine learning algorithms and generated sex-specific TdP risk classifiers. We showed that (1) classifiers trained on data reflecting male and female electrophysiological properties are built on distinct and not interchangeable sets of features; (2) male classifiers underestimate the torsadogenic risk in females; (3) female classifier predictions are robust to changes in sex hormone fluctuations during the menstrual cycle. Taken together, these results confirm the need for including sex when estimating the torsadogenic risk of a drug and provide a new tool to aid in this investigation.
4.1. Sex-specific model of ventricular electrophysiology and Ca2+ handling
The female Yang and Clancy20 model was parameterized using differences in expression levels of ion channels and transporters measured in cardiac tissue explanted from female vs. male patients, and included the effects of sex hormones on three ionic currents (IKr, IKs and ICaL).34 While the resulting parameterizations accurately recapitulate the well documented differences in AP (and QT) properties in the basic research and clinical literature,28,35 less is known about sex differences in Ca2+ handling in humans, and divergent results have been reported in animal models. Indeed, many studies in rodents revealed significantly lower diastolic [Ca2+] and/or smaller CaT amplitudes in female vs. male cardiomocytes,23 associated with a decreased excitation-contraction coupling gain,24 but studies showing no such sex-differences have also been reported.23 These discrepancies may be explained by differences in experimental conditions and/or species.23 Interestingly, L-type Ca2+ current, and SR Ca2+ content are not significantly different between sexes.24 The simulated Ca2+ levels of the original female model were unusually high, particularly at faster frequencies and compared to the simulated male Ca2+ levels. Therefore, we modified some parameters affecting the Ca2+-handling processes in the female model (see Detailed Methods in the Supplementary Materials) and obtained CaTs in line with the differences reported in animal studies. Due to scarcity of experimental data on human cardiomyocytes under normal conditions, we could only compare our model results to reported sex-dependent studies in hypertrophic patients. Intriguingly, the outputs of our updated model more closely resemble the trend of sex-differences in the Ca2+ recordings and related biomarkers acquired from isolated ventricular myocytes of hypertrophic patients (Fig 2).29–32 We did not detect sex differences in a small subset of control patients from previous publications (3 female vs. 5 males, not shown).30 Information on the sex-specific differences in Ca2+ handling from a bigger sample of non-diseased patients would be highly desirable to better constrain the sex-specific models, but we are not aware of any published data with larger cohorts.
Previous studies showed that acutely applying supra-physiological concentrations of female sex hormones to ventricular myocytes from mice, rats, guinea pigs, rabbits, and humans caused substantial reductions in Ca2+ current and CaTs.23 On the other hand, conflicting findings are reported for the impact of ovariectomy on CaT amplitude and diastolic [Ca2+].23 Given the absence of human data and discrepant animal, we could not directly compare our sex-hormone models to experimental data, and thus did not modify the original Yang and Clancy20 model formulations to describe the effects of sex hormones. Our models may be further refined should systematic examination on the effects of sex hormones on cardiomyocytes, preferably in humans, become available, along with complete information of ion channel blockade for each drug (Table S1), and of any drug-hormone interaction.20
4.2. Sex-specific TdP biomarkers
Our work is preceded by a number of important studies that utilized in silico approaches to identify biomarkers predictive for TdP.9–13,36 Here we propose for the first time the adoption of sex-specific torsadogenic risk predictors. Through an RFE algorithm, we obtained male and female classifiers built on distinct minimal sets of best performing features. Importantly, the RFE iterative process does not require any manual intervention, removing opportunity for user bias. From the analysis of predictive biomarkers (Fig 3C), we found that the male classifier resembles the SVM classifier developed by Lancaster and Sobie.13 In both cases, TdP classification is based on changes in (1) diastolic [Ca2+] and (2) one or more metrics related to AP prolongation. Diastolic [Ca2+] is an index of cell Ca2+ loading. In fact, in these models it is highly correlated with SR Ca2+ content and CaT amplitude (r = 0.9803 and 0.8476, respectively – see correlation maps in the Figure S2). The integral of IK1 used by our male classifier is correlated with APD90/AP triangulation (r = 0.8872 and 0.8946 respectively). Accordingly, our Fig 3F is strikingly similar to Fig 3b in Lancaster and Sobie, 2016.13 The female classifier, on the contrary, selected hERG IC50 and several features related to Ca2+-handling processes. Drug-induced changes in diastolic [Ca2+], the integral of the CaT, and CaT decay time are all positively associated with torsadogenic risk (Fig 3E). Altered Ca2+ homeostasis has been shown to be an important determinant of early-afterdepolarizations and TdP susceptibility. Increased whole-cell Ca2+ load is well known to be capable of destabilizing repolarization through enhanced forward mode INCX.37 This effect could be exacerbated in women, for whom NCX expression is higher in physiological conditions.38 While a detailed mechanistic interpretation of the different CaT-related features for TdP remains unclear, their importance in this work clearly suggests that further investigation is warranted.
Redfern et al. illustrated the importance of safety margins (IC50/Cmax) in predicting TdP risks.26 However, since the clinically relevant concentrations might not be known for new candidate compounds, we utilized IC50 values as candidate biomarkers and simulated various drug concentrations. Nevertheless, our results could be refined using safety margins instead.
4.3. Ground truth classification
To solve the critical lack of ground truth regarding clinical sex-specific TdP risk classification, we operated under the assumption that drugs considered safe (i.e., missing in the CredibleMeds database,27 which contains a continuously updated database of therapeutic compounds categorized for torsadogenic risk) are so in both sexes. Conversely, drugs clearly associated with TdP (i.e., “Known Risk of TdP” category), are likely to be so in both sexes. On the contrary, drugs with intermediate risk of causing TdP (i.e., “Possible” or “Conditional Risk of TdP” categories) are more likely to change risk categorization depending on sex. Those drugs were therefore not used to build our classifiers. Based on this assumed ground truth, our sex-specific TdP classifiers have shown remarkable predictive power (Fig 3D,H, Table 1). When evaluating additional sources (such as drug labels and thorough QT results) for “low-risk” drugs (Table S4), a positive TdP indication was found for prenylamine39,40 and ajmaline.41 Notably, ML models classified the two drugs as pro-arrhythmic (prenylamine for both sexes, ajmaline for females), despite the ground truth assumption of no risk.
4.4. Predictions on intermediate drugs
Our classification results with the intermediate risk drugs identified multiple therapeutic compounds that are predicted safe in men and torsadogenic in women (Fig 5C and Table S4), which is in general agreement with the increased susceptibility to TdP in females. As drug-induced TdP is a rare event, prospective studies to evaluate the TdP risk factors are difficult to design and would require very large patient cohorts. We analyzed retrospective reviews of case reports, and notably, found a nice correspondence in the predictions for some specific compounds with observations published in the clinical literature. Indeed, female sex is a risk factor for documented TdP episodes associated with the use of Risperidone,42 Sunitinib,43 Paroxetine,44 and Quetiapine,45 which are among the intermediate-risk therapeutic compounds with the largest selectivity for women based on our estimated torsadogenic risk.
It is also important to understand sex-differences in TdP outcome in high-risk drugs, which also demonstrate female sex-prevalence, as demonstrated for quinidine, amiodarone, sotalol, disopyramide, bepridil, prenylamine (but not procainamide).14
4.5. Drug concentrations
It is important to recognize that even at comparable drug dosages, drug exposure may vary between women and men owing to differences in absorption, distribution, metabolism and excretion that could explain higher TdP risk in women. The increased risk is not fully explained by sex difference in drug plasma levels,46 though cellular concentrations of a same systemic drug dose can vary across individuals and between sexes. Thus, future studies should account for both (population) pharmacokinetic and pharmacodynamic drug interactions. Experimental differences in pharmacokinetics observed between men and women have frequently been attributed to bodyweight differences and thus might be addressed by appropriate adjustment of dosage by body weight. In fact, in a cohort of more than 200 patients a statistically significant prevalence of dofetilide dose reduction or discontinuation was found in female vs. male patients mostly due to QTc prolongation, although no TdP cases were reported.47 On the other hand, the occurrence of TdP was not associated to any critical serum drug level of quinidine.48
4.6. Summary and future directions
Our results indicate the need for considering sex when developing and applying torsadogenic classifiers to obtain more accurate predictions and provide safer therapeutic treatments to patients. Of note, the current structure and size of the human thorough QT/QTc study for evaluation of proarrhythmic potential are prohibitive of sex-specific safety screening, as guidelines stipulate that only if a sign of harm is detected in a small (8–10 subjects) Phase I safety trial, then special populations (including women) should be assessed. We argue that the requirement for suitably powered sex-specific risk needs to be encoded in the early phase thorough QT/QTc study, and might be an attainable goal.
Clinical risk assessment and trials suggest that, besides sex, other patient conditions, i.e., age, disease, electrolyte imbalance, interaction with other drugs should all be taken into account in evaluating TdP risk.49 Data gathered from experiments in human induced pluripotent stem cells-derived cardiomyocytes obtained from male and female cell lines have been recently proven useful for investigating the sex-differences in torsadogenicity,50 suggesting that these patient-derived cells could be used to guide new models and paradigms for safety pharmacology accounting for patient conditions.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
While female sex is a well-known risk factor for Torsade de Pointes (TdP), it is generally ignored by current safety regulatory guidelines and most of the proarrhythmia risk prediction models.
What question did this study address?
We sought to develop and evaluate sex-specific TdP risk classifiers using mechanistic models of male and female cardiac myocytes electrophysiology.
What does this study add to our knowledge?
We found that sex-specific TdP risk classification relies on distinct and not interchangeable sets of biomarkers and is robust to fluctuations in sex hormones levels.
How might this change clinical pharmacology or translational science?
Accounting for sex-specific TdP risk is critical for pro-arrhythmic risk evaluation during drug development and can contribute to safer therapeutics.
Funding
The National Institutes of Health (NIH) Stimulating Peripheral Activity to Relieve Conditions grant 1OT2OD026580 (EG and CC); the National Heart, Lung, and Blood Institute grants R01HL131517, R01HL141214, P01HL141084 (EG), and R00HL138160 (SM); the American Heart Association Postdoctoral Fellowship 20POST35120462 (HN), Predoctoral Fellowship 20PRE35120465 (XZ), and grant 15SDG24910015 (EG); the UC Davis School of Medicine Dean’s Fellow award (EG) and Academic Senate grant (EG and US); the Health and Environmental Sciences Institute grant U01 FD006676-01 (AE).
Footnotes
Conflict of Interest: The authors declared no competing interests for this work.
References
- 1.Dessertenne F La tachycardie ventriculaire a deux foyers opposes variables. Arch. Mal. Coeur Vaiss 59, 263–272 (1966). [PubMed] [Google Scholar]
- 2.Haverkamp W et al. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Eur. Heart J 21, 1216–1231 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Sanguinetti MC, Jiang C, Curran ME & Keating MT A mechanistic link between an inherited and an acquird cardiac arrthytmia: HERG encodes the IKr potassium channel. Cell 81, 299–307 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration International Conference on Harmonisation; Guidance on S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; Availability. Fed. Regist 70, 61133–4 (2005). [PubMed] [Google Scholar]
- 5.Food and Drug Administration International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed. Regist 70, 61134–5 (2005). [PubMed] [Google Scholar]
- 6.Sager PT Key clinical considerations for demonstrating the utility of preclinical models to predict clinical drug-induced torsades de pointes. Br. J. Pharmacol 154, 1544–1549 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gintant G An evaluation of hERG current assay performance: Translating preclinical safety studies to clinical QT prolongation. Pharmacol. Ther 129, 109–119 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Sager PT, Gintant G, Turner JR, Pettit S & Stockbridge N Rechanneling the cardiac proarrhythmia safety paradigm: A meeting report from the Cardiac Safety Research Consortium. Am. Heart J 167, 292–300 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Li Z et al. Assessment of an In Silico Mechanistic Model for Proarrhythmia Risk Prediction Under the Ci PA Initiative. Clin. Pharmacol. Ther 105, 466–475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirams GR et al. Simulation of multiple ion channel block provides improved early prediction of compounds’ clinical torsadogenic risk. Cardiovasc. Res 91, 53–61 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogh-Madsen T, Jacobson AF, Ortega FA & Christini DJ Global Optimization of Ventricular Myocyte Model to Multi-Variable Objective Improves Predictions of Drug-Induced Torsades de Pointes. Front. Physiol 8, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passini E et al. Drug‐induced shortening of the electromechanical window is an effective biomarker for in silico prediction of clinical risk of arrhythmias. Br. J. Pharmacol bph.14786 (2019).doi: 10.1111/bph.14786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster MC & Sobie E Improved Prediction of Drug-Induced Torsades de Pointes Through Simulations of Dynamics and Machine Learning Algorithms. Clin. Pharmacol. Ther 100, 371–379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makkar RR, Fromm BS, Russell TS, Meissner MD & Lehmann MH Female Gender as a Risk Factor for Torsades de Pointes Associated With Cardiovascular Drugs. JAMA J. Am. Med. Assoc 270, 2590 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Chorin E et al. Female gender as independent risk factor of torsades de pointes during acquired atrioventricular block. Hear. Rhythm 14, 90–95 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Flórez-Vargas O et al. Bias in the reporting of sex and age in biomedical research on mouse models. Elife 5, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitale C et al. Under-representation of elderly and women in clinical trials. Int. J. Cardiol 232, 216–221 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Ramirez FD et al. Sex Bias Is Increasingly Prevalent in Preclinical Cardiovascular Research: Implications for Translational Medicine and Health Equity for Women. Circulation 135, 625–626 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Yang P-C, Kurokawa J, Furukawa T & Clancy CE Acute Effects of Sex Steroid Hormones on Susceptibility to Cardiac Arrhythmias: A Simulation Study. PLoS Comput. Biol 6, e1000658 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang P-C & Clancy CE In silico Prediction of Sex-Based Differences in Human Susceptibility to Cardiac Ventricular Tachyarrhythmias. Front. Physiol 3, 1–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hara T, Virág L, Varró A & Rudy Y Simulation of the Undiseased Human Cardiac Ventricular Action Potential: Model Formulation and Experimental Validation. PLoS Comput. Biol 7, e1002061 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrell SR, Ross JL & Howlett SE Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am. J. Physiol. Circ. Physiol 299, H36–H45 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Parks RJ & Howlett SE Sex differences in mechanisms of cardiac excitation–contraction coupling. Pflügers Arch. - Eur. J. Physiol 465, 747–763 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks RJ, Ray G, Bienvenu LA, Rose RA & Howlett SE Sex differences in SR Ca2+ release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J. Mol. Cell. Cardiol 75, 162–173 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Colatsky T et al. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative — Update on progress. J. Pharmacol. Toxicol. Methods 81, 15–20 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Redfern WS et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res 58, 32–45 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Woosley R, Heise C, Gallo T, Woosley R & Romero K QTDrug List. AZCERT at <https://crediblemeds.org/new-drug-list/> [Google Scholar]
- 28.Verkerk AO et al. Gender Disparities in Cardiac Cellular Electrophysiology and Arrhythmia Susceptibility in Human Failing Ventricular Myocytes. Int. Heart J 46, 1105–1118 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Fischer TH et al. Sex-dependent alterations of Ca 2+ cycling in human cardiac hypertrophy and heart failure. Europace 18, 1440–1448 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Coppini R et al. Late Sodium Current Inhibition Reverses Electromechanical Dysfunction in Human Hypertrophic Cardiomyopathy. Circulation 127, 575–584 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Ferrantini C et al. Late sodium current inhibitors to treat exercise-induced obstruction in hypertrophic cardiomyopathy: an in vitro study in human myocardium. Br. J. Pharmacol 175, 2635–2652 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coppini R et al. Electrophysiological and Contractile Effects of Disopyramide in Patients With Obstructive Hypertrophic Cardiomyopathy. JACC Basic to Transl. Sci 4, 795–813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa T & Kurokawa J Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: Implication for the gender difference in cardiac arrhythmias. Pharmacol. Ther 115, 106–115 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Gaborit N et al. Gender-related differences in ion-channel and transporter subunit expression in non-diseased human hearts. J. Mol. Cell. Cardiol 49, 639–646 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Rautaharju PM et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can. J. Cardiol 8, 690–5 (1992). [PubMed] [Google Scholar]
- 36.Yang P-C et al. A Computational Pipeline to Predict Cardiotoxicity. Circ. Res 126, 947–964 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sims C et al. Sex, Age, and Regional Differences in L-Type Calcium Current Are Important Determinants of Arrhythmia Phenotype in Rabbit Hearts With Drug-Induced Long QT Type 2. Circ. Res 102, 86–100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papp R et al. Genomic upregulation of cardiac Cav1.2α and NCX1 by estrogen in women. Biol. Sex Differ 8, 5–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamari I, Rabinowitz B & Neufeld HN Torsade de pointes due to prenylamine controlled by lignocaine. Eur. Heart J 3, 389–92 (1982). [DOI] [PubMed] [Google Scholar]
- 40.Champeroux P et al. Prediction of the risk of Torsade de Pointes using the model of isolated canine Purkinje fibres. Br. J. Pharmacol 144, 376–385 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haverkamp W et al. Torsade de pointes induced by ajmaline. Z. Kardiol 90, 586–590 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Vieweg WVR et al. Risperidone, QTc interval prolongation, and torsade de pointes: A systematic review of case reports. Psychopharmacology (Berl). 228, 515–524 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Harvey PA & Leinwand LA Oestrogen enhances cardiotoxicity induced by Sunitinib by regulation of drug transport and metabolism. Cardiovasc. Res 107, 66–77 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenzel-Seifert K, Wittmann M & Haen E QTc Prolongation by Psychotropic Drugs and the Risk of Torsade de Pointes. Dtsch. Aerzteblatt Online 108, 687–693 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasnain M et al. Quetiapine, QTc interval prolongation, and torsade de pointes : a review of case reports. Ther. Adv. Psychopharmacol 4, 130–138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darpo B et al. Are women more susceptible than men to drug-induced QT prolongation? Concentration-QT c modelling in a phase 1 study with oral rac-sotalol. Br. J. Clin. Pharmacol 77, 522–531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pokorney SD et al. Dofetilide dose reductions and discontinuations in women compared with men. Hear. Rhythm 15, 478–484 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Thompson KA, Murray JJ, Blair IA, Woosley RL & Roden DM Plasma concentrations of quinidine, its major metabolites, and dihydroquinidine in patients with torsades de pointes. Clin. Pharmacol. Ther 43, 636–642 (1988). [DOI] [PubMed] [Google Scholar]
- 49.Sauer AJ & Newton-Cheh C Clinical and Genetic Determinants of Torsade de Pointes Risk. Circulation 125, 1684–1694 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huo J, Wei F, Cai C, Lyn-Cook B & Pang L Sex-Related Differences in Drug-Induced QT Prolongation and Torsades de Pointes: A New Model System with Human iPSC-CMs. Toxicol. Sci 167, 360–374 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


