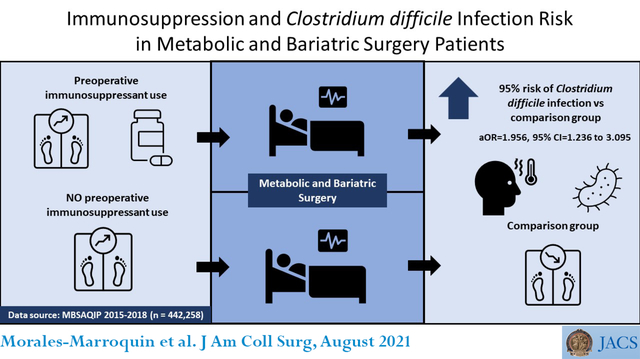
Abstract
Background:
Immunosuppressant use increases the risk of Clostridioides difficile infection. To date no studies have analyzed the relationship between immunosuppressant use and Clostridioides difficile infections after metabolic and bariatric surgery.
Methods:
A retrospective analysis of the 2015–2018 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) data was conducted. The MBSAQIP data includes information from 854 affiliated practices in the United States and Canada. Initial sample size was 760,076 MBS patients. After excluding participants due to missing variables (n= 188,106) and the use of surgical procedures other than Roux-en-Y gastric bypass and sleeve gastroplasty (n=129,712), final analyses were performed on 442,258 participants. Logistic regression models generated the odds of post-MBS Clostridioides difficile infection by immunosuppressant status (+/−).
Results:
Unadjusted logistic regression analysis showed that patients using immunosuppressants were 95% more likely to have post-operative Clostridioides difficile infection (OR=1.945, 95% CI= 1.230 to 3.075; p <0.001) versus MBS patients not taking immunosuppressants. After adjusting for age, gender, ethnicity, pre-operative BMI, diabetes status, and surgery procedure type, the association remained unaffected (aOR=1.956, 95% CI=1.236 to 3.095; p <0.01). Patients who completed the laparoscopic Roux-en-Y gastric bypass procedure had more than double the odds of developing Clostridioides difficile infection compared to those who completed the laparoscopic sleeve gastrectomy procedure (OR=2.183, 95% CI=1.842 to 2.587; p < 0.0001).
Conclusions:
Our results using a population-based sample of MBS patients show that those taking immunosuppressants have significantly higher risk of developing Clostridioides difficile infection post-operatively. These findings suggest that patients using immunosuppressants should be closely monitored both pre- and post-procedure.
Keywords: Bariatric surgery, LRYGB, Clostridioides difficile, C. difficile, weight loss surgery
Graphical Abstract
Precis
This study showed for the first time that immunosuppressant increased postmetabolic and bariatric surgery Clostridioides difficile infection risk by 95% in comparison with patients who also underwent operation but were not consuming immunosuppressant. Increased attention to metabolic and bariatric surgery patients taking immunosuppressant is needed.
INTRODUCTION
C. difficile is a spore-forming, gram-positive bacteria that causes pseudomembranous enterocolitis, which can lead to fulminant colitis (1), and as such is considered a significant public health threat (2). According to the Centers for Disease Control and Prevention there are approximately half a million C. difficile infections each year in the United States (US) (3, 4). Additionally, it is estimated that 15,000 deaths are directly attributed to C. difficile infection in the US annually, with a higher mortality risk among those aged 65 years or older (3). Focused efforts to prevent C. difficile infections resulted in a 36% decrease in health care-associated C. difficile infections from 2011 to 2017 but community-associated C. difficile infections and in-hospital death rates have not changed despite these efforts (5).
Immunosuppressants are commonly prescribed for autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, systemic vasculitis, and post-organ transplantation (6). However, immunosuppressants have been associated with long-term adverse effects including increased risk of infection, malignancy, cardiovascular disease, marrow suppression, and cytopenia, even after treatment has ceased (6). Research shows that the use of a single immunosuppressant increases the risk for opportunistic infections such as C. difficile more than 3.2 times, whereas the combination of two or more immunosuppressants increases infection risk by 6.5 times (7). The proposed mechanism of action through which immunosuppressants lead to increased C. difficile infections is by immune system response inhibition, leading to increased C. difficile toxins (8). In addition to immunosuppressants, other proposed risk factors for C. difficile infection include gastrointestinal surgeries, antibiotic use, and obesity, especially if a patient was already an asymptomatic C. difficile carrier (9–12).
Metabolic and bariatric surgery (MBS) is a safe and effective long-term treatment option for obesity (13, 14). Incidence of C. difficile infection in MBS patients is low (0.13%); however, the presence of C. difficile is associated with increased length of stay and 30-day post-operative surgical complications including anastomotic leaks, deep surgical site infection, re-operations, and re-admissions as well as increased risk of pneumonia, acute kidney injury, venous thromboembolism, sepsis, intubation, and mortality in these patients (15–17). While fecal microbial transplantation, a new innovative treatment, has shown to be effective against C. difficile infection, it is not effective in all patients (18). Therefore, the best strategy to prevent C. difficile infection is to minimize its occurrence in the first place (18).
Due to the lack of evidence evaluating the relationship between immunosuppressants use and C. difficile infection rates in MBS patients, this analysis examined this relationship in a population-based, national sample of US MBS patients.
METHODS
Design
A retrospective analysis was conducted using data from the 2015–2018 MBS Accreditation and Quality Improvement Program (MBSAQIP). The University of Texas Health Science Center institutional review board considers a retrospective analysis of public, anonymized data, such as the MBSAQIP dataset, exempt from review.
Data source
In 2012 the American College of Surgeons (ACS) and the American Society for MBS (ASMBS) merged their programs to form the MBS Accreditation and Quality Improvement Program (MBSAQIP)(19). The MBSAQIP collects prospective, risk-adjusted data, based on preoperative, intra-operative, and post-operative standardized definitions of variables that are specific for MBS. Perioperative care is standardized across centers ensuring reliable data (20). The merged 2015–2018 MBSAQIP Participant Use Data File (PUF) were used for this analysis and includes MBS patients who received their clinical care at one of the accredited centers in the USA and Canada (16). The PUF contains 173 HIPAA-compliant variables on 204,837 cases submitted from 854 centers in 2018; 200,374 from 832 centers in 2017; 186,772 from 791 centers in 2016; and 168,903 from 742 centers in 2015.
Participants
All participants from the dataset were included unless they had missing data from variables used for this analysis. The MBSAQIP database PUF contains a total sample size of 760,076 participants from years 2015–2018. After excluding participants due to missing variables (n= 188,106) and the use of other surgical procedures (n=129,712), final analyses were performed on 442,258 participants
Variables
The primary dependent (outcome) variable for this analysis was C. difficile infection and the primary independent (predictor) variable was immunosuppressant use for a chronic condition. The C. difficile infection variable included patients who developed C. difficile colitis within 30 days post-MBS. Both independent and dependent variables are dichotomous variables with yes/no categories. Covariates included age, ethnicity, pre-operative body mass index (BMI), pre-surgery diabetes status, and MBS procedure type. Three age groups were created as follows: < 30 years; 31–60 years; and > 60 years. Ethnic groups were classified as Non-Hispanic White; Non-Hispanic Black; Hispanic, and Other based on patient self-report. Pre-operative BMI was classified into four groups using the following cut points: < 35; 35 to 39; 40 to 49; and ≥ 50 kg/m2. Diabetes status was analyzed as a dichotomous variable (Yes/No) depending on pre-surgery diagnosis. Finally, surgical procedure type was classified into two groups with 1) CPT code 43775 as Laparoscopic Sleeve Gastrectomy (LSG), and 2) CPT code 43644 as Laparoscopic Roux-en-Y Gastric Bypass (LRYGB).
Statistical analysis
Descriptive analysis was performed for baseline characteristics including age, gender, ethnicity, pre-operative BMI, pre-surgery diabetes status, and surgical procedure type. Next, a comparison of patient characteristics between C. difficile positive and negative diagnosis was performed using chi-square tests. Additionally, a comparison of patient characteristics in relation to immunosuppressant use for chronic condition was also performed using chi-square tests. Finally, via logistic regression analysis, the crude odds ratio (model 1) was calculated for C. difficile infection among patients with immunosuppressant use for chronic condition (control, immunosuppressant use). A 10% rule was applied to identify any potential confounders from the list of covariates including age, gender, ethnicity, pre-operative BMI, diabetes status, and surgery type; none of these covariates were shown to be confounders. Backward selection was performed with the variables age, gender, ethnicity, pre-operative BMI (controlled for due to the conflicting findings with regards to the effect it has on C. difficile infection risk) (21–25), diabetes status, and surgical procedure type as they were all significantly associated with C. difficile infection and/or immunosuppressant use in bivariate analysis. To produce the final model (model 2), an adjusted logistic regression model controlling for the covariates from the backward selection process to determine the effect these covariates had on the potential relationship between C. difficile infection and immunosuppressant use was run. All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC). Type one error was maintained at 5%.
RESULTS
A total of 760,076 individuals who completed MBS between 2015–2018 were identified. After excluding participants due to missing variables (n= 188,106) and the use of surgical procedures other than LRYGB and LSG (n=129,712), final analyses were performed on 442,258 participants. Seventy-eight percent of the population were between 31 and 60 years old and 80% of the participants were female. The ethnic distribution was 58.33% non-Hispanic White, 16.90% non-Hispanic Black, 9.74% Hispanic, and 15.04% other ethnicities. A total of 5.34% had a BMI < 35 kg/m2, 18.73% a BMI between 35–39 kg/m2, 52.24% between 40–49 kg/m2, and 23.69% had a BMI ≥ 50 kg/m2. Almost three quarters of the participants (74.41%) reported not having diabetes diagnosis at the time of the surgery whereas 25.59% reported having the diagnosis. Likewise, nearly three quarters of the surgeries were LSG (72.84%) and 27.16% were LRYGB.
Table 1 illustrates baseline demographics based on C. difficile infection diagnosis. After excluding missing data, the total number of C. difficile cases in patients undergoing either LRYGB or LSG was 551 with an overall incidence of 0.12% within 30 days of MBS. Gender, ethnicity, and surgery type were significant. It is interesting to note that even though the total population distribution of MBS surgeries was 72.84% and 27.16% for LSG and LRYGB respectively, the distribution of participants within the C. difficile group were 54.45% and 45.55% for LSG and LRYGB, respectively.
Table 1.
Summary of Descriptive Characteristics of Selected Population (MBSAQIP, 2015–2018) by Clostridioides difficile Infection Status
| Variable | Total (n = 442258) | CDIFF + (n = 551) | CDIFF − (n = 441707) | p Value* |
|---|---|---|---|---|
| Age | 0.064 | |||
| <30 y | 52914 (11.96) | 71 (12.89) | 52843 (11.96) | |
| 31–60 y | 343122 (77.58) | 407 (73.87) | 342715 (77.59) | |
| >60 y | 46222 (10.45) | 73 (13.25) | 46149 (10.45) | |
| Sex | 0.0054† | |||
| Female | 353867 (80.01) | 467 (84.75) | 353400 (80.01) | |
| Male | 88391 (19.99) | 84 (15.25) | 88307 (19.99) | |
| Ethnicity | <0.0001† | |||
| Hispanic | 43060 (9.74) | 41 (7.44) | 43019 (9.74) | |
| NHW | 257963 (58.33) | 401 (72.78) | 257562 (58.31) | |
| NHB | 74730 (16.90) | 68 (12.34) | 74662 (16.90) | |
| Other | 66505 (15.04) | 41 (7.44) | 66464 (15.05) | |
| BMI | 0.4625 | |||
| <35 kg/m2 | 23619 (5.34) | 35 (6.35) | 23584 (5.34) | |
| 35–39 kg/m2 | 82838 (18.73) | 98 (17.79) | 82740 (18.73) | |
| 40–49 kg/m2 | 231038 (52.24) | 277 (50.27) | 230761 (52.24) | |
| ≥50 kg/m2 | 104763 (23.69) | 141 (25.59) | 104622 (23.69) | |
| Operation type | <0.0001† | |||
| LSG | 322131 (72.84) | 300 (54.45) | 321831 (72.86) | |
| LRYGB | 120127 (27.16) | 251 (45.55) | 119876 (27.14) | |
| Preoperative diabetes | 0.5579 | |||
| Yes | 113174 (25.59) | 147 (26.68) | 113027 (25.59) | |
| No | 329084 (74.41) | 404 (73.31) | 328680 (74.41) |
Data presented as n (%)
Chi-square test
Statistically significant
CDIFF, Clostridioides difficile, LRYGB, laparoscopic Roux-en-Y gastric bypass; LSG, laparoscopic sleeve gastrectomy; NHB, Non-Hispanic black; NHW, Non-Hispanic white.
Table 2 shows baseline demographics based on immunosuppressants use. A total of 7,987 participants (1.8%) were taking immunosuppressants. All covariates were significant and, in comparison to people without prior immunosuppressant use, there was a higher percentage of individuals with BMI between 35 and 39 in the group with prior immunosuppressant use (20.26 versus 18.70%).
Table 2.
Summary of the Descriptive Characteristics of Selected Population (MBSAQIP, 2015–2018), by Immunosuppressant Use
| Variable | Total (n = 442258) | Immunosuppressant use + (n = 7987) | Immunosuppressant use − (n = 434271) | p Value* |
|---|---|---|---|---|
| Age | <0.0001† | |||
| <30 y | 52914 (11.96) | 445 (5.57) | 52469 (12.08) | |
| 31–60 y | 343122 (77.58) | 6375 (79.82) | 4336747 (77.54) | |
| >60 y | 46222 (10.45) | 1167 (14.61) | 45055 (10.37) | |
| Sex | <0.0001† | |||
| Female | 353867 (80.01) | 6666 (83.46) | 347201 (79.95) | |
| Male | 88391 (19.99) | 1321 (16.54) | 87070 (20.05) | |
| Ethnicity | 0.0003† | |||
| Hispanic | 43060 (9.74) | 749 (9.38) | 42311 (9.74) | |
| NHW | 257963 (58.33) | 4667 (58.43) | 253296 (58.33) | |
| NHB | 74730 (16.90) | 1466 (18.35) | 73264 (16.87) | |
| Other | 66505 (15.04) | 1105 (13.83) | 65400 (15.06) | |
| BMI | 0.0046† | |||
| <35 kg/m2 | 23619 (5.34) | 426 (5.33) | 23193 (5.34) | |
| 35–39 kg/m2 | 82838 (18.73) | 1618 (20.26) | 81220 (18.70) | |
| 40–49 kg/m2 | 231038 (52.24) | 4066 (50.91) | 226972 (52.27) | |
| ≥50 kg/m2 | 104763 (23.69) | 1877 (23.50) | 102886 (23.69) | |
| Operation type | 0.0011† | |||
| LSG | 322131 (72.84) | 5946 (74.45) | 316185 (72.81) | |
| LRYGB | 120127 (27.16) | 2041 (25.55) | 118086 (27.16) | |
| Preoperative diabetes | <0.0001† | |||
| Yes | 113174 (25.59) | 2377 (29.76) | 110797 (25.51) | |
| No | 329084 (74.41) | 5610 (70.24) | 323474 (74.49) |
Data presented as n (%)
Chi-square test
Statistically significant
LRYGB, laparoscopic Roux-en-Y gastric bypass; LSG, laparoscopic sleeve gastrectomy; NHB, Non-Hispanic black; NHW, Non-Hispanic white
Table 3 displays the association between immunosuppressant use and C. difficile infection among MBS patients. Model 1 and Model 2 represent unadjusted and adjusted odds ratios, respectively. It was observed in all models that immunosuppressant use is associated with an increased risk of C. difficile infection. Unadjusted logistic regression models showed that patients using immunosuppressants were 95% more likely to have post-operative C. difficile infection (OR=1.945, 95% CI= 1.230 to 3.075; p <0.01) versus MBS patients not taking immunosuppressants (Table 3). After adjusting for age, gender, ethnicity, pre-operative BMI, diabetes status, and surgery procedure type, the association between immunosuppressant use and C. difficile infection remained significant with a 96% (OR= 1.956, 95% CI= 1.236 to 3.095; p <0.01) increased risk of C. difficile infection among those who used immunosuppressants prior to MBS. Patients between 31 and 60 years of age had a 17% (OR=0.826, 95% CI=0.639 to 1.067; p < 0.05) lower risk of C. difficile infection in comparison to those younger than 30 years. No significant differences in C. difficile infection risk were noted in NHB and Hispanics in comparison to NHW. Women had a 41% increased risk of C. difficile infection (OR=1.407, 95% CI=1.112 to 1.780; p < 0.01) in comparison to their male counterparts. No significant differences between BMI categories were noted. Patients who completed LRYGB had more than double the odds of developing C. difficile infection (OR=2.183, 95% CI=1.842 to 2.587; p < 0.0001) in comparison to patients who had LSG. Lastly, no differences in C. difficile risk were noted between patients previously diagnosed with diabetes versus their non-diagnosed counterparts.
Table 3.
Crude and Adjusted Logistic Regression Models Showing Association Between Clostridioides difficile Infection by Immunosuppressant Use (MBSAQIP, 2015–2018)
| Model, variable | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| 1* | |||
| Immunosuppressant use† | |||
| Non-immunosuppressant user | 1.0 (ref) | 1.0 (ref) | - |
| Immunosuppressant user | 1.945 | 1.230 – 3.075 | 0.0044‡ |
| 2§ | |||
| Immunosuppressant use† | |||
| Non-Immunosuppressant user | 1.0 (ref) | 1.0 (ref) | - |
| Immunosuppressant user | 1.956 | 1.236 – 3.095 | 0.0042‡ |
| Age group | |||
| <30 y | 1.0 (ref) | 1.0 (ref) | - |
| 31–60 y | 0.826 | 0.639 – 1.067 | 0.0375‡ |
| >60 y | 1.021 | 0.727 – 1.435 | 0.3978 |
| Ethnicity | |||
| NHW | 1.0 (ref) | 1.0 (ref) | - |
| Hispanic | 0.622 | 0.450 – 0.859 | 0.9767 |
| NHB | 0.609 | 0.469 – 0.789 | 0.8189 |
| Other | 0.400 | 0.290 – 0.553 | 0.0005‡ |
| Sex | |||
| Male | 1.0 (ref) | 1.0 (ref) | - |
| Female | 1.407 | 1.112 – 1.780 | 0.0044‡ |
| BMI | |||
| 35–39 kg/m2 | 1.0 (ref) | 1.0 (ref) | - |
| <35 kg/m2 | 1.184 | 0.804 – 1.743 | 0.4832 |
| 40–49 kg/m2 | 1.011 | 0.801 – 1.274 | 0.3515 |
| ≥50 kg/m2 | 1.133 | 0.872 – 1.472 | 0.5577 |
| Operation type | |||
| LSG | 1.0 (ref) | 1.0 (ref) | - |
| LRYGB | 2.183 | 1.842 – 2.587 | <0.0001‡ |
| Preoperative diabetes | |||
| No | 1.0 (ref) | 1.0 (ref) | - |
| Yes | 0.985 | 0.811 – 1.196 | 0.8789 |
Crude logistic regression model
Non-immunosuppressant user: Patients who did not use immunosuppressant before the operation (n = 434271); Immunosuppressant user: Patients who used immunosuppressant for chronic health condition before the operation (n = 7987)
Significant difference in comparison with reference
Adjusted logistic regression model controlling for age, race/ethnicity, sex, preoperative BMI, surgical procedure, and preoperative diabetes
LRYGB, laparoscopic Roux-en-Y gastric bypass; LSG, laparoscopic sleeve gastrectomy; NHB, Non-Hispanic black; NHW, Non-Hispanic white
DISCUSSION
This is the first nationally representative study evaluating the relationship between immunosuppressant therapy and the risk of C. difficile infection in patients following MBS. Analysis showed a remarkable 95% higher odds of C. difficile infection post-MBS among those patients taking immunosuppressants for chronic medical conditions versus MBS patients not taking immunosuppressants. This finding suggests it may be crucial for health care providers to increase C. difficile awareness post-MBS, particularly in patients taking immunosuppressants. This is relevant as C. difficile infections are associated with longer hospital stay (26), and those on immunosuppression are at a greater risk for recurrent C. difficile infection (27) and increased mortality (17). The observed overall incidence of C. difficile infection post-MBS in the present study is lower than the reported incidence of C. difficile in other surgery populations (0.13% versus 0.47%)(28).
Two previous studies have examined C. difficile infections in patients undergoing MBS. One study focused on the differences in the infection rates between surgery types whereas the other identified specific risk factors, however, none of them evaluated immunosuppressant use. The first study evaluated the impact of open or LRYGB and open or LSG on C. difficile infection (29). The authors found an 87% higher infection rate in patients that underwent open or LRYGB versus open or LSG, however, the potential explanation for this difference was not studied. The second study evaluated the MBSAQIP dataset 2015–2017 to search for risk factors for C. difficile infection in patients following MBS (16). Despite not analyzing immunosuppressants use and including a year less of data compared to the present analysis, they also found a higher risk of C. difficile infection in females. Other identified risk factors from this study included chronic kidney disease, a history of venous thromboembolism, and obstructive sleep apnea. Likewise, patients who underwent LRYGB had more than twice the risk of C. difficile infection versus patients who underwent LSG (16). These outcomes are in agreement to the results obtained in the present study as we observed a more than double increased risk of C. difficile infection rate in patients who had LRYGB versus those who had LSG. It is possible that the observed disparities are caused by the different anatomical location of the surgical incisions. LSG utilizes a gastric resection whereas LRYGB involves a gastric resection alongside two anastomoses involving the intestine: a gastrojejunostomy and a jejunojejunostomy (30). The concentration of bacteria capable of surviving the acidic environment in the stomach (pH 1–2) is quite low, whereas the concentration of bacteria normally present in the duodenum and jejunum is higher, likely due to the less acidic environment (pH 5.7–6.4) (30). Therefore, it is possible that creating a resection within the stomach where there is a lower concentration of bacteria might lead to a lower risk of infection compared to creating two anastomoses at the intestinal level, where bacterial concentrations are higher. It is also possible that the differential C. difficile risk by MBS procedure type is caused by the distinct microbial composition caused by LRYGB versus LSG (16, 31).
The only additional studies that have analyzed the effect of immunosuppressants on C. difficile infection rates in surgical patients are those involving solid organ transplants. Research in this area aligns with our findings as results show that the prevalence of C. difficile infection is higher in transplant patients consuming corticosteroids at the time of the infection (32) and during the early post-transplant period when patients are taking the highest doses of immunosuppressants (33).
Recent evidence suggests that C. difficile infection might be associated with alterations to the gut microbial composition (34). However, the specific taxa associated with a higher risk have not been fully elucidated. A systematic review published by our team (35), showed that MBS leads to changes to the gut microbiota that are different depending on the MBS procedure type. Although no studies have analyzed the gut microbial changes associated with a higher risk of C. difficile infection after MBS, it is possible that these changes could increase the risk of colonization and posterior invasion of pathogenic bacteria. For example, after RYGB surgery there is a pronounced decrease of the genera Bifidobacterium and Lactobacillus, both recognized probiotics; and a decrease in the butyrate-producing bacterial specie Faecalibacterium prausnitzii, which is the most abundant specie in the colon of healthy humans (36). It is possible that MBS, peri-surgery medication, various dietetic composition and how it interacts with environmental factors all affect gut microbial composition and affect C. difficile infection risk.”
The focus of this study was the effect of chronic immunosuppressant use on C. difficile risk; however, it is possible that short-term use of these medications might also increase the risk of C. difficile infection. During the current COVID-19 pandemic, high-dose glucocorticoids, a type of immunosuppressant, have been recommended to decrease COVID-19 severity (37, 38). Studies have shown that taking immunosuppressants <15 days before the diagnosis of C. difficile infection is associated with a two-fold increase in mortality (17). Therefore, it is possible that even short-term (2–3 weeks) treatments with glucocorticoids, such as those recommended for COVID-19, may significantly increase the risk of C. difficile infection and severity. Avoiding glucocorticoids may not be an option when treating those acutely ill with COVID-19 during the pandemic, so it is important for all healthcare providers, including MBS surgeons, to be aware of the potentially increased risk of C. difficile infection in patients who have recently been treated with glucocorticoids due to COVID-19 (8–10, 37–40). It is also important to note that the symptoms of COVID-19 and C. difficile infection overlap, with both presenting with diarrhea, nausea, vomiting, and abdominal pain, but with almost opposite responses to glucocorticoid treatment (8–10, 40–43). While immunosuppressants have been shown to decrease mortality in COVID-19 patients, they increase mortality in C. difficile infection (44). Previous studies have shown that glucocorticoid exposure triples the risk of C. difficile infection in comparison to other immunosuppressant agents such as infliximab, azathioprine, mercaptopurine, and methotrexate (7, 9, 45).
There are some limitations of the current analysis that need to be noted. First, it was not possible to differentiate between the specific immunosuppressant sub-categories, the duration of immunosuppression, the underlying conditions for which these medications were prescribed, and the previous history of C. difficile infection. Second, there was no available information regarding relevant medication such as antibiotics and proton pump inhibitors which increase C. difficile risk (10, 46), or metformin, which is related to a lower risk of C. difficile infection (47). Third, information regarding probiotic consumption was not collected and therefore we could not evaluate the effect of probiotics on C. difficile infection risk in patients taking immunosuppressants. This information is relevant as probiotics have shown to be protective in non-immunosuppressed patients but their effect on immunosuppressed patients is more debatable (48, 49). Lastly, the short follow up period of 30 days post-surgery limits any findings to within a relatively short post-MBS period. Therefore, C. difficile infections that developed beyond this time are not represented in our study. However, one of the strengths of the present study is the utilization of the MBSAQIP database, which is the largest MBS dataset in the country representing hundreds of MBS practices and hundreds of thousands of patients. The observed striking increased risk of C. difficile infection in patients taking immunosuppressive medications may lead to the development of a pre-MBS C. difficile prediction model for high-risk patients. Specifically, this pre-operative tool could help surgeons to easily identify the post-operative risk of C. difficile infection in patients who are prescribed immunosuppressants or have other factors that increase their risk of infection and disease severity. Similar models have been developed to predict the occurrence of C. difficile infection in hospitalized patients who receive antibiotics.
In summary, this is the first study evaluating C. difficile infection rates in patients who have completed MBS and who are on chronic immunosuppressant therapies. Our analysis showed a 95% higher odds of C. difficile infection in immunosuppressed patients after MBS compared with those not taking immunosuppressant medications. Given these results, it is crucial for healthcare providers, especially MBS surgeons, to be aware of the increased risk of C. difficile infection among their patients taking immunosuppressants. In light of the current COVID-19 pandemic and its overlapping presentation with C. difficile infection, providers may want to consider performing appropriate diagnostic testing, considering the differential effects of glucocorticoid treatment. Type of immunosuppressant, duration of therapy, underlying disease, other medications consumed, and type of MBS are important variables to include to create effective prediction models for post-operative C. difficile infection risk. Finally, it is paramount to investigate the role of C. difficile infection preventive strategies such as probiotics in this high-risk population.
Acknowledgments
Support: This work was supported by NIH-National Institute on Minority Health and Health Disparities grant [R01MD011686]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
REFERENCES:
- 1.Hurley BW, Nguyen CC. The spectrum of pseudomembranous enterocolitis and antibiotic-associated diarrhea. Arch Intern Med. 2002. October 28;162(19):2177–84. [DOI] [PubMed] [Google Scholar]
- 2.Prevention CfDC. Antibiotic Resistance Threats in the United States. US Department of Health and Human Services. 2013. [Google Scholar]
- 3.Prevention CfDCa. Nearly half a million Americans suffer from C. difficile infections in single year. Available at: https://www.cdc.gov/hai/dpks/deadly-diarrhea/dpk-deadly-diarrhea.html. Accessed 11/06/2020, 2020.
- 4.Lessa FC, Winston LG, McDonald LC, Emerging Infections Program CdST. Burden of Clostridioides difficile infection in the United States. N Engl J Med. 2015. June 11;372(24):2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh AY, Mu Y, Winston LG, et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med. 2020. April 2;382(14):1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu DC, Katelaris CH. Long-term management of patients taking immunosuppressive drugs. Australian Prescriber. 2009;32(3). [Google Scholar]
- 7.Gong SS, Fan YH, Han QQ, Lv B, Xu Y. Nested case-control study on risk factors for opportunistic infections in patients with inflammatory bowel disease. World J Gastroenterol. 2019. May 14;25(18):2240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridioides difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002. March;235(3):363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leekha S, Aronhalt KC, Sloan LM, Patel R, Orenstein R. Asymptomatic Clostridioides difficile colonization in a tertiary care hospital: admission prevalence and risk factors. Am J Infect Control. 2013. May;41(5):390–3. [DOI] [PubMed] [Google Scholar]
- 10.Zarowitz BJ, Allen C, O’Shea T, Strauss ME. Risk Factors, Clinical Characteristics, and Treatment Differences Between Residents With and Without Nursing Home- and Non-Nursing Home-Acquired Clostridioides difficile Infection. J Manag Care Spec Pharm. 2015. Jul;21(7):585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spigaglia P COVID-19 and Clostridioides difficile infection (CDI): Possible implications for elderly patients. Anaerobe. 2020. August;64:102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishara J, Farah R, Mograbi J, et al. Obesity as a Risk Factor for Clostridioides difficile Infection. Clinical Infectious Diseases. 2013. August 15;57:489–93. [DOI] [PubMed] [Google Scholar]
- 13.Consortium F, SH B, WC K, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009. July 30;361:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maciejewski ML, Arterburn DE, Scoyoc LV, et al. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surgery. 2016;151:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hefler J, Dang J, Modasi A, Switzer N, Birch DW, Karmali S. Effects of Chronic Corticosteroid and Immunosuppressant Use in Patients Undergoing Bariatric Surgery. Obes Surg. 2019. October;29(10):3309–15. [DOI] [PubMed] [Google Scholar]
- 16.Dang TT, Dang JT, Moolla M, et al. Clostridioides difficile and Laparoscopic Bariatric Surgery: an Analysis of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program Database. Obes Surg. 2019. June;29(6):1881–8. [DOI] [PubMed] [Google Scholar]
- 17.Das R, Feuerstadt P, Brandt LJ. Glucocorticoids Are Associated With Increased Risk of Short-Term Mortality in Hospitalized Patients With Clostridium difficile- Associated Disease. Nature. 2010. April 2010. [DOI] [PubMed] [Google Scholar]
- 18.Juul FE, Garborg K, Bretthauer M, et al. Fecal Microbiota Transplantation for Primary Clostridioides difficile Infection. N Engl J Med. 2018. June 28;378(26):2535–6. [DOI] [PubMed] [Google Scholar]
- 19.Surgery ACoSaASoMaB. Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. Available at: https://www.facs.org/quality-programs/mbsaqip.
- 20.Surgery ACoSaASoMaB. User Guide for the 2018 Participant Use Data File (PUF). Available at: https://www.facs.org/-/media/files/quality-programs/bariatric/mbsaqip_2018_puf_userguide.ashx.
- 21.Mulki R, Baumann AJ, Alnabelsi T, et al. Body mass index greater than 35 is associated with severe Clostridioides difficile infection. Aliment Pharmacol Ther. 2017. January;45(1):75–81. [DOI] [PubMed] [Google Scholar]
- 22.Bishara J, Farah R, Mograbi J, et al. Obesity as a risk factor for Clostridioides difficile infection. Clin Infect Dis. 2013. August;57(4):489–93. [DOI] [PubMed] [Google Scholar]
- 23.Chandradas S, Khalili H, Ananthakrishnan A, et al. Does Obesity Influence the Risk of Clostridioides difficile Infection Among Patients with Ulcerative Colitis? Dig Dis Sci. 2018. September;63(9):2445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punni E, Pula JL, Asslo F, Baddoura W, DeBari VA. Is obesity a risk factor for Clostridioides difficile infection? Obes Res Clin Pract. 2015. Jan-Feb;9(1):50–4. [DOI] [PubMed] [Google Scholar]
- 25.Meier K, Nordestgaard AT, Eid AI, et al. Obesity as protective against, rather than a risk factor for, postoperative Clostridioides difficile infection: A nationwide retrospective analysis of 1,426,807 surgical patients. J Trauma Acute Care Surg. 2019. June;86(6):1001–9. [DOI] [PubMed] [Google Scholar]
- 26.Marra AR, Perencevich EN, Nelson RE, et al. Incidence and Outcomes Associated With Clostridioides difficile Infections: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020. January 3;3(1):e1917597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avni T, Babitch T, Ben-Zvi H, et al. Clostridioides difficile infection in immunocompromised hospitalized patients is associated with a high recurrence rate. Int J Infect Dis. 2020. January;90:237–42. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues MA, Brady RR, Rodrigues J, Graham C, Gibb AP. Clostridioides difficile infection in general surgery patients; identification of high-risk populations. Int J Surg. 2010;8(5):368–72. [DOI] [PubMed] [Google Scholar]
- 29.Hussan H, Ugbarugba E, Bailey MT, et al. The Impact of Bariatric Surgery on Short Term Risk of Clostridioides difficile Admissions. Obes Surg. 2018. July;28(7):2006–13. [DOI] [PubMed] [Google Scholar]
- 30.Aron-Wisnewsky J, Dore J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012. October;9(10):590–8. [DOI] [PubMed] [Google Scholar]
- 31.Farin W, Onate FP, Plassais J, et al. Impact of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy on gut microbiota: a metagenomic comparative analysis. Surg Obes Relat Dis. 2020. July;16(7):852–62. [DOI] [PubMed] [Google Scholar]
- 32.Cusini A, Beguelin C, Stampf S, et al. Clostridioides difficile infection is associated with graft loss in solid organ transplant recipients. Am J Transplant. 2018. July;18(7):1745–54. [DOI] [PubMed] [Google Scholar]
- 33.Albright JB, Bonatti H, Mendez J, et al. Early and late onset Clostridioides difficile-associated colitis following liver transplantation. Transpl Int. 2007. October;20(10):856–66. [DOI] [PubMed] [Google Scholar]
- 34.Duan J, Meng X, Liu S, et al. Gut Microbiota Composition Associated With Clostridioides difficile-Positive Diarrhea and C. difficile Type in ICU Patients. Front Cell Infect Microbiol. 2020;10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales-Marroquin E, Hanson B, Greathouse L, de la Cruz-Munoz N, Messiah SE. Comparison of methodological approaches to human gut microbiota changes in response to metabolic and bariatric surgery: A systematic review. Obes Rev. 2020. August;21(8):e13025. [DOI] [PubMed] [Google Scholar]
- 36.Miquel S, Martin R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013. June;16(3):255–61. [DOI] [PubMed] [Google Scholar]
- 37.Lamontagne F, Agoritsas T, Macdonald H, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020. September 4;370:m3379. [DOI] [PubMed] [Google Scholar]
- 38.Prevention CfDCa. COVID-19 Science Update released: September 11, 2020. Available at: https://www.cdc.gov/library/covid19/091120_covidupdate.html. Accessed September 11, 2020.
- 39.Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic. JAMA. 2020. October 6;324(13):1292–5. [DOI] [PubMed] [Google Scholar]
- 40.Vaishnavi C. Established and potential risk factors for Clostridum difficile infection. Indian J Med Microbiol. 2009. Oct-Dec;27(4):289–300. [DOI] [PubMed] [Google Scholar]
- 41.Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020. July 30;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Group WHOREAfC-TW, Sterne JAC, Murthy S, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020. October 6;324(13):1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandhu A, Tillotson G, Polistico J, et al. Clostridiodes difficile in COVID-19 Patients, Detroit, Michigan, USA, March-April 2020. Emerg Infect Dis. 2020. September;26(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Horin S, Margalit M, Bossuyt P, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridioides difficile infection. Clin Gastroenterol Hepatol. 2009. September;7(9):981–7. [DOI] [PubMed] [Google Scholar]
- 45.Schneeweiss S, Korzenik J, Solomon DH, Canning C, Lee J, Bressler B. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther. 2009. August;30(3):253–64. [DOI] [PubMed] [Google Scholar]
- 46.Nasiri MJ, Goudarzi M, Hajikhani B, Ghazi M, Goudarzi H, Pouriran R. Clostridioides (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: A systematic review and meta-analysis. Anaerobe. 2018. April;50:32–7. [DOI] [PubMed] [Google Scholar]
- 47.Eliakim-Raz N, Fishman G, Yahav D, et al. Predicting Clostridioides difficile infection in diabetic patients and the effect of metformin therapy: a retrospective, case-control study. Eur J Clin Microbiol Infect Dis. 2015. June;34(6):1201–5. [DOI] [PubMed] [Google Scholar]
- 48.Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridioides difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017. December 19;12:CD006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bose DD. Probiotics for Recurrent Clostridioides difficile Infection in solid Organ Transplant Recipients. Infectious Diseases in Clinical Practice. 2013. September 2013;21. [Google Scholar]