Abstract
Purpose of review:
Breast cancer frequently metastasizes to the bone and lung, but the ability to treat metastatic tumor cells remains a pressing clinical challenge. Histone deacetylases (HDACs) and histone acetyltransferases (HATs) have emerged as promising targets since these enzymes are aberrantly expressed in numerous cancers and regulate the expression of genes that drive tumorigenesis and metastasis. This review focuses on the abnormal expression of histone modifying enzymes in cancers that have a high tropism for the bone and lung and explores the clinical use of histone deacetylase inhibitors for the treatment and prevention of metastasis to these sites.
Recent findings:
Preclinical studies have demonstrated that the role for HDACs is highly dependent on tumor type and stage of disease progression. HDAC inhibitors can induce apoptosis, senescence, cell differentiation, tumor dormancy genes, and inhibit angiogenesis, making these promising therapeutics for the treatment of metastatic disease. HDAC inhibitors are already FDA approved for hematologic malignancies and are in clinical trials with standard-of-care chemotherapies and targeted agents for several solid tumors, including cases of metastatic disease. However, these drugs can negatively impact bone homeostasis.
Summary:
Although HDAC inhibitors are not currently administered for the treatment of bone and lung metastatic disease, preclinical studies have shown that these drugs can reduce distant metastasis by targeting molecular factors and signaling pathways that drive tumor cell dissemination to these sites. Thus, HDAC inhibitors in combination with bone protective therapies may be beneficial in the treatment of bone-metastatic cancers.
Keywords: histone deacetylase, histone acetyltransferase, epigenetics, breast cancer, bone metastasis
INTRODUCTION
Despite improvements in survival and outcomes in patients with breast cancer, metastatic disease remains a leading cause of morbidity and mortality [1, 2]. The bone and lung are two of the most common sites of breast cancer metastasis, and approximately 70% of breast cancer and prostate cancer patients present with bone metastases upon autopsy [3]. Lung cancer, melanoma, renal cell carcinoma, and thyroid cancer are also reported to disseminate to the bone with relatively high (>20%) frequency [3]. Cancers that most commonly metastasize to the lung include colorectal, breast, head and neck, urologic (renal, prostate) and osteosarcoma [4, 5]. Thus, therapeutically targeting tumor cells that have metastasized to the bone and lung distant sites holds clinical importance. Patients who develop bone metastases may experience severe pain, impaired mobility, pathologic fractures, spinal cord compression and hypercalcemia [6]. Bisphosphonates and denosumab, inhibitors of osteoclast activity and bone resorption, are commonly utilized to manage metastasis-related symptoms and have been shown to prevent the development of bone metastases and improve survival in select patient populations [7]. The Early Breast Cancer Trialists’ Collaborative Group reported that adjuvant bisphosphonates reduce the rate of breast cancer recurrence in the bone and improve survival in women who were postmenopausal, but not premenopausal, at the time of treatment initiation [8]. These findings have been confirmed by several follow-up studies [9-11]. A trial by the Austrian Breast & Colorectal Study Group (ABCSG) showed that adjuvant use of denosumab with an aromatase inhibitor in postmenopausal patients with hormone receptor-positive breast cancer improves disease-free survival [12]; however, the D-CARE study found no benefit of denosumab on breast cancer patient survival [13]. Thus, there remains an urgent need to identify therapies that broadly improve breast cancer patient survival, particularly for patients with bone metastatic disease.
In the search for new therapeutic targets, genetic mutations in the nucleotide sequence that alter the expression of oncogenes and tumor suppressor genes have been investigated by many groups as potential drivers of tumorigenesis and metastasis. These studies have uncovered an important role for epigenetic alterations in the initiation and progression of cancer [14]. Heritable, functional epigenetic modifications to the genome alter gene expression without changing the nucleotide sequence through acetylation, methylation, phosphorylation, deimination, and ubiquitination of the DNA and histones. Human cancers are characterized by genome wide epigenetic changes leading to the dysregulation of genes that drive oncogenesis [15]. Global deregulation of DNA methylation, for instance, is one of the most prominent and earliest recognized epigenetic alterations in cancer cells [16]. Promoter hypermethylation may silence tumor suppressor genes while aberrant DNA hypomethylation may result in the overexpression of pro-tumorigenic genes, both driving tumorigenesis [17, 18].
It is well established that post-translational modifications of histones, and lysine acetylation in particular, regulate the expression of genes critical in tumor development and metastasis [19]. Acetylation of the ε-amino group of lysine residues within histones is a reversible process regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs) [20]. Lysine acetylation on histone tails by HATs weakens the interaction between the histone and neighboring nucleosomes to relax the chromatin structure and make target sequences accessible for transcription. Deacetylation of histones by HDACs strengthens this interaction to repress gene transcription [21]. Many nonhistone proteins including transcription factors, hormone receptors, chaperones, and cytoskeletal proteins are also reversibly acetylated, resulting in altered function, stability, localization, and protein-protein interactions [22].
The activity of HATs and HDACs is known to drive tumorigenesis and metastasis by downregulating the expression of cell cycle inhibitors and pro-apoptotic factors and upregulating proteins that promote angiogenesis, invasiveness, and migration [23]. HDACs in particular have shown great promise as therapeutic targets, and HDAC inhibitors are already FDA approved for hematologic malignancies and in clinical trials for numerous solid tumors (Table 3), including cases of advanced disease [24, 25]. While HDAC inhibitors are not indicated for treating metastases to the bone and lung, these drugs alter signaling pathways known to play key roles in tumor cell dissemination. The goal of this review is to discuss the abnormal expression of histone modifying enzymes and the potential therapeutic application of HDAC inhibitors for bone and lung metastatic cancers, and breast cancer in particular.
Table 3.
HDAC inhibitors and their targets. Light yellow = HDACs targeted by each inhibitor at the IC50; dark yellow box = HDACs targeted at ten times the IC50; gray = HDACs not targeted at either concentration; asterisk (*) = FDA approved.
| Class | Inhibitor name | FDA Use | concentrations (lowest = IC50) |
HDAC1 | HDAC2 | HDAC3 | HDAC4 | HDAC5 | HDAC6 | HDAC7 | HDAC8 | HDAC9 | HDAC10 | HDAC11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *Belinostat | T-cell lymphoma | 27nM [114]; 0.27μm | ||||||||||||
| *Panobinostat | Multiple myeloma | 5nM [115]; 50nM | ||||||||||||
| *Vorinostat | T-cell lymphoma | 1μM [116]; 5μM | ||||||||||||
| II: Short chain fatty acid | *Valproic acid | Epilepsy, bipolar disorder, migraines | 1mM [117]; 10mM | |||||||||||
| III: Benzamide | Entinostat | Phase III for breast cancer, phase II for lung cancer | 0.5μM [118]; 5μM | |||||||||||
| IV: Cyclic tetrapeptide | *Romidepsin | T-cell lymphoma | 5nM [119]; 50nM |
HISTONE DEACETYLASES IN METASTATIC CANCER
HDACs have been grouped into four classes based on their homology, sequence similarity, and expression patterns (Table 1) [26]. The class I enzymes are ubiquitously expressed in all human tissues [27]. The expression of class IIa enzymes is generally restricted to the heart, skeletal muscle, and brain, while class IIb expression is restricted to the liver, kidney and placenta. HDAC11, the only class IV enzyme, shares sequence similarity to both Class I and Class II proteins. Class I, II, and IV HDACs all belong to the arginase/ deacetylase superfamily of proteins containing arginase-like amidino hydrolases and histone deacetylases [26, 28]. Class III enzymes, the sirtuins, possess deacetylase activity but are functionally unrelated to the other HDACs and utilize nicotinamide adenine dinucleotide (NAD) instead of zinc as a cofactor [29]. The functional classification of these enzymes is of particular importance given that different HDAC inhibitors target different classes of HDACs. Thus, while there is potentially some specificity for targeting groups of HDACs, it is particularly challenging to target an individual HDAC, since these enzymes can have compensatory functions within each class [30, 31].
Table 1.
Histone deacetylase classification and cellular localization.
| Class | Informs | Cellular Localization |
|---|---|---|
| I | HDAC1,2,3,8 | nucleus |
| IIa | HDAC4,5,7,9 | cytoplasm and nucleus |
| IIb | HDAC6,10 | |
| III | SIRT1-7 | nucleus (SIRT1,3,6,7) cytoplasm (SIRT2) mitochondria (SIRT3-5) |
| IV | HDAC11 | nucleus |
Class I Enzymes
Aberrant expression of class I HDACs has been identified in breast cancer and other tumor types that have a high predilection for metastasizing to the bone and lung. In breast cancer, tumors from patients with invasive ductal cell carcinoma and ductal carcinoma in situ (DCIS) have elevated expression of HDAC1, 3 & 8 [32]. Immunohistochemical analysis of tissue microarrays from patients with primary invasive breast cancer revealed that elevated HDAC2 & 3 protein expression correlates with negative hormone receptor status, while high HDAC2 expression is associated with HER2 overexpression and lymph node metastasis [33]. HDAC1 & 3 are also significantly upregulated in prostate cancer with higher HDAC1 levels in metastatic tumors [34]. HDAC1 and 2 expression in prostate cancer also correlates with Gleason scores and tumor dedifferentiation, with high-grade tumors expressing higher levels of both isoforms [35]. This suggests that these enzymes may play a role in prostate cancer progression and metastasis. HDAC2 is upregulated in non-small cell lung cancer cells and promotes migration and invasion, cellular characteristics critical for metastasis [36]. In renal cell carcinoma, HDAC1 mRNA is upregulated in 4% of patients in The Cancer Genome Atlas data set and its expression is associated with worse overall survival [37]. This study also found that HDAC1 overexpression increases renal cell carcinoma cell invasion in vitro. Lastly, elevated HDAC1 and HDAC2 expression has been identified in osteosarcoma cells, which have a high predilection for metastasizing to the lung [38]. In this study, siRNA-mediated silencing of HDAC1 and 2 significantly reduces osteosarcoma cell growth in vitro.
These studies highlight the association of elevated class I HDAC expression with tumor progression and metastasis in multiple tumor types that frequently metastasize to the bone and lung. Of note, several of these studies implicate only one or two of the class I HDACs, but therapeutic targeting of these HDACs is likely to be broader than simply targeting, for example, HDAC1 in renal cell carcinoma. Thus, as the HDAC inhibitors move forward in clinical studies, it will be important to determine the role for the other class I HDACs in these tumor types to ensure that broad inhibition of HDACs is clinically appropriate and does not lead to unexpected detrimental outcomes. Since HDACs must be broadly targeted, it could also be argued that identifying the particular HDAC responsible for a molecular mechanism is perhaps less important therapeutically than understanding which class or classes of enzymes are involved.
Class II Enzymes
Aberrant expression of class II HDACs has also been implicated in tumor development among bone and lung metastatic cancers. One study found that HDAC7 downregulation is associated with decreased histone 3 lysine 27 acetylation (H3K27ac) at transcription start sites and super enhancers in stem-like breast cancer cells [39]. Notably, these transcriptional changes repress expression of oncogenes including C-MYC, VEGFA, SLUG and SMAD as well as multiple stem cell transcription factor genes. Self-renewing cancer stem cells (CSCs) are important drivers of tumor initiation, progression, metastasis and therapy resistance [40]. Thus, targeting HDAC7 may present an important avenue for inhibiting the CSC phenotype and activation of multiple oncogenes in breast cancer to prevent tumor development and metastasis. In prostate cancer, HDAC4 and 5 are up-regulated in primary and metastatic tumors in vivo and their expression enhances cell invasion in vitro [34]. In clear cell renal cell carcinoma HDAC6 up-regulation has been reported in a subset of patients with metastatic disease [37, 41]. Both HDAC1 and HDAC6 overexpression also increase renal cell carcinoma tumor cell invasion in vitro by increasing matrix metalloproteinase expression and promoting cell motility by decreasing acetylation of α-tubulin [37, 41]. In non-small cell lung cancer, HDAC10 is upregulated in primary and metastatic tumors and preferentially localizes to the cytoplasm of cancer cells but not in adjacent normal lung epithelial cells [42]. The same study also found that in vitro overexpression of HDAC10 and a nuclear localization-defective HDAC10 mutant significantly increases cell growth and G1/S phase cell cycle transition while HDAC10 knockdown induces G1 arrest via upregulation of the cell cycle inhibitors p21 and p27. HDAC10 knockdown also significantly downregulates the antiapoptotic protein BCL2 while upregulating the pro-apoptotic protein BAK. These data indicate that alterations in the subcellular localization of class II HDACs may also contribute to tumorigenesis and metastasis. Indeed, HDACs are known to have multiple functions outside of the nucleus. In cancer cells, HDACs may be rapidly shuttled from the nucleus to the cytoplasm, which reduces the ability of HDACs to transcriptionally repress oncogenes and deacetylate non-histone proteins in the cytoplasm that regulate tumorigenesis and metastasis. For example, excessive acetylation of α-tubulin, which is a substrate of HDAC6, stabilizes microtubules, resulting in cell cytotoxicity [43], an effect that has been leveraged in the use of taxanes as standard of care therapies for multiple cancer types. By deacetylating α-tubulin, cytoplasmic HDAC6 also regulates microtubule-mediated processes including cell division and migration that drive tumor development and metastasis [44]. The Hsp90 chaperone protein is also a substrate of HDACs acting in the cytoplasm. Increased acetylation of Hsp90 by either HDAC6 knockdown or HDAC inhibition inactivates its chaperone activity and leads to degradation of its target proteins including HER2 ErbB1, ErbB2, Akt, and c-Raf [45]. Thus, aberrant deacetylation of cytoplasmic chaperone proteins alters the expression of numerous oncogenic factors that drive tumorigenesis. The full scope of the cytoplasmic roles of HDACs in cancer remains to be fully uncovered and continued studies are needed to better understand how dysregulation of their extra-nuclear actions drives tumor development and metastasis.
While few studies have directly investigated the role of HDAC expression in the process of bone or lung metastasis, the class II enzymes in particular may drive dissemination to these sites through regulation of pathways known to promote metastasis. One of the key pathways known to regulate tumor progression, dissemination, and metastasis that is targeted by HDACs is hypoxia inducible factor (HIF) signaling [46-48] (Figure 1). Tumor cells are subject to fluctuating hypoxic conditions as solid tumors grow beyond several millimeters, which activates HIF signaling. Importantly, HIF signaling is activated by tumor cells in the bone marrow since this microenvironment is known to have regions with physiologically low oxygen tensions [3, 49]. HIF activation is also well established to promote bone colonization by tumor cells. Expression of constitutively active HIF1-α in MDA-MB-231 bone metastatic breast cancer cells enhances bone colonization in vivo, while bone metastasis is significantly reduced in mice inoculated with cells expressing dominant-negative HIF1-α [50, 51]. Similarly, knockdown of HIF1-α reduces MDA-MB-231 colonization of the bone marrow [52]. Breast cancer patients with a greater number of disseminated tumor cells in the bone marrow have 3-fold higher expression of HIF1-α in their primary tumors [47]. HIF1-α expression has also been shown to predispose the lungs for metastasis [28, 53, 54], and expression of dominant negative HIF1-α or treatment with 2-methoxyestradiol reduces lung colonization by breast cancer cells. Furthermore, knockdown of HIF1-α in the mammary fat pad, reduces lung metastasis in vivo [55]. Multiple studies have explored the effects of HDAC inhibition and silencing on HIF1-α expression. Silencing of HDAC6 reduces HIF1-α levels by disrupting its association with the chaperone Hsp90, leading to subsequent proteasomal degradation [56]. Genetic or pharmacologic HDAC9 inhibition downregulates HIF1-α in a process dependent on the eukaryotic translation initiation machinery, especially eIF4E, 4G1 and 3G subunits [57]. Lastly, HDAC7 has also been identified as a transcriptional activator of HIF1-α signaling by translocating to the nucleus under hypoxic conditions and forming a complex with HIF1-α and p300 [58]. Thus, targeting HDACs through the use of HDAC inhibitors may impact multiple pathways, including HIF, that promote tumor colonization of the bone marrow and lungs.
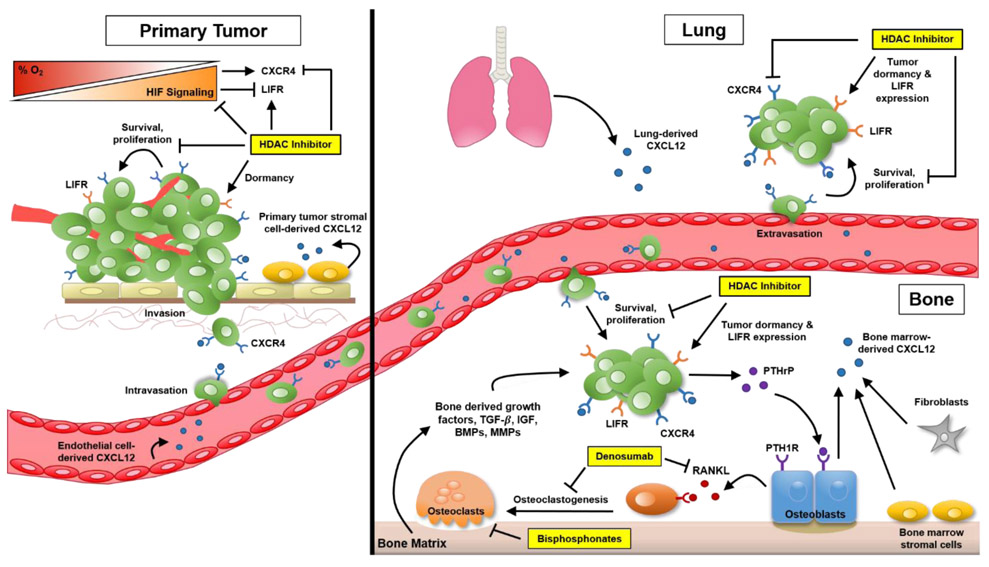
Figure 1. HDAC inhibitors in the treatment of lung and bone metastasis.
Hypoxia-inducible factor (HIF) signaling plays a critical role in cancer metastasis. Hypoxia (low-oxygen tension) and HIF expression promote metastasis to the lung, bone and other organs via multiple mechanisms including upregulating the expression of CXCR4 by tumor cells. Signaling via CXCR4 and its ligand, CXCL12, plays a key role in tumor cell dissemination to distant sites by enhancing tumor growth, invasion, angiogenesis as well as adhesion to endothelial cells to promote the early stages of metastasis. CXCL12 secreted by cells in the bone, lung and other distant organs acts as a chemoattractant to promote homing of cancer cells to metastatic sites. At the end organ, CXCR4 signaling triggers adhesion of cancer cells to endothelial cells to promote extravasation as well as proliferation. In the bone specifically, disseminated tumor cells can induce bone destruction to support their own growth by releasing PTHrP which stimulates production of RANKL by osteoblasts, resulting in osteoclastic bone resorption that releases bone derived growth factors which further stimulates tumor growth and exacerbates bone destruction. This process can be targeted with bisphosphonate or denosumab treatment to inhibit osteoclast-mediated bone resorption. Treatment with HDAC inhibitors can directly target tumor cells by reducing HIF signaling and CXCR4 expression, as well as inducing the expression of pro-dormancy genes (leukemia inhibitory factor receptor, LIFR), making them potentially effective therapeutics for the treatment and prevention of lung and bone metastasis.
Class III Enzymes / Sirtuins
The role of sirtuins has also been investigated in bone and lung metastatic tumors [59]. In prostate tumor cells, SIRT7 overexpression induces epithelial-mesenchymal transition (EMT) to promote cell migration and invasion in vitro while its depletion reduces lung metastasis in vivo [60]. SIRT7 inactivation reverses the EMT phenotype as evidenced by decreased levels of the EMT-inducing transcription factor Slug (SNAI2) and vimentin (a mesenchymal marker), as well as increased expression of E-cadherin (an epithelial marker and cell-cell adhesion molecule) and DAB2 interacting protein (DAB2IP), a tumor suppressor whose loss promotes EMT and metastasis. In breast cancer, SIRT1 plays both tumor suppressing and tumor promoting roles. Increased expression of SIRT1 in triple negative breast cancer patients is associated with lymph node metastasis, and SIRT1 knockdown in MDA-MB-231 cells, a model of metastatic cancer, suppresses invasion in vitro [61]. Latifkar et al. proposes that loss of SIRT1 expression promotes metastasis by reducing lysosomal acidification and protein degradation, which may support the release of exosomes containing extracellular matrix hydrolases that increase invasion [62]. In contrast, in hormone receptor and HER2 positive breast cancer, SIRT1 expression suppresses TGF-β driven EMT and is associated with lower risk of lymph node metastasis [63]. These studies indicate that inhibiting sirtuins may be beneficial in blocking tumor progression in prostate cancer and hormone receptor negative breast cancer, but may have the opposite effect in hormone receptor positive breast cancer, but further follow-up studies are needed. Of note, these studies did not examine bone metastasis, so it remains unknown whether these sirtuins regulate bone metastasis similar to their effects on lung and lymph node metastasis. Lastly, in renal cell carcinoma, SIRT1, 3, & 6 levels are significantly downregulated [64]. In particular, high SIRT3 expression is associated with better overall survival and greater metastasis free survival in patients. In a separate analysis of clear cell renal cell carcinoma, SIRT1, SIRT3, and SIRT5 expression is lower in tumor specimens with advanced TNM stage and poor histological grade, while SIRT6 and SIRT7 expression is higher [65]. Additional in vivo and in vitro experiments are needed to further validate the functional role of these particular sirtuins in renal cell carcinoma. The role for sirtuins in tumor progression varies widely across tumor types, and their role in bone metastasis remains unclear. It will therefore be important to examine the effect of individual inhibitors that target the class III enzymes for each tumor type and subtype.
Class IV Enzymes
Though much less heavily studied, biphasic roles for the only class IV enzyme, HDAC11, have recently been identified in tumor progression and metastasis. In breast cancer, HDAC11 expression promotes tumor growth within the lymph nodes through a mechanism involving the downregulation of factors that induce cell cycle arrest [66••] including RRM2 [67] and E2F8 [68]. HDAC11 knockdown significantly decreases the percentage and size of tumors formed by breast cancer cells injected into the axillary lymph node. Unexpectedly, this study also revealed that HDAC11 shRNA knockdown and treatment with quinsinostat, the most potent HDAC11 inhibitor [69], significantly increases metastasis to the lung in vivo and cell migration in vitro. Interestingly, quisinostat still significantly inhibited axillary lymph node tumor growth in vivo. These results suggest that increased HDAC11 expression in the lymph node promotes tumor cell survival and proliferation. However, a decline in HDAC11 expression promotes a migratory phenotype allowing tumor cell dissemination from the lymph node to distant organs. This finding indicates that HDAC11 may have opposing roles in tumor progression and metastasis, which may make it an unattractive clinical target. While hematogenous dissemination is the main route for tumor cell dissemination to the bone marrow, axillary lymph node metastases are recognized as an independent risk factor for bone metastases in breast cancer patients [70]. Due to the spectrum of HDAC11 expression along the metastatic cascade, caution should be exercised in the utilization of HDAC11 inhibition for cancer therapy since serious unintended consequences on tumor metastasis may result due to untimely initiation of treatment or poorly selected patient candidates.
A role for HDAC11 has also been uncovered in lung cancer. HDAC11 is elevated in CSC-like cells derived from non-small cell lung cancer cell lines and promotes expression and promoter activity of the stem cell transcription factors Sox2 [71]. The study also demonstrated that HDAC11 shRNA silencing and treatment with selective inhibitors (FT234 and FT895) markedly decreases self-renewal and Sox2 expression in CSC-like lung tumor cells, which is reminiscent of the effects of class II HDAC inhibition in breast cancer cells [39]. Since CSCs are hypothesized to contribute to the initiation of metastasis formation [40], the ability of HDAC11 and the class II HDACs to regulate the CSC population may be of therapeutic benefit and suggest that consideration of the therapeutic window for these drugs may be of particular importance.
Since HDAC up-regulation is frequently observed in vitro, in vivo, and in human tumors, the use of HDAC inhibitors may ultimately prove beneficial in reducing metastasis and improving patient outcomes, but as indicated above, this is likely to be tumor and subtype-dependent. HDACs target multiple signaling pathways that are known to play a role in the dissemination of tumor cells to the lung and bone, which may prove both useful and problematic in the clinical setting. Targeting multiple signaling pathways through broad HDAC inhibitors may prevent therapeutic resistance but could also lead to unintended consequences on pathways that counteract the anti-tumor mechanisms that are activated. Thus, it is critical to better understand the specific roles of the HDACs and their expression patterns in order to identify which patients are the best candidates for HDAC inhibitor treatment. In addition, the development of more isoform-specific HDAC inhibitors may offer improved treatment options with fewer adverse effects, but this will likely be challenging given HDAC redundancy [72-74].
HISTONE ACETYLTRANSFERASES IN METASTATIC CANCER
The HATs are classified into five major subfamilies (Table 2) [75, 76]. Like the HDACs, studies have not directly investigated the role of HAT expression or activity in lung or bone metastasis. However, a number of studies have found evidence highlighting the role of the HAT1 subfamily in tumor progression and metastasis among the highly bone and lung metastatic cancers. Compared with surrounding normal epithelium, lung tumors express lower levels of HAT1 as well as Fas, a death receptor required for apoptosis [77]. This study also demonstrated that restoration of HAT1 promotes Fas expression and significantly increases cancer cell death, suggesting that HAT1 may serve as a suppressor of lung tumor progression. The Gcn5/PCAF family has also been implicated in tumor proliferation. In non-small cell lung cancer, GCN5 is up-regulated and induces cell proliferation and G1/S phase cell cycle transition via increased histone H3 and H4 acetylation at the cyclin D1, cyclin E1, and E2F1 promoters [78]. These data indicate that histone modifications by HATs drive tumorigenesis by inducing the transcription of genes that promote cell cycle progression and proliferation. Reduced PCAF expression has also been shown to dysregulate cell cycle progression by impairing the acetylation of p53 and downstream p21 transcription, resulting in increased cyclin D1, phosphorylation of retinoblastoma 1, and progression through the G1/S transition [79].
Table 2.
Histone acetyltransferase classification and origin of subfamily names.
| Subfamily | Naming Origin |
|---|---|
| HAT1 | Founding member histone acetyltransferase 1 |
| Gcn5/PCAF | Founding member yeast Gcn5 and human paralog, PCAF |
| MYST | Founding member s MOZ, Ybf2/Sas3, Sas2, and TIP60 |
| P300/CBP | Human paralogs p300 and CBP |
| Rtt109 | Initials identification as regular of Ty1 transposition gene product 109 |
Altered expression of the MYST family of HATs has also been studied in some bone and lung metastatic cancers. MYST3 is amplified in 11% and up-regulated in 15% of primary breast tumors with an even higher frequency (22%) detected in the more aggressive luminal B subtype (HER2−) in patient datasets from The Cancer Genome Atlas [80]. High MYST3 expression correlates with reduced progression-free and overall survival in patients with estrogen receptor-positive (ER+) breast cancers. Furthermore, MYST3 depletion significantly reduces proliferation of ER+/MYST3− high breast cancer cells in vitro. These data suggest that MYST3 expression may promote breast tumor progression and a more aggressive cancer phenotype. In contrast, homozygous deletion of MYST4 has been identified in lung cancer cell lines and primary lung tumors [81]. The same study also found that depletion of MYST4 in vitro enhances cancer cell growth and viability while MYST4 depletion in vivo increases tumor growth and liver metastasis, indicating that this histone acetyltransferase likely serves as a tumor suppressor and metastasis suppressor in lung cancer.
Lastly, abnormal expression of the p300/CREB-binding protein (CBP) subfamily is observed in some bone and lung metastatic cancers. Breast tumors express higher levels of p300 in comparison to normal surrounding breast tissue [82]. Expression of p300 correlates with higher histological grade, advanced stage at diagnosis, tumor recurrence, and shortened overall survival. Another study demonstrated that in vitro inhibition of p300 acetyltransferase activity induces apoptosis and reduces migration and invasion of breast cancer cells [83]. In this study, in vivo inhibition also reduces metastatic lung tumor burden as well as mitotic index and Ki67 levels, indicating that p300 activity promotes breast cancer lung metastasis. Ring et al. found that expression of CBP is higher in triple negative breast cancer than other less aggressive breast tumor subtypes [84]. Targeting CBP in vivo also decreases breast tumor growth more than with paclitaxel alone [85]. These data indicate that CBP expression is associated with the development of a more aggressive tumor phenotype and that targeting p300/CPB may enhance sensitivity to standard-of-care chemotherapies. CBP is also a known transcriptional activator of β-catenin [86], a key signal transducer in the Wnt signaling pathway which is known to promote EMT and metastasis [87-90]. Wnt activation by β-catenin/T-cell factor 4 (TCF4) overexpression in lung and breast cancer cells also increases the expression of the transcription Gli2, which in turn promotes production of parathyroid hormone-related protein (PTHrP), a key driver of osteolysis in bone metastatic tumors [91]. Furthermore, Wnt signaling is also upregulated in prostate tumors that have metastasized to the bone [92, 93] and breast tumors that have metastasized to the lung [94, 95] . Collectively these data suggest that targeting p300/CPB may be beneficial in blocking tumor metastasis to the bone and lung, particularly in the case of breast cancer.
Much like the HDACs, overexpression of HATs has been implicated in tumor development and metastasis in multiple cancer types. Inhibitors of these enzymes could prove clinically beneficial for the treatment of metastatic cancer. However, unlike the HDAC inhibitors, HAT inhibitors have not produced consistent and promising results that translate from in vitro to in vivo and clinical studies [96]. This may be due, in part, to challenges such as HATs functioning in large, multi-protein complexes that regulate the enzymatic activity and substrate specificity of the acetyltransferase. It is necessary to accurately recapitulate these protein-protein interactions in vitro, otherwise the recombinant complexes may not reflect their in vivo enzymatic activity and the ability to develop effective inhibitors will be limited. Poor cell permeability and stability in vivo as well as lack of selectivity also contribute to the limited development and use of HAT inhibitors. Lastly, while p300/CPB appear to be a promising therapeutic target in breast cancer, some HATs such as MYST4 [81] display both tumor promoting and suppressive roles, indicating that the selectivity of any HAT inhibitors in clinical development will need to be rigorously examined in preclinical studies for each tumor type.
CLINICAL USE OF HDAC INHIBITORS AS CANCER THERAPEUTICS
The HDAC inhibitors are broken into four different classes based on their chemical structures: hydroxamates, aliphatic/ short chain fatty acids, benzamides, and cyclic peptides (Table 3). HDAC inhibitors are currently FDA approved for hematologic malignancies like multiple myeloma, which often has a bone osteolysis component [97], and lymphomas [98]. The HDAC inhibitor valproic acid (i.e. valproate) is also FDA-approved for the treatment of epilepsy, bipolar disorder, and migraines. Since aberrant expression of HDACs are known to drive tumorigenesis and metastasis in solid tumors by downregulating cell cycle inhibitors and pro-apoptotic factors and upregulating proteins that promote invasion, migration, and angiogenesis [23], HDAC inhibitors have also emerged as attractive therapeutics for the treatment of advanced solid tumors [99, 100]. However, despite their success in treating hematologic malignancies, single-agent HDAC inhibitor therapy has not shown the same clinical efficacy in solid tumors. In a phase II clinical trial in patients with metastatic breast cancer, vorinostat monotherapy did not induce complete or partial responses based on Response Evaluation Criteria in Solid Tumors (RECIST) [101]. Additional phase II clinical trials of vorinostat also demonstrated minimal activity for the treatment of relapsed non-small cell lung cancer, recurrent ovarian cancer [102], metastatic head and neck cancer [103] and only modest activity in patients with recurrent glioblastoma multiforme [104]. Yet another phase II trial of vorinostat in patients with metastatic castration-resistant prostate cancer who had already been pretreated with chemotherapy found no significant clinical activity as measured by rate of progression at six months [105]. In fact, all 29 participants had to be taken off therapy (400mg orally daily) before six months due to significant toxicities or disease progression. Other clinical trials of single-agent HDAC inhibitor therapy have also failed to identify any clinically meaningful anti-tumor activity. Panobinostat had no objective antitumor response in a phase I trial in patients with metastatic melanoma [106] or a phase II study in patients with castration-resistant prostate cancer [107]. A similar lack of efficacy has also been demonstrated with romidepsin [108].
Findings from these studies demonstrating poor efficacy of single-agent HDAC inhibitor therapy are likely due to multiple factors. Notably, a lack of efficient drug delivery is not likely as immunohistochemical analysis of tumor sections revealed increases in histone acetylation following drug treatment [104]. Rather, the poor efficacy could be attributed to the effects of prior adjuvant treatments received by study participants as in the aforementioned trials on vorinostat in platinum-resistant ovarian cancer [102] and conventional therapy resistant metastatic head and neck cancer [103]. Prior failure on adjuvant therapy has been associated with decreased response rates and worse outcomes with subsequent chemotherapy treatments [109], possibly due to additional acquired mutations. For this reason, there is a shift to exploring combination HDAC inhibitor therapy to overcome resistance to conventional treatments. Emerging evidence suggests that rather than acting solely as a traditional cytotoxic agent, HDAC inhibitors may function better as biological response modifiers (BRMs), particularly in modulating the immune system’s response against cancer growth [110]. Combination treatment with HDAC inhibitors and immunotherapy will be discussed further in the next sections. Lastly, commonly recognized mechanisms of resistance to HDAC inhibitors likely contribute to their poor efficacy in clinical trials. Changes in drug efflux mechanisms [111], increased expression of the antiapoptotic protein Bcl-2 [112] as well as elevated levels of thioredoxin leading to lower reactive-oxygen species (ROS)-mediated DNA damage [113] have been cited as just a few factors driving resistance to HDAC inhibitors.
The results of these clinical trials have prompted assessment of the inhibitors in combination with standard-of-care chemotherapeutic and tumor-targeted agents given that multiple mechanisms have been identified that may facilitate synergism or lead to additive drug interactions between HDAC inhibitors and cytotoxic agents including microtubule inhibitors, antifolates, and nucleoside analogs [120, 121]. Combination treatment with DNA-damaging agents has also been heavily investigated since HDAC inhibitors induce chromatin decondensation, which facilitates access of these agents to their DNA substrates to induce apoptosis [122, 123]. Abrogation of the DNA repair response seen with many cytotoxic agents, especially poly ADP ribose polyperase (PARP) inhibitors, has also been exploited as a benefit of combination vorinostat therapy [124]. In a separate study on multiple myeloma (MM), co-treatment with the mTOR inhibitor everolimus overcomes resistance to panobinostat by synergistically downregulating multiple DNA repair genes, anti-apoptotic factors, and G2/M mitotic factors, thereby suppressing DNA damage repair, inhibiting cell cycle progression, and inducing cell death [125]. Mechanistically, MM cell resistance to panobinostat monotherapy is mediated by overexpression of C-X-C motif chemokine receptor 4 (CXCR4) which in turn activates pro-tumorigenic AKT/mTOR signaling. CXCR4 normally promotes lymphocyte trafficking, hematopoietic stem cell homing to the bone marrow, and endothelial cell precursor recruitment to sites of ischemia [126-128]. However, CXCR4 is overexpressed in numerous cancers. This promotes tumor cell dissemination by enhancing chemotaxis to tissues that normally secrete high levels of its ligand, C-X-C motif chemokine ligand 12 (CXCL12), including the bone, lung, brain, and liver which are common sites of metastasis (Figure 1). Of note, CXCR4 is one of the most enriched genes in MDA-MB-231 cells human breast cancer cells that form osteolytic bone metastases in vivo and is recognized as a key driver of bone metastasis [129]. In prostate cancer, bone-disseminated tumor cells express higher CXCR4 levels than tumor cells derived from the primary tumor or other soft tissue metastases [130]. Activation of CXCL12/CXCR4 expression and signaling also enhances the development of lung metastasis in melanoma [131] and breast cancer [132]. Panobinostat has previously been shown to deplete CXCR4 expression and downregulate AKT and ERK1/2− mediated pro-survival signaling in acute myeloid leukemia (AML) cells [133]. Thus, combination HDAC inhibitor therapy targeting CXCR4 may be particularly advantageous with respect to the treatment of bone and lung metastases not only by reducing receptor expression and chemotaxis to distant organs, but also by inhibiting pro-tumorigenic signaling.
Preclinical studies have also revealed rationale for the combination of HDAC inhibitors with hormonal therapy due to the transcriptional regulation of estrogen receptor expression and signaling by several HDACs [134]. Promoter hypoacetylation and hypermethylation silences ERα expression, but this can be reversed by HDAC and DNA methyltransferase inhibition [135, 136]. In ER-negative breast cancer, re-expression of ERα induced by the HDAC inhibitor trichostatin A sensitizes the tumor cells to aromatase inhibitors and other antihormonal therapies [137, 138]. Interestingly, selective genetic ablation or HDAC inhibition in ER-positive cells transcriptionally downregulates ERα expression but upregulates expression of ERβ, which acts as a tumor suppressor in multiple cancer types [139-142]. In a preclinical study, the selective ERβ agonist LY500307 suppresses triple negative breast cancer and melanoma metastasis to the lung by inducing tumor cell IL-1β release to increase intratumoral neutrophil infiltration [143•]. It may therefore be possible that co-treatment with an HDAC inhibitor and ERβ agonist could prove beneficial for the treatment of hormone therapy insensitive metastatic breast cancer by upregulating ERβ expression and activity. This is of particular clinical importance since breast cancer patients originally diagnosed with ERα positive breast cancer frequently present with ERα negative metastases due to ERα down-regulation and this is especially prevalent in the case of bone metastasis [144, 145]. HDAC inhibitor therapy may therefore be particularly useful in patients whose metastatic tumors have converted from positive to negative ER status, expanding the available therapy options for this patient cohort. Combined treatment with an HDAC inhibitor also reverses hormone therapy resistance in breast cancer via additional mechanisms including downregulation of Akt, a kinase hyperactivated in many cancers [146, 147] as well as reversed overexpression of Bcl-2 [148]. Both effects significantly enhance cytotoxicity.
Preclinical studies have revealed mechanistic rationale for the combination of HDAC inhibitors with immunotherapies. Multiple class I HDAC inhibitors including vorinostat modulate the expression of programmed death 1 ligand (PD-L1) by melanoma tumor cells and augment the antitumor response to PD-1 blockade in vivo [122]. In vivo studies of lung adenocarcinoma demonstrated that treatment with romidepsin augments PD-1 immunotherapy response by increasing the expression of multiple T-cell chemokines, enhancing T-cell tumor infiltration, and promoting T-cell dependent tumor regression [149]. Another study demonstrated an interesting mechanism whereby PD-1 blockade enhances T-cell function and the subsequent production of interferon-gamma (IFNγ) and other pro-inflammatory cytokines as expected. However, the cytokines, in turn, activate a negative feedback response that induces melanoma tumor cell expression of PD-L1, PD-L2, and other immunosuppressive mediators and results in a significant reduction of pro-tumorigenic M2 macrophages [150]. In this study’s model, selective HDAC6 inhibition in combination with anti-PD-1 antibodies increases infiltration of CD8+ T-cells and natural killer cells and diminishes intratumoral M2 macrophages populations. Another study demonstrated that belinostat upregulates IFNγ and decreases expression of immunosuppressive regulatory T cells (Tregs) to enhance the antitumor activity of anti-CTLA-4 therapy [151]. Lastly, combining HDAC inhibitors with high dose interleukin 2 (IL-2) has also demonstrated synergistic activity by downregulating Foxp3 expression and function of Tregs as well as myeloid derived suppressor cells, both of which suppress immune clearance of tumor cells [152]. Given that neither immune checkpoint inhibitors nor HDAC inhibitors have been successful as monotherapies in breast tumors, the potential benefit of combination therapy is promising, but will need to be extensively tested in preclinical models since both drugs can cause significant patient toxicity as monotherapies [153, 154]. This will be discussed further in the section below.
Current HDAC inhibitor clinical trials
There are several class I HDAC inhibitors currently in clinical trials, including panobinostat in a phase I trial in combination with everolimus, a mammalian target of rapamycin (mTOR) inhibitor, and lcl161, a small molecule second mitochondrial activator of caspase (SMAC) mimetic, for the treatment of advanced non-small cell lung adenocarcinoma, triple negative breast cancer, renal cell carcinoma and colorectal cancer [ClinicalTrials.gov identifier: NCT02890069]. Vorinostat is in multiple clinical trials including: (I) a phase I/II study combining treatment with pembrolizumab, an anti-PD-L1 monoclonal antibody, in stage IV non-small cell lung cancer [ClinicalTrials.gov identifier: NCT02638090], (II) a phase II study combining vorinostat, carboplatin, and a paclitaxel albumin-stabilized nanoparticle formulation and as pre-operative chemotherapy [ClinicalTrials.gov Identifier: NCT00616967], (III) a phase I study combining pembrolizumab with vorinostat in patients with advanced renal cell carcinoma [ClinicalTrials.gov Identifier: NCT02619253]. Valproic acid is the only FDA approved class II HDAC inhibitor and has been tested in multiple phase I and II clinical trials as combination cancer therapy with other epigenetic modifiers, cytotoxic therapies, and immune modulators with varying results [155]. While the class III inhibitor entinostat is not yet FDA approved for clinical use, it has received ‘breakthrough designation’ status in combination with exemestane, an aromatase inhibitor, for the treatment of advanced breast cancer. This is based on the ENCORE301 phase II study that found that the combination of exemestane and entinostat improved survival in ER+ advanced breast cancer patients progressing on aromatase inhibitors [156]. A recent study also reported that patients treated with entinostat and the demethylating agent 5-azacitidine experience global reductions in DNA methylation which may increase expression of ER in patient tumors [157••], suggesting that re-introduction of endocrine therapy may be effective in some patients. Entinostat is also in numerous other clinical trials for the treatment of solid tumors including (I) a phase Ib/II study of entinostat and atezolizumab, a PD-L1 inhibitor, in advanced triple negative breast cancer [ClinicalTrials.gov identifier: NCT02708680] and (II) a phase I/II study of entinostat and aldesleukin, recombinant IL-2, in metastatic renal cell carcinoma [ClinicalTrials.gov identifier: NCT01038778]. Romidepsin is currently in a phase I/II clinical trial with cisplatin and nivolumab, another PD-L1 inhibitor, for the treatment of metastatic triple negative breast cancer and BRCA mutation-associated breast cancer [ClinicalTrials.gov identifier: NCT02393794], although this trial was recently suspended pending responses of the first several participants.
These clinical trials have advanced based on the molecular and biological mechanisms that have been identified in preclinical studies, but more work is needed to identify the most efficacious treatment combinations for patients that maximize synergy and minimize toxicities. Future studies to improve the selectivity of HDAC inhibitors to target cancer cells at lower doses and thereby reduce toxic effects on normal cells are warranted. Further identification of biomarkers for HDAC inhibitors alone and in combination with other anticancer agents is imperative to select candidate patients or predict their responses to combination regimens. This would also limit adverse effects by excluding individuals unlikely to benefit from therapy.
SYSTEMIC EFFECTS OF HDAC INHIBITORS AND THEIR IMPACT ON BONE METASTASIS
Since HDAC inhibitors are administered systemically, it is important to understand their potential adverse effects. The most common events reported from single agent trials include nausea, vomiting, and anorexia [154]. Transient thrombocytopenia, neutropenia, and anemia have been reported. HDAC inhibitor-induced thrombocytopenia, in particular, is a major dose limiting toxicity [158]. Preclinical studies have demonstrated that the thrombocytopenia is due to decreased platelet release from megakaryocytes, rather than myelosuppression or reduced platelet lifespan. Patients on chronic valproic acid therapy also have an increased risk of osteoporosis and osteomalacia [159, 160]. This is corroborated by several in vivo studies demonstrating that HDAC inhibitors negatively impact bone volume. One study found that while vorinostat significantly lowers intratibial tumor burden in SCID/NCr mouse models of mammary carcinoma and prostate cancer, the contralateral limbs of tumor bearing mice and femurs of non-tumor bearing mice treated with vorinostat exhibit 50% loss of trabecular bone density compared with controls [161]. Histochemical staining showed increased numbers of active, tartrate resistant acid phosphatase (TRAP)-positive osteoclasts in non-tumor bearing, vorinostat treated mice, indicating that HDAC inhibitors also negatively impact the activity of normal bone marrow resident cells to alter bone density. A separate study demonstrated that vorinostat causes substantial trabecular bone loss in C57BL/6 mice by inducing DNA damage and cell cycle arrest in bone marrow stromal cells, which significantly decreases mature osteoblast numbers [162]. From this study, the effect appears to be primarily due to osteoblast formation since osteoclast numbers in this study were reduced, though not statistically significant, and RANKL production was not substantially altered. Given that the HDAC inhibitors are not cell-type specific, the finding that vorinostat affects bone resident cells is not surprising, and needs to be considered in preclinical and clinical studies going forward. Contrary to these findings, a separate study found that non-tumor bearing mice administered vorinostat less frequently (100 mg/kg, i.p. every other day for 3 weeks) did not exhibit any bone loss [163]. The mice also did not exhibit an increase in osteoclasts or a decrease in osteogenic colonies, serum osteocalcin, or osteoblast numbers. The discrepant in vivo effects of vorinostat on bone loss are likely related to the frequency of treatment since in the previously mentioned studies, mice were treated at 100 mg/kg, daily for 3 or 4 weeks [161, 162]. This draws an interesting parallel with the known effects of intermittent versus continuous parathyroid hormone (PTH) effects on bone, where intermittent PTH induces bone formation [164], while continuous PTH leads to bone loss due to sustained RANKL activation [165]. Thus, careful consideration should be given to the frequency of HDAC inhibitor administration in patients. In addition to the reported effects of vorinostat on bone homeostasis, a study of chronic valproic acid (valproate) exposure in seven different mouse strains receiving the drug at varying doses consistently revealed significant reductions in trabecular bone volume in C3H/HeJ and Balb/c mice, while the A/J strain displayed resistance to valproate-associated bone loss [166]. Importantly, the authors note that the magnitude of decrease in bone density in the valproic acid sensitive strains are comparable to those seen in the most severely affected subgroups of human patients chronically treated with the drug. Together, these results suggest that inherent genetic factors may contribute to the pathogenesis of HDAC inhibitor-induced bone loss, though the specific mechanisms have not yet been fully elucidated.
To combat the effects of HDAC inhibitor-induced bone loss, co-administration of an anti-resorptive agent with HDAC inhibitors may therefore be necessary. Bisphosphonates and denosumab are already FDA approved and frequently administered to patients to reduce the risk and severity of skeletal related events due to metastatic bone disease. Thus, adding these drugs to an HDAC inhibitor treatment regimen is clinically feasible. Antiresorptive agents have been shown in multiple studies to improve outcomes for patients with breast, prostate and other cancers [167, 168]. In addition to protecting against HDAC inhibitor-induced bone loss, combination therapy regimens may also enhance the anti-tumor activity of HDAC inhibitors. Vorinostat acts synergistically with the bisphosphonate zoledronic acid to induce prostate cancer cell apoptosis by disrupting the mitochondrial transmembrane potential to activate caspase-3 and DNA fragmentation [169]. Panobinostat has also been shown to synergize with zoledronic acid in prostate cancer and multiple myeloma to inhibit proliferation and induce apoptosis by increasing reactive oxygen species production and inhibiting p38-MAPK activation [170], the latter of which has been shown to mediate acquired resistance to zoledronic acid [171]. Combined treatment with panobinostat and zoledronic acid also significantly inhibits prostate tumor growth in vivo [170]. Thus, the combinatory use of a bisphosphonate may protect against HDAC-inhibitor induced bone loss and reduce tumor growth in the bone, making this a beneficial therapy regimen for patients with bone-disseminated cancer. Given that in some models HDAC inhibitors reduce bone volume [161, 162, 166] and prolonged use of HDAC inhibitors also increases the risk of developing osteoporosis [172, 173], it is certainly worth considering combination therapy with bisphosphonates, which have a known safety profile and are well tolerated [174, 175], in patients who take HDAC inhibitors for both cancer and non-cancer indications.
Another evolving area of focus in the treatment of metastatic disease is tumor dormancy. Patients may present with clinically detectable metastases decades following primary tumor resection. This late tumor relapse / recurrence is thought to be caused by the emergence of tumor cells from a dormant state at distant metastatic [176]. In general, a non-proliferative (e.g. Ki67 or BrdU negative) disseminated tumor cell that has not grown into a micrometastasis is considered dormant [177-180]. There are currently no available therapies to prevent tumor cell exit from dormancy in the bone or lung. While the mechanisms that regulate dormancy remain incompletely understood [181], several key factors in the bone have been identified including leukemia inhibitor factor (LIFR) [182]. Loss of LIFR expression and signaling in MCF7 human breast cancer cells, which lie dormant in vivo, results in greater tumor-induced bone destruction due to increased tumor cell proliferation and reduced expression of genes that promote a dormancy phenotype including transforming growth factor-β2 (TGF-β2) [183] and tropomyosin-1 (TPM1) [184], among others. These data strongly suggest that LIFR signaling is key in regulating breast tumor dormancy in the bone. Furthermore, this study also determined that the downregulation of LIFR signaling is induced by hypoxia, in part, due to epigenetic mechanisms involving histone acetylation. Consequently, treatment with the pan-HDAC inhibitor valproic acid significantly increases expression of LIFR and other pro-dormancy genes in MCF7 cells cultured in normoxia and hypoxia. This of importance in the context of bone and lung metastatic disease since the bone marrow is a physiologically hypoxic microenvironment [185, 186] and hypoxia is evident in most solid tumors larger than a few millimeters [187]. Another study similarly found that valproate increases LIFR expression on breast cancer cells, but found that LIFR/STAT3 signaling may also result in drug resistance to HDAC inhibitors over time, which can be overcome with the addition of a JAK1 or bromodomain containing 4 (BRD4) inhibitor [188]. These findings suggest that HDAC inhibition may present an interesting viable option for maintaining disseminated tumor cells in a dormant state to prevent tumor recurrence, but will need to be evaluated for the potential for therapeutic resistance. The combination of these findings suggests that HDAC inhibitors may help promote dormancy through LIFR, but if LIFR signaling must be blocked in order to prevent therapeutic resistance then the net effect on dormancy may be lost. Further studies are needed to determine whether mitigating HDAC inhibitor therapeutic resistance also mitigates its potential beneficial effect in promoting tumor dormancy.
CONCLUSION
Bone and lung metastases are a common occurrence in patients with cancer and cause considerable morbidity and mortality. There is an urgent need to identify effective therapies for the treatment of distant metastases. HDAC inhibitors have emerged as promising cancer therapeutics since HDACs are aberrantly expressed in numerous cancers and are known to alter the expression of genes that drive tumorigenesis and metastasis. Several inhibitors are already FDA approved for hematologic malignancies and/or are currently in clinical trials for solid tumors, including for cases of advanced metastatic disease. However, they have not been specifically studied for the treatment of bone and lung metastasis, and the role of individual HDACs varies greatly between tumor types, with sometimes opposing effects. The literature suggests that HDAC inhibitors generally target the molecular mechanisms known to promote tumor metastasis to the lung and bone and may therefore be effective in mitigating metastatic progression to these sites, but these effects will need to be tested across multiple tumor types, and the effects in one cancer type are unlikely to be generalizable to another. Based on preclinical and clinical studies, combination treatment utilizing histone-modifying therapy along with standard-of-care therapies and/or bone-protective agents holds promise for successful treatment of bone and lung metastatic disease.
Table 4.
HDAC inhibitors currently in clinical trials for the treatment of bone and lung metastatic cancers.
| HDAC inhibitor | Combination anticancer therapy |
Target cancer | Clinical trial phase |
Trial identifier |
|---|---|---|---|---|
| Panobinostat | PDR001 (immune checkpoint inhibitor) | Advanced non-small cell lung adenocarcinoma, triple negative breast cancer, renal cell carcinoma, colorectal cancer | I | NCT02890069 |
| Panobinostat | Ipilimumab (anti-CTLA-4 antibody) | Unresectable stage III or IV melanoma | I | NCT02032810 |
| Vorinostat | Pembrolizumab (anti-PD-1 antibody) | Locally advanced or metastatic non-small cell lung cancer | I/II | NCT02638090 |
| Vorinostat | MLN9780 (autophagy inhibitor) | Advanced solid tumors and lymphomas with p53 mutations | I | NCT02042989 |
| Vorinostat | Carboplatin (platinum agent) + paclitaxel albumin-stabilized nanoparticle | Breast cancer | II | NCT00616967 |
| Vorinostat | Pembrolizumab | Renal cell carcinoma, urinary bladder neoplasms | I/Ib | NCT02619253 |
| Vorinostat | Carboplatin or Paclitaxel (antimicrotubule agent) | Advanced solid tumors | I | NCT01281176 |
| Valproic acid | Neratinib (EGFR/ HER2 inhibitor) | Advanced Ras-mutated solid tumors | I/II | NCT03919292 |
| Entinostat | Exemestane (aromatase inhibitor) | ER+, HER2−, locally advanced or metastatic breast cancer | III | NCT02115282 |
| Entinostat | Azacitidine (DNA methyltransferase inhibitor) | Advanced breast cancer | II | NCT01349959 |
| Entinostat | Capecitabine (antimetabolite to inhibit DNA synthesis) | Metastatic breast cancer | I | NCT03473639 |
| Entinostat | Nivolumab (anti-PD-1 antibody) with or without Ipilimumab | Advanced solid tumors | I | NCT02453620 |
| Entinostat | Enzalutamide (androgen receptor antagonist) | Castration-resistant prostate cancer | I | NCT03829930 |
| Entinostat | Atezolizumab (anti-PD-L1 antibody) | Advanced triple negative breast cancer | Ib/II | NCT02708680 |
| Entinostat | Atezolizumab and bevacizumab (VEGF inhibitor) | Advanced renal cell carcinoma | I/II | NCT03024437 |
| Entinostat | Aldesleukin (recombinant IL-2) | Metastatic renal cell carcinoma | I/II | NCT01038778 |
| Romidepsin | Various advanced solid tumors | I | NCT01638533 | |
| Romidepsin | Nivolumab | Locally recurrent or metastatic triple negative breast cancer | I/II | NCT02393794* |
Acknowledgements
R.W.J. and C.E. are supported by DoD Breakthrough Award W81XWH-18-1-0029 (R.W.J.) and R.W.J. is supported by NIH award R00CA194198 (R.W.J.).
Footnotes
Conflict of Interest
Rachelle Johnson and Courtney Edwards declare no conflict of interest. Both authors are supported by a DoD grant; Dr. Johnson is supported by an NIH grant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Chaffer CL and Weinberg RA, A perspective on cancer cell metastasis. Science, 2011. 331(6024): p. 1559–64. [DOI] [PubMed] [Google Scholar]
- 2.Tarin D, Comparisons of metastases in different organs: biological and clinical implications. Clin Cancer Res, 2008. 14(7): p. 1923–5. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RW, Schipani E, and Giaccia AJ, HIF targets in bone remodeling and metastatic disease. Pharmacology & therapeutics, 2015. 150: p. 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gok Durnali A, et al. , Outcomes of Adolescent and Adult Patients with Lung Metastatic Osteosarcoma and Comparison of Synchronous and Metachronous Lung Metastatic Groups. PLOS ONE, 2016. 11(5): p. e0152621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, et al. , Clinical characteristics and prognoses of patients treated surgically for metastatic lung tumors. Oncotarget, 2017. 8(28): p. 46491–46497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman R, Bone targeted treatments in cancer - The story so far. Journal of bone oncology, 2016. 5(3): p. 90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman R, Bone targeted treatments in cancer - The story so far. J Bone Oncol, 2016. 5(3): p. 90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet, 2015. 386(10001): p. 1353–1361. [DOI] [PubMed] [Google Scholar]
- 9.Pena RAH, et al. , Overall survival in female Medicare beneficiaries with early stage breast cancer receiving bisphosphonates or denosumab. 2018. 36(15_suppl): p. 530–530. [Google Scholar]
- 10.Rennert G, et al. , Oral Bisphosphonates and Improved Survival of Breast Cancer. Clinical Cancer Research, 2017. 23(7): p. 1684. [DOI] [PubMed] [Google Scholar]
- 11.Suarez-Almazor ME, et al. , Survival in older women with early stage breast cancer receiving low-dose bisphosphonates or denosumab. 2020. 126(17): p. 3929–3938. [DOI] [PubMed] [Google Scholar]
- 12.Gnant M, et al. , Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 2019. 20(3): p. 339–351. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RE, et al. , Adjuvant denosumab in early breast cancer: First results from the international multicenter randomized phase III placebo controlled D-CARE study. 2018. 36(15_suppl): p. 501–501. [Google Scholar]
- 14.Jones PA and Baylin SB, The fundamental role of epigenetic events in cancer. Nat Rev Genet, 2002. 3(6): p. 415–28. [DOI] [PubMed] [Google Scholar]
- 15.Egger G, et al. , Epigenetics in human disease and prospects for epigenetic therapy. Nature, 2004. 429(6990): p. 457–463. [DOI] [PubMed] [Google Scholar]
- 16.Baylin SB and Jones PA, Epigenetic Determinants of Cancer. Cold Spring Harb Perspect Biol, 2016. 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrlich M, DNA methylation in cancer: too much, but also too little. Oncogene, 2002. 21(35): p. 5400–5413. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood N and Rabbani SA, DNA Methylation Readers and Cancer: Mechanistic and Therapeutic Applications. Frontiers in oncology, 2019. 9: p. 489–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Audia JE and Campbell RM, Histone Modifications and Cancer. Cold Spring Harbor perspectives in biology. 8(4): p. a019521–a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XJ and Seto E, HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene, 2007. 26: p. 5310. [DOI] [PubMed] [Google Scholar]
- 21.Grunstein M, Histone acetylation in chromatin structure and transcription. Nature, 1997. 389(6649): p. 349–52. [DOI] [PubMed] [Google Scholar]
- 22.Yang XJ and Seto E, Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell, 2008. 31(4): p. 449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glozak MA and Seto E, Histone deacetylases and cancer. Oncogene, 2007. 26: p. 5420. [DOI] [PubMed] [Google Scholar]
- 24.Jain S and Zain J, Romidepsin in the treatment of cutaneous T-cell lymphoma. Journal of blood medicine, 2011. 2: p. 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann BS, et al. , FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist, 2007. 12(10): p. 1247–52. [DOI] [PubMed] [Google Scholar]
- 26.Seto E and Yoshida M, Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harbor perspectives in biology, 2014. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S-Y and Kim J-S, A short guide to histone deacetylases including recent progress on class II enzymes. Experimental & Molecular Medicine, 2020. 52(2): p. 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leipe DD and Landsman D, Histone deacetylases, acetoin utilization proteins and acetylpolyamine amidohydrolases are members of an ancient protein superfamily. Nucleic Acids Res, 1997. 25(18): p. 3693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley EW, et al. , Histone Deacetylases in Bone Development and Skeletal Disorders. Physiological reviews, 2015. 95(4): p. 1359–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma P, et al. , Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proceedings of the National Academy of Sciences, 2012. 109(8): p. E481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seto E and Yoshida M, Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 2014. 6(4): p. a018713–a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ververis K and Karagiannis TC, An atlas of histone deacetylase expression in breast cancer: fluorescence methodology for comparative semi-quantitative analysis. American journal of translational research, 2012. 4(1): p. 24–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Müller BM, et al. , Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer - overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer, 2013. 13(1): p. 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, et al. , Increased expression of histone deacetylaces (HDACs) and inhibition of prostate cancer growth and invasion by HDAC inhibitor SAHA. American journal of translational research, 2009. 1(1): p. 62–71. [PMC free article] [PubMed] [Google Scholar]
- 35.Weichert W, et al. , Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. British Journal Of Cancer, 2008. 98: p. 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Mei DT, and Zeng Y, HDAC2 promotes the migration and invasion of non-small cell lung cancer cells via upregulation of fibronectin. Biomed Pharmacother, 2016. 84: p. 284–290. [DOI] [PubMed] [Google Scholar]
- 37.Ramakrishnan S, et al. , HDAC 1 and 6 modulate cell invasion and migration in clear cell renal cell carcinoma. BMC Cancer, 2016. 16(1): p. 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuire JJ, et al. , Histone deacetylase inhibition prevents the growth of primary and metastatic osteosarcoma. Int J Cancer, 2020. 147(10): p. 2811–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caslini C, et al. , HDAC7 regulates histone 3 lysine 27 acetylation and transcriptional activity at super-enhancer-associated genes in breast cancer stem cells. Oncogene, 2019. 38(39): p. 6599–6614. [DOI] [PubMed] [Google Scholar]
- 40.Raman D, et al. , Editorial: The Role of Breast Cancer Stem Cells in Clinical Outcomes. Frontiers in oncology, 2020. 10: p. 299–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palazzo A, Ackerman B, and Gundersen GG, Tubulin acetylation and cell motility. Nature, 2003. 421(6920): p. 230–230. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, et al. , HDAC10 promotes lung cancer proliferation via AKT phosphorylation. Oncotarget, 2016. 7(37): p. 59388–59401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed AA, et al. , Modulating microtubule stability enhances the cytotoxic response of cancer cells to Paclitaxel. Cancer research, 2011. 71(17): p. 5806–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aldana-Masangkay GI and Sakamoto KM, The role of HDAC6 in cancer. Journal of biomedicine & biotechnology, 2011. 2011: p. 875824–875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuino L, et al. , Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther, 2003. 2(10): p. 971–84. [PubMed] [Google Scholar]
- 46.Erler JT, et al. , Lysyl oxidase is essential for hypoxia-induced metastasis. Nature, 2006. 440(7088): p. 1222–6. [DOI] [PubMed] [Google Scholar]
- 47.Woelfle U, et al. , Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res, 2003. 63(18): p. 5679–84. [PubMed] [Google Scholar]
- 48.Yang MH, et al. , Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol, 2008. 10(3): p. 295–305. [DOI] [PubMed] [Google Scholar]
- 49.Rankin EB, Giaccia AJ, and Schipani E, A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Curr Osteoporos Rep, 2011. 9(2): p. 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiraga T, et al. , Hypoxia and Hypoxia-Inducible Factor-1 Expression Enhance Osteolytic Bone Metastases of Breast Cancer. Cancer Research, 2007. 67(9): p. 4157. [DOI] [PubMed] [Google Scholar]
- 51.Lu X, et al. , In vivo Dynamics and Distinct Functions of Hypoxia in Primary Tumor Growth and Organotropic Metastasis of Breast Cancer. Cancer Research, 2010. 70(10): p. 3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunn LK, et al. , Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One, 2009. 4(9): p. e6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiterer M, et al. , Acute and chronic hypoxia differentially predispose lungs for metastases. Scientific Reports, 2019. 9(1): p. 10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin C-W, et al. , Daxx inhibits hypoxia-induced lung cancer cell metastasis by suppressing the HIF-1α/HDAC1/Slug axis. Nature Communications, 2016. 7(1): p. 13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao D, et al. , Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res, 2007. 67(2): p. 563–72. [DOI] [PubMed] [Google Scholar]
- 56.Kong X, et al. , Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Molecular and cellular biology, 2006. 26(6): p. 2019–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hutt DM, et al. , The histone deacetylase inhibitor, Vorinostat, represses hypoxia inducible factor 1 alpha expression through translational inhibition. PloS one, 2014. 9(8): p. e106224–e106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato H, Tamamizu-Kato S, and Shibasaki F, Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem, 2004. 279(40): p. 41966–74. [DOI] [PubMed] [Google Scholar]
- 59.Chalkiadaki A and Guarente L, The multifaceted functions of sirtuins in cancer. Nature Reviews Cancer, 2015. 15: p. 608. [DOI] [PubMed] [Google Scholar]
- 60.Xie D, et al. , Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A, 2010. 107(6): p. 2485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung YR, et al. , Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype. Hum Pathol, 2015. 46(7): p. 1027–35. [DOI] [PubMed] [Google Scholar]
- 62.Latifkar A, et al. , Loss of Sirtuin 1 Alters the Secretome of Breast Cancer Cells by Impairing Lysosomal Integrity. Developmental Cell, 2019. 49(3): p. 393–408.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simic P, et al. , SIRT1 Suppresses the Epithelial-to-Mesenchymal Transition in Cancer Metastasis and Organ Fibrosis. Cell Reports, 2013. 3(4): p. 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeh SU, et al. , Differential expression of the sirtuin family in renal cell carcinoma: Aspects of carcinogenesis and prognostic significance. Urol Oncol, 2017. 35(12): p. 675.e9–675.e15. [DOI] [PubMed] [Google Scholar]
- 65.Tan Y, et al. , Integrative Analysis of Sirtuins and Their Prognostic Significance in Clear Cell Renal Cell Carcinoma. Front Oncol, 2020. 10: p. 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leslie PL, et al. , Histone deacetylase 11 inhibition promotes breast cancer metastasis from lymph nodes. Nature Communications, 2019. 10(1): p. 4192.••Examines for the first time the role for HDAC11 in tumor biology
- 67.Wang N, Li Y, and Zhou J, Downregulation of ribonucleotide reductase subunits M2 induces apoptosis and G1 arrest of cervical cancer cells. Oncology letters, 2018. 15(3): p. 3719–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christensen J, et al. , Characterization of E2F8, a novel E2F-like cell-cycle regulated repressor of E2F-activated transcription. Nucleic acids research, 2005. 33(17): p. 5458–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arts J, et al. , JNJ-26481585, a Novel “Second-Generation” Oral Histone Deacetylase Inhibitor, Shows Broad-Spectrum Preclinical Antitumoral Activity. Clinical Cancer Research, 2009. 15(22): p. 6841. [DOI] [PubMed] [Google Scholar]
- 70.Chen W-Z, et al. , Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Scientific reports, 2017. 7(1): p. 11325–11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bora-Singhal N, et al. , Novel HDAC11 inhibitors suppress lung adenocarcinoma stem cell self-renewal and overcome drug resistance by suppressing Sox2. Scientific Reports, 2020. 10(1): p. 4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haberland M, et al. , Genetic dissection of histone deacetylase requirement in tumor cells. Proceedings of the National Academy of Sciences, 2009. 106(19): p. 7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jurkin J, et al. , Distinct and redundant functions of histone deacetylases HDAC1 and HDAC2 in proliferation and tumorigenesis. Cell cycle (Georgetown, Tex.), 2011. 10(3): p. 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanaja GR, Ramulu HG, and Kalle AM, Overexpressed HDAC8 in cervical cancer cells shows functional redundancy of tubulin deacetylation with HDAC6. Cell Commun Signal, 2018. 16(1): p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marmorstein R and Zhou M-M, Writers and readers of histone acetylation: structure, mechanism, and inhibition. Cold Spring Harbor perspectives in biology. 6(7): p. a018762–a018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pandey R, et al. , Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic acids research, 2002. 30(23): p. 5036–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han N, et al. , HAT1 induces lung cancer cell apoptosis via up regulating Fas. Oncotarget, 2017. 8(52): p. 89970–89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L, et al. , Lysine acetyltransferase GCN5 potentiates the growth of non-small cell lung cancer via promotion of E2F1, cyclin D1, and cyclin E1 expression. J Biol Chem, 2013. 288(20): p. 14510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu T, et al. , Epigenetically Down-Regulated Acetyltransferase PCAF Increases the Resistance of Colorectal Cancer to 5-Fluorouracil. Neoplasia (New York, N.Y.), 2019. 21(6): p. 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu L, et al. , Identification of MYST3 as a novel epigenetic activator of ERα frequently amplified in breast cancer. Oncogene, 2016. 36: p. 2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simo-Riudalbas L, et al. , KAT6B Is a Tumor Suppressor Histone H3 Lysine 23 Acetyltransferase Undergoing Genomic Loss in Small Cell Lung Cancer. Cancer Res, 2015. 75(18): p. 3936–45. [DOI] [PubMed] [Google Scholar]
- 82.Xiao XS, et al. , High Expression of p300 in Human Breast Cancer Correlates with Tumor Recurrence and Predicts Adverse Prognosis. Chin J Cancer Res, 2011. 23(3): p. 201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fermento ME, et al. , Inhibition of p300 suppresses growth of breast cancer. Role of p300 subcellular localization. Experimental and Molecular Pathology, 2014. 97(3): p. 411–424. [DOI] [PubMed] [Google Scholar]
- 84.Xiao X-S, et al. , High Expression of p300 in Human Breast Cancer Correlates with Tumor Recurrence and Predicts Adverse Prognosis. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu, 2011. 23(3): p. 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ring A, et al. , CBP/β-Catenin FOXM1 Is a Novel Therapeutic Target in Triple Negative Breast Cancer. Cancers, 2018. 10(12): p. 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hecht A, et al. , The p300 CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. The EMBO journal, 2000. 19(8): p. 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DiMeo TA, et al. , A Novel Lung Metastasis Signature Links Wnt Signaling with Cancer Cell Self-Renewal and Epithelial-Mesenchymal Transition in Basal-like Breast Cancer. Cancer Research, 2009. 69(13): p. 5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jang GB, et al. , Blockade of Wnt/beta-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep, 2015. 5: p. 12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, et al. , Wnt signaling in bone metastasis: mechanisms and therapeutic opportunities. Life Sci, 2018. 208: p. 33–45. [DOI] [PubMed] [Google Scholar]
- 90.Wu ZQ, et al. , Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci U S A, 2012. 109(41): p. 16654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson RW, et al. , Wnt signaling induces gene expression of factors associated with bone destruction in lung and breast cancer. Clin Exp Metastasis, 2014. 31(8): p. 945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, et al. , Wnt Signaling Drives Prostate Cancer Bone Metastatic Tropism and Invasion. Transl Oncol, 2020. 13(4): p. 100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen G, et al. , Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer, 2004. 101(6): p. 1345–56. [DOI] [PubMed] [Google Scholar]
- 94.DiMeo TA, et al. , A Novel Lung Metastasis Signature Links Wnt Signaling with Cancer Cell Self-Renewal and Epithelial-Mesenchymal Transition in Basal-like Breast Cancer. 2009. 69(13): p. 5364–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhuang X, et al. , Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol, 2017. 19(10): p. 1274–1285. [DOI] [PubMed] [Google Scholar]
- 96.Wapenaar H and Dekker FJJCE, Histone acetyltransferases: challenges in targeting bi-substrate enzymes. 2016. 8(1): p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghobrial IM, Myeloma as a model for the process of metastasis: implications for therapy. Blood, 2012. 120(1): p. 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duvic M and Vu J, Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Investig Drugs, 2007. 16(7): p. 1111–20. [DOI] [PubMed] [Google Scholar]
- 99.Buchwald M, Kramer OH, and Heinzel T, HDACi--targets beyond chromatin. Cancer Lett, 2009. 280(2): p. 160–7. [DOI] [PubMed] [Google Scholar]
- 100.Zucchetti B, et al. , The role of histone deacetylase inhibitors in metastatic breast cancer. The Breast, 2019. 43: p. 130–134. [DOI] [PubMed] [Google Scholar]
- 101.Luu TH, et al. , A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res, 2008. 14(21): p. 7138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Modesitt SC, et al. , A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol, 2008. 109(2): p. 182–6. [DOI] [PubMed] [Google Scholar]
- 103.Blumenschein GR Jr., et al. , Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Invest New Drugs, 2008. 26(1): p. 81–7. [DOI] [PubMed] [Google Scholar]
- 104.Galanis E, et al. , Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol, 2009. 27(12): p. 2052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bradley D, et al. , Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862). Cancer, 2009. 115(23): p. 5541–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ibrahim N, et al. , A phase I trial of panobinostat (LBH589) in patients with metastatic melanoma. Cancer Med, 2016. 5(11): p. 3041–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rathkopf DE, et al. , A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol, 2013. 72(3): p. 537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haigentz M Jr., et al. , Phase II trial of the histone deacetylase inhibitor romidepsin in patients with recurrent/metastatic head and neck cancer. Oral Oncol, 2012. 48(12): p. 1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bonneterre J and Mercier M, Response to chemotherapy after relapse in patients with or without previous adjuvant chemotherapy for breast cancer. Cancer Treatment Reviews, 1993. 19: p. 21–30. [DOI] [PubMed] [Google Scholar]
- 110.Hull EE, Montgomery MR, and Leyva KJ, HDAC Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer Therapy and Inflammatory Diseases. BioMed research international, 2016. 2016: p. 8797206–8797206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fantin VR and Richon VM, Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin Cancer Res, 2007. 13(24): p. 7237–42. [DOI] [PubMed] [Google Scholar]
- 112.Pommier Y, et al. , Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene, 2004. 23(16): p. 2934–49. [DOI] [PubMed] [Google Scholar]
- 113.Robey RW, et al. , Histone deacetylase inhibitors: emerging mechanisms of resistance. Mol Pharm, 2011. 8(6): p. 2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Plumb JA, et al. , Pharmacodynamic Response and Inhibition of Growth of Human Tumor Xenografts by the Novel Histone Deacetylase Inhibitor PXD101. Molecular Cancer Therapeutics, 2003. 2(8): p. 721. [PubMed] [Google Scholar]
- 115.Crisanti MC, et al. , The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Molecular Cancer Therapeutics, 2009. 8(8): p. 2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richon VM, et al. , A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proceedings of the National Academy of Sciences of the United States of America, 1998. 95(6): p. 3003–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Göttlicher M, et al. , Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. The EMBO journal, 2001. 20(24): p. 6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tatamiya T, et al. , Isozyme-selective activity of the HDAC inhibitor MS-275. Cancer Research, 2004. 64(7 Supplement): p. 567. [Google Scholar]
- 119.Furumai R, et al. , FK228 (Depsipeptide) as a Natural Prodrug That Inhibits Class I Histone Deacetylases. Cancer Research, 2002. 62(17): p. 4916. [PubMed] [Google Scholar]
- 120.Hurtubise A and Momparler RL, Effect of histone deacetylase inhibitor LAQ824 on antineoplastic action of 5-Aza-2'-deoxycytidine (decitabine) on human breast carcinoma cells. Cancer Chemother Pharmacol, 2006. 58(5): p. 618–25. [DOI] [PubMed] [Google Scholar]
- 121.Dowdy SC, et al. , Histone deacetylase inhibitors and paclitaxel cause synergistic effects on apoptosis and microtubule stabilization in papillary serous endometrial cancer cells. Mol Cancer Ther, 2006. 5(11): p. 2767–76. [DOI] [PubMed] [Google Scholar]
- 122.Owonikoko TK, et al. , Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells. Int J Cancer, 2010. 126(3): p. 743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim MS, et al. , Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res, 2003. 63(21): p. 7291–300. [PubMed] [Google Scholar]
- 124.Yin L, et al. , PARP inhibitor veliparib and HDAC inhibitor SAHA synergistically co-target the UHRF1/BRCA1 DNA damage repair complex in prostate cancer cells. J Exp Clin Cancer Res, 2018. 37(1): p. 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beider K, et al. , The mTOR inhibitor everolimus overcomes CXCR4-mediated resistance to histone deacetylase inhibitor panobinostat through inhibition of p21 and mitotic regulators. Biochem Pharmacol, 2019. 168: p. 412–428. [DOI] [PubMed] [Google Scholar]
- 126.Balkwill F, Cancer and the chemokine network. Nature Reviews Cancer, 2004. 4(7): p. 540–550. [DOI] [PubMed] [Google Scholar]
- 127.Wang J, Loberg R, and Taichman RS, The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev, 2006. 25(4): p. 573–87. [DOI] [PubMed] [Google Scholar]
- 128.Masuda T, et al. , ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Scientific Reports, 2015. 5: p. 9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang Y, et al. , A multigenic program mediating breast cancer metastasis to bone. Cancer Cell, 2003. 3(6): p. 537–549. [DOI] [PubMed] [Google Scholar]
- 130.Gravina GL, et al. , CXCR4 pharmacogical inhibition reduces bone and soft tissue metastatic burden by affecting tumor growth and tumorigenic potential in prostate cancer preclinical models. Prostate, 2015. 75(12): p. 1227–46. [DOI] [PubMed] [Google Scholar]
- 131.D'Alterio C, et al. , Inhibition of stromal CXCR4 impairs development of lung metastases. Cancer Immunol Immunother, 2012. 61(10): p. 1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martinez-Ordoñez A, et al. , Breast cancer metastasis to liver and lung is facilitated by Pit-1-CXCL12-CXCR4 axis. Oncogene, 2018. 37(11): p. 1430–1444. [DOI] [PubMed] [Google Scholar]
- 133.Mandawat A, et al. , Pan-histone deacetylase inhibitor panobinostat depletes CXCR4 levels and signaling and exerts synergistic antimyeloid activity in combination with CXCR4 antagonists. Blood, 2010. 116(24): p. 5306. [DOI] [PubMed] [Google Scholar]
- 134.Duong V, et al. , ERα and ERβ expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene, 2006. 25(12): p. 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Munshi A, et al. , Activation of ER-α by DNMT and HDAC inhibitors radiosensitizes ER-α negative breast cancer cells. 2007. 67(9 Supplement): p. 3104–3104. [Google Scholar]
- 136.Sharma D, et al. , Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol, 2005. 19(7): p. 1740–51. [DOI] [PubMed] [Google Scholar]
- 137.Sharma D, et al. , Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res, 2006. 66(12): p. 6370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan J, et al. , ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol, 2008. 134(8): p. 883–90. [DOI] [PubMed] [Google Scholar]
- 139.Bossard C, et al. , Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS One, 2012. 7(9): p. e44787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pinton G, Nilsson S, and Moro L, Targeting estrogen receptor beta (ERβ) for treatment of ovarian cancer: importance of KDM6B and SIRT1 for ERβ expression and functionality. Oncogenesis, 2018. 7(2): p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paruthiyil S, et al. , Estrogen Receptor β Inhibits Human Breast Cancer Cell Proliferation and Tumor Formation by Causing a G2 Cell Cycle Arrest. 2004. 64(1): p. 423–428. [DOI] [PubMed] [Google Scholar]
- 142.Chaurasiya S, et al. , Estrogen receptor β exerts tumor suppressive effects in prostate cancer through repression of androgen receptor activity. PLOS ONE, 2020. 15(5): p. e0226057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao L, et al. , Pharmacological activation of estrogen receptor beta augments innate immunity to suppress cancer metastasis. Proc Natl Acad Sci U S A. 2018. April 17;115(16):E3673–E3681.•Demonstrates that targeting ERβ may be effective even when ERα has become down-regulated, as frequently occurs in recurrent metastatic breast cancer
- 144.Schrijver WAME, et al. , Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. JNCI: Journal of the National Cancer Institute, 2018. 110(6): p. 568–580. [DOI] [PubMed] [Google Scholar]
- 145.Van Poznak C, et al. , Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol, 2015. 33(24): p. 2695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thomas S, et al. , Efficacy of Histone Deacetylase and Estrogen Receptor Inhibition in Breast Cancer Cells Due to Concerted down Regulation of Akt. PLOS ONE, 2013. 8(7): p. e68973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Testa JR and Tsichlis PN, AKT signaling in normal and malignant cells. Oncogene, 2005. 24(50): p. 7391–7393. [DOI] [PubMed] [Google Scholar]
- 148.Raha P, et al. , Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression. Breast Cancer Res, 2015. 17(1): p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng H, et al. , HDAC Inhibitors Enhance T-Cell Chemokine Expression and Augment Response to PD-1 Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res, 2016. 22(16): p. 4119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Knox T, et al. , Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Scientific Reports, 2019. 9(1): p. 6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Llopiz D, et al. , Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor Belinostat in a murine hepatocellular carcinoma model. Cancer Immunol Immunother, 2019. 68(3): p. 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pili R, et al. , Immunomodulation by Entinostat in Renal Cell Carcinoma Patients Receiving High-Dose Interleukin 2: A Multicenter, Single-Arm, Phase I/II Trial (NCI-CTEP#7870). 2017. 23(23): p. 7199–7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson DB, et al. , Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. The Lancet Oncology, 2020. 21(8): p. e398–e404. [DOI] [PubMed] [Google Scholar]
- 154.Subramanian S, et al. , Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals (Basel, Switzerland), 2010. 3(9): p. 2751–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eckschlager T, et al. , Histone Deacetylase Inhibitors as Anticancer Drugs. International journal of molecular sciences, 2017. 18(7): p. 1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yardley DA, et al. , Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2013. 31(17): p. 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Connolly RM, et al. , Combination Epigenetic Therapy in Advanced Breast Cancer with 5-Azacitidine and Entinostat: A Phase II National Cancer Institute/Stand Up to Cancer Study. Clin Cancer Res, 2017. 23(11): p. 2691–2701.••Demonstrates that treatment with entinostat in combination with 5-azacitidine may increase expression of ER, suggesting re-introduction of endocrine therapy may be beneficial in some patients
- 158.Bishton MJ, et al. , Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood, 2011. 117(13): p. 3658–68. [DOI] [PubMed] [Google Scholar]
- 159.Oner N, et al. , Bone mineral metabolism changes in epileptic children receiving valproic acid. J Paediatr Child Health, 2004. 40(8): p. 470–3. [DOI] [PubMed] [Google Scholar]
- 160.Sato Y, et al. , Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology, 2001. 57(3): p. 445–9. [DOI] [PubMed] [Google Scholar]
- 161.Pratap J, et al. , The histone deacetylase inhibitor, vorinostat, reduces tumor growth at the metastatic bone site and associated osteolysis, but promotes normal bone loss. Molecular cancer therapeutics, 2010. 9(12): p. 3210–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McGee-Lawrence ME, et al. , Suberoylanilide hydroxamic acid (SAHA; vorinostat) causes bone loss by inhibiting immature osteoblasts. Bone, 2011. 48(5): p. 1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu S, et al. , Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. Acta Pharmacologica Sinica, 2013. 34(5): p. 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jilka RL, et al. , Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone, 2009. 44(2): p. 275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao Y, et al. , T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab, 2008. 8(2): p. 132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Senn SM, et al. , Adverse effects of valproate on bone: defining a model to investigate the pathophysiology. Epilepsia, 2010. 51(6): p. 984–93. [DOI] [PubMed] [Google Scholar]
- 167.Smith MR, et al. , Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet, 2012. 379(9810): p. 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scagliotti GV, et al. , Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol, 2012. 7(12): p. 1823–1829. [DOI] [PubMed] [Google Scholar]
- 169.Sonnemann J, Bumbul B, and Beck JF, Synergistic activity of the histone deacetylase inhibitor suberoylanilide hydroxamic acid and the bisphosphonate zoledronic acid against prostate cancer cells in vitro. Molecular Cancer Therapeutics, 2007. 6(11): p. 2976. [DOI] [PubMed] [Google Scholar]
- 170.Bruzzese F, et al. , Panobinostat synergizes with zoledronic acid in prostate cancer and multiple myeloma models by increasing ROS and modulating mevalonate and p38-MAPK pathways. Cell Death Dis, 2013. 4: p. e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Milone MR, et al. , Acquired resistance to zoledronic acid and the parallel acquisition of an aggressive phenotype are mediated by p38-MAP kinase activation in prostate cancer cells. Cell Death Dis, 2013. 4(5): p. e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boluk A, et al. , The effect of valproate on bone mineral density in adult epileptic patients. Pharmacol Res, 2004. 50(1): p. 93–7. [DOI] [PubMed] [Google Scholar]
- 173.Sheth RD, et al. , Effect of carbamazepine and valproate on bone mineral density. J Pediatr, 1995. 127(2): p. 256–62. [DOI] [PubMed] [Google Scholar]
- 174.Greenspan SL, et al. , Bisphosphonates: safety and efficacy in the treatment and prevention of osteoporosis. Am Fam Physician, 2000. 61(9): p. 2731–6. [PubMed] [Google Scholar]
- 175.Pazianas M, et al. , Long-term treatment with bisphosphonates and their safety in postmenopausal osteoporosis. Ther Clin Risk Manag, 2010. 6: p. 325–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Langley RR and Fidler IJ, The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. International journal of cancer, 2011. 128(11): p. 2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pantel K, Alix-Panabieres C, and Riethdorf S, Cancer micrometastases. Nat Rev Clin Oncol, 2009. 6(6): p. 339–51. [DOI] [PubMed] [Google Scholar]
- 178.Pantel K and Brakenhoff RH, Dissecting the metastatic cascade. Nat Rev Cancer, 2004. 4(6): p. 448–56. [DOI] [PubMed] [Google Scholar]
- 179.Klein CA, Parallel progression of primary tumours and metastases. Nat Rev Cancer, 2009. 9(4): p. 302–12. [DOI] [PubMed] [Google Scholar]
- 180.Price TT, et al. , Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med, 2016. 8(340): p. 340ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sosa MS, Bragado P, and Aguirre-Ghiso JA, Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer, 2014. 14(9): p. 611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Johnson RW, et al. , Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nature cell biology, 2016. 18(10): p. 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bragado P, et al. , TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat Cell Biol, 2013. 15(11): p. 1351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim RS, et al. , Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PloS one, 2012. 7(4): p. e35569–e35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chow DC, et al. , Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J, 2001. 81(2): p. 675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Spencer JA, et al. , Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature, 2014. 508(7495): p. 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Höckel M and Vaupel P, Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. JNCI: Journal of the National Cancer Institute, 2001. 93(4): p. 266–276. [DOI] [PubMed] [Google Scholar]
- 188.Liu S, et al. , BRD4 inhibitor and histone deacetylase inhibitor synergistically inhibit the proliferation of gallbladder cancer in vitro and in vivo. Cancer science, 2019. 110(8): p. 2493–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]