Abstract
Objective:
To estimate the incidence of blood product transfusion, including red blood cells, platelets, and plasma, and characterize pre-transfusion hematologic values for infants during their initial hospitalization after birth.
Study design:
Retrospective cohort study using data from 7 geographically diverse US academic and community hospitals that participated in the National Heart Lung and Blood Institute Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) from 2013–2016. Pre-transfusion hematologic values were evaluated closest to each transfusion and no more than 24 hours prior.
Results:
Data from 60,243 infants was evaluated. The incidence of any transfusion differed by gestational age (P < .0001), with 80% (95% CI, 76–84%) transfused at <27 weeks’ gestation (n=329) and 0.5% (95% CI, 0.5–0.6) transfused at ≥37 weeks’ gestation (n=53,919). The median pre-transfusion hemoglobin was 11.2 g/dL (10th–90th percentile 8.8–14.1) for the entire cohort, ranging from 10.5 g/dL (8.8–12.3) for extremely preterm infants at <27 weeks’ gestation to 13.0 g/dL (10.5–15.5) for term infants. The median pre-transfusion platelet count (× 109/L) was 71 (10th–90th percentile 26–135) for the entire cohort, and was > 45 for all gestational age groups examined. The median pre-transfusion INR for the entire cohort was 1.7 (10th–90th percentile 1.2–2.8).
Conclusion:
There is wide variability in pre-transfusion hemoglobin, platelet count, and INR values for neonatal transfusions. Our findings suggest that a large proportion of neonatal transfusions in the U.S. are administered at thresholds higher than supported by the best available evidence and highlight an opportunity for improved patient blood management.
Keywords: Red blood cell, platelet, plasma, blood, infant, preterm
Anemia and thrombocytopenia are common in newborn infants and are often treated with red blood cell (RBC) and platelet transfusions. There are limited data describing neonatal transfusion practices in the United States (U.S.), with most studies in extremely preterm infants and less data in more mature infants. One study estimated that RBC transfusion occurs in 0.43% of US neonatal admissions, although this incidence varied substantially between complicated vs. uncomplicated births1.
In a 2012 international survey, almost half of the NICUs surveyed did not have specific RBC transfusion guidelines and clinicians reported wide variation in hemoglobin (Hb) transfusion thresholds used for extremely preterm infants2. Similar variability was reported in a survey of platelet transfusion practices among U.S. and Canadian neonatologists3, with most clinicians reporting the use of pre-transfusion platelet count thresholds ≥ 50 × 109/L in the majority of clinical scenarios presented, despite this practice not being supported by the best available evidence at the time4. Although surveys may not reflect actual transfusion practices, a retrospective multicenter cohort study among preterm infants also found a wide range of pre-transfusion platelet counts, with 65% being >50 × 109/L5. Data regarding plasma transfusion practices in newborn infants are sparse6 and evidence are lacking to support prophylactic plasma transfusions7. Italian centers reported that over half of plasma transfusions given to NICU patients were not evidence-based8. However, a comprehensive evaluation of neonatal transfusion practices in the U.S., including thresholds used for transfusion, is currently lacking.
This study characterizes the epidemiology of neonatal transfusion practices in seven U.S. hospitals, which included infants admitted after birth and cared for in the NICU, as well as other hospital areas during the initial birth hospitalization. Our primary aim was to estimate the incidence of RBC, platelet, and plasma transfusions among newborn infants and to characterize pre-transfusion hematologic thresholds by gestational age, postnatal age and the presence of major neonatal comorbidities. We hypothesized, based on evidence from preterm infant studies, that there would be significant variability in transfusion incidence and in pre-transfusion hematologic values among infants with different gestational ages and morbidities.
METHODS
The NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) data are available as a public use dataset through BioLINCC. REDS-III involved 12 academic and community hospitals from four geographic regions of the US, of which 7 included newborn infants. Hospitals included both tertiary and quaternary centers, some of which performed cardiac and non-cardiac surgery as well as extracorporeal membrane oxygenation (ECMO). The database, which covers a 4-year period beginning on January 1, 2013, has previously been described9. This dataset has been used to evaluate adult transfusion practices10, 11, but this is the first report on neonatal transfusions. Approval for data collection was obtained from the Institutional Review Board at each participating institution.
Study Population and Definitions
Infants born at participating REDS-III hospitals were followed from birth until hospital discharge, death, or one year of age if they remained hospitalized for that period (whichever occurred first) and included infants admitted to the NICU as well as to any other hospital area such as the pediatric ward or pediatric intensive care unit in the course of the birth admission. However, infants re-admitted after discharge home were not included. Gestational age (completed weeks), birth weight, and select diagnoses were determined from ICD 9/10 coding (codes available upon request). Laboratory values (Hb, platelet count, and international normalized ratio [INR]) closest in time to each transfusion and no more than 24-hours prior) were identified using a previously described approach12 to capture pre-transfusion values temporally relevant to transfusion. As the de-identified analysis dataset did not contain dates, we estimated the day of birth by using a common index medication administered at birth (Vitamin K) in combination with a “Live-born” ICD 9/10 diagnosis code. This estimation was only used for analyses evaluating pre-transfusion hematologic values by postnatal age.
Transfusion Exposures
A transfusion event was defined as the issuance of a blood product from the transfusion service. Data captured on the issued product included issue time, issue location (intensive care unit [ICU], operating room, procedure suite, or elsewhere), and a barcode (Codabar or ISBT 128) from which the product type was extracted.
Statistical Analyses
The sample size was fixed based on the REDS-III dataset. Transfusion incidence was calculated as a binomial proportion (% of infants) among birth admissions, and imprecision in estimates for this cohort were provided using corresponding 95% confidence intervals (CI). We estimated the incidence of any transfusion and of specific product types and compared the incidence by demographics, gestational age and selected diagnoses. Density estimation plots were used to show the distribution of pre-transfusion hematologic values by gestational age and diagnosis. P-values comparing median pre-transfusion hematologic values by gestational age, diagnosis, and post-natal age were calculated using the Kruskal-Wallis test, but pairwise comparisons were not performed to reduce type I error from multiple hypothesis testing. To determine the impact of center and case-mix, we tested whether there were differences in pre-transfusion hematologic values among the study centers, after adjusting for gestational age group and whether an infant underwent any surgery during hospitalization using tests of Type III effects in a multivariable linear regression. We also evaluated if location of surgery (operating room vs. non-operating room) was associated with pre-transfusion hematologic values after adjusting for gestational age group and surgery. All analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and density estimation plots were generated with R version 4.1.0 (R-project, Vienna, Austria).
RESULTS
We evaluated a total of 60,243 infants, comprising all birth admissions at participating hospitals during the years of study. The cohort was 49% female and 10.5% preterm (<37 weeks gestation), with 1.6% of infants being very low birth weight (VLBW, <1,500 g at birth) (Table Ⅰ). Among the full cohort, the incidence of any blood product transfusion was 1.6% (95% CI 1.5–1.7%), with RBCs being the most common component transfused (1.3%; 95% CI 1.2–1.4%), followed by platelets and plasma (each 0.7%; 95% CI 0.6–0.7%). Among the most immature infants (<27 weeks’ gestation), 80% (95% CI 76–84%) had at least one transfusion exposure to any blood product, with 70% (95% CI 65–75%) receiving RBCs, 34% (95% CI 29–39%) platelets, and 24% (95% CI 20–29%) plasma. The incidence of transfusion for all products decreased with increasing gestational age, and was 0.5% (95% CI 0.5–0.6%) among full-term infants ≥ 37 weeks’ gestation.
Table I.
Incidence of blood product transfusion, including specific components
| Group | Encounters | Any Transfusiona | Any RBC | Any Platelet | Any Plasma |
|---|---|---|---|---|---|
| All | 60,243 | 1.6 (1.5–1.7) | 1.3 (1.2–1.4) | 0.7 (0.6–0.7) | 0.7 (0.6–0.7) |
| Sex | |||||
| Female | 29,635 | 1.6 (1.4–1.7) | 1.3 (1.2–1.4) | 0.6 (0.5–0.7) | 0.7 (0.6–0.7) |
| Male | 30,608 | 1.7 (1.5–1.8) | 1.4 (1.3–1.5) | 0.7 (0.6–0.8) | 0.7 (0.6–0.7) |
| Gestational Ageb | |||||
| <27 weeks | 329 | 80 (76–84) | 70 (65–75) | 34 (29–39) | 24 (20–29) |
| 27 to 28 weeks | 288 | 49 (43–54) | 44 (39–50) | 12 (8–16) | 11 (7–14) |
| 29 to 32 weeks | 996 | 16 (14–18) | 13 (11–15) | 5.8 (4.4–7.3) | 4.7 (3.4–6.0) |
| 33 to 36 weeks | 4,693 | 2.8 (2.3–3.2) | 2.1 (1.7–2.5) | 1.1 (0.8–1.4) | 1.3 (1.0–1.6) |
| 37+ weeks | 53,919 | 0.5 (0.5–0.6) | 0.4 (0.3–0.5) | 0.3 (0.2–0.3) | 0.3 (0.3–0.4) |
| Hospital stay >3 days | |||||
| <27 weeks | 295 | 81 (76–85) | 71 (66–76) | 34 (28–39) | 23 (18–27) |
| 27 to 28 weeks | 277 | 48 (42–54) | 45 (39–51) | 11 (7–14) | 10 (7–14) |
| 29 to 32 weeks | 987 | 16 (14–18) | 13 (11–15) | 5.8 (4.3–7.2) | 4.6 (3.3–5.9) |
| 33 to 36 weeks | 3,063 | 4.1 (3.4–4.8) | 3.1 (2.5–3.7) | 1.6 (1.2–2.0) | 1.8 (1.3–2.3) |
| 37+ weeks | 8,199 | 3.2 (2.8–3.6) | 2.5 (2.2–2.8) | 1.7 (1.4–2.0) | 2.0 (1.7–2.3) |
| Birthweight (g)c | |||||
| <1,000 | 443 | 74 (70–78) | 64 (60–69) | 31 (27–35) | 19 (16–23) |
| 1,000 to <1,500 | 518 | 26 (22–29) | 22 (18–25) | 7.9 (5.6–10.2) | 6.2 (4.1–8.3) |
| 1,500 to <2,500 | 3,987 | 3.6 (3.0–4.2) | 2.8 (2.3–3.3) | 1.4 (1.0–1.7) | 1.4 (1.0–1.7) |
| ≥2,500 | 55,285 | 0.7 (0.6–0.7) | 0.5 (0.5–0.6) | 0.3 (0.3–0.3) | 0.4 (0.3–0.5) |
| Raced | |||||
| White | 26,441 | 1.6 (1.4–1.7) | 1.3 (1.2–1.4) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) |
| Black | 10,109 | 2.0 (1.7–2.2) | 1.6 (1.4–1.9) | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) |
| Asian | 3,257 | 1.6 (1.2–2.0) | 1.4 (1.0–1.8) | 0.6 (0.3–0.8) | 0.8 (0.5–1.1) |
| Not specified/Other | 20,436 | 1.5 (1.4–1.7) | 1.3 (1.1–1.4) | 0.7 (0.6–0.8) | 0.6 (0.5–0.8) |
| Ethnicity | |||||
| Hispanic | 9,685 | 2.2 (1.9–2.5) | 2.0 (1.7–2.2) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) |
| Non-Hispanic | 41,511 | 1.7 (1.6–1.9) | 1.4 (1.3–1.6) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) |
| Unknown | 9,047 | 0.5 (0.4–0.7) | 0.2 (0.1–0.3) | 0.3 (0.2–0.4) | 0.1 (0.1–0.2) |
All data are reported as % (95% CI), such that numbers in the table can be considered per 100 infants.
Abbreviations: CI, confidence interval; RBC, red blood cell.
Includes one infant who received cryoprecipitate without RBC, platelet or plasma transfusion.
Data not shown for 18 preterm infants for which gestational age unknown.
Term babies who are missing a birthweight are assumed to be normal birthweight. 10 subjects have a premature diagnosis, but no birthweight-related diagnosis or birth weight, thus they are excluded from this stratification.
Includes 324 patients of American Indian/Alaska Native descent, 62 of Native Hawaiian/Pacific Islander descent, 93 patients of more than one race, 9,307 identified as other race, and 10,650 of unknown race
Next, we evaluated the incidence of transfusion among infants whose birth hospitalization was greater than 3 days (Table Ⅰ), which excluded healthy newborns with a typical hospitalization duration as well as neonates with early death or transfer. Among this subset of full-term infants ≥ 37 weeks gestation with length of stay > 3 days(n=8,199), 3.2% (95% CI 2.8–3.6%) were exposed to transfusion (any blood product), with 2.5% (95% CI 2.2–2.8%) receiving RBCs, 1.7% (95% CI 1.4–2.0%) platelets, and 2% (1.7–2.0%) plasma. By contrast, the incidence of any transfusion among the infants <27 weeks’ gestation (n=329) was 80%, which was numerically similar to the 81% among the subset of <27 weeks’ gestation infants with length of stay >3 days (n=295).
Incidence of transfusion by comorbidities
We estimated transfusion exposure among infants with various neonatal comorbidities (Table Ⅱ). The incidence of any transfusion exposure ranged from 3.5% (95% CI 2.6–4.4%) among infants with hemolytic disease of the fetus and newborn to 100% among infants undergoing cardiac surgery with cardiopulmonary bypass or ECMO, with similar variation in the utilization of specific blood components. In most diagnostic subgroups, RBCs were the most frequently transfused product, followed by platelets and plasma.
Table II.
Incidence of blood product transfusion, including specific components, among infants with specific diagnoses
| Diagnosis | Encounters | Any Transfusiona | Any RBC | Any Platelet | Any Plasma |
|---|---|---|---|---|---|
| Necrotizing enterocolitisb | 111 | 77 (70–85) | 71 (63–80) | 40 (31–49) | 38 (29–47) |
| Chronic lung disease of prematurity or BPD | 344 | 70 (65–75) | 63 (58–68) | 21 (17–26) | 15 (11–19) |
| Intraventricular hemorrhage | 415 | 54 (49–59) | 45 (40–50) | 23 (19–27) | 20 (17–24) |
| Moderate or Severe Intraventricular hemorrhage | 75 | 87 (79–94) | 64 (53–75) | 49 (38–61) | 36 (25–47) |
| Sepsis | 402 | 41 (36–46) | 34 (29–38) | 23 (19–27) | 20 (16–24) |
| Retinopathy of prematurity | 314 | 64 (58–69) | 57 (52–63) | 22 (17–27) | 16 (12–20) |
| Hemolytic disease of the fetus and newborn | 1,526 | 3.5 (2.6–4.4) | 3.3 (2.4–4.2) | 1.4 (0.8–2.0) | 1.4 (0.8–2.0) |
| Meconium aspiration | 422 | 5.5 (3.3–7.6) | 1.9 (0.6–3.2) | 2.8 (1.3–4.4) | 4.3 (2.3–6.2) |
| Hypoxic-ischemic encephalopathy | 71 | 49 (38–61) | 17 (8–26) | 21 (12–31) | 42 (31–54) |
| Congenital diaphragmatic hernia | 77 | 77 (67–86) | 70 (60–80) | 30 (20–40) | 53 (42–64) |
| Congenital diaphragmatic hernia, with ECMO | 16 | 100 (100–100) | 100 (100–100) | 69 (46–91) | 100 (100–100) |
| Congenital diaphragmatic hernia, without ECMO | 61 | 70 (59–82) | 62 (50–74) | 20 (9.7–30) | 41 (29–53) |
| Persistent pulmonary hypertensionc | 203 | 53 (46–60) | 42 (36–49) | 23 (17–29) | 35 (28–42) |
| Persistent pulmonary HTN, with ECMO | 18 | 100 (100–100) | 100 (100–100) | 78 (59–97) | 94 (84–100) |
| Persistent pulmonary HTN, without ECMO | 185 | 49 (41–56) | 37 (30–44) | 18 (12–23) | 29 (23–36) |
| Acute renal failure | 95 | 79 (71–87) | 71 (61–80) | 41 (31–51) | 51 (40–61) |
| Cardiac Surgery with bypass | 93 | 100 (100–100) | 99 (97–100) | 83 (75–90) | 94 (89–99) |
| Surgery without bypass | 114 | 33 (25–42) | 32 (24–41) | 7.9 (2.9–12.8) | 12 (6–18) |
| without congenital heart disease | 65 | 15 (7–24) | 14 (5–22) | 4.6 (0.0–9.7) | 3.1 (0.0–7.3) |
| with congenital heart disease | 49 | 57 (43–71) | 57 (43–71) | 12 (3–21) | 24 (12–37) |
| Congenital heart disease, no surgery | 1,342 | 28 (25–30) | 23 (21–26) | 9 (8–11) | 11 (10–13) |
| ECMOd | 32 | 100 (100–100) | 100 (100–100) | 75 (60–90) | 94 (85–100) |
All data are reported as % (95% CI), such that numbers in the table can be considered per 100 infants.
Abbreviations: BPD, bronchopulmonary dysplasia; ECMO, extracorporeal membrane oxygenation; HTN, hypertension.
Includes one infant who received cryoprecipitate without RBC, platelet or plasma transfusion.
Includes 9 term infants (≥37 weeks’ gestation) with NEC.
Includes 76 preterm infants (<37 weeks’ gestation) with a diagnosis of persistent pulmonary HTN.
Includes the patients listed above with congenital diaphragmatic hernia and persistent pulmonary hypertension
Pre-transfusion hematologic values
In the entire cohort, a pre-transfusion Hb within 24 hours before transfusion was available for 76% (2,639) of RBC transfusions, which included multiple transfusions for some infants. The median pre-transfusion Hb was 11.2 g/dL (10–90th percentile 8.8–14.1). For those who received multiple transfusions, the pre-transfusion Hg was 10.5 g/dL for the first transfusion and 11.4 g/dL for subsequent transfusions. Pre-transfusion Hb values differed significantly by gestational age (P<0.001): Among infants <27 weeks’ gestation, the median pre-transfusion Hb was 10.5 g/dL (10–90th percentile 8.8–12.3), compared with 13.0 g/dL (10.5–15.5) among full-term infants (Figure 1 and Table Ⅲ [available at www.jpeds.com]). Pre-transfusion Hb also varied by comorbid condition, with the highest values found among infants with congenital diaphragmatic hernia (with or without ECMO), undergoing cardiopulmonary bypass, or on ECMO (Figure 2 and Table Ⅳ [available at www.jpeds.com]).
Figure 1. Pre-transfusion hematologic values, stratified by gestational age.
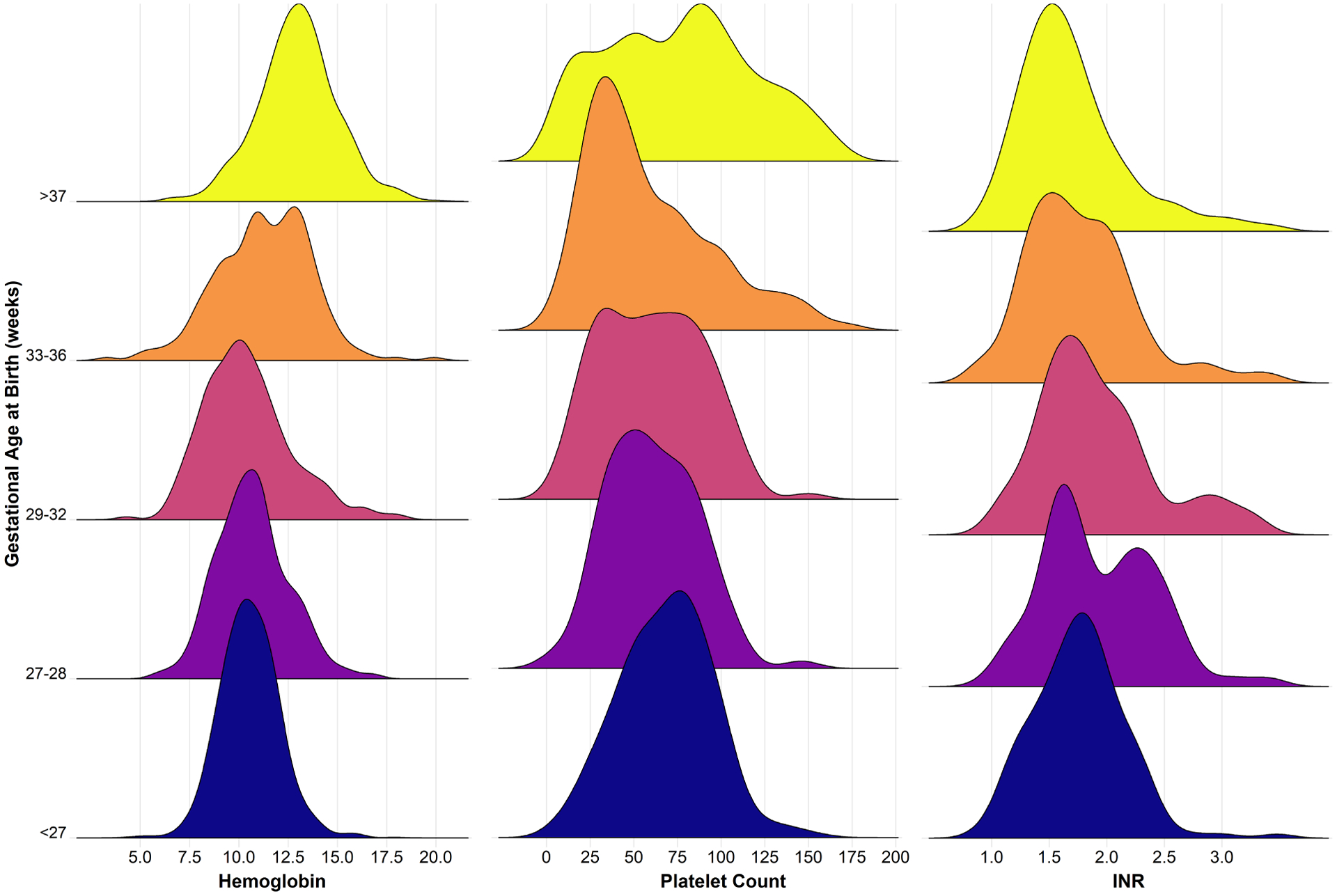
Density plots show the distribution of pre-transfusion hematologic values for hemoglobin (g/dL), platelet count (× 109/L) and INR (P<0.001 for testing for differences in median value by gestational age strata using Kruskal-Wallis test for each hematologic parameter).
Table III (online only).
Hemoglobin measured within 24 hours before RBC transfusion, by gestational and postnatal age
| Gestational and Postnatal Agea | n | 10th %tile | 25th %tile | 50th %tile | 75th %tile | 90th %tile | Pb |
|---|---|---|---|---|---|---|---|
| All | 2,639 | 8.8 | 9.8 | 11.2 | 12.7 | 14.1 | |
| <27 weeks | 1,020 | 8.8 | 9.6 | 10.5 | 11.5 | 12.3 | P<0.0001 |
| week 1 of life | 325 | 8.9 | 9.9 | 10.8 | 11.8 | 12.8 | |
| week 2 of life | 185 | 9.0 | 9.8 | 10.8 | 11.6 | 12.4 | |
| week 3 of life | 117 | 9.2 | 9.6 | 10.4 | 11.4 | 12.2 | |
| week 4 or more of life | 393 | 8.5 | 9.3 | 10.1 | 11.1 | 11.9 | |
| 27 to 28 weeks | 328 | 8.4 | 9.5 | 10.6 | 11.8 | 13.2 | 0.05 |
| week 1 of life | 93 | 9.0 | 10.0 | 11.0 | 11.7 | 13.1 | |
| week 2 of life | 51 | 9.0 | 9.6 | 10.7 | 11.6 | 12.4 | |
| week 3 of life | 43 | 8.4 | 8.8 | 9.9 | 11.1 | 13.0 | |
| week 4 or more of life | 141 | 8.2 | 9.1 | 10.5 | 12.3 | 13.3 | |
| 29 to 32 weeks | 249 | 7.9 | 8.8 | 10.2 | 11.6 | 13.5 | 0.004 |
| week 1 of life | 73 | 8.4 | 9.8 | 10.6 | 12.4 | 14.7 | |
| week 2 of life | 19 | 7.0 | 8.6 | 9.3 | 10.7 | 12.5 | |
| week 3 of life | 26 | 7.4 | 8.2 | 9.8 | 10.5 | 11.7 | |
| week 4 or more of life | 131 | 7.9 | 8.6 | 10.0 | 11.7 | 13.3 | |
| 33 to 36 weeks | 234 | 8.2 | 9.6 | 11.3 | 13.0 | 14.0 | 0.004 |
| week 1 of life | 65 | 7.9 | 9.3 | 11.0 | 12.7 | 13.9 | |
| week 2 of life | 37 | 9.6 | 11.0 | 12.3 | 13.8 | 14.7 | |
| week 3 of life | 28 | 8.5 | 10.5 | 12.2 | 13.0 | 14.0 | |
| week 4 or more of life | 104 | 8.1 | 9.3 | 11.1 | 12.8 | 13.5 | |
| 37+ weeks | 801 | 10.5 | 11.8 | 13.0 | 14.1 | 15.5 | 0.002 |
| week 1 of life | 210 | 10.3 | 11.9 | 13.2 | 14.9 | 15.9 | |
| week 2 of life | 171 | 11.1 | 12.0 | 13.3 | 14.3 | 15.4 | |
| week 3 of life | 90 | 10.8 | 11.8 | 12.8 | 14.0 | 15.7 | |
| week 4 or more of life | 330 | 9.9 | 11.5 | 12.7 | 13.9 | 15.0 |
Hemoglobin values are reported in g/dL.
Seven preterm infants with unspecified gestational age not shown.
P values report tests for differences in median values by week of life using the Kruskal-Wallis test.
Figure 2. Pre-transfusion hematologic values, stratified by diagnoses.
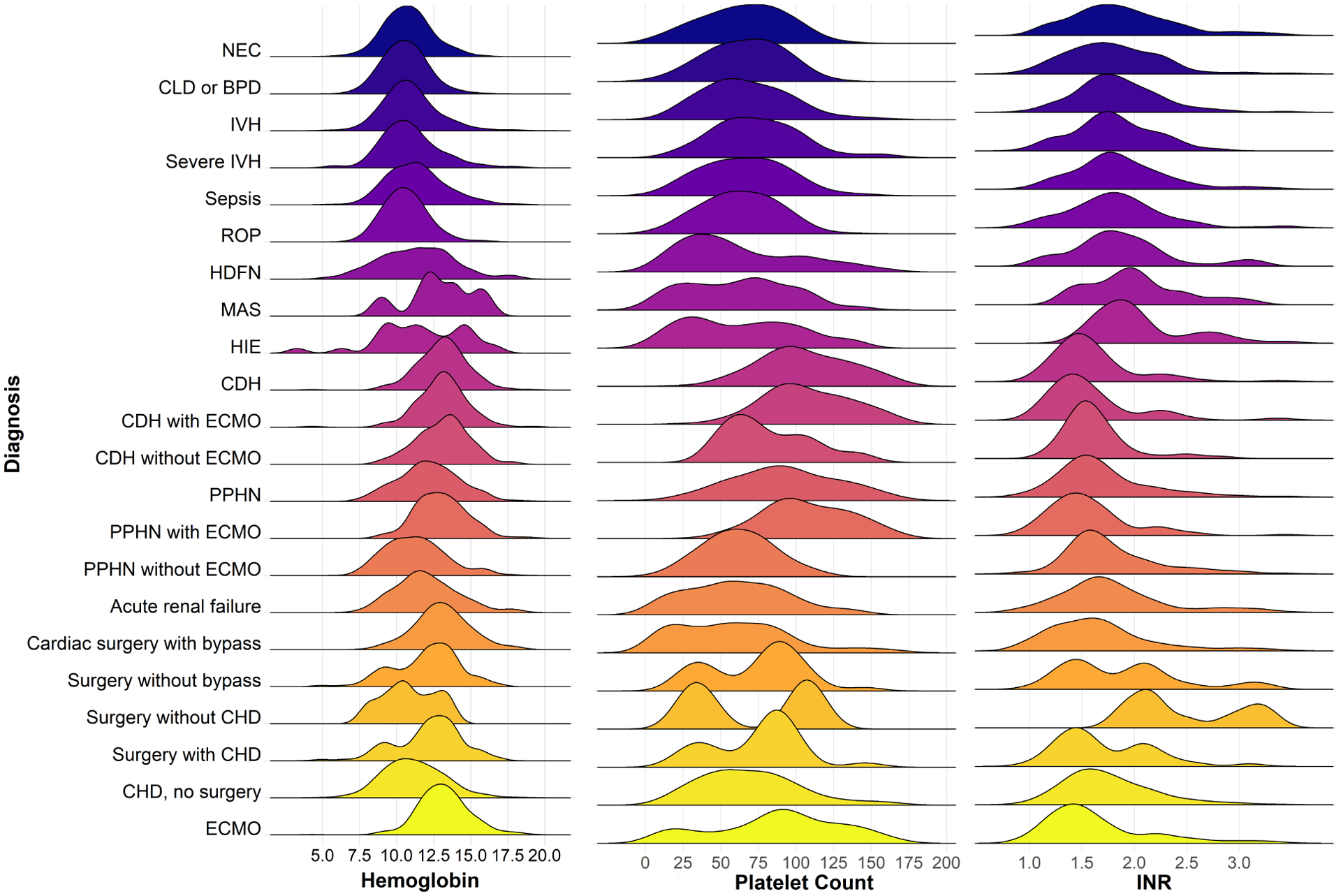
Density plot shows the distribution of pre-transfusion hematologic values for hemoglobin (g/dL), platelet count (× 109/L) and INR. Due to the skewed distribution of platelet data, the density estimation curves span negative values. P <0.0001 for test for difference in median values among groups using Kruskal-Wallis test. Abbreviations: NEC, necrotizing enterocolitis; CLD, chronic lung disease; BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; HDFN, hemolytic disease of the fetus and newborn; MAS, meconium aspiration syndrome; HIE, hypoxic-ischemic encephalopathy; CDH, congenital diaphragmatic hernia; ECMO, extracorporeal membrane oxygenation; PPHN, persistent pulmonary hypertension of the newborn; CHD, congenital heart disease.
Table IV (online only).
Hemoglobin measured within 24 hours before RBC transfusion, by diagnosis
| Diagnosis | n | 10th %tile | 25th %tile | 50th %tile | 75th %tile | 90th %tile |
|---|---|---|---|---|---|---|
| All | 2,639 | 8.8 | 9.8 | 11.2 | 12.7 | 14.1 |
| Necrotizing enterocolitis | 453 | 8.9 | 9.6 | 10.7 | 11.7 | 12.9 |
| Chronic lung disease of prematurity or BPD | 917 | 8.7 | 9.6 | 10.5 | 11.5 | 12.4 |
| Intraventricular hemorrhage | 735 | 8.8 | 9.7 | 10.8 | 11.8 | 13.3 |
| Moderate or Severe Intraventricular hemorrhage | 227 | 8.8 | 9.6 | 10.7 | 12.1 | 13.6 |
| Sepsis | 633 | 9 | 9.9 | 11.2 | 12.5 | 14.1 |
| Retinopathy of prematurity | 830 | 8.8 | 9.6 | 10.5 | 11.5 | 12.4 |
| Hemolytic disease of the fetus and newborn | 143 | 8.2 | 9.5 | 11.4 | 13.1 | 14.6 |
| Meconium aspiration | 13 | 9.3 | 11.7 | 12.5 | 14.1 | 15.7 |
| Hypoxic-ischemic encephalopathy | 30 | 8.65 | 9.5 | 11.55 | 14.5 | 15.15 |
| Congenital diaphragmatic hernia | 199 | 11.1 | 12 | 13.2 | 14.1 | 15.3 |
| Congenital diaphragmatic hernia, with ECMO | 144 | 11.1 | 12 | 13.1 | 14 | 15.1 |
| Congenital diaphragmatic hernia, without ECMO | 55 | 10.6 | 11.9 | 13.2 | 14.1 | 15.3 |
| Persistent pulmonary hypertension | 325 | 9.4 | 11 | 12.1 | 13.6 | 15 |
| Persistent pulmonary hypertension, with ECMO | 172 | 11.1 | 11.8 | 12.9 | 14.1 | 15.3 |
| Persistent pulmonary hypertension, without ECMO | 153 | 8.8 | 9.8 | 11.1 | 12.5 | 13.9 |
| Acute Renal Failure | 496 | 9.3 | 10.6 | 11.8 | 13.3 | 14.9 |
| Cardiac surgery, with cardiopulmonary bypass | 551 | 10.8 | 12 | 13.1 | 14.1 | 15.4 |
| Surgery, without cardiopulmonary bypass | 118 | 9 | 10.6 | 12.4 | 13.5 | 14.5 |
| without congenital heart disease | 13 | 8.4 | 9.5 | 10.7 | 12.2 | 13.3 |
| with congenital heart disease | 105 | 9 | 11.2 | 12.4 | 13.7 | 14.6 |
| Congenital heart disease | 871 | 8.7 | 9.7 | 11 | 12.5 | 13.8 |
| ECMOa | 421 | 11.3 | 12.1 | 13.1 | 14.1 | 15.4 |
Hemoglobin values are reported in g/dL.
Abbreviations: BPD, bronchopulmonary dysplasia; ECMO, extracorporeal membrane oxygenation; HTN, hypertension.
Includes patients listed above with congenital diaphragmatic hernia and persistent pulmonary hypertension.
Among the entire cohort, 93% (1,162) of platelet transfusions had a pre-transfusion platelet count, with a median of 71 × 109/L (10–90th percentile 26–135 × 109/L). For those who received multiple transfusions, the pre-transfusion platelet count was 70 × 109/L for the first transfusion and 71 × 109/L for subsequent transfusions. Pre-transfusion platelet counts varied significantly by gestational age (P<0.001), with a median pre-transfusion platelet count of 70 ×109/L (33–100) among infants <27 weeks’ gestation and a median platelet count of 85 ×109/L (17–185) among term infants (Figure 1 and Table Ⅴ [available at www.jpeds.com]). Among infants with different comorbidities, the highest median pre-transfusion platelet counts (>100×109/L) were found among infants on ECMO for congenital diaphragmatic hernia and/or persistent pulmonary hypertension of the newborn (Figure 2 and Table Ⅵ [available at www.jpeds.com]). The median pre-transfusion platelet count was >50×109/L for all diagnoses examined and all gestational age groups, with the exception of infants born at 33–36 weeks’ gestation.
Table V (online only).
Platelet values measured within 24 hours before platelet transfusion, by gestational and postnatal age
| Gestational and Postnatal Agea | n | 10th %tile | 25th %tile | 50th %tile | 75th %tile | 90th %tile | Pb |
|---|---|---|---|---|---|---|---|
| All | 1,162 | 26 | 44 | 71 | 96 | 135 | |
| <27 weeks | 373 | 33 | 50 | 70 | 86 | 100 | 0.0007 |
| week 1 of life | 137 | 44 | 60 | 77 | 92 | 105 | |
| week 2 of life | 72 | 33 | 50 | 67 | 85 | 96 | |
| week 3 of life | 37 | 34 | 53 | 66 | 77 | 98 | |
| week 4 or more of life | 127 | 26 | 43 | 64 | 82 | 98 | |
| 27 to 28 weeks | 95 | 30 | 40 | 58 | 81 | 93 | 0.03 |
| week 1 of life | 45 | 33 | 47 | 64 | 82 | 94 | |
| week 2 of life | 15 | 41 | 56 | 69 | 94 | 108 | |
| week 3 of life | 8 | 20 | 27 | 56 | 77 | 90 | |
| week 4 or more of life | 27 | 30 | 38 | 49 | 59 | 84 | |
| 29 to 32 weeks | 124 | 22 | 35 | 62 | 84 | 102 | 0.01 |
| week 1 of life | 62 | 22 | 32 | 53 | 82 | 94 | |
| week 2 of life | 6 | 22 | 23 | 75 | 87 | 150 | |
| week 3 of life | 10 | 9 | 17 | 39 | 74 | 89 | |
| week 4 or more of life | 46 | 31 | 52 | 74 | 96 | 114 | |
| 33 to 36 weeks | 119 | 23 | 30 | 47 | 88 | 129 | 0.21 |
| week 1 of life | 66 | 19 | 30 | 46 | 74 | 99 | |
| week 2 of life | 22 | 25 | 27 | 63 | 124 | 129 | |
| week 3 of life | 13 | 26 | 33 | 99 | 143 | 148 | |
| week 4 or more of life | 18 | 25 | 35 | 51 | 65 | 79 | |
| 37+ weeks | 450 | 17 | 47 | 85 | 124 | 185 | <0.0001 |
| week 1 of life | 189 | 25 | 50 | 90 | 134 | 206 | |
| week 2 of life | 121 | 38 | 73 | 98 | 132 | 191 | |
| week 3 of life | 47 | 9 | 17 | 83 | 125 | 180 | |
| week 4 or more of life | 93 | 13 | 29 | 58 | 85 | 113 |
Platelet values are reported as × 109/L
Two preterm infants with unspecified gestational age not shown.
P values report tests for differences in median values by week of life using the Kruskal-Wallis test.
Table VI (online only).
Platelet values measured within 24 hours before platelet transfusion, by diagnosis
| Diagnosis | n | 10th %tile | 25th %tile | 50th %tile | 75th %tile | 90th %tile |
|---|---|---|---|---|---|---|
| All | 1,162 | 26 | 44 | 71 | 96 | 135 |
| Necrotizing enterocolitis | 228 | 29 | 47 | 69 | 87 | 105 |
| Chronic lung disease of prematurity or bronchopulmonary dysplasia | 292 | 32 | 49 | 69 | 85 | 98 |
| Intraventricular hemorrhage | 287 | 30 | 47 | 64 | 87 | 105 |
| Moderate or Severe Intraventricular hemorrhage | 84 | 42 | 55 | 76 | 97 | 107 |
| Sepsis | 413 | 29 | 45 | 67 | 86 | 104 |
| Retinopathy of prematurity | 281 | 29 | 45 | 63 | 81 | 97 |
| Hemolytic disease of the fetus and newborn | 87 | 17 | 32 | 54 | 99 | 134 |
| Meconium aspiration | 34 | 15 | 34 | 68 | 93 | 109 |
| Hypoxic-ischemic encephalopathy | 40 | 18 | 32 | 64 | 94 | 113 |
| Congenital diaphragmatic hernia | 163 | 72 | 87 | 106 | 135 | 166 |
| Congenital diaphragmatic hernia, with ECMO | 151 | 74 | 89 | 108 | 138 | 170 |
| Congenital diaphragmatic hernia, without ECMO | 12 | 49 | 61 | 74 | 103 | 116 |
| Persistent pulmonary hypertension | 270 | 51 | 71 | 95 | 125 | 155 |
| Persistent pulmonary hypertension, with ECMO | 188 | 75 | 89 | 108 | 136 | 166 |
| Persistent pulmonary hypertension, without ECMO | 82 | 32 | 47 | 63 | 78 | 95 |
| Acute Renal Failure | 243 | 16 | 33 | 60 | 86 | 114 |
| Cardiac surgery, with cardiopulmonary bypass | 192 | 14 | 33 | 70 | 129 | 273 |
| Surgery, without cardiopulmonary bypass | 34 | 30 | 43 | 85 | 97 | 114 |
| without congenital heart disease | 6 | 27 | 33 | 72 | 107 | 114 |
| with congenital heart disease | 28 | 30 | 51 | 85 | 95 | 146 |
| Congenital heart disease | 441 | 30 | 46 | 68 | 92 | 121 |
| ECMOa | 302 | 23 | 65 | 95 | 126 | 157 |
Platelet values are reported as × 109/L
Includes patients listed above with congenital diaphragmatic hernia and persistent pulmonary hypertension.
For plasma transfusions, a pre-transfusion INR was available in 79% (895) of transfusions, and the median pre-transfusion INR was 1.7 (10–90th percentile 1.2–2.8) among the entire cohort, which was 1.8 for the first transfusion and 1.7 for subsequent transfusions. When assessed based on gestational age, all gestational age subgroups had a median pre-transfusion INR <2 except for infants 27–28 weeks gestation, who had a median INR of 2.0 (Figure 1 and Table Ⅶ [available at www.jpeds.com]). Similarly, infants in all diagnostic groups had median pre-transfusion INR values <2, with the exception of meconium aspiration syndrome (2.0), hypoxic-ischemic encephalopathy (2.0) and surgery without cardiopulmonary bypass without congenital heart disease (2.4) (Figure 2 and Table Ⅷ [available at www.jpeds.com]).
Table VII (online only).
INR values measured within 24 hours before plasma transfusion, by gestational and postnatal age
| Gestational and Postnatal Agea | n | 10th %tile | 25th %tile | 50th %tile | 75th %tile | 90th %tile | Pb |
|---|---|---|---|---|---|---|---|
| All | 895 | 1.2 | 1.5 | 1.7 | 2.1 | 2.8 | |
| <27 weeks | 152 | 1.2 | 1.5 | 1.8 | 2.0 | 2.3 | 0.0002 |
| week 1 of life | 77 | 1.5 | 1.7 | 1.9 | 2.2 | 2.4 | |
| week 2 of life | 39 | 1.3 | 1.6 | 1.8 | 1.9 | 2.2 | |
| week 3 of life | 5 | 1.2 | 1.5 | 1.6 | 1.8 | 3.7 | |
| week 4 or more of life | 31 | 1.1 | 1.2 | 1.4 | 1.7 | 2.2 | |
| 27 to 28 weeks | 81 | 1.5 | 1.6 | 2.0 | 2.4 | 3.4 | 0.03 |
| week 1 of life | 52 | 1.5 | 1.6 | 1.9 | 2.3 | 2.6 | |
| week 2 of life | 11 | 1.6 | 2.1 | 2.5 | 3.8 | 6.6 | |
| week 3 of life | 3 | 1.5 | 1.5 | 2.3 | 2.6 | 2.6 | |
| week 4 or more of life | 15 | 1.2 | 1.2 | 1.6 | 2.6 | 3.5 | |
| 29 to 32 weeks | 106 | 1.4 | 1.6 | 1.8 | 2.2 | 2.9 | 0.62 |
| week 1 of life | 56 | 1.5 | 1.6 | 1.9 | 2.2 | 2.8 | |
| week 2 of life | 9 | 1.2 | 1.4 | 1.8 | 1.9 | 12.1 | |
| week 3 of life | 6 | 1.4 | 1.5 | 1.8 | 3.0 | 4.0 | |
| week 4 or more of life | 35 | 1.2 | 1.5 | 1.8 | 2.2 | 2.9 | |
| 33 to 36 weeks | 115 | 1.3 | 1.5 | 1.8 | 2.1 | 3.4 | 0.04 |
| week 1 of life | 79 | 1.4 | 1.5 | 1.8 | 2.3 | 3.8 | |
| week 2 of life | 18 | 1.2 | 1.4 | 1.6 | 1.9 | 2.4 | |
| week 3 of life | 7 | 1.2 | 1.2 | 1.3 | 2.0 | 2.1 | |
| week 4 or more of life | 11 | 0.9 | 0.9 | 1.9 | 2.3 | 2.4 | |
| 37+ weeks | 438 | 1.2 | 1.4 | 1.6 | 2.0 | 2.6 | 0.0006 |
| week 1 of life | 241 | 1.3 | 1.5 | 1.7 | 2.1 | 2.7 | |
| week 2 of life | 84 | 1.2 | 1.3 | 1.5 | 1.8 | 2.1 | |
| week 3 of life | 37 | 1.2 | 1.3 | 1.5 | 1.9 | 2.6 | |
| week 4 or more of life | 76 | 1.1 | 1.4 | 1.6 | 2.0 | 3.2 |
Two values for preterm infants with unspecified gestational age not shown.
P values report tests for differences in median values by week of life using the Kruskal-Wallis test.
Table VIII (online only).
INR values measured within 24 hours before plasma transfusion, by diagnosis
| Diagnosis | n | 10th %tile | 25th %tile | 50th %tile | 75th %tile | 90th %tile |
|---|---|---|---|---|---|---|
| All | 895 | 1.2 | 1.5 | 1.7 | 2.1 | 2.8 |
| Necrotizing enterocolitis | 123 | 1.3 | 1.6 | 1.9 | 2.3 | 3.1 |
| Chronic lung disease of prematurity or BPD | 116 | 1.2 | 1.5 | 1.7 | 2.1 | 2.3 |
| Intraventricular hemorrhage | 196 | 1.4 | 1.6 | 1.9 | 2.2 | 3.0 |
| Moderate or Severe Intraventricular hemorrhage | 69 | 1.3 | 1.6 | 1.8 | 2.2 | 2.6 |
| Sepsis | 263 | 1.4 | 1.6 | 1.8 | 2.2 | 2.7 |
| Retinopathy of prematurity | 120 | 1.2 | 1.6 | 1.8 | 2.1 | 3.3 |
| Hemolytic disease of the fetus and newborn | 60 | 1.3 | 1.6 | 1.8 | 2.1 | 3.0 |
| Meconium aspiration | 43 | 1.5 | 1.8 | 2.0 | 2.8 | 3.8 |
| Hypoxic-ischemic encephalopathy | 64 | 1.6 | 1.8 | 2.0 | 2.6 | 3.8 |
| Congenital diaphragmatic hernia | 136 | 1.2 | 1.4 | 1.5 | 1.7 | 2.2 |
| Congenital diaphragmatic hernia, with ECMO | 90 | 1.2 | 1.3 | 1.5 | 1.7 | 2.2 |
| Congenital diaphragmatic hernia, without ECMO | 46 | 1.3 | 1.5 | 1.6 | 1.7 | 2.0 |
| Persistent pulmonary hypertension | 217 | 1.3 | 1.4 | 1.6 | 1.9 | 2.4 |
| Persistent pulmonary hypertension, with ECMO | 90 | 1.2 | 1.3 | 1.5 | 1.7 | 2.2 |
| Persistent pulmonary hypertension, without ECMO | 127 | 1.4 | 1.5 | 1.7 | 2.1 | 2.7 |
| Acute Renal Failure | 184 | 1.2 | 1.5 | 1.7 | 2.1 | 3.0 |
| Cardiac surgery, with cardiopulmonary bypass | 190 | 1.2 | 1.3 | 1.6 | 1.9 | 2.6 |
| Surgery, without cardiopulmonary bypass | 52 | 1.3 | 1.5 | 1.9 | 2.2 | 3.1 |
| without congenital heart disease | 12 | 1.9 | 2.1 | 2.4 | 3.2 | 3.3 |
| with congenital heart disease | 40 | 1.3 | 1.4 | 1.6 | 2.1 | 2.5 |
| Congenital heart disease | 383 | 1.3 | 1.5 | 1.7 | 2.1 | 2.6 |
| ECMOa | 180 | 1.2 | 1.3 | 1.5 | 1.9 | 2.6 |
Includes patients listed above with congenital diaphragmatic hernia and persistent pulmonary hypertension.
Postnatal age and pre-transfusion hematologic values
Next, we investigated whether pre-transfusion Hb, platelet count and INR values differed by postnatal age (Tables Ⅲ, Ⅴ, and Ⅶ). Overall, pre-transfusion Hb values varied significantly by postnatal week of life in all gestational age groups, although were not statistically significant for infants at 27–28 weeks’ gestation (Table Ⅲ). Significant differences in pre-transfusion platelet count by week of life were observed among infants of all gestational ages except <27 weeks (Table Ⅴ). Mean pre-transfusion INR varied significantly with post-natal age in all gestational age groups except 29–32 and 33–36 weeks (Table Ⅶ). The majority of pre-transfusion hematologic values were observed within the first 4 months of life, although infants undergoing surgery comprised a larger proportion of transfusions evaluated after this period (Table Ⅸ [available at www.jpeds.com]).
Table IX (online only).
Pre-transfusion hematologic values by postnatal age among infants with and without surgery
| Post-Natal Age | Hemoglobin | Platelet Count | INR | |||
|---|---|---|---|---|---|---|
| N | Median | N | Median | N | Median | |
| < 4 weeks | ||||||
| All infants | 1800 | 11.3 | 934 | 73 | 766 | 1.7 |
| Surgery | 502 | 13.1 | 306 | 103 | 246 | 1.6 |
| No surgery | 1298 | 10.8 | 628 | 61 | 520 | 1.8 |
| ≥4 weeks | ||||||
| All infants | 839 | 10.9 | 228 | 63 | 129 | 1.60 |
| Surgery | 319 | 12.7 | 96 | 75 | 85 | 1.6 |
| No surgery | 520 | 10 | 132 | 55 | 44 | 1.5 |
| < 4 months | ||||||
| All infants | 2529 | 11.1 | 1119 | 70 | 867 | 1.7 |
| Surgery | 736 | 13.1 | 361 | 97 | 303 | 1.6 |
| No surgery | 1793 | 10.5 | 758 | 60 | 564 | 1.8 |
| ≥4 months | ||||||
| All infants | 110 | 11.8 | 43 | 81 | 28 | 1.4 |
| Surgery | 85 | 12.4 | 41 | 81 | 28 | 1.4 |
| No surgery | 25 | 9.6 | 2 | 97 | 0 | --- |
Hemoglobin values are in g/dL and platelet values are reported as × 109/L.
Surgery includes infants who had cardiac and non-cardiac surgery and ECMO.
Differences in pre-transfusion hematologic values by center and location
In multivariable analyses adjusted for gestational age and surgical diagnosis, pre-transfusion Hb (P<0.001) and platelet counts (P<0.001) differed among study centers but pre-transfusion INR did not (P=0.17). Most transfusions were administered in the ICU (62%), followed by non-ICU general ward (24%) and operating room (8%). Pre-transfusion hematologic values differed between infants transfused in the OR, compared with non-OR setting, for hemoglobin (P<0.001) and platelet count (P<0.001) but not INR (P=0.20), after adjustment for surgical diagnosis and gestational age.
Number of transfusions
The mean number of transfusions per patient (among infants receiving each product type) was 4.7 for RBCs, 3.3 for platelets, and 2.9 for plasma (Table Ⅹ [available at www.jpeds.com]).
Table X (online only).
Number of blood product transfusions and duration of birth hospitalization by product type and birth weight
| Product and Birth weight | # transfusions | Length of stay (days) | |||
|---|---|---|---|---|---|
| N | Mean | Median | Mean | Median | |
| RBCs | 808 | 4.7 | 3 | 64 | 55 |
| <1,000 g | 284 | 5.3 | 4 | 81 | 82 |
| 1,000 to <1,500 g | 113 | 2.4 | 2 | 59 | 55 |
| 1,500 to <2,500 g | 111 | 3.2 | 2 | 52 | 37 |
| ≥2,500 g | 300 | 5.7 | 3 | 55 | 41 |
| Plasma | 393 | 2.9 | 2 | 54 | 29 |
| <1,000 g | 85 | 2.3 | 1 | 71 | 54 |
| 1,000 to <1,500 g | 32 | 2.9 | 2 | 44 | 33 |
| 1,500 to <2,500 g | 55 | 2.5 | 2 | 52 | 28 |
| ≥2,500 g | 221 | 3.3 | 2 | 49 | 28 |
| Platelets | 400 | 3.3 | 1 | 58 | 32 |
| <1,000 g | 138 | 3.4 | 2 | 75 | 65 |
| 1,000 to <1,500 g | 41 | 2.2 | 1 | 44 | 38 |
| 1,500 to <2,500 g | 55 | 2.7 | 1 | 52 | 28 |
| ≥2,500 g | 166 | 3.6 | 1 | 50 | 27 |
| Missing | 22 | 3.1 | 2 | 68 | 73 |
DISCUSSION
This study found marked variation in neonatal transfusion practices among a cohort of infants born and cared for in seven U.S. hospitals. Our findings suggested that many transfusions are administered at thresholds higher than supported by the best available evidence.
Our study cohort was unique in that it included newborn infants of all gestational ages admitted to the NICU as well as other hospital areas. Infants in this cohort were born at participating hospitals between 2013 and 2016, and thus our findings likely reflect contemporary transfusion practices. The incidence of any transfusion in our study was 1.6% (1.5–1.7), compared with 1.1% in another population-based study of neonatal and pediatric patients1. However, these overall population-based estimates for newborns mask the large variation in transfusion incidence among newborn infants of different gestational ages. In our study, the most immature preterm infants had the highest incidence of transfusion for all blood products.
Prior to 2013–2016, there were two randomized trials to guide RBC transfusion in preterm infants. The multicenter Canadian Premature Infants in Need of Transfusion (PINT) trial, which enrolled 451 extremely low birth weight infants (weighing < 1000 g at birth), did not find a significant difference in mortality or short-term morbidity with the use of more liberal, compared with conservative, RBC transfusion thresholds13. In this trial, the highest transfusion threshold in the restrictive/lower arm was a Hb of 11.5 g/dL. In our study, approximately a quarter of infants ≤ 28 weeks’ gestation had pre-transfusion Hb values greater than this threshold, suggesting that a substantial proportion of infants in this cohort received RBC transfusions using a liberal Hb threshold. Long-term follow up of the PINT trial suggested the possibility of worse cognitive outcomes among infants in the more restrictive transfusion arm14 and other studies have raised concerns regarding the risks of severe anemia on NEC15, 16. In another single-center randomized trial, there was some evidence of worse short-term brain injury among preterm infants randomized to a conservative, compared with a liberal, transfusion threshold,17 although long-term brain growth and neurodevelopment were paradoxically worse in infants randomized to the liberal Hb threshold18. These conflicting findings highlight the historical uncertainty regarding optimal RBC transfusion thresholds in preterm infants, and may potentially explain the wide variation in Hb transfusion thresholds observed in our cohort.
The Effects of Liberal vs Restrictive Transfusion Thresholds on Survival and Neurocognitive Outcomes in Extremely Low-Birth-Weight Infants (ETTNO) trial enrolled 1013 infants with a birth weight < 1000 g from 36 neonatal units in Europe and found no difference in either short-term morbidity or long-term survival without neurodevelopmental impairment among infants randomized to higher vs. lower Hb transfusion thresholds19. Another recently published multicenter trial conducted by the NICHD Neonatal Research Network randomized 1824 extremely preterm infants to higher vs. lower hemoglobin thresholds and reported no significant difference in survival without neurodevelopmental impairment between study arms20. Both of these more recent trials, published after REDS-III was completed, are likely to support the use of lower Hb transfusion thresholds for preterm infants given the lack of benefit observed with use of higher transfusion thresholds.
For platelet transfusions, the incidence was highest among the most preterm infants (34% among <27 weeks’ gestation). There have been a limited number of trials investigating platelet transfusion practices in preterm infants, and none conducted in the U.S. A multicenter Canadian trial published in 1993 found no benefit in the use of platelet transfusion thresholds >50×109/L to prevent the incidence or progression of intracranial bleeding in preterm infants4. In our study, however, over half of preterm infants received platelet transfusions at thresholds >50×109/L. More recently, a multicenter randomized trial conducted in Europe (PlaNeT-2) found evidence of harm, with an increased risk of death or serious bleeding associated with the use of a platelet transfusion threshold of 50×109/L, compared with 25×109/L, among infants <34 weeks’ gestation21. Although 39% of infants in that trial received platelet transfusions before enrollment, these data generally support more conservative platelet transfusion practices and suggest that platelet transfusion exposure could be decreased substantially among U.S. infants with the adoption of the lower threshold of 25×109/L. However, it is important to note that our study included a more heterogeneous population of term and preterm infants, than was studied in the PlaNeT-2 trial, and therefore pre-transfusion thresholds may not be directly comparable.
Trial data to guide prophylactic plasma transfusion in term and preterm infants are lacking. There are limited data in this population to identify INR values above which bleeding risk increases, with two studies reporting that INR and fibrinogen were associated with bleeding risk among infants with hypoxic-ischemic encephalopathy22, 23 and one suggesting that maintaining an INR <2 could prevent bleeding in this population. In our study, we found large variation in pre-transfusion INR among infants with hypoxic-ischemic encephalopathy, with the 10th to 90th percentile ranging from 1.6 to 3.8.
We believe our study supports the application of patient blood management to the neonatal population. Increased efforts to support the translation of evidence into practice may be needed to promote evidence-based neonatal transfusion practices. One past study highlighted the importance of monitoring compliance with transfusion guidelines and reported improvements in outcomes following such efforts24. Patient blood management could be particularly useful in the preterm population, where a sufficient body of evidence is available to guide practice. However, data for term infants, including those undergoing surgery or ECMO, are limited and largely derived from observational studies. One study suggested that the use of a hematocrit of 35% (corresponding to a Hb of ~11.7 g/dL), instead of 40% (corresponding to a Hb of 13.3 g/dL), for transfusion during ECMO could reduce RBC transfusions without worse outcomes25. In our study, the median pre-transfusion Hb among infants on ECMO was 13.1 g/dL, suggesting that evaluation of the safety and efficacy of more conservative thresholds is warranted, although we were unable to differentiate thresholds for blood products used to prime an ECMO circuit with those used for transfusion once an infant was on ECMO. Another study noted a higher risk of mortality among infants on ECMO with greater RBC and platelet transfusion exposure, even after controlling for illness severity,26 and similar findings regarding the adverse effects of platelet transfusion were reported in a recent multicenter study of pediatric ECMO patients27. By contrast, among infants undergoing surgery, one study found that a pre-operative hematocrit <40% (corresponding to a Hb of 13.3 g/dl) was associated with greater odds of post-operative mortality28, although residual confounding may have led to bias29. In addition to infants on ECMO, newborn infants undergoing cardiac surgery with cardiopulmonary bypass had the highest incidence of transfusions and highest transfusion thresholds in our study. Recent studies have described an association between RBC transfusions after stage 1 palliation and worse clinical outcomes30 and between platelet transfusions during bypass rewarming and improved neonatal outcomes31. Taken together, these findings highlight the need for additional studies to guide transfusion decisions in the term, surgical and ECMO populations of infants, who are currently transfused at the highest Hb and platelet count thresholds.
Our study has several limitations. Our goal was to estimate neonatal transfusion practices and not how they relate to specific outcomes; therefore, we were unable to determine the benefits or harms from the various pre-transfusion hematologic values. We believe randomized trials are better suited for such evaluations. In addition, we could not determine if transfusions were administered prophylactically or in response to bleeding or other circumstances that may explain the variation we observed in pre-transfusion hematologic values. Finally, we relied on ICD 9/10 determination of gestational age, birth weight and comorbid conditions, and some misclassification is possible. Although outside the scope of this study, we also recognize that there is substantial center-to-center variability in the characteristics and modifications of the blood products transfused, such as the type of anticoagulant preservative solution for RBCs, the storage duration, the use and timing of irradiation, the use of pathogen inactivation technology for platelets, and ABO matching, among others. Additional studies are needed to investigate this product variability and the potential effects of these variables on neonatal outcomes. Finally, the centers that comprised this cohort may not be generalizable to all types of settings in which newborn infants are cared for.
In conclusion, our study demonstrated wide variability in neonatal transfusion practices and suggests that a high percentage of transfusions given to infants in the U.S. may be administered at thresholds higher than supported by the best available evidence. Our findings highlight the need to translate the existing evidence into patient blood management in the neonatal population, to reduce unnecessary transfusion exposures and associated risks while potentially improving short- and long-term outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the following individuals from the NHLBI Recipient Epidemiology Donor Evaluation Study – IV – Pediatric (REDS-IV-P) domestic program:
Hubs
A.E. Mast, Versiti Wisconsin, Milwaukee, WI
E.A. Hod, Columbia University Medical Center, New York, NY
B.S. Custer, Vitalant Research Institute, San Francisco, CA and E.P. Vichinsky, Benioff Children’s Hospital Oakland, Oakland, CA
B.R. Spencer, American Red Cross, Dedham, MA
Data coordinating center
S.M. Mathew and D.R. Harris, Westat, Rockville, MD
Central laboratory
M.P. Busch and P.J. Norris, Vitalant Research Institute, San Francisco, CA
Publications Committee Chairman
P.M. Ness, Johns Hopkins University, Baltimore, MD
Steering Committee Chairpersons
S.H. Kleinman, University of British Columbia, Victoria, BC, Canada
National Institute of Child Health and Human Development (NICHD)
R. Tamburro
National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health
S.A. Glynn and K. Malkin
Supported by research contracts from the National Heart, Lung, and Blood Institute (NHLBI Contracts HHSN 75N92019D00032, HHSN 75N92019D00034, 75N92019D00035, HHSN 75N92019D00036, and HHSN 75N92019D00037). Additional funding was provided by the National Institute of Child Health and Human Development (NICHD). R.P. received funding from NHLBI (K23 HL128942). The funding source designated an investigator-led steering committee, which independently oversaw the design and conduct of the study and interpretation of the data, preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The authors declare no conflicts of interest.
LIST OF ABBREVIATIONS
- RBC
red blood cell
- U.S.
United States
- Hb
hemoglobin
- NICU
neonatal intensive care unit
- REDS-III
Recipient Epidemiology and Donor Evaluation Study-III
- ICD
international classification of diseases
- INR
international normalized ratio
- CI
confidence interval
- VLBW
very low birth weight
- ECMO
extracorporeal membrane oxygenation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented at the AABB Annual Meeting, << >>, 2020, << >>; and at the Pediatric Academic Societies annual meeting, May 4, 2021 (virtual).
REFERENCES
- [1].Goel R, Josephson CD, Patel EU, Petersen MR, Packman Z, Gehrie E, et al. Individual- and hospital-level correlates of red blood cell, platelet, and plasma transfusions among hospitalized children and neonates: a nationally representative study in the United States. Transfusion. 2020;60:1700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guillen U, Cummings JJ, Bell EF, Hosono S, Frantz AR, Maier RF, et al. International survey of transfusion practices for extremely premature infants. Semin Perinatol. 2012;36:244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Josephson CD, Su LL, Christensen RD, Hillyer CD, Castillejo MI, Emory MR, et al. Platelet transfusion practices among neonatologists in the United States and Canada: results of a survey. Pediatrics. 2009;123:278–85. [DOI] [PubMed] [Google Scholar]
- [4].Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr. 1993;123:285–91. [DOI] [PubMed] [Google Scholar]
- [5].Sparger KA, Assmann SF, Granger S, Winston A, Christensen RD, Widness JA, et al. Platelet Transfusion Practices Among Very-Low-Birth-Weight Infants. JAMA Pediatr. 2016;170:687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Poterjoy BS, Josephson CD. Platelets, frozen plasma, and cryoprecipitate: what is the clinical evidence for their use in the neonatal intensive care unit? Semin Perinatol. 2009;33:66–74. [DOI] [PubMed] [Google Scholar]
- [7].Keir AK, Stanworth SJ. Neonatal Plasma Transfusion: An Evidence-Based Review. Transfus Med Rev. 2016;30:174–82. [DOI] [PubMed] [Google Scholar]
- [8].Motta M, Del Vecchio A, Perrone B, Ghirardello S, Radicioni M. Fresh frozen plasma use in the NICU: a prospective, observational, multicentred study. Arch Dis Child Fetal Neonatal Ed. 2014;99:F303–8. [DOI] [PubMed] [Google Scholar]
- [9].Karafin MS, Bruhn R, Westlake M, Sullivan MT, Bialkowski W, Edgren G, et al. Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion. 2017;57:2903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Edgren G, Murphy EL, Brambilla DJ, Westlake M, Rostgaard K, Lee C, et al. Association of Blood Donor Sex and Prior Pregnancy With Mortality Among Red Blood Cell Transfusion Recipients. JAMA. 2019;321:2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Roubinian NH, Escobar GJ, Liu V, Gardner MN, Carson JL, Kleinman SH, et al. Decreased red blood cell use and mortality in hospitalized patients. JAMA Intern Med. 2014;174:1405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gottschall J, Wu Y, Triulzi D, Kleinman S, Strauss R, Zimrin AB, et al. The epidemiology of platelet transfusions: an analysis of platelet use at 12 US hospitals. Transfusion. 2020;60:46–53. [DOI] [PubMed] [Google Scholar]
- [13].Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7. [DOI] [PubMed] [Google Scholar]
- [14].Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–13. [DOI] [PubMed] [Google Scholar]
- [15].Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. JAMA. 2016;315:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh R, Visintainer PF, Frantz ID 3rd, Shah BL, Meyer KM, Favila SA, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011;165:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Franz AR, Engel C, Bassler D, Rudiger M, Thome UH, Maier RF, et al. Effects of Liberal vs Restrictive Transfusion Thresholds on Survival and Neurocognitive Outcomes in Extremely Low-Birth-Weight Infants: The ETTNO Randomized Clinical Trial. JAMA. 2020;324:560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or Lower Hemoglobin Transfusion Thresholds for Preterm Infants. N Engl J Med. 2020;383:2639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, et al. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med. 2019;380:242–51. [DOI] [PubMed] [Google Scholar]
- [22].Forman KR, Diab Y, Wong EC, Baumgart S, Luban NL, Massaro AN. Coagulopathy in newborns with hypoxic ischemic encephalopathy (HIE) treated with therapeutic hypothermia: a retrospective case-control study. BMC Pediatr. 2014;14:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pakvasa MA, Winkler AM, Hamrick SE, Josephson CD, Patel RM. Observational study of haemostatic dysfunction and bleeding in neonates with hypoxic-ischaemic encephalopathy. BMJ Open. 2017;7:e013787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baer VL, Henry E, Lambert DK, Stoddard RA, Wiedmeier SE, Eggert LD, et al. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion. 2011;51:264–9. [DOI] [PubMed] [Google Scholar]
- [25].Sawyer AA, Wise L, Ghosh S, Bhatia J, Stansfield BK. Comparison of transfusion thresholds during neonatal extracorporeal membrane oxygenation. Transfusion. 2017;57:2115–20. [DOI] [PubMed] [Google Scholar]
- [26].Keene SD, Patel RM, Stansfield BK, Davis J, Josephson CD, Winkler AM. Blood product transfusion and mortality in neonatal extracorporeal membrane oxygenation. Transfusion. 2020;60:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cashen K, Dalton H, Reeder RW, Saini A, Zuppa AF, Shanley TP, et al. Platelet Transfusion Practice and Related Outcomes in Pediatric Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med. 2020;21:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goobie SM, Faraoni D, Zurakowski D, DiNardo JA. Association of Preoperative Anemia With Postoperative Mortality in Neonates. JAMA Pediatr. 2016;170:855–62. [DOI] [PubMed] [Google Scholar]
- [29].Higgins RD, Patel RM, Josephson CD. Preoperative Anemia and Neonates. JAMA Pediatr. 2016;170:835–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mille FK, Badheka A, Yu P, Zhang X, Friedman DF, Kheir J, et al. Red Blood Cell Transfusion After Stage I Palliation Is Associated With Worse Clinical Outcomes. J Am Heart Assoc. 2020;9:e015304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gautam NK, Pierre J, Edmonds K, Pawelek O, Griffin E, Xu Z, et al. Transfusing Platelets During Bypass Rewarming in Neonates Improves Postoperative Outcomes: A Randomized Controlled Trial. World J Pediatr Congenit Heart Surg. 2020;11:71–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


