Abstract
Background and Purpose:
Clinicians will increasingly encounter adult patients who were born preterm and will need to understand their long-term sequelae. Adult survivors of preterm birth have been reported to have increased risks of hypertension and other stroke risk factors. However, their stroke risks have seldom been examined and the findings are discrepant, possibly due to small sample sizes, insufficient follow-up, or survivor bias. We examined whether preterm birth is associated with stroke in a large population-based cohort.
Methods:
A national cohort study was conducted of all 2,140,866 singletons born in Sweden during 1973–1994 who survived to age 18 years, who were followed up for first-time stroke through 2015 (maximum age 43). Cox regression was used to examine stroke risks associated with gestational age at birth, adjusting for other perinatal and parental factors. Co-sibling analyses assessed for potential confounding by shared familial (genetic and/or environmental) factors.
Results:
In 28.0 million person-years of follow-up, 4,861 (0.2%) persons were diagnosed with stroke. At ages 18–43 years, the adjusted hazard ratio (aHR) for stroke associated with preterm birth (<37 weeks) was 1.26 (95% CI, 1.12–1.43; P<0.001), and further stratified was 1.42 (1.11–1.81; P=0.005) for early preterm (22–33 weeks) and 1.22 (1.06–1.40; P=0.004) for late preterm (34–36 weeks), compared with full-term (39–41 weeks). Positive associations were found with both hemorrhagic stroke (early preterm: aHR, 1.42; 95% CI, 1.04–1.94; any preterm: 1.15; 0.97–1.35) and ischemic stroke (early preterm: 1.33; 0.87–2.03; any preterm: 1.31; 1.07–1.60). These findings were similar in men and women and only partially explained by shared determinants of preterm birth and stroke within families.
Conclusions:
In this large national cohort, preterm birth was associated with increased risks of both hemorrhagic and ischemic stroke in adulthood. Preterm birth survivors need early preventive evaluation and long-term clinical follow-up to reduce their lifetime risk of stroke.
Keywords: cerebrovascular disorders, hemorrhagic stroke, ischemic stroke, premature birth, preterm birth, stroke
Graphical Abstract
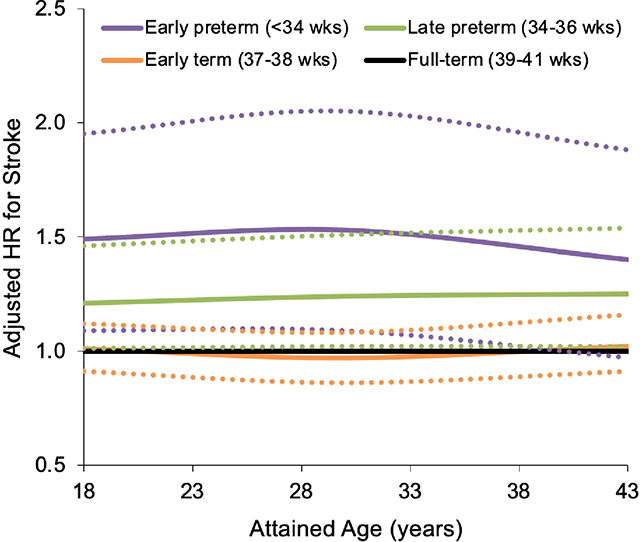
Adjusted hazard ratios (aHRs) for first-time stroke at ages 18-43 years by gestational age at birth, Sweden, 1973–2015 (dotted lines represent 95% CI). Early preterm (22–33 weeks) and late preterm (34–36 weeks) birth were associated with 1.4-fold (aHR, 1.42; 95% CI, 1.11-1.81; P=0.005) and 1.2-fold (aHR, 1.22; 1.06–1.40; P=0.004) risks of stroke, respectively, compared with full-term birth (39–41 weeks). Preterm birth should be recognized as a risk factor for stroke later in life. Preterm birth survivors may need early preventive evaluation and long-term clinical follow-up to reduce their lifetime risk of stroke.
INTRODUCTION
Preterm birth (gestational age <37 completed weeks) has a worldwide prevalence of nearly 11%, affecting 15 million births annually.1 Over 95% of preterm infants who receive modern neonatal and pediatric care now survive into adulthood,2, 3 and hence their long-term health outcomes have a growing clinical and public health importance. Adult survivors of preterm birth have been reported to have increased risks of hypertension,4–6 diabetes,7, 8 hyperlipidemia,9, 10 and ischemic heart disease,11 which are major risk factors for stroke.12 However, their long-term risks of stroke have seldom been examined and the findings are discrepant.13–17 Because stroke is a leading cause of death and disability worldwide,18 a better understanding of stroke risks is needed to guide long-term clinical care and prevention in the growing population of preterm birth survivors.
Some13–15 but not all16, 17 prior studies have suggested that preterm birth is associated with increased stroke incidence or mortality in adulthood. However, prior discrepant findings may be related to several methodologic issues, including small sample sizes, insufficient follow-up into adulthood, and/or potential survivor bias in the earliest birth cohorts. Most studies have been unable to assess risks of specific major types of stroke (hemorrhagic or ischemic). Furthermore, it is unclear whether previously reported associations might be due to confounding by shared familial (genetic and/or environmental) determinants of preterm birth and stroke, as opposed to direct effects of preterm birth. To our knowledge, this possibility has never been examined.
To address these knowledge gaps, we conducted a national cohort study of more than 2 million persons in Sweden. Our aims were to: (1) determine population-based risk estimates for stroke and its major types associated with gestational age at birth after up to 43 years of follow-up; (2) examine whether these associations vary according to sex, fetal growth, or birth year; and (3) assess for potential confounding by shared familial (genetic and/or environmental) factors using co-sibling analyses. We hypothesized that preterm birth is associated with increased risks of stroke and its major types in adulthood, and that these associations are largely independent of shared familial factors.
METHODS
Study Population
The Swedish Birth Register contains prenatal and birth information for nearly all births nationwide since 1973. Using this register, we identified all 2,242,969 singleton live births in Sweden during 1973–1994. These birth years were chosen to allow sufficient follow-up into adulthood. Singleton births were selected to improve internal comparability given the higher prevalence of preterm birth and its different underlying causes among multiple births. We excluded 1,213 (0.05%) persons who had a stroke diagnosis before age 18 years, 94,244 (4.2%) others who were no longer living in Sweden at age 18 years, and 6,646 (0.3%) others who had missing information for gestational age at birth. A total of 2,140,866 persons (95.4% of the original cohort) remained for inclusion in the study. This study was approved by the Regional Ethical Review Board in Lund, Sweden (No. 2020/627). Participant consent was not required as this study used only pseudonymized registry-based secondary data. Due to legal concerns, the supporting data (which come from a large portion of the Swedish population) cannot be made openly available. Further information about the data registers is available from the Swedish National Board of Health and Welfare (https://www.socialstyrelsen.se/en/statistics-and-data/registers/).
Gestational Age at Birth Ascertainment
Gestational age at birth was identified from the Swedish Birth Register based on maternal report of last menstrual period in the 1970s and ultrasound estimation starting in the 1980s and onward (>60% of the cohort). This was analyzed alternatively as a continuous variable or categorical variable with 5 groups based on the number of completed weeks of gestation: early preterm (22–33 weeks), late preterm (34–36 weeks), early term (37–38 weeks), full-term (39–41 weeks, used as the reference group), and post-term (≥42 weeks). In addition, the first 2 groups were combined to provide summary estimates for preterm birth (<37 weeks). Early term birth (37–38 weeks) was examined because it has previously been associated with increased risks of hypertension,4 diabetes,7 hyperlipidemia,9 ischemic heart disease,11 and cardiovascular mortality3, 19 in adulthood compared with full-term birth, but has not been examined in relation to stroke risk. There were too few adults born extremely preterm (22–27 weeks) and an insufficient number of strokes to allow separate analysis of this subgroup.
Stroke Ascertainment
The study cohort was followed up for the earliest diagnosis of stroke from age 18 years through December 31, 2015 (maximum age 43 years). Stroke was identified using International Classification of Diseases (ICD) codes from all primary and secondary diagnoses in the Swedish Hospital and Outpatient Registries and all deaths attributed to stroke in the Swedish Death Register (Table I in Supplemental Material). “Any stroke” was examined as the primary outcome, and hemorrhagic or ischemic strokes were examined separately as secondary outcomes. The Hospital Register started in 1964 and includes all primary and secondary hospital discharge diagnoses with nationwide coverage starting in 1987; these diagnoses have a reported positive predictive value and sensitivity for first-time stroke of >90%.20–22 The Swedish Outpatient Register contains all outpatient diagnoses from specialty clinics nationwide starting in 2001. The Swedish Death Register includes all deaths and causes of death for all persons registered in Sweden since 1960, with compulsory reporting nationwide.
Covariates
Other perinatal and parental characteristics that may be associated with gestational age at birth and stroke risk were identified using the Swedish Birth Register and national census data, which were linked using a pseudonymized personal identification number. Age was adjusted for in all analyses as the Cox model time scale (as described below). Covariates included the following: sex, birth year (continuous and categorical by decade), birth order (1, 2, ≥3), maternal and paternal age (continuous), maternal and paternal education level (<9, 10–11, ≥12 years), maternal birth country or region (Sweden, other Europe/US/Canada, Asia/Oceania, Africa, Latin America, other/unknown), maternal body mass index (BMI; continuous), maternal smoking (0, 1–9, ≥10 cigarettes/day), and maternal preeclampsia, other hypertensive disorders, or diabetes (gestational or pregestational types 1 or 2) (Table I in Supplemental Material).
Maternal BMI and smoking were assessed at the beginning of prenatal care starting in 1982 and were available for 36.9% and 55.6% of births, respectively. Data were >99% complete for all other variables. Missing data for each covariate were multiply imputed with 20 imputations using all other covariates and stroke as predictors.23 As alternatives to multiple imputation, sensitivity analyses were performed that (1) restricted to births with complete data for all covariates (N=744,083), or (2) coded missing data for each covariate as a separate category using a missing data indicator.
Statistical Analysis
Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between gestational age at birth and incident stroke and its major types (hemorrhagic or ischemic) at ages 18–43 years. Attained age was used as the Cox model time axis with age 18 years as “time zero”. Individuals were censored at death from causes other than stroke as identified in the Swedish Death Register (n=15,839; 0.7%) or emigration as determined by absence of a Swedish residential address in census data (n=131,532; 6.1%). Analyses were conducted both unadjusted and adjusted for covariates (as above). The proportional hazards assumption was assessed by examining log-log plots,24 and no substantial departures were found.
To assess changes in risk during adulthood, associations between gestational age at birth and stroke risk were examined in narrower intervals of attained age (18–29 and 30–43 years) among persons still living in Sweden without a prior diagnosis of stroke at the beginning of the respective interval. Sex-specific differences were assessed by performing sex-stratified analyses and examining potential interactions between preterm birth and sex on both the additive and multiplicative scale. Attributable fraction in the exposed (AFe) and population attributable fraction (PAF) also were computed for each gestational age group compared with full-term.
Co-sibling analyses were performed to assess for potential confounding by unmeasured shared familial (genetic and/or environmental) factors.3 Shared environmental factors in families may include lifestyle factors such as diet and physical activity, or ambient exposures such as passive smoking and air pollution. These analyses included all 1,797,851 (84.0%) individuals who had at least one sibling. Stratified Cox regression was used with a separate stratum for each set of siblings as identified by their mother’s pseudonymized identification number. In the stratified Cox model, each set of siblings had its own baseline hazard function reflecting their shared genetic and environmental factors, and thus associations between gestational age at birth and stroke were examined within the family. In addition, these analyses were further adjusted for the same covariates as in the main analyses. In a secondary analysis, stroke risks were assessed by comparing only the first preterm-born individual in each family (90.0% of all persons born preterm) to their full-term siblings.
Other secondary analyses were performed to examine: (1) whether associations between preterm birth and stroke risks are independent of fetal growth (defined as number of SD from the mean birth weight for gestational age and sex); (2) potential interactions between preterm birth and small for gestational age (SGA; defined as birth weight <10th percentile for gestational age) or birth year (by decade or < vs. ≥ 1985); and (3) stroke risks associated with spontaneous or medically indicated preterm birth (systematically recorded starting in 1990 [N=578,431 births], allowing follow-up to age 26 years). All statistical tests were 2-sided and used an α-level of 0.05. All analyses were conducted using Stata version 15.1.
RESULTS
Table 1 reports perinatal and parental characteristics by gestational age at birth. Preterm infants were more likely than full-term infants to be male or first-born, and their mothers were more likely to be at the extremes of age or foreign-born, have low education level or obesity, to smoke, or have preeclampsia, other hypertensive disorders, or diabetes during their pregnancy.
Table 1.
Characteristics of study participants by gestational age at birth (1973–1994), Sweden.
| Early preterm | Late preterm | Early term | Full-term | Post-term | |
|---|---|---|---|---|---|
| (<34 weeks) | (34–36 weeks) | (37–38 weeks) | (39–41 weeks) | (≥42 weeks) | |
| N=21,722 | N=80,183 | N=361,006 | N=1,478,910 | N=199,045 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Offspring characteristics | |||||
| Sex | |||||
| Male | 11,920 (54.9) | 44,027 (54.9) | 189,294 (52.4) | 750,452 (50.7) | 103,633 (52.1) |
| Female | 9,802 (45.1) | 36,156 (45.1) | 171,712 (47.6) | 728,458 (49.3) | 95,412 (47.9) |
| Birth year | |||||
| 1973–1979 | 5,497 (25.3) | 22,087 (27.5) | 90,517 (25.1) | 453,668 (30.7) | 86,040 (43.2) |
| 1980–1989 | 10,081 (46.4) | 37,661 (47.0) | 171,963 (47.6) | 638,928 (43.2) | 73,413 (36.9) |
| 1990–1994 | 6,144 (28.3) | 20,435 (25.5) | 98,526 (27.3) | 386,314 (26.1) | 39,592 (19.9) |
| Birth order | |||||
| 1 | 10,878 (50.1) | 38,727 (48.3) | 143,703 (39.8) | 605,838 (41.0) | 95,690 (48.1) |
| 2 | 6,146 (28.3) | 24,045 (30.0) | 131,017 (36.3) | 556,883 (37.6) | 66,345 (33.3) |
| ≥3 | 4,698 (21.6) | 17,411 (21.7) | 86,286 (23.9) | 316,189 (21.4) | 37,010 (18.6) |
| Maternal characteristics | |||||
| Age (years) | |||||
| <20 | 1,393 (6.4) | 4,516 (5.6) | 14,673 (4.1) | 57,993 (3.9) | 10,340 (5.2) |
| 20–24 | 5,531 (25.5) | 20,843 (26.0) | 86,189 (23.9) | 376,207 (25.4) | 56,179 (28.2) |
| 25–29 | 6,852 (31.5) | 27,259 (34.0) | 128,622 (35.6) | 563,712 (38.1) | 75,277 (37.8) |
| 30–34 | 4,982 (22.9) | 17,849 (22.3) | 87,613 (24.3) | 345,310 (23.4) | 42,202 (21.2) |
| 35–39 | 2,390 (11.0) | 7,947 (9.9) | 36,027 (10.0) | 116,791 (7.9) | 13,084 (6.6) |
| ≥40 | 574 (2.6) | 1,769 (2.2) | 7,882 (2.2) | 18,897 (1.3) | 1,963 (1.0) |
| Education (years) | |||||
| ≤9 | 4,215 (19.4) | 14,649 (18.3) | 59,633 (16.5) | 220,680 (14.9) | 32,700 (16.4) |
| 10–12 | 11,301 (52.0) | 41,085 (51.2) | 180,946 (50.1) | 736,965 (49.8) | 98,703 (49.6) |
| >12 | 6,206 (28.6) | 24,449 (30.5) | 120,427 (33.4) | 521,265 (35.3) | 67,642 (34.0) |
| Birth country or region | |||||
| Sweden | 18,838 (86.7) | 70,445 (87.9) | 316,185 (87.6) | 1,322,747 (89.4) | 179,536 (90.2) |
| Other Europe/US/Canada | 1,961 (9.0) | 6,622 (8.3) | 28,902 (8.0) | 108,628 (7.4) | 14,307 (7.2) |
| Asia/Oceania | 600 (2.8) | 2,120 (2.6) | 11,096 (3.1) | 31,902 (2.2) | 3,187 (1.6) |
| Africa | 123 (0.6) | 381 (0.5) | 1,725 (0.5) | 6,211 (0.4) | 988 (0.5) |
| Latin America | 113 (0.5) | 437 (0.5) | 2,476 (0.7) | 7,254 (0.5) | 717 (0.4) |
| Other/unknown | 87 (0.4) | 178 (0.2) | 622 (0.2) | 2,168 (0.1) | 310 (0.2) |
| Body mass index (kg/m2) | |||||
| <18.5 | 501 (2.3) | 2,565 (3.2) | 11,433 (3.2) | 36,603 (2.3) | 2,624 (1.3) |
| 18.5–24.9 | 19,898 (91.6) | 72,105 (89.9) | 322,661 (89.4) | 1,341,706 (90.7) | 183,877 (92.4) |
| 25.0–29.9 | 992 (4.6) | 4,265 (5.3) | 21,485 (5.9) | 83,021 (5.6) | 9,906 (5.0) |
| ≥30.0 | 331 (1.5) | 1,248 (1.6) | 5,427 (1.5) | 19,580 (1.3) | 2,638 (1.3) |
| Smoking (cigarettes/day) | |||||
| 0 | 12,665 (58.3) | 48,132 (60.0) | 229,283 (63.5) | 940,499 (63.6) | 115,956 (58.3) |
| 1–9 | 7,155 (32.9) | 25,962 (32.4) | 106,887 (29.6) | 460,183 (31.1) | 75,409 (37.9) |
| ≥10 | 1,902 (8.8) | 6,089 (7.6) | 24,836 (6.9) | 78,228 (5.3) | 7,680 (3.9) |
| Preeclampsia | 3,121 (14.4) | 7,973 (9.9) | 22,974 (6.4) | 66,215 (4.5) | 9,486 (4.8) |
| Other hypertensive disorders | 297 (1.4) | 824 (1.0) | 3,029 (0.8) | 8,285 (0.6) | 806 (0.4) |
| Diabetes mellitus | 631 (2.9) | 2,288 (2.8) | 6,838 (1.9) | 11,742 (0.8) | 1,167 (0.6) |
| Paternal characteristics | |||||
| Age (years) | |||||
| <25 | 3,715 (17.1) | 13,308 (16.6) | 50,393 (14.0) | 211,219 (14.3) | 33,221 16.7) |
| 25–29 | 6,538 (30.1) | 25,608 (31.9) | 114,865 (31.8) | 505,278 (34.2) | 71,051 (35.7) |
| 30–34 | 5,605 (25.8) | 21,697 (27.1) | 105,724 (29.3) | 440,978 (29.8) | 56,008 (28.1) |
| 35–39 | 3,293 (15.2) | 11,646 (14.5) | 56,100 (15.5) | 209,203 (14.1) | 24,783 (12.5) |
| 40–44 | 1,430 (6.6) | 4,701 (5.9) | 21,464 (6.0) | 71,926 (4.9) | 8,549 (4.3) |
| ≥45 | 659 (3.0) | 2,157 (2.7) | 9,160 (2.5) | 29,464 (2.0) | 3,615 (1.8) |
| Unknown | 482 (2.2) | 1,066 (1.3) | 3,300 (0.9) | 10,842 (0.7) | 1,818 (0.9) |
| Education (years) | |||||
| ≤9 | 5,743 (26.4) | 21,109 (26.3) | 89,082 (24.7) | 355,101 (24.0) | 51,383 (25.8) |
| 10–12 | 10,414 (47.9) | 38,407 (47.9) | 172,609 (47.8) | 702,457 (47.5) | 92,574 (46.5) |
| >12 | 5,074 (23.4) | 19,624 (24.5) | 96,114 (26.6) | 410,792 (27.8) | 53,275 (26.8) |
| Unknown | 491 (2.3) | 1,043 (1.3) | 3,201 (0.9) | 10,560 (0.7) | 1,813 (0.9) |
In 28.0 million person-years of follow-up, 4,861 (0.2%) persons were diagnosed with stroke at ages 18–43 years. The median age at stroke diagnosis was 29.4 (mean 29.4 ± 6.2) and at end of follow-up was 31.9 (mean 32.5 ± 6.6) years. Stroke incidence rates by gestational age at birth are reported in Table 2.
Table 2.
Associations between gestational age at birth (1973–1994) and risk of any stroke (1991–2015), Sweden.
| Unadjusted | Adjusted* | AFe† | PAR‡ | ||||
|---|---|---|---|---|---|---|---|
| Cases | Rate§ | HR (95% CI) | HR (95% CI) | P | (%) | (%) | |
| Attained ages 18–43 years | |||||||
| Preterm (<37 wks) | 283 | 21.9 | 1.31 (1.16, 1.48) | 1.26 (1.12, 1.43) | <0.001 | 21.8 | 1.7 |
| Early preterm (<34 wks) | 66 | 24.7 | 1.49 (1.17, 1.90) | 1.42 (1.11, 1.81) | 0.005 | 30.7 | 0.6 |
| Late preterm (34–36 wks) | 217 | 21.2 | 1.26 (1.10, 1.45) | 1.22 (1.06, 1.40) | 0.004 | 19.1 | 1.2 |
| Early term (37–38 wks) | 767 | 17.1 | 1.04 (0.96, 1.12) | 1.01 (0.94, 1.10) | 0.75 | 0.0 | 0.0 |
| Full-term (39–41 wks) | 3,303 | 17.1 | Reference | Reference | |||
| Post-term (≥42 wks) | 508 | 17.3 | 0.95 (0.86, 1.04) | 0.96 (0.87, 1.05) | 0.40 | 1.2 | 0.2 |
| Per additional week (trend) | 0.97 (0.95, 0.98) | 0.97 (0.96, 0.99) | <0.001 | ||||
| Attained ages 18–29 years | |||||||
| Preterm (<37 wks) | 161 | 16.4 | 1.31 (1.12, 1.54) | 1.26 (1.07, 1.48) | 0.005 | 23.8 | 2.0 |
| Early preterm (<34 wks) | 40 | 19.6 | 1.57 (1.15, 2.15) | 1.48 (1.08, 2.03) | 0.01 | 35.9 | 0.8 |
| Late preterm (34–36 wks) | 121 | 15.6 | 1.25 (1.04, 1.50) | 1.20 (1.00, 1.45) | 0.05 | 19.8 | 1.3 |
| Early term (37–38 wks) | 449 | 13.0 | 1.04 (0.94, 1.16) | 1.01 (0.91, 1.12) | 0.89 | 3.8 | 0.8 |
| Full-term (39–41 wks) | 1,790 | 12.5 | Reference | Reference | |||
| Post-term (≥42 wks) | 229 | 11.3 | 0.89 (0.78, 1.02) | 0.93 (0.81, 1.07) | 0.30 | (9.5)|| | (1.2)# |
| Per additional week (trend) | 0.96 (0.94, 0.98) | 0.97 (0.95, 0.99) | 0.004 | ||||
| Attained ages 30–43 years | |||||||
| Preterm (<37 wks) | 122 | 38.9 | 1.30 (1.08, 1.56) | 1.27 (1.06, 1.53) | 0.01 | 22.4 | 1.7 |
| Early preterm (<34 wks) | 26 | 41.3 | 1.38 (0.93, 2.03) | 1.34 (0.91, 1.98) | 0.14 | 27.0 | 0.5 |
| Late preterm (34–36 wks) | 96 | 38.3 | 1.28 (1.04, 1.57) | 1.25 (1.02, 1.54) | 0.03 | 21.1 | 1.3 |
| Early term (37–38 wks) | 318 | 30.6 | 1.02 (0.91, 1.16) | 1.02 (0.91, 1.16) | 0.70 | 1.5 | 0.3 |
| Full-term (39–41 wks) | 1,513 | 30.2 | Reference | Reference | |||
| Post-term (≥42 wks) | 279 | 30.5 | 1.00 (0.88, 1.14) | 0.99 (0.87, 1.12) | 0.86 | 1.2 | 0.2 |
| Per additional week (trend) | 0.98 (0.95, 1.00) | 0.98 (0.95, 1.00) | 0.04 | ||||
Adjusted for child characteristics (age, sex, birth year, birth order), maternal characteristics (age, education, birth country or region, BMI, smoking, preeclampsia, other hypertensive disorders, diabetes), and paternal characteristics (age, education).
Attributable fraction among the exposed.
Population attributable fraction.
Stroke incidence rate per 100,000 person-years.
Prevented fraction among the exposed.
Population prevented fraction.
Associations Between Gestational Age at Birth and Stroke
Low gestational age at birth was associated with significantly higher risks of stroke at ages 18–43 years. Adjusted HRs for any stroke associated with early preterm (22–33 weeks) or late preterm (34–36 weeks) birth were 1.42 (95% CI, 1.11–1.81: P=0.005) and 1.22 (1.06–1.40; P=0.004), respectively, compared with full-term. Early term birth (37–38 weeks) was not associated with increased risk. Each additional week of gestation was associated with a 3% lower risk of stroke on average (adjusted HR per additional week, 0.97; 95% CI, 0.96–0.99; P<0.001; Table 2). In addition, adjusted HRs appeared stable across different intervals of attained age in adulthood (e.g., adjusted HR at ages 18–29 years comparing preterm vs. full-term: 1.26; 95% CI, 1.07–1.48; P=0.005; ages 30–43 years: 1.27; 1.06–1.53; P=0.01; Table 2). Most adjusted HRs were only slightly lower than unadjusted HRs (Table 2).
Associations between gestational age at birth and stroke risk were similar among men and women (Table II in Supplemental Material). No interactions were found between preterm birth and sex on either the additive (P=0.93) or multiplicative (P=0.96) scale (Table III in Supplemental Material), and hence the main results were not stratified by sex. The Graphic Abstract shows adjusted HRs and 95% CIs for any stroke at ages 18–43 years by gestational age group. At these attained ages, an estimated 21.8% of all strokes among persons born preterm, and 1.7% of all strokes in this population, were related to preterm birth (Table 2, AFe and PAR).
Hemorrhagic and Ischemic Stroke
Low gestational age at birth was associated with higher risks of both hemorrhagic stroke (early preterm: adjusted HR, 1.42; 95% CI, 1.04–1.94; any preterm: 1.15; 0.97–1.35) and ischemic stroke (early preterm: 1.33; 0.87–2.03; any preterm: 1.31; 1.07–1.60), compared with full-term birth (Table 3). The associations with hemorrhagic and ischemic stroke were overall similar in magnitude. Risk estimates for hemorrhagic and ischemic stroke did not differ significantly from each other when comparing any preterm (P=0.33) or early preterm (P=0.06) vs. full-term birth.
Table 3.
Associations between gestational age at birth (1973–1994) and risk of hemorrhagic or ischemic stroke (1991–2015), Sweden.
| Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|
| Cases | Rate† | HR (95% CI) | HR (95% CI) | P | |
| Hemorrhagic stroke | |||||
| Preterm (<37 wks) | 156 | 12.1 | 1.20 (1.02, 1.42) | 1.15 (0.97, 1.35) | 0.10 |
| Early preterm (<34 wks) | 40 | 15.0 | 1.51 (1.10, 2.06) | 1.42 (1.04, 1.94) | 0.03 |
| Late preterm (34–36 wks) | 116 | 11.3 | 1.13 (0.93, 1.36) | 1.08 (0.89, 1.30) | 0.44 |
| Early term (37–38 wks) | 469 | 10.5 | 1.05 (0.95, 1.17) | 1.02 (0.92, 1.13) | 0.73 |
| Full-term (39–41 wks) | 1,199 | 10.2 | Reference | Reference | |
| Post-term (≥42 wks) | 319 | 10.8 | 1.00 (0.89, 1.13) | 1.01 (0.90, 1.14) | 0.83 |
| Per additional week (trend) | 0.96 (0.94, 0.99) | 0.98 (0.96, 1.00) | 0.07 | ||
| Ischemic stroke | |||||
| Preterm (<37 wks) | 104 | 8.0 | 1.35 (1.11, 1.66) | 1.31 (1.07, 1.60) | 0.009 |
| Early preterm (<34 wks) | 22 | 8.2 | 1.40 (0.92, 2.14) | 1.33 (0.87, 2.03) | 0.18 |
| Late preterm (34–36 wks) | 82 | 8.0 | 1.34 (1.07, 1.68) | 1.30 (1.04, 1.63) | 0.02 |
| Early term (37–38 wks) | 271 | 6.0 | 1.04 (0.91, 1.19) | 1.02 (0.89, 1.16) | 0.78 |
| Full-term (39–41 wks) | 1,180 | 6.1 | Reference | Reference | |
| Post-term (≥42 wks) | 174 | 5.9 | 0.89 (0.76, 1.04) | 0.90 (0.76, 1.05) | 0.18 |
| Per additional week (trend) | 0.96 (0.94, 0.99) | 0.97 (0.94, 0.99) | 0.01 | ||
Adjusted for child characteristics (age, sex, birth year, birth order), maternal characteristics (age, education, birth country or region, BMI, smoking, preeclampsia, other hypertensive disorders, diabetes), and paternal characteristics (age, education).
Incidence rate per 100,000 person-years.
Co-Sibling Analyses
Co-sibling analyses to control for unmeasured shared familial factors resulted in moderate (<50%) attenuation of risk estimates for any stroke (Table 4). For example, comparing preterm with full-term birth, the adjusted HRs for any stroke at ages 18–43 years were 1.26 (95% CI, 1.12–1.43) in the primary analysis vs. 1.16 (0.92–1.47) in the co-sibling analysis. The co-sibling analyses had reduced statistical power compared with the main analyses, resulting in wider confidence intervals.
Table 4.
Co-sibling analyses for gestational age at birth (1973–1994) in relation to stroke risk (1991–2015), Sweden.
| Cases | HR (95% CI)* | |
|---|---|---|
| Any stroke | ||
| Preterm (<37 wks) | 215 | 1.16 (0.92, 1.47) |
| Early term (37–38 wks) | 598 | 1.07 (0.92, 1.24) |
| Full-term (39–41 wks) | 2,509 | Reference |
| Per additional week (trend) | 0.98 (0.95, 1.01) | |
| Hemorrhagic stroke | ||
| Preterm (<37 wks) | 117 | 1.05 (0.77, 1.45) |
| Early term (37–38 wks) | 365 | 1.06 (0.88, 1.29) |
| Full-term (39–41 wks) | 1,526 | Reference |
| Per additional week (trend) | 1.00 (0.96, 1.04) | |
| Ischemic stroke | ||
| Preterm (<37 wks) | 79 | 1.17 (0.79, 1.73) |
| Early term (37–38 wks) | 207 | 1.09 (0.83, 1.43) |
| Full-term (39–41 wks) | 869 | Reference |
| Per additional week (trend) | 0.96 (0.91, 1.01) |
Adjusted for shared familial (genetic and/or environmental) factors in addition to specific child characteristics (age, sex, birth year, birth order), maternal characteristics (age, education, birth country or region, BMI, smoking, preeclampsia, other hypertensive disorders, diabetes), and paternal characteristics (age, education).
Co-sibling analyses of hemorrhagic and ischemic stroke also resulted in moderate attenuation of risk estimates (Table 4). For example, comparing preterm with full-term births, the adjusted HRs for hemorrhagic stroke were 1.15 (95% CI, 0.97–1.35) in the primary analysis vs. 1.05 (0.77–1.45) in the co-sibling analysis, and for ischemic stroke were 1.31 (1.07–1.60) vs. 1.17 (0.79–1.73). Overall, these findings suggest that the associations observed in the main analyses were only partially explained by familial (genetic and/or environmental) factors that are shared determinants of preterm birth and stroke.
Secondary Analyses
In sensitivity analyses that examined alternatives to multiple imputation for missing data, all results were similar to the main findings and the conclusions were unchanged. Results from these and all other secondary analyses are reported in Supplemental Results and Tables IV–VII in Supplemental Material.
DISCUSSION
In this large national cohort, preterm birth was associated with increased risk of first-time stroke in adulthood. Early preterm (22–33 weeks) and late preterm (34–36 weeks) birth were associated with 1.4- and 1.2-fold risks, respectively, at ages 18–43 years. Similar associations were found in men and women and for both hemorrhagic and ischemic stroke. These findings were independent of covariates and only partially explained by shared genetic or environmental determinants of preterm birth and stroke within families, suggesting important direct effects of preterm birth.
To our knowledge, this is the largest study to date of preterm birth in relation to stroke risks, and the first with sufficient power to assess stroke types or potential familial confounding in co-sibling analyses. Prior studies have yielded inconsistent findings, possibly due to smaller sample sizes, different follow-up times, or other methodologic differences. The largest previous study included 1.3 million persons born in Sweden in 1983–1995 who were followed up through 2010 (maximum age 28 years).13 That study reported a non-significant 1.8-fold risk of stroke among those born at <32 weeks (adjusted HR, 1.81; 95% CI, 0.90–3.65) but no increased risk at 32–36 weeks compared with 37–41 weeks, and did not examine specific types of stroke.13 A cohort study of 10,803 women born in Scotland in 1950–1956 who were followed up through 2003 reported a significant inverse linear association between gestational age at birth and stroke risk (adjusted HR per additional week of gestation, 0.79; 95% CI, 0.71–0.88), but did not examine specific gestational age groups compared with full-term.14 In contrast, no association between gestational age at birth and stroke risk was found in a Finnish cohort of 12,439 persons born in 1924–1934 who were followed up through 2003,16 nor in an overlapping cohort of 19,015 persons born in 1924–1944 who were followed up through 2010.17 These studies of earlier birth cohorts preceding modern neonatal care may potentially be affected by survivor bias, wherein the infants who survived may have been healthier and less susceptible to stroke than more recent preterm birth survivors. However, a cohort study of 14,193 persons born in Sweden in 1915–1929 who were followed up through 2001 (maximum age 87 years) reported that low gestational age at birth was associated with increased mortality from stroke and specifically ischemic stroke.15
The present study extends prior evidence by determining stroke risks in a large national cohort with >5-fold as many strokes as in previous studies, affording the statistical power needed to assess hemorrhagic and ischemic stroke risks, sex-specific differences, and familial confounding. Our findings are consistent with previously reported associations between preterm birth and cardiometabolic disorders that are known risk factors for stroke, including hypertension,4–6 diabetes,7, 8 and hyperlipidemia,9, 10 as well as ischemic heart disease11 and cardiovascular mortality3, 25 in adulthood. We also found that associations between preterm birth and stroke risk were slightly stronger among persons born more recently (Table V in Supplemental Material). This trend may be partially related to survivor bias among those born in earlier years, and/or improved diagnosis of stroke or more complete ascertainment of diagnoses among those born in later years.
Multiple underlying mechanisms may potentially link preterm birth with increased stroke risks. Preterm birth interrupts fetal angiogenesis during a critical developmental period, leading to reduced capillary density and increased arterial stiffness.26, 27 Preterm birth also has been associated with persistently elevated levels of antiangiogenic factors (e.g., soluble endoglin [sENG] and soluble fms-like tyrosine kinase-1 [sFlt-1]), which are correlated with blood pressure.28 Increases in placental-derived sENG and sFlt-1 have been linked with capillary rarefaction,29, 30 which is a major determinant of vascular resistance and may lead to hypertension,31, 32 a major risk factor for both hemorrhagic and ischemic stroke.12 Co-sibling analyses in the present study further suggest a partial role of shared familial factors. Family- and twin-based studies have suggested that genetic factors inherited primarily from the mother influence gestational age at birth, with an estimated heritability of 25–40%.33–35 Substantial maternal genetic correlation also has been reported between birth weight (a composite of gestational age and fetal growth) and stroke risk in the offspring.36 Additional clinical and genetic studies are needed to further delineate the mechanisms linking preterm birth with later development of stroke, which may eventually facilitate new preventive interventions in this higher-risk population.
The prevalence of preterm birth is currently 5–9% in most European countries, 10% in the US, and nearly 11% worldwide.1 Even a modestly increased risk of stroke in adults who were born preterm may have important clinical and public health implications. Our findings suggest that preterm birth survivors need early preventive evaluation and long-term clinical follow-up into adulthood to reduce their lifetime risk of stroke. More aggressive reduction of other modifiable risk factors may be needed, including obesity, hypertension, diabetes, hyperlipidemia, smoking, and physical inactivity. In patients of all ages, medical records and history-taking should routinely include gestational age at birth to facilitate preventive actions (including healthy lifestyle counseling) across the life course in those born preterm.37
Strengths and Limitations
A key strength of this study was the ability to examine stroke risks associated with preterm birth in a large national cohort of adults who were born during the modern neonatal care era, using highly complete birth and medical registry data. This study design minimizes potential selection or ascertainment biases. The large sample size afforded the statistical power needed to assess narrowly defined gestational age groups and major stroke types. The results were controlled for many perinatal and parental factors, as well as unmeasured shared familial exposures using co-sibling analyses.
Limitations include the lack of detailed clinical records needed to verify stroke diagnoses, although positive predictive values >90% have been reported using Swedish registry data.20–22 Maternal report of last menstrual period (used mainly in the 1970s) may overestimate gestational age by an average of ~2 days,38 causing a slight conservative bias to risk estimates. Lifestyle factors later in life may be important modifiers of stroke risk after preterm birth and should be explored in future studies with access to this information. Paternal smoking and obesity information also was unavailable and may be useful to assess in future studies. Additional follow-up to older ages when stroke is more common will be needed in this or other large cohorts. Lastly, the present study was limited to Sweden and will need replication in other countries when feasible, including racially diverse populations to explore for heterogeneity of findings in racial/ethnic subgroups.
Summary
In this large national cohort, preterm birth was associated with increased risks of both hemorrhagic and ischemic stroke among men and women at ages 18–43 years. These associations were only partially explained by shared familial factors, suggesting important direct effects of preterm birth. Preterm birth should be recognized as a risk factor for stroke later in life. Preterm birth survivors need early preventive evaluation and long-term clinical follow-up to reduce their lifetime risk of stroke.
Supplementary Material
ACKNOWLEDGMENTS
Sources of Funding: This work was supported by the National Heart, Lung, and Blood Institute at the NIH [R01 HL139536 to C.C. and K.S.]; the Swedish Research Council; the Swedish Heart-Lung Foundation; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the writing of the manuscript or the decision to submit it for publication.
Non-standard Abbreviations and Acronyms
- AFe
attributable fraction in the exposed
- BMI
body mass index
- HR
hazard ratio
- ICD
International Classification of Diseases
- PAR
population attributable risk
Footnotes
Disclosures: None.
REFERENCES
- 1.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump C, Winkleby MA, Sundquist J, Sundquist K. Prevalence of survival without major comorbidities among adults born prematurely. JAMA. 2019;322:1580–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crump C, Sundquist J, Winkleby MA, Sundquist K. Gestational age at birth and mortality from infancy into mid-adulthood: A national cohort study. Lancet Child Adolesc Health. 2019;3:408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump C, Sundquist J, Sundquist K. Risk of hypertension into adulthood in persons born prematurely: A national cohort study. Eur Heart J. 2020;41:1542–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of hypertension among young adults who were born preterm: A swedish national study of 636,000 births. Am J Epidemiol. 2011;173:797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: A national cohort study. Diabetologia. 2020;63:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of diabetes among young adults born preterm in sweden. Diabetes Care. 2011;34:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump C, Sundquist J, Sundquist K. Association of preterm birth with lipid disorders in early adulthood: A swedish cohort study. PLoS Med. 2019;16:e1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: A systematic review and meta-analysis. Pediatrics. 2013;131:e1240–1263 [DOI] [PubMed] [Google Scholar]
- 11.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr. 2019;173:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation. 2020;141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 13.Ueda P, Cnattingius S, Stephansson O, Ingelsson E, Ludvigsson JF, Bonamy AK. Cerebrovascular and ischemic heart disease in young adults born preterm: A population-based swedish cohort study. Eur J Epidemiol. 2014;29:253–260 [DOI] [PubMed] [Google Scholar]
- 14.Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: Findings from the aberdeen children of the 1950s prospective cohort study. Circulation. 2005;112:1414–1418 [DOI] [PubMed] [Google Scholar]
- 15.Koupil I, Leon DA, Lithell HO. Length of gestation is associated with mortality from cerebrovascular disease. J Epidemiol Community Health. 2005;59:473–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: The helsinki birth cohort study. Stroke. 2007;38:264–270 [DOI] [PubMed] [Google Scholar]
- 17.Kajantie E, Osmond C, Eriksson JG. Coronary heart disease and stroke in adults born preterm - the helsinki birth cohort study. Paediatr Perinat Epidemiol. 2015;29:515–519 [DOI] [PubMed] [Google Scholar]
- 18.Global Burden of Disease 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:439–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crump C, Sundquist K, Winkleby MA, Sundquist J. Early-term birth (37–38 weeks) and mortality in young adulthood. Epidemiology. 2013;24:270–276 [DOI] [PubMed] [Google Scholar]
- 20.Koster M, Asplund K, Johansson A, Stegmayr B. Refinement of swedish administrative registers to monitor stroke events on the national level. Neuroepidemiology. 2013;40:240–246 [DOI] [PubMed] [Google Scholar]
- 21.Merlo J, Lindblad U, Pessah-Rasmussen H, Hedblad B, Rastam J, Isacsson SO, Janzon L, Råstam L. Comparison of different procedures to identify probable cases of myocardial infarction and stroke in two swedish prospective cohort studies using local and national routine registers. Eur J Epidemiol. 2000;16:235–243 [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 24.Grambsch PM. Goodness-of-fit and diagnostics for proportional hazards regression models. Cancer Treat Res. 1995;75:95–112 [DOI] [PubMed] [Google Scholar]
- 25.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA. 2011;306:1233–1240 [DOI] [PubMed] [Google Scholar]
- 26.Bonamy AK, Andolf E, Martin H, Norman M. Preterm birth and carotid diameter and stiffness in childhood. Acta Paediatr. 2008;97:434–437 [DOI] [PubMed] [Google Scholar]
- 27.Bonamy AK, Martin H, Jorneskog G, Norman M. Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med. 2007;262:635–642 [DOI] [PubMed] [Google Scholar]
- 28.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, et al. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614 [DOI] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891 [DOI] [PubMed] [Google Scholar]
- 31.Shore AC, Tooke JE. Microvascular function in human essential hypertension. J Hypertens. 1994;12:717–728 [PubMed] [Google Scholar]
- 32.Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003;89:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000;107:375–381 [DOI] [PubMed] [Google Scholar]
- 34.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: Maternal and fetal contributions. Am J Epidemiol. 2008;167:474–479 [DOI] [PubMed] [Google Scholar]
- 35.Svensson AC, Sandin S, Cnattingius S, Reilly M, Pawitan Y, Hultman CM, Lichtenstein P. Maternal effects for preterm birth: A genetic epidemiologic study of 630,000 families. Am J Epidemiol. 2009;170:1365–1372 [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Tang Z, Yu X, Gao Y, Guan F, Li C, Huang S, Zheng J, Zeng P. Birth weight and stroke in adult life: Genetic correlation and causal inference with genome-wide association data sets. Front Neurosci. 2020;14:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crump C Medical history taking in adults should include questions about preterm birth. BMJ. 2014;349:g4860. [DOI] [PubMed] [Google Scholar]
- 38.Hogberg U, Larsson N. Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand. 1997;76:907–912 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


