Abstract
Background:
Relapse after allogeneic hematopoietic cell transplantation (HCT) leads to poor survival in patients with acute myeloid leukemia (AML). A second HCT (HCT2) may achieve durable remission.
Objectives:
To determine the outcomes of patients who received an HCT2 for relapsed AML and to evaluate the predictors of overall survival (OS) and progression-free survival (PFS).
Study Design:
We retrospectively reviewed medical records of adult patients who underwent an HCT2 for relapsed AML at our institution during 2000–2019.
Results:
Ninety-one patients were identified with a median age of 44 years (range, 18–73) at HCT2. Donor types were HLA-identical sibling (n=37, 41%), HLA-matched-unrelated (n=34, 37%), haploidentical (n=19, 21%), and cord-blood (n=1, 1%). Donors were different at HCT2 in 53% of patients. The majority of patients received reduced intensity conditioning (n=71, 78%) and were in remission (n=56, 61%) at HCT2. The median remission duration after HCT1 was 8.4 months (range, 1–70) and the median time between transplants was 14 months (range, 3–73). The median follow-up of surviving patients after HCT2 was 66 months (range, 2–171), with 32% alive at time of analysis. The most common cause of death was disease recurrence (n=45, 73%). At 2 years, the rates of OS, PFS, progression, and non-relapse mortality were 36%, 27%, 42%, and 18%, respectively. The development of chronic GVHD after first HCT and HCT comorbidity index (HCT-CI) ≥2 at HCT2 were associated with inferior PFS and OS after HCT2.
Conclusion:
A second HCT is feasible in selected patients with AML who have relapsed after HCT1. Long-term survival benefit is possible in patients without chronic GVHD after HCT1 and HCT-CI <2 at HCT2.
Keywords: Acute myeloid leukemia, second allogeneic stem cell transplantation, survival
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative option for acute myeloid leukemia (AML). Although patients can achieve long-term disease-free remissions after HCT, disease relapse remains a major cause of treatment failure and generally leads to poor survival, with only 10–20% of patients in relapse surviving beyond two years1. However, a second HCT can achieve durable remissions in a subset of patients with relapsed AML2, 3.
Several studies have reported the major predictors of outcome after a second HCT are the duration of remission after first HCT and the status of disease at time of second HCT4–7. Age at second HCT, development of graft versus host disease (GVHD) after first HCT, and donor type have also been reported as predictors of outcomes1, 4, 8, 9. Notably, these studies included both adult and pediatric patients, included a variety of acute and chronic leukemias, and largely used human leukocyte antigen (HLA)-matched sibling donors. Moreover, they included patients transplanted over a decade ago (Supplemental Table I). Throughout the last decade, donor choice has changed with increased use of HLA-haploidentical donors, more therapeutic agents for relapsed AML have become available, and maintenance therapy following transplant has become more common. Herein, we retrospectively analyzed the outcomes of relapsed AML patients who received a second HCT at our institution within the last two decades and additionally sought to investigate any potential impact of time-period on transplant outcomes.
Materials and Methods
Patient and study design
The University of Texas MD Anderson Cancer Center Institutional Review Board approved this retrospective analysis. All patients provided written informed consent for transplantation in accordance with the Declaration of Helsinki. Adult patients ≥ 18 years of age who received a second HCT for relapsed AML from January 1, 2000 until August 1, 2019 were identified through a retrospective review of our clinical database. All relevant demographic, clinical, laboratory and pathologic data were retrospectively abstracted.
Definitions and clinical end points
AML was diagnosed according to the 2016 World Health Organization (WHO) criteria for hematological malignancies10. Cytogenetic risk stratification was based on the 2017 European LeukemiaNet (ELN) guidelines11. The response assessment was based on the revised criteria defined by the International Working Group for AML12.
Haploidentical donors were defined as having two or more mismatches from a related donor. Myeloablative (MAC) and reduced intensity conditioning regimens (RIC) were defined according to the Center for International Blood and Marrow Transplant Research (CIBMTR) criteria13. Acute GVHD was staged and graded according to the criteria published by Przepiorka et al14. Chronic GVHD was reported as limited and/or extensive based on the criteria published by Sullivan et al15.
Evaluation of outcomes and statistical analysis
The primary outcome of interest was overall survival (OS), defined by the time from second HCT to death or last known follow-up. Secondary outcomes included cumulative incidence of progression, progression free survival (PFS), defined as disease progression or death following the second HCT, non-relapse mortality (NRM), defined as death without recurrent or progressive disease after second HCT, and acute and chronic GVHD. Actuarial OS and PFS were estimated using the Kaplan-Meier method. Observations were censored at the time of last follow-up or third HCT (n=3), when applicable. The cumulative incidence of disease progression, NRM, and GVHD were estimated to account for competing risks. Univariate analyses were performed for PFS, OS, NRM and progression; multivariate analyses were performed for OS and PFS using Cox proportional hazard models. Backward elimination was used to develop multivariate prognostic models. First degree interaction effects were evaluated and accounted for in the regression analysis, as indicated. Characteristics were compared using Chi-square and Fisher’s exact tests for categorical variables, and Wilcoxon’s rank-sum test was used for continuous variables. Statistical significance was defined at the .05 level and statistical analyses were primarily performed using Stata 9.0 (College Station, TX).
Results
Patient characteristics at second HCT
A total of 91 patients were included in this study. Each received an HCT for AML and subsequently received a second HCT for relapsed disease from January 2000 through August 2019 at our institution (Supplemental Figure 1). Patient, disease, and transplant characteristics at time of first and second HCT are presented in Table I. The median age at time of second HCT was 43 years (range, 18–73 years) and 48 (53%) of the patients were male. Fifty-six patients (61%) were in complete remission (CR) with or without hematologic recovery (CRi) at the time of second HCT, whereas 22 (24%) were transplanted with active disease. A total of 13 (14%) patients had either aplastic marrow (n=8, 9%) or received salvage treatment following the most recent marrow evaluation, precluding disease evaluation (n=5, 5%). The HCT comorbidity index (HCT-CI) was 2 or higher in approximately half of the patients. The median remission duration after the first HCT was 8.4 months (range, 1–70 months) and patients proceeded to a second HCT at a median of 14 months (range, 3–73 months) following the first HCT.
Table I.
Baseline patient and transplant characteristics, N=91.
| Variables | First HCT, N (%) | Second HCT, N (%) |
|---|---|---|
| Median age at HCT in years (range) | 43 (18–67) | 44 (18–73) |
| Males | 55 (60) | 48 (53) |
| Disease status at HCT | ||
| CR/CRi | 58 (64) | 56 (61) |
| Persistent disease | 29 (32) | 22 (24) |
| Unevaluable* | 4 (4) | 13 (15) |
| HCT Comorbidity index | ||
| 0–1 | - | 45 (49) |
| ≥2 | - | 46 (51) |
| Remission duration of first HCT, months (range) | 8.4 (1–70) | NA |
| Median time from first to second HCT, months (range) | NA | 14 (3–73) |
| Change in donors | NA | 48 (53) |
| Median calendar year of HCT (range) | 2009 (2000–2018) | 2010 (2000–2019) |
| Conditioning intensity | ||
| MAC (Bu>4000) | 61 (67) | 20 (22) |
| RIC/NMA | 30 (33) | 71 (78) |
| Bu<4000 | 5 (5) | 3 (3) |
| Mel | 23 (25) | 59 (65) |
| 100 | 4 (4) | 16 (18) |
| 140 | 19 (21) | 43 (47) |
| Other | 2 (3) | 9 (10) |
| Graft source | ||
| Peripheral blood | 51 (56) | 71 (78) |
| Bone marrow | 34 (37) | 19 (21) |
| Umbilical cord blood | 6 (7) | 1 (1) |
| Donor relation | ||
| Mismatched unrelated (cord) | 6 (7) | 1 (1) |
| Matched sibling donor | 42 (46) | 37 (41) |
| 10/10 Matched unrelated donor | 33 (36) | 32 (35) |
| 9/10 Matched unrelated donor | 4 (4) | 2 (2) |
| Mismatched family (haploidentical) | 6 (7) | 19 (21) |
| GVHD prophylaxis | ||
| Tacrolimus + MTX | 75 (82) | 63 (69) |
| PTCy +/− Tacrolimus +/− MMF | 15 (17) | 25 (28) |
| Other | 1 (1) | 3 (3) |
| Acute GVHD | 39 (43) | 44 (48) |
| Grade 2–4 | 18 (20) | 32 (35) |
| Grade 3–4 | 4 (4) | 13 (14) |
| Chronic GVHD | 17 (19)** | 25 (27) |
Abbreviations: HCT=hematopoietic cell transplantation; CR=complete response; CRi=complete response with incomplete hematologic recovery; MAC=myeloablative conditioning; RIC=reduced intensity conditioning; NMA=non-myeloablative conditioning; BU=busulfan; Mel=melphalan; GVHD=graft-versus-host disease; MTX=methotrexate; MMF=mycophenolate mofetil; PTCy=posttransplant cyclophosphamide.
Includes patients with 1) aplastic marrow insufficient for analysis and/or 2) patients who received a salvage treatment following the most recent disease evaluation.
Includes 9 patients with limited and 8 patients with extensive chronic GVHD.
Transplant characteristics at second HCT
The majority of patients (78%) received a RIC regimen consisting largely of melphalan (100–140 mg/m2) as a single dose with fludarabine (40 mg/m2) given for 4 days (n=59) or intravenous busulfan at a fixed dose of 100 mg/m2 or targeting an area under the concentration versus time curve (AUC) of 4,000 μMol-min ±10% (n=3). Twenty patients (22%) received a MAC preparative regimen consisting of intravenous busulfan either at a dose calculated to target an AUC of 5,000–6,000 μMol-min ±10%, or 130 mg/m2 in combination with fludarabine (40 mg/m2) given daily for 4 days.
Forty-eight (53%) patients switched to a different donor for the second HCT (Table I). Most donors were matched siblings (41%) or matched (9/10 or 10/10) unrelated donors (37%). The majority of mismatched donors were haploidentical (21% of the total patient population). The primary donor source was peripheral blood stem cells (78%), followed by bone marrow (21%) and cord blood (1%). GVHD prophylaxis was primarily (n=85, 97%) tacrolimus-based. In addition, one-third (n=25, 28%) of the patients received post-transplant cyclophosphamide prophylaxis. A median of 4 cycles (range, 1–12) of maintenance therapy following transplant was administered in 14% of patients, which consisted mainly of azacitidine (32 mg/m2/day) subcutaneously for 5 days every 28 days (n=11) or sorafenib (n=2).
A comparison of patient, disease, and transplant characteristics between the previous (2000–2010) and more recent decade (2011–2019) is shown in Table II. Notably, no patients with active disease were transplanted in the recent decade, compared with 22 (48%) who were transplanted with active disease during the previous decade. Furthermore, in the recent decade a different donor was selected more frequently (67% vs 39%; p=0.008), haploidentical donors were used more often (38% vs 4%, p=0.0002), and more patients received maintenance therapy (27% vs 2%, p=0.0007).
Table II.
Comparison of disease and transplant characteristics between the last two decades.
| Variables | Second HCT between 2000–2010 N=46 (100%) |
Second HCT between 2011 and 2019 N=45 (100%) |
p value |
|---|---|---|---|
| Age at AML diagnosis, median (range) | 41 (18–67) | 43 (17–66) | 0.47 |
| Males, n (%) | 23 (50) | 25 (56) | 0.67 |
| Therapy related disease, n (%) | 3 (6) | 3 (6) | 1.00 |
| Cytogenetic risk at diagnosis (per ELN) | 0.44 | ||
| Favorable | 2 (5) | 3 (7) | |
| Intermediate | 27 (64) | 22 (50) | |
| Adverse | 13 (31) | 19 (43) | |
| Not evaluable | 4 | 1 | |
| Cytogenetic risk at time of second HCT (per ELN) | 0.78 | ||
| Favorable | 0 | 0 | |
| Intermediate | 30 (75) | 31 (79) | |
| Adverse | 10 (25) | 8 (21) | |
| Not evaluable | 6 | 6 | |
| Age at second HCT, median(range) | 43.5 (18–68) | 47 (21–73) | 0.39 |
| Duration of remission after first HCT, months, median (range) | 8 (1–49) | 9 (1–71) | 0.61 |
| ≤6 months | 14 (30) | 15 (33) | 0.8 |
| >6 months | 32 (70) | 30 (67) | |
| Median time from relapse to second HCT, months (range) | 3.1 (0.3–31) | 4.1 (2–40) | 0.01 |
| Median time from first to second HCT, months (range) | 12.1 (2.6–51.5) | 14.9 (6.5–74.2) | 0.15 |
| Salvage treatment after relapse following first HCT, n (%) | 35 (76) | 44 (98) | 0.003 |
| High dose cytarabine based | 26 (57) | 21 (47) | |
| High dose cytarabine based plus DLI | 1 (2) | 2 (5) | |
| Hypomethylating-based | 6 (13) | 15 (33) | |
| Hypomethylating-based plus DLI | 1 (2) | 6 (13) | |
| DLI only | 1 (2) | 0 | |
| None | 11 (24) | 1 (2) | |
| Remission status at second HCT | <.0001 | ||
| CR/CRi | 20 (43) | 36 (80) | |
| Persistent disease | 22 (48) | 0 | |
| Unevaluable* | 4 (9) | 9 (20) | |
| MRD status a second HCT | |||
| Positive | 1 (5) | 8 (22) | |
| Negative | 4 (20) | 19 (53) | |
| Not evaluable | 15 (75) | 9 (25) | |
| HCT Comorbidity index | 0.21 | ||
| 0–1 | 26 (57) | 19 (42) | |
| ≥2 | 20 (43) | 26 (58) | |
| Donor selection | 0.008 | ||
| Same donor | 28 (61) | 15 (33) | |
| Different donor | 18 (39) | 30 (67) | |
| Donor relation | 0.0002 | ||
| Umbilical cord | 0 | 1 (2) | |
| Matched sibling | 26 (57) | 11 (24) | |
| Matched unrelated (9/10, 10/10) | 18 (37) | 16 (33) | |
| Mismatched family (haploidentical) | 2 (4) | 17 (38) | |
| Graft type | 0.44 | ||
| Peripheral blood | 37 (80) | 34 (76) | |
| Bone marrow | 8 (18) | 11 (24) | |
| Umbilical cord blood | 1 (2) | 0 | |
| Conditioning intensity | 1.00 | ||
| MAC (Bu>4000) | 10 (22) | 10 (22) | |
| RIC/NMA | 36 (78) | 35 (78) | |
| Maintenance following second HCT, n (%) | 1 (2) | 12 (27) | 0.0007 |
| HMA based maintenance | 1 (2) | 9 (20) | |
| Targeted maintenance | 0 | 3 (7) | |
| None | 45 (98) | 33 (73) | |
Abbreviations: HCT=hematopoietic cell transplantation; AML=acute myeloid leukemia; ELN=European Leukemia Network; CR=complete response; CRi=complete response with incomplete hematologic recovery; MAC=myeloablative conditioning; RIC=reduced intensity conditioning; NMA=non-myeloablative conditioning; BU=busulfan; Mel=melphalan; HMA=hypomethylating agent.
Includes patients with 1) aplastic marrow insufficient for analysis and/or 2) patients who received a salvage treatment following the most recent disease evaluation.
Study outcomes
Following the second HCT, 85 patients (93%) engrafted. Among these, all achieved neutrophil engraftment and 68 (80%) achieved platelet engraftment at a median of 12 days (range, 9–40 days) and 14 days (range, 8–54), respectively. The cumulative incidence of day 28 neutrophil and platelet engraftment was 99% and 65%, respectively. The cumulative incidence of grades 3–4 acute GVHD at 100 days was 11% (95% CI 6–20%) and the cumulative incidence of chronic GVHD at 2 years was 18% (95% CI 10–32%).
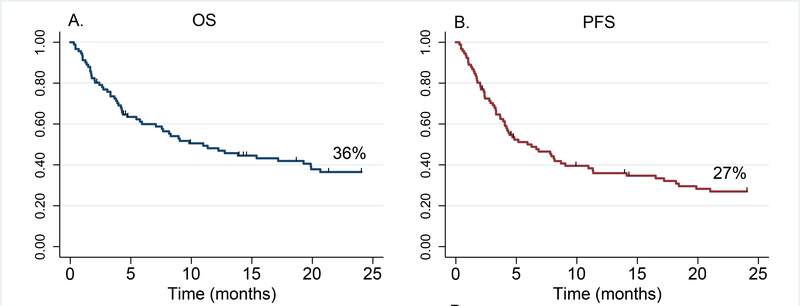
A total of 29 patients (32%) were alive at the time of this analysis. The median follow-up among survivors was 66 months (range, 2–171 months) and median OS was 11 months. Forty patients (44%) had disease relapse/progression at a median of 6 months after the second HCT (range, 1–86 months) and 17 patients experienced NRM. The cumulative incidence of progression and NRM at 2 years was 42% (95 CI 32–54) and 18% (95% CI 12–28%), respectively. OS and PFS at 2 years were 36% (95% CI 26–47%) and 27% (95% CI 18–37%), respectively (Figure 1). Causes of death were disease recurrence (n=45, 73%), infections (n=6, 10%), GVHD (n=4, 6%), and other causes of NRM (n=7, 11%).
Figure 1.
Overall (left) and progression-free (right) survival of patients who received second HCT. OS=Overall Survival; PFS=Progression-Free Survival.
Through our univariate analysis (Table III), we found that HCT-CI ≥2 and development of chronic GVHD after first HCT were associated with significantly inferior PFS and OS following the second HCT. The only significant univariable covariate for NRM was older age (>60 years) and none of the covariates were associated with significant disease progression.
Table III.
Univariate analysis of factors for outcomes after second HCT
| Variables | Progression Free Survival | Overall Survival | Progression | Non Relapse Mortality | |||||
|---|---|---|---|---|---|---|---|---|---|
| N vs N | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Second HCT before vs after 2011 | 46 vs 45 | 1.4 (0.8–2.4) | 0.2 | 1.2(0.75–2.02) | 0.4 | 0.9 (0.4–1.6) | 0.6 | 1.1 (0.4–2.8) | 0.9 |
| Age at second HCT ≥60 vs <60 years | 16 vs 75 | 1.5 (0.7–3.6) | 0.3 | 1.5 (0.7–3.3) | 0.3 | 0.9 (0.3–2.9) | 0.8 | 3.8 (1.2–12) | 0.02 |
| Age at second HCT ≥40 vs <40 years | 54 vs 37 | 1.7 (0.96–2.9) | 0.07 | 1.5 (0.9–2.5) | 0.09 | 1.4 (0.7–2.6) | 0.4 | 1.4 (0.5–3.7) | 0.5 |
| Female vs Male | 43 vs 48 | 1.5 (0.9–2.6) | 0.1 | 1.2 (0.7–1.9) | 0.5 | 0.8 (0.4–1.6) | 0.5 | 0.9 (0.3–2.4) | 0.8 |
| HCT-CI ≥2 vs 0–1 | 46 vs 45 | 1.8 (1.0–3.2) | 0.05 | 1.7 (0.99–2.9) | 0.05 | 1.01 (0.5–1.9) | 0.9 | 1.3 (0.5–3.4) | 0.6 |
| Primary vs t-AML | 85 vs 6 | 1.7 (0.7–4.3) | 0.3 | 1.9 (0.8–4.4) | 0.1 | 1.4 (0.4–4.6) | 0.6 | 0.9 (0.1–5.6) | 0.9 |
| Response before second HCT | |||||||||
| CR/CRi vs Persistent disease* | 56 vs 22 | 0.8 (0.4–1.5) | 0.5 | 0.7 (0.4–1.2) | 0.2 | 0.6 (0.3–1.2) | 0.1 | 1.5 (0.4–5.4) | 0.5 |
| Duration of remission after first HCT | |||||||||
| ≤6 months vs >6 months | 28 vs 63 | 1.4 (0.8–2.4) | 0.3 | 1.4 (0.8–2.4) | 0.2 | 0.6 (0.3–1.2) | 0.1 | 1.5 (0.4–5.4) | 0.5 |
| Haploidentical vs another donor | 19 vs 72 | 0.8 (0.4–1.6) | 0.5 | 0.86 (0.5–1.6) | 0.6 | 0.8 (0.4–1.7) | 0.6 | 0.9 (0.2–3.2) | 0.9 |
| Haploidentical vs MUD or MRD | 19 vs 32 / 37 |
0.8 (0.4–1.6) | 0.5 | 0.9 (0.5–1.6) | 0.7 | 0.8 (0.4–1.7) | 0.6 | 0.9 (0.3–3.3) | 0.9 |
| MUD vs MRD | 32 vs 37 | 0.7 (0.4–1.4) | 0.3 | 0.8 (0.5–1.5) | 0.5 | 0.9 (0.5–1.9) | 0.9 | 2.5 (0.7–8.2) | 0.1 |
| Same vs different donor | 43 vs 48 | 0.9 (0.5–1.5) | 0.7 | 0.8 (0.5–1.3) | 0.3 | 1.1 (0.6–2.2) | 0.7 | 0.5 (0.2–1.4) | 0.2 |
| MAC vs RIC for second HCT | 20 vs 71 | 1.1 (0.6–2.1) | 0.6 | 1.3 (0.7–2.3) | 0.4 | 1.2 (0.6–2.6) | 0.6 | 1.1 (0.4–3.4) | 0.8 |
| Graft source | |||||||||
| Peripheral blood | 71 | 1.0 (0.6–1.8) | 0.9 | 0.8 (0.4–1.6) | 0.6 | 1.6 (0.7–3.6) | 0.3 | 0.6 (0.2–1.7) | 0.3 |
| Bone marrow | 19 | Ref. | Ref. | Ref. | Ref. | ||||
| Cord blood | 1 | Ref. | Ref. | Ref. | Ref. | ||||
| GVHD prophylaxis for second HCT | |||||||||
| PTCy vs no PTCy | 25 vs 66 | 0.6 (0.3–1.2) | 0.2 | 0.7 (0.4–1.2) | 0.2 | 0.8 (0.3–1.6) | 0.4 | 0.6 (0.2–2.2) | 0.5 |
| Acute GVHD grade 3–4 after first HCT | 4 vs 87 | 2.8 (0.9–9) | 0.09 | 2.2 (0.7–6.9) | 0.2 | 0.9 (0.4–2.2) | 0.9 | 1.7 (0.2–15) | 0.6 |
| Chronic GVHD after first HCT | 17 vs 74 | 3.03 (1.7–5.5) | <0.001 | 2.6 (1.5–4.8) | 0.001 | 0.9 (0.4–2.4) | 0.9 | 1.7 (0.5–5.2) | 0.4 |
| Cytogenetic risk per ELN at time of second HCT | |||||||||
| Adverse vs intermediate | 18 vs 61 | 1.3 (0.7–2.3) | 0.4 | 0.9 | 0.4 | 2.0 (0.9–4.2) | 0.06 | 0.5 (0.1–2.5) | 0.4 |
Abbreviations: HCT=hematopoietic cell transplantation; HR=hazards ratio; MRD=measurable residual disease; CI=confidence interval; t-AML=therapy related acute myeloid leukemia; CR=complete response; CRi=complete response with incomplete hematologic recovery; MRD=matched related donor; MUD=matched unrelated donor; CB=cord blood; MAC=myeloablative conditioning; RIC=reduced intensity conditioning; ELN=European Leukemia Network; GVHD=graft-versus-host disease; PTCy=posttransplant cyclophosphamide.
Unevaluable patients excluded (unevaluable include patients with 1) aplastic marrow insufficient for analysis and/or 2) patients who received a salvage treatment following the most recent disease evaluation.
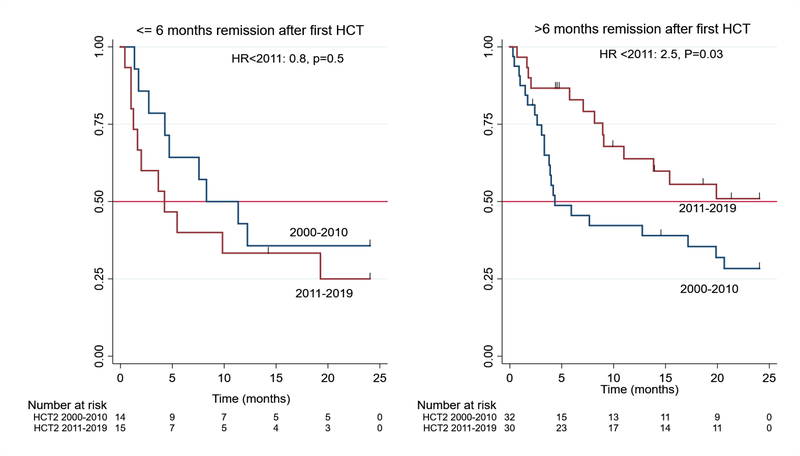
Evaluation of the predictors of OS and PFS stratified by decade was also completed through univariate analysis (data not shown). This analysis revealed a significant (p=0.04) interaction effect for the impact of the duration of remission after first HCT on OS. We found that a short (≤ 6 months) remission duration after the first HCT was associated with worse OS in the more recent (HR=2.5, 95% CI 1.1–5.5, p=0.03), but not in the previous (HR=0.8, 95% CI 0.4–1.7, p=0.5) decade. There were no interaction effects associated with PFS. Because of the skewed distribution by decade of remission status and donor type, we performed subset analyses to evaluate the impact of each factor within the relevant time period. Consistent with results for the overall cohort, there was no association with OS or PFS for either factor.
In the multivariate analysis (Table IV), we found that inferior OS after the second HCT was associated with chronic GVHD after the first HCT (HR 2.9 (95% CI, 1.5–5.7; p=0.001)), HCT-CI ≥2 at second HCT (HR 2.6 (95% CI, 1.4–4.9; p=0.003)), relapse within 6 months of first HCT (limited to patients who received a second HCT between 2011 and 2019) (HR 2.6 (95% CI, 1.1–5.8; p=0.02)), and second HCT before 2011 (limited to patients with >6 months remission duration after first HCT) (HR 2.5 (95% CI, 1.2–5.2; p=0.02)). Among these, development of chronic GVHD after first HCT (HR 3.4 (95% CI, 1.8–6.4; p <0.001)) and HCT-CI ≥2 (HR 2.1 (95% CI, 1.2–3.7; p=0.01)) were also significantly associated with worse PFS.
Table IV.
Multivariate analysis of factors for progression-free and overall survival after second HCT.
| Variables | Progression Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| History of cGVHD after first HCT | 3.4 | 1.8–6.4 | <0.001 | 2.9 | 1.5–5.7 | 0.001 |
| HCT-CI≥2 | 2.1 | 1.2–3.7 | 0.01 | 2.6 | 1.4–4.9 | 0.003 |
| ≤6 months remission after first HCT | 1.2 | 0.7–2.2 | 0.4 | 2.6* | 1.1–5.8 | 0.02 |
| Second HCT before 2011 | 1.2 | 0.7–1.9 | 0.5 | 2.4* | 1.1–5.1 | 0.02 |
Abbreviations: HCT=hematopoietic cell transplantation; HR=hazards ratio; CI=confidence interval; cGVHD=chronic graft-versus-host disease; HCT-CI=hematopoietic cell transplantation comorbidity index; NS=not significant.
Hazard ratios reflect adjustment for a significant interaction effect between transplant decade and duration of remission of first transplant.
Discussion
In this study, we retrospectively analyzed the outcomes of adult patients who underwent a second HCT for relapsed AML over the last two decades at our institution and investigated the impact of time on our management and outcomes.
In our study, 36% of the patients were alive 2 years after their second HCT and 27% were relapse free, while 20% died from NRM. These results are in line with previous studies that demonstrated comparable OS and PFS following second HCT8, 16, 17. Furthermore, patients who received a second HCT in the recent decade, and particularly those who had a long remission after their first HCT, demonstrated substantial improvement in overall survival compared to those who underwent transplantation in the prior decade (Figure 2). These results support previously published findings18.
Figure 2.
Overall survival of patients who received second HCT ≤ 6 months (left) or > 6 months (right) after first HCT, stratified by decade second HCT was received.
Identifying prognostic factors associated with survival after second HCTs informs clinicians on the potential benefit of recommending a second HCT in relapsed AML. Published retrospective studies demonstrated that the biological aggressiveness of the underlying leukemia, as reflected by the duration of remission after first HCT and ability to achieve a remission prior to second HCT, were important predictors of outcomes after second HCT4, 5, 19. Consistent with published data, we confirmed a significant association between survival and the time from first HCT to relapse. Perhaps due to small sample size, we did not find a statistically significant association between persistence of disease at time of second transplant and transplant outcomes.
We also found that the occurrence of chronic GVHD after the first HCT was an unfavorable predictive factor for overall and progression free survival after the second HCT. These findings agree with a recent study by Ruutu et al., who completed a retrospective registry analysis of 2632 patients with various relapsed hematologic malignancies and found that chronic GVHD after first HCT was an adverse prognostic factor following the second HCT9. The study also reported higher NRM and lower OS after second HCT in patients who developed chronic GVHD after first HCT. However, not all studies reported a similar effect of chronic GVHD, and some have even reported beneficial effects of chronic GVHD in reducing relapse after second HCT8. Further studies are needed to clarify this interaction.
Remarkably, advanced age as a dichotomized variable was not a significant predictor detected through our univariate analysis, though there was a trend towards inferior outcomes. In general, age is a commonly used prognostic parameter in transplantation. However, a more thorough classification of patients under the aspect of prognosis was demonstrated by including parameters like physical capacity, nutritional status, and comorbidities in several studies of hematological malignancies20. The HCT-CI was developed to better define and assess pre-existing comorbidities in hematological malignancies21. It is widely used and is predictive of survival in transplant recipients22. In the present study, an increased comorbidity burden (HCT-CI ≥2) was an independent predictor of clinical outcomes, which is in line with results from previous studies conducted in HCT recipients23, 24. To the best of our knowledge, this is the first study reporting the relevance of HCT-CI as a poor prognosticator in the setting of second HCT in relapsed AML.
Another factor that has been reported to impact the outcome after second HCT is the development of grade 3–4 acute GVHD after first HCT25. In our cohort, only four patients with a history of acute GVHD underwent a second HCT and we identified a statistically insignificant trend towards inferior survival in these patients. The prognostic impact of other factors such as donor sex, conditioning regimen and graft source are unclear, and we did not find any impact of these factors on subsequent survival after second HCT. Importantly, changing donors for the second transplant was also not beneficial, corroborating an earlier CIBMTR and more recent European Group for Blood and Marrow Transplant analyses5, 6, 9. Furthermore, although recent data has shown a benefit for using HLA-haploidentical donors in patients with relapsed leukemia after a first HCT26, 27, we failed to show better outcomes. These findings are in line with the EBMT registry analysis16.
Our analysis has several limitations including its retrospective nature with its inherent biases. Similar to previous studies, ours is limited by its relatively small sample size and by the heterogeneity of the transplant regimens used. Additionally, most patients in our study did not have molecular mutations evaluable at time of second HCT, which may impact prognosis28.
In summary, a second transplant may be beneficial in a select group of patients. Careful consideration of patient characteristics and review of outcomes after the first transplant can be used to inform decision making regarding the completion of second HCTs in patients with AML.
Supplementary Material
Highlights.
Second hematopoietic stem cell transplants may be beneficial for select AML cases.
GVHD and HCT-CI ≥2 were associated with worse outcomes after a second transplant.
Donor change or using a haploidentical donor does not affect outcomes.
Acknowledgment:
We wish to thank Mark Tanner for assistance with manuscript preparation.
Footnotes
Conflicts of Interest
None of the authors declare any conflicts of interest.
References
- 1.Bejanyan N, Oran B, Shanley R, Warlick E, Ustun C, Vercellotti G, et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transplant. 2014;49(8):1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moukalled NM, Kharfan-Dabaja MA. What is the role of a second allogeneic hematopoietic cell transplant in relapsed acute myeloid leukemia? Bone Marrow Transplant. 2020;55(2):325–31. [DOI] [PubMed] [Google Scholar]
- 3.Weisdorf D The role of second transplants for leukemia. Best Pract Res Clin Haematol. 2016;29(4):359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosing C, Saliba RM, Shahjahan M, Estey EH, Couriel D, Giralt S, et al. Disease burden may identify patients more likely to benefit from second allogeneic hematopoietic stem cell transplantation to treat relapsed acute myelogenous leukemia. Bone Marrow Transplantation. 2005;36(2):157–62. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34(8):721–7. [DOI] [PubMed] [Google Scholar]
- 6.Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhäuser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31(26):3259–71. [DOI] [PubMed] [Google Scholar]
- 7.Michallet M, Tanguy ML, Socié G, Thiébaut A, Belhabri A, Milpied N, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Société Française de Greffe de moelle (SFGM). Br J Haematol. 2000;108(2):400–7. [DOI] [PubMed] [Google Scholar]
- 8.Yaniv I, Krauss AC, Beohou E, Dalissier A, Corbacioglu S, Zecca M, et al. Second Hematopoietic Stem Cell Transplantation for Post-Transplantation Relapsed Acute Leukemia in Children: A Retrospective EBMT-PDWP Study. Biol Blood Marrow Transplant. 2018;24(8):1629–42. [DOI] [PubMed] [Google Scholar]
- 9.Ruutu T, de Wreede LC, van Biezen A, Brand R, Mohty M, Dreger P, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant. 2015;50(12):1542–50. [DOI] [PubMed] [Google Scholar]
- 10.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. [DOI] [PubMed] [Google Scholar]
- 11.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 13.CIBMTR. Pre-HCT Preparative Regimen (Conditioning). 2020;form 2400:Available from: https://www.cibmtr.org/manuals/fim/1/en/topic/q155-315-pre-hct-preparative-regimen-conditioning.
- 14.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 15.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267–76. [PubMed] [Google Scholar]
- 16.Shimoni A, Labopin M, Finke J, Ciceri F, Deconinck E, Kröger N, et al. Donor selection for a second allogeneic stem cell transplantation in AML patients relapsing after a first transplant: a study of the Acute Leukemia Working Party of EBMT. Blood Cancer J. 2019;9(12):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Y, Artz AS, van Besien K, Stock W, Larson RA, Odenike O, et al. Outcomes following second allogeneic stem cell transplant for disease relapse after T cell depleted transplant correlate with remission status and remission duration after the first transplant. Exp Hematol Oncol. 2019;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canaani J, Beohou E, Labopin M, Ghavamzadeh A, Beelen D, Hamladji RM, et al. Trends in patient outcome over the past two decades following allogeneic stem cell transplantation for acute myeloid leukaemia: an ALWP/EBMT analysis. J Intern Med. 2019;285(4):407–18. [DOI] [PubMed] [Google Scholar]
- 19.Lund TC, Ahn KW, Tecca HR, Hilgers MV, Abdel-Azim H, Abraham A, et al. Outcomes after Second Hematopoietic Cell Transplantation in Children and Young Adults with Relapsed Acute Leukemia. Biol Blood Marrow Transplant. 2019;25(2):301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamaker ME, Prins MC, Stauder R, Sorror ML, Maris MB, Storb R, et al. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Leuk Res. 2014;38(3):275–83. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller JW, Andreadis C, Damon LE, Kaplan LD, Martin TG, Wolf JL, et al. Hematopoietic cell transplantation comorbidity index (HCT-CI) is predictive of adverse events and overall survival in older allogeneic transplant recipients EBMT risk score predicts outcome of allogeneic hematopoietic stem cell transplantation in patients who have failed a previous transplantation procedure Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Geriatr Oncol. 2014;5(3):238–44. [DOI] [PubMed] [Google Scholar]
- 23.Rezvani K, Kanfer EJ, Marin D, Gabriel I, Rahemtulla A, Taylor A, et al. EBMT risk score predicts outcome of allogeneic hematopoietic stem cell transplantation in patients who have failed a previous transplantation procedure. Biol Blood Marrow Transplant. 2012;18(2):235–40. [DOI] [PubMed] [Google Scholar]
- 24.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352(9134):1087–92. [DOI] [PubMed] [Google Scholar]
- 25.Radich JP, Sanders JE, Buckner CD, Martin PJ, Petersen FB, Bensinger W, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol. 1993;11(2):304–13. [DOI] [PubMed] [Google Scholar]
- 26.Yeh SP, Lin CC, Lin CH, Lo WC, Chen TT, Lo WJ, et al. Second haploidentical peripheral blood stem cell transplantation for treatment of acute leukemia with relapse after first allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2015;50(7):1001–3. [DOI] [PubMed] [Google Scholar]
- 27.Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transplant. 2014;49(7):895–901. [DOI] [PubMed] [Google Scholar]
- 28.Rautenberg C, Stepanow S, Lauseker M, Germing U, Jäger P, Geyh S, et al. Impact of Somatic Mutations and Pretransplant Strategies on the Outcome of Patients Allografted for MDS or Secondary AML. Blood. 2019;134:4612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.