Abstract
Orienting attention in the space around us is a fundamental prerequisite for willed actions. On Earth, at 1 g, orienting attention requires the integration of vestibular signals and vision, although the specific vestibular contribution to voluntary and automatic components of visuospatial attention remains largely unknown. Here, we show that unweighting of the otolith organ in zero gravity during parabolic flight, selectively enhances stimulus-driven capture of automatic visuospatial attention, while weakening voluntary maintenance of covert attention. These findings, besides advancing our comprehension of the basic influence of the vestibular function on voluntary and automatic components of visuospatial attention, may have operational implications for the identification of effective countermeasures to be applied in forthcoming human deep space exploration and habitation, and on Earth, for patients’ rehabilitation.
Subject terms: Neuroscience, Neurological manifestations, Human behaviour
Introduction
Spatial attention on Earth, i.e. the capacity to allocate our attentional resources to specific regions in the space around us, enhances our ability to select relevant objects and to anticipate expected events, in order to coherently act in our environment. Neuropsychological studies in stroke patients with spatial awareness impairments and in patients with vestibular disorders, together with vestibular manipulations in healthy individuals, show that orienting attention in space not only requires the interaction between automatic (i.e. bottom-up, stimulus-driven, or exogenous) and voluntary (i.e. top-down, goal-directed, or endogenous) components of visuospatial attention1–4 but also engages the vestibular system5–7. However, the specific contribution of vestibular signals to automatic and voluntary attention remains largely unknown.
On Earth, the vestibular system, situated in the inner ear, encodes head position and enables postural balance by computing the crystals orientation in otolith organs with respect to gravity. In zero gravity, the weight of otolith (utricular) afferent inputs is absent or greatly reduced8,9. Thus zero gravity represents a unique condition to unweight the otolith organ and test its contribution to visuospatial attention. Studies in microgravity induced by free-fall conditions of parabolic and orbital space flights (see9), suggested that this condition increases the weight of visual information over vestibular signals, enhancing visual processing10,11. This in turn might affect attentional processes. However, the evidence on how microgravity might affect visuospatial attention is scant and controversial10–12 and no data exist on its effects on the two distinct attentional components (automatic vs. voluntary).
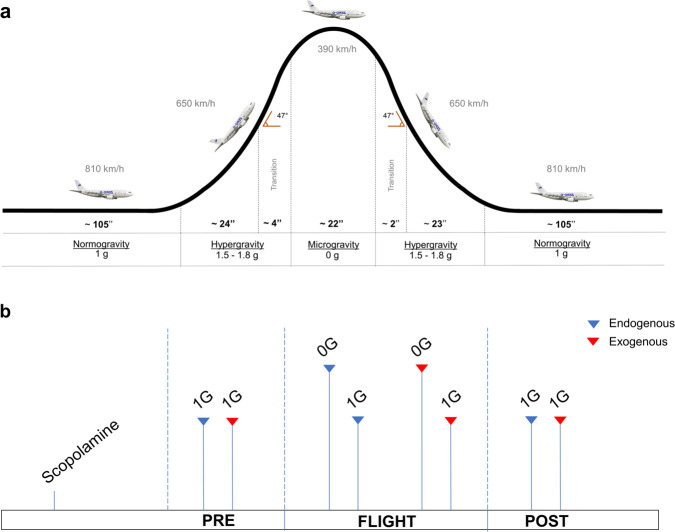
We took advantage of short periods of microgravity (~22 s), obtained during parabolic flights (72nd ESA Parabolic Flights Campaign, Fig. 1a), to investigate whether temporary unweighting of the otolith organ may differently affect automatic and voluntary orienting of visuospatial attention. Because in microgravity there is a decrease of vestibular weight in favour of vision, we predicted that increased saliency of visual stimuli might boost automatic capture of stimulus-driven attention, without affecting spatial orienting of voluntary attention, which is driven by internal goals.
Fig. 1. Overview of our parabolic flight experiment.
Flight trajectory during the parabolic manoeuvre (a) and study timeline (b).
To test these hypotheses we asked seven healthy participants to perform modified versions of the cue-to-target Posner task4 in which subjects had to detect visual targets, appearing in their left or right hemifield. In Posner’s procedures, central or peripheral visual cues indicate the position of the upcoming target. It is well established that peripheral, non-informative (i.e. non-predictive) cues orient attention exogenously, whereas central informative (i.e. predictive) cues orient attention endogenously4. In our study, peripheral cues exogenously captured attention, while central cues endogenously oriented attention. In valid trials, the cue indicated where the target would actually appear. In invalid trials, the cue indicated the side opposite to the upcoming target. Subjects’ reaction times (RTs) are typically faster in valid than in invalid trials (i.e. ‘validity effect’). Depending on the different types of trials, this procedure allowed measuring the engagement of attention to the target, disengagement of attention from its current focus, and attentional shift. Participants’ performed the tasks in the following conditions: 1 g before (PRE) flight, 0 g (0 G) and 1 g (1 G) during flight, 1 g after (POST) flight (Fig. 1b, Methods).
Results
Accuracy
One participant suffered from motion sickness during the first block of parabolas and his data were not considered in the analysis (see details in the Procedure). Participants were highly accurate in both the exogenous (mean accuracy > 94%) and the endogenous task (mean accuracy > 93%), as indexed by the high percentage of correct responses (see Supplementary Table 1 for details). The repeated-measures ANOVAs on participants’ accuracy for both tasks did not show any significant effect or interaction.
Reaction times
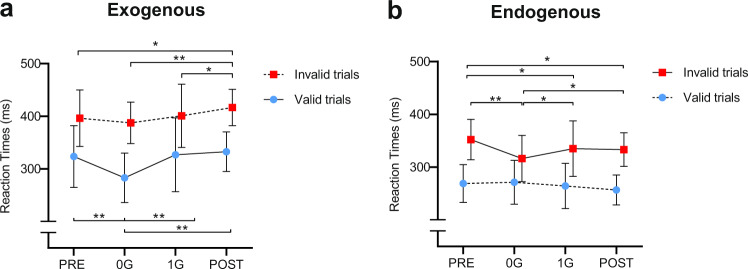
By measuring participants’ RTs, we observed a validity effect (i.e. faster RTs for valid compared to invalid trials) for both exogenous [F1,4 = 90.149; P = 0.001; partial η2 = 0.958] and endogenous [F1,4 = 18.712; P = 0.012; partial η2 = 0.824] tasks and a significant validity by condition interaction for both tasks. Consistently with the previous studies4, participants showed faster RTs for valid trials compared to invalid trials for both exogenous (valid: mean = 328.58 ± 51.19; invalid: mean = 388.39 ± 41.35) and endogenous (valid: mean = 272.01 ± 35.36; invalid: mean = 331.32 ± 40.05) tasks. Interestingly, Newman–Keuls post hoc analyses13 revealed, for the interaction of the exogenous task [F3,12 = 4.688; P = 0.022; partial η2 = 0.540], faster RTs for valid trials in 0 G compared to PRE (P = 0.0002), 1 G (P = 0.0003), and POST (P = 0.0002). In addition, invalid trials were slower in POST compared to PRE (P = 0.027), 0 G (P = 0.005), and 1 G (P = 0.040). For the interaction of the endogenous task [F3,12 = 6.809; P = 0.006; partial η2 = 0.630], Newman–Keuls post hoc analyses showed faster RTs for invalid trials in 0 G compared to PRE (P = 0.0008), 1 G (P = 0.033), and POST (P = 0.022), besides, invalid trials were slower in PRE compared to 1 G (P = 0.020) and POST (P = 0.030). No significant differences were found for valid trials (Fig. 2).
Fig. 2. Mean reaction times (RTs) and SD (n = 5) for valid and invalid trials for the two tasks across gravity conditions.
a Exogenous task: valid trials were faster in 0 g compared to 1 g conditions; invalid trials were slower in POST compared to all other conditions. b Endogenous task: invalid trials were faster in 0 g compared to 1 g conditions; invalid trials were slower in PRE compared to 1 G and POST. There were no significant differences for valid trials. Newman-Keuls post-hoc analyses, *P < 0.05 and **P < 0.01. PRE = 1 g before flight, 0 G = 0 g on the flight, 1 G = 1 g on the flight, POST = 1 g after the flight.
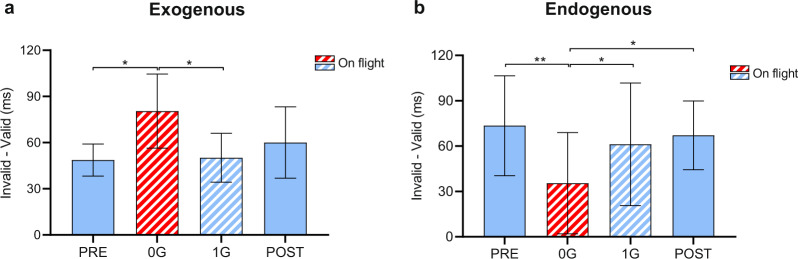
When looking at the validity effect (invalid—valid trials), Newman–Keuls post hoc analyses showed, for the exogenous task, a larger validity effect in 0 G compared to PRE (P = 0.027) and 1 G (P = 0.021) conditions, and a trend in the same direction compared to POST (P = 0.055). This last finding may be explained by the increased RTs of invalid trials in the POST condition with respect to all other conditions, likely due to fatigue effects. In contrast, for the interaction of the endogenous task, post hoc analyses revealed a smaller validity effect in 0 G compared to PRE (P = 0.006), 1 G (P = 0.015), and POST (P = 0.011) conditions (Fig. 3 and Supplementary Fig. 1).
Fig. 3. Validity effect for the two tasks across conditions.
Mean values and SD (n = 5) are reported. a Exogenous task: the validity effect was larger in 0 g compared to PRE and 1 G, and there was a similar trend for POST (P = 0.055); b endogenous task: the validity effect was smaller in 0 g compared to all 1 g conditions. Newman–Keuls post hoc analyses, *P ≤ 0.05 and **P ≤ 0.01. PRE = 1 g before the flight, 0 G = 0 g on the flight, 1 G = 1 g on the flight, POST = 1 g after the flight.
To sum up, our results show that microgravity increased the effect of exogenous cueing, but decreased the effect of endogenous cueing, speeding up the engagement of automatic attention by peripheral cues in the exogenous task and disengagement of voluntary attention in the endogenous task.
Discussion
The observation of the validity effect in microgravity indicates that attentional systems still operate when unweighting the otolith organs. The finding that microgravity increased the validity effect for the exogenous cueing, but decreased it for the endogenous cueing, strongly suggests that otolith signals differentially affect the two attentional systems. The analyses revealed that 0 g speeded up attentional engagement to valid exogenously cued locations (targets), while quickening disengagement from invalid endogenously cued locations. In other words, 0 g might have enhanced, as we predicted, stimulus saliency and, consequently, stimulus-driven attentional capture, in the exogenous task. Conversely, the results of the endogenous task suggest that 0 g might have impaired the maintenance of voluntary attention at cued locations. These data indicate that microgravity, rather than enhancing the overall visual performance, might boost attention to peripheral salient visual stimuli with a resulting detriment of top-down control. It is worth noting that in the present study, attentional processes in 1 g were studied before and after 0 g, ruling out the impact of various dynamic processes, such as, for example, adaptation to parabolic flight (PF), practice with the task, or scopolamine pharmacodynamics.
The finding that microgravity differentially affects automatic and voluntary attention is consistent with the evidence of separate circuits underlying voluntary and automatic components of spatial attention3,14 and the existence of distinct vestibular projections reaching different structures of the two attention systems. A bilateral dorsal network (including the intraparietal sulcus and frontal eye fields) is mainly involved in goal-directed attention, while a right-lateralized ventral network (including the temporoparietal junction or TPJ, and the ventral frontal cortex) in stimulus-driven attention14–17. Some areas of the vestibular system overlap with posterior regions of the ventral network, such as TPJ and areas, such as supramarginal gyrus and angular gyrus5,18,19, which mainly process the space/environment around us using egocentric reference frames, i.e. centred on the viewer’s head/trunk/eyes20. Other vestibular projections reach the intermediate layers of the superior colliculus (iSC), which carry out multisensory integration in egocentric space and are engaged by the dorsal attention system6,21.
It is well-known that vestibular signals anchor visuospatial processing to egocentric frames of reference (see for example22). We may speculate that, in the present study, reduced otolith input to brain regions overlapping with the ventral attention system might have weakened egocentric reference frames23 in favour of allocentric reference frames (in which stimuli in space are represented in terms of coordinate systems independent of the subjects’ viewpoint), which are processed by more posterior and ventral areas (i.e. posterior inferior temporal and lateral occipital areas, see20). The reduced weight of target spatial position with reference to the subjects’ body/head might have led to stimulus distance underestimation and/or stimulus size overestimation (which are observed in microgravity24) enhancing stimulus salience and stimulus-driven attentional capture, as shown by findings of the exogenous task. Moreover, reduced otolith input to iSC might have weakened multisensory integration in egocentric space, impairing maintenance of voluntary attention at cued locations within this space, as suggested by finding of the endogenous task. Weakening of egocentric and strengthening of stimulus-centred coordinates systems, in 0 g, might have enhanced automatic attentional orienting to peripheral salient stimuli, with detriment effects on voluntary goal-directed attention. Since in our study egocentric and, allocentric reference frames were aligned, future studies disentangling the two coordinates systems (see for example ref. 25) are necessary to test and validate our hypothesis.
One of the limitations of PF experiments might be the unspecific effects of the weightlessness experience that may potentially distract the subject from task performance. However, distraction is expected to negatively affect the overall performance26—increasing errors and RTs of both valid and invalid trials in both types of exogenous and endogenous tasks—rather than selectively facilitating responses to valid exogenously cued locations (targets) and to invalid endogenously cued locations, as it occurred in our PF experiments.
Although these findings warrant further in-depth investigation, they may have operational implications for defining new countermeasures to be applied in forthcoming human deep space exploration and habitation8,27, where humans will experience different g. Indeed, the efficiency of human performance in microgravity is crucial to the success of long-term space stations and interplanetary missions8,11,28,29. We may speculate that enhanced reflexive attentional capture by upcoming environmental visual stimuli and weakened ability to voluntarily maintaining attention at specific regions of the egocentric space might contribute to some altered perceptions experienced by the astronauts during spaceflights6,27,28. For example, in microgravity astronauts misperceive objects size (i.e. height and depth overestimation) and objects distance (i.e. distance underestimation) and may manifest the illusion that the wall or the floor is looming over them when they are actually approaching it24,30 as if the enhanced visual salience of objects in the exogenous system prevails over the subjects’ voluntary control of viewer’s centred events. Moreover, strengthened automatic attentional orienting in 0 g compared to 1 g, might potentially explain the increased cognitive fatigue reported by astronauts when operating in microgravity27,28. Indeed, heightened automatic reorienting towards upcoming stimuli might distract from engagement in ongoing (goal-directed) activities, increasing the cognitive load.
By shedding new light on the basic influence of the vestibular function on attentional processes, the present findings may offer a theoretical framework not only for space research but also, on Earth, for the design of innovative cognitive rehabilitation strategies in individuals with vestibular disorders30,31 or with disorders of spatial awareness due to a stroke (such as unilateral spatial neglect and related disorders32–35).
Methods
Participants
Seven healthy right-handed volunteers (5 males and 2 females; mean age 35.83 ± 11.2 years; mean education = 19 years ± 1.4) with no previous experience with Parabolic Flights participated in the study. They had a normal or corrected-to-normal vision. Handedness was estimated using the Edinburgh Handedness Inventory36 test, which ranges from −100 (completely left-handed) to +100 (completely right-handed). All participants had passed the Parabolic Flight Medical Certificate (DM-2016- en-ed3) equivalent of an Air Force Class III medical examination. All participants provided a signed informed consent form. The study was approved by the Ethics Committees of the University of Turin (Italy) and the French Ethics Committee of the University of Caen Normandie (France) in compliance with French legislation and the Declaration of Helsinki for human participants. One participant suffered from motion sickness during the first block of parabolas and his data were not considered in the analysis (see the Statistical Analysis section for details). Thus the presented data come from 6 participants (4 males and 2 females; mean age 35.00 ± 11.90 years; mean education = 19 years ± 1.5; Edinburgh Handedness Inventory mean score = 76 ± 16.9).
Procedure
The experiment was performed during the 72nd European Space Agency (ESA) Parabolic Flights Campaign (PFC), in November 2019, onboard the Airbus A310 Zero-G and took place at Bordeaux-Merignac airport (France). The flights were run by Novespace (http://www.novespace.fr). One campaign consists of three parabolic flights (PFs) on three consecutive days. Each flight has a duration of two to three hours and includes 30 parabolic manoeuvres, divided into 6 series of 5 parabolas each. There is a 5 min (1 g) interval between the series of parabolas, with a longer interval (8 min) between the first three series and the last three series of parabolas. The reduced-gravity environment is obtained by carrying out, with the A310 Zero-G airplane, a PF manoeuvre (see Fig. 1a) producing periods of weightlessness for approximately 22 s. The air pressure in the cabin is maintained at approximately 800 millibars during the parabolas, which corresponds to an altitude of about 2000 m. The temperature is between 20 and 25 °C. Each parabola starts with a pull-up and ends with a pull-out at 1.8 g (hypergravity). These phases last about 23–24 s. On each flight, we tested two participants, each participant taking part in only one flight. They performed each attentional task (endogenous or exogenous) during the 0 g phase of a sequence of 5 parabolas and during the 1 g 5 min-interval following the same sequence of parabolas. Participants performed the two tasks during two consecutive series of parabolas with the order of the two tasks balanced across subjects. Since on each flight we had equipped only one experimental setting for the present study (a second experimental setting was equipped for a different study, here not reported), the two participants underwent the present study following a sequential order: the first participant performed the two attentional tasks during the first two series of five parabolas and the following 1 g intervals, whereas the second participant performed the study during the fourth and fifth series of five parabolas and the subsequent intervals. This order was counterbalanced across participants. During the flight, participants were sitting cross-legged and firmly strapped to the floor at 60 cm from the screen (17” monitor) of a laptop, that was attached to the floor and centred on their sagittal mid-plane. An operator in charge of administering the experimental task and supervising its execution was positioned on the right side of the subject, slightly further back so as not to interfere with task execution (Fig. 4). The authors affirm that human research participants provided informed consent, for publication of the image in Fig. 4.
Fig. 4. Photo of the experimental set-up in flight.
The participant was sitting cross-legged and firmly strapped to the floor at 60 cm from the screen of a laptop that was attached on the floor and centred on the sagittal mid-plane. The experimenter in charge of administering the experimental task and supervising its execution was positioned on the right side of the participant, slightly further back so as not to interfere with task execution.
Before the flight, participants received a muscarinic receptor antagonist (scopolamine: 0.25 mg/1 mL; 0.7 mL for males and 0.5 mL for females), known to alleviate motion sickness37. Nonetheless, one participant suffered from motion sickness during the first series of parabolas, during the performance of the endogenous task, and his data were not considered in the analysis. In the second series of parabolas, a backup participant replaced him, performing the exogenous task (following the scheduled order of task administration).
The exogenous and endogenous tasks were administered in four conditions, starting soon after the scopolamine injection (Fig. 1b): at 1 g before the parabolic flight (PRE), at 0 g (0 G) and 1 g (1 G) onboard the flight and at 1 g, soon after the flight (POST). During the ground sessions, the same setting of 0 g and 1 g in-flight sessions were reproduced, i.e. participants were sitting cross-legged on the floor at 60 cm from the screen of an identical laptop, with the operator sitting in the same position as during the flight. Visual stimuli presentation and data collection were performed using E-prime software. The order of exogenous and endogenous conditions was counterbalanced across subjects.
Exogenous attention task
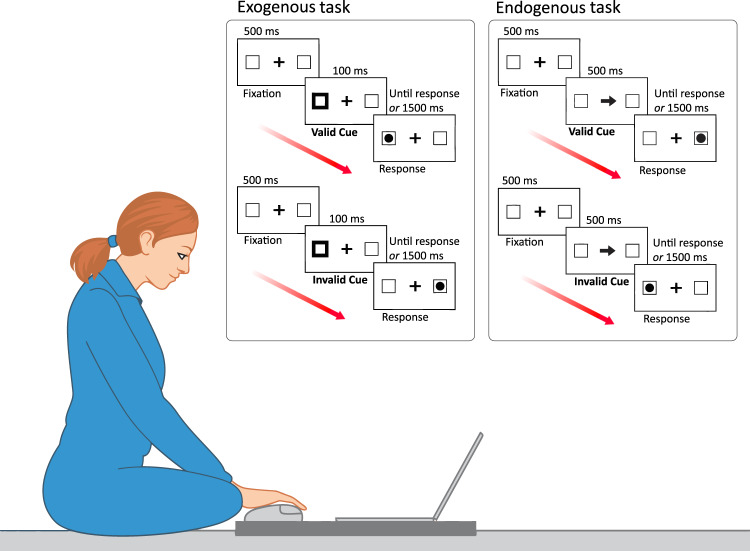
The sequence of experimental events is presented in Fig. 5. Each trial began with a central fixation cross (size: 0.4° × 0.4°) and two lateral boxes (size: 1° × 1°), centred 8° of visual angle to the left and the right of the central fixation. This “Fixation” period lasted 500 ms and was followed by a “Cue” period. Cues consisted of a thickening (from 0.05° to 0.14°) of the contour of one box. Stimulus onset asynchrony (SOA) between cue and target was 100 ms, with the visual target preceded by a valid cue (signalling where the target could appear) or an invalid cue (signalling the spatial position opposite to where the target could appear). Participants were asked to report, as quickly and accurately as possible, the presence of a peripheral visual target (a dot, 0.3° in diameter), presented inside of one of the two boxes, by pressing the left or right button of the mouse. The mouse was fixed on the floor in front of the participant, centred on her/his sagittal midplane. The target remained visible until a response was made or until 1500 ms had elapsed. Participants were instructed to respond only to the targets and not to the orienting cues. They were asked to keep their eyes on the central fixation cross during the entire block of trials38. It is well established that peripheral, non-informative (non-predictive) cues orient attention exogenously4. For this reason, valid trials were 50% of all trials, as it is done in this type of paradigm2,4. Participants were informed about the non-informative value of the cues.
Fig. 5. Schematic of the experimental set-up.
Examples of visual stimuli (original proportions are not preserved in the figure), and the experimental timeline for the Exogenous and Endogenous Tasks.
Stimuli were administered in sessions of five blocks. Within each block 8 valid and 8 invalid trials were presented in random order. For the 0 G condition, each block was administered in correspondence of the parabola 0 g phase and for the 1 G condition, the 5 blocks were presented during the 5 min interval occurring between series of parabolas. Participants’ RTs and accuracy were recorded and constituted the dependent variables.
Endogenous attention task
The sequence of experimental events is presented in Fig. 5. Each trial began with a central fixation cross (size: 0.4° × 0.4°) and two lateral boxes (size: 1° × 1°), presented at 8° of visual angle to the left and the right of the central fixation cross. The “Fixation” period lasted 500 ms and was followed by a “Cue” period in which an arrow took the place of the central fixation cross. The SOA between cue and target was 500 ms, with the visual target preceded by a valid (signalling where the target could appear) or an invalid (signalling the spatial position opposite to where the target could appear) cue. Participants were asked to report, as quickly as possible and accurately as possible, the presence of a peripheral visual target (a dot, 0.3° in diameter) presented inside one of the two boxes, by pressing the left or right button of the mouse that was fixed on the floor. The target remained visible until a response was made or until 1500 ms had elapsed. Participants were instructed to respond only to the targets and not to the orienting cues. They were also asked to keep their eyes on the central fixation cross during the entire block of trials38. It is well established that central informative (i.e. predictive) cues orient attention endogenously4. To make the cue informative, trials were 80% valid and 20% invalid, as it is done in this type of experimental paradigm2,4. Participants were informed about the informative value of the cues. Stimuli were presented in sessions of 5 blocks. Within each block, 14 trials were presented in random order. For the 0 G condition, each block was administered in correspondence of the 0 g phase and for the 1 G condition, the 5 blocks were presented during the 5-min interval occurring between series of parabolas. Participants’ Reaction Times and Accuracy were recorded and constituted the dependent variables.
Statistical analysis
For each participant, RTs faster than 100 ms (i.e. anticipatory responses) and slower than 1000 ms were classified as outliers39. For technical reasons, the data of one subject (S6) in the PRE condition of the exogenous task were not complete and therefore they were not included in the analyses. Given that also data of the endogenous task for the subject who got sick (S1) were not available (and the back-up subject S1_BK only performed the exogenous task), the main statistical analyses were performed on five subjects for all four gravity conditions of the exogenous and endogenous task.
For both the exogenous and endogenous tasks, data were normally distributed as assessed by the Shapiro–Wilk test. Therefore, we performed separate repeated-measures ANOVAs for the two tasks on accuracy and RTs for correct responses with validity (valid and invalid) and condition (PRE, 0 G, 1 G, POST) as within-subject factors. Statistical analyses were performed using SPSS (IBM, Version 26.0) and STATISTICA (Version 12.0) with alpha set at 0.05 (two-tailed). All data are presented as means with the SD. Effect sizes are indicated for significant effects.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We would like to thank all participants who participated in this study. Our special thanks go to the parabolic flight staff at Novespace Bordeaux for the cooperative support before and during the data collection. In addition, we thank Prof. Rosalba Rosato for her valuable suggestions during the paper revision of the statistical issues. This study was funded by the European Space Agency (ESA) Parabolic Flight (PF) programme (Grant CORA-PFC-2018-003). The authors thank the Belgian Federal Science Policy Office (BELSPO) for the provision of financial support in the framework of the PRODEX Programme of the European Space Agency (ESA) under contract number [4000134275]. This work was also supported by MIUR (RICR_RILO_17_01, RICR_RILO_18_02) grants to R.R.
Author contributions
A.S., S.T.C. R.R. coordinated the study. R.R., A.S., S.T.C., A.B., M.S.G., D.R.R. and A.M. designed the study. A.S., R.G., C.I., J.L., D.S. and R.R. performed the experiments. A.S. and R.R. supervised data analyses. R.G., C.I., J.L. and D.S. analysed the data. A.S., R.R., A.B., R.G. and C.I. wrote the paper, S.T.C., M.S.G., D.R.R. and A.M. critically reviewed the paper; all authors approved the final version of the paper; R.R., A.S. and S.T.C. obtained funding designed for the study.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The data are not publicly available due to containing information that could compromise research participant privacy. Please email authors to request de-identified data and we will respond to any reasonable requests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Claudio Iacono, Roberto Gammeri.
Contributor Information
Adriana Salatino, Email: adriana.salatino@uclouvain.be.
Raffaella Ricci, Email: raffaella.ricci@unito.it.
Supplementary information
The online version contains supplementary material available at 10.1038/s41526-021-00159-3.
References
- 1.James, W. Psychology, Briefer Course (Macmillan and Co, London,1892)
- 2.Posner MI. Orienting of attention. Q. J. Exp. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 3.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 4.Chica AB, Martín-Arévalo E, Botta F, Lupiáñez J. The Spatial Orienting paradigm: how to design and interpret spatial attention experiments. Neurosci. Biobehav. Rev. 2014;40:35–51. doi: 10.1016/j.neubiorev.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Dieterich M, Brandt T. The parietal lobe and the vestibular system. Handb. Clin. Neurol. 2018;151:119–140. doi: 10.1016/B978-0-444-63622-5.00006-1. [DOI] [PubMed] [Google Scholar]
- 6.McAuliffe J, Johnson MJ, Weaver B, Deller-Quinn M, Hansen S. Body position differentially influences responses to exogenous and endogenous cues. Atten. Percept. Psychophys. 2013;75:1342–1346. doi: 10.3758/s13414-013-0553-7. [DOI] [PubMed] [Google Scholar]
- 7.Kaliuzhna M, Serino A, Berger S, Blanke O. Differential effects of vestibular processing on orienting exogenous and endogenous covert visual attention. Exp. Brain Res. 2018;237:401–410. doi: 10.1007/s00221-018-5403-3. [DOI] [PubMed] [Google Scholar]
- 8.Clément G, et al. Challenges to the central nervous system during human spaceflight missions to Mars. J. Neurophysiol. 2020;123:2037–2063. doi: 10.1152/jn.00476.2019. [DOI] [PubMed] [Google Scholar]
- 9.Karmali F, Shelhamer M. The dynamics of parabolic flight: flight characteristics and passenger percepts. Acta Astronaut. 2008;63:594–602. doi: 10.1016/j.actaastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clément G, Demel M. Perceptual reversal of bi-stable figures in microgravity and hypergravity during parabolic flight. Neurosci. Lett. 2012;507:143–146. doi: 10.1016/j.neulet.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Clément G, et al. Long-duration spaceflight increases depth ambiguity of reversible perspective figures. PLoS ONE. 2015;10:e0132317. doi: 10.1371/journal.pone.0132317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De la Torre GG. Cognitive neuroscience in space. Life. 2014;4:281–294. doi: 10.3390/life4030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHugh ML. Multiple comparison analysis testing in ANOVA. Biochem. Med. 2011;21:203–209. doi: 10.11613/BM.2011.029. [DOI] [PubMed] [Google Scholar]
- 14.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiebaut De Schotten M, et al. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 16.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mengotti P, Käsbauer AS, Fink GR, Vossel S. Lateralization, functional specialization, and dysfunction of attentional networks. Cortex. 2020;132:206–222. doi: 10.1016/j.cortex.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Bottini G, et al. Identification of the central vestibular projections in man: a positron emission tomography activation study. Exp. Brain Res. 1994;99:164–169. doi: 10.1007/BF00241421. [DOI] [PubMed] [Google Scholar]
- 19.Lopez C, Blanke O, Mast FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience. 2012;212:159–179. doi: 10.1016/j.neuroscience.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Medina J, et al. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. J. Cogn. Neurosci. 2009;21:2073–2084. doi: 10.1162/jocn.2008.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyberg S, Sinn P, Engbert R, Sommer W. Revising the link between microsaccades and the spatial cueing of voluntary attention. Vis. Res. 2017;133:47–60. doi: 10.1016/j.visres.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Pavlidou A, Ferrè ER, Lopez C. Vestibular stimulation makes people more egocentric. Cortex. 2018;101:302–305. doi: 10.1016/j.cortex.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Barrett DJ, Bradshaw MF, Rose D, Everatt J, Simpson PJ. Reflexive shifts of covert attention operate in an egocentric coordinate frame. Perception. 2001;30:1083–1091. doi: 10.1068/p3165. [DOI] [PubMed] [Google Scholar]
- 24.Clément G, Skinner A, Lathan C. Distance and size perception in astronauts during long-duration spaceflight. Life. 2013;3:524–537. doi: 10.3390/life3040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruotolo F, et al. Neural correlates of egocentric and allocentric frames of reference combined with metric and non-metric spatial relations. Neuroscience. 2019;409:235–252. doi: 10.1016/j.neuroscience.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Friesen CK, Moore C, Kingstone A. Does gaze direction really trigger a reflexive shift of spatial attention? Brain Cognit. 2005;57:66–69. doi: 10.1016/j.bandc.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Musso G, Capra R, Ricci R, Salatino A. Human factors engineering activities for past, present and future manned space habitats. Adv. Intell. Syst. Comput. 2019;786:173–182. [Google Scholar]
- 28.Musso G, et al. Habitability issues in long duration space missions far from Earth. Adv. Intell. Syst. Comput. 2018;597:145–154. [Google Scholar]
- 29.Roberts DR, Stahn AC, Seidler RD, Wuyts FL. Towards understanding the effects of spaceflight on the brain. Lancet Neurol. 2020;19:808. doi: 10.1016/S1474-4422(20)30304-5. [DOI] [PubMed] [Google Scholar]
- 30.Clément G, Ngo-Anh JT. Space physiology II: adaptation of the central nervous system to space flight—past, current, and future studies. Eur. J. Appl. Physiol. 2012;113:1655–1672. doi: 10.1007/s00421-012-2509-3. [DOI] [PubMed] [Google Scholar]
- 31.Bigelow RT, Agrawal Y. Vestibular involvement in cognition: visuospatial ability, attention, executive function, and memory. J. Vestib. Res. 2015;25:73–89. doi: 10.3233/VES-150544. [DOI] [PubMed] [Google Scholar]
- 32.Bartolomeo P, Thiebaut De Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb. Cortex. 2007;17:2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- 33.Bottini G, Gandola M. Beyond the non-specific attentional effect of caloric vestibular stimulation: evidence from healthy subjects and patients. Multisens. Res. 2015;28:591–612. doi: 10.1163/22134808-00002504. [DOI] [PubMed] [Google Scholar]
- 34.Ricci R, Calhoun J, Chatterjee A. Orientation bias in unilateral neglect: representational contributions. Cortex. 2000;36:671–677. doi: 10.1016/S0010-9452(08)70544-6. [DOI] [PubMed] [Google Scholar]
- 35.Gammeri R, Iacono C, Ricci R, Salatino A. Unilateral spatial neglect after stroke: current insights. Neuropsychiatr. Dis. Treat. 2020;16:131–152. doi: 10.2147/NDT.S171461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Lochner M, Thompson A. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT3 receptors. Neuropharmacology. 2016;108:220–228. doi: 10.1016/j.neuropharm.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connell, C., Thompson, B., Kuhn, G. & Gant, N. Exercise-induced fatigue and caffeine supplementation affect psychomotor performance but not covert visuo-spatial attention. PLoS ONE10.1371/journal.pone.0165318 (2016). [DOI] [PMC free article] [PubMed]
- 39.Hayward, D. A. & Ristic, J. Measuring attention using the Posner cuing paradigm: the role of across and within trial target probabilities. Front. Hum. Neurosci. 10.3389/fnhum.2013.00205 (2013). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The data are not publicly available due to containing information that could compromise research participant privacy. Please email authors to request de-identified data and we will respond to any reasonable requests.