Abstract
Introduction:
Contemporary national trends in repair of ruptured abdominal aortic aneurysms and intact abdominal aortic aneurysms are relatively unknown. Furthermore, screening is only covered for patient’s 65 to 75 years old with a family history or men with a smoking history. It is unclear what proportion of patients who present with a ruptured aneurysm would have been candidates for screening.
Methods:
Using the National Inpatient Sample from 2004 to 2015, we identified rupture and intact AAA admissions and repairs based on International Classification of Diseases codes._We generated the screening eligible cohort using previously identified proportions of male smokers (87%) and all patients with a family history of aneurysm (10%) and applied these proportions to patients aged 65-75. We accounted for those who may have had a prior AAA diagnosis (17%) either from screening or incidental detection in patients over age 75 presenting with rupture. The primary outcomes were treatment and in-hospital mortality stratified by patients meeting criteria for screening versus those who did not.
Results:
We evaluated 65,125 admissions for ruptured AAA and 461,191 repairs for intact AAA. Overall, an estimated 45,037 (68%) of patients admitted and 25,777 (59%) of patients undergoing repair for ruptured AAA did not meet criteria for screening. Of the patients who did not qualify; 27,653 (63%) were older than 75 years old; 10,603 (24%) were younger than 65 years old; and 16,103 (36%) were females. EVAR use increased for ruptured AAA from 10% in 2004 to 55% in 2015 (P<0.001) with an operative mortality of 35%, and for intact AAA from 45% in 2004 to 83% in 2015 (P<0.001) with an operative mortality of 2.0%.
Conclusions:
The majority of patients who underwent repair for ruptured AAA did not qualify for screening. EVAR is the primary treatment for both ruptured AAA and intact AAA with a relatively low in-hospital mortality. Therefore, expansion of screening criteria to include selected women and a wider age range should be considered.
Table of Contents Summary
In this analysis of a large population-based dataset, we found that EVAR is associated with lower in-hospital mortality. The majority of patients admitted for ruptured aneurysm are not included in the current screening guidelines. Therefore, expansion of screening criteria to include selected women and a wider age range should be considered.
INTRODUCTION
Abdominal aortic aneurysms (AAA) are the 15th leading cause of death in the United States.1 Mortality remains high in the setting of a ruptured aneurysm despite the introduction of minimally invasive endovascular abdominal aortic aneurysm repair (EVAR).2 Prophylactic repair offers much lower mortality and complication rates compared with repair of ruptured aneurysms.3, 4 US Preventative Task Force (USPTF) recommends screening men between the ages 65 to 75 years with a caveat to selectively screen men with no smoking history.5 In 2007, the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAVE) Act was implemented. Consequently, the Centers for Medicare and Medicaid Services (CMS) initiated reimbursement for a one-time aortic ultrasound for men aged 65 to 75 who have a smoking history and for men or women in this age group with a family history of AAA.6 While the screening policy has likely contributed to the decrease in the incidence of ruptured AAA, the proportion of ruptured AAA occurring in patients currently excluded from screening is unknown.7 Existing data suggest that excluded populations might also benefit from screening.8, 9
We therefore, analyzed data from the National (Nationwide) Inpatient Sample (NTS) Database from 2004 to 2015 to identify the proportion of patients that present for aortic repair that were not eligible for screening. Additionally, we investigated the contemporary trends in admissions, treatment patterns, and outcomes for ruptured and intact AAA repair.
METHODS
Data Source
We performed a retrospective cohort analysis of ruptured AAA diagnosis and repair as well as intact AAA repair using the National Inpatient Sample, formerly known as the Nationwide Inpatient Sample (NIS). The NTS was developed and maintained by the Healthcare Cost and Utilization Project and the Agency for Healthcare Research and Quality (AHRQ). NIS currently collects in-hospital diagnoses and procedures for 20% of all discharges of non-veterans hospitals in participating states (48/50). Prior to 2012, the NIS collected all hospital discharges from 20% of hospitals. The NIS stratifies the hospitals and applies discharge weights to the sample by using information from the National Census Bureau to generate the national estimate. A list of participating states as well as more information can be found online: www.hcup-us.ahrq.gov/nisoverview.jsp. This manuscript was written according to the STROBE guidelines.10
Study population
We included admissions from 2004 to 2015. Because the diagnosis codes changed from International Classification of Diseases (ICD) ICD-9 to ICD-10 at the end of 2015, we excluded the last quarter of 2015 to reduce coding variability. We defined the study cohort as admissions with both an diagnosis of AAA (ICD-9: 441.3-Abdominal Aneurysm with Rupture and 441.4-Abdominal Aneurysm Without Rupture) and procedure of AAA repair (ICD-9: 38.34-Aorta resection and Anastomosis, 39.25 Aorta-Iliac femoral bypass, 38.44-Replacement of Abdominal Aorta, 38.64-Excision of Aorta, 39.52-Other repair of Aneurysm, 39.71-Endovascular Abdominal Aorta Repair).
Clinical and Outcome Variables
For all patients, we collected age, sex, and used the predefined Elixhauser covariates to identify comorbidities.11 The primary outcomes were the admission, treatment, and in-hospital mortality for patients eligible for screening compared to those who were ineligible. Secondary outcomes included trends in the proportion of EVAR and open repair annually, for both intact and ruptured AAA as well as trends in mortality. Outcomes were compared between EVAR and open repair for both intact and ruptured AAA separately. We performed an additional analysis evaluating patients older than 65 and patients with the primary payer as Medicare in order to directly assess the number and proportion of patients covered by Medicare.
Study Population
For the ruptured aneurysms, we estimated a screening eligible and not eligible cohort based on national estimates, as the NIS does not collect information on smoking status or family history. Published data from the Vascular Quality Initiative (representing over 550 institutions from the USA and Canada) registry reported that 10% of both men and women who underwent AAA repair had a family history of AAA, and 87% of male patients who undergo an AAA repair have a smoking history.12, 13 To estimate the number of male patients who would qualify for screening, we used 87% of the number of male patients between the age of 65 and 75, based on presumed smoking history. Of the remaining 13% we estimated that 10% would qualify based on a presumed family history, prior analysis showed their smoking rates were similar for familial and sporadic AAA.12 We assumed 10% of female patients aged 65 to 75 had a family history and would thus be included. We took the summation of these populations to create the estimated screening eligible cohort for ruptured aneurysms. According to a prior Medicare analysis 17% of patients who presented with ruptured aneurysm had a previous diagnosis of AAA.14 We therefore included 17% of the patients over 75 years to account for those with a prior diagnosis that mya have come from a screening study. This assumption that 17% of the patients had a prior diagnosis from screening is almost certainly an overestimation since it is likely that many of these patients had the AAA detected incidentally. However, given the lack of definitive data, we wanted to show the best-case scenario
Statistical Analysis
Categorical variables are presented as percentages and compared using Pearson’s χ2 tests. We performed a Wald test to assess the difference of means for continuous variables. We performed a univariate regression analysis with time as a continuous dependent variable and outcomes as independent variables to test for trends in outcomes over time. For the graphs reporting absolute numbers by year we extrapolated numbers for 2015 based on the estimates in the first three quarters of that year. All variables had less than <3% missing data.
NIS uses de-identified data and therefore the Institutional Review Board of the Beth Israel Deaconess Medical Center waived the requirement of an informed consent. All statistical analyses were performed using Stata 15.1 (StataCorp, College Station, TX, USA).
RESULTS
Ruptured AAA Repair
From 2004 to 2015, there were 65,125 patients admitted with a diagnosis of ruptured AAA, of which 69% underwent repair. There was a significant decrease in the number of admissions, (6,461 in 2004 to 4,848 in 2015; P<.001) operations for ruptured AAA, (4,445 to 3,283; P<.001) and proportion of patients undergoing repair (71% to 67% P<.001) over the study period (Figure 1a). Of all patients undergoing repair, 14,012 (31%) underwent EVAR and 31,693 (69%) underwent open repair (Table 1). In 2004, 10% of all ruptured AAA repairs were performed with EVAR, but EVAR surpassed open repair by 2014, and 55% of all repairs were performed using EVAR in 2015 (P<.001; Figure 1a).
Figure 1a:
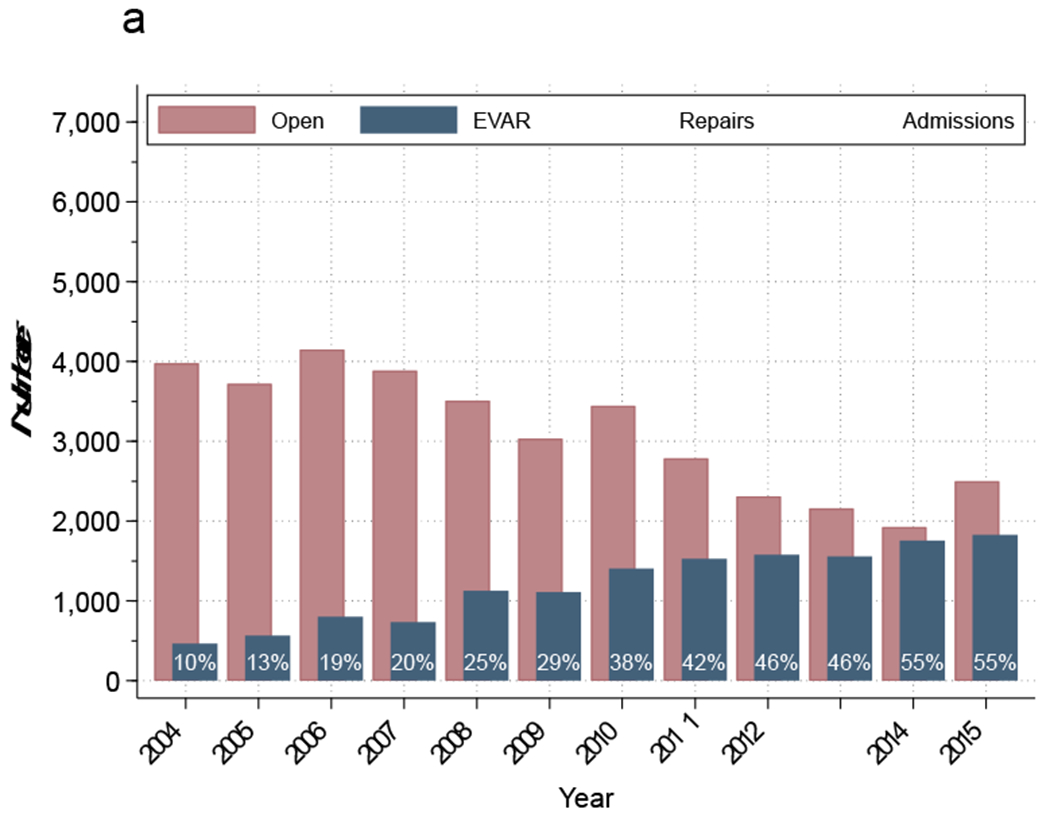
Proportion of ruptured abdominal aortic aneurysms stratified by treatment annually
Table 1:
Baseline demographics and outcomes for Ruptured AAA by repair type
| Ruptured AAA | ||||
|---|---|---|---|---|
| Repair | EVAR | Open | P-value | |
| Total | 45,117 | 14,011 | 31,693 | |
| Mean Age (SD) | 73.1 (.11) | 73.8 (.19) | 72.7 (.13) | <0.001 |
| Female | 23% | 21% | 24% | 0.02 |
| White Race | 86% | 86% | 86% | 0.50 |
| Congestive Heart Failure | 2.0% | 1.6% | 2.1% | 0.08 |
| Diabetes Mellitus | 13% | 15% | 12% | <0.001 |
| Renal Failure | 17% | 20% | 15% | <0.001 |
| Obesity | 8.7% | 10% | 8.0% | <0.001 |
| Income <50% of median household | 52% | 52% | 51% | 0.62 |
| Insurance | ||||
| Medicare | 72% | 74% | 72% | 0.07 |
| Medicaid | 3.0% | 2.7% | 3.1% | 0.25 |
| Private Insurance | 19% | 18% | 20% | 0.15 |
| Self Pay | 3.3% | 2.7% | 3.5% | 0.10 |
| No Charge | 0.3% | 0.4% | 0.2% | 0.13 |
| Other | 2.3% | 2.5% | 2.2% | 0.26 |
| Ruptured AAA Outcomes | ||||
| Overall | EVAR | Open | P-Value | |
| In-Hospital Mortality | 35% | 25% | 40% | <0.001 |
Ruptured AAA Outcomes
Overall in-hospital mortality following repair of ruptured AAA was 35%. However, there was a decrease in mortality over the study period, from 42% in 2004 to 28% in 2015 (P<.001). For the entire study period, patients undergoing EVAR had lower rates of in-hospital mortality compared to open repair (25% vs. 40%, P<.001) (Table 1). The mortality after EVAR decreased over time (30% to 21%, P=0.05). There was also a decrease in mortality following open repair over time (44 vs. 36%, P<.001) (Figure 1b).
Figure 1b:
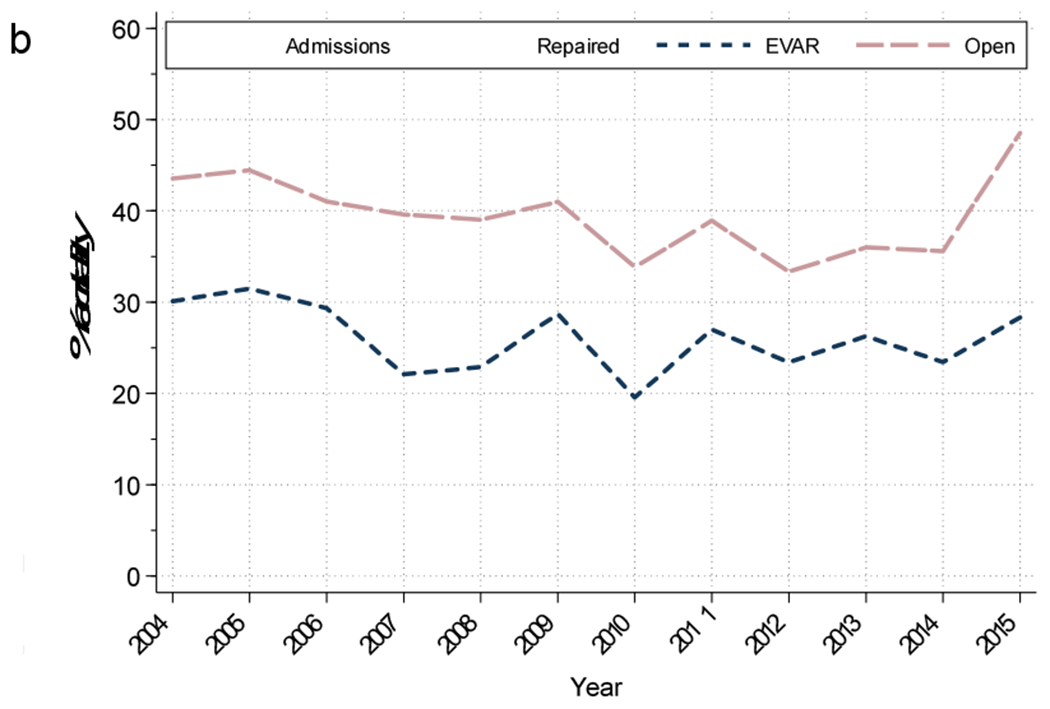
Mortality of those with ruptured abdominal aortic aneurysms who were admitted, underwent repair, and stratified by repair each year. EVAR, Endovascular abdominal aortic aneurysm repair.
Ruptured AAA by Screening Eligibility
Of the 65,125 patients admitted with a diagnosis of rupture, 44,155 (68%) would have been ineligible for screening. Figure 2 outlines the breakdown of the screening eligibility cohort. There were 18,755 women admitted with rupture, 16,103 (86%) of whom would be ineligible for screening due to age or no presumed family history or prior diagnosis. Of the 4,623 women between the ages of 65 and 75, we would expect 10% to have a family history resulting in a total of 462 women to be included in the screening eligible. Furthermore, to account for those patients with a prior diagnosis, we included 17% of women greater than 75 years (2,147 women) in the screening eligible cohort. Thus, the vast majority (86%) of women with rupture were in the screening ineligible cohort. There were 46,371 men admitted with rupture, 29,715 (64%) of whom would be ineligible for screening due to age. Of the 16,655 men between the ages of 65 and 75, we predict 87% (14,490) to be eligible for screening due to a smoking history. Of the remaining 2,165 (13%), we estimated that 217 (10%) of those patients could be eligible for screening due to a positive family history. To estimate the total number of patients with a prior diagnosis, we included 17% of all patients greater than age 75 (33,455) to result in 5,802 patients. We then added the estimates, for a total of 20,971 (32%) patients who qualified for screening (Figure 2).
Figure 2:
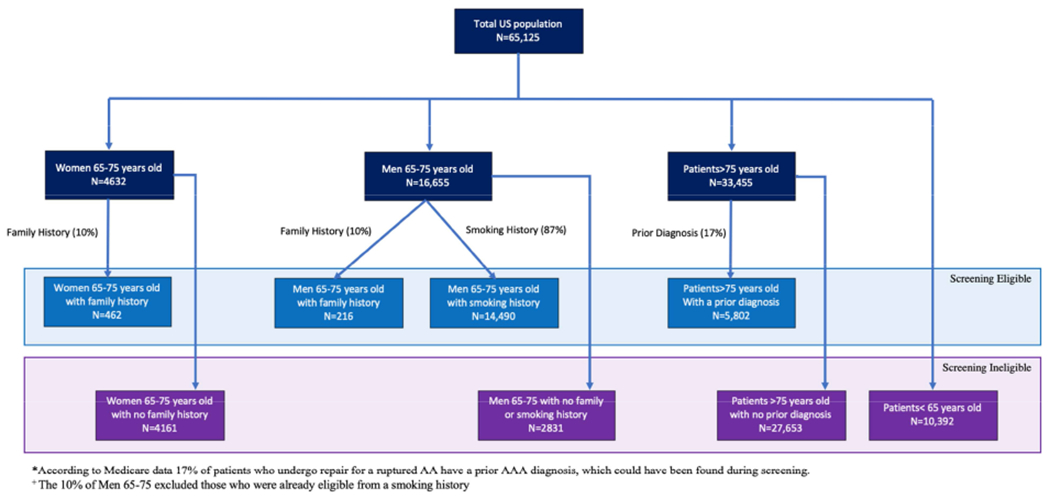
Flow chart showing inclusion criteria for patients who met the screening criteria for ruptured abdominal aortic aneurysms (AA, AAA).
Patients older than 75 years constituted 63% of the screening ineligible population, whereas patients younger than 65 accounted for 24% of the cohort, and women comprised 36% (some of whom were also below 65 or above 75 years old), and women 65-75 made up 13% of the cohort. Of those patients admitted with a rupture who died, 70% were screening ineligible. Among patients admitted with a ruptured AAA, those who were ineligible for screening had a higher hospital mortality rate than eligible patients (45% vs. 34%, P<0.001). Perioperative mortality for the screening eligible cohort was 39% compared to 30% (P<0.001) for the screening ineligible. However, since the majority (59%) of patients taken to the operating room for repair were not eligible for screening, ineligible patients comprised the majority of postoperative deaths (65%) (Figure 3).
Figure 3:
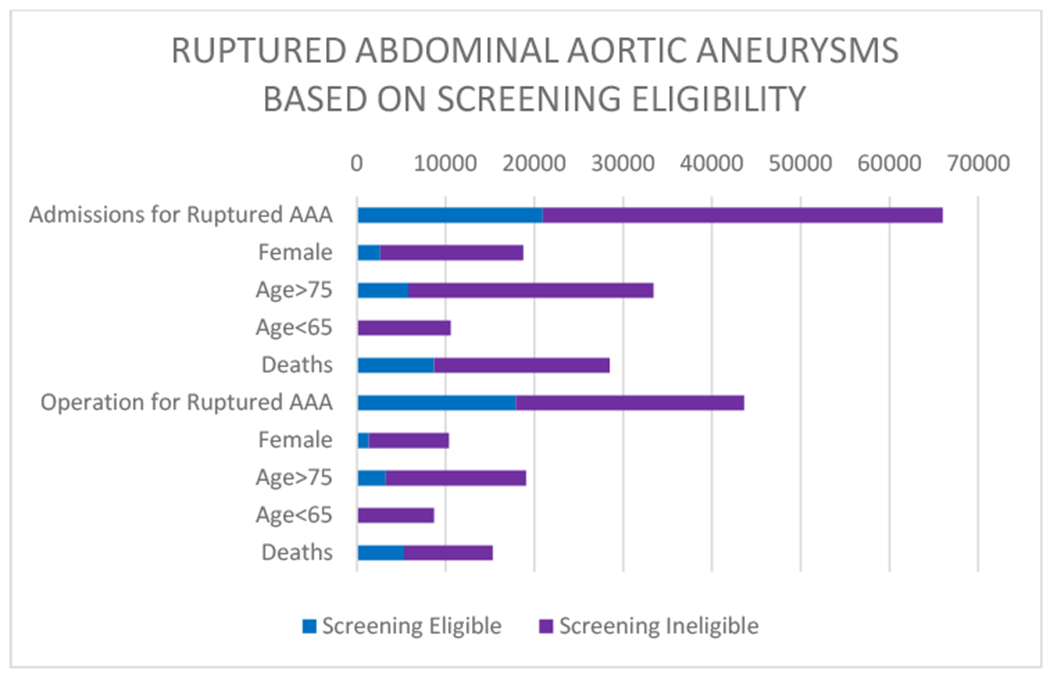
Graph showing ruptured abdominal aortic aneurysms (AAAs) stratified by screening eligibility.
Ruptured AAA in Women
There were 65,125 patients admitted for ruptured AAA, and 18,755 (29%) were women. Of the 45,117 (69%) patients admitted with ruptured AAA who underwent repair, 10,388 (23%) were women of which 29% underwent EVAR. Female patients accounted for disproportionately high percentages of in-hospital mortality, comprising 35% of deaths in all comers with ruptures, and 27% of deaths following repair. Following repair, women had a 41% postoperative mortality (31% EVAR vs. 45% open; P<0.001)
Ruptured AAA in Patients Younger than 65 Years
Patients younger than 65 years old represented 16% of all ruptured AAA admissions, and 11% of all deaths for those admitted with ruptured AAA. Younger patients represented 19% of those who underwent an operation for ruptured AAA (30% EVAR), and 11% of the postoperative deaths. These patients experienced a postoperative mortality of 20%, (13% EVAR vs. 23% open; P<0.001) where 13% of these younger patients were female.
Ruptured AAA in Patients Older than 75 Years
Patients older than 75 years old represented 51% of those patients admitted with ruptured AAA but accounted for 64% of deaths. These older patients made up 42% of those who went to the operating room (33% EVAR) and represented 55% of the postoperative deaths. Older patients who went to the operating room, experienced a 45% postoperative in-hospital mortality (33% EVAR vs. 51% open) where 29% were female.
Intact AAA Repair
We identified 461,191 patients who underwent repair of intact AAA, of which 70% were performed using EVAR and 30% open repair (Table 2). There was a significant decrease in the number of operations for intact AAA repair over the study period (40,225 in 2004 to 33,488 in 2015; P<.001) (Figure 4a). Of all patients undergoing repair, 322,111 (73%) underwent EVAR and 143,538(27%) underwent open repair (Table 2). In 2005, EVAR surpassed the number of open repairs for intact AAA repair, while the proportion of EVAR reached 85% by 2015 (Figure 4a).
Table 2:
Baseline demographics and outcomes for Intact AAA by repair type
| Intact AAA | ||||
|---|---|---|---|---|
| Repair | EVAR | Open | P-value | |
| Total | 461,191 | 322111 | 143538 | - |
| Mean Age (SD) | 72.6 (.05) | 73.6 (.05) | 70.3 (.08) | <0.001 |
| Female | 21% | 19% | 27% | <0.001 |
| White Race | 88% | 88% | 88% | 0.15 |
| Congestive Heart Failure | 0.4% | 0.3% | 0.7% | <0.001 |
| Diabetes Mellitus | 16% | 17% | 14% | <0.001 |
| Renal Failure | 12% | 11% | 12% | 0.58 |
| Obesity | 7.9% | 8.3% | 7.0% | <0.001 |
| Income <50% of median household | 51% | 51% | 52% | 0.49 |
| Insurance | ||||
| Medicare | 77% | 80% | 70% | <0.001 |
| Medicaid | 2.0% | 1.6% | 2.5% | <0.001 |
| Private Insurance | 19% | 16% | 24% | <0.001 |
| Self Pay | 0.9% | 0.7% | 1.4% | <0.001 |
| No Charge | 0.1% | 0.1% | 0.2% | <0.001 |
| Other | 1.5% | 1.4% | 1.7% | <0.001 |
| Intact AAA Outcomes | ||||
| Overall | EVAR | Open | P-Value | |
| In-Hospital Mortality | 2.0% | 0.9% | 4.7% | <0.001 |
Figure 4a:
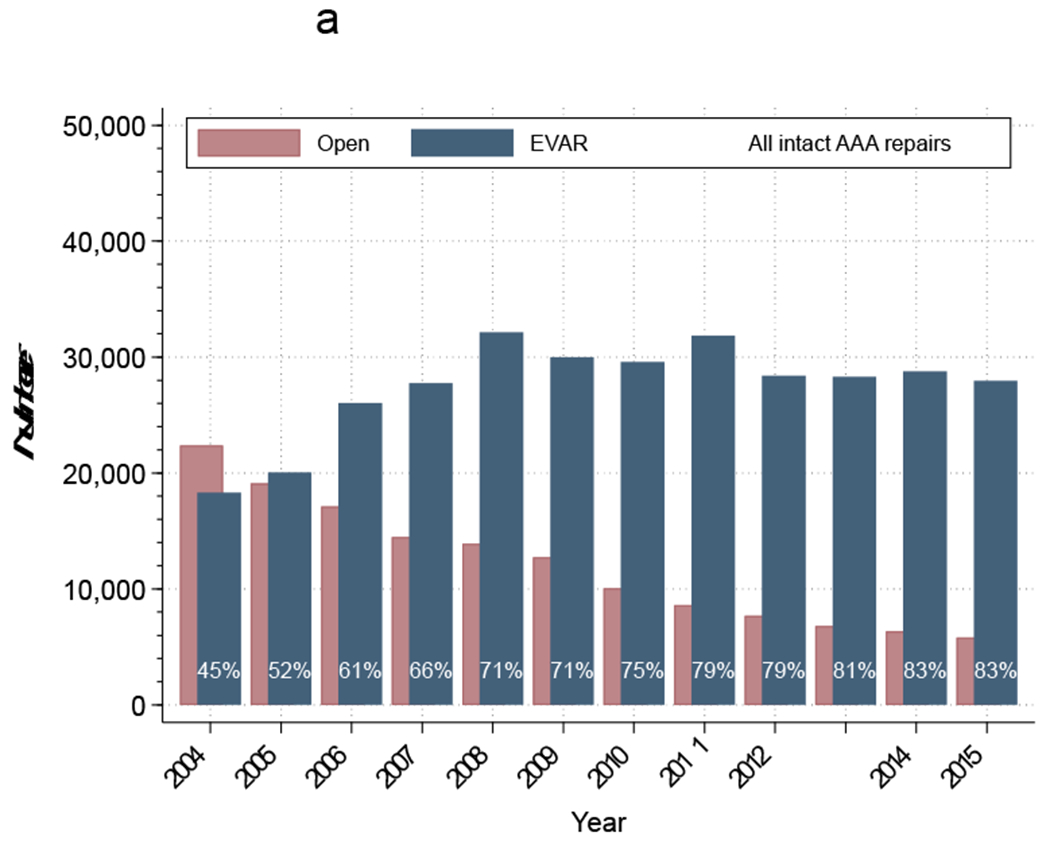
Proportion of intact abdominal aortic aneurysms (AAAs) treated annually
Intact AAA Repair Outcomes
The in-hospital mortality rate of all admissions following intact repair with either EVAR or open was 2.0%, representing a decrease from 2.8% in 2004 to 1.6% in 2015 (P<.001; Figure 4b). The overall mortality following EVAR was 0.9%, which was lower than the 4.7% following open repair (P<.001; Table 2). The mortality following EVAR decreased over the study period from 0.9% to 0.8% (P<.001), while the mortality following elective open repair increased from 4.4% to 6.0% (P=0.02; Figure 4b).
Figure 4b:
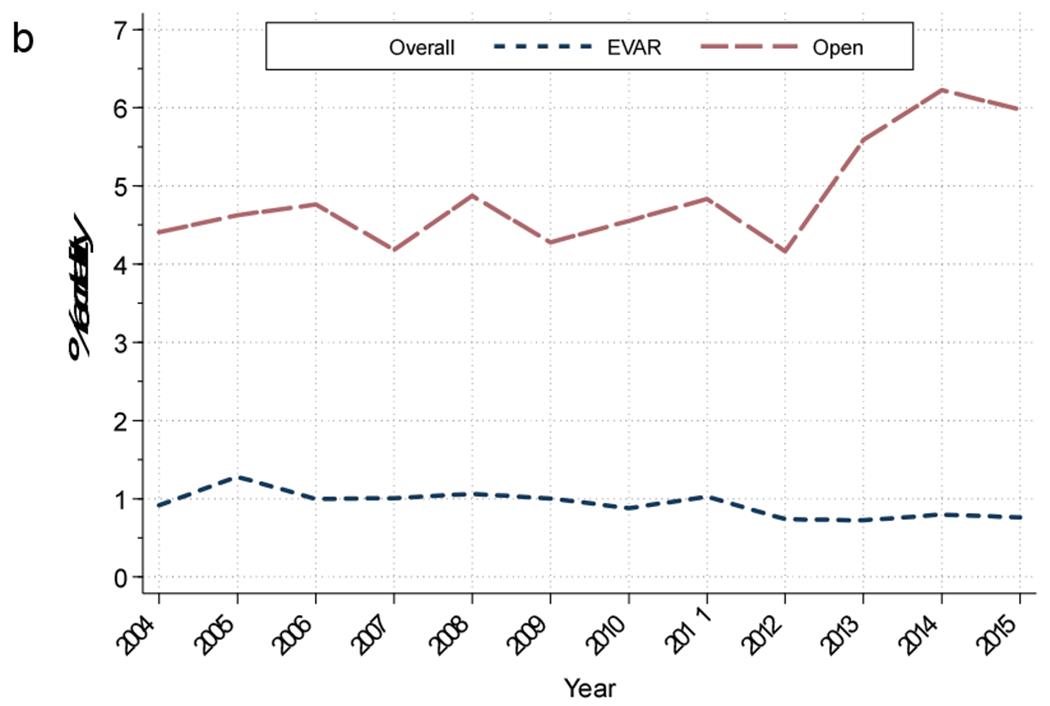
Mortality of those admitted for intact AAA repair. EVAR, Endovascular abdominal aortic aneurysm repair.
Intact AAA in Women
Women comprised 21% (96,815) of all patients treated for intact AAA (62% EVAR and 38% open) but made up 37% of all patients who died following intact repair with a perioperative mortality of 3.6% (2.0% EVAR and 6.6% open repair). The average age of women undergoing intact repairs was 74 compared with 72 years old for men.
Intact AAA in Patients Younger than 65 Years
Patients younger than 65 years old comprised 17% of patients treated for an intact AAA (58% EVAR and 42% open), with a lower proportion of women (15% vs. 22%, P<0.001). Of the patients who died following intact repair young patients comprised about 11% with a perioperative mortality of 1.3% (0.5% EVAR and 2.4% open).
Intact AAA in Patients Older than 75 Years
Patients older than 75 years old represented 40% (184,258) of those who underwent repair for intact AAA (77% EVAR and 23% open), and these patients made up 53% of those who died post operatively. There were 25% women in this age cohort. Overall, patients older than 75 years old experienced a postoperative mortality of 2.7% (1.3% EVAR and 7.5% open).
DISCUSSION
We found that 68% of patients admitted for ruptured AAA were not candidates for screening. The majority of patients who did not qualify for screening were older than 75 years old (61%), 24% were younger than 65, 9% were women without a family history between the ages 65 and 75, and the remaining 6% were men between 65 and 75 years old without a smoking or family history. We confirmed the increasing dominance of EVAR, the decreasing overall repair mortality, and the consistent lower mortality after EVAR compared to open repair. The overall mortality of intact repair remains low, even for the elderly when using EVAR, although the mortality with open repair is rising.
When Congress passed the SAAVE Act, the USPSTF also issued their first recommendations for AAA screening, recommending a one-time ultrasound for men ages 65 to 75 with a smoking history.15, 16 However, the current USPSTFs’ guidelines, updated in 2019 to include men ages 65 to 75 with a family history, continue to recommend against screening women.5 Furthermore, both sets of guidelines are based primarily on four randomized clinical trials, which studied almost exclusively open repair in men, the majority of whom were age 65-75.17–20 We found that EVAR became the primary treatment modality for ruptured AAA in 2014, and for intact aneurysm repair in 2005, reaching 85% of all intact AAAs repaired by 2015.
In guidelines that are not representative of the current experience, the advantage of EVAR is missed when calculating which patients would benefit from this low risk procedure. Patients over the age of 75 constitute over half of the patients admitted with rupture and represent a critical and growing screening ineligible population. This population has a particularly high benefit of undergoing intact repair given the difference in mortality rate of 45% for rupture repair vs 1.3% for intact repair. While there are certain high-risk patients who may not benefit from EVAR, validated risk prediction models can be used to aid in the preoperative clinical decision-making.21 A study evaluating elective EVAR for patients aged 75 years or older recently found a 1.4% perioperative mortality and an 88% five-year survival.8 With the increasing use of EVAR, formerly higher-risk patients now have a robust option for repair, and we believe that the screening guidelines should be expanded to reflect this notion. Given the life-expectancy of 12 years of those patients who live to 75 and low operative mortality with EVAR, it may be inappropriate to withhold screening for these patients.22
Our data corroborate published data that approximately 20% of the patients who ruptured are currently younger than 65 years old.23 Younger patients have excellent one-year survival of 97% when AAA is repaired in an elective setting.24 Furthermore, cost prediction models demonstrate an improvement in quality-adjusted life years and suggest a potential cost saving by repairing younger patients.25 Discerning which patients younger than 65 should qualify for screening is complex. Patients with a family history of AAA have higher rates of rupture and some have questioned if these patients would benefit from earlier screening as their pathology appears to be more aggressive.26, 27 Other studies have identified modifiable risk factors that increase the odds of AAA, which may help further identify which of these patients may benefit from earlier screening.28 This question should be an area of further investigation.
Few studies have assessed the clinical and cost benefit of screening women and those that do contain significant limitations. The one randomized controlled trial was underpowered, and the most recent cost analysis used data comprised of disproportionate numbers of open repairs resulting in a high operative mortality.29,30 These data lead to the current screening guidelines. Medicare only reimburses screening in women with a family history which according to these data is only 2% of those women who rupture. The USPSTF guidelines, which recommend against screening women entirely, exclude approximately 30% of the patients presenting with a ruptured AAA, according to our study.31
Because the clinical picture and decision-making differs between the sexes, the same criteria used to justify screening in men may not be appropriate for women. However, the United Kingdom’s National Institute for Health Care and Excellent guidelines for AAA diagnosis and management, found benefit for AAA screening for a prevalence as low as 0.35%.32 It would therefore be reasonable to conclude that screening women who have a prevalence of 1.7% (described in women with a smoking history) would be cost-effective, as was recommended in the NICE guidelines.33–35 Furthermore, there is evidence that women with ectatic infrarenal aortas may benefit from up to five year follow-up with ultrasounds.36 While there are data showing that women not only rupture at smaller aneurysm diameters but also experience a higher mortality when repaired in the urgent setting the disproportionate outcomes are mitigated when AAA are repaired in the elective setting.37, 38 Based on these data elective repair optimizes the outcomes and reduces the disparity between the sexes.
Mortality following intact open AAA repair has increased over time from 4.3% in 2004 to 5.5% in 2015. This may be an aggregate effect of the increased use of EVAR and selection bias, as patients with more complex anatomy unsuited for EVAR are more likely to undergo open repair. While the endovascular options for patients with more complex anatomy are increasing, not all hospitals participate in complex endovascular repair, leaving patients with open repair as the only option.39 It is not clear that endovascular repair of complex aneurysms is superior to open repair as long-term data are lacking. In addition, there is a well-established association of higher-volume centers and surgeons having lower operative mortality following open repair.40 These data support the SVS guidelines that open repair should be limited to centers performing ten or more procedures annually.41 Additionally, with only 15% of intact AAA repairs performed with open surgery, there is concern that trainees will not be prepared to perform open aneurysm repairs in practice.42 Further research needs to be done to explore ways to supplement the education of trainees.
The numbers of ruptured AAA admissions decreased overtime (6,461 in 2004 to 4,848 in 2015; P<.001). We do hypothesize that screening and the evolution of EVAR are partly responsible for this reduction as well as decreased rates of smoking.43, 44 However, the number of repairs for ruptured AAA remained largely unchanged which calls to question if screening is being fully utilized. Other studies have demonstrated that screening rates remain low even after enacting the SAAVE act.45 European countries have effectively expanded their screening programs with a 90% inclusion rate.46 If the US were able to improve adherence to screening criteria expanding criteria to include patients older than 75 would be superfluous.
There are limitations to this study, which must be interpreted in the context of the design and source of the data. NIS does not include information about family history, smoking status, or prior AAA diagnosis. We applied previously described proportions to this population to estimate those patients who would be eligible for screening. Furthermore we are unable to determine which patients over 75 years old had a screening ultrasound but were considered unfit for elective repair.
CONCLUSION
The majority of patients with a ruptured AAA did not meet criteria for screening, suggesting a need for reconsideration of the current screening paradigm. Of those admitted with a ruptured AAA, over half were older than 75 and almost a quarter were younger than 65 and with women making up a significant portion of the population. Based on prior risk factors such as age, cardiovascular disease and tobacco use, further studies are needed to design a more sensitive screening algorithm to capture the high-risk patients in these excluded populations. In addition, the current guidelines should reflect the current management of AAA where EVAR is the predominant treatment and is associated with a low post-operative mortality.
Article Highlights.
Type of Research:
A retrospective analysis of prospectively collected data from a national administrative database
Key Findings:
We identified a total of 65,125 admissions for ruptured abdominal aortic aneurysms (AAAs) and 461,191 repairs for intact AAAs. Of the patients who had presented with ruptured AAAs and those who had undergone repair of reputed AAAs. 68% and 59% had not qualified for screening, even after accounting for patients with a previous diagnosis.
Take Home Message:
The majority of patients who were admitted and underwent repair for ruptured AAA did not meet criteria for screening. Endovascular repair overtook open repair as the primary treatment for ruptured and intact AAA and mortality of reptured AAA decreased over the study time period.
Acknowledgement
Would like to acknowledge Harvard Catalyst Program for help in the statistical analysis.
Sources of Funding:
KD and PL are supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734-22
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
MLS : Abbott, Cook, Medtronic, Endologix
References
- 1.Prevention CfDCa. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race, and sex. United States: 2015. [Google Scholar]
- 2.Abdulameer H, Al Taii H, Al-Kindi SG, Milner R. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378–384.e372. [DOI] [PubMed] [Google Scholar]
- 3.Mani K, Bjorck M, Lundkvist J, Wanhainen A. Improved long-term survival after abdominal aortic aneurysm repair. Circulation. 2009;120:201–211. [DOI] [PubMed] [Google Scholar]
- 4.Dua A, Kuy S, Lee CJ, Upchurch GR Jr., Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59:1512–1517. [DOI] [PubMed] [Google Scholar]
- 5.Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary Care Screening for Abdominal Aortic Aneurysm: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2019;322:2219–2238. [DOI] [PubMed] [Google Scholar]
- 6.Services. USCfMM. Abdominal aortic aneurysm screenings. 2019; Available from: https://www.medicare.gov/coverage/abdominal-aortic-aneurysm-screenings.
- 7.Ali MU, Fitzpatrick-Lewis D, Miller J, Warren R, Kenny M, Sherifali D, et al. Screening for abdominal aortic aneurysm in asymptomatic adults. J Vasc Surg. 2016;64:1855–1868. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell TFX, Wade JE, Liang P, Li C, Swerdlow NJ, DeMartino RR, et al. Endovascular aneurysm repair in patients over 75 is associated with excellent 5-year survival, which suggests benefit from expanded screening into this cohort. J Vasc Surg. 2019;69:728–737. [DOI] [PubMed] [Google Scholar]
- 9.Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML, et al. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg. 2013;57:1261–1268, 1268, e1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12.Ryer EJ, Garvin RP, Zhou Y, Sun H, Pham A, Orlova K, et al. Outcomes of familial abdominal aortic aneurysm repair in the Vascular Quality Initiative. J Vasc Surg. 2019;69:717–727.e711. [DOI] [PubMed] [Google Scholar]
- 13.Zettervall SL, Buck DB, Soden PA, Cronenwett JL, Goodney PP, Eslami MH, et al. Regional variation exists in patient selection and treatment of abdominal aortic aneurysms. J Vasc Surg. 2016;64:921–927.e921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards ST, Schermerhorn ML, O’Malley AJ, Bensley RP, Hurks R, Cotterill P, et al. Comparative effectiveness of endovascular versus open repair of ruptured abdominal aortic aneurysm in the Medicare population. J Vasc Surg. 2014;59:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Congress tU. Deficit Reduction Act of 2005. 2005.
- 16.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for Abdominal Aortic Aneurysm: A Best-Evidence Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:203–211. [DOI] [PubMed] [Google Scholar]
- 17.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. [DOI] [PubMed] [Google Scholar]
- 18.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82:1066–1070. [DOI] [PubMed] [Google Scholar]
- 19.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindholt JS, Sorensen J, Sogaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826–834. [DOI] [PubMed] [Google Scholar]
- 21.Neal D, Beck AW, Eslami M, Schermerhorn ML, Cronenwett JL, Giles KA, et al. Validation of a preoperative prediction model for mortality within 1 year after endovascular aortic aneurysm repair of intact aneurysms. J Vasc Surg. 2019;70:449–461.e443. [DOI] [PubMed] [Google Scholar]
- 22.Living AfC. 2017 Profile of Older Americans. Administration on Aging, Services USDoHaH; 2018. [Google Scholar]
- 23.Laine MT, Vanttinen T, Kantonen I, Halmesmaki K, Weselius EM, Laukontaus S, et al. Rupture of Abdominal Aortic Aneurysms in Patients Under Screening Age and Elective Repair Threshold. Eur J Vasc Endovasc Surg. 2016;51:511–516. [DOI] [PubMed] [Google Scholar]
- 24.Liang NL, Reitz KM, Makaroun MS, Malas MB, Tzeng E. Comparable perioperative mortality outcomes in younger patients undergoing elective open and endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2018;67:1404–1409.e1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant SW, Sperrin M, Carlson E, Chinai N, Ntais D, Hamilton M, et al. Calculating when elective abdominal aortic aneurysm repair improves survival for individual patients: development of the Aneurysm Repair Decision Aid and economic evaluation. Health Technol Assess. 2015;19:1–154, v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Luijtgaarden KM, Verhagen HJ. What a vascular surgeon should know about familial abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2015;50:137–138. [DOI] [PubMed] [Google Scholar]
- 27.Sakalihasan N, Defraigne JO, Kerstenne MA, Cheramy-Bien JP, Smelser DT, Tromp G, et al. Family members of patients with abdominal aortic aneurysms are at increased risk for aneurysms: analysis of 618 probands and their families from the Liege AAA Family Study. Ann Vasc Surg. 2014;28:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. [DOI] [PubMed] [Google Scholar]
- 29.Sweeting MJ, Masconi KL, Jones E, Ulug P, Glover MJ, Michaels JA, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002;89:283–285. [DOI] [PubMed] [Google Scholar]
- 31.Quality AfHRa. Guide to Clinical Preventive Services, 2014. 2014. [cited 2019 August 19]; Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening.
- 32.National Institute for Health and Care Excellence Abdominal aortic aneurysm: diagnosis and management Evidence review A: Risk factors for predicting presence of an abdominal aortic aneurysm. 2018. [PubMed] [Google Scholar]
- 33.Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673–681.e671. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell TFX, Landon BE, Schermerhorn ML. AAA Screening Should Be Expanded. Circulation. 2019;140:889–890. [DOI] [PubMed] [Google Scholar]
- 35.Derubertis BG, Trocciola SM, Ryer EJ, Pieracci FM, McKinsey JF, Faries PL, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg. 2007;46:630–635. [DOI] [PubMed] [Google Scholar]
- 36.Söderberg P, Wanhainen A, Svensjö S. Five Year Natural History of Screening Detected Sub-Aneurysms and Abdominal Aortic Aneurysms in 70 Year Old Women and Systematic Review of Repair Rate in Women. Eur J Vasc Endovasc Surg. 2017;53:802–809. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell TFX, Verhagen HJ, Pratesi G, Pratesi C, Teijink JAW, Vermassen FEG, et al. Female sex is associated with comparable 5-year outcomes after contemporary endovascular aneurysm repair despite more challenging anatomy. J Vasc Surg. 2020;71: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo RC, Lu B, Fokkema MT, Conrad M, Patel VI, Fillinger M, et al. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg. 2014;59:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suckow BD, Goodney PP, Columbo JA, Kang R, Stone DH, Sedrakyan A, et al. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. J Vasc Surg. 2018;67:1690–1697.e1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zettervall SL, Schermerhorn ML, Soden PA, McCallum JC, Shean KE, Deery SE, et al. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;65:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77 e72. [DOI] [PubMed] [Google Scholar]
- 42.Sutzko DC, Andraska E, Boniakowski A, Coleman DM, Osborne NH. Decline of Open Abdominal Aortic Aneurysm Repair among Vascular Surgery Training Programs in the United States. J Am Coll Surg. 2017;225:e114–e115. [Google Scholar]
- 43.Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg. 2012;43:161–166. [DOI] [PubMed] [Google Scholar]
- 44.Schermerhorn ML, Bensley RP, Giles KA, Hurks R, O’Malley AJ, Cotterill P, et al. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995-2008: a retrospective observational study. Ann Surg. 2012;256:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herb J, Strassle PD, Kalbaugh CA, Crowner JR, Farber MA, McGinigle KL. Limited adoption of abdominal aortic aneurysm screening guidelines associated with no improvement in aneurysm rupture rate. Surgery. 2018;164:359–364. [DOI] [PubMed] [Google Scholar]
- 46.Wanhainen A, Björck M. The Swedish experience of screening for abdominal aortic aneurysm. J Vasc Surg. 2011;53:1164–1165. [DOI] [PubMed] [Google Scholar]


