Abstract
Introduction:
KEAP1/NFE2L2 mutant NSCLCs are chemoradiation-resistant and at high-risk for local-regional failure (LRF) after concurrent chemoradiation (cCRT). To elucidate the impact of durvalumab on local-regional control, we assessed LRF in NSCLC patients treated with cCRT with and without durvalumab.
Methods:
Patients with stage III NSCLC treated with cCRT or cCRT and durvalumab who underwent tumor genomic profiling were examined. The incidence of LRF and outcomes of patients with and without KEAP1/NFE2L2 mutant tumors were assessed.
Results:
We analyzed 120 consecutive patients (cCRT alone, n=54; cCRT and durvalumab, n=66). Patients treated with cCRT alone had significantly more LRF events compared to those treated with cCRT and durvalumab, with 12-month LRF incidence of 39% (95% CI:24–54%) and 18% (95% CI:8–28%), respectively (p=0.002). Among patients treated with cCRT alone and cCRT and durvalumab, 20 patients (37%) and 18 patients (27%), respectively, had KEAP1/NFE2L2 mutant tumors. In patients treated with cCRT alone, KEAP1/NFE2L2 mutant tumors had worse local-regional control (p=0.015), and on multivariate analysis, KEAP1/NFE2L2 mutation predicted for LRF (HR, 3.9, 95% CI:1.6–9.8, p=0.003). However, patients with and without KEAP1/NFE2L2 mutant tumors had similar LRF outcomes (p=0.541) when treated with cCRT and durvalumab, and mutational status did not predict for LRF (p=0.545). Among KEAP1/NFE2L2 mutant tumors, cCRT and durvalumab significantly reduced the incidence of LRF compared to cCRT alone: 12-month LRF incidence of 62% (95% CI:40–84%) vs. 25% (95% CI:4–46%), respectively (p=0.021).
Conclusion:
Durvalumab after cCRT significantly improves local-regional control and reduces LRF in chemoradiation-resistant KEAP1/NFE2L2 mutant NSCLC tumors.
Keywords: durvalumab, stage III NSCLC, chemoradiation, KEAP1, NFE2L2
Introduction:
The addition of durvalumab to the management of unresectable locally-advanced non-small cell lung cancer (NSCLC) has improved disease control and overall survival (1, 2). However, the impact of durvalumab on local-regional control and the implications on radiotherapeutic remain poorly understood. Control of local-regional disease has been independently associated with survival outcomes among patients with NSCLC (3, 4). However, controlling local-regional disease is a challenge with data finding nearly 50% of patients to have local-regional progression within two years when treated with chemoradiation alone (5, 6).
Mutations in the KEAP1-NFE2L2 pathway, which plays a key role in cellular stress response, have been postulated as a major mechanism behind chemoradiation treatment failures in NSCLC (7, 8). Mutations in either KEAP1 or NFE2L2 are found in approximately 25% of NSCLCs and are associated with potentially half of all local-regional failures, presumably by interfering with chemotherapy and radiation-induced DNA damage (9–12). The association between KEAP1/NFE2L2 mutations and outcomes in patients treated with concurrent chemoradiation and durvalumab remains unknown as prior studies assessing the impact of these mutations on immunotherapy outcomes have been limited to patients with advanced NSCLC and have had conflicting results (13).
To assess the impact of durvalumab on local-regional control, we evaluted patients with stage III NSCLC who completed tumor genomic testing and were treated with concurrent chemoradiation with or without durvalumab. We compared local-regional control outcomes and the association between KEAP1/NFE2L2 mutations and local-regional failure among both patient cohorts. We postulated that we would best elucidate the role of durvalumab in treating local-regional disease by assessing outcomes in tumors with mutations known to confer chemoradiation resistance and hypothesized that given its mechanisms independent of inducing DNA damage (14), durvalumab would improve local-regional control regardless of KEAP1/NFE2L2 mutational status.
Methods:
Patients and Treatment:
We retrospectively examined consecutive patients with American Joint Committee on Cancer (AJCC) 8th edition stage III NSCLC treated between November 2013 and March 2020 who received curative intent cCRT alone or cCRT and at least one dose of durvalumab and gave informed consent, and underwent, targeted next generation sequencing (MSK-IMPACT; Integrated Mutation Profiling of Actionable Cancer Targets) (15, 16). Next generation sequencing was performed off available tissue from the primary tumor or regional nodal metastases. This research was conducted in accordance with the US Common Rule, and this study was Institutional Review Board approved.
Standard pre-treatment evaluation included a physical examination, computed tomography (CT) scan of the chest, abdomen and pelvis and/or whole-body fluorine-18 fluorodeoxyglucose positron emission tomography (PET), and magnetic resonance imaging (MRI) of the head. Patients were treated with curative-intent radiation therapy most commonly to 60Gy (range: 54Gy to 70Gy) in 2Gy fractions, and treatment was standardly delivered using intensity-modulated radiation therapy in both cCRT alone and cCRT and durvalumab cohorts.
Treatment planning included a 4-dimensional CT simulation, wherein the gross tumor volume was contoured on the free-breathing CT scan using guidance from the diagnostic PET as previously described (17). Patients were treated as per standard of care with platinum-based doublet chemotherapy concurrent with radiation. Patients treated after November 2017 standardly received durvalumab (10mg/kg) every two weeks for up to 12 months, as clinically indicated. Imaging with chest CT was performed every 2 – 4 months, or more frequently as clinically warranted. All patients suspected of disease progression underwent PET/CT imaging, and whenever feasible, biopsy.
Analysis:
Age, sex, stage, tumor volume, smoking history, histology, Eastern Cooperative Oncology Group (ECOG) performance status, programmed death-ligand 1 (PD-L1) expression, KRAS, STK11, PBRM1, SMARCA4 mutational status, KEAP1/NFE2L2 mutational status, and time to durvalumab start from end of radiotherapy were collected. Mutations in KEAP1 and NFE2L2 were considered as one group as previously described (8) given that they result in the same phenotype of NFE2L2 overexpression (18).
Baseline characteristics between patients with and without KEAP1/NFE2L2 tumor mutations were compared using the chi-square test, Fisher’s exact or the Wilcoxon test. We assessed for association between patient and tumor characteristics and local-regional failure using univariate and multivariate Cox proportional hazards modeling. In patients treated with cCRT and durvalumab, the association between tumors with coexisting mutations in KEAP1, STK11, PBRM1 and SMARCA4 with local-regional control was also assessed given data finding these commutations to associate with immunotherapy response in advanced NSCLC (19). Variables with p < 0.05 on univariable analysis were analyzed in multivariate analysis. PD-L1 was evaluated as a categorical variable, at ≥ 1% and ≥ 50% expression. PD-L1 immunohistochemistry was evaluated using the E1L3N antibody (Cell Signaling Technology, Danvers, MA), which has been validated against a 22C3 kit performed in a commercial laboratory with comparable results (20).
Progression-free survival was defined from the start of radiotherapy to any disease-progression or death. Local and distant failures were defined from the start of radiotherapy to disease progression, with distant failure defined as metastatic disease progression per AJCC 8th edition staging (21). Patients were censored from analysis at time of their first progression event. Local-regional failure was further classified as in-field failure if a component of disease progression occurred within or adjacent to the 90% isodose volume, and marginal failure if within or adjacent to ≥50% isodose volume. Kaplan-Meier analysis was used to determine 12-month progression-free survival and the 12-month cumulative incidence of distant and local-regional failure and 95% confidence intervals (95% CI). The log-rank test was used to compare progression-free survival and local-regional failure between patients treated with cCRT alone and cCRT and durvalumab as well as to compare the incidence of local-regional and distant failures between tumors with and without KEAP2/NFE2L2 mutations. Differences were described as statistically significant for p-values < 0.05. All statistical computations were performed using SPSS software Version 27 (IBM, Armonk, NY).
Results:
Patient and Treatment Characteristics:
We identified 120 patients with stage III NSCLC treated with definitive intent therapy who had tumor genomic testing completed. Fifty-four of these patients were treated prior to United States Food and Drug Administration approval of durvalumab and received cCRT alone, whereas the remaining 66 patients were treated with cCRT and at least one cycle of durvalumab.
Among patients treated with cCRT alone (n = 54), median patient age was 64 years, 54% (n = 29) were male, 63% (n = 34) were ECOG 0, 87% (n = 47) were ever smokers, 78% (n = 42) had stage IIIB or IIIC disease, 80% (n = 43) had tumors with adenocarcinoma histology and PD-L1 was available in 16 (30%) patients of which 5 had tumors with PD-L1 ≥ 1%. Patients treated with cCRT received a median of 60 Gy and were followed for a median of 30 months (IQR: 13 – 44 months). Among patients treated cCRT alone (n = 54), 20 patients (37%) had tumors that carried a mutation in either KEAP1 (n = 14) or NFE2L2 (n = 6) (Supplemental Table 1A). Patients with tumors with or without identified KEAP1/NFE2L2 mutations were similar in age, performance status, stage and histology (Table 1A). The oncoprint of the cCRT cohort is shown in Supplemental Figure 1A.
Table 1A.
cCRT Patient Characteristics
| Characteristic | No. of Patients (%) |
p – value | |
|---|---|---|---|
| KEAP1 / NFE2L2 wt (n = 34) |
KEAP1 / NFE2L mt (n = 20) |
||
| Median age, range | 63 (44 – 81) | 65 (47 – 82) | 0.99 |
| Sex | 0.48 | ||
| Female | 17 (50) | 8 (40) | |
| Male | 17 (50) | 12 (60) | |
| Performance Status | 0.35 | ||
| ECOG 0 | 23 (67) | 11 (55) | |
| ECOG 1 | 11 (33) | 9 (45) | |
| Gross Tumor Volume (cc) | |||
| Median, Range | 102 (12.8 – 324.8) | 122.5 (19.6 – 522.1) | 0.23 |
| Histology | 0.26 | ||
| Adenocarcinoma | 28 (82) | 15 (75) | |
| Squamous Cell | 4 (12) | 5 (25) | |
| Other | 2 (6) | 0 | |
| AJCC 8th Overall Stage | 0.57 | ||
| IIIA | 9 (26) | 3 (15) | |
| IIIB | 19 (56) | 14 (70) | |
| IIIC | 6 (18) | 3 (15) | |
| Smoking History | 0.03 | ||
| Yes | 27 (80) | 20 (100) | |
Among patients treated with cCRT and durvalumab (n = 66), median patient age was 67 years, 58% (n = 38) were male, 59% (n = 39) were ECOG 0, 94% (n = 62) were ever smokers, 70% (n = 46) had stage IIIB or IIIC disease, 70% (n= 46) had tumors with adenocarcinoma histology and PD-L1 was available in 55 (83%) patients of which 34 had tumors with PD-L1 ≥ 1%. Patients treated with cCRT and durvalumab received a median of 60 Gy and were followed for a median of 15 months (IQR: 11 – 24 months). Patients received a median of 5.3 months (IQR: 2 – 11 months) of durvalumab therapy that started a median of 1.4 months (IQR: 0.8 – 1.8 months) after the completion of radiation. In total, 20 patients (30%) discontinued durvalumab therapy due to treatment-related adverse events. Among patients treated with cCRT alone (n = 66), 18 patients (27%) had tumors that carried a mutation in either KEAP1 (n = 15) or NFE2L2 (n = 3) (Supplemental Table 1B). Patients with tumors with or without identified KEAP1/NFE2L2 mutations were similar in age, performance status, stage, histology and proportion of patients PD-L1 ≥1% or PD-L1 ≥50% (Table 1B).
Table 1B.
cCRT + Durvalumab Patient Characteristics
| Characteristic | No. of Patients (%) | p – value | |
|---|---|---|---|
| KEAP1 / NFE2L2 wt (n = 48) |
KEAP1 / NFE2L mt (n = 18) |
||
| Median age, range | 66 (45 – 81) | 69 (65 – 85) | 0.14 |
| Sex | 0.72 | ||
| Female | 21 (44) | 7 (39) | |
| Male | 27 (56) | 11 (61) | |
| Performance Status | 0.36 | ||
| ECOG 0 | 30 (63) | 9 (50) | |
| ECOG 1 | 18 (37) | 9 (50) | |
| Gross Tumor Volume (cc) | |||
| Median, Range | 109.7 (7 – 600.7) | 143.4 (13.5 – 392.6) | 0.98 |
| Histology | 0.39 | ||
| Adenocarcinoma | 34 (71) | 12 (67) | |
| Squamous Cell | 11 (23) | 3 (16) | |
| Other | 3 (6) | 3 (16) | |
| PDL1 Expression | 0.76 | ||
| < 1% | 15 (31) | 6 (33) | |
| ≥ 1% | 24 (50) | 10 (56) | |
| Not available | 9 (19) | 2 (11) | |
| PDL1 ≥50% | 0.41 | ||
| < 50% | 26 (54) | 13 (72) | |
| ≥ 50% | 13 (27) | 3 (17) | |
| Not available | 9 (19) | 2 (11) | |
| AJCC 8th Overall Stage | 0.59 | ||
| IIIA | 15 (31) | 5 (28) | |
| IIIB | 25 (52) | 8(44) | |
| IIIC | 8 (17) | 5 (28) | |
| Smoking History | 0.45 | ||
| Yes | 45 (94) | 18 (100) | |
Impact of Durvalumab on Progression-Free Survival and Local-Regional Control.
Patients treated with cCRT and durvalumab had significantly longer progression-free survival compared to patients treated with cCRT alone (Figure 1A). The 12-month PFS rate was 59% (95% CI, 47 –71%) in the cCRT and durvalumab group compared to 42% (95% CI, 29 – 55%) in the cCRT alone group (p = 0.019). Among patients treated with cCRT alone (n = 54), there were 24 (44%) local-regional failure events occurring at a median of 9 months (IQR: 8 – 13 months) of which 22 were in-field failures and 2 were marginal. Among patients treated with cCRT and durvalumab (n = 66), there were 12 (18%) local-regional failure events occurring at median of 8 months (IQR: 6 – 11 months) of which 7 were classified as in-field and 4 were marginal. Patients treated with cCRT and durvalumab had a significantly lower incidence of local-regional failure compared to patients treated with cCRT alone. The 12-month cumulative incidence of local-regional failure was 39% (95% CI: 24 –54%) among patients treated with cCRT compared to 18% (95% CI: 8 – 28%) in patients treated with cCRT and durvalumab (p = 0.002) (Figure 1B).
Figure 1.
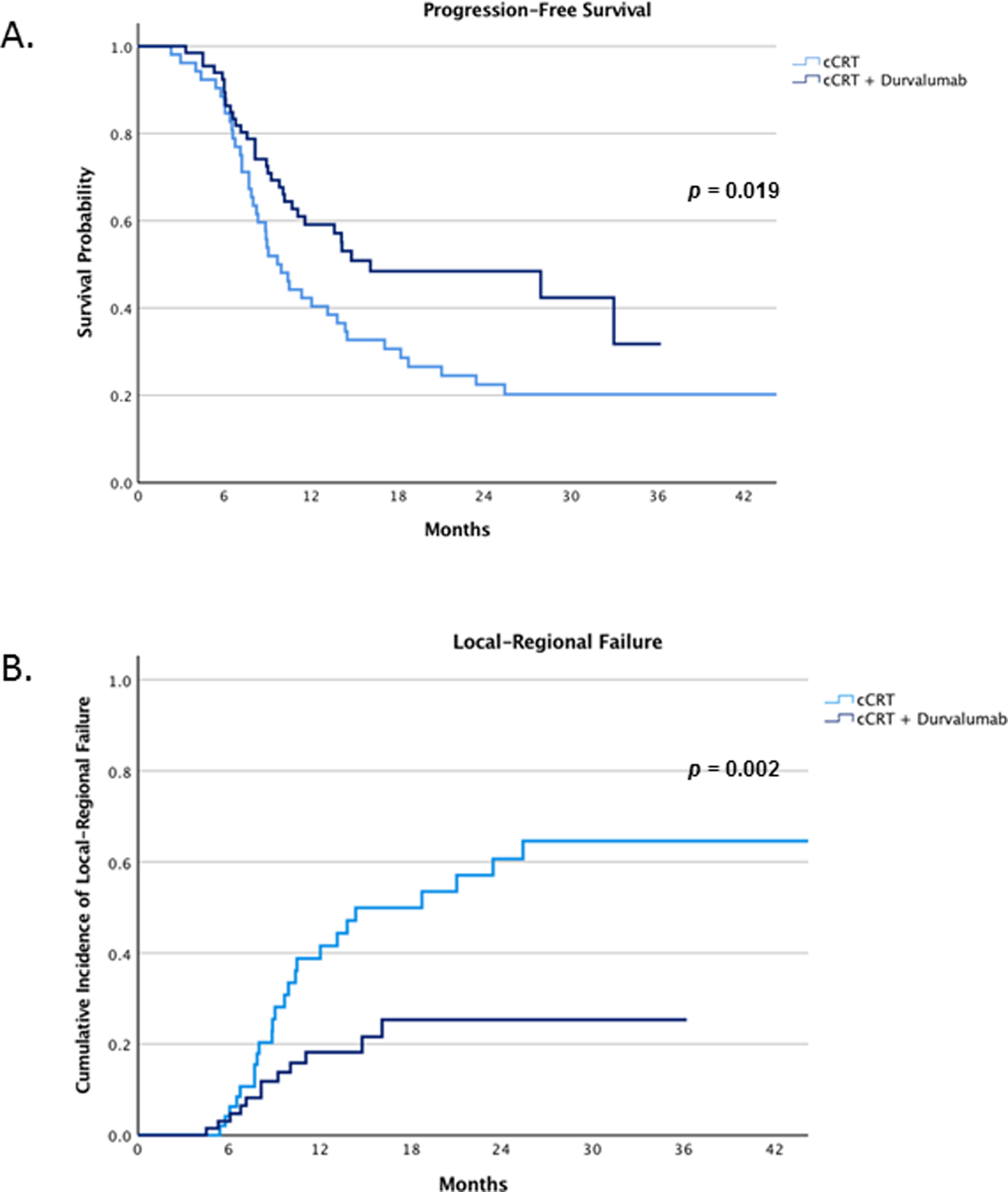
Progression-free survival (A) and incidence of local-regional failures between patients treated with cCRT alone and cCRT plus durvalumab (B).
Impact of Durvalumab on Association Between KEAP1 / NFE2L2 Mutational Status and Local-Regional Control Outcomes.
Among patients with KEAP1/NFE2L2 tumor mutations treated with cCRT alone, the 12-month cumulative incidence of local-regional failure in patients was 62% (95% CI, 40 – 84%) compared to 25% (95% CI, 9 – 41%) in patients without KEAP1/NFE2L2 mutant tumors (p = 0.015) (Figure 2A). Of the 24 patients with local-regional failure, 13 (54%) had tumors with an identified KEAP1/NFE2l2 mutation. On univariate analysis, patients with stage IIIC disease and those with KEAP1/NFE2L2 tumor mutations had inferior local-regional control. On multivariate analysis, KEAP1/NFE2L2 tumor mutation [hazards ratio (HR), 3.9, 95% CI, 1.6 – 9.8, p = 0.003] and stage IIIC disease (HR, 2.2, 95% CI, 1.3–3.6, p = 0.003) independently associated with inferior local-regional control. KRAS mutations did not associate with local-regional outcomes (p = 0.47). Additionally, the incidence of distant metastasis in patients with and without identified KEAP1/NFE2L2 mutations were similar when treated with cCRT alone (p = 0.452) (Supplemental Figure 2A).
Figure 2.
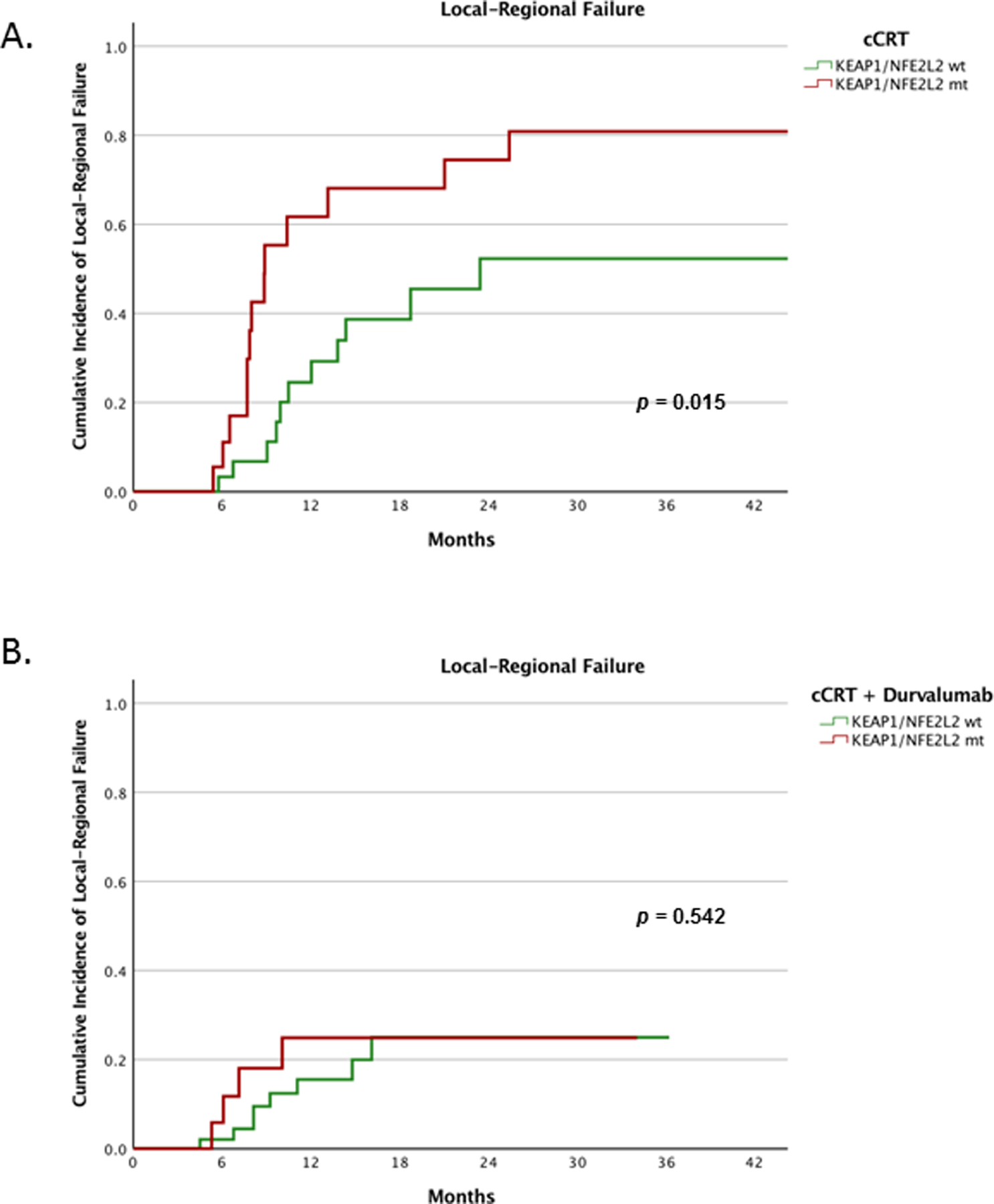
Comparison of local-regional outcomes among patients with and without KEAP1/NFE2L2 tumor mutations treated with cCRT alone (A) and cCRT plus durvalumab (B).
Among patients treated with cCRT and durvalumab who had an identified KEAP1/NFE2L2 tumor mutation, the 12-month cumulative incidence of local-regional failure was 25% (95% CI, 4 – 46%) compared to 16% (95% CI, 5 – 27%) in patients without KEAP1/NFE2L2 mutant tumors (p = 0.542) (Figure 2B). On univariate analysis, KEAP1/NFE2L2 tumor mutational status did not associate with local-regional control outcomes (p = 0.545). Additionally, PDL1 ≥ 1%, PDL1 ≥ 50%, KRAS mutation and the length of time between the end of radiation to starting durvalumab did not associate with local-regional control. The presence of a coexisting KEAP1, STK11, PBRM1 and SMARCA4 mutations, nor a KRAS mutation associated with local-regional outcomes (p = 0.75 and p = 0.73, respectively) (Table 2B). Patients with identified KEAP1/NFE2L2 tumor mutations treated with cCRT and durvalumab had a significantly lower rate of local-regional failure compared to those treated with cCRT alone (p = 0.021) (Figure 3). Patients with and without KEAP1/NFE2L2 tumor mutations had similar incidence of distant metastasis (p = 0.695) (Supplemental Figure 2B).
Table 2B.
Predictors for Local-Regional Failure with cCRT and Durvalumab
| Univariate | ||
|---|---|---|
| HR (95% CI) | p - value | |
| Age | 1.0 (0.93 – 1.07) | 0.98 |
| Sex | 1.03 (0.33 – 3.26) | 0.96 |
| ECOG 0 vs ECOG 1 | 0.91 (0.27 – 3.04) | 0.88 |
| Histology* | 1.76 (0.56 – 5.56) | 0.33 |
| Gross Tumor Volume | 1.003 (0.99 – 1.01) | 0.15 |
| Stage IIIC vs IIIA/IIIB | 1.46 (0.39 – 5.41) | 0.57 |
| KRAS mt | 1.23 (0.37 – 4.14) | 0.73 |
| KEAP1/STK11/PBRM1/SMARCA4 CoMt | 0.78 (0.17 – 3.58) | 0.75 |
| KEAP1/NFE2L2 mt | 1.45 (0.44 – 4.83) | 0.56 |
| PD-L1 ≥ 50% | 1.07 (0.29 – 4.05) | 0.96 |
| PD-L1 ≥ 1% | 1.05 (0.31 – 3.59) | 0.94 |
| Time to Durvalumab Start | 0.82 (0.41 – 1.66) | 0.54 |
Adenocarcinoma vs Squamous/Other
Figure 3.
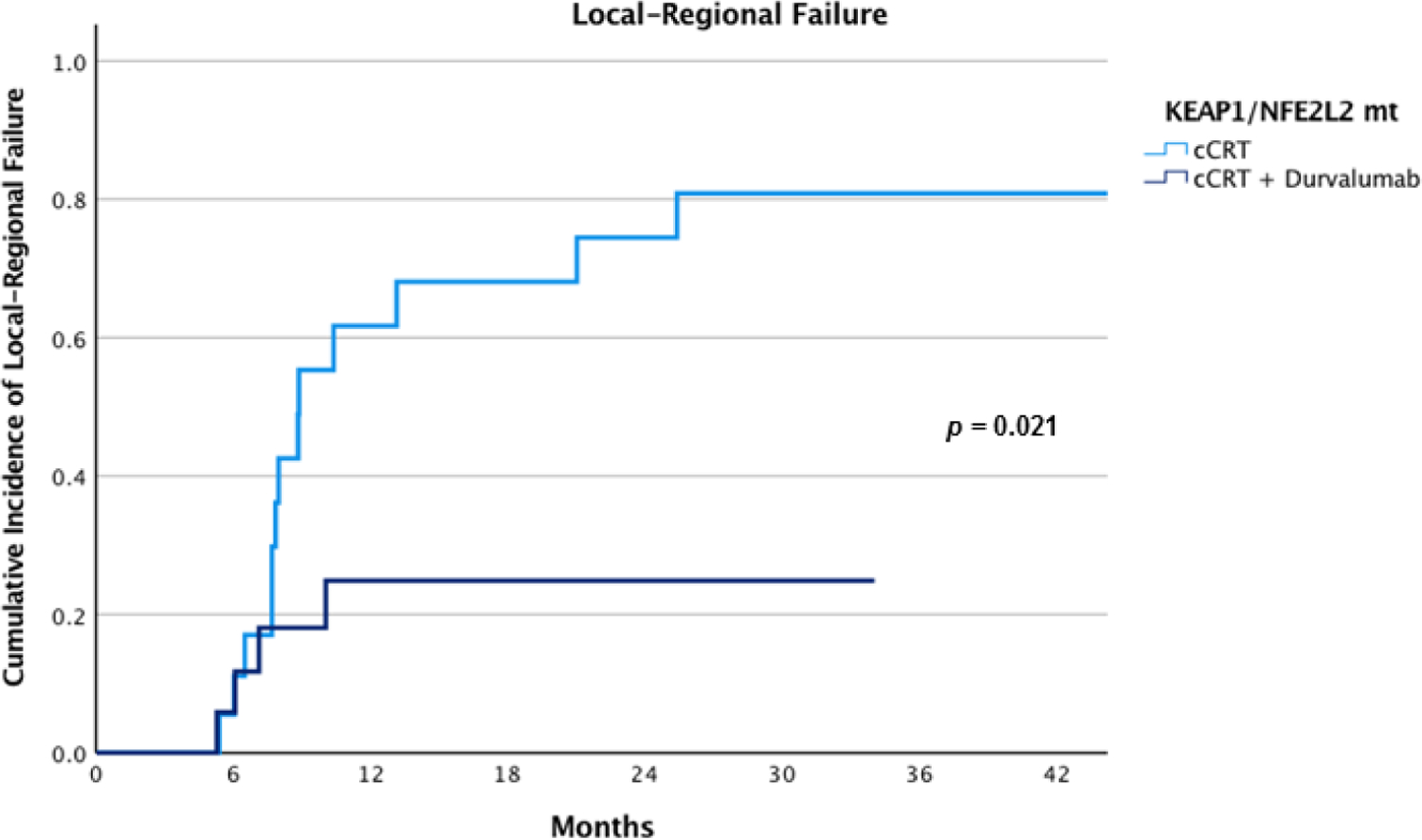
Comparison of local-regional failures among patients with KEAP1/NFE2L2 mutant tumors treated with cCRT and cCRT plus durvalumab
Discussion:
While the PACIFIC trial demonstrated that durvalumab improved disease control and overall survival in unresected stage III NSCLC, the impact of durvalumab on local-regional disease control and the implications on radiotherapeutic management has been less clear. In this study, we found patients treated with cCRT and durvalumab had a significantly lower incidence of local-regional failures compared to patients treated with cCRT alone. Consistent with other reports, we found patients with KEAP1/NFE2L2 mutated tumors, a chemoradiation-resistant phenotype, to have worse local-regional control outcomes when treated with cCRT alone (8).
However, the influence of KEAP1/NFE2L2 mutation status on locoregional control in NSCLC patients treated with cCRT and durvalumab has not been previously characterized, and we did not find KEAP1/NFE2L2 mutated tumors to have worse control outcomes when treated with cCRT and durvalumab. Furthermore, we found treatment with cCRT and durvalumab to result in a striking improvement in local-regional control in patients with KEAP1/NFE2L2 mutant tumors compared to cCRT alone. These data provide the strongest evidence to date on the role of durvalumab in treating local-regional disease, including chemoradiation-resistant KEAP1/NFE2L2 mutant tumors.
Prior to the PACIFIC study, previous studies with standard cCRT found approximately 40% of patients to fail within the radiation field at first relapse (22). This high rate of failure and the association between local-regional disease control and survival led to studies evaluating radiation dose-escalation in patients with unresected NSCLC (23). In contrast to these reports in the pre-immunotherapy era, we observed a nearly 50% reduction in the incidence of local-regional failures in patients treated with standard cCRT and durvalumab compared to cCRT alone. These data suggest that local-regional control can be substantially impacted independent of radiation dosing. Furthermore, while there has been a greater understanding in how tumor and host genetic factors impact radiation sensitivity (24, 25), the impact of durvalumab should also be computed in future strategies personalizing radiation dose. Moreover, the striking impact of durvalumab on local-regional control found herein suggests that one potential underlying mechanism by which durvalumab improves patient survival is through its direct actions on local-regional disease.
The KEAP1/NFE2L2 pathway plays a critical role in regulating cellular stress and the oxidative stress response. NFE2L2 is a transcription factor that drives the transcription of antioxidant genes and is negatively regulated by KEAP1 (18, 26). Mutations in KEAP1/NFE2L2 genes can lead to NFE2L2 overexpression and thereby protect cancer cells from the effects of radiation and cytotoxic chemotherapy (7, 8, 11). Work by Binkley and Jeon et al established the predictive value of KEAP1/NFE2L2 mutations on radiation resistance in a series of 232 NSCLC patients, of whom 47 were treated cCRT. That study found KEAP1/NFE2L2 mutant tumors are at an increased risk of local-regional failure, with two-year recurrence rates of 50% vs 17% in tumors without KEAP1/NFE2L2 mutations (8). Consistent with these data, we also found a significantly greater incidence of local-regional failure among KEAP1/NFE2L2 mutated tumors after cCRT alone, further validating this subgroup to be chemoradiation resistant.
However, we did not find KEAP1/NFE2L2 mutant tumors to have inferior local-regional failure when treated with cCRT and durvalumab, suggesting that durvalumab plays a significant role in treating local-regional disease. Among patients with KEAP1/NFE2L2 mutant tumors, we found a marked reduction in local-regional failures in patients treated with cCRT and durvalumab compared to cCRT alone, with 12-month failure rates of 62% vs 25%, respectively. These data suggest the importance of durvalumab in this chemoradiation-resistant subgroup. Unlike platinum chemotherapy and radiation, immune checkpoint inhibition (ICI) results in cancer cell death through mechanisms mostly independent of DNA damage, supporting our finding that durvalumab improved local-regional outcomes in chemoradiation-resistant tumors. There have been multiple reports assessing the predictive impact of KEAP1 mutations on ICI outcomes in metastatic NSCLC with mixed findings. An exploratory analysis of KEYNOTE-042, which compared pembrolizumab with platinum doublet chemotherapy in PD-L1-positive advanced NSCLC, suggested pembrolizumab can improve outcomes compared to chemotherapy regardless of KEAP1 mutational status (27). On the other hand, a report examining the impact of KEAP1 and co-mutations with STK11, PBRM1 and SMARCA4 in advanced lung adenocarcinoma found poor outcomes with ICIs that was hypothesized to be secondary to an immunologically cold tumor microenvironment (19). However, these reports did not assess the impact of KEAP1 mutations in patients with cCRT exposure prior to ICI initiation, and there are data suggesting that cCRT favorably modulates the tumor microenvironment in NSCLC (28, 29). We did not find patients with previously described co-mutations to have inferior local-regional outcomes when treated with ICI, further suggesting that cCRT can modify the response to ICI.
The interpretation of the study is constrained by its retrospective nature and its inclusion of a single high-volume tertiary cancer center. Furthermore, although the median patient follow-up in both cohorts was greater than 12-months and marked differences in disease outcomes were noted quite early, longer follow-up will be necessary to confirm these findings, particularly in the later cohort of patients treated with cCRT and durvalumab. Additionally, this report studied a higher risk patient population than in recent multicenter trials given that over 70% of patients in this study were stage IIIB or IIIC (1, 23) and although patients treated with cCRT alone and cCRT plus durvalumab were non-contemporaneous, beyond the addition of durvalumab, chemoradiation management has remained essentially unchanged. Patients in this study were additionally limited to those that underwent next generation sequencing, however disease outcomes reported in this selected patient population are consistent with outcomes from unselected patients with stage III NSCLC from the same cancer center (17). Furthermore, while the mutations in KEAP1/NFE2L2 found within our patient cohorts were not functionally evaluated, this limitation was present in both cohorts. Overall, this study providers the most robust data to date on the impact of durvalumab on local-regional disease outcomes and in KEAP1/NFE2L2 mutant tumors. With a growing understanding of both the toxicity of radiotherapy on thoracic organs and the heterogeneity of radiation sensitivity among tumors and patients (25, 30, 31), appreciating the role of durvalumab on local-regional control can aide future trial development investigating radiation dose and volume personalization in stage III NSCLC.
In conclusion, in this assessment of patients with unresected stage III NSCLC treated with cCRT and cCRT and durvalumab we found durvalumab to play a significant role in controlling local-regional disease, even in patients with KEAP1/NFE2L2 tumor mutations, a known chemoradiation-resistant phenotype. Future strategies of precision radiotherapeutic management in stage III NSCLC that build upon the understanding of tumor and host chemoradiation sensitivity and that also recognize the contributions of durvalumab are warranted.
Supplementary Material
Table 2A.
Predictors for Local-Regional Failure with cCRT
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p - value | HR (95% CI) | p - value | |
| Age | 1.0 (0.96 – 1.06) | 0.70 | ||
| Sex | 0.68 (0.44 – 1.04) | 0.08 | ||
| ECOG 0 vs ECOG 1 | 1.56 (0.64 – 3.70) | 0.33 | ||
| Histology* | 1.1 (0.67 – 1.81) | 0.70 | ||
| Gross Tumor Volume | 1.003 (1 – 1.01) | 0.07 | ||
| Stage IIIC vs IIIA/IIIB | 1.66 (1.06 – 2.59) | 0.03 | 2.17 (1.31 – 3.58) | 0.003 |
| KRAS mt | 1.49 (0.51 – 4.41) | 0.47 | ||
| KEAP1/NFE2L2 mt | 2.64 (1.17 – 5.88) | 0.02 | 3.95 (1.56 – 9.81) | 0.003 |
Adenocarcinoma vs Squamous/Other
Acknowledgement:
We are thankful to the Molecular Diagnostics Service in the Department of Pathology, and the Marie-Josee and Henry R. Kravis Center for Molecular Oncology.
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements:
N. Shaverdian: Reports research funding from Novartis.
M. Offin: Reports advisory role for PharMar, Novartis and Targeted Oncology. Reports honoraria from Bristol-Myers Squibb and Merck Sharp & Dohme.
A. F. Shepard: Reports honoraria from ASCO.
C. B. Simone II: Reports honoraria from Varian Medical Systems.
D. Y. Gelblum: No COI to report.
A. J. Wu: Reports research support from CivaTech Oncology, Inc., non-financial support from AlphaTau Medical, personal fees from MoreHealth, and personal fees from AstraZeneca.
M. D. Hellmann: Reports personal fees from Genentech, grants, personal fees and non-financial support from Bristol Myers Squibb, personal fees from Merck, personal fees and non-financial support from AstraZeneca, personal fees from Mirati, personal fees from Syndax, personal fees and equity from Shattuck Labs, personal fees and equity from Immunai, personal fees from Nektar, personal fees from Blueprint Medicines. In addition, Dr. Hellmann has a pending patent Determinants of Cancer Response to Immunotherapy by PD-1 Blockade (PCT/US2015/062208) licensed to PGDx.
P. K. Paik: personal fees outside the submitted work from the following: Boehringer Ingelheim, Takeda, Celgene, EMD Serono, Calithera, AstraZeneca, AbbVie, and Lilly Oncology.
A. Rimner: Reports grants from Varian Medical Systems, grants from Boehringer Ingelheim, grants from Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Merck, personal fees from Research to Practice, personal fees from Cybrexa, non-financial support from Philips/Elekta, personal fees from MoreHealth.
J. E. Chaft: Reports both research funding and consulting roles with Bristol-Myers Squibb, Merck, Genentech and AstraZeneca.
D. R. Gomez: Reports honoraria from Merck, BMS, AstraZeneca, Reflexion, Medscape, Vindico, US Oncology, and Varian. Reports research support from Merck, BMS, AstraZeneca, and Varian. Serves on advisory board for AstraZeneca.
References:
- 1.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine. 2018;379(24):2342–50. [DOI] [PubMed] [Google Scholar]
- 3.Machtay M, Paulus R, Moughan J, Komaki R, Bradley JE, Choy H, et al. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma. J Thorac Oncol. 2012;7(4):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. The Lancet. 2009;374(9687):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(9):953–62. [DOI] [PubMed] [Google Scholar]
- 6.Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, et al. Higher Biologically Effective Dose of Radiotherapy Is Associated With Improved Outcomes for Locally Advanced Non–Small Cell Lung Carcinoma Treated With Chemoradiation: An Analysis of the Radiation Therapy Oncology Group. International Journal of Radiation Oncology*Biology*Physics. 2012;82(1):425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, et al. Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov. 2017;7(1):86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binkley MS, Jeon Y-J, Nesselbush M, Moding EJ, Nabet BY, Almanza D, et al. KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discovery. 2020;10(12):1826–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collisson EA, Campbell JD, Brooks AN, Berger AH, Lee W, Chmielecki J, et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150(6):1107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong Y, Hellyer JA, Stehr H, Hoang NT, Niu X, Das M, et al. Role of KEAP1/NFE2L2 Mutations in the Chemotherapeutic Response of Patients with Non–Small Cell Lung Cancer. Clinical Cancer Research. 2020;26(1):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goeman F, De Nicola F, Scalera S, Sperati F, Gallo E, Ciuffreda L, et al. Mutations in the KEAP1-NFE2L2 Pathway Define a Molecular Subset of Rapidly Progressing Lung Adenocarcinoma. Journal of Thoracic Oncology. 2019;14(11):1924–34. [DOI] [PubMed] [Google Scholar]
- 13.Hellyer JA, Padda SK, Diehn M, Wakelee HA. Clinical Implications of KEAP1-NFE2L2 Mutations in NSCLC. Journal of Thoracic Oncology. [DOI] [PubMed]
- 14.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discovery. 2018;8(9):1069–86. [DOI] [PubMed] [Google Scholar]
- 15.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature medicine. 2017;23(6):703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD. 2015;17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offin M, Shaverdian N, Rimner A, Lobaugh S, Shepherd AF, Simone CB 2nd, et al. Clinical outcomes, local-regional control and the role for metastasis-directed therapies in stage III non-small cell lung cancers treated with chemoradiation and durvalumab. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2020. [DOI] [PMC free article] [PubMed]
- 18.Best SA, Sutherland KD. “Keaping” a lid on lung cancer: the Keap1-Nrf2 pathway. Cell Cycle. 2018;17(14):1696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinelli D, Mazzotta M, Scalera S, Terrenato I, Sperati F, D’Ambrosio L, et al. KEAP1-driven co-mutations in lung adenocarcinoma unresponsive to immunotherapy despite high tumor mutational burden. Annals of Oncology. 2020;31(12):1746–54. [DOI] [PubMed] [Google Scholar]
- 20.Gaule P, Smithy JW, Toki M, Rehman J, Patell-Socha F, Cougot D, et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA oncology. 2017;3(2):256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC cancer staging manual: Springer; New York; 2010. [Google Scholar]
- 22.Senan S, Brade A, Wang L-h, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non–Small-Cell Lung Cancer. 2016;34(9):953–62. [DOI] [PubMed] [Google Scholar]
- 23.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. The Lancet Oncology. 2015;16(2):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitter KL, Casey DL, Lu YC, Hannum M, Zhang Z, Song X, et al. Pathogenic ATM Mutations in Cancer and a Genetic Basis for Radiotherapeutic Efficacy. J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed]
- 25.Scott JG, Sedor G, Scarborough JA, Kattan MW, Peacock J, Grass GD, et al. Personalizing Radiotherapy Prescription Dose Using Genomic Markers of Radiosensitivity and Normal Tissue Toxicity in NSCLC. Journal of Thoracic Oncology. [DOI] [PMC free article] [PubMed]
- 26.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. [DOI] [PubMed] [Google Scholar]
- 27.Cho BC, Lopes G, Kowalski DM, Kasahara K, Wu Y-L, Castro G, et al. Abstract CT084: Relationship between STK11 and KEAP1 mutational status and efficacy in KEYNOTE-042: pembrolizumab monotherapy versus platinum-based chemotherapy as first-line therapy for PD-L1-positive advanced NSCLC. Cancer research. 2020;80(16 Supplement):CT084-CT. [Google Scholar]
- 28.Yoneda K, Kuwata T, Kanayama M, Mori M, Kawanami T, Yatera K, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemoradiotherapy for non-small cell lung cancer. British Journal of Cancer. 2019;121(6):490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirasawa M, Yoshida T, Matsumoto Y, Shinno Y, Okuma Y, Goto Y, et al. Impact of chemoradiotherapy on the immune-related tumour microenvironment and efficacy of anti-PD-(L)1 therapy for recurrences after chemoradiotherapy in patients with unresectable locally advanced non-small cell lung cancer. European Journal of Cancer. 2020;140:28–36. [DOI] [PubMed] [Google Scholar]
- 30.Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. International journal of radiation oncology, biology, physics. 2019;105(2):346–55. [DOI] [PubMed] [Google Scholar]
- 31.Atkins KM, Chaunzwa TL, Lamba N, Bitterman DS, Rawal B, Bredfeldt J, et al. Association of Left Anterior Descending Coronary Artery Radiation Dose With Major Adverse Cardiac Events and Mortality in Patients With Non–Small Cell Lung Cancer. JAMA oncology. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


