Abstract
Purpose:
Obesity increases the risk of cancer recurrence and death in survivors of breast cancer. This study tested the hypothesis that exercise alone, diet alone, and the combination of exercise plus diet reduce body weight and improve body composition in survivors of breast cancer.
Methods:
In this 2×2 factorial trial, 351 survivors of breast cancer with overweight or obesity were randomized to one of four treatment groups for 52 weeks: control, exercise alone, diet alone, or exercise plus diet. Endpoints included body weight and body composition measured by dual-energy x-ray absorptiometry.
Results:
After 52 weeks, compared with control, diet alone [−5.39 kg (95% CI: −7.24, −3.55); −6.0% (95% CI: −8.0, −3.9)] and exercise plus diet [−6.68 kg (95% CI: −8.46, −4.90); −7.4% (95% CI: −9.4, −5.4)] reduced body weight; exercise alone did not change body weight. Compared with control, diet alone [−3.59 kg (95% CI: −5.00, −2.17)] and exercise plus diet [−4.28 kg (95% CI: −5.71, −2.84)] reduced fat mass; exercise alone did not change fat mass. Compared with control, diet alone [−0.82 kg (95% CI: −1.50, −0.15)] and exercise plus diet [−1.24 kg (95% CI: −1.92, −0.56)] reduced lean mass; exercise alone did not change lean mass. Compared with control, exercise alone, diet alone, and exercise plus diet did not change bone mineral density.
Conclusion:
In survivors of breast cancer with overweight or obesity, diet alone or diet plus exercise produced clinically meaningful weight loss at week 52. The majority of weight loss was fat mass.
Keywords: adipose tissue, bone density, diet therapy, exercise, muscle
INTRODUCTION
Obesity is a chronic, relapsing, and progressive disease that is characterized by excess adiposity [1]. One-in-three survivors of breast cancer has obesity [2]. Obesity increases the risk of cancer recurrence and death in survivors of breast cancer [3, 4]. Moreover, obesity increases the risk of competing causes of morbidity and mortality, such as type 2 diabetes and cardiovascular disease [5-7].
Multimodal lifestyle interventions that include diet modification, increased physical activity, and lifestyle modification instruction are the foundation of obesity care [8]. Multimodal lifestyle interventions report 3–5% weight loss in survivors of breast cancer [9, 10]. In the general population, hypocaloric diets reduce body fat but catabolize lean tissue [11, 12]. Quantifying how fat and lean tissues change in response to lifestyle intervention is clinically relevant as excess fat mass and low lean mass are each independently associated with an increased risk of death in survivors of breast cancer [13]. Excess fat mass increases the risk of cancer recurrence, type 2 diabetes, and cardiovascular disease [14, 15]. In contrast, low lean mass increases the risk of frailty, falls, and functional decline [16]. Moreover, weight loss may reduce bone mineral density [17].
These observations provided the rationale to test the hypothesis that exercise alone, diet alone, and the combination of exercise plus diet reduce body weight and improve body composition, as compared with control, in survivors of breast cancer. To our knowledge, this study is the first to report the effects of distinct lifestyle interventions on measures of body composition in survivors of breast cancer with overweight or obesity. We previously reported that exercise and diet did not improve the primary endpoint of breast cancer-related lymphedema assessed by interlimb volume difference [18]. This trial used a 2×2 factorial design, which allowed the comprehensive examination of exercise and diet. This trial was sponsored by the National Cancer Institute (NCI) as part of the Transdisciplinary Research on Energetics and Cancer (TREC) consortium [19].
METHODS
Study Design
This study was a 52-week, randomized, 2×2 factorial trial. The study was conducted in accordance with Good Clinical Practice and the ethical principles originating in the Declaration of Helsinki. The Institutional Review Board approved the protocol and the informed consent document. All participants provided written informed consent and approval from their physician to enroll in the study. The study is registered on ClinicalTrials.gov as NCT01515124. The trial design is described in detail elsewhere [20].
Participants
Eligible participants had stage I-III breast cancer; completed surgery, chemotherapy, radiotherapy, and targeted therapy ≥6 months before study enrollment (concurrent endocrine therapy was allowed); had a body mass index (BMI) of 25–50 kg/m2; had breast cancer related lymphedema, defined using the Common Terminology Criteria for Adverse Events (CTCAE; version 4) [21], or a prior clinical diagnosis of lymphedema; and were aged 18–80 years. In addition, eligible participants had no evidence of residual or recurrent cancer; no medical conditions that would preclude participation in an exercise or diet program; were not engaging in any resistance exercise or ≥3 bouts of aerobic exercise of moderate intensity (e.g., brisk walking) weekly over the prior 52 weeks; were not using any medications for the purpose of weight loss; had no weight loss ≥4.5 kg in the previous 12 weeks; and had no history of bariatric or metabolic surgery. Participants were recruited using a variety of active and passive outreach methods [22].
Randomization and Blinding
Participants were assigned in an equal ratio to one of four treatment groups for 52 weeks: control, exercise alone, diet alone, or exercise plus diet. Participants were stratified by age, the number of lymph nodes resected during breast cancer surgery, receipt of radiotherapy, lymphedema severity, and body mass index and then randomized using a computerized covariate adaptive procedure [23]. Participants were not blinded to treatment assignment. Endpoint measures were obtained by certified staff members who were blinded to treatment assignment.
Control Treatment Condition
Participants assigned to the control group were instructed to refer to their physician regarding what types of exercise or diet would be safe and effective. No other guidance regarding exercise or diet was provided.
Exercise Treatment Condition
Participants assigned to the exercise group performed a combination of in-person and home-based exercise. Exercise modalities included resistance and aerobic activity, consistent with recommendations from the American College of Sports Medicine [24]. In-person exercise, supervised by an exercise oncology professional, occurred weekly in the first six weeks of the study, and once per month thereafter in groups of 2–6 participants. Participants performed resistance exercise using adjustable dumbbell weights (PowerBlock, Inc) that were provided by the study and were delivered to participant’s residence. The resistance program included nine exercises that were performed twice weekly for 2–3 sets using a weight that permitted 10 repetitions with proper form and didn’t exacerbate lymphedema symptoms; the resistance exercise prescription was adapted from the ‘Physical Activity and Lymphedema’ (PAL) trial [25, 26]. Moderate-intensity aerobic exercise was prescribed to a goal of 180 minutes weekly distributed over 3–6 days per week (e.g., 30 minutes on most days of the week). The detailed exercise treatment plan is published elsewhere [20].
Diet Treatment Condition
Participants assigned to the diet group attended 24 weekly sessions of lifestyle modification instruction led by a registered dietitian in groups of 2–12 participants. The goal of the diet was a 10% loss of body weight. Weekly counseling sessions included a weigh-in, review of the week, and behavioral modification lesson (e.g., self-monitoring, goal setting, stimulus control). During the first 20 weeks, participants followed a meal replacement program (Nutrisystem, Inc) that also included seven daily servings of fruits and vegetables, consistent with the American Cancer Society recommendations [27]. During weeks 21–24, the focus shifted to applying the behavioral modification techniques to food shopping and preparation. During weeks 24–52, the groups met in-person monthly for additional behavioral modification lessons (e.g., problem-solving, relapse prevention). Behavior modification lessons were adapted from the ‘Improving the Management of Obesity in Primary Care’ (POWER-UP) trial [28]. The detailed diet treatment plan is published elsewhere [20].
Combined Exercise Plus Diet Treatment Condition
Participants assigned to the exercise plus diet group started with six weeks of exercise instruction. At week seven, they began receiving the diet intervention in addition to the exercise intervention. Thereafter, participants received the exercise and diet interventions simultaneously.
Body Composition Endpoint Measures
Assessment of anthropometric and body composition measures followed standardized procedures. Participants wore a medical gown and were asked to remove their shoes, and all jewelry or other personal effects. Height was measured using a wall-mounted stadiometer. Weight was measured in duplicate using a calibrated digital scale; a third measurement was obtained if the difference of the first two measures exceeded 0.5 kg. Body composition was measured with whole-body dual-energy x-ray absorptiometry (DXA; Hologic Inc). All images were reviewed for quality assurance by a certified DXA technician who was blinded to treatment assignment. The DXA scanner was calibrated at regular intervals using spine and whole-body tissue phantoms. The DXA analysis was used to calculate fat mass (kg), visceral adipose tissue (cm2), subcutaneous adipose tissue (cm2), lean mass (kg), appendicular lean mass (kg), and bone mineral density (g/cm2) using APEX v.13.5 software. Appendicular lean mass is a surrogate for muscle mass because lean tissue in the arms and legs is striated and does not include cardiac and smooth muscle or other organs (which is included in the calculation of total lean mass) [29]. The validation and precision of DXA against whole-body computed tomography and magnetic resonance imaging has been reported [30, 31].
Other Measures
Demographic characteristics, including age, race, and education, were self-reported. Clinical characteristics, including time since breast cancer diagnosis, cancer stage, number of resected lymph nodes, and treatment types were abstracted from a combination of pathology reports and other physician records. Arm volume was measured by perometry [32].
Statistical Analysis
The sample size was selected to provide sufficient statistical power to detect change in the primary endpoint of breast cancer-related lymphedema interlimb volume difference [18]. Based on estimates from the Action for Health in Diabetes (Look AHEAD) [11] and the Nutrition and Exercise in Women (NEW) trials [33], this study had 80% statistical power to detect standardized mean difference effect sizes of ≥0.18 using two-sided 0.05-level tests.
The statistical analysis included all participants who were randomly assigned, and all available in-trial data at week 52 were included in accordance with the intention-to-treat principle. Intrial data at week 52 included both adherent participants and retrieved participants who prematurely discontinued their assigned intervention. Missing data at week 52 were imputed using predictive mean matching multiple imputation with 20 imputation replicates [34]. The primary contrast quantified the effect of each of the three intervention groups (exercise alone, diet alone, and exercise plus diet) with the control group, using a sequentially rejective testing procedure [35]. Continuous endpoints were analyzed using an analysis of covariance model that included group-by-time interaction terms, dependent variable’s baseline value, and randomization stratification factors [36]. Relative change in body weight was calculated using the estimated absolute treatment effect and mean baseline body weight. The categorical endpoints of ≥5% and ≥10% loss of body weight were analyzed using a logistic regression model with multiple imputation. All statistical testing was two-sided. Analyses were done using Stata/MP v.15.1 (StataCorp, LLC).
RESULTS
Between December 2011 and April 2015, 351 participants were randomized, with endpoint data collection ending in May 2016. Study participants had a mean (SD) age of 59.4 (8.7) years, 133 (38%) were non-white, and 165 (47%) completed a four-year college degree (Table 1).
Table 1.
Baseline characteristics by randomized group
| Characteristic | Control (n=90) |
Exercise (n=87) |
Diet (n=87) |
Exercise & Diet (n=87) |
|---|---|---|---|---|
| Age, y | 59.0 (8.5) | 59.1 (8.1) | 59.4 (9.2) | 60.0 (9.0) |
| Race, n (%) | ||||
| White | 66 (73.3%) | 50 (57.5%) | 52 (59.8%) | 50 (57.5%) |
| Black | 22 (24.4%) | 36 (41.4%) | 32 (36.8%) | 32 (36.8%) |
| Other | 2 (2.2%) | 1 (1.1%) | 3 (3.3%) | 5 (5.8%) |
| Education, n (%) | ||||
| High school or less | 19 (21.1%) | 15 (17.2%) | 12 (13.8%) | 18 (20.7%) |
| Some college | 28 (31.1%) | 29 (33.3%) | 36 (41.4%) | 29 (33.3%) |
| College degree or more | 43 (47.8%) | 43 (49.4%) | 39 (44.9%) | 40 (46.0%) |
| Time since cancer diagnosis, mo. | 97.5 (61.3) | 92.6 (64.3) | 89.9 (66.9) | 87.8 (61.7) |
| Cancer stage, n (%) | ||||
| Ductal carcinoma in situ | 10 (11.1%) | 6 (6.9%) | 5 (5.8%) | 3 (3.4%) |
| I | 19 (21.1%) | 24 (27.6%) | 17 (19.5%) | 14 (16.0%) |
| II | 23 (25.6%) | 24 (27.6%) | 29 (33.3%) | 28 (32.2%) |
| III | 16 (17.8%) | 13 (14.9%) | 20 (23.0%) | 21 (24.1%) |
| Unknown | 22 (24.4%) | 20 (23.0%) | 16 (18.4%) | 21 (24.1%) |
| No. of nodes removed, n | 12.7 (9.5) | 12.6 (9.4) | 12.5 (9.8) | 12.0 (8.3) |
| Cancer treatments, n (%) | ||||
| Chemotherapy | 74 (82.2%) | 65 (74.7%) | 71 (81.6%) | 79 (90.8%) |
| Radiotherapy | 73 (81.1%) | 73 (83.9%) | 69 (79.3%) | 73 (83.9%) |
| Tamoxifen | 10 (11.1%) | 10 (11.5%) | 10 (11.5%) | 6 (6.9%) |
| Aromatase inhibitor | 31 (34.4%) | 25 (28.7%) | 22 (25.3%) | 24 (27.6%) |
| Arm volume difference, % | 9.6 (14.4) | 8.8 (16.6) | 8.7 (13.5) | 7.6 (13.7) |
| Body mass index, kg/m2 | 34.0 (5.7) | 34.0 (6.2) | 33.8 (5.6) | 34.2 (6.3) |
| 25.0–29.9 kg/m2 | 26 (28.9%) | 31 (35.6%) | 26 (29.9%) | 25 (28.7%) |
| 30.0–34.9 kg/m2 | 30 (33.3%) | 21 (24.1%) | 24 (27.6%) | 27 (31.0%) |
| ≥35.0 kg/m2 | 34 (37.8%) | 35 (40.2%) | 37 (42.5%) | 35 (40.2%) |
Values are mean ± standard deviation or n (%). Percentages may not sum to 100.0% due to rounding error.
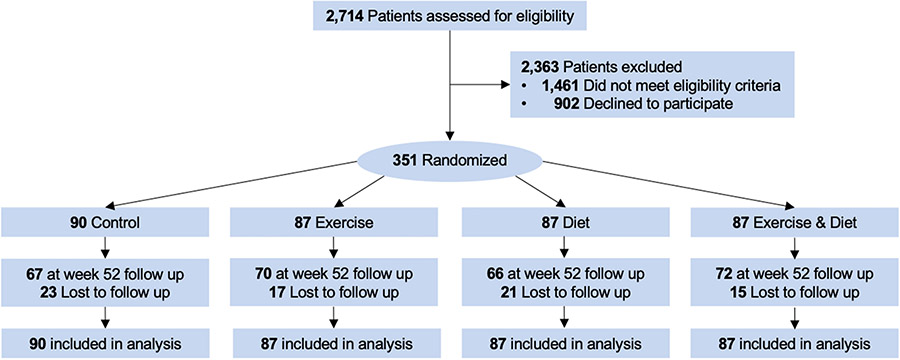
At baseline, the mean body weight was 90.0 (16.4) kg, and BMI was 34.0 (5.9) kg/m2; 243 (69%) participants had obesity (BMI ≥30 kg/m2), of whom 141 (58%) had severe obesity (BMI ≥35 kg/m2). At 52 weeks, 275 (78%) of participants provided body composition endpoint data (Figure 1). Participants who did not provide endpoint data were more likely to be recently diagnosed with cancer [multivariable-adjusted odds ratio: 1.03 per five-year interval (95% CI: 1.00, 1.07)]; no other measured baseline factors, including randomized group assignment (P=0.46) and body weight (P=0.74), were associated with missing week 52 endpoint data. Participants randomized exercise alone or exercise plus diet attended 84% of in-person supervised exercise sessions; adherence did not differ between exercise alone or exercise plus diet [Δ: −1.0% (95% CI: −7.3, 5.3)]. Participants randomized to diet alone or exercise plus diet attended 74% of in-person lifestyle modification sessions; adherence did not differ between diet alone or exercise plus diet [Δ: 0.9% (95% CI: −7.3, 9.1)].
Figure 1.
Flow of participants and ascertainment of body composition endpoint measures at week 52 by randomized group
Body Weight
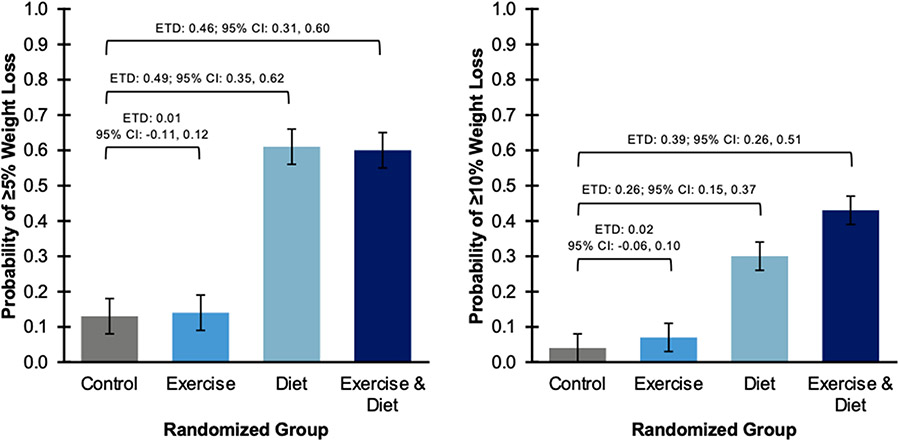
At 52 weeks, compared with control, diet alone [−5.39 kg (95% CI: −7.24, −3.55); −6.0% (95% CI: −8.0, −3.9); −2.1 kg/m2 (95% CI: −2.8, −1.4)] and exercise plus diet [−6.68 kg (95% CI: −8.46, −4.90); −7.4% (95% CI: −9.4, −5.4); −2.5 kg/m2 (95% CI: −3.2, −1.8)] reduced body weight (Table 2); exercise alone did not change body weight [−0.06 kg (95% CI: −1.85, 1.72); −0.1% (95% CI: −2.1, 1.9); −0.1 kg/m2 (95% CI: −0.7, 0.7)]. Compared with control, diet alone [0.49 (95% CI: 0.35, 0.62)] and exercise plus diet [0.46 (95% CI: 0.31, 0.60)] increased the probability of achieving ≥5% loss of baseline body weight (Figure 2); exercise alone did not increase the probability of achieving ≥5% loss of baseline body weight [0.01 (95% CI: −0.11, 0.12)]. Compared with control, diet alone [0.26 (95% CI: 0.15, 0.37)] and exercise plus diet [0.39 (95% CI: 0.26, 0.51)] increased the probability of achieving ≥10% loss of baseline body weight; exercise alone did not increase the probability of achieving ≥10% loss of baseline body weight [0.02 (95% CI: −0.06, 0.10)].
Table 2.
Change in body composition by randomized group
| Endpoint | Randomized Group |
Baseline Mean (SD) |
52 Week Mean Change (SE) |
Estimated Treatment Difference (95% CI) |
|---|---|---|---|---|
| Body weight, kg | Control | 89.3 (16.9) | −0.27 (0.63) | 0.00—Reference |
| Exercise | 89.7 (16.2) | −0.33 (0.66) | −0.06 (−1.85, 1.72) | |
| Diet | 89.9 (16.5) | −5.66 (0.69)a | −5.39 (−7.24, −3.55)b | |
| Exercise & Diet | 91.2 (16.3) | −6.95 (0.67)a | −6.68 (−8.46, −4.90)b | |
| Fat mass, kg | Control | 40.9 (10.7) | −0.94 (0.51) | 0.00—Reference |
| Exercise | 40.3 (10.9) | −0.79 (0.52) | 0.14 (−1.30, 1.59) | |
| Diet | 40.6 (10.3) | −4.52 (0.52)a | −3.59 (−5.00, −2.17)b | |
| Exercise & Diet | 41.7 (10.2) | −5.22 (0.53)a | −4.28 (−5.71, −2.84)b | |
| Visceral adipose tissue, cm2 | Control | 158.6 (57.2) | −6.59 (3.63) | 0.00—Reference |
| Exercise | 164.8 (75.5) | −12.09 (3.90)a | −5.50 (−15.73, 4.73) | |
| Diet | 158.2 (62.2) | −27.87 (3.68)a | −21.28 (−31.37, −11.19)b | |
| Exercise & Diet | 155.0 (69.6) | −26.46 (3.67)a | −19.87 (−30.03, −9.70)b | |
| Subcutaneous adipose tissue, cm2 | Control | 515.2 (128.0) | −5.05 (7.17) | 0.00—Reference |
| Exercise | 491.9 (133.2) | 2.64 (7.16) | 7.69 (−12.20, 27.58) | |
| Diet | 507.2 (125.9) | −44.59 (7.40)a | −39.53 (−59.85, −19.23)b | |
| Exercise & Diet | 502.7 (136.1) | −50.24 (7.46)a | −45.19 (−65.14, −25.23)b | |
| Lean mass, kg | Control | 49.1 (7.6) | −0.06 (0.25) | 0.00—Reference |
| Exercise | 50.1 (6.6) | 0.36 (0.25) | 0.42 (−0.25, 1.10) | |
| Diet | 49.9 (7.3) | −0.88 (0.24)a | −0.82 (−1.50, −0.15)b | |
| Exercise & Diet | 50.4 (7.3) | −1.30 (0.24)a | −1.24 (−1.92, −0.56)b | |
| Appendicular lean mass, kg | Control | 21.4 (3.8) | −0.05 (0.11) | 0.00—Reference |
| Exercise | 22.2 (3.7) | 0.18 (0.11) | 0.23 (−0.08, 0.54) | |
| Diet | 22.0 (3.8) | −0.37 (0.11)a | −0.32 (−0.63, −0.01)b | |
| Exercise & Diet | 22.3 (3.8) | −0.51 (0.11)a | −0.46 (−0.77, −0.15)b | |
| Bone mineral density, g/cm2 | Control | 1.12 (0.12) | −0.007 (0.004) | 0.000—Reference |
| Exercise | 1.12 (0.13) | −0.007 (0.004) | −0.001 (−0.012, 0.010) | |
| Diet | 1.16 (0.13) | −0.002 (0.004) | 0.005 (−0.006, 0.016) | |
| Exercise & Diet | 1.15 (0.14) | −0.003 (0.004) | 0.003 (−0.008, 0.014) |
Note: Unobserved data were multiply imputed using predictive mean matching and analyzed using a repeated measures analysis of covariance model. Models are adjusted for the baseline value of the dependent variable, and randomization stratification factors including age, receipt of radiotherapy, number of lymph nodes resected, lymphedema severity, and body mass index.
P<0.05 (two-sided) compared with baseline (within group).
P<0.05 (two-sided) compared with control, adjusted for multiplicity.
Figure 2.
Proportion of study participants who achieved a weight loss ≥5% (left panel) or ≥10% (right panel) of baseline body weight at week 52 by randomized group
Note: Unobserved data were multiply imputed using a parametric logistic regression imputation method and analyzed using generalized linear model that was adjusted for randomization stratification factors including age, receipt of radiotherapy, number of lymph nodes resected, lymphedema severity, and body mass index. ETD, Estimated Treatment Difference; CI, Confidence Interval.
Adipose Tissue
At 52 weeks, compared with control, diet alone [−3.59 kg (95% CI: −5.00, −2.17)] and exercise plus diet [−4.28 kg (95% CI: −5.71, −2.84)] reduced fat mass; exercise alone did not change fat mass [0.14 kg (95% CI: −1.30, 1.59)]. Compared with control, diet alone [−21.28 cm2 (95% CI: −31.37, −11.19)] and exercise plus diet [−19.87 cm2 (95% CI: −30.03, −9.70)] reduced visceral adipose tissue area; exercise alone did not change visceral adipose tissue area [−5.50 cm2 (95% CI: −15.73, 4.73)]. Compared with control, diet alone [−39.53 cm2 (95% CI: −59.85, −19.23)] and exercise plus diet [−45.19 cm2 (95% CI: −65.14, −25.23)] reduced subcutaneous adipose tissue area; exercise alone did not change subcutaneous adipose tissue area [7.69 cm2 (95% CI: −12.20, 27.58)].
Lean Mass
At 52 weeks, compared with control, diet alone [−0.82 kg (95% CI: −1.50, −0.15)] and exercise plus diet [−1.24 kg (95% CI: −1.92, −0.56)] reduced lean mass; exercise alone did not change lean mass [0.42 kg (95% CI: −0.25, 1.10)]. Compared with control, diet alone [−0.32 kg (95% CI: −0.63, −0.01)] and exercise plus diet [−0.46 kg (95% CI: −0.77, −0.15)] reduced appendicular lean mass; exercise alone did not change appendicular lean mass [0.23 kg (95% CI: −0.08, 0.54)].
Bone Density
At 52 weeks, compared with control, exercise alone [−0.001 g/cm2 (95% CI: −0.012, 0.010)], diet alone [0.005 g/cm2 (95% CI: −0.006, 0.016)], and exercise plus diet [0.003 g/cm2 (95% CI: −0.008, 0.014)] did not change bone mineral density.
Adverse Events
No serious or unexpected adverse events were reported; nonserious adverse events have been described [18].
DISCUSSION
In this 2×2 factorial trial of survivors of breast cancer with overweight or obesity, randomization to 52 weeks of diet or exercise plus diet produced statistically significant and clinically meaningful reductions in body weight, total body fat, and visceral adipose tissue. The majority of weight loss consisted of fat mass. Bone mineral density did not worsen in any intervention group over the 52-week treatment period, compared to the control group. The effects of exercise alone on body weight and body composition were nominal, and when combined with diet, did not mitigate the loss of lean mass.
The observed weight loss of 6.0% and 7.4% in the diet alone and exercise plus diet groups can be compared with the Lifestyle Intervention in Adjuvant Treatment of Early Breast Cancer (LISA) and Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trials [9, 10]. The LISA trial reported that a telephone-based multimodal lifestyle intervention reduced body weight by 4.8% at week 52 [10]. The ENERGY trial reported that a group-based lifestyle intervention reduced body weight by 4.5% at week 52 [9]. The current trial used a meal replacement plan, which in the general population yield greater weight loss than lifestyle modification instruction alone [28].
The observed magnitude of weight loss may be clinically meaningful [8]. Each 5 kg/m2 increase in BMI is associated with an 8–14% increase in the risk of disease recurrence or death in survivors of breast cancer [37, 38]. A hypothesis-generating analysis of the LISA trial demonstrated that randomization to the multimodal lifestyle intervention was associated with a numerically lower risk of cancer recurrence or death, as compared with control [HR: 0.71 (95% CI: 0.41, 1.24)] [39]. Weight loss ≥5% is associated with a 58% lower risk of developing type 2 diabetes [40], and weight loss ≥10% is associated with a 20% lower risk of experiencing a composite endpoint of major adverse cardiovascular events, including myocardial infarction, stroke, hospitalization for angina, and cardiovascular death [41].
In the general population, hypocaloric diets reduce body fat but catabolize lean tissue [12]. The Look AHEAD trial demonstrated that among patients with type 2 diabetes and obesity, reductions in body weight were composed of ≈70% fat mass [11]. In the current study, reductions in body weight were composed of 66% and 64% fat mass in the diet alone and the exercise plus diet groups, respectively. In a prior six-month study of weight loss in survivors of breast cancer, lean mass was not statistically significantly reduced from baseline, however the magnitude of weight loss was smaller (4.4%) than in our study making direct comparisons challenging [42]. These results confirm that the relative contribution of fat mass to weight loss in survivors of breast cancer is like other populations. Exercise, when combined with caloric restriction, did not substantively change the fraction of weight loss as fat mass, which is consistent with other studies [12].
Measures of body composition may offer additional information to quantify disease risk beyond that of body weight or BMI alone. Central adiposity, including visceral and subcutaneous adipose tissue, are strong predictors of adverse oncologic, metabolic, and cardiovascular outcomes [43]. Among survivors of breast cancer, excess subcutaneous adipose tissue is associated with a higher risk of all-cause death [44], and excess visceral adipose tissue is associated with a higher risk of myocardial infarction, stroke, and cardiovascular death [7]. The current trial demonstrated that diet alone and exercise plus diet induced statistically significant reductions in visceral and subcutaneous adipose tissue.
Critically low levels of lean mass, such as that occurring in old age, are associated with an increased risk of frailty, falls, and functional decline [16]. Over 52 weeks, participants in the diet alone and exercise plus diet groups lost 0.82 kg and 1.24 kg of lean mass, respectively; the relative changes were similar when restricted to appendicular lean mass as a surrogate for muscle mass. In randomized clinical trials of older adults with obesity, weight loss, with or without exercise, is associated with an improvement in objectively measured physical functioning [45, 46]. There were no detrimental effects of diet on bone mineral density. An ongoing randomized phase III trial will provide critical data needed to support the hypothesis that the overall health benefits of intentional and judicious weight loss in survivors of breast cancer outweigh the adverse effects of lean mass catabolism [47].
There are limitations to this trial. This trial was designed to evaluate the effects of two distinct, but complementary, lifestyle interventions; other trials included in the TREC consortium compared various combinations of exercise or diet with pharmacotherapy in survivors of cancer, thus we are unable to comment on lifestyle-pharmacotherapy synergy [19]. Unlike other modalities of body composition measurement, such as whole-body magnetic resonance imaging, DXA is unable to quantify intermuscular, hepatic, and pancreatic adipose tissue. Although the exercise group performed resistance and aerobic activities, the exercise program may have been of insufficient physiologic stimulus to meaningfully change body composition. Prior randomized trials of exercise that provided sufficient physiologic stimulus to change body composition outcomes included more supervision by exercise professionals, including the PAL trial [25, 26]. Missing endpoint data did not differ between randomized groups and was not associated with baseline body weight and body composition, however participants who did not provide endpoint data may differ in other ways that are not known. Although this study recruited participants with lymphedema, there is no reason to believe that these findings cannot be generalized to the broader population of survivors of breast cancer with overweight or obesity.
There are strengths to this trial. The randomized design and use of two distinct lifestyle modification interventions allowed for a time- and cost-efficient comparison of causal effects. The large sample size allowed us to detect small but potentially clinically important effect size differences. The diverse study sample improves the generalizability of study findings. The intervention was deployed using a combination of in-person and home-based methods. The diet program utilized a commercially available meal replacement program that is available throughout the United States. The use of home-based exercise reduced known barriers of participation associated with supervised gym-based programs.
Among survivors of breast cancer with overweight or obesity, diet alone or diet plus exercise produced clinically meaningful weight loss at week 52, with the majority of weight loss as fat mass. In conclusion, these data provide physiological support to the potential clinical benefits of weight management in survivors of breast cancer, for which phase III studies are ongoing [47].
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U54-CA155850, UL1-TR001878, P30-CA016520, P30-CA006927. Compression garments were donated by BSN Medical, and discounted meal replacements were provided by Nutrisystem, Inc. Dr. Brown is supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R00-CA218603 and R25-CA203650, the National Institute of General Medicine Sciences of the National Institutes of Health under Award Number U54-GM104940, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30-DK072476, the Susan G. Komen Foundation, and the American Institute for Cancer Research. Dr. Sarwer is supported by the National Institute for Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01-DK108628. Dr. Sturgeon is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1-TR002014, UL1-TR000003, and KL2-TR002015.
Footnotes
Conflicts of Interest/Competing Interests
Dr. Brown reports receiving grants from the National Institutes of Health, American Institute for Cancer Research, and the Susan G. Komen Foundation. Dr. Sarwer reports receiving grants from the National Institutes of Health and receiving personal fees from Ethicon and Novo Nordisk. Dr. Troxel reports receiving grants from the National Institutes of Health. Dr. DeMichele reports receiving grants from Novartis, Pfizer, Genentech, Calithera, and Menarini. Dr. Sturgeon reports receiving grants from the National Institutes of Health. Dr. Denlinger reports receiving grants from Agios Pharmaceuticals, Amgen Pharmaceuticals, Array BioPharma, Astra Zeneca, Bristol Myer Squibb, BeiGene, Genmab A/S, Loxo Oncology, and Zymeworks Inc. and honoraria from Bristol Myer Squibb, Merck, Exelixis, Taiho Oncology, and BeiGene. Dr. Schmitz reports receiving grants from the National Institutes of Health and nonfinancial support from BSN Medical, personal fees from Klose Training, and a licensed patent for a Strength After Breast Cancer course. No other disclosures were reported.
Availability of data and material
None
Code availability
None
Ethnics Approval
Yes
Consent to Participate
Yes
Consent for Publication
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Bray GA, Kim KK, Wilding JPH, World Obesity F: Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 2017, 18(7):715–723. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY: Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol 2016, 34(26):3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmann AE, Soldera SV, Pimentel I, Ribnikar D, Ennis M, Amir E, Goodwin PJ: Association of Obesity with Breast Cancer Outcome in Relation to Cancer Subtypes: A Meta-Analysis. J Natl Cancer Inst 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, Salati M, Dottorini L, Iaculli A, Varricchio A et al. : Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2021, 4(3):e213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, Chang YJ, Choi KS, Jung SY, Woo SM et al. : Incidence of Diabetes After Cancer Development: A Korean National Cohort Study. JAMA Oncol 2018, 4(8):1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cespedes Feliciano EM, Kwan ML, Kushi LH, Weltzien EK, Castillo AL, Caan BJ: Adiposity, post-diagnosis weight change, and risk of cardiovascular events among early-stage breast cancer survivors. Breast Cancer Res Treat 2017, 162(3):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cespedes Feliciano EM, Chen WY, Bradshaw PT, Prado CM, Alexeeff S, Albers KB, Castillo AL, Caan BJ: Adipose Tissue Distribution and Cardiovascular Disease Risk Among Breast Cancer Survivors. J Clin Oncol 2019, 37(28):2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF et al. : 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014, 129(25 suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, Wolin KY, Elias A, Krontiras H, Liu J et al. : Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J Clin Oncol 2015, 33(28):3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, Blackburn GL, Findlay B, Gralow JR, Mukherjee S et al. : Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol 2014, 32(21):2231–2239. [DOI] [PubMed] [Google Scholar]
- 11.Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, Hill J, Kahn SE, Nathan DM, Schwartz AV et al. : Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2015, 23(3):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D: Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev 2014, 15(4):310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, Quesenberry CP, Weltzien EK, Castillo AL, Olobatuyi TA et al. : Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol 2018, 4(6):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE: Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 2014, 10(8):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oikonomou EK, Antoniades C: The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 2019, 16(2):83–99. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Jentoft AJ, Sayer AA: Sarcopenia. The Lancet 2019, 393(10191):2636–2646. [DOI] [PubMed] [Google Scholar]
- 17.Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, Nguyen TV, Sainsbury A: Does Diet-Induced Weight Loss Lead to Bone Loss in Overweight or Obese Adults? A Systematic Review and Meta-Analysis of Clinical Trials. J Bone Miner Res 2015, 30(12):2168–2178. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz KH, Troxel AB, Dean LT, DeMichele A, Brown JC, Sturgeon K, Zhang Z, Evangelisti M, Spinelli B, Kallan MJ et al. : Effect of Home-Based Exercise and Weight Loss Programs on Breast Cancer-Related Lymphedema Outcomes Among Overweight Breast Cancer Survivors: The WISER Survivor Randomized Clinical Trial. JAMA Oncol 2019, 5(11):1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz KH, Gehlert S, Patterson RE, Colditz GA, Chavarro JE, Hu FB, Neuhouser ML, Sturgeon KM, Thornquist M, Tobias D et al. : TREC to WHERE? Transdisciplinary Research on Energetics and Cancer. Clin Cancer Res 2016, 22(7):1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winkels RM, Sturgeon KM, Kallan MJ, Dean LT, Zhang Z, Evangelisti M, Brown JC, Sarwer DB, Troxel AB, Denlinger C et al. : The women in steady exercise research (WISER) survivor trial: The innovative transdisciplinary design of a randomized controlled trial of exercise and weight-loss interventions among breast cancer survivors with lymphedema. Contemp Clin Trials 2017, 61:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health UDo, Services H: Common terminology criteria for adverse events (CTCAE) version 4.0. National Cancer Institute; 2009(09-5410). [Google Scholar]
- 22.Sturgeon KM, Hackley R, Fornash A, Dean LT, Laudermilk M, Brown JC, Sarwer DB, DeMichele AM, Troxel AB, Schmitz KH: Strategic recruitment of an ethnically diverse cohort of overweight survivors of breast cancer with lymphedema. Cancer 2018, 124(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taves DR: Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 1974, 15(5):443–453. [DOI] [PubMed] [Google Scholar]
- 24.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH et al. : Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 2019, 51(11):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP: Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med 2009, 361(7):664–673. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, Bryan CJ, Williams-Smith CT, Chittams J: Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA 2010, 304(24):2699–2705. [DOI] [PubMed] [Google Scholar]
- 27.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M et al. : Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012, 62(4):243–274. [DOI] [PubMed] [Google Scholar]
- 28.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R et al. : A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011, 365(21):1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, Friend J: Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol 2015, 16(1):108–116. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB: Body composition by DXA. Bone 2017, 104:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toombs RJ, Ducher G, Shepherd JA, De Souza MJ: The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring) 2012, 20(1):30–39. [DOI] [PubMed] [Google Scholar]
- 32.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR: Validation of an optoelectronic limb volumeter (Perometer). Lymphology 1997, 30(2):77–97. [PubMed] [Google Scholar]
- 33.Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL, McTiernan A: Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012, 20(8):1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little RJ: Missing-data adjustments in large surveys. Journal of Business & Economic Statistics 1988, 6(3):287–296. [Google Scholar]
- 35.Holm S: A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics 1979, 6(2):65–70. [Google Scholar]
- 36.Fitzmaurice GM, Laird NM, Ware JH: Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 37.Ligibel JA, Cirrincione CT, Liu M, Citron M, Ingle JN, Gradishar W, Martino S, Sikov W, Michaelson R, Mardis E et al. : Body Mass Index, PAM50 Subtype, and Outcomes in Node-Positive Breast Cancer: CALGB 9741 (Alliance). J Natl Cancer Inst 2015, 107(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T: Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014, 25(10):1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, Findlay B, Gralow JR, Mukherjee SD, Levine M et al. : The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. NPJ Breast Cancer 2020, 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research G: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002, 346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Look ARG, Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C et al. : Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016, 4(11):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, Playdon M, Li F, Irwin ML: Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol 2016, 34(7):669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown JC, Cespedes Feliciano EM, Caan BJ: The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle 2018, 9(7):1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradshaw PT, Cespedes Feliciano EM, Prado CM, Alexeeff S, Albers KB, Chen WY, Caan BJ: Adipose Tissue Distribution and Survival Among Women with Nonmetastatic Breast Cancer. Obesity (Silver Spring) 2019, 27(6):997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K: Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011, 364(13):1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, Armamento-Villareal R, Qualls C: Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med 2017, 376(20):1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ligibel JA, Barry WT, Alfano C, Hershman DL, Irwin M, Neuhouser M, Thomson CA, Delahanty L, Frank E, Spears P et al. : Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer 2017, 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]