Abstract
Background:
Cancer patients with chemotherapy-induced peripheral neuropathy (CIPN) are at increased risk of falls and developing fear of falling (FoF). Although FoF may continue to impair motor performance and increase the risk of falling even further, this association remains unexplored in CIPN.
Research question:
Does high FoF in patients with CIPN further deteriorate motor performance beyond the impairment from CIPN-related sensory deficits?
Methods:
In this secondary analysis of data collected from two clinical trials, gait parameters during habitual walking condition and postural sway parameters during 30-second quiet standing (eye-open and eyes-closed) were compared among older participants (≥ 65 years) with CIPN and high FoF (CIPN FoF+; n = 16), older participants with CIPN and low FoF (CIPN FoF−; n = 19) and normal older controls (i.e., non-cancer, non-diabetic, non-neurologic, and non-orthopedic; n = 16). We measured gait and postural sway parameters using wearable sensors (BioSensics, Newton, MA, USA), and FoF severity using the Falls Efficacy Scale-International.
Results:
The largest between-group differences were found in gait speed. The CIPN FoF+ group had significantly slower gait speed (0.78 ± 0.21 m/s) than the CIPN FoF− (0.93 ± 0.17 m/s) and normal control groups (1.17 ± 0.13 m/s) (all p < .05; effect sizes = 0.79 and 2.23, respectively). We found a significant association between gait speed and FoF severity (R2 = 0.356; p < .001) across all participants with CIPN. Among participants with CIPN, no significant differences in postural sway parameters were found between the CIPN FoF+ and CIPN FoF− groups.
Significance:
Our results suggest that gait performance further deteriorates in patients with CIPN and high FoF beyond the impairment from CIPN-related sensory deficits. Our results also suggest further research is needed regarding FoF, and fall risk, as FoF is a simple tool that healthcare providers can use in clinical practice.
Keywords: Chemotherapy-induced peripheral neuropathy, fear of falling, gait speed, postural sway, wearable sensors
1. Introduction
In the United States, more than 10 million older adults (≥65 years) are cancer survivors, and the number is projected to increase to nearly 15 million by 2030 [1]. Although advances in chemotherapy have improved survival outcomes for many of these patients [2], chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of many chemotherapeutic agents [3]. CIPN presents as a loss of sensation, tingling, and pain in the extremities and may occur as soon as one month after receiving neurotoxic chemotherapy [4]. Nearly 50% of patients with CIPN will experience long-term side effects, even if the drug is stopped [5].
Increased risk of falling is a significant problem associated with CIPN due to loss of sensation and proprioception in the foot [5]. Nearly 20% of patients with CIPN fall during or after chemotherapy [6], and the frequency of falls in patients with CIPN was three times higher than those without CIPN [7]. Previous studies have shown that slower gait speed and poorer postural sway is directly associated with increased risk of falling [8, 9]. Additionally, the increased fall risk may lead to a fear of falling (FoF), a well-known surrogate measure for falls and functional decline [10-12], which reportedly affects more than half of patients with CIPN [13]. The presence of FoF may further restrict mobility, worsen gait performance and postural control, and inevitably increases the risk of falling even further [14]. For example, in non-clinical cohorts, older adults with high FoF had slower gait speed and poorer postural control than those with low FoF [15-17]. In a clinical cohort of patients with diabetic peripheral neuropathy, which has a similar sensory loss as CIPN, gait speed decreased with increased FoF in patients with diabetic peripheral neuropathy [18].
Despite the prevalence of FoF in patients with CIPN and its possible association with poor gait performance and postural sway beyond sensory deficits as evidenced by cohorts of diabetic peripheral neuropathy, it is uncertain whether patients with CIPN and high FoF have poorer gait performance and postural sway than patients with CIPN and low FoF. Accordingly, the purpose of this study was to investigate the association between FoF, gait performance and postural sway in cancer patients with CIPN. Based on previous reports in non-CIPN cohorts [15-18], we hypothesized that participants with CIPN and high FoF would have poorer gait performance and postural sway compared to participants with CIPN and low FoF and normal controls.
2. Methods
2.1. Participants
Participants in this study were subsets of two clinical trials, ClinicalTrials.gov identifiers NCT02773329 (for patients with CIPN) and NCT01880229 (for normal controls). The CIPN group consisted of 35 ambulatory older patients with CIPN. Eligible individuals were ≥ age 65 years, had confirmed CIPN according to their oncologists based on either participant’s electronic chart review when available or participant’s self-description about loss of sensation, tingling, and pain in the foot. All participants in the CIPN group had daily symptoms of numbness in the foot, which was assessed in both subjective (self-description) and objective (described below) ways. Individuals who had a history of a neurodegenerative disease such as Parkinson’s disease, stroke and dementia, or an active foot ulcer or infection were excluded. Patients taking non-cancer medications that might affect gait performance and postural sway were also excluded (e.g., Parkinsonian medications).
The normal control group (age-, sex-, and body mass index [BMI]-matched) consisted of 16 adults aged 65 years and over who did not have FoF as determined by the Falls Efficacy Scale-International (FES-I) described below [11]. This group had no history of cancer, diabetes, neurodegenerative disease or orthopedic condition in the lower extremity, and no fall history in the past 12 months. All participants were able to walk without a walking aid for at least 15 meters, and signed a written informed consent that was approved by the local Institutional Review Board.
2.2. Demographic and clinical measures
We collected demographic information such as age, height, and weight. We assessed a history of fall in the past 12 months using a self-reported, single-item question (yes/no): Have you had an accidental fall in the past year? We assessed FoF using the 16-item FES-I [11], a validated self-report questionnaire that assesses degree of concern about falling during daily activities (e.g., “going up or down stairs”) on a Likert-type scale from 1 (“not at all concerned”) to 4 (“very concerned”). Scores are summed to yield a total score ranging from 16 to 64. A cut-off of 28 indicating high FOF has been established [19].
Peripheral neuropathy severity (i.e., foot numbness) was objectively assessed based on vibration perception threshold (VPT), which was measured from the plantar surface of both feet (the first and fifth metatarsal heads, and heel) using a Biothesiometer (Bio-Medical Instrument, Newbury, OH, USA), as previously described [20-22].
2.3. Gait and postural sway measures
We evaluated gait performance and postural sway using validated wearable sensors (BioSensics, Newton, MA, USA) [23-25]. Each sensor consisted of an accelerometer and a gyroscope and collected linear acceleration and angular velocity of a body segment to which the sensor was attached with a sampling rate of 100 Hz. For evaluating gait performance, we attached two sensors to each shin, and asked participants to walk over 40 feet (approximately 12 meters) at their habitual speed. Outcome measures for the gait performance were gait speed, stride length, cadence and stride time that were calculated using a commercial gait analysis software (LEGSys™, BioSensics, Newton, MA, USA).
Postural sway was evaluated by an additional pair of sensors attached to the lower back and shin in the dominant leg, and participants were asked to stand quietly with arms crossing the chest for 30 seconds with eyes-open and eyes-closed conditions. Outcome measures for the postural sway were body sway in the hip, ankle and center-of-mass (CoM) that were calculated using another commercial balance analysis software (BalanSens™, BioSensics, Newton, MA, USA). Higher values in any postural sway parameters indicate poorer postural control.
2.4. Statistical analysis
The primary outcome measures were gait performance and postural sway. We classified the CIPN group into two groups based on FES-I scores: CIPN with high FoF (CIPN FoF+; FES-I≥28; N=16) and CIPN with low FoF (CIPN FoF−; FES-I≤27; N=19). We first tested the normality of all outcome measures using Shapiro-Wilk tests. For demographic and clinical measures, among the CIPN FoF+, CIPN FoF− and normal control groups, we compared age and FES-I scores using one-way analysis of variance (ANOVA; parametric variables), BMI using a Kruskal-Wallis test (non-parametric variable), and the number of women and men and the number of individuals who had fallen in the past 12 months (categorical variables) using Chi-square tests. For the CIPN FoF+ and CIPN FoF− groups, we compared VPT and time since a cancer diagnosis (non-parametric variables) using Mann Whitney U tests, and the number of diabetic individuals, the number of individuals whose chemotherapy was active, and types of cancer (categorical variables) using Chi-square tests.
For the gait and postural sway parameters, we first performed cross-sectional comparisons among the CIPN FoF+, CIPN FoF− and normal control groups for all gait parameters (parametric variables) using one-way ANOVA, and all postural sway parameters (non-parametric variables) using Kruskal-Wallis tests. If the one-way ANOVA or Kruskal-Wallis test found a significant difference among the three groups, we performed a follow-up multiple comparisons to determine which groups’ means differ. For multiple comparisons, p-values were adjusted using Bonferroni correction. For a gait or postural sway parameter that had a significant difference between the CIPN FoF+ and CIPN FoF− groups, we performed a regression analysis to investigate the association between FoF severity (i.e., FES-I) and the parameters across the participants with CIPN. For the regression analysis, we used both a simple linear regression model (i.e., unadjusted) and a multivariate regression model adjusting for confounding factors including age, sex, BMI, VPT and time since cancer diagnosis.
A p-value<.05 was considered statistically significant. Effect size was calculated using Cohen’s d. All statistical analyses were performed using SPSS version 26 (IBM, Armonk, NY, USA).
3. Results
3.1. Demographic and clinical characteristics
We summarized demographic and fall-related clinical characteristics for each group in Table 1. There was no significant differences in demographic variables (all p≥.05) but fall-related clinical variables were significantly different (all p<.05).
Table 1.
Demographic and fall-related clinical characteristics for each group.
| Measures | CIPN FoF+ (N = 16) |
CIPN FoF− (N = 19) |
Normal control (N = 16) |
P-value |
|---|---|---|---|---|
| Age, years | 74.1 ± 5.8 | 72.1 ± 4.6 | 75.3 ± 6.8 | .240 |
| Body-mass index, kg/m2 | 27.25 ± 4.67 | 24.57 ± 3.46 | 24.51 ± 2.59 | .441 |
| Women, N (%) | 6 (37.5%) | 9 (47.4%) | 12 (75.0%) | .087 |
| Fall history, N (%) | 7 (43.8%) | 4 (21.1%) | 0 (0.0%) | .011 |
| FES-I, no unit | 40.2 ± 10.2 | 21.9 ± 3.0 | 19.1 ± 2.0 | < .001 |
Note: Values are expressed as mean ± standard deviation.
Abbreviations: CIPN = chemotherapy-induced peripheral neuropathy, FoF = fear of falling, FES-I = Falls Efficacy Scale-International.
We summarized cancer-related and other clinical characteristics for the CIPN groups in Table 2. Between the CIPN FoF+ and CIPN FoF− groups, there was no significant differences in cancer-related and other clinical measures (all p≥.05).
Table 2.
Cancer-related and other clinical characteristics for the CIPN groups.
| Measures | CIPN FoF+ | CIPN FoF− | P-value |
|---|---|---|---|
| Peripheral neuropathy severity (VPT; volts) | 41.5 ± 10.7 | 36.6 ±14.1 | .441 |
| Time since cancer diagnosis, years | 5.5 ± 6.1 | 4.6 ± 5.1 | .736 |
| Chemo active, N (%) | 4 (25.0%) | 11 (57.9%) | .050 |
| Types of cancer, N (%) | |||
| Multiple myeloma | 5 (31.3%) | 3 (15.8%) | .278 |
| Lung | 4 (25.0%) | 4 (21.1%) | .782 |
| Breast | 3 (18.8%) | 1 (5.3%) | .212 |
| Colorectal | 0 (0.0%) | 3 (15.8%) | .096 |
| Ovarian | 0 (6.3%) | 1 (0.0%) | .352 |
| Pancreatic | 1 (6.3%) | 1 (5.3%) | .900 |
| Melanoma | 0 (0.0%) | 2 (10.5%) | .181 |
| Lymphoma | 1 (6.3%) | 1 (5.3%) | .900 |
| Other | 2 (12.5%) | 3 (15.8%) | .782 |
| Diabetes, N (%) | 3 (18.8%) | 6 (31.6%) | .387 |
Note: Values are expressed as mean ± standard deviation.
Abbreviations: CIPN = chemotherapy-induced peripheral neuropathy; FoF = fear of falling; FES-I = Falls Efficacy Scale-International; VPT = vibration perception threshold.
3.2. Gait performance and postural sway
We summarized gait characteristics for each group in Table 3. We found significant group differences for all gait parameters among the three groups (all p<.001). Gait speed was 16.1% and 33.3% slower for the CIPN FoF+ group than for the CIPN FoF− and normal control groups, respectively (all p<.05; 95% confidence interval [CI] = [−0.3084,−0.0058] and [−0.5526,−0.2415], respectively; Cohen’s d=0.79 and 2.23, respectively), and was 20.5% slower for the CIPN FoF− group than for the normal control group (p<.05; 95% CI = [−0.3886,−0.0913]; Cohen’s d=1.56). Stride length was 12.4% and 21.4% shorter for the CIPN FoF+ group than for the CIPN FoF− and normal control groups (all p<.05; 95% CI = [−0.2814,−0.0004] and [−0.4132,−0.1244], respectively; Cohen’s d=0.73 and 1.58, respectively). Stride length was 10.3% shorter for the CIPN FoF− group than for the normal control group but the difference did not reach the statistical significance level (p≥.05). The CIPN FoF+ and CIPN FoF− groups had 15.9% and 11.7% slower cadence, respectively, than the normal control group (all p<.05; 95% CI = [3.9435,13.8742] and [1.7777,11.2717], respectively; Cohen’s d=1.51 and 1.38, respectively). The CIPN FoF+ and CIPN FoF− groups had 21.3% and 14.8% greater stride time, respectively, than the normal control group (all p<.05; 95% CI = [−0.3516,−0.0903] and [−0.2778,−0.0279], respectively; Cohen’s d=1.42 and 1.51, respectively).
Table 3.
Comparison of gait performance.
| Measures | CIPN FoF+ | CIPN FoF− | Normal control |
P-value (Group difference) |
|---|---|---|---|---|
| Gait speed (m/s) | 0.78 ± 0.21 *,† | 0.93 ± 0.17 ‡ | 1.17 ± 0.13 |
P < .001 F(2,46) = 20.549 |
| Stride length (meters) | 0.99 ± 0.22 *,† | 1.13 ± 0.15 | 1.26 ± 0.10 |
P < .001 F(2,46) = 10.699 |
| Cadence (strides/min) | 47.09 ± 7.06 * | 49.47 ± 4.99 ‡ | 56.00 ± 4.48 |
P < .001 F(2,46) = 10.840 |
| Stride time (seconds) | 1.31 ± 0.21 * | 1.24 ± 0.12 ‡ | 1.08 ± 0.09 |
P < .001 F(2,46) = 9.386 |
Note: Values are expressed as mean ± standard deviation.
Significant pairwise differences (p < .05) between the CIPN FoF+ and normal control groups.
Significant pairwise differences (p < .05) between the CIPN FoF+ and CIPN FoF− groups.
Significant pairwise differences (p < .05) between the CIPN FoF− and normal control groups.
Abbreviations: CIPN = chemotherapy-induced peripheral neuropathy; FoF = fear of falling; m/s = meters/second, min = minutes.
We summarized postural sway characteristics for each group in Table 4. Significant pairwise differences were found in some of the postural sway parameters. For the eyes-open condition, the CIPN FoF+ and CIPN FoF− groups had 2.08 deg2 and 1.33 deg2 larger ankle sway, respectively, than the normal control group (all p<.05; Cohen’s d=0.72 and 1.49, respectively), and hip sway and CoM sway were 1.18 deg2 and 0.21 cm2 larger, respectively, for the CIPN FoF− group than for the normal control group (all p<.05; Cohen’s d=0.91 and 1.41, respectively). For the eyes-closed condition, ankle sway was 1.61 deg2 larger for the CIPN FoF− group than for the normal control group (p<.05; Cohen’s d=0.99). The CIPN FoF+ and CIPN FoF− groups had 3.58 cm2 and 1.98 cm2 larger CoM sway for the eyes-closed condition, respectively, than the normal control group (all p<.05; Cohen’s d=0.53 and 0.97, respectively). There were no significant differences between the CIPN FoF+ and CIPN FoF− groups in any postural sway parameters.
Table 4.
Comparison of postural sway.
| Measures | CIPN FoF+ | CIPN FoF− | Normal control |
P-value (Group difference) |
|---|---|---|---|---|
| Eyes-open condition | ||||
| Ankle sway (deg2) | 2.60 ± 4.05 * | 1.85 ± 1.22 ‡ | 0.52 ± 0.34 |
P = .001 X2(2) = 14.659 |
| Hip sway (deg2) | 1.84 ± 2.71 | 1.77 ± 1.79 ‡ | 0.59 ± 0.37 |
P = .036 X2(2) = 6.672 |
| CoM sway (cm2) | 0.49 ± 0.87 | 0.31 ± 0.19 ‡ | 0.10 ± 0.09 |
P = .003 X2(2) = 11.684 |
| Eyes-closed condition | ||||
| Ankle sway (deg2) | 4.79 ± 6.44 | 2.98 ± 2.02 ‡ | 1.37 ± 1.09 |
P = .039 X2(2) = 6.499 |
| Hip sway (deg2) | 2.45 ± 2.31 | 3.45 ± 3.34 | 1.32 ± 0.77 |
P = .076 X2(2) = 5.152 |
| CoM sway (cm2) | 3.83 ± 9.50 * | 2.23 ± 2.88 ‡ | 0.25 ± 0.19 |
P = .001 X2(2) = 14.181 |
Note: Values are expressed as mean ± standard deviation.
Significant pairwise differences (p < .05) between the CIPN FoF+ and normal control groups.
Significant pairwise differences (p < .05) between the CIPN FoF+ and CIPN FoF− groups.
Significant pairwise differences (p < .05) between the CIPN FoF− and normal control groups.
Abbreviations: CIPN = chemotherapy-induced peripheral neuropathy; FoF = fear of falling; deg = degrees; cm = centimeters; CoM = center of mass.
3.3. Correlation between FES-I and gait speed
Gait speed and stride length were significantly different between the CIPN FoF+ and CIPN FoF− groups, and thus we investigated correlation between FES-I scores and gait speed, and between FES-I scores and stride length as follow-up analyses (Figure 1). Linear regression revealed significant correlations between FES-I scores and gait speed (p<.001; R2=0.356), and between FES-I scores and stride length (p=.001; R2=0.299).
Figure 1.
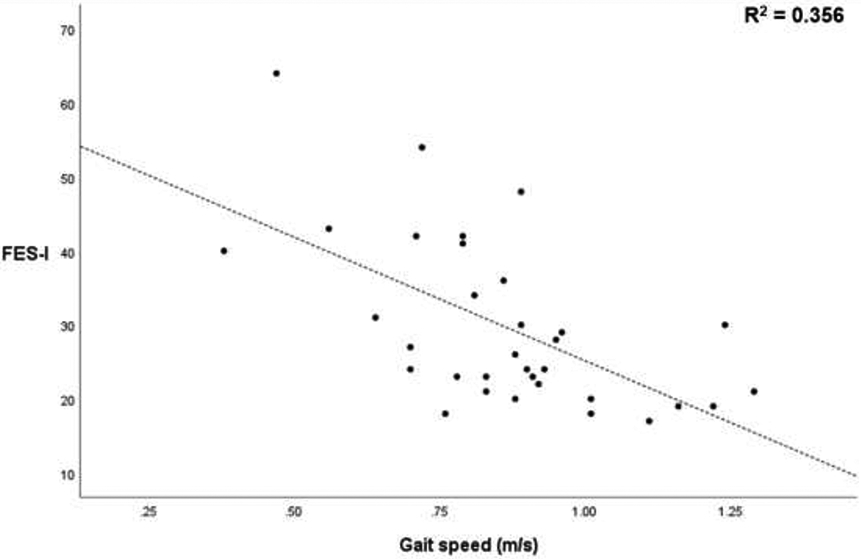
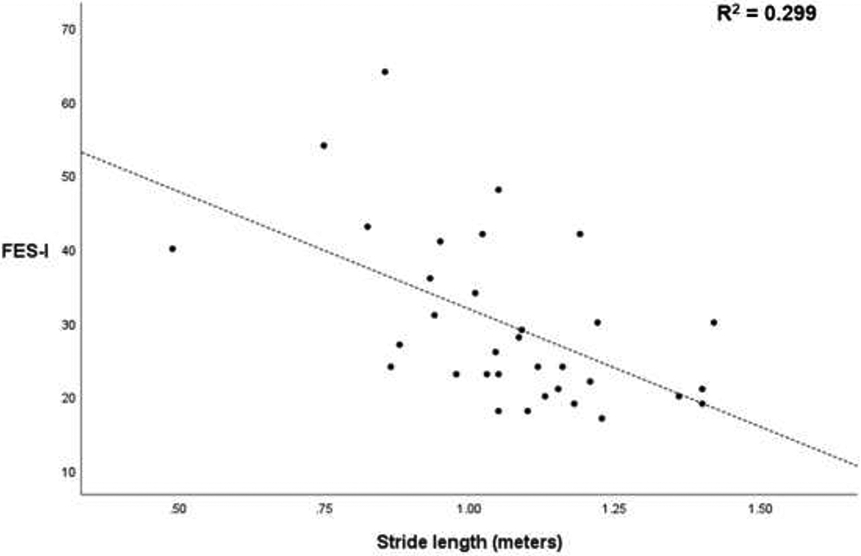
Correlations between gait speed and Falls-Efficacy Scale-International (FES-I), and between stride length and FES-I.
For multivariate regression analysis, we checked multicollinearity of independent variables using variance inflation factor, and confirmed independency among the independent variables. A multivariate regression model based on age, sex, BMI, VPT, time since cancer diagnosis, and gait speed significantly predicted FES-I scores (p<.05; F[6,26]=3.574; R2=0.452). Another multivariate regression model based on age, sex, BMI, VPT, time since cancer diagnosis, and stride length significantly predicted FES-I scores (p<.05; F[6,26]=3.011; R2=0.410).
4. Discussion
We investigated the association between FoF and gait performance and postural sway in older patients with CIPN. The primary findings were that the CIPN FoF+ group had slower gait speed and shorter stride length relative to the CIPN FoF− and normal control groups. Gait speed and stride length were significantly correlated with FoF severity, even after accounting for confounding factors. To our knowledge, this is the first study to demonstrate that FoF may be associated with worse gait performance in patients with CIPN.
Although CIPN is a known risk factor for gait impairment due to sensory deficits in the foot, FoF can induce a more cautious gait pattern [26]. These findings are consistent with those of previous studies obtained in non-CIPN cohorts. Bryant and colleagues found that gait speed was significantly slower in Parkinsonian patients with high FoF than those with low FoF [27]. Rosén and colleagues reported that gait speed was significantly correlated with FoF severity in stroke survivors [28]. Our results extend previous findings from these studies and confirm that FoF caused a more cautious gait pattern as manifested by slower gait speed and shorter stride length across disease conditions. Furthermore, FoF may have a detrimental impact on gait performance in patients with CIPN beyond sensory deficits alone. Based on the direct association between poorer gait speed and increased of falling, our results suggest the presence of FoF further increases risk of falling.
Postural sway did not demonstrate significant differences in any parameters between the CIPN FoF+ and CIPN FoF− groups. Our results for postural sway were not in agreement with those of other clinical cohorts. For example, Kalron and Achiron reported that the degree of body sway during quiet standing was significantly correlated with FoF severity in patients with multiple sclerosis [29]. It may be that a combination of FoF and peripheral nervous system dysfunction may act on balancing during quiet standing differently than a combination between FoF and central nervous system dysfunction. However, the cause of these differences is still unclear, which should be addressed in future studies.
Our multivariate regression analysis revealed no significant effect from peripheral neuropathy severity (VPT) on FoF severity (FES-I). This result is interesting because previous CIPN studies reported by Tofthagen and colleagues and Kolb and colleagues have shown an association between peripheral neuropathy severity and fall risk [6, 7]. However, our results indicate that there is no association between VPT and FoF severity. A similar result was reported by Kelly and colleagues in a clinical cohort with diabetic peripheral neuropathy. In that study, VPT in diabetic patients was not related to FES-I [18]. A potential reason for this inconsistency could be the difference in measuring peripheral neuropathy severity as argued by Zhi and colleagues [30]. We measured peripheral neuropathy severity objectively using VPT, which has been shown significantly correlated with motor deterioration among cancer survivors suffering from CIPN [26], but the primary measure of this method is the plantar numbness and thus may provide a single-domain of CIPN symptoms. Although the methods used in Tofthagen and colleagues and Kolb and colleagues are subjective, they may provide multi-domains of CIPN symptoms. Together with the previous study by Kelly and colleagues, our results suggest that caution should be taken when using peripheral neuropathy severity as a predictor for FoF development and progression in patients with CIPN.
One implication of our findings is that FoF may be a significant covariate for mobility outcomes in patients with CIPN [31]. Zimmer and colleagues and McCrary and colleagues reported mixed results of effects of exercise on mobility for CIPN patients: no significant improvement reported by Zimmer and colleagues but significant improvement reported by McCrary and colleagues [32, 33]. FoF was not measured in their studies thus, it is not certain whether FoF could have impacted their results. However, when taken in context with our data, it may be that the possible presence of FoF interfered with gait performance, thus explaining the mixed results in these prior studies. We suggest considering FoF in future clinical trials that target mobility in CIPN.
Limitations of this study should be acknowledged. First, our sample size was small, and future studies with a large sample size are needed to generalize our results. However, given the large effect size, particularly for gait performance, similar findings may emerge in large samples. Second, most of our participants in the CIPN groups had severe foot numbness. It may be that the effect of FoF on motor performance in those with relatively mild foot numbness (e.g., VPT<25 volts) is not the same as the current cohort. Additionally, some of participants in the CIPN groups were receiving chemotherapy at the time of this study, which might have an acute impact on motor performance regardless of FoF severity. Future trials to study the association between FoF, and motor performance during ongoing chemotherapy are needed. Also, antitumor therapies could have caused decrease in physical performance regardless of CIPN and FoF severity [34], which might have affected our results. Our study was limited to a secondary analysis of a retrospective study, and future prospective studies to find the association between FoF severity and actual falls are recommended. We also acknowledge that VPT may not provide a comprehensive view on our participants’ impairment.
Nevertheless, our findings are noteworthy and propose future clinical and research directions. One primary direction is to screen patients with CIPN regarding high FoF as a surrogate measure of falls. Also, targeted interventions to mitigate FoF should be evaluated. For example, some physical and psychological interventions could be effective in mitigating FoF in older adults [35]. In addition, low-dose of foot and ankle exercise may have potential to improve FoF in patients with CIPN [24]. Interventions for appropriate chemotherapy dose modification to minimize the development and progression of FoF are also warranted.
5. Conclusion
Although long-term survival for cancer patients continues to improve, many patients receive neurotoxic chemotherapy that can increase the risk of falls and decrease quality of life. Our study investigated whether FoF impacted gait performance and postural sway in older cancer patients with CIPN, yielding important insights. Our findings show that FoF in patients with CIPN is associated with worse gait performance, which suggests that FoF may impair mobility beyond sensory deficits from CIPN. Further studies are needed to evaluate how researchers and healthcare providers could utilize FoF as a risk stratification tool for gait impairment, as well as potential interventions to mitigate fall risk and preserve mobility in cancer survivors.
Table 5.
Multivariate regression model to predict FES-I scores from a model composed of age, sex, BMI, VPT, time since cancer diagnosis and gait speed (Model 1), and another model composed of age, sex, BMI, VPT, time since cancer diagnosis and stride length (Model 2).
| Independent variables | β | P-value | 95% CI | VIF |
|---|---|---|---|---|
| Model 1 | ||||
| Age | −0.014 | 0.929 | −0.727, 0.666 | 1.195 |
| Sex | 0.219 | 0.163 | −2.150, 12,126 | 1.099 |
| BMI | 0.249 | 0.119 | −0.193, 1.601 | 1.133 |
| Peripheral neuropathy severity (VPT) | 0.080 | 0.616 | −0.505, 0.836 | 1.191 |
| Time since cancer diagnosis | 0.105 | 0.523 | −0.202, 0.388 | 1.244 |
| Gait speed | −0.517 | 0.004 | −47.693, −10.281 | 1.250 |
| Model 2 | ||||
| Age | 0.009 | 0.956 | −0.700, 0.739 | 1.184 |
| Sex | 0.169 | 0.315 | −3.882, 11.595 | 1.200 |
| BMI | 0.219 | 0.186 | −0.318, 1.555 | 1.148 |
| Peripheral neuropathy severity (VPT) | 0.130 | 0.444 | −0.190, 0.421 | 1.235 |
| Time since cancer diagnosis | 0.215 | 0.179 | −0.216, 1.100 | 1.065 |
| Stride length | −0.453 | 0.011 | −46.168, −6.690 | 1.192 |
Note: P-values correspond to the standardized coefficients (β).
Abbreviations: FES-I = Falls-Efficacy Scale-International; VIF = variance inflation factor; BMI = body mass index; VPT = vibration perception threshold; CI = confidence interval.
Highlights.
Fear of falling worsens gait speed beyond sensory deficits.
Fear of falling worsens stride length beyond sensory deficits.
Gait speed was significantly associated with fear of falling severity.
Stride length was significantly associated with fear of falling severity.
Postural sway was not associated with fear of falling severity.
Acknowledgement
We thank Ana Enriquez, Manuel Gardea, and Ivan Marin for their help with participant recruitment, and data collection and data analysis. This study received partial supports from the National Cancer Institute (award number 1R21CA190933), the National Heart, Lung, and Blood Institute (award number 5T32HL139430) and the National Institutes of Health/National Institute on Aging (award numbers SB1AG032748 and R42AG060853). However, the funding sources had no role in study design, data collection and analysis, and the presentation of the results.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. , Cancer treatment and survivorship statistics, 2019, CA: a cancer journal for clinicians 69(5) (2019) 363–385. [DOI] [PubMed] [Google Scholar]
- [2].DeVita VT, Chu E, A history of cancer chemotherapy, Cancer research 68(21) (2008) 8643–8653. [DOI] [PubMed] [Google Scholar]
- [3].Starobova H, Vetter I, Pathophysiology of chemotherapy-induced peripheral neuropathy, Frontiers in molecular neuroscience 10 (2017) 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. , Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis, PAIN® 155(12) (2014) 2461–2470. [DOI] [PubMed] [Google Scholar]
- [5].Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, et al. , Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy, Journal of Clinical Oncology 35(23) (2017) 2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tofthagen C, Overcash J, Kip K, Falls in persons with chemotherapy-induced peripheral neuropathy, Supportive care in cancer 20(3) (2012) 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kolb NA, Smith AG, Singleton JR, Beck SL, Stoddard GJ, Brown S, et al. , The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling, JAMA neurology 73(7) (2016) 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Espy DD, Yang F, Bhatt T, Pai Y-C, Independent influence of gait speed and step length on stability and fall risk, Gait & posture 32(3) (2010) 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lajoie Y, Gallagher S, Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers, Archives of gerontology and geriatrics 38(1) (2004) 11–26. [DOI] [PubMed] [Google Scholar]
- [10].Tinetti ME, Richman D, Powell L, Falls efficacy as a measure of fear of falling, Journal of gerontology 45(6) (1990) P239–P243. [DOI] [PubMed] [Google Scholar]
- [11].Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C, Development and initial validation of the Falls Efficacy Scale-International (FES-I), Age and ageing 34(6) (2005) 614–619. [DOI] [PubMed] [Google Scholar]
- [12].Niederer D, Schmidt K, Vogt L, Egen J, Klingler J, Hübscher M, et al. , Functional capacity and fear of falling in cancer patients undergoing chemotherapy, Gait & posture 39(3) (2014) 865–869. [DOI] [PubMed] [Google Scholar]
- [13].Yamamoto S, Fujikawa N, Asano K, Toki M, Takao A, Arao H, Assessment of fall-related self-efficacy: characteristics that influence the perception of patients with chemotherapy-induced peripheral neuropathy, Asia-Pacific journal of oncology nursing 7(2) (2020) 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kang GE, Najafi B, Sensor-Based Daily Physical Activity: Towards Prediction of the Level of Concern about Falling in Peripheral Neuropathy, Sensors 20(2) (2020) 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Reelick MF, van lersel MB, Kessels RP, Rikkert MGO, The influence of fear of falling on gait and balance in older people, Age and ageing 38(4) (2009) 435–440. [DOI] [PubMed] [Google Scholar]
- [16].Najafi B, Piot-Ziegler C, Demierre M, Aminian K, Relationship between fear of falling and spatio-temporal parameters of gait in elderly persons, in: Gantchev N (Ed.), From basic motor control to functional recovery III, St. Kliment Ohridski University Press; 2003, pp. 152–158. [Google Scholar]
- [17].Rochat S, Martin E, Piot-Ziegler C, Najafi B, Aminian K, Büla CJ, Falls self-efficacy and gait performance after gait and balance training in older people, Journal of the American Geriatrics Society 56(6) (2008) 1154–1156. [DOI] [PubMed] [Google Scholar]
- [18].Kelly C, Fleischer A, Yalla S, Grewal GS, Albright R, Berns D, et al. , Fear of falling is prevalent in older adults with diabetes mellitus but is unrelated to level of neuropathy, Journal of the American Podiatric Medical Association 103(6) (2013) 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Delbaere K, Close JC, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR, The falls efficacy scale international (FES-I). A comprehensive longitudinal validation study, Age and ageing 39(2) (2010) 210–216. [DOI] [PubMed] [Google Scholar]
- [20].Kang GE, Zahiri M, Lepow B, Saleem N, Najafi B, The effect of daily use of plantar mechanical stimulation through micro-mobile foot compression device installed in shoe insoles on vibration perception, gait, and balance in people with diabetic peripheral neuropathy, Journal of diabetes science and technology 13(5) (2019) 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Najafi B, Crews RT, Wrobel JS, A novel plantar stimulation technology for improving protective sensation and postural control in patients with diabetic peripheral neuropathy: a double-blinded, randomized study, Gerontology 59(5) (2013) 473–480. [DOI] [PubMed] [Google Scholar]
- [22].Kang GE, Zhou H, Varghese V, Najafi B, Characteristics of the gait initiation phase in older adults with diabetic peripheral neuropathy compared to control older adults, Clinical Biomechanics 72 (2020) 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aminian K, Najafi B, Büla C, Leyvraz P-F, Robert P, Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes, Journal of biomechanics 35(5) (2002) 689–699. [DOI] [PubMed] [Google Scholar]
- [24].Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B, Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial, Gerontology 62(5) (2016) 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Najafi B, Horn D, Marclay S, Crews RT, Wu S, Wrobel JS, Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology, Journal of diabetes science and technology 4(4) (2010) 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zahiri M, Chen KM, Zhou H, Nguyen H, Workeneh BT, Yellapragada SV, et al. , Using wearables to screen motor performance deterioration because of cancer and chemotherapy-induced peripheral neuropathy (CIPN) in adults-Toward an early diagnosis of CIPN, Journal of geriatric oncology 10(6) (2019) 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bryant MS, Rintala DH, Hou J-G, Protas EJ, Influence of fear of falling on gait and balance in Parkinson’s disease, Disability and rehabilitation 36(9) (2014) 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rosén E, Sunnerhagen KS, Kreuter M, Fear of falling, balance, and gait velocity in patients with stroke, Physiotherapy theory and practice 21(2) (2005) 113–120. [DOI] [PubMed] [Google Scholar]
- [29].Kalron A, Achiron A, Postural control, falls and fear of falling in people with multiple sclerosis without mobility aids, Journal of the neurological sciences 335(1-2) (2013) 186–190. [DOI] [PubMed] [Google Scholar]
- [30].Zhi WI, Chen P, Kwon A, Chen C, Harte SE, Piulson L, et al. , Chemotherapy-induced peripheral neuropathy (CIPN) in breast cancer survivors: a comparison of patient-reported outcomes and quantitative sensory testing, Breast cancer research and treatment 178(3) (2019) 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tofthagen C, Visovsky C, Berry DL, Strength and balance training for adults with peripheral neuropathy and high risk of fall: current evidence and implications for future research, Oncology nursing forum, NIH Public Access, 2012, p. E416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zimmer P, Trebing S, Timmers-Trebing U, Schenk A, Paust R, Bloch W, et al. , Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial, Supportive Care in Cancer 26(2) (2018) 615–624. [DOI] [PubMed] [Google Scholar]
- [33].McCrary JM, Goldstein D, Sandler CX, Barry BK, Marthick M, Timmins HC, et al. , Exercise-based rehabilitation for cancer survivors with chemotherapy-induced peripheral neuropathy, Supportive Care in Cancer 27(10) (2019) 3849–3857. [DOI] [PubMed] [Google Scholar]
- [34].Monfort SM, Pan X, Loprinzi CL, Lustberg MB, Chaudhari AM, Exploring the roles of central and peripheral nervous system function in gait stability: preliminary insights from cancer survivors, Gait & posture 71 (2019) 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Parry SW, Bamford C, Deary V, Finch TL, Gray J, MacDonald C, et al. , Cognitive-behavioural therapy-based intervention to reduce fear of falling in older people: therapy development and randomised controlled trial-the Strategies for Increasing Independence, Confidence and Energy (STRIDE) study, Health Technology Assessment (Winchester, England) 20(56) (2016) 1–206. [DOI] [PMC free article] [PubMed] [Google Scholar]


