Abstract
Microstructural adaptation of bone in response to mechanical stimuli is diminished with estrogen deprivation. Here we tested in vivo whether ovariectomy (OVX) alters the acute response of osteocytes, the principal mechanosensory cells of bone, to mechanical loading in mice. We also used super resolution microscopy (Structured Illumination microscopy or SIM) in conjunction with immunohistochemistry to assess changes in the number and organization of “osteocyte mechanosomes” - complexes of Panx1 channels, P2X7 receptors and CaV3 voltage-gated Ca2+ channels clustered around αvβ3 integrin foci on osteocyte processes. Third metatarsals bones of mice expressing an osteocyte-targeted genetically encoded Ca2+ indicator (DMP1-GCaMP3) were cyclically loaded in vivo to strains from 250 to 3000 με and osteocyte intracellular Ca2+ signaling responses were assessed in mid-diaphyses using multiphoton microscopy. The number of Ca2+ signaling osteocytes in control mice increase monotonically with applied strain magnitude for the physiological range of strains. The relationship between the number of Ca2+ signaling osteocytes and loading was unchanged at 2 days post-OVX. However, it was altered markedly at 28 days post-OVX. At loads up to 1000 με, there was a dramatic reduction in number of responding (i.e. Ca2+ signaling) osteocytes; however, at higher strains the numbers of Ca2+ signaling osteocytes were similar to control mice. OVX significantly altered the abundance, make-up and organization of osteocyte mechanosome complexes on dendritic processes. Numbers of αvβ3 foci also staining with either Panx 1, P2X7R or CaV3 declined by nearly half after OVX, pointing to a loss of osteocyte mechanosomes on the dendritic processes with estrogen depletion. At the same time, the areas of the remaining foci that stained for αvβ3 and channel proteins increased significantly, a redistribution of mechanosome components suggesting a potential compensatory response. These results demonstrate that the deleterious effects of estrogen depletion on skeletal mechanical adaptation appear at the level of mechanosensation; osteocytes lose the ability to sense small (physiological) mechanical stimuli. This decline may result at least partly from changes in the structure and organization of osteocyte mechanosomes, which contribute to the distinctive sensitivity of osteocytes (particularly their dendritic processes) to mechanical stimulation.
Keywords: osteocytes, Ca2+ signaling, in vivo, estrogen loss, mechanosensing
INTRODUCTION
Bone response to mechanical loading is regulated by many hormones, with estrogen the most extensively studied. Loss of circulating estrogen or depletion of its receptors (both ERα & ERβ) not only increase bone resorption, but also markedly reduce the adaptive response of bone to mechanical loading[1–5]. Higher forces are required in estrogen depleted animals to initiate the same level of bone formation that is normally triggered at lower load levels when estrogen levels are normal. Conversely, exogenous PTH and teriparatide have been shown to potentiate the mechanoresponsiveness of bone, such that low levels of mechanical loading evoke larger than expected bone formation responses[6,7]. These pronounced hormonal effects have been demonstrated largely from studies performed on the ultimate skeletal effector cells, osteoblasts and osteoclasts. The fundamental issue of how such hormonal perturbations affect osteocytes is unresolved, despite their role as mechanosensitive “first responders”.
Calcium (Ca2+) signaling is a ubiquitous first messenger in cells. Its detection with synthetic fluorescein derivatives has been widely used in in vitro osteocyte studies to delineate acute responses to mechanical stimulation[8–13]. Our group recently reported a method for real time observation of osteocyte Ca2+ signaling events in vivo. We created a mouse strain with an osteocyte-targeted genetically encoded calcium indicator (GECI) to study acute Ca2+ signaling in these cells in vivo and used it to acquire first insights regarding how osteocyte Ca2+ signaling responds to mechanical loading in living tissues. Our studies revealed that there is a strong positive, frequency dependent correlation between the number of Ca2+ signaling osteocytes and strain magnitude; however, Ca2+ signaling intensity among the responding cell population is not altered. Thus, osteocytes encode mechanical load magnitude by the number of recruited osteocytes, with each cell acting effectively in an OFF/ON manner.
The mechanisms responsible for triggering rises in intracellular Ca2+ in response to mechanical stimulation of osteocytes have also been the subject of increasing investigation. Based on a theoretical framework developed by Weinbaum and colleagues[14–17], a growing body of experimental evidence has supported the concept that osteocyte dendritic processes are by far the most mechanosensitive part of the cell[12,18,19]. This remarkable mechanical sensitivity is attributable to recently discovered “osteocyte mechanosome” complexes, comprised of Panx1 ATP channels, associated P2X7 receptos (P2X7R) and CaV3.2 voltage-gated Ca2+ channels centered on αvβ3 integrin matrix attachment sites localized along dendritic processes [12,20–22]. These αvβ3 foci are not typical integrins associated with focal adhesion sites, as they lack the cytoplasmic component (i.e., vinculin, paxillin, FAK). Rather, these osteocyte integrin foci focally elevate strains in the nearby cell membrane during loading, which in turn triggers the mechanically activated channels[22]. Recent studies indicate that estrogen withdrawal impairs mechanosensation of MLO-Y4 osteocytes in vitro and this loss is associated with decreased αvβ3 integrin attachments[23]. Whether similar changes occur for osteocytes in vivo when estrogen is depleted is not yet known, nor is it known if this hormone loss might affect the composition or organization or topology of the osteocyte mechanosome complex.
This new understanding of osteocyte mechanosensation provides a useful conceptual framework to test how hormone perturbations affect osteocyte response to mechanical loading in vivo. In the current studies, we examined how osteocyte Ca2+ signaling in vivo responds to estrogen loss. We tested the hypothesis that loss of estrogen alters the previously demonstrated relationship between mechanical loading to the number of Ca2+ signaling osteocytes. Using our established system of controlled mechanical loading and in vivo multiphoton microscopy in mice expressing GCaMP3, in osteocytes, we examined differences in osteocyte Ca2+ signaling changes between intact and estrogen-depleted animals. In addition, we used a super resolution microscopy technique recently developed by our laboratory to assess alterations of the osteocyte mechanosome complexes located on cell processes after estrogen loss[12,22].
MATERIALS AND METHODS
A. In vivo osteocyte mechanical loading studies
Mouse osteocyte GECI model –
As previously reported by our group, OtGP3 mice exhibiting osteocyte-targeted expression of the GECI GCaMP3 were achieved by crossing Ai38 mice B6;129S-Gt(ROSA) 26Sortm38(CAG-GCaMP3)Hze/J; [JAX Labs], which contain GCaMP3 DNA behind a Lox-STOP-Lox codon[24], with DMP1-Cre mice [B6N.FVB-Tg(Dmp1-cre)1Jqfe/BwdJ; [JAX Labs] [25]. Mice were then bred onto a C57BL/6 background. GCaMP3 is a recombinant protein construct containing calmodulin (CaM), the CaM binding myosin light chain kinase fragment (M13), and a green fluorescent protein (GFP). Binding of Ca2+ to CaM causes subsequent binding of CaM to M13 and a conformational change of GFP, which results in increased fluorescence intensity. Initial studies with this model showed expression of GCaMP3 in essentially all cortical osteocytes[26]. Studies used young adult female mice that were 16-wks old at the start of the studies. All procedures were approved by the Institutional Animal Care and Use Committees at both Albert Einstein College of Medicine and City College of New York.
Estrogen depletion studies –
Surgical ovariectomy (OVX) was performed on 16 week old female OtGP3 mice to deplete estrogen from the system and induce the physiological conditions of menopause[27], following procedures detailed elsewhere [28]. Animals were allowed to recover and provided with food and water ad libitum. Osteocyte responses to in vivo mechanical loading were examined in metatarsal bone cortices of 2d and 28d post-OVX and intact controls (n=4 mice/group) to examine effects of depleting circulating estrogen on osteocyte Ca2+ signaling. Estrogen decreases throughout the first few days after OVX and is at a steady-state of depletion by 1–2 weeks post-OVX[29,30]. OVX success was confirmed from reduction (~65%) in uterine weight at 28d post-OVX (OVX: 0.036 ± 0.014g vs. Control: 0.061 ± 0.011g).
μCT studies
μCT studies were performed to assess diaphyseal changes in the MT3 bone after OVX. Bones were scanned using the SkyScan 1172 system (Bruker, Belgium) at a nominal isotropic voxel resolution of 6.7 μm. Images were acquired using a 10-MP detector, 10W power energy setting (100 KV and 100 mA) and a 0.5 mm aluminum filter to minimize beam hardening effects by filtering low-energy photons. An alignment procedure and flat-field detector calibration were performed prior to the scan to minimize ring artifacts and increase signal-to-noise ratio. 3D reconstructions were obtained using a customized back-projection with post-alignment compensation to optimize the center of rotation, smoothing correction kernel size of 1 pixel for asymmetrical boxcar window, ring artifact correction set to 10, beam hardening correction (40%). All settings were kept constant throughout the scan reconstruction and global thresholding was used to define the cortical bone boundaries. Ct Analyzer software (v. 1.13) was used to measure total bone area (T.Ar,) cortical bone (B.Ar), marrow area (Ma.Ar), cortical thickness (Ct.Th) and Polar moment of inertia (MMI) of the mid-diaphyseal region.
In Vivo Mechanical Loading –
Osteocyte Ca2+ signaling in response to mechanical loading in vivo was examined in right third metatarsal (MT3) diaphyses of OtGP3 mice with an in vivo loading model, as recently reported by our laboratory ([26] and Fig. 1). MT3 bones were loaded in 3-point bending at 1Hz to mid-diaphyseal strain levels of 250, 500, 1000, 2000 and 3000με, based on the loading calibration curve reported previously [26]. These levels encompass the range of strains that have reported during physiological activities from in vivo strain gage studies, with strains up to 2000με characteristic of habitual activities, while strains on the order of 3000με are seen in extreme activities [31,32]. All loading procedure were carried out in mice anesthetized with isoflurane. First, the MT3 dorsal surface was accessed by a single scalpel incision through the skin and extensor aponeurosis, with care taken to isolate and avoid the dorsal foot arteries. The lower stainless steel 100 μm diameter cylindrical fulcrum pin of the 3-point bending loading device was inserted beneath the MT3 mid-diaphysis, thus functionally isolating the bone. The pin was placed in its anchor bracket and the entire foot positioned in the 37°C PBS bath of the loading apparatus. The upper stainless steel loading contact points were then positioned on the dorsal bone surface and a nominal tare strain (~100με) was applied to prevent the bone from moving during loading. The entire apparatus was set onto the stage of a Multiphoton microscope (MPM, Ultima II, Bruker, Madison, WI) equipped with a tunable Ti-sapphire laser light source for in vivo osteocyte imaging studies Bones were loaded cyclically under displacement control to achieve the desired strain level using a haversine waveform at 1Hz using a custom loading device; coefficients of variation for each target strain level were previously determined to be ~15%. The duration of each strain-loading bout was 60 sec and was performed with simultaneous osteocyte imaging under MPM. The loading bout was followed by 15 min of rest. The loading and rest procedure was then repeated for the next test strain level until the complete test strain range (250–3000 με) was completed. After 15 min rest, the entire loading and imaging procedure was repeated in reverse order (3000 – 250 με) in an adjacent mid-diaphyseal region of interest (ROI).
Figure 1:
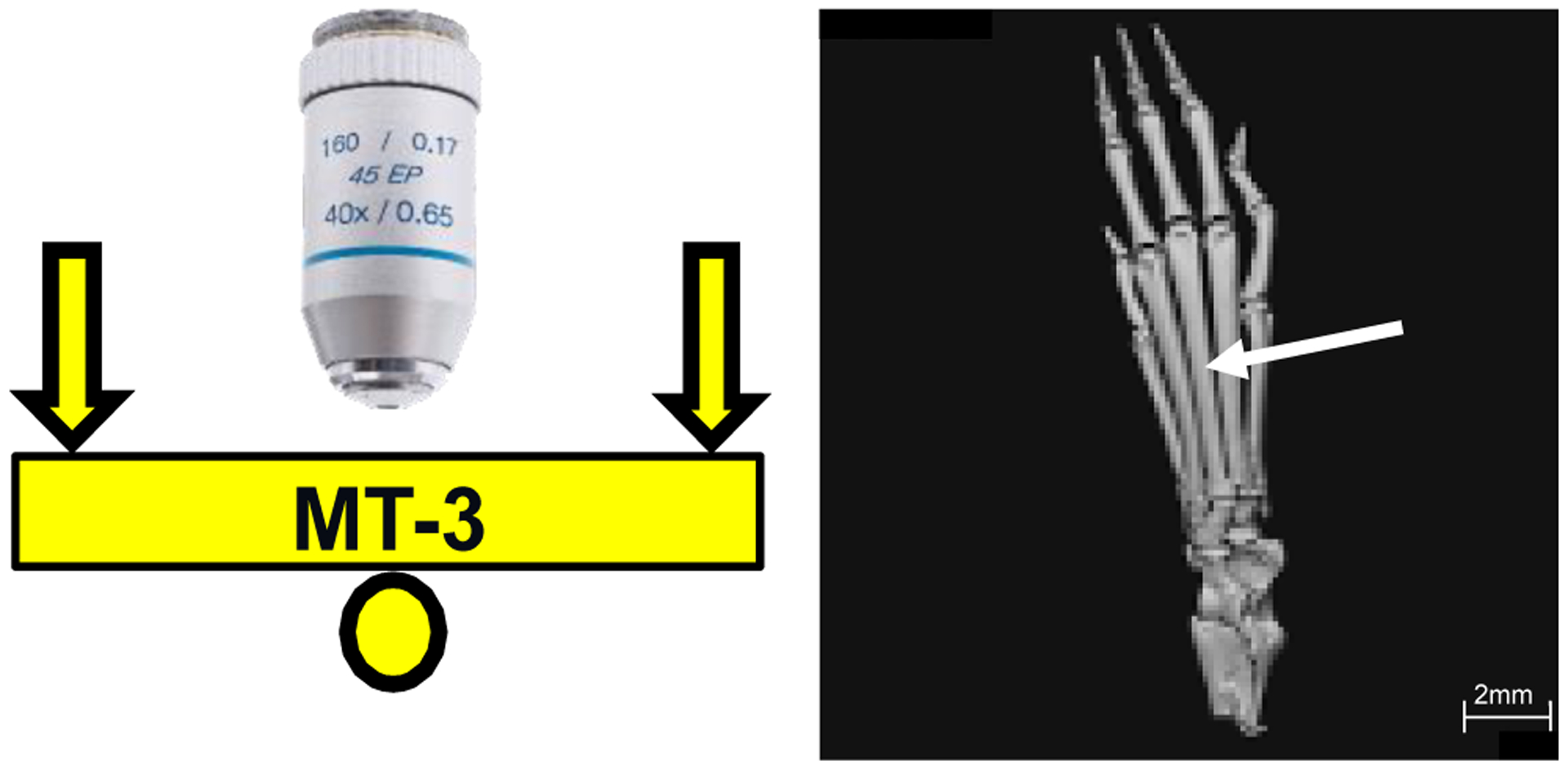
Schematic (left side) of microscope objective and loading configuration for the third metatarsal bone (MT3); b) μCT image (right side) of mouse hind paw showing MT3 (arrow)
Image Acquisition and Analysis –
Multiphoton imaging (MPM) was performed at the dorsal mid-shaft region of the MT3 in vivo, sampling osteocytes in a plane located ~ 20μm below the periosteal surface. Osteocyte Ca2+ imaging was performed using a 40x magnification water immersion objective (Olympus LUMPLFLN 40XW, NA = 0.8; working distance = 3.3 mm) focused at the mid-diaphysis. Excitation was at 920nm wavelength and a 490–560nm bandpass filter was used for detection. Time series images were acquired at a rate of 6 frames per second. The sampling ROIs at the magnification used were 250μm2 located immediately on either side of the mid-diaphysis. Ca2+ intensity measurements were performed by post-processing time series images using ImageJ (NIH). Individual osteocytes were delineated and mean pixel intensity values were collected in each frame before and during loading. The intensities for each cell of interest were normalized to the mean baseline intensity for that cell over a 30s period prior to the start of cyclic loading. Responding osteocytes were defined as those cells showing a >25% increase in normalized fluorescence intensity during loading. Figure 2 shows an example of the fluorescence intensity changes typically seen in cells that respond to mechanical loading. In addition to counting the number of cells, we also analyzed the fold increase in mean intensity during loading compared to non-loaded baseline.
Figure 2:
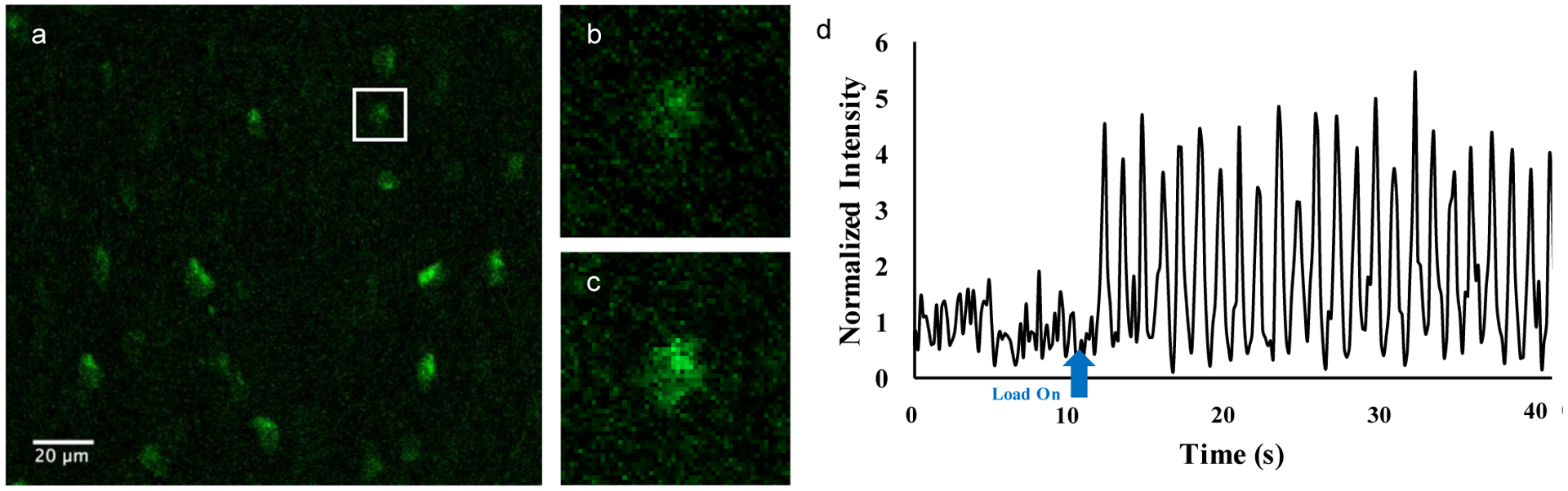
An example image of OtGP3 osteocytes fluorescing at baseline (a). One cell was selected and enlarged to show the difference between (b) baseline fluorescence and (c) increases seen during mechanical loading. (d) Representative trace of a single osteocyte Ca2+ signaling fluorescence intensity both before and during loading.
B. Super Resolution Microscopy of Osteocyte Mechanosomes
Super resolution microscopy was used to assess osteocyte mechanosome components at osteocyte processes from control and OVX mice, following procedures detailed in our previous studies Structured Illumination Microscopy (SIM) and other similar Super Resolution Microscopy imaging approaches (e.g., Airy disc imaging, STED) are applicable to conventional tissue sections and use conventional fluorophores. They employ imaging processing approaches to break through the Abbe resolution limit. SIM permits X-Y resolution on the order of ~ 90nm depending on the fluorophore being imaged. Our group previously used this approach to visualize cell process components within osteocyte canaliculi, where a combination SIM imaging and double staining immunohistochemistry (IHC) was used to test for and assess the degree of co-localization for osteocyte mechanosome proteins (β3 integrin, Panx1, P2X7R, CaV3) on osteocyte processes. In the current studies, we followed procedures detailed in Cabahug-Zuckerman et al[22]. Cross-sections (5 μm) were cut from decalcified femoral mid-diaphyses of the OVX and control mice embedded in an ethyl methacrylate (EMA) resin, which provides both the excellent retention of microstructure required for high resolution SIM studies and effective IHC staining properties[33]. The mouse MT3 diaphysis is extremely small (<1 mm in diameter) and comprised mostly cortical bone; we found EMA-embedded cross-sections of this small bone did not adhere to slides sufficiently well during IHC to allow adequate SIM imaging of mechanosomes on osteocyte processes. Larger diameter mouse long bones such as femurs do not exhibit this problem, presumably because of larger surface area and marrow cavity, so these were used assess how osteocyte mechanosomes change in response to estrogen loss. The use of different long bones for super resolution microscopy and loading studies introduces the potential for some differences in osteocyte responses. However, osteocytes appear to respond in a similar physiological manner to a wide range of systemic challenges - irrespective of location (e.g., estrogen loss, lactation, hibernation, immobilization hyperparathyroidism), though quantitative differences may exist[34].
Immunohistochemistry for β3 integrin, Panx1, P2X7R and CaV3.2 was performed as detailed by Cabahug-Zuckerman et al[22]. Briefly, deplasticized sections were immersed sequentially in 0.3% TritonX100, 10% EDTA, proteinase K (#S3020, Dako Agilent Technologies) and then protein block (#X0909, Dako) for 10 minutes each at room temperature. Sections were then incubated in primary antibodies overnight at 4°C in a humidified chamber. Sections were probed (“double-stained”) with two primary antibodies each in Dako Antibody Diluent (#S3022,). Each section was incubated with anti-β3 integrin (#ab20146, Abcam) and either anti-P2X7R (#ab77413, Abcam), anti-Panx1 (#ab139715, Abcam) or anti-CaV3.2 (#PA5-77313, Invitrogen), all at 1:200. β3 integrin foci are present on osteocyte processes and are absent from osteocyte cell bodies in situ[21,22]. Thus, β3 integrin foci in these very thin (110 nm) optical fluorescence sections provided an internal reference for establishing the localization when normal morphological features are difficult to discern (See Cabahug-Zuckerman et al 2017 for detailed discussion). Primary antibodies were detected using secondary antibodies labeled with AlexaFluor488 (or channel proteins or AlexaFluor568 for β3 integrin (1:700 dilution, incubated at room temperature for 30 min, all AlexoFluor dyes from ThermoFisher). Non-immune serum was used as the negative control and brain tissue neurons, brain microglial cells and sealing zones of osteoclasts in bone served as positive controls for both Panx1 and CaV3, P2X7R and β3 integrin, respectively.
SIM was used to acquire images at nominal X-Y resolution of 90 nm. Imaging was performed using Zeiss ELYRA S.1 Structured Illumination Microscope and 1–2 μm z-axis stacks of SIM images acquired in 110 nm optical sections using a 63X magnification oil immersion objective (NA 1.4). Images were acquired using two alternating channels to avoid potential cross-talk between fluorescent outputs, alternating between a red channel (excitation at 561 nm image capture using a 570–620 nm bandpass (BP) filter) and a green channel (excitation at 488 nm, image capture using a 495–550 BP filter). Each image set acquired by SIM was de-convolved with the Zeiss Zen Black software, using raw values of 16-bit image intensities. SIM images were used for high resolution mapping of β3 integrins and membrane proteins. 7–8 osteocytes were typically visible in each optical section from a given animal. Data were collected for all osteocyte processes within a concentric sampling region (1 μm radius) distal to the margin of a given lacuna, established using the conventional fluorescence microscopy mode of the microscope. Number and mean area of fluorescently stained foci for each membrane protein on osteocyte processes in the ROI were measured using ImageJ (NIH).
Statistical Analyses:
To test our hypothesis that estrogen loss alters the number of Ca2+ signaling osteocytes in response to mechanical load, primary comparisons between treatment groups using the variables applied strain and number of responding cells were examined using regression analysis. Differences between applied strain and signaling intensity among responding cells were assessed using two-way ANOVA and Tukey‟s post-hoc test)., , IHC-protein foci numbers and areas from SIM studies were compared between OVX and control using multiple t-tests and the two-stage Benjamini, Krieger and Yekutiele procedure to control the false discovery rate. Analyses were performed using Prism (GraphPad, San Diego, CA). Data are shown as mean ±SD
RESULTS
Ovariectomy caused a significant ~15% reduction in cortical bone area of the MT3 mid-diaphysis at 28 days, due to marrow cavity expansion (~30%) and cortical thinning (~16%). OVX did not alter total bone area, indicating that bone loss at this cortical site occurred endocortically. Moment of inertia was also unchanged.
In vivo osteocyte mechanical loading studies
In control mice, the number of Ca2+ signaling osteocytes increased monotonically with increasing strain levels and the same relationship was observed at 2d post-OVX (Fig 3). There was no significant difference in either the slope or intercept between control and 2d OVX groups. In contrast, at 28d post OVX the relationship between strain magnitude and the number of Ca2+ signaling osteocytes was profoundly different. There was a lag in response, with few osteocytes exhibiting Ca2+ signaling responses to loading at 250, 500 and 1000με; however, numbers of Ca2+ signaling osteocytes at 2000 με and 3000 με were similar to control mice (Fig 3). Linear regression analyses of both components of the 28d post-OVX response curves showed a dramatically reduced slope vs control mice for strains up to 1000με, but a regression slope at high strains was similar to control.
Figure 3:
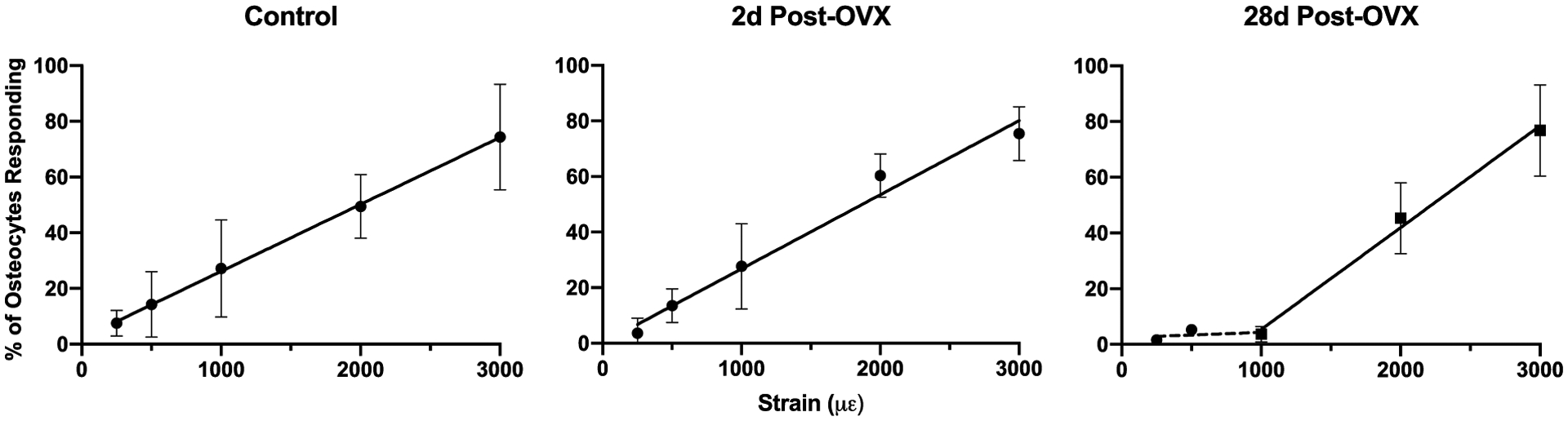
Percentages of Ca2+ signaling osteocytes increased with increasing strain levels for Control, 2d post-OVX and 28d OVX mice. Control and 2d post-OVX mice show similar strong increases in number of responding osteocytes with increasing strain, with no significant difference between regression lines (p=0.3881, ANCOVA). The loading-osteocyte response relationship changed markedly at 28d post-OVX, with few osteocytes exhibiting Ca2+ signaling responses to loading at 250, 500 and 1000με, while numbers of Ca2+ signaling osteocytes at 2000 με and 3000 με were similar to control. Data are shown fit to a two-phase model, with the first phase representing 250–1000 με (dotted line, slope not significantly different from zero) and the second phase from 1000–3000 με (solid line, slope similar to control, p=0.1), indicating a return to normal recruitment patterns.
Regression equations: Control: y = 0.02403x + 2.144; 2d Post-OVX: y = 0.02666x + 0.1290 28d Post-OVX: dashed line: y = 0.001917x + 2.427, solid line: y = 0.03655x − 31.17.
Ca2+ signaling intensity in individual osteocyte did not differ between control and OVX groups at any strain level tested, even among the few responding osteocyte in the low strain 28d OVX bones (Fig 4); however, at 3000με both control and OVX groups showed significant elevations in Ca2+ signaling intensity compared to lower strain levels.
Figure 4:
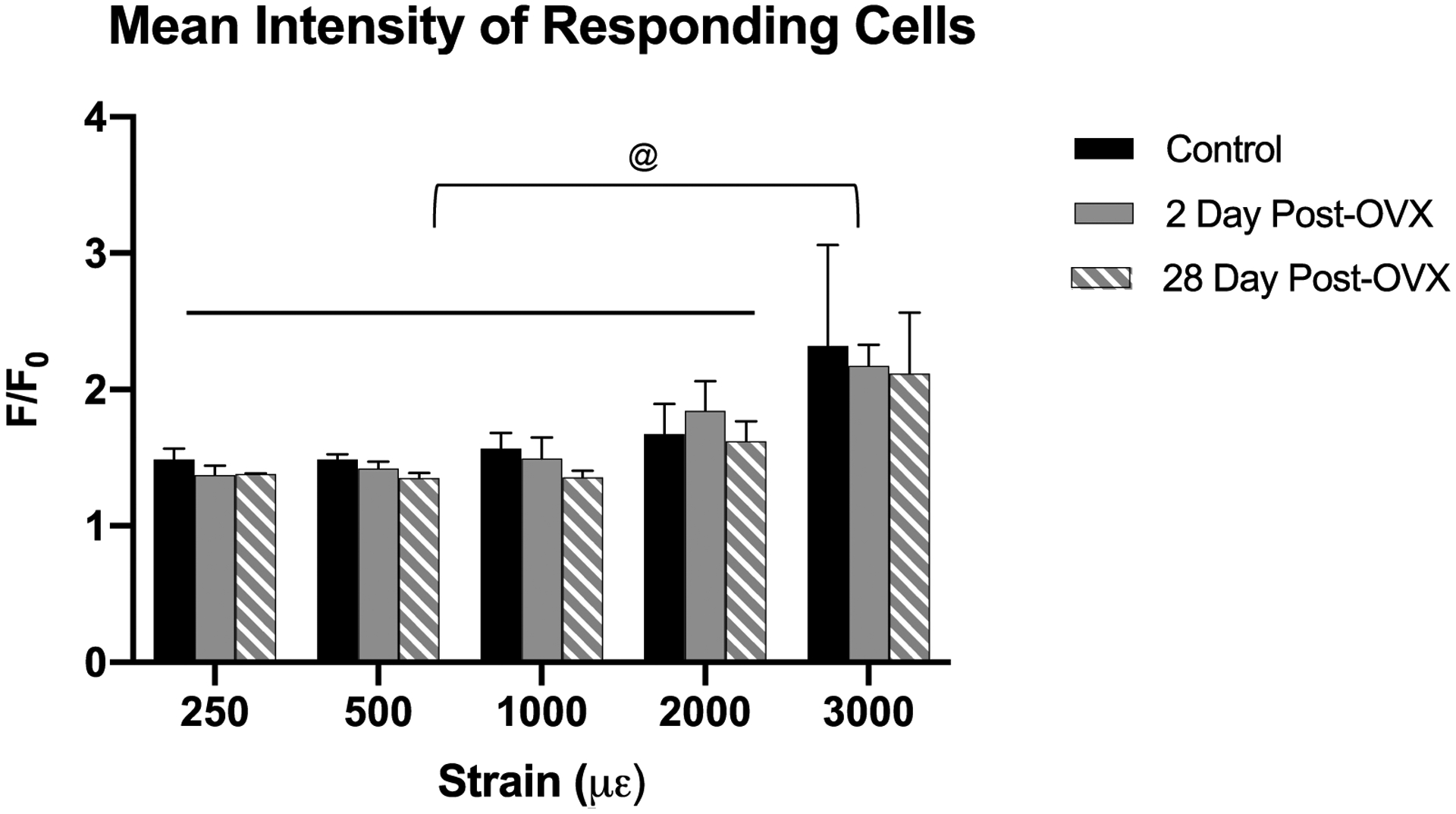
This figure shows the Ca2+ signaling intensity increase over baseline among the responding population of osteocytes for each strain value. Osteocyte Ca2+ signaling intensity was elevated in bones loaded to 3000 με. However, there were not significant differences among control, 2d and 28d OVX groups for any strain level examined, indicating that loss of estrogen does not impact the magnitude of Ca2+ signaling in osteocytes (@, p<0.05 for 3000 με vs other strain groups.
Super Resolution Microscopy: Osteocyte mechanosome components
OVX significantly altered the abundance, composition and organization of osteocyte mechanosome complexes on dendritic processes (Fig 5). The number of β3 integrin staining foci, as well as foci for Panx1, P2X7R and CaV3 were reduced significantly, by some ~40–50% in OVX vs control osteocytes. Conversely, the sizes of the remaining IHC-stained foci were increased significantly with OVX.
Figure 5:
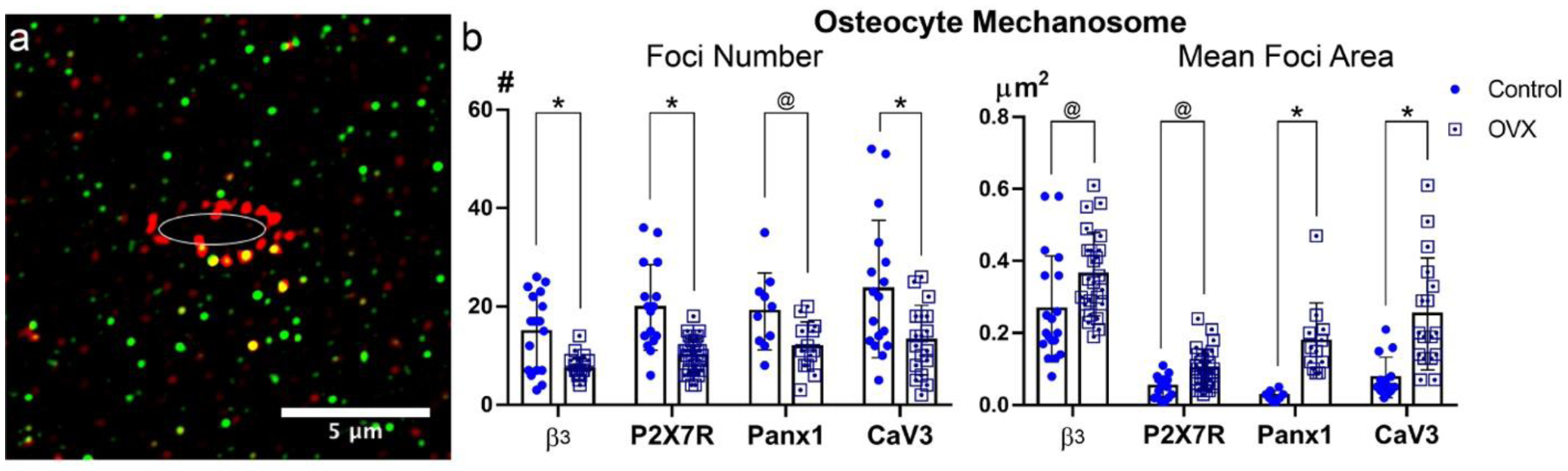
Osteocyte mechanosome components in control and OVX cortical bone. (a) Representative SIM image (110 nm optical section) showing IHC-stained foci for β3 integrin (red) and P2X7R (green) on osteocyte processes within canaliculi. Note that in these very thin optical images canaliculi go in and out the plane of section, resulting in a discontinuous appearance for processes analogous to what occurs in electron microscopy images. The ellipse in the image represents the position of the lacunar edge; protein staining foci were measured within the 1 μm wide sampling region outside of that edge (see text for detail). (b) Number and mean area of stained foci for osteocyte mechanosome components from Control and OVX mice. (@ p<0.05, * p<0.01).
DISCUSSION
The results of this study demonstrate that estrogen loss profoundly alters the acute response of osteocytes to controlled mechanical loading in vivo. Specifically, established (28d) estrogen loss dramatically blunted the normal recruitment of Ca2+ signaling osteocytes in response to low-level mechanical strains (up to 1000με) characteristic of low to moderate physiological activities[35]. In contrast, the numbers of Ca2+ signaling osteocytes in bone loaded to higher strains characteristic of more vigorous physiological activities were similar to that of control mouse bones. Consistent with our previous observations[26], the cells that exhibited a response to loading across the range of habitual physiological strains range (i.e., up to 2000 με) – including those few responding osteocytes in the low strain loaded-OVX groups – showed the same intensity of Ca2+ signal. Surprisingly, this signaling behavior appeared to be unaffected by that estrogen loss. These data suggest that the defect brought about by the hormonal challenge of OVX was not in the fundamental mechanisms of acute response (i.e., elevation of cytoplasmic Ca2+ signaling) but rather the mechanisms by which cells perceive load.
Systemic hormones strongly affect bone response to mechanical loading, with estrogen, androgen and PTH among the best-studied factors. The majority of studies of estrogen effects on bone response to loading to date have assessed the effector cells that carry out bone adaptation (i.e., osteoblast and osteoclast) and mediate their respective effects on bone formation and resorption. Estrogen plays significant roles in bone adaptation by increasing osteoblast differentiation and decreasing osteoclast activity. Numerous investigators have established that estrogen sensitizes bone to mechanical loading, permitting responses to everyday levels of mechanical stimulation to stimulate bone formation or resorption[4,5,36–38]. Estrogen acts via two types of nuclear receptors (ERα and ERβ) to regulate gene expression[39–41] and has also been reported to exert nongenomic effects[42–46]. Both ERα and ERβ receptors have been implicated in mediating the bone formation responses to mechanical signals in bone [1,3–5,47–50]. Indeed, selective estrogen modulators (SERMs) prevent OVX-induced bone loss and synergize with mechanical loading to increase bone formation bone [3–5,48,49].
How osteocytes, the mechanosensory cells that also orchestrate both osteoblast and osteoclast activation, are influenced by systemic modulators of adaptation is not well understood. Estrogen has been shown in a variety of osteoblast and osteocyte models to modulate the expression and/or activity of acute response elements in osteocytes such as ion channels[51–53] and adhesion receptors (e.g. αvβ3 integrins) [54,55]. Estrogen regulation of connexin43 gap junctions has been shown in MLO-Y4 osteocyte in vitro and may affect cell mechanosensitivity [56]. However, little is known about how hormonal perturbations might affect osteocytes in vivo, despite their central role in mechanosensing and consequent signaling to and regulation of osteoblasts and osteoclasts[28,57–60].
Recent studies from our laboratory demonstrate that osteocyte processes are a uniquely mechanosensitive part of the cell[12,18]. Mechanical stimulation of the osteocyte dendritic process can trigger Ca2+ signaling at a small fraction (<10%) of the loads needs to elicit responses from osteocyte cell bodies. This remarkable mechanical sensitivity is attributable to the recently discovered “osteocyte mechanosome” complexes located only on the osteocyte cell processes [12,21,22]. Thi et al in our group demonstrated that the first event at mechanically stimulated osteocyte mechanosomes is ATP release (from mechanically activated Panx1) and triggering of an ATP-dependent Ca2+ pore (P2X7) leading to Ca2+ influx[12]. ATP is the obligate first signal; if extracellular ATP is depleted, Ca2+ signal from the osteocyte process to the cell body is prevented. This initial Ca2+ current appears to trigger the associated voltage activated calcium (CaV type) channels that then propagate and likely amplify the Ca2+ wave as it moves from osteocyte process to cell body to initiate overall osteocyte Ca2+ signaling.
Several recent in vitro studies demonstrated changes in osteocyte mechanosomes with estrogen loss. Voisin and McNamara[54] demonstrated that estrogen withdrawal leads to decreased αvβ3 integrin attachments in osteoblast lineage cells and suggested that such alterations in osteocytes would diminish their mechanosensation. Estrogen withdrawal impairs mechanosensation of MLO-Y4 osteocytes in vitro [23] and estrogen loss also attenuates Ca2+ signaling response to fluid flow mechanical loading in MLO-Y4 osteocytes[23,61].
Our current super-resolution microscopy studies revealed marked changes to the composition or organization of osteocyte mechanosomes occur in vivo after OVX. The number of β3 integrin staining foci, as well the numbers of foci for Panx1, the P2X7R and CaV3 were reduced with estrogen depletion. However, the sizes of remaining foci for osteocyte mechanosome components were substantially increased in area after OVX, consistent with more protein at these sites as shown in Figure 6. These observations point to an overall loss of osteocyte mechanosomes, but also indicate compensatory changes of the remaining foci. The basis for increases in the sizes of the remaining stained foci for mechanosome components are not yet clear. While they could be due in part to re-aggregation of proteins released upon disruption of other mechanosomes (the ones that are lost), they could also be driven by increased expression of the proteins. Future gene expression studies are needed to confirm this idea. Studies of the neuroprotective/anti-ischemic effects of estrogen show that the hormone increased Panx1 gene expression, while other studies of mammary gland development during lactation also implicate estrogen in the modulation of Panx1 expression[62,63]. That estrogen can regulate T-type calcium (e.g. Cav3) channel expression is well-established in the literature. However, the up- or downregulation of these channels by estrogen or its loss is dependent on the type of cells and tissue[64,65], and this has not been established for osteocytes or osteoblast lineage cells. The mechanisms by which such specific changes in channel expression affect osteocyte Ca2+signaling are not known. However, it is clear from these studies that estrogen loss alters local expression of these key Ca2+ signaling components on osteocyte processes and points to a dysregulation of the osteocyte mechanosome complex. We speculate that these alterations of osteocyte mechanosome components underlie the changes in osteocyte mechanosensitivity after estrogen loss observed in these in vivo studies.
Figure 6:
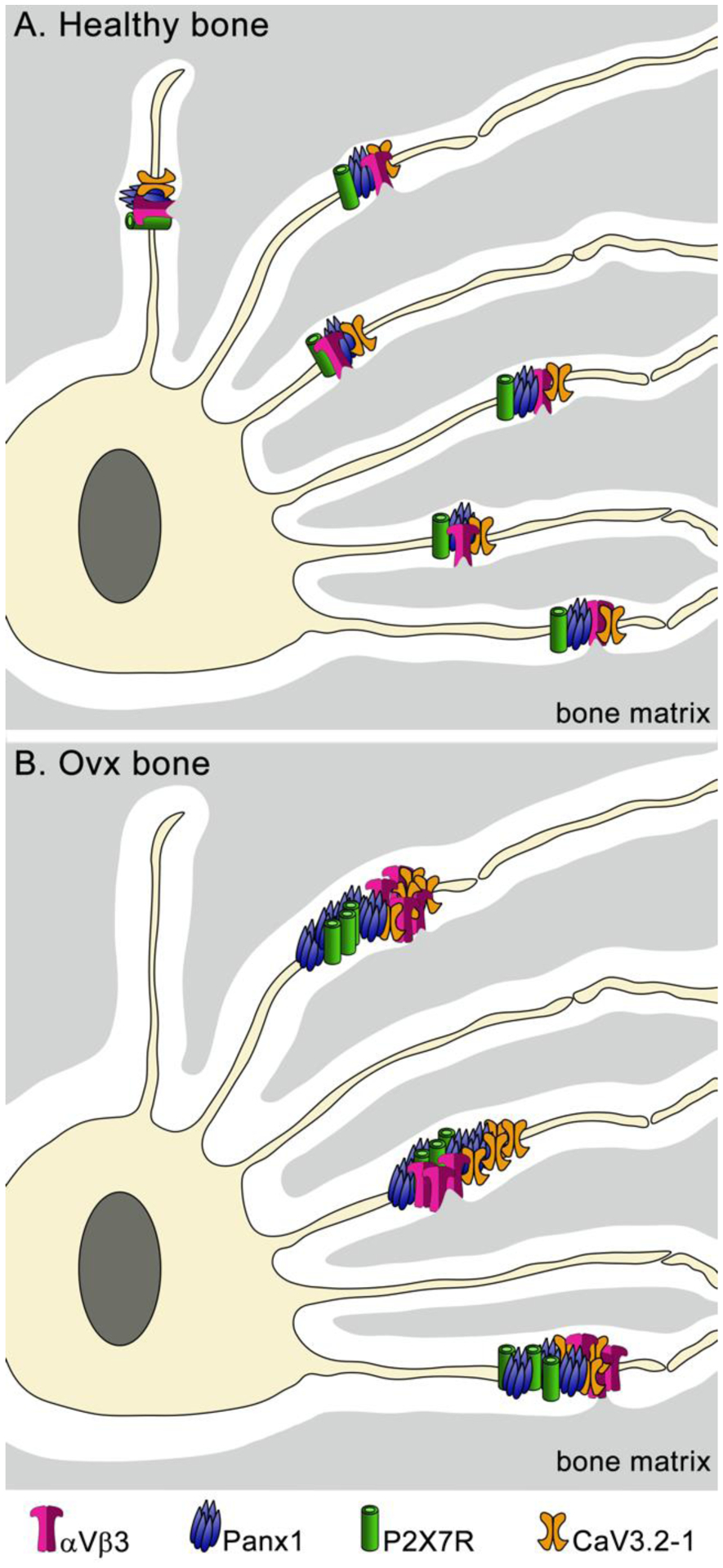
Schematic representation of osteocyte mechanosome components under healthy (a) and OVX (b) conditions. The LCS in OVX is shown enlarged as reported from in vivo studies [66]
In addition to potential cellular-level changes, other osteocyte changes resulting from long-term OVX have been shown and may also be implicated in the reduced osteocyte responses observed in these in vivo studies. Estrogen loss has been shown to cause expansion of the lacunar and canalicular space (LCS) [66–68]. Such increases in LCS dimensions would be expected to alter loading-induced fluid flow rates within the LCS for a level of mechanical loading[17]. Furthermore, changing the geometry of the space between the process cell membrane and the bony LCS wall could alter the fundamental nature of attachments and associated transduction channels. It seems plausible that this increase in canalicular size post-OVX drives a compensatory increase in mechanosome size. This idea requires further investigations in future studies. Recent studies by Zhang et al[69] reported some loss of osteocyte process at several weeks post OVX in a rats, which might trigger compensatory changes in the mechanosomes of the remaining processes. However, Sharma et al reported no change in osteocyte canaliculi after OVX[66]. In the current IHC- SIM imaging studies, we did not count number of osteocyte dendrites as osteocyte process cytoskeleton using our approach. Thus, the question of whether or not osteocytes lose processes after long-term OVX, and the potential for such a loss to affect osteocyte mechanosomes await further studies.
How the changes observed in osteocyte mechanosome make-up and size relate to specific changes in osteocyte Ca2+ signaling observed in vivo, in particular the loss of response to low level mechanical loads, is not yet known[70,71]. However, attenuation of mechanosensory responses to low level mechanical input occurs in a range of physiological systems (e.g., hearing, cutaneous mechanoreceptors muscle reflexes) with disease and aging. Interestingly, estrogen has been implicated in hearing and sound mechanotransduction by maintaining mechanosensitive elements of the inner ear[72]. The current studies revealed that estrogen loss results in alterations of the osteocyte mechanosomes in vivo. We found that OVX leads to fewer, larger mechanosome complexes on osteocyte dendrites, with αvβ3 integrin foci that form the core of osteocyte mechanosomes ~ 75% greater in area after long-term estrogen loss. The αvβ3 foci on osteocyte process are clusters of integrins which during bone loading cause focally elevated strains in the nearby cell membrane (i.e., “strain amplification”) [20,21]. These locally elevated membrane strains trigger the mechanically activated channels located close-by[12,22]. The normal stiffness of αvβ3 attachment foci on osteocyte dendrites is a key to its normal mechanical function (i.e. localized strain amplification), which allows activation of associated channels to initiate this facet of osteocyte mechanotransduction. It seems reasonable to posit that the larger mechanosome complex seen with estrogen loss in vivo alter local membrane mechanical properties, and these changes in local membrane mechanics underlie the blunted osteocyte response to low loads that we observed. Recruitment of focal adhesion proteins has been shown to result in local elastic stiffness increase[73]. Thus, the much larger osteocyte mechanosome complexes we observed after OVX (nearly 2-fold increase in integrin area) will have increased stiffness and would also reduce the focal strain amplification effects. We speculate that the fluid flow stresses in canaliculi generated at low loads are insufficient to deform these more rigid complexes sufficiently to trigger Ca2+ signaling[74]. In contrast, higher load activities generate higher fluid stresses in canaliculi and these higher stress can overcome the increased mechanical resistance of these larger mechanosomes.
The ‘Mechanostat Theory’ is the seminal concept put forth by Frost over three decades ago to explain bone adaptation to mechanical loading[75]. At its core, it models bone adaptation as a feedback system with: 1) osteoblast activity (i.e., bone formation) and osteoclast activity (i.e., bone resorption) as the response arms or actuators and 2) mechanical sensors, now known to be osteocytes, that have a set-point or threshold level of trigger adaptation called the minimum effective strain (MES), such that loads (strains) above or below this „set-point‟ trigger bone formation or resorption leading to an adaptive increase or decrease in bone geometry and strength. Experimental clinical evidence supporting the Mechanostat is extensive and has been reviewed elsewhere [76–78]. Among the most fundamental concepts of the Mechanostat is that analogous to other engineering controller systems like thermostats there is a set-point for the goal of mechanical loading that the system senses and drives the responses of the system actuators. And as with thermostats and other controllers, set-points can be changed. Thus, Frost speculated that key biological signaling molecules, such as hormones, act to change the set-point of the mechanical sensors, making the sensors more or less sensitive to external loads applied to the bone. Intriguingly, Frost posited that loss of estrogen at menopause would reduce sensor sensitivity and thus increase the set-point such that normal loading would not be perceived as sufficient to maintain bone mass. The current studies support that hypothesis. We found that most osteocytes in estrogen-deficient bone do not initiate normal Ca2+ signaling in response to low strain mechanical loading, but they do so normally in response to higher loads, which is consistent with the idea of an offset in osteocyte strain set-point.
Exercise as a means of increasing loading on bone is widely appreciated to stimulate bone formation in the growing skeleton. In the adult skeleton, exercise is primarily anti-resorptive, though some stimulation of bone formation with exercise has been suggested even in adults[79]. A complete review of the wide range of studies for exercise effects in the adult bone, and post-menopausal and aging bone in particular is beyond the scope of this paper[79–86]. What is clear from studies of exercise effects on osteoporotic bone is that high load and high loading rate exercises slow bone loss and may stimulate some bone formation. In contrast, low or non-impact aerobic activities such as cycling, swimming or slow-walking have been demonstrated to have little or no effect on preventing bone loss in postmenopausal women. Thus, it appears that low level or customary loads (strains) on bones are not sufficient to exceed the required threshold for skeletal adaptation to mechanical loading after menopause. The current studies revealed that osteocytes fail to respond effectively to mechanical loading once estrogen is lost. Specifically, most osteocytes in estrogen -deficient bone fail to initiate normal primary Ca2+ signaling in response to low strain loading (i.e., strains characteristic of most daily activities). However, osteocytes in estrogen deficient bone respond normally to higher loads, where strains are more typical of rapid walking, running, jumping and other vigorous activities. These current studies provide the first direct evidence that the insufficiency of low level or habitual daily strains on bones to stimulate skeletal adaptation correlates with fundamental signaling changes in the mechanosensors of bone, and may help explain why specific mechanical loading-exercise types are most effective in stimulating adaptation of osteoporotic bone.
This study has certain limitations. For practical reasons detailed above, the analysis of osteocyte mechanosome components by super-resolution microscopy was carried out on different long bones than the studies of osteocyte calcium signaling, raising a concern that osteocyte responses at these two locations may be dissimilar. We feel it unlikely that such differences would be large, given that numerous studies have demonstrated the similarity of osteocyte responses in different bones, or at different sites within a bone, to a wide range of systemic challenges [34]. Since osteocyte mechanosomes are implicated in the initiation of mechanically-induced calcium signaling [12,18,20–22], it seems reasonable to posit that the changes that we observed in osteocyte mechanosomes and in calcium signaling due to altered estrogen status were similarly linked. However, further studies will be needed to confirm a causal relationship, and to determine the underlying regulatory mechanisms.
In conclusion, these studies revealed that estrogen depletion in mice leads to an apparent desensitization of cortical bone osteocytes to low-level mechanical stimuli (i.e., moderate strains) that characterize activities of daily loading. The ability of osteocytes to respond to higher, strain levels is not affected. This loss of osteocyte sensing to moderate strains may help explain why post-menopausal bone loss is not attenuated by activities of daily living or by low load exercises. We speculate that drugs that can selectively improve the mechanical sensitivity of osteocytes would be beneficial in restoring bone health.
Table 1:
μCT based measurements of MT3 mid-diaphyses at 28 days post-OVX
| p value | |||
|---|---|---|---|
| T.Ar (mm2) | 0.261 ± 0.012 | 0.256 ± 0.013 | P >0.6 |
| B.Ar (mm2) | 0.228 ± 0.009 | 0.195 ± 0.015 | p< 0.01 |
| Ma.Ar (mm2) | 0.033 ± 0.005 | 0.043 ± 0.008 | p< 0.001 |
| Ct.Th (mm) | 0.170 ± 0.003 | 0.143 ± 0.003 | p< 0.001 |
| MMI (mm4) | 0.0110 ± 0.001 | 0.0106 ± 0.002 | P >0.4 |
Highlights.
Estrogen loss blunts osteocyte Ca2+ signaling response to low mechanical loads in vivo.
Ca2+ signaling response to loading initiates at osteocyte mechanosomes.
Estrogen loss altered structure and composition of osteocyte mechanosomes in vivo.
Acknowledgement:
Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institute of Aging and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Awards Number AR041210, AR070547 (MBS and DCS), AR073475 (MMT), AG056397 (MBS), DK091466 (MMT) and NS092466 (DCS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Damien Laudier provided guidance with histology and IHC studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Li CY, Jee WS, Chen JL, Mo A, Setterberg RB, Su M, et al. , Estrogen and “Exercise” Have a Synergistic Effect in Preventing Bone Loss in the Lumbar Vertebra and Femoral Neck of the Ovariectomized Rat, Calcif Tissue Int. 72 (2003) 42–49. doi: 10.1007/s00223-001-1086-y. [DOI] [PubMed] [Google Scholar]
- [2].Saxon LK, Turner CH, Estrogen receptor β: the antimechanostat? Bone. 36 (2005) 185–192. doi: 10.1016/j.bone.2004.08.003. [DOI] [PubMed] [Google Scholar]
- [3].Saxon LK, Galea G, Meakin L, Price J, Lanyon LE, Estrogen Receptors α and β Have Different Gender-Dependent Effects on the Adaptive Responses to Load Bearing in Cancellous and Cortical Bone, Endocrinology. 153 (2012) 2254–2266. doi: 10.1210/en.2011-1977. [DOI] [PubMed] [Google Scholar]
- [4].Galea GL, Price JS, Lanyon LE, Estrogen receptors‟ roles in the control of mechanically adaptive bone (re)modeling, BoneKEy Reports. 2 (2013) 1–7. doi: 10.1038/bonekey.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Windahl SH, Saxon LK, Börjesson AE, Lagerquist MK, Frenkel B, Henning P, et al. , Estrogen receptor-α is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2, J Bone Miner Res. 28 (2013) 291–301. doi: 10.1002/jbmr.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chow JW, Fox S, Jagger CJ, Chambers TJ, Role for parathyroid hormone in mechanical responsiveness of rat bone, Am. J. Physiol 274 (1998) E146–54. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9458760&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [7].Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, et al. , Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice, Bone. 43 (2008) 238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- [8].Mullender M, El Haj AJ, Yang Y, van Duin MA, Burger EH, Klein-Nulend J, Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue, Med Biol Eng Comput. 42 (2004) 14–21. [DOI] [PubMed] [Google Scholar]
- [9].Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ, Oscillating fluid flow activation of gap junction hemichannels induces atp release from MLO-Y4 osteocytes, J. Cell. Physiol 212 (2007) 207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoey DA, Kelly DJ, Jacobs CR, A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells, Biochemical and Biophysical Research Communications. 412 (2011) 182–187. doi: 10.1016/j.bbrc.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu XL, Huo B, Park M, Guo XE, Calcium response in osteocytic networks under steady and oscillatory fluid flow, Bone. 51 (2012) 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thi MM, Suadicani SO, Schaffler MB, Weinbaum S, Spray DC, Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αVβ3 integrin, Proc. Natl. Acad. Sci. U.S.A 110 (2013) 21012–21017. doi: 10.1073/pnas.1321210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Morrell AE, Brown GN, Robinson ST, Sattler RL, Baik AD, Zhen G, et al. , Mechanically induced Ca2+ oscillations in osteocytes release extracellular vesicles and enhance bone formation, Bone Res. 6 (2018) 1–11. doi: 10.1038/s41413-018-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weinbaum S, Cowin SC, Zeng Y, A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses, Journal of Biomechanics. 27 (1994) 339–360. [DOI] [PubMed] [Google Scholar]
- [15].You L, Cowin SC, Schaffler MB, Weinbaum S, A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix, Journal of Biomechanics. 34 (2001) 1375–1386. [DOI] [PubMed] [Google Scholar]
- [16].Weinbaum S, Tarbell JM, Damiano ER, The Structure and Function of the Endothelial Glycocalyx Layer, Annu. Rev. Biomed. Eng 9 (2007) 121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- [17].Fritton SP, Weinbaum S, Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction, Annual Review of Fluid Mechanics. 41 (2009) 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu D, Schaffler MB, Weinbaum S, Spray DC, Matrix-dependent adhesion mediates network responses to physiological stimulation of the osteocyte cell process, Proc. Natl. Acad. Sci. U.S.a 110 (2013) 12096–12101. doi: 10.1073/pnas.1310003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, et al. , Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels, Proceedings of the National Academy of Sciences. 107 (2010) 13648–13653. doi: 10.1073/pnas.1009382107/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Y, McNamara LM, Schaffler MB, Weinbaum S, A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proceedings of the National Academy of Sciences. 104 (2007) 15941–15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB, Attachment of Osteocyte Cell Processes to the Bone Matrix, Anat Rec. 292 (2009) 355–363. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cabahug-Zuckerman P, Stout RF Jr., Majeska RJ, Thi MM, Spray DC, Weinbaum S, et al. , Potential role for a specialized β 3integrin-based structure on osteocyte processes in bone mechanosensation, Journal of Orthopaedic Research. 118 (2017) 733–11. doi: 10.1002/jor.23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Geoghegan IP, Hoey DA, McNamara LM, Estrogen deficiency impairs integrin αvβ3-mediated mechanosensation by osteocytes and alters osteoclastogenic paracrine signalling, Scientific Reports. 9 (2019) 4654. doi: 10.1038/s41598-019-41095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, et al. , A Cre-Dependent GCaMP3 Reporter Mouse for Neuronal Imaging In Vivo, Journal of Neuroscience. 32 (2012) 3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ, DMP1-targeted Cre Expression in Odontoblasts and Osteocytes, Journal of Dental Research. 86 (2007) 320–325. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- [26].Lewis KJ, Frikha-Benayed D, Louie J, Stephen S, Spray DC, Thi MM, et al. , Osteocyte calcium signals encode strain magnitude and loading frequency in vivo, Proc. Natl. Acad. Sci. U.S.a 114 (2017) 11775–11780. doi: 10.1073/pnas.1707863114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kalu DN, Liu CC, Salerno E, Hollis B, Echon R, Ray M, Skeletal response of ovariectomized rats to low and high doses of 17 beta-estradiol, Bone Miner. 14 (1991) 175–187. doi: 10.1016/0169-6009(91)90021-q. [DOI] [PubMed] [Google Scholar]
- [28].Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, et al. , Osteocyte apoptosis and control of bone resorption following ovariectomy in mice, Bone. 46 (2010) 577–583. doi: 10.1016/j.bone.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Puca GA, Bresciani F, Interactions of 6,7–3H-17beta-estradiol with mammary gland and other organs of the C3H mouse in vivo, Endocrinology. 85 (1969) 1–10. doi: 10.1210/endo-85-1-1. [DOI] [PubMed] [Google Scholar]
- [30].Nelson JF, Felicio LS, Osterburg HH, Finch CE, Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice, Biol Reprod. 24 (1981) 784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- [31].Rubin CT, Lanyon LE, Regulation of bone formation by applied dynamic loads, J Bone Joint Surg Am. 66 (1984) 397–402. [PubMed] [Google Scholar]
- [32].Burr DB, Milgrom C, Fyhrie DP, Forwood MR, Nyska M, Finestone A, et al. , In vivo measurement of human tibial strains during vigorous activity, Bone. 18 (1996) 405–410. [DOI] [PubMed] [Google Scholar]
- [33].Kennedy OD, Sun H, Wu Y, Courtland H-W, Williams GA, Cardoso L, et al. , Skeletal Response of Male Mice to Anabolic Hormone Therapy in the Absence of the IgfalsGene, Endocrinology. 155 (2014) 987–999. doi: 10.1210/en.2013-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tsourdi E, Jähn K, Rauner M, Busse B, Bonewald LF, Physiological and pathological osteocytic osteolysis, J Musculoskelet Neuronal Interact. 18 (2018) 292–303. [PMC free article] [PubMed] [Google Scholar]
- [35].Rubin CT, Lanyon LE, Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling, J. Theor. Biol 107 (1984) 321–327. [DOI] [PubMed] [Google Scholar]
- [36].Aguirre JI, Plotkin LI, Gortazar AR, Millan MM, O’Brien CA, Manolagas SC, et al. , A Novel Ligand-independent Function of the Estrogen Receptor Is Essential for Osteocyte and Osteoblast Mechanotransduction*, J. Biol. Chem 282 (2007) 25501–25508. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- [37].Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, et al. , Osteocytes Use Estrogen Receptor α to Respond to Strain but Their ERα Content Is Regulated by Estrogen, J Bone Miner Res. 21 (2006) 1297–1306. doi: 10.1359/jbmr.060504. [DOI] [PubMed] [Google Scholar]
- [38].Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, et al. , Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual, J. Clin. Invest 123 (2012) 394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Leitman D, Paruthiyil S, Yuan C, Herber C, Olshansky M, Tagliaferri M, et al. , Tissue-Specific Regulation of Genes by Estrogen Receptors, Semin Reprod Med. 30 (2012) 14–22. doi: 10.1055/s-0031-1299593. [DOI] [PubMed] [Google Scholar]
- [40].Das SK, Estrogen Targets Genes Involved in Protein Processing, Calcium Homeostasis, and Wnt Signaling in the Mouse Uterus Independent of Estrogen Receptor-alpha and -beta, Journal of Biological Chemistry. 275 (2000) 28834–28842. doi: 10.1074/jbc.M003827200. [DOI] [PubMed] [Google Scholar]
- [41].Carnesecchi J, Vanacker J-M, Estrogen-Related Receptors and the control of bone cell fate, Molecular and Cellular Endocrinology. 432 (2016) 37–43. doi: 10.1016/j.mce.2015.07.019. [DOI] [PubMed] [Google Scholar]
- [42].Jacenik D, Cygankiewicz AI, Krajewska WM, The G protein-coupled estrogen receptor as a modulator of neoplastic transformation, Molecular and Cellular Endocrinology. 429 (2016) 10–18. doi: 10.1016/j.mce.2016.04.011. [DOI] [PubMed] [Google Scholar]
- [43].Soltysik K, Czekaj P, Membrane estrogen receptors - is it an alternative way of estrogen action? J. Physiol. Pharmacol 64 (2013) 129–142. [PubMed] [Google Scholar]
- [44].Wu T-W, Chen S, Brinton RD, Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons, Brain Research. 1379 (2011) 34–43. doi: 10.1016/j.brainres.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Arnold S, Estrogen suppresses the impact of glucose deprivation on astrocytic calcium levels and signaling independently of the nuclear estrogen receptor, Neurobiology of Disease. 20 (2005) 82–92. doi: 10.1016/j.nbd.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [46].Nilsen J, Chen S, Brinton RD, Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway, Brain Research. 930 (2002) 216–234. [DOI] [PubMed] [Google Scholar]
- [47].Bord S, Horner A, Beavan S, Compston J, Estrogen receptors alpha and beta are differentially expressed in developing human bone, The Journal of Clinical Endocrinology & Metabolism. 86 (2001) 2309–2314. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- [48].Lee KCL, Jessop H, Suswillo R, Zaman G, Lanyon LE, The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta, J. Endocrinol 182 (2004) 193–201. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15283680&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [49].Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L, Endocrinology: bone adaptation requires oestrogen receptor-alpha, Nature. 424 (2003) 389–389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- [50].Kondoh S, Inoue K, Igarashi K, Sugizaki H, Shirode-Fukuda Y, Inoue E, et al. , Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice, Bone. 60 (2014) 68–77. doi: 10.1016/j.bone.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Farkas I, Sárvári M, Aller M, Okada N, Okada H, Likó I, et al. , Estrogen receptor alpha and beta differentially mediate C5aR agonist evoked Ca2+-influx in neurons through L-type voltage-gated Ca2+ channels, Neurochemistry International. 60 (2012) 631–639. doi: 10.1016/j.neuint.2012.02.024. [DOI] [PubMed] [Google Scholar]
- [52].Vega-Vela NE, Osorio D, Avila-Rodriguez M, Gonzalez J, García-Segura LM, Echeverria V, et al. , L-Type Calcium Channels Modulation by Estradiol, Molecular Neurobiology. 20 (2016) 1–12. doi: 10.1007/s12035-016-0045-6. [DOI] [PubMed] [Google Scholar]
- [53].Turner CH, Warden SJ, Bellido TM, Plotkin LI, Kumar N, Jasiuk I, et al. , Mechanobiology of the Skeleton, Science Signaling. 2 (2009) pt3–pt3. doi: 10.1126/scisignal.268pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Voisin M, McNamara LM, Differential β 3and β 1Integrin Expression in Bone Marrow and Cortical Bone of Estrogen Deficient Rats, Anat Rec. 298 (2015) 1548–1559. doi: 10.1002/ar.23173. [DOI] [PubMed] [Google Scholar]
- [55].Li C-F, Ross FP, Cao X, Estrogen enhances alpha v beta 3 integrin expression by avian osteoclast precursors via stabilization of beta 3 integrin mRNA, Molecular Endocrinology. 9 (1995) 805–813. doi: 10.1210/me.9.7.805. [DOI] [PubMed] [Google Scholar]
- [56].Ma L, Hua R, Tian Y, Cheng H, Fajardo RJ, Pearson JJ, et al. , Connexin 43 hemichannels protect bone loss during estrogen deficiency, Bone Res. 7 (2019) 1–12. doi: 10.1038/s41413-019-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schaffler MB, Cheung W-Y, Majeska RJ, Kennedy O, Osteocytes: Master Orchestrators of Bone, Calcif Tissue Int. 94 (2013) 5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, et al. , Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs, J Bone Miner Res. 31 (2016) 1356–1365. doi: 10.1002/jbmr.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, et al. , Osteocyte Apoptosis Is Induced by Weightlessness in Mice and Precedes Osteoclast Recruitment and Bone Loss, J Bone Miner Res. 21 (2006) 605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- [60].Bonewald LF, The amazing osteocyte, J Bone Miner Res. 26 (2011) 229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Deepak V, Kayastha P, McNamara LM, Estrogen deficiency attenuates fluid flow‐induced [Ca 2+] ioscillations and mechanoresponsiveness of MLO‐Y4 osteocytes, The FASEB Journal. 31 (2017) 3027–3039. doi: 10.1096/fj.201601280R. [DOI] [PubMed] [Google Scholar]
- [62].Freitas-Andrade M, Bechberger JF, MacVicar BA, Viau V, Naus CC, Pannexin1 knockout and blockade reduces ischemic stroke injury in female, but not in male mice, Oncotarget. 8 (2017) 36973–36983. doi: 10.18632/oncotarget.16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Stewart MKG, Plante I, Penuela S, Laird DW, Loss of Panx1 Impairs Mammary Gland Development at Lactation: Implications for Breast Tumorigenesis, PLoS ONE. 11 (2016) e0154162–23. doi: 10.1371/journal.pone.0154162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bosch MA, Hou J, Fang Y, Kelly MJ, RØnnekleiv OK, 17β-estradiol regulation of the mRNA expression of t-type calcium channel subunits: Role of estrogen receptor α and estrogen receptor β, J. Comp. Neurol 512 (2009) 347–358. doi: 10.1002/cne.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Banciu A, Banciu D, Mustaciosu C, Radu M, Cretoiu D, Xiao J, et al. , Beta-Estradiol Regulates Voltage-Gated Calcium Channels and Estrogen Receptors in Telocytes from Human Myometrium, Ijms. 19 (2018) 1413–18. doi: 10.3390/ijms19051413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sharma D, Ciani C, Marin PAR, Levy JD, Doty SB, Fritton SP, Alterations in the osteocyte lacunar–canalicular microenvironment due to estrogen deficiency, Bone. 51 (2012) 488–497. doi: 10.1016/j.bone.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ciani C, Sharma D, Doty SB, Fritton SP, Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone, Bone. 59 (2014) 229–234. doi: 10.1016/j.bone.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Verbruggen SW, Vaughan TJ, McNamara LM, Mechanisms of osteocyte stimulation in osteoporosis, Journal of the Mechanical Behavior of Biomedical Materials. 62 (2016) 158–168. doi: 10.1016/j.jmbbm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- [69].Zhang D, Miranda M, Li X, Han J, Sun Y, Rojas N, et al. , Retention of osteocytic micromorphology by sclerostin antibody in a concurrent ovariectomy and functional disuse model, Annals of the New York Academy of Sciences. 1442 (2018) 91–103. doi: 10.1111/nyas.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shaffer SW, Harrison AL, Aging of the somatosensory system: a translational perspective, Phys Ther. 87 (2007) 193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- [71].García-Piqueras J, García-Mesa Y, Cárcaba L, Feito J, Torres-Parejo I, Martín-Biedma B, et al. , Ageing of the somatosensory system at the periphery: age-related changes in cutaneous mechanoreceptors, J Anat. 234 (2019) 839–852. doi: 10.1111/joa.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A, Sahlin L, et al. , Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice, J Endocrinol. 201 (2009) 397–406. doi: 10.1677/JOE-09-0060. [DOI] [PubMed] [Google Scholar]
- [73].Matthews BD, Overby DR, Alenghat FJ, Karavitis J, Numaguchi Y, Allen PG, et al. , Mechanical properties of individual focal adhesions probed with a magnetic microneedle, Biochemical and Biophysical Research Communications. 313 (2004) 758–764. doi: 10.1016/j.bbrc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [74].Fritton SP, Weinbaum S, Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction, Annu. Rev. Fluid Mech 41 (2009) 347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Frost HM, The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents, Bone Miner. 2 (1987) 73–85. [PubMed] [Google Scholar]
- [76].Cianferotti L, Brandi ML, Muscle–bone interactions: basic and clinical aspects, Endocrine. 45 (2013) 165–177. doi: 10.1007/s12020-013-0026-8. [DOI] [PubMed] [Google Scholar]
- [77].Hughes JM, Petit MA, Biological underpinnings of Frost’s mechanostat thresholds: the important role of osteocytes, J Musculoskelet Neuronal Interact. 10 (2010) 128–135. [PubMed] [Google Scholar]
- [78].Hughes JM, Castellani CM, Popp KL, Guerriere KI, Matheny RW, Nindl BC. The Central Role of Osteocytes in the Four Adaptive Pathways of Bone’s Mechanostat, Exerc Sport Sci Rev. 48 (2020) 140–148. doi: 10.1249/JES.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Taaffe DR, Daly RM, Suominen H, Galvão DA, Bolam KA, Chapter 29 - Physical ctivity and Exercise in the Maintenance of the Adult Skeleton and the Prevention of Osteoporotic Fractures, in: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA (), Osteoporosis (Fourth Edition), Academic Press, San Diego, 2013: pp. 683–719. doi: 10.1016/B978-0-12-415853-5.00029-7. [DOI] [Google Scholar]
- [80].Greenway KG, Walkley JW, Rich PA, Does long-term swimming participation have a deleterious effect on the adult female skeleton? Eur J Appl Physiol. 112 (2012) 3217–3225. doi: 10.1007/s00421-011-2305-5. [DOI] [PubMed] [Google Scholar]
- [81].Ma D, Wu L, He Z, Effects of walking on the preservation of bone mineral density in perimenopausal and postmenopausal women, Menopause. 20 (2013) 1216–1226. doi: 10.1097/GME.0000000000000100. [DOI] [PubMed] [Google Scholar]
- [82].Beck BR, Daly RM, Singh MAF, Taaffe DR, Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis, Journal of Science and Medicine in Sport. 20 (2016) 1–8. doi: 10.1016/j.jsams.2016.10.001. [DOI] [PubMed] [Google Scholar]
- [83].Gianoudis J, Bailey CA, Sanders KM, Nowson CA, Hill K, Ebeling PR, et al. , Osteo-cise: strong bones for life: protocol for a community-based randomised controlled trial of a multi-modal exercise and osteoporosis education program for older adults at risk of falls and fractures, BMC Musculoskelet Disord. 13 (2012) 78. doi: 10.1186/1471-2474-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gianoudis J, Bailey CA, Ebeling PR, Nowson CA, Sanders KM, Hill K, et al. , Effects of a Targeted Multimodal Exercise Program Incorporating High-Speed Power Training on Falls and Fracture Risk Factors in Older Adults: A Community-Based Randomized Controlled Trial, J Bone Miner Res. 29 (2013) 182–191. doi: 10.1002/jbmr.2014. [DOI] [PubMed] [Google Scholar]
- [85].Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW, Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription, Brazilian Journal of Physical Therapy. 23 (2019) 170–180. doi: 10.1016/j.bjpt.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Daly RM, Gianoudis J, Kersh ME, Bailey CA, Ebeling PR, Krug R, et al. , Effects of a 12-Month Supervised, Community-Based, Multimodal Exercise Program Followed by a 6-Month Research-to-Practice Transition on Bone Mineral Density, Trabecular Microarchitecture, and Physical Function in Older Adults: A Randomized Controlled Trial, J Bone Miner Res. 35 (2020) 419–429. doi: 10.1002/jbmr.3865. [DOI] [PubMed] [Google Scholar]


