Abstract
Background:
Damage associated molecular patterns (DAMPs) stimulate endothelial syndecan-1 shedding and neutrophil extracellular traps (NET) formation. The role of NETs in trauma and trauma-induced hypercoagulability is unknown. We hypothesized that trauma patients with accelerated thrombin generation would have increased NETosis and syndecan-1 levels.
Methods:
In this pilot study, we analyzed 50 citrated plasma samples from 30 trauma patients at 0h (n=22) and 6h (n=28) from time of injury (TOI) and 21 samples from healthy volunteers, for a total of 71 samples included in analysis. Thrombin generation was quantified using calibrated automated thrombogram (CAT) and reported as lag time (LT), peak height (PH), and time to peak (ttPeak). Nucleosome calibrated (H3NUC) and free histone standardized (H3Free) ELISAs were used to quantify NETs. Syndecan-1 levels were quantified by ELISA. Results are presented as median [interquartile range] and Spearman rank correlations.
Results:
Plasma levels of H3NUC were increased in trauma patients as compared to healthy volunteers both at 0h (89.8 ng/mL [35.4, 180.3]; 18.1 ng/mL [7.8, 37.4], p=0.002) and at 6h (86.5 ng/mL [19.2, 612.6]; 18.1 ng/mL [7.8, 37.4], p=0.003) from TOI. H3Free levels were increased in trauma patients at 0h (5.74 ng/mL [3.19, 8.76]; 1.61 ng/mL [0.66, 3.50], p=0.002) and 6h (5.52 ng/mL [1.46, 11.37]; 1.61 ng/mL [0.66, 3.50], p=0.006). Syndecan-1 levels were greater in trauma patients (4.53 ng/mL [3.28, 6.28]; 2.40 ng/mL [1.66, 3.20], p<0.001) only at 6h from TOI. H3Free and syndecan-1 levels positively correlated both at Oh (0.376, p=0.013) and 6h (0.583, p<0.001) from TOI. H3NUC levels and syndecan-1 levels were positively correlated at 6h from TOI (0.293, p=0.041). TtPeak correlated inversely to H3 NUC (−0.358, p=0.012) and syndecan-1 levels (−0.298, p=0.038) at 6h from TOI.
Conclusions:
Our pilot study demonstrates that trauma patients have increased NETosis, measured by H3NUC and H3Free levels, increased syndecan-1 shedding, and accelerated thrombin generation kinetics early after injury.
Keywords: Neutrophil extracellular traps, endotheliopathy, syndecan-1, trauma, thrombin, inflammation
Introduction:
The physiologic response to trauma is characterized by systemic inflammation and dysregulation of coagulation. Damage associated molecular patterns (DAMPs) released into the circulation after trauma increase endothelial permeability and activate neutrophil-endothelial cell interactions (1). Recent studies have shown links between blood clotting, neutrophil priming, and inflammatory tissue injury after trauma (2). Neutrophils have reemerged as an important first responder in innate immunity via formation of neutrophil extracellular traps (NETs), which were first identified as a defense mechanism against bacterial infection and virulence factors (3). Since their discovery, NETs have been shown to play a role in sepsis associated intravascular coagulation, autoimmune disease, ischemia-reperfusion injury, metastasis and cancer associated thrombus, deep vein thrombosis (DVT), and most recently the novel SARS-CoV 2 virus (COVID-19) infection (4–11). The role of NETs in trauma patients remains largely unknown.
An early stage of NETosis involves the activation of peptidyl-arginine deiminase 4 (PAD4) and citrullination of histone N-terminal tails, which weakens histone-DNA interactions and leads to the unfolding of tightly wrapped chromatin to form NETs (12). Citrullinated histone H3 (H3Cit), in particular, is well studied and has been used as a marker of NETosis (13, 14). However, the lack of standardized assays to quantify H3Cit levels has led to variable results and remains a barrier to studying NETs in the clinical setting (15). Similarly, measurements of circulating cell-free DNA (cfDNA) are not specific to NETs and cell death by NETosis, as cfDNA is also released through other methods of cell death. Increased levels of cfDNA regulated by deoxyribonuclease (DNAse) have been demonstrated in trauma patients but the origin of this cfDNA is uncertain (16–18). In our study, two independent assays, a histone-protein calibrated assay (H3Free) and a novel nucleosome-based assay (H3NUC) were used in to quantify H3Cit in trauma patients and to better understand the potential role of NETs in trauma (15). Activated endothelial cells are known to induce NET formation, and NETs in turn are able to trigger further endothelial damage, leading to a cycle of NETosis and endothelial injury (19). Syndecan-1 is a component of the endothelial glycocalyx which maintains endothelial integrity and prevents thrombosis (20). Syndecan-1 is shed into the circulation after trauma, and higher levels of syndecan-1 are associated with alterations in coagulation and increased mortality (21–23). Trauma patients are known to display early hypo- or hypercoagulability depending on severity of injury, degree of hemorrhage, and resuscitation methods (24). Additionally, endothelial injury is a known mediator of venous thromboembolism (VTE) (25).
Our group has previously used the calibrated automated thrombogram (CAT) assay to quantify plasma thrombin generation kinetics in response to tissue factor (26, 27). This represents a global marker of an individual's plasma coagulome (26–30). Thrombin generation after trauma, particularly shortened time to peak (the time to maximal thrombin generation), is an independent predictor of VTE development after trauma (28). NETosis and syndecan-1 shedding have not previously been described in trauma and trauma-induced hypercoagulability. In our pilot study, we hypothesized that trauma patients with accelerated thrombin generation will have increased levels of NETs and syndecan-1 early after time of injury (TOI).
Methods:
Study Design and Database
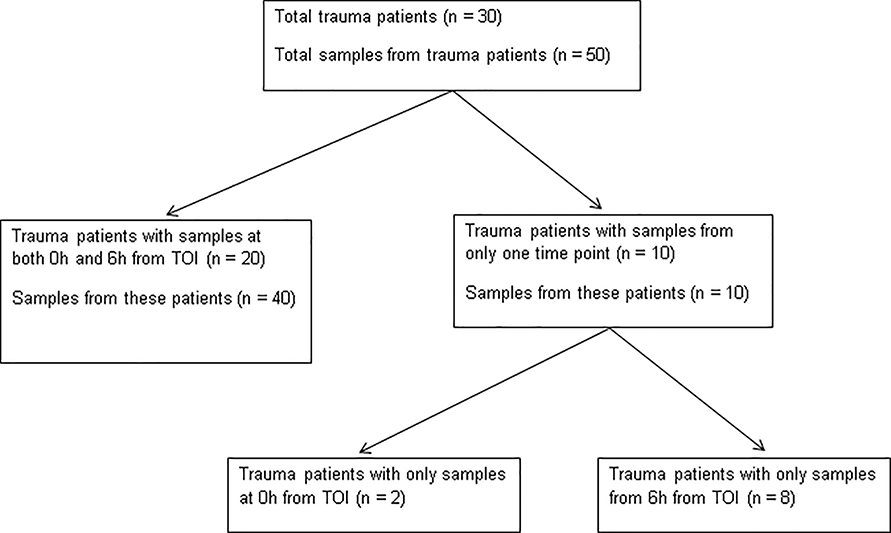
All level one and two trauma patients admitted to Mayo Clinic in Rochester, MN, a Level One Trauma center, were considered for inclusion in this study, as previously described (28). Exclusion criteria were: refusal or inability to obtain informed consent from a patient or their Legal Authorized Representative, therapeutic anticoagulation (e.g. dabigatran etexilate, rivaroxaban, apixaban), an inherited or acquired coagulation disorder, presentation > 12 hours after injury, active cancer, sepsis, renal failure, or burns. We analyzed a total of 71 citrated plasma samples from the existing bio-repository collected from a previously published parent study (28). Only samples from time points early after TOI were included in this pilot study. Fifty samples were collected from 30 trauma patients, and 21 samples were collected from 21 healthy volunteers. Of these 30 trauma patients, 20 had samples analyzed from Oh and 6h from TOI with details of sample times shown in Figure 1. The remaining 10 patients had samples from only one of the time points. In total, 22 samples were obtained at 6h from TOI and 28 samples were obtained at 6h from TOI. A secure electronic REDCap database was constructed to house data on patient demographics, transfusion, surgical interventions, and hospital complications including infections and VTE from inpatient and outpatient medical records. This study was approved by the Mayo Clinic Institutional Review Board.
Figure 1:
Description of Samples from Trauma Patients Obtained Oh and 6h from Time of Injury (TOI)
Sample Collection, Processing, and Storage
Whole blood was collected by venipuncture or via Existing indwelling catheters into 4.5 mL citrated Vacutainer tubes (0.105M buffered sodium citrate, 3.2% Becton Dickinson, Plymouth, UK) and processed (within 1 hour of collection) to platelet poor (cell-free) plasma by double centrifugation (3000g, 15 minutes), and stored in multiple aliquots at −80 degrees Celsius until analysis.
Calibrated Automated Thrombogram (CAT)
Thrombin generation was measured with the Calibrated Automated Thrombogram (CAT, Thrombinoscope BV. Maastricht, Netherlands), utilizing a Fluoroskan Ascent plate reader (390 nm excitation, 460 nm emission, Thermo Electron Corp, Vantaa, Finland), as previously described (31, 32). Assays of trauma patient samples were performed in triplicate. Corn trypsin inhibitor (50 μg/mL final concentration) was added to each plasma sample prior to analysis. Thrombin generation was initiated using two different reagents: 20 μL of PPP (5 pM re-lipidated human tissue factor and 4 μM phospholipids, Diagnostica Stago Inc., Parsippany, NJ) reagent. Then 80 μL of platelet poor (cell-free) plasma was added to each well of U-bottom 96-well microtiter plates (Nunc, Thermo Fischer Scientific, Rochester, NY) using a single channel pipette. After incubation for 10 minutes at 37°C, 20 μL of warmed FluCa reagent (FluCa kit, Diagnostica Stago Inc., Parsippany, NJ), which contains the fluorogenic substrate and CaCl2 was added to each well via an automated dispenser. Thrombin generation curves were recorded continuously for 90 minutes at a rate of three readings per minute. Separate wells containing the thrombin calibrator, which corrects for inner filter effects and quenching variation among individual plasmas were also measured in parallel. A dedicated software program, Thrombinoscope (Thrombinoscope BV, Maastricht, The Netherlands) was used to calculate thrombin activity over time. The parameters derived were: lag time (LT) in minutes, peak height (PH) in nM, and time to peak (ttPeak) in minutes.
Markers of NETosis (H3NUC and H3Free)
A modified version of the previously described nucleosome-based H3Cit ELISA was used to quantify H3NUC using the H3R8Cit ELISA Capture Kit (EpiCypher Catalog No. R&D143001) (15). All reagents were equilibrated to room temperature prior to usage. Monoclonal detection antibody to H3R8Cit (EpiCypher Catalog No. 13–0046) at a concentration of 2 μg/mL was used to coat High Bind Clear 96-well microplates (Thermo Fisher Scientific Catalog No. 3855) in IX PBS overnight at 4°C. Plates were washed three times using wash buffer (50 mM Tris-HCl pH 7.5, 0.01% (w/v) BSA, 0.01% (v/v) Tween-20) followed by blocking for 1 hour at room temperature (IX PBS, 1% (w/v) BSA) and three additional washes. Calibration standards were prepared by diluting H3R2,8,17Cit recombinant designer nucleosome (dNucs, EpiCypher Catalog No. 16–1362) in 2-fold decrements from 500 – 7.8 ng/mL including a 0 ng/mL (blank) assay buffer control (50 mM Tris-HCl pH 7.5, 300 mM NaCl, 0.01% (w/v) BSA, 0.01% (v/v) Tween-20) and 100 μL were added to each plate in duplicate. Each plasma sample was run in duplicate at four serial dilutions (1:2, 1:4, 1:8, 1:16) in assay buffer. Samples were incubated for 2 hours at room temperature and washed three times. Biotinylated H3R8Cit was used as detection antibody at 1:200 dilution in assay buffer. After an hour incubation, the plates were washed three times with signal detection performed using Pierce™ High Sensitivity Streptavidin-HRP (Thermo Fisher Scientific Catalog No. 21130), 1-Step™ Ultra TMB-ELISA Substrate Solution (Thermo Fisher Scientific Catalog No. 34028), and Stop Solution for TMB Substrates (Thermo Fisher Scientific Catalog No. N600) according to the manufacturer's directions. Signal (absorbance at 450 nm) was read using EnVision™ Multilabel Plate Reader (Perkin Elmer). H3R8Cit recoveries were determined by selecting the plasma dilution within the assay dynamic range (standard curve R2 > 0.95) and interpolating onto the plate-specific standard curve using a non-linear fit (sigmoidal 4PL regression analysis for absorbance vs. log ([H3R2,8,17Cit dNuc], ng/mL) and finally correcting for the dilution factor.
Citrullinated Histone H3 (CitH3) ELISA kit was used (Cayman Chemical, Clone 11D3) to measure H3Free following the manufacturer's instructions at 1:4 dilutions in assay buffer.
Measurement of Syndecan-1 levels
Syndecan-1 levels were measured by ELISA according to the manufacturer's instructions (Diaclone: Besancon, France) and performed by the University of Maryland Cytokine Core Laboratory.
Statistical Analyses
Data analysis was performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). Descriptive statistics are presented as median values and interquartile ranges (IQR). Kruskal-Wallis test was used to detect differences in values between trauma patients and healthy volunteers. Correlations between CAT parameters, markers of NETosis, and syndecan-1 were assessed with Spearman rank correlation analysis. Analyses comparing trauma patients and healthy volunteers as well as correlation analyses were performed using only samples obtained at 0h from TOI and repeated using only samples obtained at 6h from TOI. P< 0.05 was considered significant.
Results:
Baseline Characteristics
Baseline characteristics of trauma patients and healthy volunteers are shown in Table 1. Detailed patient characteristics and injury patterns can be found in Table 2. A total of 22 samples were obtained at Oh from TOI and 28 samples were obtained at 6h from TOI. Of the 30 patients included in this study, 18 required transfusion of blood products early after TOI.
Table 1:
Baseline Characteristics
| Trauma (n= 30) | Healthy Volunteers (n = 21) | P-value | |
|---|---|---|---|
| Age (years) | 56.5 | 43 | 0.13 |
| Sex (% male) | 18 (60%) | 9 (43%) | 0.36 |
| ISS | 25.5 | - | - |
Age and injury severity score (ISS) reported as median values.
Table 2:
Detailed Patient Characteristics
| Patient ID | Time of samples relative to TOI | Major injuries | Transfusion (Y/N) | ISS |
|---|---|---|---|---|
| 1 | 0h, 6h | TBI (multi-compartment hemorrhage) | N | 26 |
| 2 | 6h | TBI (subdural hematoma), Facial fractures | N | 24 |
| 3 | 0h, 6h | TBI (diffuse axonal injury with cerebral edema), Hemorrhagic shock, Rib fractures, L scapula fracture, L clavicle fracture, Pelvic fracture, T and L spine fractures | Y | 50 |
| 4 | 0h, 6h | TB (subarachnoid and subdural hemorrhage requiring craniectomy), Skull fracture | N | 26 |
| 5 | 6h | TBI (concussion), Skull base fracture, C and T spine fractures | N | 17 |
| 6 | 6h | TBI (intraparenchymal hemorrhage), Open skull fracture | Y | 29 |
| 7 | 6h | TBI (subdural hemorrhage) | N | 29 |
| 8 | 0h, 6h | TBI (epidural hematoma needing craniotomy), LB fx (femur) with vascular injury, Facial degloving injury | N | 38 |
| 9 | 6h | TBI (subarachnoid hemorrhage), Facial lacerations, Skull fractures, LB fx (R humerus/R ulna/R radius) | N | 24 |
| 10 | 0h, 6h | TBI (subdural hemorrhage), Open R ankle fracture | Y | 27 |
| 11 | 0h, 6h | TBI (subdural and subarachnoid hemorrhage requiring craniotomy) | Y | 26 |
| 12 | 6h | TBI (subarachnoid and intraparenchymal hemorrhage requiring craniotomy), Skull fracture, Spinal fractures, LB fx (tibia/fibula) | Y | 33 |
| 13 | 0h, 6h | TBI (subdural and epidural hematoma requiring craniectomy), Skull base fractures, Spinal fractures | Y | 33 |
| 14 | 0h, 6h | LB fx (L tibia and fibula), Mild concussion | N | 9 |
| 15 | 0h, 6h | LB fx (open L radius), Ankle fracture, Spinal fracture | N | 14 |
| 16 | 0h, 6h | LB fx (L acetabulum/L radius fracture), Splenic laceration, Rib fractures | N | 22 |
| 17 | 0h, 6h | LB fx (bilateral femur fractures) | Y | 9 |
| 18 | 6h | LB fx (tibia and fibula), Ankle fracture | N | 4 |
| 19 | 6h | LB fx (bilateral tibia and R fibula), Lumbar and sacral fractures | N | 17 |
| 20 | 0h, 6h | Cardiac injury from stab wound, Hemorrhagic shock | Y | 26 |
| 21 | 0h, 6h | Splenic/hepatic/renal lacerations, Hemorrhagic shock, TBI (concussion), Spinal fractures with cord injury | Y | 50 |
| 22 | 0h, 6h | Hemorrhagic shock, Concussion, Spinal fractures, LB fx (femoral neck), Ankle fracture, Rib fractures | Y | 22 |
| 23 | 0h, 6h | Hemorrhagic shock, Multiple lacerations from stab wounds | Y | 5 |
| 24 | 0h, 6h | Hemorrhagic shock, Splenic laceration, mesenteric injury, TBI (subgaleal hematoma), Lumbar and sacral fractures, Rib fractures | Y | 22 |
| 25 | 0h, 6h | Hemorrhagic shock, LB fx (R tibia and fibula), R thigh hematoma, R wrist fracture, R scapular fracture, rib fractures | Y | 14 |
| 26 | 0h, 6h | Spinal fractures with cord injury, Rib fractures | Y | 25 |
| 27 | 0h, 6h | Hemorrhagic shock, TBI (subarachnoid hemorrhage) LB fx (bilateral femur), | Y | 34 |
| 28 | 0h | Hemorrhagic shock, Aortic injury, LB fx (L femur and R humerus), Ankle fracture, Pelvic fracture, Spinal fractures, Rib fractures, TBI (concussion), | Y | 34 |
| 29 | 0h | Hemorrhagic shock, Liver laceration | Y | 21 |
| 30 | 0h, 6h | Hemorrhagic shock, TBI (Subarachnoid and subdural hemorrhage), Spinal fractures with cord injury, Rib fractures | Y | 75 |
Characteristics and injury patterns of individual patients. TOI = time of injury, TBI = traumatic brain injury, LB fx = long bone fracture, ISS = injury severity score.
Thrombin Generation, Syndecan-1, and NETosis in Trauma Patients Compared to Healthy Volunteers
A total of 50 samples from the 30 trauma patients were analyzed for thrombin generation, syndecan-1 levels, and markers of NETosis. Samples obtained Oh from TOI and samples obtained 6h from TOI were compared separately with the samples from 21 healthy volunteers. As shown in Table 3, trauma patients had significantly greater PH and shorter ttPeak at both Oh and 6h from TOI as compared to healthy volunteers. H3NUC and H3Free methods to quantify NETosis showed significantly greater H3Cit levels in trauma patients at both Oh and 6h from TOI compared to healthy volunteers. Syndecan-1, a marker of endotheliopathy, was significantly greater in trauma patients at 6h from TOI as compared to healthy volunteers. Syndecan-1 levels in trauma patients at Oh from TOI trended toward increased levels as compared to healthy volunteers, but this did not reach statistical significance.
Table 3:
Thrombin Generation, NETosis, Syndecan-1 Measurements for Trauma Patients vs. Healthy Volunteers
| Healthy Volunteers (n=21) | Trauma (n=22) | P-value | ||
|---|---|---|---|---|
| Including only samples obtained 0h from TOI for trauma patients | LT (min) | 4.00 (3.33– 4.67) | 3.67 (3.29–4.67) | 0.336 |
| PH (nm) | 184.0 (142.6–224.0) | 277.8 (206.7–299.8) | 0.006 | |
| ttPeak (min) | 8.33 (7.00–9.33) | 6.56 (5.67–7.83) | 0.003 | |
| H3NUC (ng/mL) | 18.1 (7.8–37.4) | 89.8 (35.4–180.3) | 0.002 | |
| H3Free (ng/mL) | 1.61 (0.66–3.50) | 5.74 (3.19–8.76) | 0.002 | |
| Syndecan-1 (ng/mL) | 2.40 (1.66–3.20) | 3.20 (2.40–5.86) | 0.069 | |
| Trauma (n = 28) | ||||
| Including only samples obtained 6h from TOI for trauma patients | LT (min) | 4.00 (3.33– 4.67) | 4.34 (3.62–4.97) | 0.716 |
| PH (nm) | 184.0 (142.6–224.0) | 226.1 (201.3–254.9) | 0.009 | |
| ttPeak (min) | 8.33 (7.00–9.33) | 7.33 (6.34–8.49) | 0.036 | |
| H3NUC (ng/mL) | 18.1 (7.8–37.4) | 86.5 (19.2–612.6) | 0.003 | |
| H3Free (ng/mL) | 1.61 (0.66–3.50) | 5.52 (1.46–11.37) | 0.006 | |
| Syndecan-1 (ng/mL) | 2.40 (1.66–3.20) | 4.53 (3.28–6.28) | <0.001 | |
Thrombin generation (reported as lagtime (LT), peak height (PH), time to peak (ttPeak), markers of NETosis (citrullinated H3 nucleosomes [H3NUC] and citrullinated histones [H3Free]), and syndecan-1 levels in samples from healthy volunteers compared to samples obtained 0h and 6h from time of injury (TOI). All values expressed as median and interquartile range (IQR). Kruskal Wallis test, p<0.05 considered significant.
Correlation of Thrombin Generation, Syndecan-1, and NETosis
Correlation between markers of NETosis. endotheliopathy, and thrombin generation were assessed, as shown in Table 4. When samples from healthy volunteers and those obtained from trauma patients at 0h from TOI were included in analysis as shown in Table 4a, we found a positive correlation between the two markers of NETosis, H3NUC and H3Free. Syndecan-1 and H3Free were positively correlated with each other, but neither syndecan-1 nor markers of NETosis correlated to of thrombin generation. Analysis was repeated including samples from healthy volunteers and samples obtained from trauma patients 6h from TOI as shown in Table 4b. We again found a positive correlation between H3NUC and H3Free levels. Syndecan-1 levels were positively correlated to both H3NUC and H3Free levels. H3NUC and syndecan-1 levels were inversely correlated to ttPeak.
Table 4:
Correlation among Markers of NETosis, Endotheliopathy, and Thrombin Generation
| a. | |||||
| LT (min) | PH (nm) | ttPeak (min) | H3NUC (ng/mL) | H3Free (ng/mL) | |
| H3NUC (ng/mL) | −0.031 (p=0.842) | −0.136 (p=0.387) | −0.166 (p=0.288) | - | - |
| H3Free (ng/mL) | −0.079 (p=0.615) | 0.034 (p=0.828) | −0.189 (p=0.226) | 0.445 (p=0.003) * | - |
| Syndecan-1 (ng/mL) | −0.066 (p=0.672) | 0.193 (p=0.214) | −0.196 (p=0.208) | 0.039 (p=0.803) | 0.376 (p=0.013) * |
| b. | |||||
| LT (min) | PH (nm) | ttPeak (min) | H3NUC (ng/mL) | H3Free (ng/mL) | |
| H3NUC (ng/mL) | −0.189 (p=0.194) | 0.205 (p=0.158) | −0.358 (p=0.012) * | - | - |
| H3Free (ng/mL) | −0.193 (p=0.185) | −0.046 (p=0.756) | −0.189 (p=0.193) | 0.438 (p=0.002) * | - |
| Syndecan-1 (ng/mL) | −0.150 (p=0.302) | 0.140 (p=0.336) | −0.298 (p=0.038) * | 0.293 (p=0.041) * | 0.583 (p<0.001) * |
Spearman correlation coefficients between markers of thrombin generation, NETosis, and syndecan-1 for (a) samples (total n = 43) from volunteers (n = 21) and trauma patients obtained 0h after TOI (n = 22) and (b) samples (total n = 49) from volunteers (n = 21) and trauma patients obtained 6h after TOI (n = 28). All values log-transformed except PH.
p<0.05 considered significant.
Discussion:
This is the first study to demonstrate the presence of circulating NETs in trauma patients utilizing a nucleosome based assay (H3NUC). Using two independent assays, H3Free and H3NUC, a novel nucleosome based assay, we observed increased levels of citrullinated histones in trauma patients as compared to healthy controls (15). Prior studies showing increased levels of cfDNA in trauma patients have concluded that circulating cfDNA is released from injured or necrotic cells and is not a specific marker of NETosis (33). It has also been proposed that cell-free mitochondrial DNA (cf-mtDNA) is the primary structural component of NETs released after trauma, but the molecular mechanisms for neutrophil release of cf-mtDNA are unknown (34–36). Our study identifies the presence of H3Cit in trauma patients through a nucleosome-based assay, indicating that NETosis is increased in these patients (12, 13, 16). Published literature does not always distinguish nucleosomes from free histones in circulation, but several studies have shown that nucleosomes are stable in circulation whereas free histones are not (13, 14, 37). C-reactive protein, an acute phase reactant, can form complexes with and potentially stabilize free histones in circulation (38). These histone complexes could conceivably be detected using the current H3NUC assay (H3R8Cit capture antibody, H3R8Cit detection antibody), making it less specific. Despite slight differences in H3NUC and H3Free results, the two assays were positively correlated, suggesting that both captured PAD4-dependent histone citrullination, a necessary step in NETosis.
This pilot study is the first to identify a correlation between syndecan-1 shedding, which is indicative of endothelial glycocalyx disruption, and markers of NETosis, specifically H3Free, in trauma patients (22). Recently, NETosis and glycocalyx disruption have been implicated as contributors to vascular injury in diabetes (39, 40). Hemorrhagic shock is a known clinical contributor to glycocalyx disruption, and this effect is partially reversed by the transfusion of plasma (41). NETs, themselves, have been shown to contribute to endothelial barrier dysfunction (42). The mechanisms of glycocalyx disruption in trauma are incompletely understood, and the role of NETosis and syndecan-1 shedding in trauma-induced hypercoagulability is unknown. In murine models, and NET derived DNA and histones serve as scaffolding for the formation of platelet-rich thrombi in cancer-associated thrombosis and DVTs (8, 43). Post-mortem studies suggest that NETs promote organization and maturation of human thromboemboli in a similar fashion (44). In vitro studies have shown that NETs enhance thrombin generation in platelet poor plasma (45). Targeting NETosis has the potential to reduce VTE burden after trauma by affecting the observed hypercoagulability, including thrombin generation, without carrying the risk of bleeding associated with traditional VTE prophylaxis (i.e. low-molecular weight heparin). In animal models, DNAse decreases NET-mediated thrombus and prevents intravascular occlusion by NETs (43, 46–48). Recent studies have also described a targeted anti-citrullinated protein antibody (tAPCA) with the ability to suppress NET formation and ameliorate tissue damage in a murine model of inflammatory arthritis (49). Further studies are needed to determine the mechanisms by which NETosis and syndecan-1 shedding may contribute to dysregulated coagulation after trauma.
Our study has several limitations. First, this is a pilot study with a small number of patients from a single center with almost exclusively blunt trauma. Because of this, our results cannot be generalized to all trauma patients. Patients on anticoagulant therapy were excluded from the study, so we cannot draw any conclusions about endothelial disruption or NETosis in these patients. Of the 50 samples drawn from 30 trauma patients, all were drawn early after injury (within 6h from TOI), making it difficult to assess temporal trends in syndecan-1 levels or NETosis after injury. In our study, we also did not quantify syndecan-1 shedding before and after transfusion, which is known to impact the degree of glycocalyx disruption (41). Markers of endotheliopathy and cfDNA have previously been shown to correlate to long term outcomes (50). In our pilot study, we were not able to assess correlations of NETosis and syndecan-1 shedding to disease severity or long term endpoints. Though we used a highly specific, novel, internally validated nucleosome-based ELISA for detecting NETosis, the best means of quantifying NETosis in plasma samples has yet to be determined (15).
Conclusion:
Trauma patients have increased NETosis, as measured by quantifying both H3NUC and H3Free, increased syndecan-1 shedding, and accelerated thrombin generation kinetics early after injury as compared to healthy volunteers.
Acknowledgments:
The authors gratefully acknowledge the Clinical Research Unit (CRU) of the Center for Translational Science Activities (CTSA) at Mayo Clinic for their 24 hour support in sample collection. We are grateful for the technical assistance received from Tammy L. Price-Troska and Timothy M. Hailing for sample analysis.
Funding: This project was supported by T32 AG049672 from the National Institute of Aging (NIA) and Robert and Arlene Kogod Center for Aging, Mayo Clinic (JG), R38HL150086 Stimulating Access to Research in Residency (TAM) from the National Heart, Lung, and Blood Institute (NHLBI), HL146508 from the NHLBI (MA), ROl GM 129533 (RAK) from the National Institute of General Medical Sciences (NIGMS), ROl GM 126086-03 (MSP) from NIGMS, UM1 HL120877-06 (MSP) by the Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC), 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), the NIH Roadmap for Medical Research and by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). EpiCypher (ALJ) is supported by the NIH under award numbers R43AI134162 from the National Institute of Allergy and Infectious Diseases (NIAID), R43GM131560 (NIGMS), and R44AI134162 (NIAID). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCRR.
Footnotes
Conflict of Interest: EpiCypher is a commercial developer and supplier of platforms similar to those used in this study: recombinant semi-synthetic nucleosomes (dNucs), antibody validation platforms, and nucleosome-based H3Cit assays (e.g. EpiCypher Catalog No. R&D143001). ALJ and NWH are inventors on patents covering use of recombinant nucleosomes carrying histone or DNA modifications for antibody validation and assay quantification. All other authors declare no conflict of interest.
Awards: This abstract received the Travel Award for the 43rd Annual Conference on Shock 2020.
References:
- 1.Sun S, Sursal T, Adibnia Y, Zhao C, Zheng Y, Li H, Otterbein LE, Hauser CJ, Itagaki K. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One 8(3):e59989, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett CD, Hsu AT, Ellson CD, B YM, Kong YW, Greenwood JD, Dhara S, Neal MD, Sperry JL, Park MS, et al. Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clin Exp Immunol 194(1): 103–117, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303(5663): 1532–5, 2004. [DOI] [PubMed] [Google Scholar]
- 4.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, Jenne CN. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129(10): 1357–1367, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apel F, Zychlinsky A, Kenny EF. The role of neutrophil extracellular traps in rheumatic diseases. Nat Rev Rheumatol 14(8):467–475, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 62(2):600–14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tohme S, Yazdani HO, Al-Khafaji AB. Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res 76(6): 1367–80, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 109(32): 13076–81, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Goswami J, Varley P, van der Windt DJ, Ren J, Loughran P, Yazdani H, Neal MD, Simmons RL, Zhang J, et al. Hepatic Surgical Stress Promotes Systemic Immunothrombosis That Results in Distant Organ Injury. Front Immunol 11:987, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 5(11), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyer MR, Chen Q, Haldeman S, Yazdani H, Hoffman R, Loughran P, Tsung A, Zuckerbraun BS, Simmons RL, Neal MD. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep 8(1):2068, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184(2):205–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thalin C, Lundstrom S, Seignez C, Daleskog M, Lundstrom A, Henriksson P, Helleday T, Phillipson M, Wallen H, Demers M. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One 13(l):e0191231, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekaney ML, Otto GP, Sossdorf M, Sponholz C, Boehringer M, Loesche W, Rittirsch D, Wilharm A, Kurzai O, Bauer M, et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care 18(5):543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thalin C, Aguilera K, Hall NW, Marunde MR, Burg JM, Rosell A, Daleskog M, Mansson M, Hisada Y, Meiners MJ, et al. Quantification of citrullinated histones: Development of an improved assay to reliably quantify nucleosomal H3Cit in human plasma. J Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng W, Paunel-Gorgulu A, Flohe S, Witte I, Schadel-Hopfner M, Windolf J, Logters TT. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm 2012:149560, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumore PM, Steinman CR. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J Clin Invest 86(l):69–74, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan KC, Zhang J, Chan AT, Lei KI, Leung SF, Chan LY, Chow KC, Lo YM. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res 63(9):2028–32, 2003. [PubMed] [Google Scholar]
- 19.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 584(14):3193–7, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Chignalia AZ, Yetimakman F, Christiaans SC, Unal S, Bayrakci B, Wagener BM, Russell RT, Kerby JD, Pittet JF, Dull RO. The Glycocalyx and Trauma: A Review. Shock 45(4):338–48., 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang W, Zaske AM, Menge T, Kozar RA. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One 6(8):e23530, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg 254(2): 194–200, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Ostrowski SR, Haase N, Muller RB, Moller MH, Pott FC, Pemer A, Johansson PI. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: a prospective study. Crit Care 19:191, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care 11(6):590–7, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Qi H, Yang S, Zhang L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front Immunol 8:928, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MS, Owen BA, Ballinger BA, Sarr MG, Schiller HJ, Zietlow SP, Jenkins DH, Ereth MH, Owen WG, Heit JA. Quantification of hypercoagulable state after blunt trauma: microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery 151(6): 831–6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park MS, Xue A, Spears GM, Hailing TM, Ferrara MJ, Kuntz MM, Dhillon SK, Jenkins DH, Harmsen WS, Ballman KV, et al. Thrombin generation and procoagulant microparticle profiles after acute trauma: A prospective cohort study. J Trauma Acute Care Surg 79(5):726–31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park MS, Spears GM, Bailey KR, Xue A, Ferrara MJ, Headlee A, Dhillon SK, Jenkins DH, Zietlow SP, Harmsen WS, et al. Thrombin generation profiles as predictors of symptomatic venous thromboembolism after trauma: A prospective cohort study. J Trauma Acute Care Surg 83(3):381–387, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cimenti C, Mangge H, Haidl H, Zach D, Muntean W. Thrombin generation in severely obese children. J Thromb Haemost 4(8): 1834–6, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Haidl H, Cimenti C, Leschnik B, Zach D, Muntean W. Age-dependency of thrombin generation measured by means of calibrated automated thrombography (CAT). Thromb Haemost 95(5):772–5, 2006. [PubMed] [Google Scholar]
- 31.Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost 96(5):553–61, 2006. [PubMed] [Google Scholar]
- 32.Hemker HC. Recollections on thrombin generation. J Thromb Haemost 6(2):219–26, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Jackson Chornenki NL, Coke R, Kwong AC, Dwivedi DJ, Xu MK, McDonald E, Marshall JC, Fox-Robichaud AE, Charbonney E, Liaw PC. Comparison of the source and prognostic utility of cfDNA in trauma and sepsis. Intensive Care Med Exp 7(1):29, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mcllroy DJ, Jamicki AG, Au GG, Lott N, Smith DW, Hansbro PM, Balogh ZJ. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care 29(6): 1133 el-5, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Thurairajah K, Briggs GD, Balogh ZJ. The source of cell-free mitochondrial DNA in trauma and potential therapeutic strategies. Eur J Trauma Emerg Surg 44(3):325–334, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ 16(11): 1438–44, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Marsman G, Zeerleder S, Luken BM. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis 7(12):e2518, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrams ST, Zhang N, Dart C, Wang SS, Thachil J, Guan Y, Wang G, Toh CH. Human CRP defends against the toxicity of circulating histones. J Immunol 191(5):2495–502, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Hirota T, Levy JH, Iba T. The influence of hyperglycemia on neutrophil extracellular trap formation and endothelial glycocalyx damage in a mouse model of type 2 diabetes. Microcirculation:el2617,2020. [DOI] [PubMed] [Google Scholar]
- 40.Bryk AH, Prior SM, Plens K, Konieczynska M, Hohendorff J, Malecki MT, Butenas S, Undas A. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: associations with a prothrombotic state and hypofibrinolysis. Cardiovasc Diabetol 18(1):49, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial gly cocalyx in a rodent model of hemorrhagic shock. Anesth Analg 112(6): 1289–95, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meegan JE, Yang X, Beard RS Jr., Jannaway M, Chatterjee V, Taylor-Clark TE, Yuan SY. Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun 503(3): 1498–1502, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 10(1): 136–44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savchenko AS, Martinod K, Seidman MA, Wong SL, Borissoff JI, Piazza G, Libby P, Goldhaber SZ, Mitchell RN, Wagner DD. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost 12(6): 860–70, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vase Biol 34(9): 1977–84, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vase Biol 32(8): 1777–83, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Timiceriu A, Coletti R, Kollnberger M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 209(4):819–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jimenez-Alcazar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renne C, Renne T, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358(6367): 1202–1206, 2017. [DOI] [PubMed] [Google Scholar]
- 49.Chirivi RGS, van Rosmalen JWG, van der Linden M, Euler M, Schmets G, Bogatkevich G, Kambas K, Hahn J, Braster Q, Soehnlein O, et al. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naumann DN, Hazeldine J, Dinsdale RJ, Bishop JR, Midwinter MJ, Harrison P, Hutchings SD, Lord JM. Endotheliopathy is associated with higher levels of cell-free DNA following major trauma: A prospective observational study. PLoS One 12(12):e0189870, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]