Abstract
Adolescent intermittent ethanol (AIE) exposure in the rat results in a retention of adolescent-like responsiveness to ethanol into adulthood characterized by enhanced sensitivity to socially facilitating and decreased sensitivity to socially suppressing and aversive effects. Similar pattern of responsiveness to social and aversive effects of the selective glutamate NMDA NR2B receptor antagonist ifenprodil is evident in adolescent rats, suggesting that AIE would also retain this pattern of ifenprodil sensitivity into adulthood. Social (Experiment 1) and aversive measured via conditioned taste aversion (Experiment 2) effects of ifenprodil were assessed in adult male and female rats following AIE exposure. Sensitivity to the social and aversive effects of ifenprodil was not affected by AIE exposure. Experiment 3 assessed protein expression of vesicular transporters of GABA (vGAT) and glutamate (vGlut2) within the prelimbic cortex and nucleus accumbens in adolescents versus adults and in AIE adults versus controls. vGlut2 expression was higher in adolescents relative to adults within the PrL, but lower in the NAc. AIE adults did not retain these adolescent-typical ratios. These findings suggest that AIE is not associated with the retention of adolescent-typical sensitivity to NR2B receptor antagonism, along with no AIE-induced shift in vGlut2 to vGAT ratios.
Keywords: ifenprodil, adolescent intermittent ethanol exposure, vesicular transporters of GABA and glutamate, social interaction, conditioned taste aversion
Introduction
Binge drinking is the most common pattern of alcohol use in the United States, particularly during adolescence (Witt, 2010). According to recent Monitoring the Future survey results, 45% of 12th graders report at least one occasion of being drunk in their life (Johnston et al., 2019). As adolescence is a critical period of neural, hormonal and behavioral maturation, emerging evidence from both studies in humans and laboratory animals suggests that adolescents may be especially vulnerable to neural disruptions associated with alcohol consumption (Spear, 2018). Using an animal model of adolescence in the rat, greater alcohol (ethanol) consumption has been reported in adolescents than adults (Doremus et al., 2005; Vetter et al., 2007). These elevated levels of ethanol intake may be related in part to adolescent-typical sensitivities to ethanol that include dampened aversive responses to ethanol relative to adults (Anderson et al., 2010; Saalfield & Spear, 2016; Welzl et al., 2001), as well as marked ethanol-induced social facilitation that is evident in adolescent but not adult rats (Varlinskaya & Spear, 2002, 2015). In turn, excessive consumption of ethanol repeatedly during adolescence may result in lasting cognitive and behavioral alterations (Spear, 2018) as well as increased risk for developing alcohol use disorder (Nixon & McClain, 2010) later in life.
Long-lasting consequences of adolescent intermittent ethanol (AIE) exposure are extensively investigated in humans and animal models (see Spear, 2018 for review). In animal studies, ethanol has been administered to adolescent rodents through a variety of routes, including intragastric (i.g.) gavage, with intermittent exposure in a dose range of 3.5 – 5.0 g/kg ethanol i.g. during adolescence producing blood ethanol concentrations (BECs) in the range of 130–200 mg/dL (see Fernandez et al., 2017; Kim et al., 2019; Liu & Crews, 2015). In rats, among consequences of AIE exposure is the persistence of adolescent-typical sensitivities to ethanol into adulthood (Crews et al., 2019; Spear & Swartzwelder, 2014). For instance, adolescent rats intermittently exposed to ethanol between postnatal days (P) 25 and 45 (3.5 g/kg ethanol, i.g.) exhibit increases in social behavior when challenged with low doses of ethanol in adulthood – social facilitation that is not normally evident in adult rats (Varlinskaya et al., 2014), but is reminiscent of the notable sensitivity of adolescents to the social facilitating effects of ethanol (Varlinskaya & Spear, 2015). It has also been shown that following adolescent ethanol exposure, adults are less sensitive to the aversive effects of ethanol than their ethanol-naïve counterparts (Anderson et al., 2010; Saalfield & Spear, 2015; Vetter-O’Hagen et al., 2009), with AIE animals requiring higher doses of ethanol to induce a conditioned taste aversion (CTA) relative to controls. These findings are also similar to the insensitivity to the aversive effects of ethanol that is typically evident in adolescents relative to adults when indexed via CTA (Anderson et al., 2010; Saalfield & Spear, 2016).
Molecular contributors to AIE-associated preservation of adolescent-typical ethanol sensitivity into adulthood remain to be characterized. Among the neural systems undergoing marked changes through adolescence are the major excitatory and inhibitory neurotransmitter systems, glutamate and GABA respectively, that are thought to contribute to overall greater levels of excitability early in development. This increased excitability continues through adolescence in certain brain regions such as the prefrontal cortex (PFC; Caballero et al., 2014), with this overall greater excitability diminishing with maturity as the window of plasticity closes (Selemon, 2013). Animal studies have shown that repeated exposure to ethanol during adolescence in an intermittent pattern of with “binge”-like levels of ethanol exposure that bring blood ethanol concentration (BEC) to 80 mg% and higher alter neuronal maturation (Crews et al., 2019; Guerri & Pascual, 2010; Spear, 2018), particularly in regions of the brain that regulate sensitivity to reward and drugs of abuse (Alaux-Cantin et al., 2013). Therefore, the brain regions undergoing significant maturation during adolescence such as the PFC (Gass et al., 2014) or related to reward such as the nucleus accumbens (NAc; Griffin III et al., 2014) may be especially vulnerable to the effects of AIE.
There is ample evidence demonstrating that ethanol exerts both an antagonist-like action on the glutamatergic system (Allgaier, 2002; Krystal et al., 2003; Lovinger et al., 1989; Möykkynen et al., 2003; Wirkner et al., 2000) as well as an agonist-like effect on the GABAergic system (Enoch, 2008; Fleming et al., 2007; Koob, 2004; Suzdak et al., 1986). Given that during adolescence there is a shift in the balance between these two systems (e.g., Selemon, 2013), we hypothesize that lasting behavioral alterations produced by AIE may be due to disruptions in normal maturational decreases in glutamate excitation and increases in GABA inhibition that occur during adolescence (Thomases et al., 2013). Glutamate levels in the NAc have been reported to be significantly higher in animals exposed to ethanol chronically during adolescence relative to adult controls (Pascual et al., 2009), possibly due to compensatory mechanisms following repeated exposure to the glutamate antagonistic effects of ethanol (Valenzuela, 1997). Such compensatory mechanisms may be a consequence of ethanol exposure specifically during adolescence, given that chronic adolescent, but not adult ethanol exposure downregulates NMDA NR2B receptors (Pascual et al., 2009). Based on these findings, we hypothesized that the adolescent-typical shift in balance between the glutamate and GABA systems toward higher functional activity of the glutamate system may be maintained into adulthood following AIE, with AIE-exposed rats exhibiting adolescent-typical sensitivities in adulthood not only to ethanol (e.g., see Saalfield & Spear, 2015; Varlinskaya et al., 2014) but also to other drugs with glutamate antagonist effects.
Several similarities between adolescent-typical behavioral responses to ethanol and the selective NMDA NR2B receptor antagonist, ifenprodil suggest that these adolescent characteristic sensitivities to ethanol may be mediated through antagonism of NMDA NR2B receptors. Similar to low doses of ethanol (Varlinskaya & Spear, 2002, 2015), ifenprodil increases social behaviors in adolescents, but not adults (Morales et al., 2013). Adolescent rats are also less sensitive than adults to the socially suppressing effects of higher doses of ifenprodil (Morales & Spear, 2014) as well as ifenprodil-induced CTA (Dannenhoffer & Spear, 2019) – adolescent insensitivities analogous to those previously reported with ethanol (Anderson et al., 2010; Saalfield & Spear, 2016; Vetter-O’Hagen et al., 2009).
To the extent that retention of adolescent-typical pattern of responsiveness to ethanol following AIE into adulthood (Spear & Swartzwelder, 2014) is associated with preservation of adolescent-characteristic alterations in the glutamate system, one might predict that adults exposed to AIE would also exhibit a pattern of ifenprodil sensitivity more similar to that seen normally during adolescence than in adulthood. This suggestion led to two hypotheses that were tested in the present set of experiments: (1) in adulthood, AIE-exposed animals will be less sensitive to the aversive effects of ifenprodil, but more sensitive to the social facilitation induced by ifenprodil, relative to water-exposed controls; and (2) adolescents as well as AIE-exposed animals tested in adulthood will exhibit alterations in the glutamate/GABA balance. The first hypothesis was addressed in Experiments 1 and 2 by testing adult male and female rats with a history of AIE or water-exposed controls for their responsiveness to the NMDA NR2B receptor antagonist ifenprodil in social interaction (Varlinskaya & Spear, 2002, 2006, 2008) and CTA (Anderson et al., 2010; Saalfield & Spear, 2015, 2016) tests. The second hypothesis was assessed in Experiment 3 by examining baseline levels of protein expression of the vesicular transporters for glutamate (vGlut2) and GABA (vGAT) in the prelimbic cortex (PrL) and NAc of drug-naïve adolescents and adults as well as in adult animals that were exposed to ethanol or water during adolescence. These vesicular transporters were used as an index of presynaptic excitatory/inhibitory balance (De Gois et al., 2005).
Methods
Subjects
Male and female Sprague Dawley rats born and reared in our colony at Binghamton University were housed in a temperature-controlled (22°C) vivarium maintained on a 12-/12-hr light/dark cycle with lights on at 0700h and ad libitum access to water and food (Purina Rat Chow, Lowell, MA). On P1, litters were culled to 8–10 pups. Animals were weaned on P21 and pair-housed with same-sex non-littermates. All animal handling and maintenance followed the guidelines for animal care established by the National Institutes of Health, using Binghamton University’s IACUC approved protocols.
Experimental Design
Designs for Experiment 1 (social interaction) and Experiment 2 (CTA) were 2 sex (male, female) × 2 adolescent exposure (water, ethanol) × 5 ifenprodil dose (0, 0.75, 1.5, 3.0 and 6.0 mg/kg) factorial, with 8–10 animals tested within each of the 20 groups specified by this factorial design in each experiment and an equal number of non-manipulated animals included as social test partners in Experiment 1. Brains from animals injected with vehicle in Experiment 1 were used in Experiment 3 (see below). Assignment to exposure and drug dose was semi-random, with each housing pair assigned to the same exposure and drug condition, and no more than one animal per sex per litter placed in any given adolescent exposure/drug group (Holson & Pearce, 1992; Zorrilla, 1997).
Adolescent Intermittent Ethanol (AIE) Exposure
Males and females were exposed intragastrically (i.g.) to 4 g/kg of 25% ethanol solution in tap water (v/v) every other day during adolescence (P25–45 for a total of 11 exposures). Control animals were given an isovolumetric amount of tap water i.g. on these exposure days.
Drug
The non-competitive NMDA receptor antagonist ifenprodil hemitartrate ((1R*,2S*)-erythro-2-(4-Benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol hemi-(DL)-tartrate) was purchased from Tocris Bioscience. Ifenprodil was dissolved in double-distilled water (ddH2O) and injected intraperitoneally (i.p.) at a volume of 2 ml/kg body weight. Control animals were given an equivalent volume of ddH2O vehicle.
Social Interaction Test
Social interaction chambers were designed from Plexiglas (Binghamton Plate Glass, Binghamton, NY), with each consisting of two compartments (45 cm x 30 cm x 20 cm) and an aperture (9 cm x 7 cm) in the wall dividing the two sides to allow crosses from one side to the other. All testing were conducted under dim light (15–20 lux) between 12:00 and 15:00 hr. on a single test day, which occurred between P70–73. Each animal was injected with the selected dose of ifenprodil and immediately placed alone in the testing apparatus for a 30-min habituation period. A social partner, naïve to both the test apparatus and experimental animal, was then introduced for a 10-min social interaction test. Partners were same-sex, age- and weight-matched non-littermates. A marker was used to make a line on the back of the experimental animal to differentiate it from the partner during testing. Social behaviors scored during the 10-min videotaped sessions included (see Varlinskaya & Spear, 2002, 2006, 2008) social investigation, social contact, and play fighting. Social investigation was scored as the frequency with which the experimental animal sniffed the partner. Social contact was scored as the frequency of crawling over or under the partner as well as grooming the partner. Play fighting was scored as the frequency with which the experimental animal pounced or chased the partner, attacked the partner’s nape, or pinned the partner. Social behaviors were summed to get an overall social activity measure that was used in our previous studies for the assessment of socially facilitating and socially suppressing effects of ifenprodil (Morales & Spear, 2014; Morales et al., 2013). Total number of crosses from one side of the chamber during the 10-min test session was determined for each experimental animal and used as an index of general ambulation under social test circumstances. Overall social activity and number of crosses were transformed to percent change from vehicle control within each adolescent exposure/sex condition in order to eliminate baseline differences associated with sex and/or AIE.
Conditioned Taste Aversion
A palatable tastant (“supersac”: 3% sucrose and 0.125% saccharin in water; e.g., Dannenhoffer & Spear, 2016) was used as the conditioned stimulus (CS) in the CTA paradigm. Conditioning and testing were conducted in the animals’ home cage using a wire mesh divider to separate the cage mates, thereby allowing assessment of individual intakes while avoiding the stress of social isolation (Hall, 1998).
Animals were 50% water restricted prior to both conditioning (P70) and test days (P72) in order to encourage sufficient consumption of the tastant (Anderson et al., 2010; Dannenhoffer & Spear, 2016). Water restriction was accomplished by measuring water intake of each pair of cage mates for 24 hrs (P68–69), and then providing 50% of that intake over the next 24 hours (P69–70), with conditioning occurring immediately thereafter. Animals were then given 24 hrs of free access to water (P71), followed by another 50% water deprivation period prior to testing (P72).
On both conditioning and test days, animals were weighed and placed back into their home cages with the divider in place. Fifteen minutes thereafter, animals were given a 100 ml bottle filled with supersac for a 30-min drinking session. On conditioning day (P70), following the 30-min supersac exposure, bottles were removed, animals were injected with an assigned dose of ifenprodil, and were returned to their divided home cage for 15 more minutes before the divider was removed. Consumption on both conditioning and test day were measured in mls. In order to ensure sufficient consumption of the tastant to assess the development of a CTA, animals that did not consume at least 0.5 ml (n=10) on conditioning day were removed from the study.
Behavioral Data Analysis
Overall social activity and total number of crosses indexed via percent change relative to vehicle were assessed via separate 2 (adolescent exposure: ethanol, water) × 2 (sex) × 5 (ifenprodil dose) ANOVAs. In the CTA study, intake in ml on conditioning day was analyzed using a 2 (adolescent exposure: ethanol, water) × 2 (sex) × 5 (ifenprodil dose) ANOVA to determine if there were differences in pre-conditioning intake of the tastant across the groups. Test day data were transformed into % change relative to vehicle-injected control animals within each sex and exposure group and analyzed using a 2 (adolescent exposure: ethanol, water) × 2 (sex) × 5 (ifenprodil dose) ANOVA. Fisher’s post hoc analyses were used to determine the locus of significant main effects and interactions.
Western blotting
The brains for Experiment 3.1 were collected from animals tested in a previous study investigating the effect of NBQX and THIP on social behavior in adolescent (P35) and adult (P70) rats; however, the tissue collected were derived only from vehicle controls (Dannenhoffer et al., 2018b). The brains collected for Experiment 3.2 were derived from the animals of Experiment 1 that received vehicle at testing. Two days after testing, animals were removed from the home cage and rapidly decapitated; this post-test interval allowed us to avoid the possibility of the transporter data being influenced by acute manipulations associated with social behavioral testing. Brains were flash frozen in isopentane and stored at −80°C until analysis. The PrL of the PFC and the NAc (core and shell) were collected via tissue punches that were 1.2 mm wide × 1.0 mm deep and 2.0 mm wide × 1.0 mm deep, respectively (see Figure 1 for localization of tissue collection). The design for Experiment 3.1 was a 2 sex (male; female) × 2 age (adolescent; adult) factorial. The design for Experiment 3.2 was a 2 sex (male; female) × 2 adolescent exposure (water; alcohol) factorial.
Figure 1:
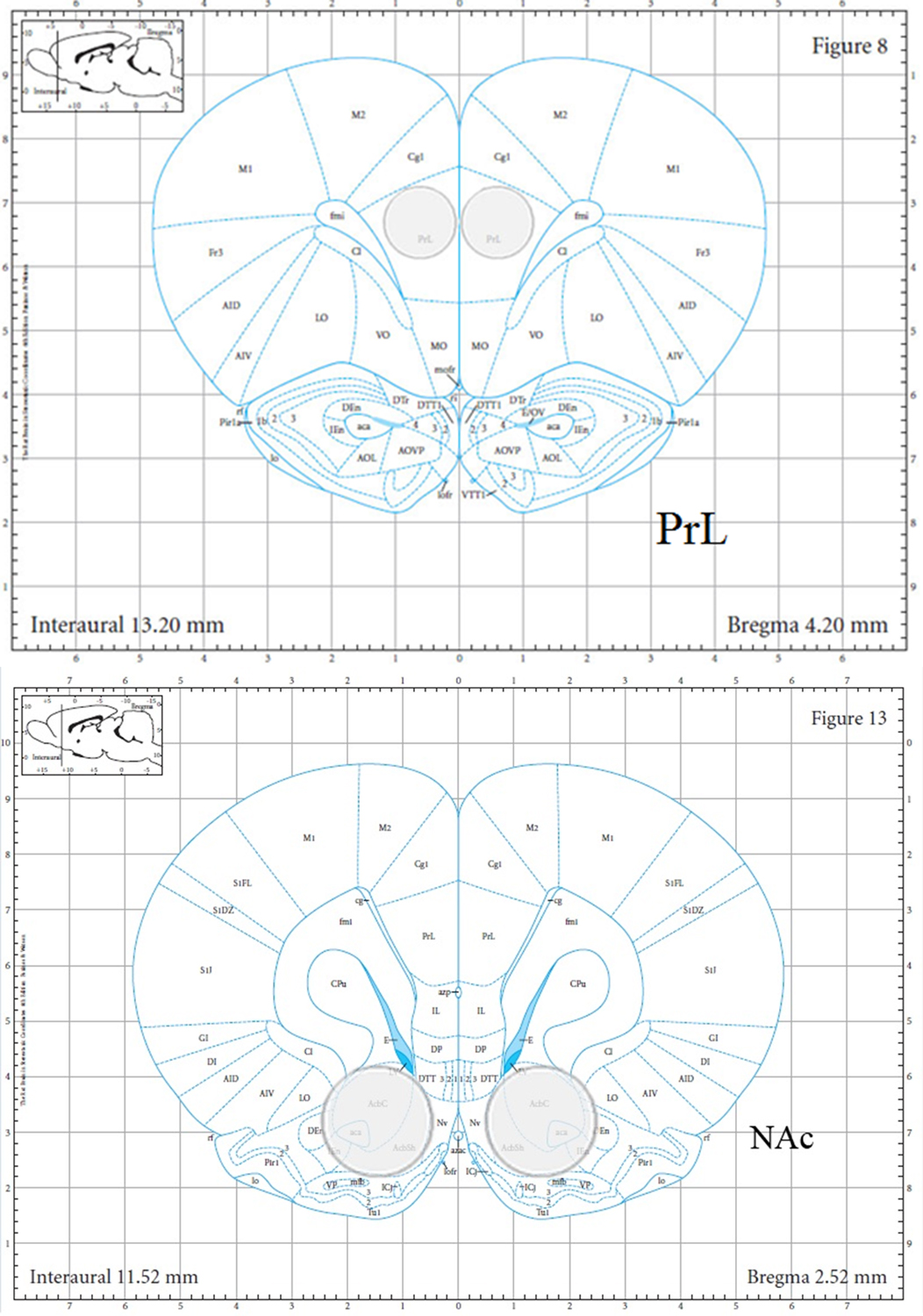
Tissue collection locations. Tissue punches were used to collect samples. Prelimbic sections were 1.2 mm wide and 1.0 mm deep. NAc punches were 2.0 mm wide and 1.0 mm deep.
Sample preparation:
Western blotting on whole cell homogenates was processed as we have done previously (e.g., see Santerre et al., 2014). Whole cell protein concentrations were determined using the BCA protein assay (Pierce, USA). Electrophoresis was conducted on whole cell homogenates in Novex Tris-Glycine gels (8–16%) sodium-dodecyl-sulfate polyacrylamide gels, then samples were transferred from the gels to polyvinylidene difluoride membranes (Invitrogen Life Technologies, Carlsbad, CA), and placed on a rocker for 24 hours with 1% BSA blocking buffer. Antibodies directed against vGlut2 (AB2251; Millipore) and vGAT (AB5062P; Millipore) were both applied at 1:1000 dilutions. Following appropriate secondary antibody application, blots were exposed to enhanced chemiluminescence (GE Healthcare, Piscataway, NJ), and exposed to X-ray film. Blots were then exposed to the housekeeper, β-actin antibody (1:10000 dilutions; EMD Millipore, Billerica, MA) for verification that proteins were loaded and transferred equally. All blots were quantified using ImageJ (NIH), by calculating optical densities of the bands following calibration.
Western blot Data Analysis
Optical densities were analyzed as a percent change from adult males (Experiment 3.1 %Control) and water-exposed males (Experiment 3.2 %Control). The ratio of vesicular transporter to the housekeeper was calculated as (vGlut2 OD/actin OD) or (vGAT OD/actin OD), and then each sample was calculated as %Control = ((sample/average of Control group) × 100). vGlut2 to vGAT ratios were calculated in a similar fashion (vGlut2/vGAT sample/average vGlut2 to vGAT control group) as has been done elsewhere (Fung et al., 2011; Marcello et al., 2013; Navarro et al., 2019). Both sets of experiments were then analyzed as factorial ANOVAs (Experiment 3.1: 2 age × 2 sex; Experiment 3.2: 2 sex × 2 exposure) using Fisher’s post hoc analyses to assess group differences. As initial analyses revealed no sex differences, male and female data were collapsed, and new %Control values were calculated (Experiment 3.1: all adults; Experiment 3.2: all water-exposed adults). Unpaired t-tests were used to assess differences between adolescents and adults (3.1) and water- versus alcohol-exposed adults (3.2).
Results
Experiment 1: Ifenprodil and social interaction in adulthood following AIE
AIE did not change sensitivity to the social effects of ifenprodil in adulthood. Overall social activity was affected by ifenprodil regardless of AIE and sex, as evidenced by a significant main effect of ifenprodil dose, F (4, 167) = 3.377, p < 0.05, with the doses of 3.0 (p = 0.02) and 6.0 mg/kg (p = 0.001) significantly suppressing overall social activity (Table 1). The dose of 0.75 mg/kg that has been shown to produce social facilitation in adolescent rats (Morales et al., 2013) was ineffective in AIE animals. Total crosses used as an index of locomotor activity under social test circumstances also differed only as a function of ifenprodil dose, F (4, 167) = 3.289, p < 0.05, with the highest dose of 6.0 mg/kg (p = 0.001) significantly suppressing this measure (Table 2).
Table 1:
Effects of Ifenprodil on Overall Social Activity. Overall social activity did not differ across doses in any of the four exposure × sex groups; however, when collapsed across both variables, overall activity was significantly lower following ifenprodil doses 3.0 and 6.0 mg/kg. Data are presented as a % change from controls.
| Ifenprodil Dose (mg/kg) | Overall Social Activity (% change versus 0 mg/kg dose) | ||||
|---|---|---|---|---|---|
| Male | Female | Collapsed across exposure, sex | |||
| Water | Ethanol | Water | Ethanol | ||
| 0 | 100.0 ± 17.1 | 100.0 ± 14.5 | 100.0 ± 19.3 | 100.0 ± 11.8 | 100.0 ± 7.7 |
| 0.75 | 92.6 ± 7.5 | 86.5 ± 7.3 | 108.9 ± 9.7 | 77.2 ± 13.1 | 91.8 ± 5.0 |
| 1.5 | 80.5 ± 10.5 | 91.5 ± 11.4 | 109.3 ± 10.0 | 86.0 ± 10.1 | 92.0 ± 5.3 |
| 3.0 | 63.3 ± 8.5 | 85.6 ± 10.8 | 86.3 ± 12.9 | 81.5 ± 13.5 | 79.2 ± 5.8 * |
| 6.0 | 62.5 ± 13.0 | 75.1 ± 18.5 | 72.9 ± 12.4 | 66.9 ± 20.6 | 69.2 ± 7.9 * |
Data are expressed as mean ± SEM
denotes significant difference from vehicle-injected controls (0 mg/kg dose), p < 0.05.
Table 2:
Total Test Crosses assessed as a % change from controls. Test chamber crosses did not differ across doses in any of the exposure × sex groups; however, the highest dose of ifenprodil (6.0 mg/kg) significantly reduced test crosses when collapsed across both sex and exposure. Data are presented as a % change from controls.
| Ifenprodil Dose (mg/kg) | Total Test Crosses (% change versus 0 mg/kg dose) | ||||
|---|---|---|---|---|---|
| Male | Female | Collapsed across exposure, sex | |||
| Water | Ethanol | Water | Ethanol | ||
| 0 | 100.0 ± 17.0 | 100.0 ± 11.8 | 100.0 ± 16.3 | 100.0 ± 10.8 | 100.0 ± 6.9 |
| 0.75 | 115.5 ± 15.6 | 65.7 ± 7.7 | 93.5 ± 15.8 | 90.5 ± 16.6 | 92.0 ± 7.6 |
| 1.5 | 93.2 ± 15.9 | 69.4 ± 8.5 | 86.4 ± 8.5 | 110.5 ± 12.7 | 89.9 ± 6.2 |
| 3.0 | 66.7 ± 8.0 | 71.4 ± 8.7 | 94.9 ± 19.5 | 97.4 ± 16.3 | 83.0 ± 7.2 |
| 6.0 | 76.3 ± 12.8 | 59.4 ± 13.0 | 72.0 ± 12.1 | 61.3 ± 17.8 | 67.5 ± 6.9 * |
Data are expressed as mean ± SEM
denotes significant difference from vehicle-injected controls (0 mg/kg dose), p < 0.05.
Experiment 2: Ifenprodil-induced CTA in adulthood following AIE
Intake of “supersac” on conditioning day differed as a function of sex, F (1, 179) = 60.115, p < 0.0001, and adolescent exposure, F (1, 179) = 11.596, p < 0.001. Females ingested significantly less “supersac” solution than males, whereas ethanol-exposed animals of both sexes ingested less “supersac” than their water-exposed counterparts (Figure 2a). In contrast, responsiveness to the aversive effects of ifenprodil was not affected by AIE. The ANOVA revealed a significant ifenprodil dose by sex interaction, F (4, 179) = 2.957, p < 0.05. Adult males demonstrated insensitivity to the aversive effects of ifenprodil (Figure 2b), whereas females showed significant CTA following the doses of 3.0 (p = 0.04) and 6.0 mg/kg (p = 0.003) when collapsed across adolescent exposure conditions (Figure 2c).
Figure 2:
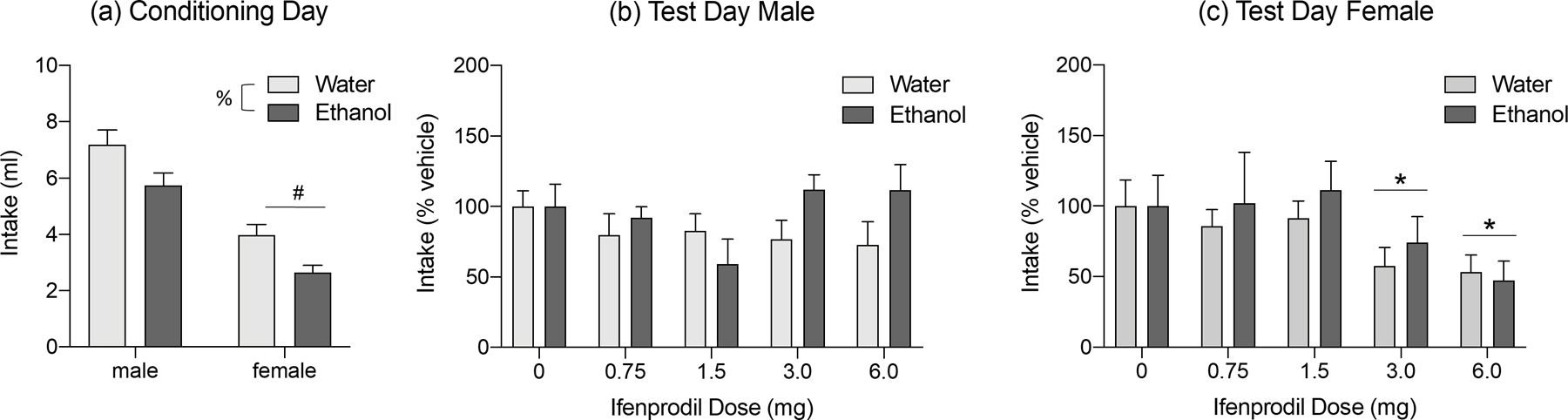
Effects of adolescent intermittent ethanol exposure (AIE) on ‘supersac” intake on conditioning day (a) and ifenprodil-induced conditioned taste aversion (CTA) in male (b) and female (c) rats. On conditioning day, females ingested less “supersac” than males (marked by #, p <0.05, collapsed across adolescent exposure condition) and rats exposed to ethanol during adolescence ingested less than their water-exposed counterparts (marked by %, p <0.05, collapsed across adolescent exposure). No doses of ifenprodil induced a CTA in males, regardless of adolescent exposure. In females, the two highest doses produced a significant aversion (marked by *, p < 0.05) when collapsed across adolescent exposure.
Experiment 3. Expression of vGlut2 and vGAT vesicular transporters in PrL and NAc: Impact of age and AIE.
Age Differences.
Prior analyses revealed no sex differences; therefore data were collapsed across sex prior to conducting unpaired t-tests to further explore differences in these variables and their ratios between adolescents and adults. Within the PrL (Figure 3a), adolescents expressed higher vGlut2 relative to adults [t (27) = 4.515; p < 0.001]; in contrast there was no age difference in vGAT expression (Figure 3b). Analysis of vGlut2/vGAT ratios in the PrL revealed significantly higher ratios in adolescents than adults [t (25) = 3.837; p < 0.01], suggestive of overall greater excitatory tone in this region during adolescence (Figure 3c). Adolescents and adults also differed in their vGlut2 expression in the NAc [t (26) = 7.796; p < 0.001]; however, the pattern of age differences was opposite to that evident in the PrL (Figure 3d), with again no age differences emerging in vGAT expression (Figure 3e). Overall, adolescents showed lower vGlut2/vGAT ratios in the NAc relative to adults [t (24) = 3.161; p < 0.01; Figure 3f].
Figure 3:
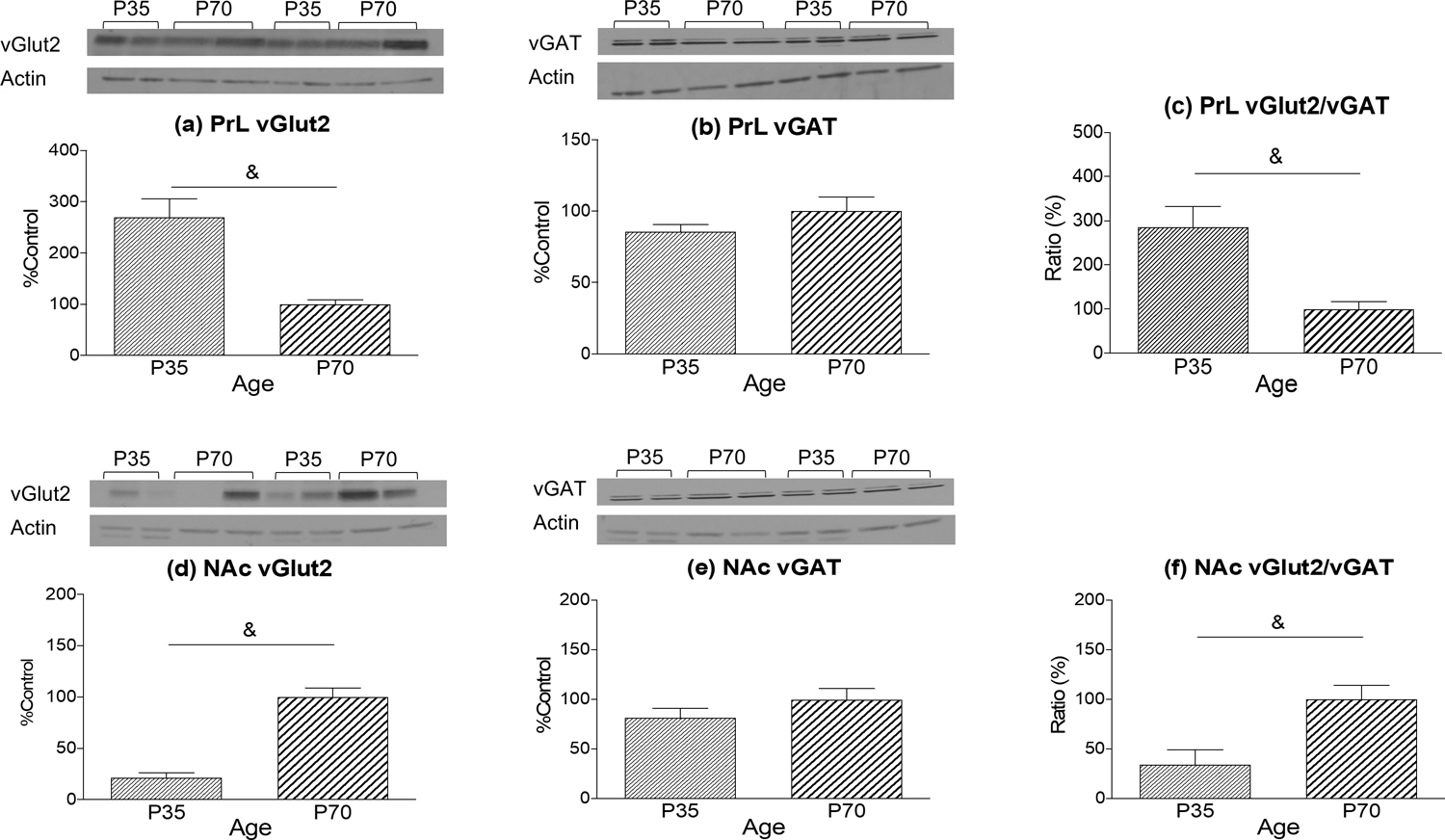
Expression of vGlut2, vGAT, and their ratio in the PrL (a, b, c) and NAc (d, e, f) of adolescent (P35) and adult (P70) rats. Expression of vGlut2 within the PrL (a) was significantly higher in adolescents relative to adults resulting in a greater vGlut2/vGAT ratio (c). Expression of vGlut2 within the NAc was significantly lower in adolescents relative to adults (d) resulting in a smaller vGlut2/vGAT ratio. Representative blots against actin are included. Significant (p < 0.05) age differences are marked with &.
AIE Effects.
Analysis of the AIE data revealed no significant differences in vGlut2 or vGAT expression or ratios in either the PrL or NAc (Figure 4a,c,d,e,f). The only significant difference that emerged was in vGAT expression in PrL (Figure 4b), with vGAT being significantly lower in AIE animals relative to their water-exposed counterparts [t (27) = 2.625; p < 0.05]. This difference, however, did not alter PrL vGlut2/vGAT ratio (Figure 4f).
Figure 4:
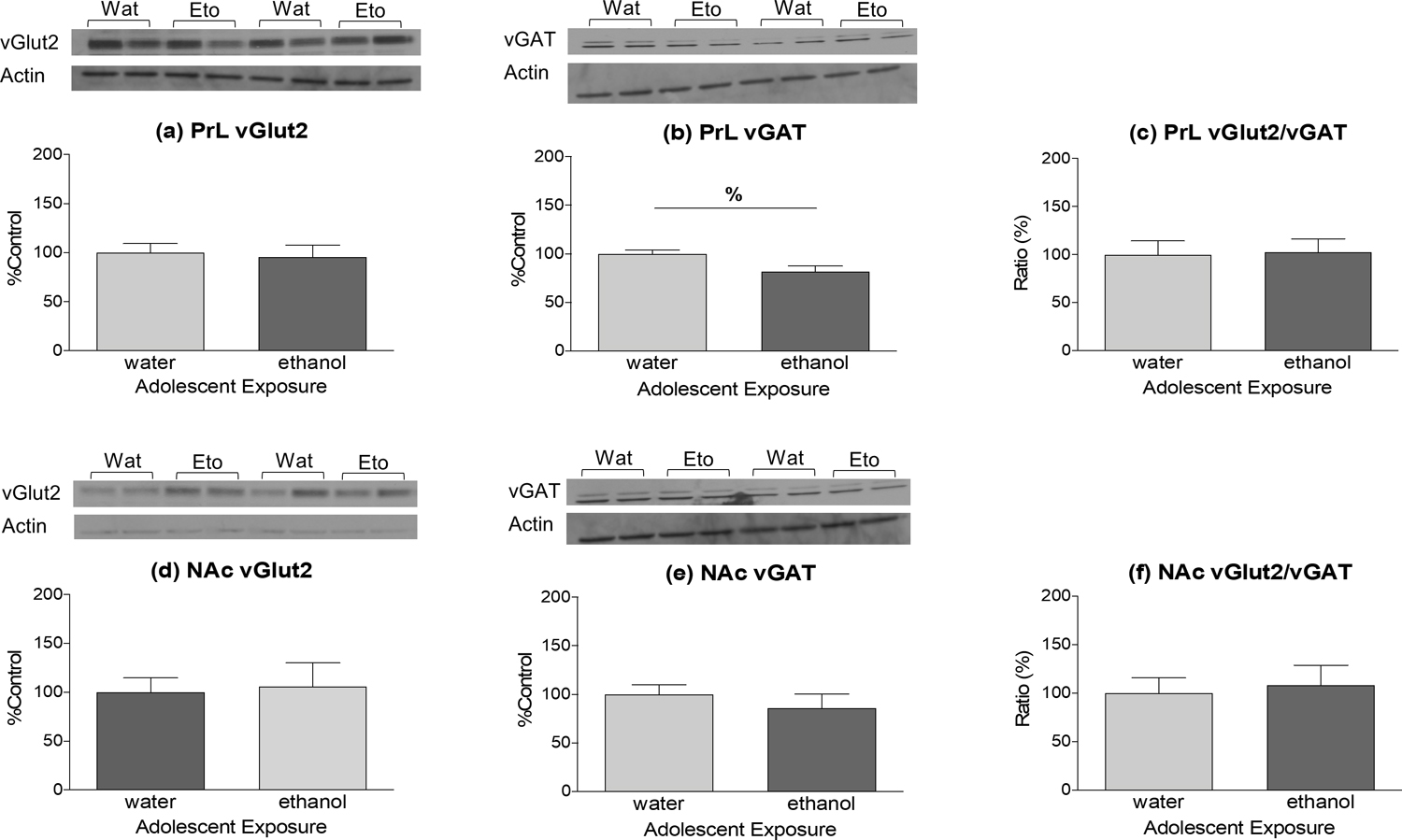
Effects of AIE on expression of vGlut2, vGAT, and their ratio in the PrL (a, b, c) and NAc (d, e, f) of adult rats. AIE produced a significant decrease (marked with %, p < 0.05) in vGAT expression within the PrL (b); however, this change did not influence vGlut2/vGAT ratio (c). No changes in vGlut2, vGAT, or their ratio were evident in the NAc (d, e, f).
Discussion
AIE exposure results in preservation of adolescent-typical sensitivity to social facilitation induced by low doses of ethanol that is not normally evident in adults and adolescent-characteristic attenuated sensitivities to the social inhibitory and aversive effects of higher doses of ethanol (Alaux-Cantin et al., 2013; Diaz-Granados & Graham, 2007; Saalfield & Spear, 2015; Varlinskaya & Spear, 2015). These adolescent-typical propensities for social facilitation and attenuated sensitivity to social inhibitory and aversive effects are evident not only when adolescents are compared with adults following acute challenge with ethanol (Varlinskaya & Spear, 2015), but also when challenged with the NR2B antagonist ifenprodil (Dannenhoffer & Spear, 2019; Morales & Spear, 2014; Morales et al., 2013). The present study tested the hypothesis that, reminiscent of the retention of adolescent-typical responsiveness to ethanol after AIE exposure (Varlinskaya et al., 2014), AIE animals would also exhibit a similar retention of adolescent-typical responsiveness to ifenprodil. The results of the present study did not confirm this hypothesis. Assessment of vGlut2/vGAT ratios in the PrL and NAc revealed age- and region-specific effects; but similar to behavioral responses, adolescent-typical expression patterns were not retained into adulthood after AIE.
Responsiveness to the social effects of ifenprodil was not affected by AIE exposure, with adult-typical social suppression evident at higher doses of ifenprodil. The lowest dose of this selective NR2B antagonist that has been shown to produce social facilitation (significant increases in overall social activity) in adolescent animals (Morales et al., 2013) had no effect on social behavior regardless of adolescent exposure. In contrast, the doses of 3.0 and 6.0 mg/kg ifenprodil produced social suppression in males and females. These findings demonstrate that adolescent-typical ifenprodil-induced social facilitation (Morales et al., 2013) and attenuated sensitivity to social inhibitory effects of ifenprodil were not retained into adulthood following AIE. Although BECs were not assessed at any time during AIE exposure, consistency in BECs across investigators from our lab and the BEC range reported by others (Fernandez et al., 2017; Kim et al., 2019) suggests that possible attenuation of ethanol exposure level is less likely a reason for the negative results. Future studies with ethanol versus ifenprodil challenge in AIE-exposed rats can further mitigate this concern. Irrespective, the lack of adolescent-typical responding to the social effects of ifenprodil (Morales & Spear, 2014; Morales et al., 2013) in AIE-exposed animals suggests that AIE-associated alterations in sensitivity to the social effects of ethanol (Dannenhoffer et al., 2018a; Varlinskaya et al., 2020; Varlinskaya et al., 2014) are modulated through non-NMDA NR2B receptor adaptations. Experiment 2 was designed to test whether AIE-exposed rats would exhibit an attenuated aversion to ifenprodil in adulthood relative to their water-exposed counterparts, reminiscent of the adolescent-typical insensitivity to ifenprodil-induced CTA (Dannenhoffer & Spear, 2019). The results again did not confirm our hypothesis. Insensitivity to ifenprodil in the CTA paradigm evident in adolescent females in our prior work (Dannenhoffer & Spear, 2019) was not retained into adulthood following AIE in females, given that they demonstrated CTA at 6 mg/kg ifenprodil regardless of adolescent exposure condition (Dannenhoffer & Spear, 2019). Among males, CTA to ifenprodil was not evident in either ethanol- or water-exposed animals at any ifenprodil dose. These effects were unexpected, given that ifenprodil has previously been found to be aversive in experimentally naïve adult males (Dannenhoffer & Spear, 2019). The resistance to ifenprodil’s aversive effects seen in AIE and water-exposed control males suggests some alterations in the NMDA receptor system associated with experimental manipulations during adolescence (i.e. gavage procedure) rather than exposure to ethanol; however, an inclusion of non-gavaged control group can address this point in future studies.
Although not demonstrating any alterations in sensitivity to the aversive effects of the selective NR2B antagonist, AIE-exposed males and females ingested significantly less “supersac” than their water-exposed counterparts. Suppressed intake of sweet solutions has been frequently used as an index of anhedonia (Anisman & Matheson, 2005; Henningsen et al., 2009; Moreau et al., 1995; Willner, 1997) – one of the core symptoms of depression (Heshmati & Russo, 2015). This finding clearly demonstrating that males and females were affected by AIE was not surprising, since adolescent ethanol exposure is associated with affective alterations later in adulthood (Crews et al., 2019). Our previous study has also shown that exposure to a stressor suppressed “supersac” intake following AIE exposure, with no such stress effect evident in water-exposed controls (Varlinskaya et al., 2017).
Ethanol is well-known antagonist of the glutamatergic system. Indeed, human alcoholics display similar subjective responses to the effects of ethanol and the NMDA receptor antagonist ketamine (Krystal et al., 1998). Chronic ethanol exposure is likely to upregulate particular NMDA receptors following the cessation of ethanol consumption as a compensatory response (Dodd et al., 2000; Krystal et al., 1998; Valenzuela, 1997). Studies have also shown that ethanol-dependent patients exhibit reduced sensitivity to the aversive effects of the NMDA receptor antagonist, ketamine, suggesting a critical role for NMDA receptors in the development of alcohol dependence (Krystal et al., 2003). Chronic ethanol exposure, through NMDA receptors, has also been suggested to contribute to the retention of adolescent-like synaptic plasticity after ethanol exposure (Kroener et al., 2012). More research is needed to understand the similarities and differences in adolescent-typical and AIE-induced sensitivities to ethanol’s aversive effects through glutamatergic modulation.
In Experiment 3, we first assessed age differences in vGlut2 and vGAT expression within the PrL and NAc (Experiment 3.1). As anticipated, adolescents showed greater vGlut2 levels than adults and suggestive greater excitation (indexed via enhanced vGlut2/vGAT ratios) within the PrL. Conversely, in the NAc, an opposite ontogenetic pattern was observed, with lower vGlut2/vGAT ratios in adolescents than adults being primarily driven by lower vGlut2 levels. Surprisingly, vGAT expression did not differ in either region. To determine if AIE maintained these adolescent-typical patterns into adulthood (Spear & Swartzwelder, 2014), we assessed the same measures in adult animals following AIE and in water controls (Experiment 3.2). Null effects were obtained, with no changes in vGlut2 or the ratio in either brain region in adult animals exposed to AIE. A slight but significant decrease was noted in the PrL for vGAT expression that partially supports a shift towards enhanced excitability, but overall differences were negligible with regard to vGlut2/vGAT ratios. Overall, these findings suggest that AIE does not retain adolescent-typical neuronal excitability into adulthood as measured by vesicular transporters; but other targets such as those related to intrinsic excitability remain to be examined. More direct approaches to assessing excitatory/inhibitory balance are needed in that vesicular transporters of glutamate and GABA are just one indirect measure of presynaptic vesicular neurotransmitter loading.
Across human and rodent studies, alcohol dependence results in a shift in excitatory/inhibitory balance (Kroener et al., 2012; Michalak & Biała, 2016; Valenzuela, 1997). One possibility for the lack of shifts in ratios following AIE could be that our AIE regimen does not produce physical dependent measures to alcohol at the time vesicular transporters were assessed. These animal models of alcohol dependence typically use a vapor model that exposes the animal to ethanol vapor for 16 hours/day through several rounds of exposure and withdrawal phases (Kroener et al., 2012) resulting in changes in synaptic plasticity and overall glutamatergic action within cortical areas required for executive functions. Moreover, animal studies have shown changes in alcohol-dependence during withdrawal via hyperexcitation and hypoinhibition (Crews et al., 1996; Grobin et al., 1998) which contribute to anxiety and tolerance. Thus, it is possible that while AIE produces intoxication levels in the brain along with transient withdrawal, the lack of persistent changes in excitatory/inhibitory balance into adulthood may be due to our sub-chronic AIE paradigm or length in time following the last exposure.
It has been hypothesized that enhanced functional activity of the glutamate system versus the GABA system declines across ontogeny as adulthood approaches, contributing to the closing the window of adolescent plasticity, particularly in prefrontal areas (Crews et al., 2007), with GABA inhibition playing a particularly critical role in curbing this plasticity (Hensch, 2005). Much of the support for this hypothesis stems from assessment of postsynaptic receptor expression and function. However, the results of the present study demonstrated that age differences in vGlut2 expression and vGlut2/vGAT ratios are region-specific, suggesting that ontogenetic shifts may be related to presynaptic glutamate availability, but more functional assessments are needed to confer this functional contribution. Irrespective, projections from cortical areas to the NAc that are characterized as glutamatergic and may regulate various forms of ethanol-related behaviors (Scofield et al., 2016), and there is some evidence to suggest that opposite patterns of glutamatergic signaling in the PrL and NAc may be involved in reward responses; although it is important to point out that the NAc has incoming conduits from multiple brain regions. Glutamatergic-related plasticity in reward circuitry involving the PFC and NAc are regulated in part by brain-derived neurotrophic factor (BDNF; Cooper et al., 2017). Increased BDNF in the NAc stimulates cocaine-seeking while in the PFC attenuates intake of the same substance (McGinty et al., 2010). Although it remains to be determined whether similar findings would be obtained with ethanol-seeking behavior, when taken together with present findings, it is speculative that regional differences in BDNF within this pathway may drive ontogenetic and related behavioral phenotypes, especially as BDNF expression is epigenetically regulated following AIE (Sakharkar et al., 2016).
Overall, the findings from the present studies do not support (1) selective alterations in NR2B sensitivity as a contributor to AIE-induced retention of adolescent-typical responsiveness to the socially facilitating, socially inhibitory or aversive effects of acute ethanol challenge; or (2) shifts in glutamate/GABA ratio seen in adolescents relative to adults is not retained into adulthood following AIE. Thus, it is likely that other systems besides NMDA NR2B receptors are involved in AIE-associated retention of adolescent-typical sensitivities to ethanol into adulthood.
Acknowledgments
The work presented in this manuscript was funded by NIH grant U01 AA019972 (NADIA Project) and T32 Training Grant 1T32AA025606-01 at Binghamton University.
Footnotes
The authors have no conflicts of interest to disclose.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, & Naassila M (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology, 67, 521–531. [DOI] [PubMed] [Google Scholar]
- Allgaier C (2002). Ethanol sensitivity of NMDA receptors. Neurochemistry international, 41(6), 377–382. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, & Spear LP (2010). Ethanol-induced conditioned taste aversion in male sprague-dawley rats: Impact of age and stress. Alcoholism: Clinical and Experimental Research, 34(12), 2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, & Matheson K (2005). Stress, depression, and anhedonia: caveats concerning animal models. Neuroscience & Biobehavioral Reviews, 29(4–5), 525–546. [DOI] [PubMed] [Google Scholar]
- Caballero A, Thomases DR, Flores-Barrera E, Cass DK, & Tseng KY (2014). Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology, 231(8), 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Robison A, & Mazei-Robison MS (2017). Reward circuitry in addiction. Neurotherapeutics, 14(3), 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, & Hodge C (2007). Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior, 86(2), 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Morrow AL, Criswell H, & Breese G (1996). Effects of ethanol on ion channels. In International review of neurobiology (Vol. 39, pp. 283–367): Elsevier. [DOI] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, … Vetreno RP (2019). Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcoholism: Clinical and Experimental Research, 43(9), 1806–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer CA, Kim EU, Saalfield J, Werner DF, Varlinskaya EI, & Spear LP (2018a). Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology, 235(10), 3065–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer CA, & Spear LP (2016). Age differences in conditioned place preferences and taste aversions to nicotine. Developmental Psychobiology, 58(5), 660–666. [DOI] [PubMed] [Google Scholar]
- Dannenhoffer CA, & Spear LP (2019). Excitatory/inhibitory balance across ontogeny contributes to age-specific behavioral outcomes of ethanol-like challenge in conditioned taste aversion. Developmental Psychobiology, 61(8), 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer CA, Varlinskaya EI, & Spear LP (2018b). Effects of AMPA receptor antagonist, NBQX, and extrasynaptic GABAA agonist, THIP, on social behavior of adolescent and adult rats. Physiology & behavior, 194, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gois S, Schäfer MK-H, Defamie N, Chen C, Ricci A, Weihe E, … Erickson JD (2005). Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. Journal of Neuroscience, 25(31), 7121–7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Granados JL, & Graham DL (2007). The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcoholism: Clinical and Experimental Research, 31(12), 2020–2027. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, & Wilce PA (2000). Glutamate-mediated transmission, alcohol, and alcoholism. Neurochemistry international, 37(5–6), 509–533. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, & Spear LP (2005). Factors Influencing Elevated Ethanol Consumption in Adolescent Relative to Adult Rats. Alcoholism: Clinical & Experimental Research, 29(10), 1796–1808. 10.1097/01.alc.0000183007.65998.aa [DOI] [PubMed] [Google Scholar]
- Enoch M-A (2008). The role of GABAA receptors in the development of alcoholism. Pharmacology Biochemistry and Behavior, 90(1), 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GM, Lew BJ, Vedder LC, & Savage LM (2017). Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience, 348, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, & Swartzwelder HS (2007). Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. Journal of neurophysiology, 97(5), 3806–3811. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, & Weicker CS (2011). Expression of VGluT1 and VGAT mRNAs in human dorsolateral prefrontal cortex during development and in schizophrenia. Brain research, 1388, 22–31. [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, … Chandler LJ (2014). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology, 39(11), 2570–2583. 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC III, Haun HL, Hazelbaker CL, Ramachandra VS, & Becker HC (2014). Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology, 39(3), 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, & Morrow AL (1998). The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology, 139(1–2), 2–19. [DOI] [PubMed] [Google Scholar]
- Guerri C, & Pascual M (2010). Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol, 44(1), 15–26. [DOI] [PubMed] [Google Scholar]
- Hall FS (1998). Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Critical Reviews™ in Neurobiology, 12(1–2). [DOI] [PubMed] [Google Scholar]
- Henningsen K, Andreasen JT, Bouzinova EV, Jayatissa MN, Jensen MS, Redrobe JP, & Wiborg O (2009). Cognitive deficits in the rat chronic mild stress model for depression: relation to anhedonic-like responses. Behavioural Brain Research, 198(1), 136–141. [DOI] [PubMed] [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience, 6(11), 877–888. [DOI] [PubMed] [Google Scholar]
- Heshmati M, & Russo SJ (2015). Anhedonia and the brain reward circuitry in depression. Current behavioral neuroscience reports, 2(3), 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson R, & Pearce B (1992). Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and teratology, 14(3), 221–228. [DOI] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2019). Monitoring the Future National Survey Results on Drug Use, 1975–2018: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research. [Google Scholar]
- Kim EU, Varlinskaya EI, Dannenhoffer CA, & Spear LP (2019). Adolescent intermittent ethanol exposure: Effects on pubertal development, novelty seeking, and social interaction in adulthood. Alcohol, 75, 19–29. [DOI] [PubMed] [Google Scholar]
- Koob GF (2004). A role for GABA mechanisms in the motivational effects of alcohol. Biochemical pharmacology, 68(8), 1515–1525. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, & Chandler LJ (2012). Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One, 7(5), e37541. 10.1371/journal.pone.0037541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D’Souza DC, … Charney DS (2003). Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology, 28(11), 2020–2028. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, … Charney DS (1998). Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Archives of general psychiatry, 55(4), 354–360. [DOI] [PubMed] [Google Scholar]
- Liu W, & Crews F (2015). Adolescent intermittent ethanol exposure enhances ethanol activation of the nucleus accumbens while blunting the prefrontal cortex responses in adult rat. Neuroscience, 293, 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, & Weight FF (1989). Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science, 243(4899), 1721–1724. [DOI] [PubMed] [Google Scholar]
- Marcello L, Cavaliere C, Colangelo A, Bianco M, Cirillo G, Alberghina L, & Papa M (2013). Remodelling of supraspinal neuroglial network in neuropathic pain is featured by a reactive gliosis of the nociceptive amygdala. European Journal of Pain, 17(6), 799–810. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW Jr, & Berglind WJ (2010). Brain-derived neurotrophic factor and cocaine addiction. Brain research, 1314, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak A, & Biała G (2016). Alcohol dependence--neurobiology and treatment. Acta poloniae pharmaceutica, 73(1), 3–12. [PubMed] [Google Scholar]
- Morales M, & Spear LP (2014). The effects of an acute challenge with the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, on social inhibition in adolescent and adult male rats. Psychopharmacology, 231(8), 1797–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, & Spear LP (2013). Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behavioural Brain Research, 250, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau J. a., Scherschlicht R, Jenck F, & Martin J (1995). Chronic mild stress-induced anhedonia model of depression: sleep abnormalities and curative effects of electroshock treatment. Behavioural pharmacology. [PubMed]
- Möykkynen T, Korpi ER, & Lovinger DM (2003). Ethanol inhibits α-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. Journal of Pharmacology and Experimental Therapeutics, 306(2), 546–555. [DOI] [PubMed] [Google Scholar]
- Navarro DV, Alvarado M, Figueroa A, Salas-Lucia F, Pacheco P, Gonzalez-Liencres C, … Berbel P (2019). Distribution of GABAergic Neurons and VGluT1 and VGAT Immunoreactive Boutons in the Ferret (Mustela putorius) Piriform Cortex and Endopiriform Nucleus. Comparison With Visual Areas 17, 18 and 19. Frontiers in Neuroanatomy, 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, & McClain JA (2010). Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Current opinion in psychiatry, 23(3), 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, & Guerri C (2009). Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of neurochemistry, 108(4), 920–931. [DOI] [PubMed] [Google Scholar]
- Saalfield J, & Spear L (2015). Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Developmental cognitive neuroscience, 16, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfield J, & Spear L (2016). The ontogeny of ethanol aversion. Physiology & behavior, 156, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, & Pandey SC (2016). A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Structure and Function, 221(9), 4691–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre JL, Gigante ED, Landin JD, & Werner DF (2014). Molecular and behavioral characterization of adolescent protein kinase C following high dose ethanol exposure. Psychopharmacology, 231(8), 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield M, Heinsbroek J, Gipson C, Kupchik Y, Spencer S, Smith A, … Kalivas P (2016). The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacological reviews, 68(3), 816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD (2013). A role for synaptic plasticity in the adolescent development of executive function. Translational psychiatry, 3(3), e238–e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews Neuroscience, 19(4), 197. [DOI] [PubMed] [Google Scholar]
- Spear LP, & Swartzwelder HS (2014). Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev, 45, 1–8. 10.1016/j.neubiorev.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak PD, Crawley J, Schwartz R, Skolnick P, & Paul S (1986). A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science, 234(4781), 1243–1247. [DOI] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, & Tseng KY (2013). Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. Journal of Neuroscience, 33(1), 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF (1997). Alcohol and neurotransmitter interactions. Alcohol health and research world, 21(2), 144. [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Hosová D, Towner T, Werner DF, & Spear LP (2020). Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behavioural Brain Research, 378, 112292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Kim EU, & Spear LP (2017). Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain research, 1654, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2002). Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research, 26(10), 1502–1511. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2006). Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 48(2), 146–161. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2008). Social interactions in adolescent and adult Sprague–Dawley rats: impact of social deprivation and test context familiarity. Behavioural Brain Research, 188(2), 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2015). Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiology & behavior, 148, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, & Spear LP (2014). Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol, 48(5), 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, & Spear L (2009). Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol & Alcoholism, 44(6), 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, & Spear LP (2007). Time Course of Elevated Ethanol Intake in Adolescent Relative to Adult Rats Under Continuous, Voluntary-Access Conditions. Alcoholism: Clinical and Experimental Research, 31(7), 1159–1168. 10.1111/j.1530-0277.2007.00417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzl H, D’Adamo P, & Lipp H-P (2001). Conditioned taste aversion as a learning and memory paradigm. Behavioural Brain Research, 125(1–2), 205–213. [DOI] [PubMed] [Google Scholar]
- Willner P (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology, 134(4), 319–329. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, & Illes P (2000). Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn-Schmiedeberg’s archives of pharmacology, 362(6). [DOI] [PubMed] [Google Scholar]
- Witt ED (2010). Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol, 44(1), 119–124. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP (1997). Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 30(2), 141–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


