Graphic abstract
Hybrid systems of nanoparticles and polymers have emerged as a new material in the biomedical field. To date, various kinds of hybrid systems have been introduced and applied to drug delivery, regenerative medicine, therapeutics, disease diagnosis, and medical implantation. Among them, the hybridization of nanostructured porous silicon nanoparticles (pSiNPs) and biocompatible polymers has been highlighted due to its unique biological and physicochemical properties. This review focuses on the recent advances in the hybrid systems of pSiNPs and biocompatible polymers from an engineering aspect and its biomedical applications. Representative hybrid formulations, (i) Polymer-coated pSiNPs, (ii) pSiNPs-embedded polymeric nanofibers, are outlined along with their preparation methods, biomedical applications, and future perspectives. We believe this review provides insight into a new hybrid system of pSiNPs and biocompatible polymers as a promising nano-platform for further biomedical applications.
Recently developed and representative hybrid systems of porous silicon nanoparticles and biocompatible polymers and their biomedical applications are introduced.
Keywords: Porous silicon, Organic–inorganic hybrid, Controlled release, Polymer scaffold, Nanofiber
Introduction
Hybrid systems of nanoparticles and polymers aim to develop new materials with more advanced features. The hybrid system maximizes the synergistic effect of each material’s unique features, enabling the design of fine-tuned nanomaterials by changing their chemical, optical, and even electrical properties. The merits of these hybrid systems include (i) overcoming the limitations of each system, (ii) being simply designable into a diversified composite, and (iii) being comprehensively applicable into the fields of lab-on chips, biological/chemical sensors, drug delivery, and therapeutic agents, by taking advantage of their multi-functionality [1, 2].
To date, various kinds of hybrid systems have been developed and utilized. Among them, the hybridization of nanostructured porous silicon nanoparticles (pSiNPs) and biocompatible polymers has been highlighted due to its unique biological and physicochemical properties such as (i) diminish the burst release of a loaded substance (drug, protein, peptide, etc.) by encapsulating porous structure of nanoparticles, (ii) enhance the biocompatibility of nano-formulation by the polymer coating, (iii) double-shield protection of loaded substance from undesired environments, (iv) controlled release of loaded substance under specific stimuli (pH, temperature, enzyme activity, light, etc.) [3–5]. Porous silicon (pSi) materials have been extensively studied within biomedical fields due to their universal and tunable properties [6, 7]. According to the research over the past decade, pSi showed several attractive properties such as (i) extensive surface area, (ii) superior loading capacity with various substances (chemical drugs, dyes, proteins, DNA, RNA, etc.), (iii) targeted delivery, and (iv) controlled release with tunable degradation potency [8–13]. In particular, the nano-sized pSi, such as pSiNPs, is worth investigating and has gained much attraction in expanding the scope of research, mostly in drug delivery and controlled-release system, on account of its superior properties such as a high loading yield of drugs, facile surface engineering for targeted delivery, self-reporting by intrinsic photoluminescence at the near-infrared region, and high biocompatibility [14–19].
However, there are some drawbacks of pSiNPs being used in biomedical applications, including highly fragile chemical stability in both corrosive or aqueous media, the lack of mechanical robustness when the loaded materials are released from silicon-based nanoparticles and films, and an undesired structural/chemical degeneration of loaded sensitive biomolecules during the deposition and patterning process. To overcome such critical points, hybrid formulations with biocompatible polymers have been introduced such as polymer-coated pSiNPs and pSiNPs embedded within polymeric nanofibers that enable securing the loaded susceptible biomolecules (DNA, RNA, Protein, etc.), and release them under specific conditions while maintaining the activity of loaded biomolecules. Biologically compatible polymer and polymeric nanofiber are recognized as promising materials in the field of biomedical engineering, owing to cost-effectiveness and the controlled manufacturing, with similarity to native biological structures [6, 20, 21].
In this review, we give an overview of recently developed hybrid systems of pSiNPs and polymer and highlight two representative formulations; (i) pSiNPs coated within a polymer, (ii) pSiNPs embedded within polymeric nanofiber, with their biomedical application. The first formulation is a representative and universal approach of the pSiNPs-polymer hybrid system, and the second formulation is a recently reported advanced hybrid system. Our systematic outlines and fundamental information for further development of the hybrid system will allow for more practical applications in various fields including basic research, industrial science, and translational medicine.
Hybrid system of pSiNPs and polymers
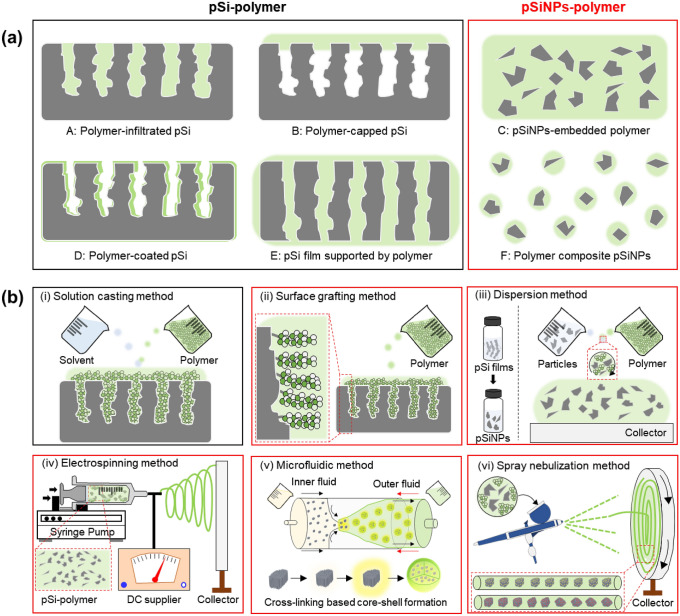
For the past decade, various hybrid systems of pSiNPs and polymers have been introduced with their preparation methods and applications. The pSi-polymer and pSiNPs-polymer hybrid system can be generated in various forms (Fig. 1a). Amongst many, the simplest method is the formulation of polymer-infiltrated pSi (A), where the polymer invades the entire pore of pSi [22–25]. After the nano-sized pores are filled with polymers, the formulations can be intensified by the covalent attachment between the pore walls and polymers. Polymer-capped pSi (B), on the other hand, is a more sophisticated formulation with a thin layer of polymer covering only the top layer of porous silicon nanoparticles [26–29]. The notable advantage of this method is a high proportion of the remaining pSi pore volume. However, the synthesis process is tricky because the percentage of polymer penetrating the pores should be regulated. The pSiNPs-embedded polymer (C) is also one of the hybrid formulations that are embedded with different nano sizes of particles in the polymer matrix by encapsulating the entire porous silicon nanoparticles with a polymer [30–33]. The polymer-coated pSi (D) formulation has a similar design to the polymer-infiltrated pSi method [34–37]. However, in the case of the polymer-coated pSi method, the polymer forms a uniform monolayer on both the outer and inner walls of pSi, building a hybrid composite in the form of an open pore structure. The pSi film supported by polymer (E) is commonly prepared from the polymer and the pSi film, which is crushed and separated from the bulk Si using the electropolishing method [38, 39]. Lastly, polymer composite pSiNPs (F) are much more challenging formulations than the encapsulated ones because each particle needs to be compressed into a polymer layer [1, 2, 40–44].
Fig. 1.
a Schematic illustration of the hybrid systems of porous silicon (pSi) and polymer (black-box: pSi-polymer, red-box: pSiNPs-polymer). b Schematic illustration of the conventional fabrication methods for the hybrid systems of pSi-polymer (black) and pSiNPs-polymer (red). Gray color: pSi and pSiNPs. Green color: polymer. (Color figure online)
The preparation methods of the pSi-polymer and pSiNPs-polymer hybrid systems are summarized in Fig. 1b; (i) solution casting, (ii) surface grafting, (iii) dispersion, (iv) electrospinning, (v) microfluidic, and (vi) spray nebulization method. The formulation of polymer-infiltrated pSi (A), polymer-capped pSi (B), and pSi film supported by polymer (F) is generally produced by the solution casting method [1, 45–48]. [Solution casting (or melting casting) method] This method is based on interfacial interactions (not a chemical reaction) between pSi and polymer and has been widely used as the facile route to design pSi-polymer formulations [49]. In this method, a polymer solution is prepared and poured onto pSi, and then, the excessive polymer solution is removed and dispersed via the spin-coating step. The penetration of a polymer into a porous silicon-based scaffold is determined by several factors (molecular weight of polymer, solution viscosity, surface tension, pore size and morphology, surface chemistry of pSi pore walls). The positive edge of this method relies on its relative simplicity, which does not require any complicated synthesis steps or experimental equipment. [Surface grafting method] As one of the most representative methods, the surface grafting method builds a polymer chain, which is linked to a complementary functional group located on the surface of pSi or pSiNPs by chemical reactions such as a covalent bond between the polymer and surface of pSiNPs [50]. The grafting method allows the surface engineering of pSiNPs with a broad range of reactive groups based on the versatile capability of Si/SiO2 in porous silicon nanoparticles. Polymer composite pSiNPs (F) are generally produced with this method which produces a well-organized layer using pre-performed polymer but has limitations in grafting density. [Dispersion method] This method is the most straightforward synthetic technique that generates hybrid pSiNPs-polymer formulations such as pSiNPs-embedded polymer (C) and polymer-coated pSi (D) by dispersion pSiNPs into the polymer solution. For this case, an additional step such as coating step, lithograph step, or fabrication step is involved for further applications of the hybrid formulation. [Electrospinning method] This method is the conventional method for fabricating hybrid pSiNPs-polymer formulations in the form of fibers by adopting the diffusion or melting of the hybrid formulation through the squeeze nozzle under a strong electric field. The high voltage utilized in this synthesis changes the shape of the hybrid formulation into a cone. The produced nanofibers are largely regulated by electronic conductivity, electrode separation, temperature, concentration, and other properties of the polymer. [Microfluidic method and the spray nebulization method] These methods also build on the in situ hybrid polymerization developed by the dispersion method. In this review article, we focused on the in-situ polymer-coated pSiNPs formulations by a microfluidic system, which is a type of dispersion method, and recently introduced pSiNPs-embedded polymeric nanofibers by an airbrush spray nebulization method [1, 2].
Polymer-coated pSiNPs
The fabrication of polymer-coated pSiNPs can be categorized into two methods. The first method integrates the preformed polymer with the pSiNPs scaffold, and the second one uses an in situ polymerization using various polymers from inside or outside of pSiNPs. Recently reported hybrid polymer-coated pSiNPs formulations are incorporated via (i) microfluidic system (polymer emulsion) using two or three different features of polymers and (ii) entire coating of the nanoparticle by employing commercially available polymers. Microfluidic technology enables the fabrication and engineering of nanomaterials for drug delivery utilizing accurate fluid control and rapid large-scale combining. Hybrid nanomaterials produced by the microfluidic method typically display distinctive properties, such as enhanced mono-dispersity, a highly efficient drug encapsulation, and an increased blood circulation time compared to the conventional bench-scale synthetic methods. Polymer-coated pSiNPs produced by microfluidic technology contain all the advantages of the microfluidic technique to maximize the synergistic effects between polymer and pSiNPs. The high drug loading efficiency, sustained-release profile of the encapsulated drug, and controlled-release of drug within the specific environment are the representative synergetic effect of this formulation, and it can induce maximization of therapeutic effects and minimization of any side effects [51]. Also, it is possible to load a multifarious type of drug by applying a divergent phase of polymer (hydrophobic or hydrophilic) under this system [52–57].
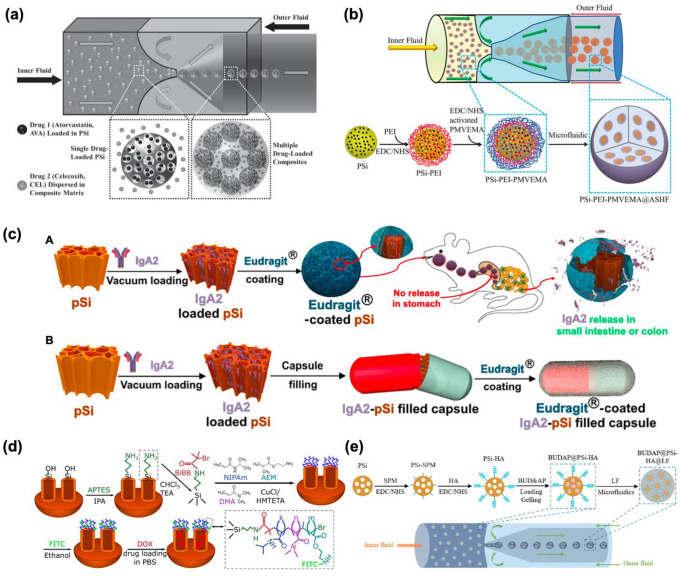
In 2014, Santos and co-workers reported that microfluidic assembly between pSiNPs and polymer could be useful for payloads, targeted drug delivery, and controlled drug release at a certain pH condition. They demonstrated two advanced drug delivery platforms by adopting a similar fabrication concept of microfluidics [58]. In their first study, a combination of pSiNPs with pH-responsive polymer, hypromellose acetate succinate (M and F grade fine powders; MF and HF), was applied to synergistic colon cancer therapy. Two different model drugs, atorvastatin (AVA) and celecoxib (CEL), were loaded into pSiNPs using the immersion method, and then fabricated with a polymer (inner oil fluid: MF, HF or their mixtures in ethyl acetate, outer fluid: Poloxamer 407 in aqueous media) (Fig. 2a). In their follow-up study, the same microfluidic template was used with a slightly different component for the orally administrated drug delivery system [59]. As model drugs, fluorouracil (5-FU; hydrophilic) and celecoxib (CEL; hydrophobic) were chosen for potential multifunctional colon cancer therapy. 5-FU was loaded into the pSiNPs-polymer composite, which was (i) fabricated with mucoadhesive poly(methyl vinyl ether-co-maleic acid, PMVEMA) with a polyethyleneimine (PEI) liker; (ii) encapsulated with pH-responsive hydroxypropyl-methylcellulose acetate succinate (ASHF) including CEL (inner fluid), and then fabricated with Poloxamer 407 (outer fluid) (Fig. 2b). The major advantages of this fabrication method using the microfluidic instrument are (i) the potential to produce a monodisperse structure, (ii) high flexibility for material combination, (iii) high encapsulation efficiency, and (iv) targeted delivery/controlled release for precision medicine.
Fig. 2.
Schematic illustration of the pSiNPs encapsulation within polymer shells. a Preparation steps for the biocompatible pH-responsive polymer-coated pSiNPs shell containing multiple drugs (pSi-AVA@MFHF-CEL) [58], and b PEI-PMVEMA-coated pSiNPs shell containing multiple drugs (pSi-PEI-PMVEMA@ASHF) based on the microfluidic assembly technique [59]. c Preparation steps for A: Eudragit-coated pSiNPs containing IgA2, and B: Eudragit-coated IgA2-loaded pSiNPs in a gelatin capsule [37]. d Preparation steps for temperature-responsive coating of the TR-NDA PSiNPs [36] and e hierarchical structured and programmable responsive vehicles (BUDAP@PSi-HA@LF) [60]. Copyright (2014) Wiley–VCH, (2020) American Chemical Society, (2016) ELSEVIER, (2018) ELSEVIER
Recently, a case of applying pSiNPs-polymer hybrid using a simpler method (dispersion method), which called the entire coating of nanoparticles, has been reported. This method of loading protein or peptide onto porous silicon nanoparticles and then coating the outside of the particle with a commercialized polymer has the certain advantage of being easier to prepare a hybrid formulation or to deliver a drug. In the following study, this entire coating method was also applied for oral delivery, and the polymer coating of pSiNPs prevents denaturation or inactivation of proteins or peptides through low loading efficiency and controlled release characteristics, and by protecting the loaded material. Above all, commercialized polymers have been verified a lot, and pH is the main stimulus for oral delivery, and the following paper also introduces a system in which drugs are released at a specific pH, enabling site-selective release.
In 2020, Sailor and co-workers introduced the polymer-coated pSiNPs system for distinct oral delivery of IgA antibodies. In this study, two approaches were described (Fig. 2c); (i) model protein Immunoglobulin A-2 (IgA2) antibodies were loaded into pSiNPs, and then each pSiNPs was coated with pH-responsive polymers (Eudragit L100, S100, and L30-D55), (ii) IgA2 antibodies-loaded pSiNPs were encapsulated into a gelatin capsule and then coated with pH-responsive polymer for latent cancer therapy [37]. They showed the possibility of delivering biologically unstable proteins to the specific organs (small intestine or colon) by oral uptake through the hybridization of FDA-approved Eudragit polymer and pSiNPs. The polymer-coated pSiNPs system and its application for the targeted delivery need to be further scaled up in the future.
By combining two different materials, which named polymer-coated pSiNPs, it can be overcome the conventional limitation of each material and conclude the synergistic effects such as (i) minimizing the expected side effect by reducing burst release of drugs, (ii) maximizing the blood circulation time with solid secure of loaded cargo (drugs, RNA, DNA, peptide, etc.), (iii) preventing aggregation of pSiNPs, (iv) high stability in biological condition, (v) more sophisticated control-release via two different stimuli such as pH, temperature, and light.
Among the benefits of these pSiNPs-polymer hybrid formulations, the most effective one is their high biocompatibility and the ability of control-release by different stimuli such as pH or temperature. As an additional research case, there are pSiNPs-hybrid formulations that release substances in response to various stimuli such as temperature and pH, respectively. In 2016, Lehto and co-workers developed a polymer that can only be decomposed vaguely above 37 °C or higher to pSiNPs and loaded DOX (33 wt.%) as an externally triggered hybrid formulation by infrared or radiofrequency radiation and applied to cancer therapy. The designed hybrid formulation is named TR-NDA pSiNPs by enclosing various polymers such as NIPAm, DMA, and AEM to pSiNPs and proposed its property only works at specific temperatures (Fig. 2d) [36].
In 2018, Santos and co-workers reported pH-responsive polymer-based pSiNPs hybrid formulations named BUDAP@PSi-HA and BUDAP@PSi-HA@LF, which can orally deliver Budesonide (BUD), a glucocorticoid, for the treatment of inflammatory bowel disease (IBD). Hyaluronic acid (HA) was functionalized onto pSiNPs, and using the different solubility of ascorbyl palmitate (AP) and hydroxypropyl methylcellulose acetate succinate (HPMCAS), the drug was released only at a specific pH in the local spot. The loading efficiency was improved. 24.5 ± 0.1% and 3.8 ± 0.3%, respectively (Fig. 2e). The response-based release of pSiNPs-polymer hybrid formulations with stimuli (temperatures, pHs) have been proposed, and many of these systems need to keep evolving for improvement of the field of biomedical engineering [60].
pSiNPs-embedded polymeric nanofibers
Nanofibers are the fiber that has a nanometer diameter with a fibrous structure. For decades, the importance of nanofibers has been discussed due to their interesting features in biomedical science. In particular, nanofibers have exhibited high potential across the fields for the miniaturization of electronic devices, elevation of device functions, and application in tissue engineering, and have been widely adopted for the technology of the normal devices and equipment as well as chemical engineering. Generally, polymeric nanofibers, which are produced by the electrospinning method, have several features; (i) a large surface area per unit mass with high flexibility, (ii) a large number of micro-cavities between fibers, allowing fusion with other materials, and (iii) a high degree of dispersion against external stress. Furthermore, these advantages can be applied in the biomedical field as a carrier for drug delivery and controlled-release, a platform for cell culture, a blocking agent for adhesion prevention, a wound treatment agent, and sensors [61–63]. The polymeric nanofiber scaffolds have also been used as a valuable tool for tissue engineering, regenerative medical engineering, and various in vitro applications because of their remarkable properties, such as an ability to imitate the topographical characteristics in specific biological environments (mostly cellular system), facile fabrication, payloads of therapeutic components and targeted release [64–66]. However, they are inevitably limited in clinical applications as it is difficult to fabricate them solely with biomolecules (proteins, DNA, RNA, etc.). Other crucial drawbacks of nanofibers include (i) complicated preparation (electrospinning formation using a high voltage for sufficient electric force generation) and (ii) use of organic solvents for loading various cargos, which could reduce the biocompatibility of materials and stability of the payload. The hybridization of nanofibers with pSiNPs (pSiNPs-embedded polymeric nanofibers) ushered in a new era in the biomedical application of nanofibers, and the development of noble preparation methods which are simple, efficient, biocompatible, broadly applicable, and capable of securing the activity of a payload has been highly noted [67–70].
Recently, a new preparation method of pSiNPs-embedded polymeric nanofibers based on the airbrushing spray nebulization was introduced with its biomedical applications. The airbrush spray nebulization method is a solution blow spinning (SBS) technique to generate nanofiber composites with in-situ synthesis and stable loading of sensitive biomolecules. The advantages of this technology include the potential to utilize nanofiber mat and to fabricate scaffold in-situ, as mentioned above. Also, only three components are needed for synthesis: (i) commercially available airbrushes, (ii) concentrated polymer solutions, and (iii) compressed gas sources (N2, CO2, and Ar). To be specific, the small diameter of the polymer-based nanofibers, which almost match extracellular matrix (ECM) properties, made the hybrid formulation of the pSiNPs-embedded nanofiber feasible to be used as a biomimetic scaffold with useful multi-functions: a higher surface area to volume ratios is ideal for the adhering of the cells (tissue engineering and regeneration medicine) and drug loading. Hybrid nanofiber formulations of pSiNPs and biocompatible polymers have been proposed in the biomedical field for tissue engineering, bio-imaging, and drug delivery applications. The pSiNPs-embedded polymeric nanofibers have complemented the drawbacks of each material, demonstrating higher drug loading efficiency than a single system, reliable biomolecule securing, and sustained drug release, as well as proving the potential of in vivo and clinical trials [62, 63, 71].
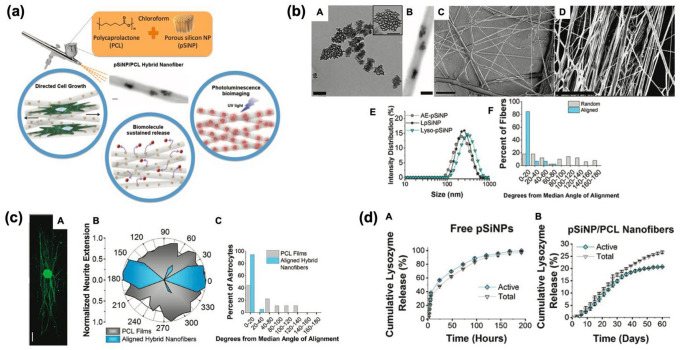
Sailor and co-workers prepared the hybrid spray-nebulized nanofibers based on a polycaprolactone (PCL) or poly(lactide-co-glycolide) matrix including pSiNPs for the stable protein delivery and release [30]. The hybrid pSiNPs/PCL nanofiber was produced by spray nebulization of chloroform solution containing PCL or poly(lactide-co-glycolide), using an airbrush (Fig. 3a). The hybrid pSiNPs/PCL nanofibers showed an average diameter of approximately 500–600 nm, and the containment of pSiNPs in hybrid nanofiber was verified by transmission/scanning electron microscope (TEM/SEM). Compared with the non-aligned fiber, the angles of uniaxially aligned spray-nebulized hybrid nanofibers were quantified (Fig. 3b). For the directed growth study of a single astrocyte cell, which is a central nervous system glial cell, the cells were cultured on the hybrid pSiNPs-PCL nanofibers and followed fluorescent staining signals from rat dorsal root ganglion. As a result, the astrocytes well adhered to the hybrid nanofiber for 96 h with an average angle of 6 ± 8° (Fig. 3c). As a model protein, lysozyme was loaded into pSiNPs, and then lysozyme-loaded hybrid pSiNPs-embedded nanofibers (3.6% by mass lysozyme) were tested by releasing the protein within pH 7.4 buffer at 37 °C. The release of lysozyme was quantified for 60 days, which is longer compared with free pSiNPs (Fig. 3d). In this study, the spray nebulization method of hybrid nanofiber was introduced as an alternative to the conventional method to facilitate simple and diverse material fusion in biomedical applications. The combined pSiNPs/PCL polymer exhibited an enhanced tuning ability of loading/release, long-stable photoluminescence signals in vitro.
Fig. 3.
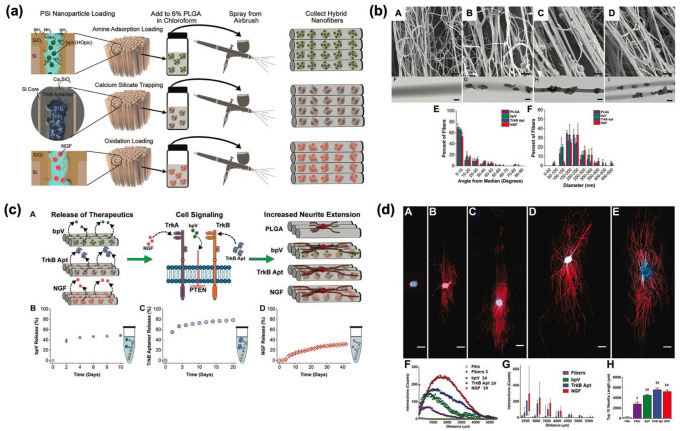
Polymer (PCL) scaffold containing pSiNPs. a Schematic illustration of pSiNPs/PCL hybrid nanofiber prepared by spray nebulization. b Transmission electron microscope (TEM) images of the hybrid formulations (A: as-prepared pSi, B: pSiNPs-embedded hybrid nanofiber, C: non-aligned hybrid nanofiber, D: aligned hybrid nanofiber) and their characterization results (E: diameter distribution of hybrid nanofibers, F: average angle of deviation of fibers from the median alignment angle of non-aligned-/aligned-nanofibers). c Directed growth study of a single astrocyte cell on the hybrid pSiNPs-PCL nanofibers, and fluorescent staining signal analysis of rat dorsal root ganglion. A: Fluorescent microscopy image of the whole dorsal root ganglia (DRG) incubated with aligned PCL nanofibers containing neurofilament (NF200)-loaded pSiNPs (incubation time: 72 h, scale bar = 500 μm). B: Normalized polar histogram of neurite growth with the aligned hybrid nanofibers (blue) and control PCL films (gray). C: the average angle of deviation of astrocyte from the median alignment angle of aligned hybrid nanofibers (blue) and control PCL films (gray). d The cumulative analysis of lysozyme amount (gray) and activity (blue) released from the nanofibers containing lysozyme-loaded pSiNPs. A: lysozyme-loaded pSiNPs without polymer hybrid, B: PCL nanofibers containing lysozyme-loaded pSiNPs. The Copyright (2018) Wiley–VCH [30]. (Color figure online)
Sailor’s group demonstrated that RNA aptamer and DNA-based oligonucleotides could also be another excellent delivery candidate using the same hybrid nanofiber technique. Three different types of therapeutic substances were loaded into hybrid nanofibers such as (i) bpV(HOpic): a drug which inhibits phosphatase (loading method: electrostatic adsorption), (ii) tensin homolog (PTEN, TrkB aptamer): an RNA aptamer for tropomyosin-related kinase receptor type B (loading method: calcium silicate condensation), (iii) protein (NGF): a nerve growth factor (loading method: oxidative trapping) (Fig. 4a; [top] The inner pore walls of pSi were modified to a positive charge by amine functionalization for the loading bpV(Hopic), which is a negative charge drug, using electrostatic interactions, [middle] Negatively charged TrkB aptamer was loaded by calcium trapping method, [bottom] NGF protein was loaded into oxidized pores by physical trapping) [32]. The average diameter and charge of the final individual hybrid nanofiber were identified using dynamic light scattering (DLS), zeta potential (PLGA only: 209 ± 60 nm, bpV(HOpic) loaded nanofiber: 211 ± 78 nm, TrkB loaded nanofiber: 213 ± 60 nm, NGF loaded nanofiber: 250 ± 79 nm). Each of uniaxially aligned nanofibers was analyzed by scanning electron microscopy, and average angles were collected for PLGA(12.3 ± 17.6°), bpV(HOpic)(9.6 ± 11.3°), TrkB aptamer (12.6 ± 15.6°), NGF(15.3 ± 16.7°) nanofibers (Fig. 4b). To demonstrate the wide delivery ability of the hybrid nanofibers, in vitro release profile test was conducted in PBS buffer at 37 °C on a time-dependent basis. The most effective release among the combined pSiNPs/PLGA nanofibers was from NGF-loaded hybrid nanofibers (over 6 weeks). A common factor from all the formulations was a burst release within the first 2 days, and the release was continued for a long period as an advantage of hybrid nanofiber (Fig. 4c). Lastly, the ability of the neurotrophic APIs was evaluated to confirm the neurite extension on hybrid nanofibers. The result showed that DRG explants on any nanofibers induced neurite extension compared with the pure PLGA films. Also, the statistical results showed that neurite extension on each nanofiber exhibited longer than the previously reported study (Fig. 4d).
Fig. 4.
Polymer (PLGA) scaffold containing protein-/peptide-loaded pSiNPs. a Schematic illustration of the preparation of PLGA scaffold containing amine-functionalized (top)/aptamer-loaded (middle)/protein-loaded (bottom) pSiNPs. b Scanning electron microscope (SEM) images of nanofibers (A: PLGA nanofibers, B: bpV(Hopic)-hybrid nanofibers, C: TrkB aptamer-hybrid nanofibers, D: NGF-hybrid nanofibers), (E) Deviation from the median alignment angle from the nanofibers, and (F) diameters calculated from each image. c Release profiles of the three neurotrophic agents from hybrid nanofibers. A: Schematic illustration of the three different agents releasing mechanism. B–D: Release plot of the three types of hybrid nanofibers in PBS buffer solution at 37 °C (B: bpV, C: TrkB aptamer, D: NGF). d Increment of neurite extension induced by therapeutics release from DRG explant on hybrid nanofibers. A: PLGA film, B: PLGA nanofibers, C: bpV(Hopic)-pSiNPs hybrid nanofibers, D: TrkB aptamer-pSiNPs hybrid nanofibers, E: NGF-pSiNPs hybrid nanofibers. F–H: The range of neurite extensions and the average length of the ten longest neurites on the hybrid nanofibers. The Copyright (2020) Wiley–VCH [32]
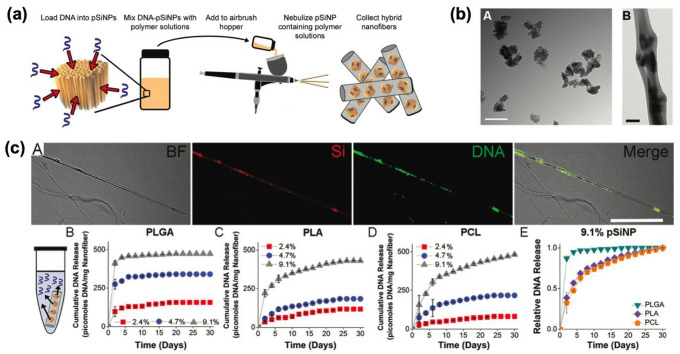
Similarly, Ricci and co-workers developed hybrid porous silicon-PLGA/PLA/PCL nanofiber for the sustained release of synthetic DNA-based oligonucleotides [33]. The nanofibers were generated with the specific ratios of polymer mixed with DNA-loaded pSiNPs (loading method: calcium silicate condensation/trapping) using an airbrush hopper (Fig. 5a). The morphological change of DNA-loaded pSiNPS before and after the blending with PLGA polymer was observed by transmission electron microscopy (Fig. 5b). For a clear comparison of three polymers, time-gated imaging was measured, and pSiNPs/PLGA hybrid nanofibers(16-fold than PLA, 120-fold than PCL) displayed the highest signal increase. This study suggests that DNA-based molecules can also be utilized using hybrid nanofiber systems fabricated using a straightforward and cost-effective airbrush. In addition, the experiment results of various hybrid nanofibers produced from easily available polymers indicate that airbrush-based hybrid nanofibers can be a prospective approach for nanotechnology, tissue engineering, including regenerative medical applications.
Fig. 5.
Polymer (PLGA/PLA/PCL) scaffold containing DNA-loaded pSiNPs. a Schematic illustration of the preparation of hybrid nanofiber containing DNA-loaded pSiNPs. b TEM images of A: DNA-loaded pSiNPs, B: PLGA hybrid nanofiber containing DNA-loaded pSiNPs (Scale bar – A: 200 nm, B: 2 μm). c Bright-field (BF) image and fluorescence images of PCL nanofibers. A: Si—pSiNPs image, DNA—FAM signal within DNA, Merge—the merged image of BF, Si, and DNA. Scale bar: 10 μm. B–E: Release profile of hybrid nanofiber elution in PBS at 37 °C. The supernatant was collected and changed every 48 h. B: PLGA-based nanofibers, C: PLA-based nanofibers, D: PCL-based nanofibers. Inset percent: DNA-concentration within pSiNPs. E: The comparison of the DNA release rate from the three types of nanofibers containing DNA-containing (9.1%) pSiNPs. The Copyright (2020) The Royal Society of Chemistry [33]
Summary and perspectives
In this focused review, we have introduced the recent development of a hybrid system of pSiNPs and biocompatible polymers with their biomedical applications. We outlined the preparation methods and analyzed their pros and cons from an engineering viewpoint. Encapsulation or embedding of pSiNPs within a polymer shell or nanofiber could allow to enhance and amplify the innate properties of each material, such as simply tunable morphology, extended multi-functions, stable loading and maintaining with susceptible molecules, and varying response-based release within stimuli in various pH, temperatures, etc. We believed that the hybrid pSiNPs-polymer materials and their preparation methods hold great potential for practical applications and can open a new chapter within a wide variety of industrial fields, as well as in basic research.
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) of Korea funded by the Ministry of Science & ICT (NRF-2019-M3A9H1103783). This research was also supported by the Basic Science Research Program through the NRF of Korea funded by the Ministry of Education (NRF-2018-R1A6A1A03025124, NRF-2018-R1D1A1B07043383).
Declaration
Conflict of interest
The authors declare that they have no conflict of interest and Jung Y declares that s/he has no conflict of interest in relation to the work in this article. Kim D declares that s/he has no conflict of interest in relation to the work in this article
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Segal E, Krepker M, Polymer-porous silicon composites. In: Handbook of porous silicon. Springer International Publishing Switzerland; 2014: 187–198.
- 2.Bonanno LM, Segal E. Nanostructured porous silicon–polymer-based hybrids: from biosensing to drug delivery. Nanomedicine. 2011;6(10):1755–70. doi: 10.2217/nnm.11.153. [DOI] [PubMed] [Google Scholar]
- 3.de Moraes Porto ICC. Polymer biocompatibility. Polymerization Croatia. INTECH. 2012;2012:47–63. [Google Scholar]
- 4.Soares DCF, Domingues SC, Viana DB, Tebaldi ML. Polymer-hybrid nanoparticles: current advances in biomedical applications. Biomed Pharmacother. 2020;131:110695. doi: 10.1016/j.biopha.2020.110695. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D-X, Esser L, Vasani RB, Thissen H, Voelcker NH. Porous silicon nanomaterials: recent advances in surface engineering for controlled drug-delivery applications. Nanomedicine. 2019;14(24):3213–30. doi: 10.2217/nnm-2019-0167. [DOI] [PubMed] [Google Scholar]
- 6.Canham L. Handbook of porous silicon. Berlin: Springer; 2014. [Google Scholar]
- 7.Sailor MJ. Porous silicon in practice: preparation, characterization and applications. Hoboken: John Wiley & Sons; 2012. [Google Scholar]
- 8.Harraz FA. Porous silicon chemical sensors and biosensors: a review. Sens Actuator B-Chem. 2014;202:897–912. doi: 10.1016/j.snb.2014.06.048. [DOI] [Google Scholar]
- 9.Santos HA, Hirvonen J. Nanostructured porous silicon materials: potential candidates for improving drug delivery. Nanomedicine. 2012;7(9):1281–4. doi: 10.2217/nnm.12.106. [DOI] [PubMed] [Google Scholar]
- 10.Park J-H, Gu L, Von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8(4):331–6. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tieu T, Alba M, Elnathan R, Cifuentes-Rius A, Voelcker NH. Advances in porous silicon–based nanomaterials for diagnostic and therapeutic applications. Adv Ther. 2019;2(1):1800095. doi: 10.1002/adtp.201800095. [DOI] [Google Scholar]
- 12.Salonen J, Lehto V-P. Fabrication and chemical surface modification of mesoporous silicon for biomedical applications. Chem Eng J. 2008;137(1):162–72. doi: 10.1016/j.cej.2007.09.001. [DOI] [Google Scholar]
- 13.Santos HA, Mäkilä E, Airaksinen AJ, Bimbo LM, Hirvonen J. Porous silicon nanoparticles for nanomedicine: preparation and biomedical applications. Nanomedicine. 2014;9(4):535–54. doi: 10.2217/nnm.13.223. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Kang JS, Kim D. A mini review: recent advances in surface modification of porous silicon. Materials. 2018;11(12):2557. doi: 10.3390/ma11122557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung Y, Huh Y, Kim D. Recent advances in surface engineering of porous silicon nanomaterials for biomedical applications. Microporous Mesoporous Mater. 2021;310:110673. doi: 10.1016/j.micromeso.2020.110673. [DOI] [Google Scholar]
- 16.Kang RH, Lee SH, Kang S, Kang J, Hur JK, Kim D. Systematic degradation rate analysis of surface-functionalized porous silicon nanoparticles. Materials. 2019;12(4):580. doi: 10.3390/ma12040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertucci A, Kim K-H, Kang J, Zuidema JM, Lee SH, Kwon EJ, Kim D, Howell SB, Ricci F, Ruoslahti E. Tumor-targeting, microRNA-silencing porous silicon nanoparticles for ovarian cancer therapy. ACS Appl Mater Interfaces. 2019;11(27):23926–37. doi: 10.1021/acsami.9b07980. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Liu Z, Fontana F, Ding Y, Liu D, Hirvonen JT, Santos HA. Tailoring porous silicon for biomedical applications: from drug delivery to cancer immunotherapy. Adv Mater. 2018;30(24):1703740. doi: 10.1002/adma.201703740. [DOI] [PubMed] [Google Scholar]
- 19.Kang RH, Jang J-E, Huh E, Kang SJ, Ahn D-R, Kang JS, Sailor MJ, Yeo SG, Oh MS, Kim D. A brain tumor-homing tetra-peptide delivers a nano-therapeutic for more effective treatment of a mouse model of glioblastoma. Nanoscale Horiz. 2020;5(8):1213–25. doi: 10.1039/D0NH00077A. [DOI] [PubMed] [Google Scholar]
- 20.Luu Y, Kim K, Hsiao B, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J Control Release. 2003;89(2):341–53. doi: 10.1016/S0168-3659(03)00097-X. [DOI] [PubMed] [Google Scholar]
- 21.Burnham MR, Turner JN, Szarowski D, Martin DL. Biological functionalization and surface micropatterning of polyacrylamide hydrogels. Biomaterials. 2006;27(35):5883–91. doi: 10.1016/j.biomaterials.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Massad-Ivanir N, Friedman T, Nahor A, Eichler S, Bonanno LM, Sa'ar A, Segal EJSM. Hydrogels synthesized in electrochemically machined porous Si hosts: effect of nano-scale confinement on polymer properties. Soft Matter. 2012;8(35):9166–76. doi: 10.1039/c2sm25966d. [DOI] [Google Scholar]
- 23.Krepker MA, Segal E. Dual-functionalized porous Si/hydrogel hybrid for label-free biosensing of organophosphorus compounds. Anal Chem. 2013;85(15):7353–60. doi: 10.1021/ac4011815. [DOI] [PubMed] [Google Scholar]
- 24.Robbiano V, Paternò GM, La Mattina AA, Motti SG, Lanzani G, Scotognella F, Barillaro G. Room-temperature low-threshold lasing from monolithically integrated nanostructured porous silicon hybrid microcavities. ACS Nano. 2018;12(5):4536–44. doi: 10.1021/acsnano.8b00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumeria T, Wang J, Chan N, Harris TJ, Sailor MJ. Visual sensor for sterilization of polymer fixtures using embedded mesoporous silicon photonic crystals. ACS Sens. 2018;3(1):143–50. doi: 10.1021/acssensors.7b00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Sailor MJ. Chitosan hydrogel-capped porous SiO2 as a pH responsive nano-valve for triggered release of insulin. Adv Funct Mater. 2009;19(5):733–41. doi: 10.1002/adfm.200800921. [DOI] [Google Scholar]
- 27.Vasani RB, Szili EJ, Rajeev G, Voelcker NH. On-demand antimicrobial treatment with antibiotic-loaded porous silicon capped with a pH-responsive dual plasma polymer barrier. Chem Asian J. 2017;12(13):1605–14. doi: 10.1002/asia.201700427. [DOI] [PubMed] [Google Scholar]
- 28.Tong WY, Alnakhli M, Bhardwaj R, Apostolou S, Sinha S, Fraser C, Kuchel T, Kuss B, Voelcker NH. Delivery of siRNA in vitro and in vivo using PEI-capped porous silicon nanoparticles to silence MRP1 and inhibit proliferation in glioblastoma. J Nanobiotechnol. 2018;16(1):38. doi: 10.1186/s12951-018-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang D-X, Yoshikawa C, Welch NG, Pasic P, Thissen H, Voelcker NH. Spatially controlled surface modification of porous silicon for sustained drug delivery applications. Sci Rep. 2019;9(1):1367. doi: 10.1038/s41598-018-37750-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuidema JM, Kumeria T, Kim D, Kang J, Wang J, Hollett G, Zhang X, Roberts DS, Chan N, Dowling CJAM. Oriented nanofibrous polymer scaffolds containing protein-loaded porous silicon generated by spray nebulization. Adv Mater. 2018;30(12):1706785. doi: 10.1002/adma.201706785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia B, Zhang W, Shi J, Li J, Chen Z, Zhang Q. NIR light-triggered gelling in situ of porous silicon nanoparticles/PEGDA hybrid hydrogels for localized combinatorial therapy of cancer cells. J Appl Polym Sci. 2019;136(17):47443. doi: 10.1002/app.47443. [DOI] [Google Scholar]
- 32.Zuidema JM, Dumont CM, Wang J, Batchelor WM, Lu YS, Kang J, Bertucci A, Ziebarth NM, Shea LD, Sailor MJ. Porous silicon nanoparticles embedded in poly (lactic-co-glycolic acid) nanofiber scaffolds deliver neurotrophic payloads to enhance neuronal growth. Adv Funct Mater. 2020;30(25):2002560. doi: 10.1002/adfm.202002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuidema JM, Bertucci A, Kang J, Sailor MJ, Ricci FJN. Hybrid polymer/porous silicon nanofibers for loading and sustained release of synthetic DNA-based responsive devices. Nanoscale. 2020;12(4):2333–9. doi: 10.1039/C9NR08474F. [DOI] [PubMed] [Google Scholar]
- 34.Gongalsky MB, Kharin AY, Osminkina LA, Timoshenko VY, Jeong J, Lee H, Chung BH. Enhanced photoluminescence of porous silicon nanoparticles coated by bioresorbable polymers. Nanoscale Res Lett. 2012;7(1):446. doi: 10.1186/1556-276X-7-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Thapa R, Liu D, Nissinen T, Granroth S, Närvänen A, Suvanto M, Santos HA, Lehto VP. Smart porous silicon nanoparticles with polymeric coatings for sequential combination therapy. Mol Pharm. 2015;12(11):4038–47. doi: 10.1021/acs.molpharmaceut.5b00473. [DOI] [PubMed] [Google Scholar]
- 36.Tamarov K, Xu W, Osminkina L, Zinovyev S, Soininen P, Kudryavtsev A, Gongalsky M, Gaydarova A, Närvänen A, Timoshenko V. Temperature responsive porous silicon nanoparticles for cancer therapy–spatiotemporal triggering through infrared and radiofrequency electromagnetic heating. J Controll Release. 2016;241:220–8. doi: 10.1016/j.jconrel.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Kumeria T, Wang J, Kim B, Park J-H, Zuidema JM, Klempner M, Cavacini L, Wang Y, Sailor MJJABS. Engineering: enteric polymer-coated porous silicon nanoparticles for site-specific oral delivery of IgA antibody. ACS Biomater. Sci. Eng. 2020; just accepted. [DOI] [PMC free article] [PubMed]
- 38.DeLouise LA, Fauchet PM, Miller BL, Pentland AA. Hydrogel-supported optical-microcavity sensors. Adv Mater. 2005;17(18):2199–203. doi: 10.1002/adma.200500261. [DOI] [Google Scholar]
- 39.El-Zohary SE, Shenashen M, Allam NK, Okamoto T, Haraguchi MJJoN. Electrical characterization of nanopolyaniline/porous silicon heterojunction at high temperatures. J. Nanomater. 2013;2013: 568175.
- 40.Shahbazi M-A, Almeida PV, Mäkilä EM, Kaasalainen MH, Salonen JJ, Hirvonen JT, Santos HAJB. Augmented cellular trafficking and endosomal escape of porous silicon nanoparticles via zwitterionic bilayer polymer surface engineering. Biomaterials. 2014;35(26):7488–500. doi: 10.1016/j.biomaterials.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Shahbazi MA, Almeida PV, Mäkilä E, Correia A, Ferreira MP, Kaasalainen M, Salonen J, Hirvonen J, Santos HA. Poly (methyl vinyl ether-alt-maleic acid)-functionalized porous silicon nanoparticles for enhanced stability and cellular internalization. Macromol Rapid Commun. 2014;35(6):624–9. doi: 10.1002/marc.201300868. [DOI] [PubMed] [Google Scholar]
- 42.Correia A, Shahbazi M-A, Mäkilä E, Almeida S, Salonen J, Hirvonen J, Santos HA. Cyclodextrin-modified porous silicon nanoparticles for efficient sustained drug delivery and proliferation inhibition of breast cancer cells. ACS Appl Mater Interfaces. 2015;7(41):23197–204. doi: 10.1021/acsami.5b07033. [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Zhang H, Mäkilä E, Fan J, Herranz-Blanco B, Wang C-F, Rosa R, Ribeiro AJ, Salonen J, Hirvonen JJB. Microfluidic assisted one-step fabrication of porous silicon@ acetalated dextran nanocomposites for precisely controlled combination chemotherapy. Biomaterials. 2015;39:249–59. doi: 10.1016/j.biomaterials.2014.10.079. [DOI] [PubMed] [Google Scholar]
- 44.Schiattarella C, Moretta R, Defforge T, Gautier G, Della Ventura B, Terracciano M, Tortiglione C, Fardella F, Maddalena P, De Stefano L. Time-gated luminescence imaging of positively charged poly-l-lysine-coated highly microporous silicon nanoparticles in living Hydra polyp. J Biophotonics. 2020;13(12):e202000272. doi: 10.1002/jbio.202000272. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee P, Whitehead MA, Senter RA, Fan D, Coffer JL, Canham LT. Biorelevant mesoporous silicon/polymer composites: directed assembly, disassembly, and controlled release. Biomed Microdevices. 2006;8(1):9–15. doi: 10.1007/s10544-006-6377-7. [DOI] [PubMed] [Google Scholar]
- 46.Li YY, Cunin F, Link JR, Gao T, Betts RE, Reiver SH, Chin V, Bhatia SN, Sailor MJ. Polymer replicas of photonic porous silicon for sensing and drug delivery applications. Science. 2003;299(5615):2045–7. doi: 10.1126/science.1081298. [DOI] [PubMed] [Google Scholar]
- 47.Park JS, Meade SO, Segal E, Sailor MJ. Porous silicon-based polymer replicas formed by bead patterning. Phys Status Solidi (a) 2007;204(5):1383–7. doi: 10.1002/pssa.200674351. [DOI] [Google Scholar]
- 48.Li YY, Kollengode VS, Sailor MJ. Porous-silicon/polymer nanocomposite photonic crystals formed by microdroplet patterning. Adv Mater. 2005;17(10):1249–51. doi: 10.1002/adma.200401760. [DOI] [Google Scholar]
- 49.Oksman K, Aitomäki Y, Mathew AP, Siqueira G, Zhou Q, Butylina S, Tanpichai S, Zhou X, Hooshmand S. Review of the recent developments in cellulose nanocomposite processing. Compo Part A Appl Sci Manuf. 2016;83:2–18. doi: 10.1016/j.compositesa.2015.10.041. [DOI] [Google Scholar]
- 50.Wang S, Wang Z, Li J, Li L, Hu W. Surface-grafting polymers: from chemistry to organic electronics. Mater Chem Front. 2020;4(3):692–714. doi: 10.1039/C9QM00450E. [DOI] [Google Scholar]
- 51.Valencia PM, Farokhzad OC, Karnik R, Langer RJN-EMA. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat Nanotechnol. 2012;7:623-9. [DOI] [PMC free article] [PubMed]
- 52.Zhang L, Chen Q, Ma Y, Sun J. Microfluidic methods for fabrication and engineering of nanoparticle drug delivery systems. ACS Appl Bio Mater. 2019;3(1):107–20. doi: 10.1021/acsabm.9b00853. [DOI] [PubMed] [Google Scholar]
- 53.Shrimal P, Jadeja G, Patel S. A review on novel methodologies for drug nanoparticle preparation: microfluidic approach. Chem Eng Res Des. 2020;153:728–56. doi: 10.1016/j.cherd.2019.11.031. [DOI] [Google Scholar]
- 54.RoyChaudhuri C. A review on porous silicon based electrochemical biosensors: beyond surface area enhancement factor. Sens Actuator B-Chem. 2015;210:310–23. doi: 10.1016/j.snb.2014.12.089. [DOI] [Google Scholar]
- 55.Mahdavi Z, Rezvani H, Moraveji MK. Core–shell nanoparticles used in drug delivery-microfluidics: a review. RSC Adv. 2020;10(31):18280–95. doi: 10.1039/D0RA01032D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lei KF. Microfluidic systems for diagnostic applications: a review. J Lab Autom. 2012;17(5):330–47. doi: 10.1177/2211068212454853. [DOI] [PubMed] [Google Scholar]
- 57.Martins JP, Torrieri G, Santos HA. The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert Opin Drug Deliv. 2018;15(5):469–79. doi: 10.1080/17425247.2018.1446936. [DOI] [PubMed] [Google Scholar]
- 58.Liu D, Zhang H, Herranz-Blanco B, Mäkilä E, Lehto VP, Salonen J, Hirvonen J, Santos HA. Microfluidic assembly of monodisperse multistage pH-responsive polymer/porous silicon composites for precisely controlled multi-drug delivery. Small. 2014;10(10):2029–38. doi: 10.1002/smll.201303740. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Liu D, Shahbazi MA, Mäkilä E, Herranz-Blanco B, Salonen J, Hirvonen J, Santos HA. Fabrication of a multifunctional Nano-in-micro drug delivery platform by microfluidic templated encapsulation of porous silicon in polymer matrix. Adv Mater. 2014;26(26):4497–503. doi: 10.1002/adma.201400953. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Li Y, Liu Z, Kerdsakundee N, Zhang M, Zhang F, Liu X, Bauleth-Ramos T, Lian W, Mäkilä EJB. Hierarchical structured and programmed vehicles deliver drugs locally to inflamed sites of intestine. Biomaterials. 2018;185:322–32. doi: 10.1016/j.biomaterials.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Panthi G, Park M, Kim H-Y, Park S-J. Electrospun polymeric nanofibers encapsulated with nanostructured materials and their applications: a review. J Ind Eng Chem. 2015;24:1–13. doi: 10.1016/j.jiec.2014.09.011. [DOI] [Google Scholar]
- 62.Tutak W, Sarkar S, Lin-Gibson S, Farooque TM, Jyotsnendu G, Wang D, Kohn J, Bolikal D, Simon CG., Jr The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials. 2013;34(10):2389–98. doi: 10.1016/j.biomaterials.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 63.Lim CT. Nanofiber technology: current status and emerging developments. Prog Polym Sci. 2017;70:1–17. doi: 10.1016/j.progpolymsci.2017.03.002. [DOI] [Google Scholar]
- 64.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev. 2011;17(5):349–64. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghafoor B, Aleem A, Ali MN, Mir M. Review of the fabrication techniques and applications of polymeric electrospun nanofibers for drug delivery systems. J Drug Deliv Sci Technol. 2018;48:82–7. doi: 10.1016/j.jddst.2018.09.005. [DOI] [Google Scholar]
- 66.Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–53. doi: 10.1016/S0266-3538(03)00178-7. [DOI] [Google Scholar]
- 67.Daristotle JL, Behrens AM, Sandler AD, Kofinas P. A review of the fundamental principles and applications of solution blow spinning. ACS Appl Mater Interfaces. 2016;8(51):34951–63. doi: 10.1021/acsami.6b12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stojanovska E, Canbay E, Pampal ES, Calisir MD, Agma O, Polat Y, Simsek R, Gundogdu NS, Akgul Y, Kilic A. A review on non-electro nanofibre spinning techniques. RSC Adv. 2016;6(87):83783–801. doi: 10.1039/C6RA16986D. [DOI] [Google Scholar]
- 69.Song J, Li Z, Wu H. Blowspinning: a new choice for nanofibers. ACS Appl Mater Interfaces. 2020;12(30):33447–64. doi: 10.1021/acsami.0c05740. [DOI] [PubMed] [Google Scholar]
- 70.Singh R, Ahmed F, Polley P, Giri J. Fabrication and characterization of core–shell nanofibers using a next-generation airbrush for biomedical applications. ACS Appl Mater Interfaces. 2018;10(49):41924–34. doi: 10.1021/acsami.8b13809. [DOI] [PubMed] [Google Scholar]
- 71.Tutak W, Gelven G, Markle C, Palmer XL. Rapid polymer fiber airbrushing: Impact of a device design on the fiber fabrication and matrix quality. J Appl Polym Sci. 2015;132(47):42813. doi: 10.1002/app.42813. [DOI] [Google Scholar]