Abstract
Cell-based cancer immunotherapy is mainly performed to re-stimulate or boost the anti-tumor immunity by leveraging the anti-tumoral functions of infused cells. Although conventional adoptive cell therapy with T cells and DC vaccines had potentiated the use of ex vivo engineered cells for cancer immunotherapy, these approaches had a low success rate and some off-target side effects. Recent developments on this intervention are adopting nanoengineering to overcome limitations imposed by the environment the therapeutic cells would be in and the natural characteristics of the cells; thus, enhancing the efficacy of therapies. For this purpose, T cells, NK cells, DCs, and macrophages are engineered to either maintain anti-tumoral phenotypes, target tumor efficiently, or improve the innate functionalities and viability.
Keywords: Adoptive immunotherapy, Cancer, Immune cells, Nanoengineering, Targeting
Cancer immunotherapy
Cancer immunotherapy has opened a new paradigm for cancer treatment by providing durable clinical responses. The purpose of cancer immunotherapy is to re-stimulate or boost the anti-tumor immunity of a patient to combat cancer. The anti-tumor immunity is based on acquiring tumor-specific antigens by antigen-presenting cells (APCs) within the tumor tissues to prime and activate T cells in lymph nodes [1]. By doing so, activated cytotoxic T lymphocytes (CTLs) infiltrate into tumors and kill the antigen-specific tumor cells. As tumor progresses, such immunity cycle is interrupted with factors associated with proliferating tumor cells [2]. The factors range from the direct and indirect effects of pro-tumoral cells to tumor cells themselves [3–6]. These factors create the environment in a complex manner to favor tumor progression. For this reason, it is necessary to alleviate the inhibition of the anti-tumor immunity in a multiplex and simultaneous manner for successful cancer immunotherapy.
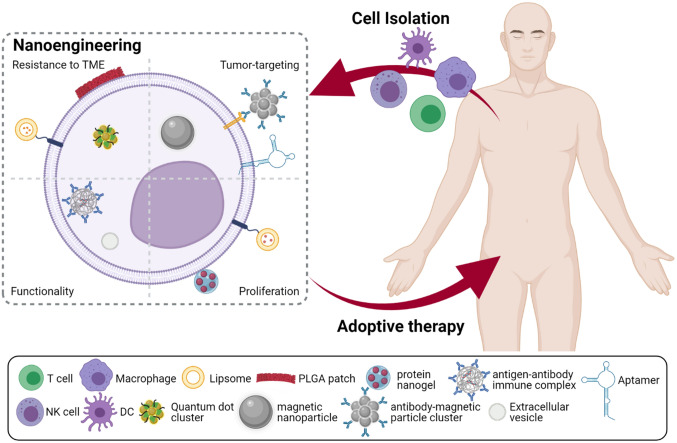
Initial studies on cancer immunotherapy focused on blocking checkpoint proteins on either T cells or cancer cells which prevent immune responses when bound so that T cells in the tumor tissues are able to recognize the tumor cells as “foreign” and be activated. However, the efficacy of immune checkpoint inhibitors (ICI) is solely dependent on the presence of tumor antigen-specific cytotoxic lymphocytes in the tumor tissues; ICI treatment cannot play its part in tumor regression if the frequency of antigen-specific T cells is scarce. For this reason, the focus of studies on cancer immunotherapy moved to adoptive cell therapy (ACT) involving ex vivo stimulation and proliferation of antigen-specific cytotoxic lymphocytes [7–9]. Transfusion of ex vivo engineered effectors cells can be advantageous since specific engineering of cells for enhanced or novel functionality. After the development of early ACT, T cell-based therapies evolved by adopting T cell receptor (TCR) engineering and chimeric antigen receptor (CAR) for increased specificity towards tumor-associated antigens (TAAs) [10]. Based on the success of immunotherapy with transfusion of ex vivo engineered therapeutic cells, the advantage of employing effector cells for appropriate uses into immunotherapy. For this purpose, similar methods were applied to engineering natural killer cells (NK cells) and macrophages for more effective immunotherapy [11–15]. Immunotherapy utilizing engineered effector cells such as T cells and dendritic cells (DCs) have been acknowledged and approved by FDA for their anti-tumor efficacy and a growing number of studies are being performed to develop more effective platforms (Fig. 1).
Fig. 1.
Overview of different strategies utilized for engineering immune cells with nanomaterials to improve adoptive cell therapy. Created with BioRender.com
Immune cells for effective adoptive cell therapy
T cell
T cells have been considered to be the most important effector cells for restoring anti-tumor immunity [16]. For this reason, the majority of studies on cell-based therapy were focused on utilizing T cells. Early studies on T cell-based adoptive cell therapy were initiated by isolating tumor infiltrated lymphocytes (TILs) and re-stimulating or expanding the cells to transfuse them back into the body. Then the focus moved to ex vivo transduction with either T cell receptor (TCR) or chimeric antigen receptor (CAR) onto peripheral T cells. These attempts were made for either re-gaining the cytotoxicity or enhancing the ability to recognize specific antigens. However, T cell-based therapies pose shortcomings of possible cytokine release syndrome and the fact that these are MHC-restricted and antigens are rarely known.
NK cell
NK cells are effector cells similar to CTLs in terms of exerting cytotoxicity by producing a large number of cytokines but more effective than CTLs in terms of recognizing abnormal cells [17]. The ability to distinguish abnormal cells from healthy cells can be advantageous since it reduces off-target toxicity, often suggested as a limitation of T cell-based therapies [18, 19]. Current studies on NK cell-based immunotherapy are focused on cytokine supplement, modification of internal signal pathway for in vivo intervention, and adoptive transfer of genetically engineered NK cells [17]. In recent clinical trials, CAR-NK cells are being developed and have shown potential in lysing target cells similarly to CAR-T cells but with fewer side effects [20]. However, the development of effective NK cell-based therapies still requires enhanced targeting ability, recognition, and resistance against the TME.
DC
DC is another key compartment of the anti-tumor immunity for its role in presenting antigens and T cell activation and differentiation. Naturally, DCs capture and process antigens to present them on the MHC molecules so that antigen-specific T cells are primed and expanded for effective T cell-mediated immunity [21]. In order to utilize the function of antigen-presenting, ex vivo engineering of DCs is routinely performed by loading TAAs into immature DCs (iDCs) or partially matured DCs. For this purpose, iDCs are derived from peripheral blood CD14 + precursors with granulocyte-macrophage colony-stimulating factor and interleukin-4. Such pulsed DCs have been referred to as DC vaccines and expected to stimulate and educate naïve T cells by introducing the loaded antigens.
Macrophage
Macrophage is another antigen-presenting cell with the ability to secrete pro-inflammatory cytokines and chemokines that create an immunostimulatory environment [22]. In addition, pro-inflammatory macrophages are capable of releasing tumoricidal agents such as nitric oxide (NO). As antigen-presenting cells, macrophages can also phagocytose dead tumor cells to process tumor-associated antigens. For this reason, macrophages were believed to kill tumor cells, process tumor-associated antigens, and activate adaptive anti-tumor immunity upon tumor cell encounter. Since these tumoricidal functions were observed with pro-inflammatory macrophages, early studies were conducted by transfusing ex vivo generated pro-inflammatory macrophages [12, 23–25]. However, the plasticity of macrophages led to re-polarizing into anti-inflammatory phenotypes when the macrophages end up in the TME, even though pre-conditioned into pro-inflammatory phenotypes.
Limitations of adoptive cell therapy and windows for improvement
Environment: immunosuppressive tumor microenvironment
Transfusion of isolated and engineered T cells faces a critical obstacle that the immunosuppressive TME inhibits the infiltration of T cells into tumor tissues. Such hardship applies to all immune cell transfusions for therapy, since macrophages, T cells, DCs, and NK cells are all in need of encountering tumor cells for them to play their roles in the anti-tumor immunity. Tumors can be classified into various states in terms of infiltration of immune cells into the tissues.
Especially when the target tumor is classified as an immune-excluded tumor, infused immune cells are highly likely to encounter hardship in terms of infiltrating into tumor tissues. Immune-excluded tumors are known to inhibit T cell infiltration and limit contact of T cells and tumor cells [26]. Such barriers are created by altered chemokine networks and tumor vasculature [27]. For example, successful T cell recruitment to peripheral tissue requires inflammatory chemokines such as CCL19, CCL21, CXCL12, CCR5, and CXCR3. After recruitment, infiltration into tumors is also necessary and this requires expression of CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10. However, immune-excluded tumors lack these chemokines and tend to secrete more chemokines that attract pro-tumoral suppressor cells such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T (Treg) cells.
Moreover, tumor cells or the tumor microenvironment (TME) often induce apoptosis of cells or alter the function of these cells. It is widely known that the immunosuppressive TME can repolarize pro-inflammatory macrophages into anti-inflammatory phenotypes, as known as tumor-associated macrophages (TAMs) [28]. This tendency is also observed for NK cells; the immunosuppressive TME can induce polarization of transfused NK cells into tumor-promoting phenotypes [29]. Tumor-induced dysfunction can occur for T cells and DCs as well, undermining the anti-tumor responses of the transfused cells. Tumor-derived gangliosides and cytokines such as high mobility groupbox-1 (HMGB1) exemplify components that inhibit the function of DCs or induce apoptosis of the cells [30].
Delivery: tumor targeting and recognition
For effective cell-based therapies, tumor-targeting and recognizing ability of therapeutic cells is another important aspect. Therapeutic cells should accumulate and remain in tumor sites for a certain period of time—long enough for the cells to exert their functions. Also, precise localization within tumor sites is necessary for reduced off-target responses and optimal clinical outcomes. Although some therapeutic cells are capable of active recruitment or circulating until they encounter cancer cells, others have limited ability to migrate towards tumor cells. For example, monocytes and neutrophils are easily recruited toward tumor sites since they are circulating leukocytes and are known to be highly infiltrative [31, 32]. Specifically, monocytes are specialized for circulating and detecting environment changes and differentiating into DCs and macrophages in target areas. Such cells can be somewhat limited in terms of efficient recruitment so that engineering them to have improved migrating ability was adopted. Li et al. have introduced macrophages decorated with hyaluronic acid-coated superparamagnetic iron oxide nanoparticles which outperform normal macrophages in terms of targeting efficacy and intratumoral retention [33]. This work has demonstrated that increasing the delivery efficiency can improve adoptive cell therapy by showing that the engineered macrophages are more effective in eliminating tumors compared to normal macrophages.
Along with precise and efficient localization of therapeutic cells in target areas, how well the cells recognize their target cells is another important aspect for effective adoptive therapy. NK cells are potential effector cells for cancer therapy since they can be safer than T cells in terms of side effects but they lack cell-specific receptors [34]. Attempts to increase the targeting ability have been performed by either strengthening the activating receptors or enhancing the affinity [35]. It has been demonstrated that empowering the targeting ability improves clinical outcomes. For example, Liu et al. engineered NK cells with CARs to enhance the engagement with target cells and showed improved homing ability to a tumor and tumoricidal ability as a result compared with NK cells without CARs [36]. For these reasons, an increasing number of studies underscore the importance of effective targeting and recognition of tumor cells by therapeutic cells and develop methods to improve this.
Cell: proliferation and expansion
Expansion and proliferation of therapeutic effector cells have been considered essential for therapies leveraged by T cell immunity [37]. In the case of T cell-mediated adoptive cell therapies, it is important to generate a sufficient amount of T cells ex vivo for significant clinical activity of therapeutic T cells [38]. Moreover, ex vivo pre-conditioning for expansion and proliferation is suggested to correlate with the persistence of CAR-T cells and consequently inversely with clinical remissions with cancers [39]. However, expansion of T cells before infusion can be problematic for a limited number of harvested TILs or naïve T cells [40]. Next, in the case of APC-mediated adoptive cell therapies, its role in inducing T cell activation, proliferation, and expansion is considered crucial for significant clinical outcomes [41]. However, T cell activation, proliferation, or function is often inhibited by inhibitory cells or receptors, such as Treg cells, CTLA4, and PD-1 [37]. Specifically, CTLA4 restrains T cell activation via disturbing T cell-APC immune conjugation, and PD-1 binds with PD-L1 on APCs to induce T cell exhaustion. This may indicate that the efficacy of adoptive therapies utilizing APCs, such as DCs and macrophages, is also highly dependent on how efficiently T cells can be activated and proliferated.
Cell: innate functionality
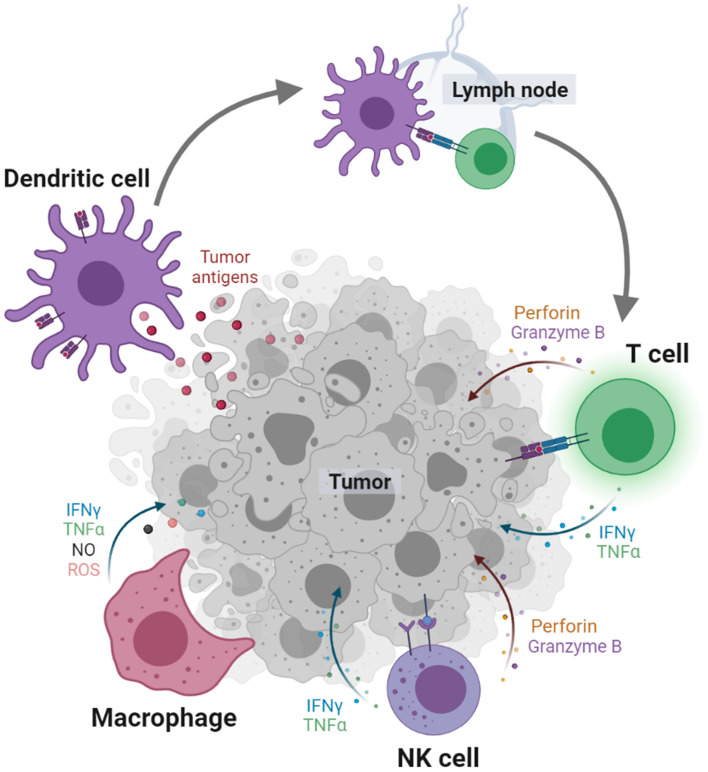
Functions of transfused cells must be maintained or enhanced within target areas for effective anti-tumor immunity. This is important since adoptive immunotherapy is solely dependent on the innate functions of transfused immune cells for tumoricidal responses as presented in Fig. 2. T cells and NK cells are responsible for cytotoxic responses against tumor cells [14]. T cells and NK cells are similar in terms of cytotoxic responses but they are different in terms of activation and recognition mechanism; T cells are activated and primed by DCs in lymph nodes and recognize tumor cells with antigens while NK cells are activated based on the balance between activating and inhibitory signals and recognizing “missing self” [34]. DCs are especially important for adaptive immunity in terms of activating and priming the effector cells [21, 30]. They uptake and process tumor-associated antigens to present them as MHC-peptide complexes. Then these antigen-presenting DCs migrate to lymph nodes for T cell education. Macrophages are for killing tumor cells both directly and indirectly [22]. Especially, the pro-inflammatory macrophages are capable of engulfing the tumor cells and secreting tumoricidal cytokines and chemokines such as nitric oxide (NO), reactive oxygen species (ROS), TNF-α, and IFN-γ. These major roles of the therapeutic cells can be utilized for the remission of tumors but may be minute for significant effect. For this reason, several approaches have demonstrated that enhancing the functionalities of cells can improve therapeutic efficacy [42, 43].
Fig. 2.
Major roles of immune cells in anti-tumor immunity. Dendritic cells uptake, process, and present tumor antigens as MHC-peptide complexes to educate T cells in lymph nodes. Activated T cells and NK cells kill tumor cells by secreting perforin, granzyme B, IFN-γ, and TNF-α. Macrophages engulf tumor cells or kill them with IFN-γ, TNF-α, nitric oxide (NO), and reactive oxygen species (ROS). Created with BioRender.com
Nanoengineering for enhanced functionalities of transferred cells
T cell
T cell engineering for improved cancer immunotherapy has been focused on improving viability, ability to expand and proliferate, and tumor targeting (Table 1). In 2010, Stephan et al. developed synthetic nanoparticle-conjugated T cells for improved viability and proliferation as well as function [44]. Nanoparticles were loaded with IL-15Sa and IL-21, and conjugated to the surfaces of T cells via cell surface thiols for engineering. It was demonstrated that the engineered T cells proliferated robustly in vivo, and eradicated B16 melanomas effectively. Another work by Stephan et al., proposed in 2012, conjugated maleimide-functionalized nanoparticles to the cell surface in attempts to promote a greater T-cell expansion in tumor sites [45]. It demonstrated that the Shp1 and Shp2 inhibitors, encapsulated in the conjugated nanoparticles, modulated the activation and fate of the engineered T cells for more effective cancer immunotherapy.
Table 1.
Overview of different strategies utilized for engineering immune cells with nanomaterials to improve adoptive cell therapy
| Purpose | Cell type | Engineering | Expected outcome | |
|---|---|---|---|---|
| Resistance to the immunosuppressive TME | T cell | ShpI-loaded liposome conjugated on the cell surface | Blocked tumor-induced suppression in the synapse for expansion and inhibited TRP-SIY prostate tumor | [45] |
| T cell | PD-1 antibody-armed magnetic nanocluster on the cell surface | Efficiently blocked PD-1 and inhibited 4T1 carcinomas | [46] | |
| T cell | SCH-58261 encapsulating multilamellar liposome conjugated on the cell surface | Rescued hypofunctional cells in SKOV3.CD19 tumors and reduced the tumors | [49] | |
| DC | Intracellular loaded quantum dot-CpG complex | Enhanced DC activation and stimulation and reduction of EG7-OVA tumor | [57] | |
| Macrophage | IFN-γ encapsulating polymer patch on the cell surface | Pro-inflammatory phenotype maintenance and reduction of 4T1 carcinomas | [61] | |
| Macrophage | Intracellular loaded hyaluronic acid-decorated superparamagnetic iron oxide nanoparticles (HIONs) | Repolarized TAMs and reduction of 4T1 carcinomas | [33] | |
| Tumor targeting/recognition ability | T cell | PD-1 antibody-armed magnetic nanocluster on the cell surface | Enhanced T cell recruitment in tumors and inhibited 4T1 carcinomas | [46] |
| T cell | Aptamer conjugated on the cell surface | Target and bind to SFC-7901 gastric cancer cell and CT26 colon carcinoma cell for tumor regression | [48] | |
| T cell | Magnetic nanoparticle on the cell surface | Enhanced T cell homing to lymph nodes | [47] | |
| NK cell | Aptamer conjugated on the cell surface | Target and bind to lymphoma cells for tumor regression | [52] | |
| NK cell | Cationic nanoparticle on the cell surface | Recognize MDA-MB-231 cells for tumor regression | [53] | |
| NK cell | Antibody conjugated on the cell surface | Target and bind to BT474, SKBR3, and MDA-MB-435/ HER2 + by Herceptin-labeled NK-92MI | [54] | |
| NK cell | Fe3O4/SiO2/shell nanoparticles conjugated on the cell surface | Infiltrated into RPMI8226 human B cell lymphoma for tumor regression | [63] | |
| NK cell | Intracellular loaded Fe3O4@polydopamine nanoparticle | Infiltrated into A549 adenocarcinoma for tumor regression | [56] | |
| Macrophage | Intracellular loaded hyaluronic acid-decorated superparamagnetic iron oxide nanoparticles (HIONs) | Infiltrated into 4T1 carcinoma for tumor regression | [33] | |
| Proliferation and expansion | T cell | IL-15Sa and IL-21 encapsulating liposome conjugated on the cell surface | Robustly proliferated in vivo and eradicated established B16 melanomas | [44] |
| T cell | ShpI-loaded liposome conjugated on the cell surface | Blocked tumor-induced suppression in the synapse for expansion and inhibited TRP-SIY prostate tumor | [45] | |
| T cell | IL-14Sa nanogel on the cell surface | Expanded 16-fold more and inhibited B16F10 melanoma | [50] | |
| T cell | IL-2/Fc nanogel on the cell surface | Expanded 80-fold more and inhibited B16F10 melanoma | [51] | |
| Enhanced innate functionality | T cell | Aptamer conjugated on the cell surface | Enhanced perforin, granzyme B, CD107a, CD69, and FasL expression and inhibited SGC-7901 gastric cancer and CT26 colon carcinoma | [48] |
| T cell | IL-2/Fc nanogel on the cell surface | Induced increased CD8 + memory precursor differentiation | [51] | |
| DC | Intracellular loaded immune complex mimicking polymer nanoparticle | Enhanced cross-presentation and inhibited EG7-OVA tumor growth | [58] | |
| DC | Intracellular loaded glycan-modified extracellular vesicles (EVs) | Enhanced cross-presentation and inhibited U87 and GBM8 glioblastoma | [59] | |
| DC | Intracellular loaded gp100 peptide-decorated liposomes | Induce enhanced CTL responses and inhibited B16F10 melanoma | [60] | |
| Macrophage | Intracellular loaded polyhydroxylated fullerenols | Enhanced phagocytosis and cytokine secretion and inhibited B16 tumor metastasis | [62] |
Enhancing the tumor-targeting ability of adoptively transferred T cells has been another area of interest for developing T cell-based tumor immunotherapies (Table 1). In 2019, Nie et al. utilized PD-1 antibodies-armed magnetic nanoclusters to decorate CTLs or TILs for a more effective adoptive T cell therapy [46]. Conjugation of magnetic nanoclusters enabled improved recruitment towards tumor sites upon magnetization and superparamagnetism with MRI guidance. Additional to overcoming the limitation of tumor targeting, the spatiotemporal effect of the adoptive T cells and PD-1 antibodies inhibited tumor growth by alleviating the suppressiveness; thus, enhancing the T cell infiltration. Engineering T cells for adoptive transfer with magnetic nanoparticles was performed by Sanz-Ortega et al. as well for enhanced tumor-targeting ability and retention in the region of interest [47]. Magnetic nanoparticle-loaded T cells were specifically targeted by an external magnetic field so that T cells accumulate in the lymph nodes. The study potentiated the possible modulation of immune responses in any regions of interest by targeting via an external magnetic field. On the other hand, in 2020, Liu et al. have introduced the method that utilizes aptamer for targeting and binding to tumor cells [48]. The aptamer-T cells induced significant regression of tumor because the enhanced targeting ability allowed the transferred cells to become enriched in the TME. Moreover, it was demonstrated that the aptamer-conjugated T cells have a strong anti-tumor effect with enhanced perforin, granzyme B, CD107a, and FasL expression.
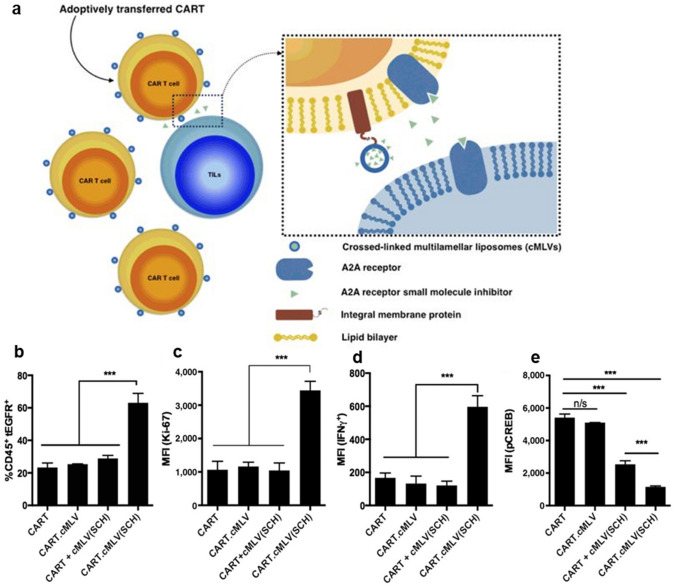
CAR-T cell therapy is an improved platform of adoptive T cell therapy which is endowed with enhanced ability to recognize certain tumor antigens (Table 1). However, the suppressive TME easily causes hypofunction of CAR-T cells, and studies are being conducted to develop CAR-T cells with enhanced abilities to proliferate, expand, and kill tumor cells. To this end, in 2018, Siriwon et al. engineered the cell surface by conjugating nanoparticles, in which A2aR-specific small molecule antagonist SCH-58261 is encapsulated as shown in Fig. 3a [49]. The SCH-loaded multilamellar liposomal vesicles increased proliferation (Fig. 3b and c) and prevented CAR-T cell hypofunction (Fig. 3d and e). Consequently, adoptive therapy of engineered CAR-T cells suppressed tumor growth when compared to conventional CAR-T cells. Moreover, for increased efficiency of expansion and specific tumor-killing ability, Tang et al. employed protein nanogels as backpacks for ex vivo engineered CAR-T cells [50]. For engineering, synthesized IL-14Sa-containing nanogels were conjugated via CD45. It was demonstrated that the method allowed a greater expansion of the transferred T cells and secretion of granzyme and effector cytokines. A similar application with nanogels was adopted by Xie et al. for IL-2 backpacking [51]. To adjuvant anti-tumor adoptive T-cell immunotherapy, IL-2/Fc nanogels were synthesized and backpacked to the cell surface of adoptively transferred T cells. By doing so, transferred tumor-reactive T cells expanded at a greater rate than those without backpacks. Also, it was demonstrated that the sustained release of IL-2/Fc promoted memory precursor differentiation and induced less T cell exhaustion.
Fig. 3.
Engineering T cells with liposomes to inhibit T cell hypofunction. a Schematic diagram of A2AR small-molecule inhibitors-loaded cMLV conjugated on the surface of CAR-T cells. Enhanced proliferation represented by b the percentage of CART.tEGFR cells and c the expression level of Ki-67 in CART.tEGFR. Ameliorated hypofunction by d the expression level of IFNγ and e the expression level of CREB in CART.tEGFR. Mean ± SD (n/s: not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
Reprinted from Cancer Immunol Res, 6(7), Siriwon N, Kim YJ, Siegler E, Chen XH, Rohrs JA, Liu YR, Wang P, CAR-T Cells Surface-Engineered with Drug-Encapsulated Nanoparticles Can Ameliorate Intratumoral T-cell Hypofunction, 812–24 [49]
NK cell
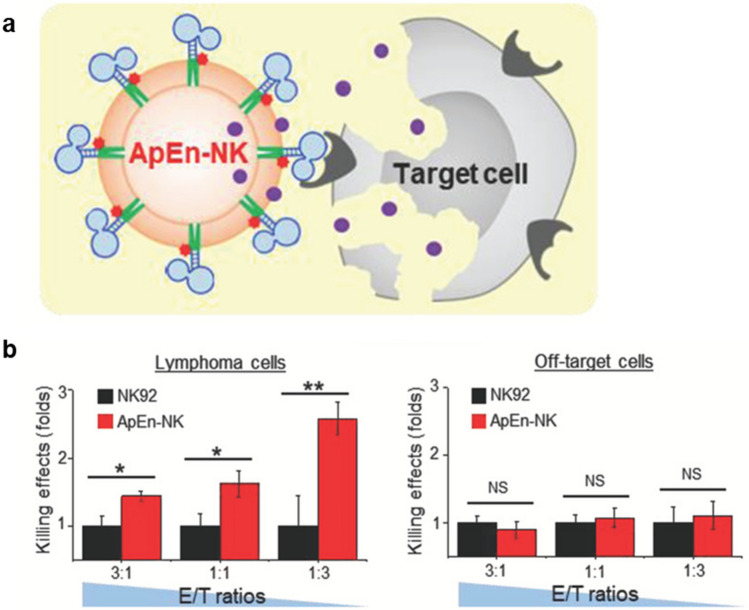
For more effective NK cell-based therapies, the focus of engineering has been on improving tumor-targeting ability (Table 1). Since NK cells lack the innate ability to target tumor cells specifically, it is important to endow the ability to localize in the region of interest and interact with target cells. Yang et al. engineered NK cells with aptamers as shown in Fig. 4a so that the NK cells can bind to the aptamer-specific tumor cells [52]. The resulting specificity allowed NK cells to trigger higher apoptosis of targeted tumor cells than those without aptamer engineering (Fig. 4b). For the same purpose, Kim et al. [53] and Li et al. [54] developed cationic nanoparticle-loaded NK cells and antibody-decorated NK cells, respectively. The cationic nanoparticles activated NK cells for enhanced recognition ability of tumor cells so that the cytotoxicity of NK cells can eliminate tumor cells more efficiently. Similarly, antibody-decorated NK cells allowed specific targeting of tumor cells, and the efficacy of lysing antibody-specific tumor cells was increased.
Fig. 4.
Engineering NK cells with aptamers to endow tumor-targeting ability. a Schematic diagram of ApEn-NK effects on target cells. b Specific killing effect on lymphoma cell lines and no significant killing effect on off-target cells. Mean ± SD (NS: not significant, *P < 0.05, **P < 0.01).
Reprinted from Small, 15(22), Yang SH, Wen JG, Li H, Xu L, Liu YT, Zhao NX, Zeng ZH, Qi JJ, Jiang WQ, Han W, Zu YL, Aptamer-Engineered Natural Killer Cells for Cell-Specific Adaptive Immunotherapy [52]
Targeting tumors can be done not only by providing a means to bind but also by utilizing magnetic particles and external magnetic fields (Table 1). Jang et al. conjugated Fe3O4/SiO2/shell nanoparticles so that limited infiltration into target tumor tissues can be ameliorated [55]. By doing so, NK cells are capable of being enriched in the region of interest and consequently exert cytotoxic responses against tumor cells with less off-target activities. Wu et al. pointed out that a higher infiltration rate of NK cells can be clinically beneficial and thus developed Fe3O4@polydopamine nanoparticle-loaded NK cells [56]. It was demonstrated that the NK cells were able to accumulate in the tumor site with an external magnetic field and inhibited tumor growth as a result.
DC
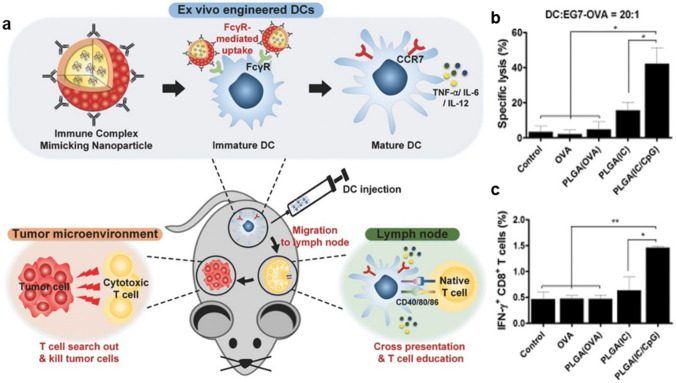
Engineering DCs for enhanced anti-tumor immunotherapy is focused on improving immunomodulatory effect, migration, and innate functionality as APCs (Table 1). In order for DC vaccines to modulate the immunosuppressive TME which impairs the anti-tumor efficacy, Liu et al. employed quantum dots to pulse the DC vaccines [57]. To this end, the quantum dots were engineered as fluorescence nanoprobes, immunomodulatory adjuvants, and nanocarriers of tumor antigens and Toll-like receptor 9 agonists and internalized into DCs. The engineered DC vaccines alleviated the immunosuppressive TME and promoted improved antigen presentation and T cell activation. Enhancing the anti-tumoral efficacy can also be achieved by improving the migration or targeting ability of DCs. Additional to the quantum dot-pulsed DC vaccine, chloroquine was administrated in combination to repolarize TAMs. Thus, the combination therapy synergistically amplified the efficacy of cancer immunotherapy. Kim et al. developed synthetic vaccine nanoparticles containing antigen for uptake, and adjuvant for immunostimulation to improve migration and cross-presentation at the same time as in Fig. 5a [58]. As a result, tumor regression and prolonged survival were observed owing to enhanced cytokine secretion, lymph node homing, and cross-presentation. Specifically, significant enhancements were observed in CTL activity (Fig. 5b) and proliferation of antigen-specific CD8+ T cells (Fig. 5c). Dusoswa et al. engineered DCs for improved cross-presentation with glycan-modified extracellular vesicles (EVs) [59]. Glycoengineering of EVs was performed so that DCs can uptake sources of antigens followed by antigen presentation more efficiently. Introducing antigens can also be performed via nanoparticles for more efficient ex vivo antigen pulsing [60]. Yazdani et al. synthesized peptide-decorated liposomes to pulse DC vaccine and demonstrated enhanced anti-tumor immunity based on the infiltration of antigen-specific CD4+ and CD8+ T cells. The engineered DC vaccine was shown to be more effective in inducing specific CTL responses. This approach also demonstrated the enhanced efficacy of cell-based intervention and PD-1 antibody combination therapy.
Fig. 5.
Engineering DCs with polymer nanoparticles to enhance cross-presentation. a Schematic diagram of ex vivo engineered DCs for efficient anti-tumor immunity. b CTL activity represented by the mean percentage of tumor cell lysis. c Proliferation of antigen-specific CD8 + T cells. Mean ± SD (*P < 0.05, **P < 0.01).
Reprinted from Adv Funct Mater, 26(44), Kim SY, Phuengkham H, Noh YW, Lee HG, Um SH, Lim YT, Immune Complexes Mimicking Synthetic Vaccine Nanoparticles for Enhanced Migration and Cross-Presentation of Dendritic Cells, 8072–82 [58]
Macrophage
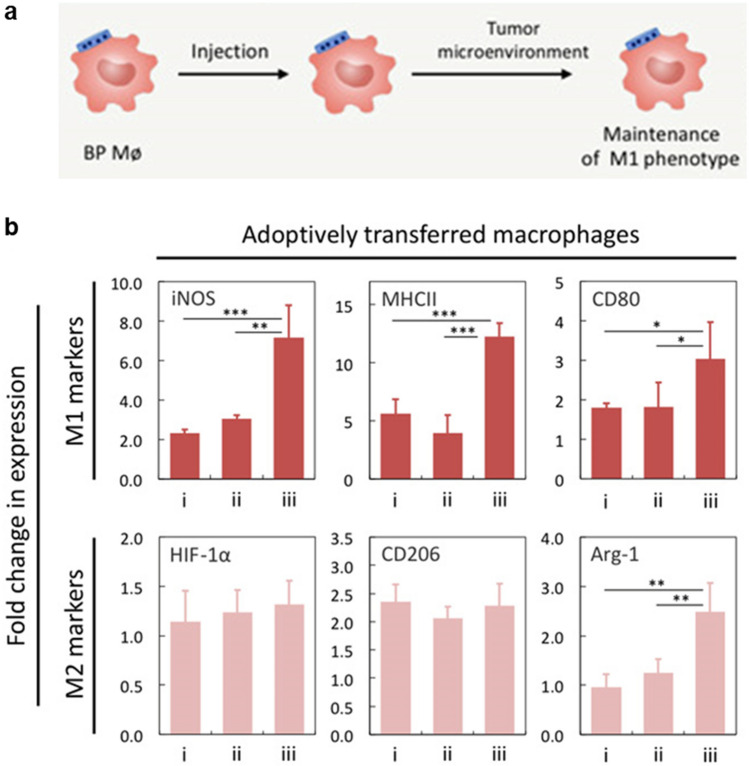
Macrophage engineering has been performed to overcome the limitations of its plasticity within the immunosuppressive TME by maintaining the anti-tumoral phenotype for a prolonged time period (Table 1). Shields et al. developed cellular backpacks which adhere to the surface of macrophages and aid phenotype regulation in vivo [61]. The backpacks were synthesized with microcontact printed biodegradable polymers, in which interferon-γ (IFN-γ) was encapsulated as in Fig. 6a. The particle-based engineering allowed adoptively transferred pro-inflammatory macrophages to maintain their phenotypes in the immunosuppressive milieu of solid tumors (Fig. 6b). Hence, with the backpack-loaded macrophages, tumor growth suppression and overall survival improvement were demonstrated.
Fig. 6.
Engineering macrophages with polymer backpack for phenotype maintenance in the TME. a Schematic diagram of macrophage and polymer patch loaded with IFN-γ. b Highly expressed M1 markers on adoptively transferred macrophages bound to backpacks. BMDMs were polarized ex vivo with IFN-γ (i), unpolarized and injected with free IFN-γ (ii) or unpolarized, bound to IFN-γ backpacks and injected (iii). Mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001).
Reprinted from Sci Adv, 6(18), Shields CWt, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, Zhao Z, Pusuluri A, Ukidve A, Pan DC, Mitragotri S, Cellular backpacks for macrophage immunotherapy [61]
Alongside the vulnerability to the immunosuppressive TME, another limitation suggested for the adoptive transfer of macrophages is that macrophages have limited tumor-targeting ability (Table 1). To provide macrophages with abilities to target tumors and to generate tumoricidal components more efficiently, Li et al. have engineered patient-derived macrophages with hyaluronic acid-decorated superparamagnetic iron oxide nanoparticles (HIONs) [33]. HION-loaded macrophages exhibited tumor-specific cytostatic effect and became more resistant to the immunosuppressive TME. Interestingly, adoptively transferred engineered macrophages repolarized in situ anti-inflammatory macrophages into pro-inflammatory phenotypes in a paracrine-like manner. Thus, loading HION enabled macrophages to reprogram the TME and suppress tumor growth with the enhanced functionalities. Similarly, Tang et al. regulated macrophages via particle-based engineering with polyhydroxylated fullerenols for more effective adoptive immunotherapy [62]. Fullerenols enhanced mitochondrial metabolism, phagocytosis, and cytokine secretion of engineered macrophages so that the activated macrophages inhibit tumor growth and suppress metastasis. It was clearly demonstrated that loading nanoparticles for adoptive macrophage therapy improved the functionality and consequently enhanced the efficacy of cancer immunotherapy.
Concluding remarks
An increasing number of studies on cell-based cancer immunotherapy are focusing on adopting nanomedicine-based interventions to overcome limitations imposed by the environment and nature of the therapeutic cells. Gene-based engineering is also performed for this purpose mainly by editing genetic information of the cells permanently to produce therapeutic agents, such as anti-tumoral cytokines [64]. It also faces safety issues where the location of gene insertion and consequent mutagenesis cannot be controlled easily [65]. On the other hand, nanoparticle-based engineering is considered advantageous over genetic engineering of cells in terms of its specificity and simplicity in engineering processes. It includes conjugation of nanoparticles onto the surface of cells and intracellular-loading of nanoparticles to enhance the efficacy of cell-based therapies. To this end, the engineered therapeutic cells—T cells, NK cells, DCs, and macrophages—are expected to either maintain anti-tumoral phenotypes, target tumor efficiently, or improve the innate functionalities and viability. Some recent works have employed multi-functional nanoparticles for simultaneous and synergistic improvement of clinical outcomes where nanoparticles can influence the therapeutic cells as well as their surroundings [33, 46, 48, 51] and some have introduced particle-based engineered therapeutic cells in combinations with conventional anti-tumor therapies such as anti-PD-1 therapy [57, 60]. It is believed that growing interests in cell-based cancer immunotherapy and attempts to combine effective therapies for synergy will allow the development of more robust nanoengineered cell-based therapies.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation (NRF-2017R1E1A1A01074847) funded by the Ministry of Science and ICT, Republic of Korea.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17-A new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135(5):1178–86. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- 4.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immun. 2010;59(10):1593–600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Niu GL, Kortylewski M, Burdelya L, Shain K, Zhang SM, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10(2):209–209. doi: 10.1038/nm0204-209b. [DOI] [PubMed] [Google Scholar]
- 6.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Can Res. 2001;61(12):4756–60. [PubMed] [Google Scholar]
- 7.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9(5):619–24. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Muul LM, Solomon D, Rosenberg SA. Expansion of human-tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987;102(1):127–41. doi: 10.1016/S0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma—a preliminary-report. New Engl J Med. 1988;319(25):1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 10.Rohaan MW, Wilgenhof S, Haanen JBAG. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474(4):449–61. doi: 10.1007/s00428-018-2484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WL, Liu L, Su HF, Liu Q, Shen J, Dai HR, Zheng W, Lu Y, Zhang WJ, Bei YC, Shen PP. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Brit J Cancer. 2019;121(10):837–45. doi: 10.1038/s41416-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreesen R, Scheibenbogen C, Brugger W, Krause S, Meerpohl HG, Leser HG, Engler H, Lohr GW. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res. 1990;50(23):7450–6. [PubMed] [Google Scholar]
- 13.Wang WX, Jiang JT, Wu CP. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett. 2020;472:175–80. doi: 10.1016/j.canlet.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri L, Mancusi A, Capanni M, Martelli MF, Velardi A. Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Curr Opin Immunol. 2005;17(2):211–7. doi: 10.1016/j.coi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karschnia P, Jordan JT, Forst DA, Arrillaga-Romany IC, Batchelor TT, Baehring JM, Clement NF, Gonzalez Castro LN, Herlopian A, Maus MV, Schwaiblmair MH, Soumerai JD, Takvorian RW, Hochberg EP, Barnes JA, Abramson JS, Frigault MJ, Dietrich J. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–21. doi: 10.1182/blood-2018-12-893396. [DOI] [PubMed] [Google Scholar]
- 19.Hirayama AV, Turtle CJ. Toxicities of CD19 CAR-T cell immunotherapy. Am J Hematol. 2019;94(S1):42–9. doi: 10.1002/ajh.25445. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects. Cancer Lett. 2020;472:175–80. doi: 10.1016/j.canlet.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Shurin MR. Dendritic cells presenting tumor antigen. Cancer Immunol Immunother. 1996;43(3):158–64. doi: 10.1007/s002620050317. [DOI] [PubMed] [Google Scholar]
- 22.Belgiovine C, Digifico E, Anfray C, Ummarino A, Torres Andón F. Targeting tumor-associated macrophages in anti-cancer therapies: convincing the traitors to do the right thing. J Clin Med. 2020;9(10):3226. doi: 10.3390/jcm9103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han X, Wilbanks GD, Devaja O, Ruperelia V, Raju KS. IL-2 enhances standard IFNgamma/LPS activation of macrophage cytotoxicity to human ovarian carcinoma in vitro: a potential for adoptive cellular immunotherapy. Gynecol Oncol. 1999;75(2):198–210. doi: 10.1006/gyno.1999.5557. [DOI] [PubMed] [Google Scholar]
- 24.Andreesen R, Scheibenbogen C, Brugger W, Krause S, Leser HG, Kopf S, Engler H, Schumichen C, Lohr GW. A New Approach to Adoptive Immunotherapy of Cancer Using Tumorcytotoxic Macrophages Grown from Peripheral-Blood Monocytes. Cancer Detect Prev. 1991;15(5):413–21. [PubMed] [Google Scholar]
- 25.Andreesen R, Hennemann B, Krause SW. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukocyte Biol. 1998;64(4):419–26. doi: 10.1002/jlb.64.4.419. [DOI] [PubMed] [Google Scholar]
- 26.Pai SI, Cesano A, Marincola FM. The paradox of cancer immune exclusion: immune oncology next frontier. Cancer Treat Res. 2020;180:173–95. doi: 10.1007/978-3-030-38862-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanitis E, Dangaj D, Irving M, Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol. 2017;28:18–32. doi: 10.1093/annonc/mdx238. [DOI] [PubMed] [Google Scholar]
- 28.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Dhar P, Wu JD. NK cell plasticity in cancer. J Clin Med. 2019;8(9):1492. doi: 10.3390/jcm8091492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4(1):36–44. doi: 10.7150/jca.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018;371(3):551–65. doi: 10.1007/s00441-017-2753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li CX, Zhang Y, Dong X, Zhang L, Liu MD, Li B, Zhang MK, Feng J, Zhang XZ. Artificially Reprogrammed macrophages as tumor-tropic immunosuppression-resistant biologics to realize therapeutics production and immune activation. Adv Mater. 2019;31(15):1807211. doi: 10.1002/adma.201807211. [DOI] [PubMed] [Google Scholar]
- 34.Woan KV, Miller JS. Harnessing natural killer cell antitumor immunity: from the bench to bedside. Cancer Immunol Res. 2019;7(11):1742–7. doi: 10.1158/2326-6066.CIR-19-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med. 2019;7(5):105. doi: 10.21037/atm.2019.01.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, Orange J, Wan X, Lu X, Reynolds A, Gagea M, Banerjee P, Cai R, Bdaiwi MH, Basar R, Muftuoglu M, Li L, Marin D, Wierda W, Keating M, Champlin R, Shpall E, Rezvani K. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–31. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, Behera M, Wu H, McCausland M, Chen Z, Zhang C, Khuri FR, Owonikoko TK, Ahmed R, Ramalingam SS. Proliferation of PD-1 + CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114(19):4993–8. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jafarzadeh L, Masoumi E, Fallah-Mehrjardi K, Mirzaei HR, Hadjati J. Prolonged persistence of chimeric antigen receptor (CAR) T cell in adoptive cancer immunotherapy: challenges and ways forward. Front Immunol. 2020;11:702. doi: 10.3389/fimmu.2020.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–67. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saibil SD, Ohashi PS. Targeting T cell activation in immuno-oncology. Curr Oncol. 2020;27(Suppl 2):98–105. doi: 10.3747/co.27.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn ZS, Mac J, Wang P. T cell immunotherapy enhanced by designer biomaterials. Biomaterials. 2019;217:119265. doi: 10.1016/j.biomaterials.2019.119265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM. Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 2018;39(2):135–50. doi: 10.1016/j.it.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035-U135. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephan MT, Stephan SB, Bak P, Chen JZ, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33(23):5776–87. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nie WD, Wei W, Zuo LP, Lv CL, Zhang F, Lu GH, Li F, Wu GH, Huang LL, Xi XB, Xie HY. Magnetic nanoclusters armed with responsive PD-1 antibody synergistically improved adoptive T-cell therapy for solid tumors. ACS Nano. 2019;13(2):1469–78. doi: 10.1021/acsnano.8b07141. [DOI] [PubMed] [Google Scholar]
- 47.Sanz-Ortega L, Rojas JM, Marcos A, Portilla Y, Stein JV, Barber DF. T cells loaded with magnetic nanoparticles are retained in peripheral lymph nodes by the application of a magnetic field. J Nanobiotechnol. 2019;17:1–20. doi: 10.1186/s12951-018-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CG, Wang Y, Liu P, Yao QL, Zhou YY, Li CF, Zhao Q, Liu GH, Zhang XL. Aptamer-T cell targeted therapy for tumor treatment using sugar metabolism and click chemistry. Acs Chem Biol. 2020;15(6):1554–65. doi: 10.1021/acschembio.0c00164. [DOI] [PubMed] [Google Scholar]
- 49.Siriwon N, Kim YJ, Siegler E, Chen XH, Rohrs JA, Liu YR, Wang P. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol Res. 2018;6(7):812–24. doi: 10.1158/2326-6066.CIR-17-0502. [DOI] [PubMed] [Google Scholar]
- 50.Tang L, Zheng YR, Melo MB, Mabardi L, Castano AP, Xie YQ, Li N, Kudchodkar SB, Wong HC, Jeng EK, Maus MV, Irvine DJ. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat Biotechnol. 2018;36(8):707-+. doi: 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie YQ, Arik H, Wei LX, Zheng YR, Suh H, Irvine DJ, Tang L. Redox-responsive interleukin-2 nanogel specifically and safely promotes the proliferation and memory precursor differentiation of tumor-reactive T-cells. Biomater Sci-Uk. 2019;7(4):1345–57. doi: 10.1039/C8BM01556B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang SH, Wen JG, Li H, Xu L, Liu YT, Zhao NX, Zeng ZH, Qi JJ, Jiang WQ, Han W, Zu YL. Aptamer-engineerednatural killer cells for cell-specific adaptive immunotherapy. Small. 2019;15(22):1900903. doi: 10.1002/smll.201900903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KS, Han JH, Choi SH, Jung HY, Park JD, An HJ, Kim SE, Kim DH, Doh J, Han DK, Kim IH, Park W, Park KS. Cationic nanoparticle-mediated activation of natural killer cells for effective cancer immunotherapy. ACS Appl Mater Inter. 2020;12(51):56731–40. doi: 10.1021/acsami.0c16357. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Chen MK, Liu ZL, Zhang LD, Felding BH, Moremen KW, Lauvau G, Abadier M, Ley K, Wu P. A single-step chemoenzymatic reaction for the construction of antibody-cell conjugates. Acs Central Sci. 2018;4(12):1633–41. doi: 10.1021/acscentsci.8b00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Im S, Jang D, Saravanakumar G, Lee JS, Kang Y, Lee YM, Lee JY, Doh J, Yang ZY, Jang MH, Kim WJ. Harnessingthe formation of natural killer-tumor cell immunological synapses for enhancedtherapeutic effect in solid tumors. Adv Mater. 2020;32(22):2000020. doi: 10.1002/adma.202000020. [DOI] [PubMed] [Google Scholar]
- 56.Wu LY, Zhang FQ, Wei ZH, Li XY, Zhao H, Lv HY, Ge R, Ma H, Zhang H, Yang B, Li J, Jiang JL. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater Sci-Uk. 2018;6(10):2714–25. doi: 10.1039/C8BM00588E. [DOI] [PubMed] [Google Scholar]
- 57.Liu F, Sun JL, Yu WQ, Jiang QY, Pan M, Xu Z, Mo FY, Liu XQ. Quantum dot-pulsed dendritic cell vaccines plus macrophage polarization for amplified cancer immunotherapy. Biomaterials. 2020;242:119928. doi: 10.1016/j.biomaterials.2020.119928. [DOI] [PubMed] [Google Scholar]
- 58.Kim SY, Phuengkham H, Noh YW, Lee HG, Um SH, Lim YT. Immune complexes mimicking synthetic vaccine nanoparticles for enhanced migration and cross-presentation of dendritic cells. Adv Funct Mater. 2016;26(44):8072–82. doi: 10.1002/adfm.201603651. [DOI] [Google Scholar]
- 59.Dusoswa SA, Horrevorts SK, Ambrosini M, Kalay H, Paauw NJ, Nieuwland R, Pegtel MD, Wurdinger T, Van Kooyk Y, Garcia-Vallejo JJ. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J Extracell Vesicles. 2019;8(1):1648995. doi: 10.1080/20013078.2019.1648995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yazdani M, Gholizadeh Z, Nikpoor AR, Hatamipour M, Alani B, Nikzad H, Roshan NM, Verdi J, Jaafari MR, Noureddini M, Badiee A. Vaccination with dendritic cells pulsed ex vivo with gp100 peptide-decorated liposomes enhances the efficacy of anti PD-1 therapy in a mouse model of melanoma. Vaccine. 2020;38(35):5665–77. doi: 10.1016/j.vaccine.2020.06.055. [DOI] [PubMed] [Google Scholar]
- 61.Shields CWt, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, Zhao Z, Pusuluri A, Ukidve A, Pan DC, Mitragotri S. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020;6(18):eaaz6579. doi: 10.1126/sciadv.aaz6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang JL, Chen ZY, Sun BY, Dong JQ, Liu J, Zhou HG, Wang LM, Bai R, Miao Q, Zhao YL, Chen CY, Liu Y. Polyhydroxylated fullerenols regulate macrophage for cancer adoptive immunotherapy and greatly inhibit the tumor metastasis. Nanomed-Nanotechnol. 2016;12(4):945–54. doi: 10.1016/j.nano.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 63.Jang ES, Shin JH, Ren G, Park MJ, Cheng K, Chen XY, Wu JC, Sunwoo JB, Cheng Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials. 2012;33(22):5584–92. doi: 10.1016/j.biomaterials.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Q, Cheng H, Peng HS, Zhou H, Li PY, Langer R. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv Drug Deliver Rev. 2015;91:125–40. doi: 10.1016/j.addr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]